Abstract
Late-onset forms of GM2 gangliosidosis―mainly, Tay-Sachs disease and Sandhoff disease―are under-recognized in clinical practice. In these rare lysosomal storage disorders, deficiency of β-hexosaminidase A results in excessive accumulation of GM2 ganglioside primarily within neurons, leading to cell death and progressive neurodegenerative symptoms, including ataxia, dysarthria, muscle weakness, tremors, atrophy, and psychosis. Presentation is variable and often mimics more common neurodegenerative disorders. We conducted semi-structured interviews on GM2 gangliosidoses diagnosis and treatment with five experts, 30 neurologists, and 28 patients and caregivers. Symptom onset occurred during adolescence/early adulthood in 92% of patients (median age: 14 years). Patients first visited a healthcare provider at a median age of 20 years and received a GM2 diagnosis at a median age of 26 years. Nearly all patients reported problems with their legs and balance starting from symptom onset. Problems with memory, attention span, speech and fatigue were reported more after diagnosis. Patients visited an average of eight healthcare providers before receiving a diagnosis; 64% were diagnosed by a neurologist. Four neurologists (13%) in our sample were aware that there are late-onset forms of GM2 gangliosidosis. The path to diagnosis is long for this late-onset form of a classically fatal infantile disease.
Keywords: Late-onset GM2 gangliosidoses, Tay-Sachs disease, Sandhoff disease, β-hexosaminidase A, Diagnosis, Patient perspective
1. Introduction
The GM2 gangliosidoses are a group of rare, autosomal-recessive, lysosomal storage disorders that includes Tay-Sachs disease and Sandhoff disease. These diseases are caused by pathogenic variants in the genes encoding for β-hexosaminidase A (HEXA [α-β dimer]and HEXB [β-β dimer]), the enzyme responsible for lysosomal degradation of the glycosphingolipid GM2 ganglioside. The α subunit is encoded by the HEXA gene, whereas the β subunit is encoded by the HEXB gene. Tay-Sachs disease results from a deficiency of β-hexosaminidase A activity and Sandhoff disease results from a combined deficiency of β-hexosaminidase A and B activities [1]. An even more rare GM2 gangliosidosis (known as AB variant) is caused by pathogenic variants in the GM2A gene encoding the GM2-activator protein leading to deficiency of this protein [2].
In both Tay-Sachs and Sandhoff diseases (herein, GM2 gangliosidoses), β-hexosaminidase A deficiency results in excessive accumulation of GM2 ganglioside primarily within neurons, leading to cell death and progressive neurodegenerative symptoms, including ataxia, dysarthria, muscle weakness, tremors, atrophy, and psychosis [[3], [4], [5]]. The two diseases are viewed clinically as one entity because they share many symptoms in common, though differences are being increasingly recognized [6,7]. Diagnosis is made by a blood sample showing low hexosaminidase(s) enzymatic activity and confirmed by genetic testing [8,9]. There are three clinical subtypes according to age of onset: the classic acute infantile form with rapid symptom progression and death before the age of 4 years, a subacute juvenile form with symptom onset in early childhood and death commonly in the second decade, and the chronic adult form with first symptoms during adolescence or adulthood (i.e. late-onset form) that has slower progression and longer survival [10].
Late-onset GM2 gangliosidoses often go unrecognized or misdiagnosed in the clinical setting due to their rarity, onset long after infancy, heterogeneous phenotype, and variable presentation that may mimic other well-known neurodegenerative disorders, such as amyotrophic lateral sclerosis, spino-cerebellar ataxias, spinal muscular atrophy and peripheral neuropathy [5,11,12]. Late-onset patients most often present with clumsiness, ataxia, and leg muscle weakness. Cognitive ability is often preserved initially; however, cognitive dysfunction affects nearly half of patients and up to 40% develop psychiatric manifestations that mimic bipolar disorder or psychosis that can be a presenting feature [12,13].
Currently, there are no approved therapies for GM2 gangliosidoses. Management is symptomatic (i.e., ambulatory support, physical therapy, speech therapy, psychiatric medications) [6] by an interdisciplinary team of specialists, including neurologists, speech pathologists, and occupational and physical therapists, among others. Gene therapy and substrate reduction therapy are being investigated as potential disease-modifying therapies. Gene therapy aims to replace the defective HEXA gene with a functioning gene that will enable production of active enzyme to break down GM2 ganglioside and prevent its accumulation in the brain [12]. Substrate reduction therapy aims to reduce the overall burden of substrate (i.e., ganglioside) that needs degradation by reducing its synthesis [12].
As late-onset GM2 gangliosidoses are often under-recognized, we sought to better understand the diagnostic process by querying disease state experts, frontline physicians, and patients, regarding early disease manifestations, first symptoms, common symptoms, differential diagnosis, common misdiagnoses, physician referral patterns, and current disease management.
2. Participants and methods
2.1. Study design and data collection
A qualitative research design using semi-structured, 60-min, individual interviews with disease state experts, neurologists, and patients and caregivers was used to elicit multiple perspectives on the GM2 diagnosis and treatment experience. Interviews and analysis of interviews were conducted by Fulcrum Research Group (Waltham, MA, USA) on behalf of Sanofi (Cambridge, MA, USA). Computer-assisted telephone interviews with screen sharing were conducted in the participants' primary language between March 2, 2020 and March 11, 2021. Moderators were healthcare-focused expert moderators and all interviews were conducted in the native language of the respondent. Interviews were audio recorded and transcribed and translated to English if conducted in another language. Detailed notes were also captured for all English-language interviews, with selected interviews transcribed to ensure clarity.
As this was a market research study and all data were de-identified prior to acquisition and analysis, formal ethical approval and Institutional Review Board approval were not required. Patient identity and confidentiality were protected throughout the research according to standard market research practice. All patients provided informed consent to participate in the interview discussion and for their interviews to be transcribed. Patients also consented to publication of the aggregate findings.
2.2. Expert interviews
The experts were physicians recruited from a market research panel that gathered information about practice and specialty to match participants with research opportunities based on their specialties. Physicians eligible for this study had diagnosed at least one late-onset GM2 patient within the past five years. They were interviewed on the following topics: background and practice information; referral patterns leading to patient(s) diagnosed with late-onset GM2; experience diagnosing late-onset GM2, including symptoms, tests, and challenges; and experience managing late-onset GM2 patients. The information gathered from these interviews was used to guide development of a multi-layered hypothetical patient case for diagnosing late-onset GM2 for use in subsequent interviews with neurologists.
2.3. Patient and caregiver interviews
Patients and caregivers were recruited with assistance from the National Tay-Sachs and Allied Diseases Association in the United States, Cure and Action for Tay-Sachs Foundation in the United Kingdom, Hand in Hand gegen Tay-Sachs und Sandhoff in Germany, Acción y Cura para Tay-Sachs in Spain, and local organizers in Brazil. Eligible participants were patients ≥18 years of age with a late-onset GM2 diagnosis or caregivers of patients with a late-onset GM2 diagnosis.
Patients and caregivers participated in a 15-min online survey (Supplementary Appendix A) to capture information on their symptoms before and after their late-onset GM2 diagnosis, the number and types of physicians they visited before diagnosis, whether they received a misdiagnosis and what that misdiagnosis was. This information was used to facilitate a more detailed discussion of these topics during a computer-assisted telephone interview with screen sharing (Supplementary Appendix B). Interview topics included early disease manifestations and key symptoms (before diagnosis); the diagnostic journey (specialists seen, experiences, misdiagnosis (if any), time to diagnosis, referral patterns, and receiving the diagnosis); post-diagnosis management and treatment; an emotional image exercise in which patients were shown images to help them express emotions about symptoms and diagnosis (patient could use or not use these images if they found it helpful).
2.4. Neurologist interviews
Neurologists were recruited from the same type of market research panel as the experts, and recruitment was blind to the topic of late-onset GM2. Eligible neurologists were general neurologists or neuromuscular specialists who spend at least 75% of their time in direct patient care (vs. teaching, research, or administration), spend at least 20% of their time on neuromuscular disorders; do not spend >15% of their time on multiple sclerosis (i.e., not a multiple sclerosis specialist); and have at least 50% adult patients. Neurologists practiced in a mix of community and academic practice settings.
Neurologists were interviewed about their background and practice information and were asked to evaluate a multi-layered, hypothetical patient case that was developed with input from the interviews with disease experts and patients/caregivers (Supplementary Appendix C). The patient demographics, presenting symptoms, and history (Fig. 1A) were provided first, followed by questions and discussion, including initial tests the physician would order. Next, test results including visual and text description of test results (Fig. 1B) were presented followed by discussion of test results and possible diagnoses at each step without revealing the correct diagnosis. Neurologists were also asked about their referral patterns (i.e., when they would refer such a patient and to which specialty type). At the end of the case study, late-onset GM2 was revealed as the disease in the hypothetical patient and the neurologists were asked about awareness of late-onset GM2, lysosomal storage disorders more broadly, and key challenges in diagnosis.
Fig. 1.
Case discussed with neurologists. Demographics, history and presenting symptoms were provided first (A). After discussion and questions, test results were provided (B). See Appendix C for the full interview discussion guide.
Of note, the patient case for neurologists in the United States differed slightly from the case for the neurologists in the United Kingdom, Germany, and Spain based on differing results of the patient/caregiver interviews in these regions; patients outside the US reported more problems with arms and speech. Results of the case did not differ meaningfully between US and non-US neurologists and these differences did not impact the ability of neurologists to diagnose the case.
2.5. Data analytics and statistics
Participant data from physicians and patients/caregivers were de-identified according to standard market research practice. Interviews were analyzed qualitatively using a thematic analysis to identify key themes, issues, and concerns. Patient pre-interview survey data were analyzed in Excel.
3. Results
3.1. Expert perspective
Five experts were interviewed between March 2 and 31, 2020, including two from the United States and one each from the United Kingdom, Germany and Spain (Table 1). The group comprised one geneticist, three metabolic disease specialists, and one internal medicine physician. The experts drew upon their experience diagnosing late-onset GM2 to describe symptom presentation, patient history, and the impact of the disease on the lives of patients. This information guided the creation of the hypothetical patient case for diagnosing late-onset GM2 for use in interviews with neurologists.
Table 1.
Participant characteristics.
| United States | United Kingdom | Germany | Spain | Brazil | Total | |
|---|---|---|---|---|---|---|
| Key opinion leader sample, n | 2 | 1 | 1 | 1 | – | 5 |
| Geneticist | 1 | 0 | 0 | 0 | – | 1 |
| Metabolic disease specialist | 1 | 1 | 1 | 0 | – | 3 |
| Primary care, general practitioner, internal medicine | 0 | 0 | 0 | 1 | – | 1 |
| Neurologist sample, n | 12 | 6 | 6 | 6 | – | 30 |
| General neurologist | 3 | 3 | 3 | 2 | – | 11 |
| Neuromuscular specialist | 9 | 3 | 3 | 4 | – | 19 |
| Community practice | 8 | 1 | 3 | 1 | – | 13 |
| Academic practice | 4 | 5 | 3 | 5 | – | 17 |
| Average years in practice | 20 | 18 | 17 | 17 | – | 18 |
| Average % of time devoted to neuromuscular disorders | 46% | 28% | 39% | 38% | – | 39% |
| Average % of time dedicated to direct patient care | 89% | 83% | 92% | 82% | – | 87% |
| Average % adult patients | 88% | 92% | 87% | 100% | – | 91% |
| Patient and caregiver sample, n | 18 | 4 | 2 | 2 | 2 | 28 |
| Patients | 9 | 1 | 0 | 0 | 0 | 10 |
| Caregivers | 1 | 3 | 2 | 1 | 2 | 9 |
| Patient and caregiver dyads | 8 | 0 | 0 | 1 | 0 | 9 |
| Diagnosis | ||||||
| Late-onset Tay-Sachs | 16 | 2 | 1 | 2 | 1 | 22 |
| Late-onset Sandhoff | 2 | 2 | 1 | 0 | 1 | 6 |
| Female/male patients | 11/5 | 0/1 | 0/0 | 1/0 | 0/1 | 12/7 |
| Female/male caregivers | 8/1 | 3/0 | 2/0 | 2/0 | 1/1 | 16/2 |
| Average age at first symptoms (years) | 17 | 8 | 9 | 8 | 7 | N/A |
| Other family members with GM2 diagnosis | 44% | 25% | 0% | 100% | 50% | 39% |
3.2. Patient and caregiver perspective
A total of 28 interviews were conducted between July 20, 2020 and March 11, 2021, of which 10 involved only a patient, 9 involved only a caregiver, and 9 included both a patient and caregiver (i.e., a patient-caregiver dyad). Patients and caregivers were located in the United States (n = 18), United Kingdom (n = 4), Germany (n = 2), Spain (n = 2), and Brazil (n = 2) (Table 1). Twenty-two patients (79%) had late-onset Tay-Sachs disease and six (21%) had late-onset Sandhoff disease; reported symptoms and diagnosis data did not differ between Tay-Sachs and Sandhoff patients. Most patients (12 of 19) and caregivers (16 of 18) were female, and 39% had other family members with a GM2 diagnosis. Patients in this study were the index case in their family, with the exception of two patients and one caregiver of a patient that were not the index case.
3.2.1. Disease symptoms
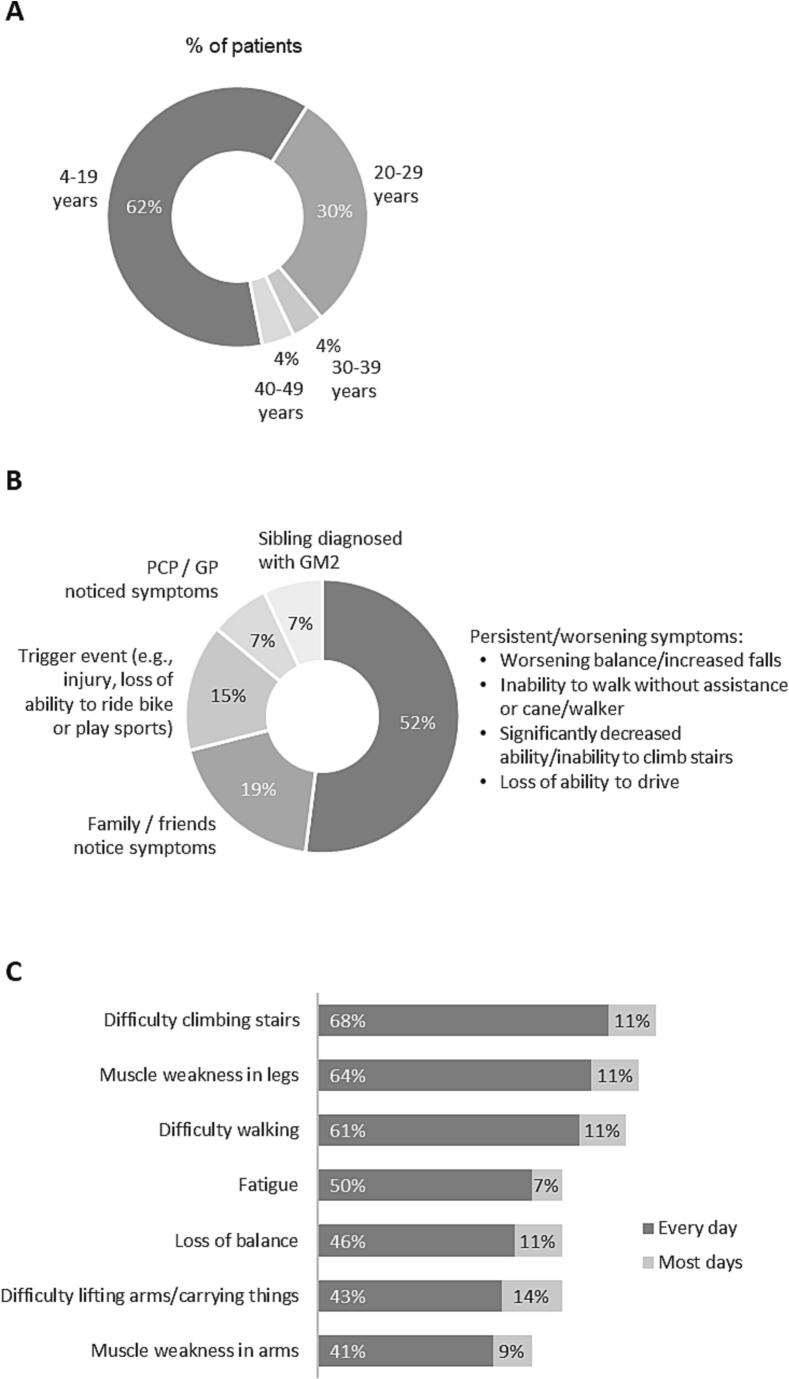
For most patients (92%), symptom onset occurred during adolescence or early adulthood (Fig. 2A) with a median onset age of 14 years. Nearly all patients reported issues with their legs and balance starting from symptom onset to the present; many also reported arm weakness, fatigue and mental health issues, including depression, hallucinations, anxiety, dramatic changes in mood, and difficulty focusing (Fig. 3). Overall, speech problems were reported less often than other symptoms; however, patients in the United Kingdom, Germany, Spain and Brazil reported more speech problems than patients in the United States.
Fig. 2.
Patient/caregiver reported age at onset of GM2 symptoms (A), trigger for initial visit to a healthcare provider (B), and symptoms impacting every/most days (C) (includes symptoms experienced every day by 40% or more of patients).
Fig. 3.
Overview of late-onset GM2 symptoms reported by patients and caregivers (N = 28).
Table 2 shows the high symptom burden of leg (71% of patients) and arm (50%) weakness leading to difficulty walking (68%), difficulty climbing stairs (71%), and loss of balance (68%) starting before diagnosis and continuing to the present (82%, 46%, 79%, 79%, and 75%, respectively). Problems with memory (57%), attention span (54%), speech (61%) and fatigue (75%) were reported by more patients after diagnosis and continuing to the present. Depression and dramatic mood changes were reported by fewer patients, but still a substantial minority, at all time-points. Most patients reported that muscle weakness (75%), difficulty walking (68%), difficulty climbing stairs (79%), difficulty carrying things (57%) and loss of balance (57%) were present every day or most days. Difficulty swallowing, tremors, coughing, anxiety, and headache occurred in relatively few patients in our sample and thus are captured within the Other category in Table 2.
Table 2.
Frequency and timing of symptoms reported by patients and caregivers.
| All patients/caregivers (N = 28) |
||||
|---|---|---|---|---|
| Which types of symptoms did you experience both before and after diagnosis? | Experienced before diagnosis | Experienced after diagnosis | Continue to experience | Never experience |
| Muscle weakness in legs | 71% | 46% | 82% | 0% |
| Muscle weakness in arms | 50% | 43% | 46% | 21% |
| Loss of balance | 68% | 46% | 75% | 4% |
| Difficulty walking | 68% | 50% | 79% | 4% |
| Difficulty climbing stairs | 71% | 50% | 79% | 0% |
| Difficulty lifting arms / carrying things | 32% | 57% | 64% | 18% |
| Difficulty with memory | 25% | 36% | 57% | 32% |
| Short attention span | 36% | 18% | 54% | 36% |
| Difficulty speaking | 39% | 32% | 61% | 29% |
| Depression | 54% | 36% | 39% | 29% |
| Fatigue | 39% | 50% | 75% | 14% |
| Dramatic changes in mood | 29% | 18% | 46% | 39% |
| Hallucinations | 14% | 7% | 7% | 75% |
| Other⁎ | 25% | 14% | 25% | 7% |
| How often do you experience each symptom? | Every day | Most days | Some days | Rarely |
| Muscle weakness in legs | 64% | 11% | 4% | 4% |
| Muscle weakness in arms | 32% | 14% | 0% | 0% |
| Loss of balance | 46% | 11% | 18% | 0% |
| Difficulty walking | 61% | 7% | 7% | 4% |
| Difficulty climbing stairs | 68% | 11% | 0% | 0% |
| Difficulty lifting arms / carrying things | 43% | 14% | 7% | 0% |
| Difficulty with memory | 18% | 11% | 29% | 0% |
| Short attention span | 29% | 11% | 14% | 0% |
| Difficulty speaking | 32% | 7% | 21% | 7% |
| Depression | 50% | 7% | 14% | 4% |
| Fatigue | 4% | 7% | 29% | 7% |
| Dramatic changes in mood | 0% | 0% | 4% | 4% |
| Hallucinations | 4% | 0% | 4% | 7% |
| Other⁎ | 11% | 11% | 0% | 0% |
Difficulty swallowing, tremors, coughing, anxiety, and headaches.
3.2.2. Reaching a diagnosis
Patients first visited a healthcare provider at a median age of 20 years (range: 4–40). For approximately half of patients, the first healthcare provider visit was prompted by persistent or worsening symptoms, such as worsening balance or increased falls, inability to walk without assistance or a cane/walker, significantly decreased ability or inability to climb stairs, and loss of ability to drive (Fig. 2B). Nearly 20% visited a healthcare provider because family or friends noticed symptoms and 15% did so due to a trigger event, such as injury or loss of ability to ride a bike or play sports.
Patients received a GM2 diagnosis at a median age of 26 years (range: 16–67). The median time from symptom onset to diagnosis was 14 years (range: 1–32). Before they received a diagnosis of late-onset GM2, 60% of patients visited their primary care/general physician first before seeing a specialist. On average, patients first visited a specialist 8 years after symptom onset and they visited eight healthcare providers before they received a diagnosis. The physicians who ultimately diagnosed patients with late-onset GM2 were most often neurologists (64%), followed by geneticists (14%), internal medicine/primary care physicians (8%), and metabolic disease specialists (4%); 10% of patients did not recall the diagnosing physician's specialty. During their diagnostic journey, patients reported being misdiagnosed with amyotrophic lateral sclerosis (ALS, n = 7), multiple sclerosis (MS, n = 5), spinal muscular atrophy (SMA, n = 4), ataxia (n = 3), and cerebral palsy (n = 2).
3.2.3. Healthcare providers managing the disease
Neurologists were the primary managers/treaters of late-onset GM2 patients, managing neurological symptoms, monitoring progression, and referring patients to therapy, such as occupational therapy or physical therapy (Table 3). Primary care physicians and psychiatrists were often involved to lesser degree. Occupational therapists (71%), speech pathologists (39%), dieticians (29%), psychologists (71%), and social workers (21%) were also involved in specific aspects of care (Table 3).
Table 3.
Health care providers involved in care of late-onset GM2 patients.
| All patients/caregivers (N = 28) |
||||||
|---|---|---|---|---|---|---|
| US (n = 18) | UK (n = 4) | Germany (n = 2) | Spain (n = 2) | Brazil (n = 2) | Total (n = 28) | |
| Healthcare providers managing GM2 patient care | ||||||
| Neurologist | 72% | 100% | 100% | 100% | 0% | 75% |
| PCP | 33% | 75% | 50% | 0% | 0% | 36% |
| Psychiatrist | 11% | 50% | 50% | 0% | 100% | 25% |
| Geneticist | 17% | 50% | 0% | 0% | 0% | 18% |
| Metabolic Disease Specialist | 0% | 100% | 0% | 0% | 0% | 14% |
| Endocrinologist | 6% | 0% | 0% | 0% | 0% | 4% |
| Rheumatologist | 6% | 0% | 0% | 0% | 0% | 0% |
| Ophthalmologist | 0% | 0% | 0% | 0% | 100% | 11% |
| Other | 1% | 25% | 0% | 0% | 0% | 25% |
| Other health professionals involved in specific aspects of care | ||||||
| Occupational Therapist | 61% | 100% | 50% | 100% | 100% | 71% |
| Speech Pathologist | 33% | 50% | 50% | 0% | 100% | 39% |
| Dietician | 17% | 50% | 50% | 0% | 100% | 29% |
| Psychologist | 22% | 50% | 0% | 0% | 100% | 29% |
| Social Worker | 0% | 75% | 50% | 100% | 0% | 21% |
| NP / PA | 6% | 50% | 0% | 0% | 0% | 11% |
| RN | 6% | 0% | 0% | 0% | 0% | 4% |
| Other | 6% | 0% | 0% | 0% | 0% | 4% |
| None | 22% | 0% | 0% | 0% | 0% | 14% |
3.2.4. Living with late-onset GM2
Patients reported that they experienced an average of 4.8 symptoms every day and that their symptoms impacted their lives on every/most days. Issues relating to the legs and mobility had the greatest day-to-day impact on patients, followed by fatigue and arm weakness (Fig. 2C). Over time, initial symptoms worsened and new issues arose, with significant variability for each patient. Patients often changed their daily life and routine to adjust to progression of symptoms (e.g., moving back home, finding a single-floor home, and changing to less demanding jobs or leaving the workforce).
Figure 4 illustrates how progression of mobility and speech problems had substantial impacts on patients' lives. According to patients, loss of mobility had the greatest impact on their daily lives. For patients with speech deficiencies, speech issues impacted every aspect of life, leading to feelings of isolation (Fig. 4).
Fig. 4.
Progression of mobility and speech problems and their impact on patients' lives.
3.3. Neurologist awareness of late-onset GM2
Thirty neurologists were interviewed between January 19 and February 12, 2021, including 19 neuromuscular disease specialists and 11 general neurologists located in the United States (n = 12), United Kingdom (n = 6), Germany (n = 6), and Spain (n = 6) (Table 1). Thirteen were in community practice and 17 were in an academic practice setting. On average, neurologists had been practicing for 18 years with currently 39% of their time devoted to neuromuscular disorders, 87% of time spent on direct patient care, and 91% of time spent treating adult patients.
3.3.1. Case study background presentation
On reading the case (Fig. 1), neurologists initially focused on the signs and symptoms of proximal muscle weakness. Neurologists located outside of the United States also focused on arm weakness and speech issues, which is consistent with the results of the patient/caregiver interviews described above. Neurologists also noted that proximal muscle weakness initially points to a neuromuscular issue, clumsiness suggests a possible central nervous system component, and that the patient's symptoms began in childhood and have progressed. They noted that the patient was young for an acquired neuromuscular disease, suggesting a high likelihood of a congenital or genetic disorder, rather than an acquired disorder, such as ALS or MS.
3.3.2. Case study test results
Next, the neurologists were presented with the following test results for this patient (Fig. 1): elevated creatine kinase of 618 U/L (normal: 171 U/L); normal results for nerve conduction, repetitive nerve stimulation, and complete blood count. Electromyography (EMG)showed pseudomyotonic discharge, polyphasic muscle action potentials, and incomplete interference pattern during maximal contraction. Muscle biopsy showed severe neurogenic muscular atrophy with secondary additional myopathic changes, but no evidence of a metabolic or myotonic myopathy or an inflammatory cause such as vasculitis or myositis. For interpretation of the EMG and muscle biopsy results, most neurologists relied on the written descriptions rather than the imaging and many reported that they rely on specialists to conduct and interpret these results. Neurologists were accustomed to reviewing MRIs and easily identified the presence of cerebellar atrophy.
At this point, most neurologists thought the totality of evidence pointed to a genetic disorder. The most frequently mentioned suspected diagnoses were metabolic/mitochondrial issue (unknown), spinocerebellar ataxia (SCA), and SMA. Some form of myopathy was mentioned with moderate frequency. Friedreich's ataxia, myopathic dystrophy and GM2 gangliosidosis were mentioned infrequently.
3.3.3. Case study conclusion
Prior to any mention of lysosomal storage disorders by the moderators, <10% of neurologists considered lysosomal storage disorders at all. When presented with lysosomal storage disorders as a possible diagnosis, most neurologists said they had not considered them for this patient. Once lysosomal storage disorders were suggested as a possible cause, the neurologists believed that lysosomal storage disorders made sense because these disorders involve multisystemic symptoms and may include cerebellar atrophy in some cases. However, late-onset GM2 gangliosidoses were not among the lysosomal storage disorders they mentioned (i.e., Gaucher, Fabry and Pompe diseases). Only four of 30 (13%) neurologists (two in the United States and two in Germany) were aware that there are late-onset forms of GM2 gangliosidoses.
4. Discussion
These qualitative studies of the patient/caregiver and neurologist experiences with late-onset GM2 gangliosidoses illustrate the extensive disease burden and long journey to diagnosis for these patients and highlight a lack of awareness of the late-onset form of this rare disease. Patients and their caregivers reported that symptoms typically began during adolescence or early adulthood, starting with leg weakness and balance problems; many also reported arm weakness, fatigue, speech problems and mental health issues. Their symptoms impacted their lives on every/most days, and loss of mobility and speech deficiencies were the most devastating disease symptoms. Reaching a diagnosis took a median 14 years from symptom onset and visits to an average of eight healthcare providers. Neurologists were the diagnosing physicians for 64% of patients and managed GM2 care for 75% of patients. However, when neurologists were presented with a case study of a patient with late-onset GM2, most did not consider lysosomal storage disorders and most were unaware that there are late-onset forms of GM2 gangliosidoses.
Qualitative research of this kind should be interpreted with caution because the findings represent the experiences and opinions of a selected group of respondents and may not be broadly applicable to the populations studied, especially considering the small sample sizes in our studies. Our patient data were collected from surveys and interviews with patients/caregivers, and we did not have access to their medical records to provide more detailed information about their symptoms and diagnostic testing.
Our interviews with patients, caregivers and neurologists indicate several potential areas for improving the diagnosis and management of late-onset GM2 gangliosidoses. Increased awareness and education about the symptoms of late-onset GM2 gangliosidoses among neurologists, geneticists, internal medicine/primary care physicians, and metabolic disease specialists could help to shorten the time to diagnosis. In addition, early and broad-based genetic testing for suspected genetic disorders would likely lead to earlier diagnosis. Both diagnosis and care of patients with GM2 gangliosidoses could be improved by encouraging prompt referral of patients with multisystemic symptoms to specialists or centers of excellence for the management of lysosomal storage disorders. Finally, patient quality of life could be enhanced by providing treatment to alleviate problems relating to mobility and speech.
4.1. Conclusions
For patients who are the index case in the family, the path to a diagnosis of late-onset GM2 gangliosidoses is long and difficult due to the rarity and multisystem nature of the disease and the lack of healthcare provider awareness of this late-onset form of a classically fatal infantile disease.
Funding
Sanofi, Cambridge, MA, USA funded this study. Fulcrum, Waltham, MA, USA designed and conducted the study and analyzed the data. All authors agreed to submit the article for publication.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Mariah C. Lopshire: Project administration, Visualization, Writing – original draft, Writing – review & editing. Cynthia Tifft: Visualization, Writing – original draft, Writing – review & editing. John Burns: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. Rebecca Gould: Conceptualization, Methodology, Investigation, Formal analysis, Writing – review & editing. Riliang Zheng: Supervision, Writing – review & editing. Isabela Batsu: Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
MCL, RZ, and IB are employees of Sanofi and hold Sanofi stock or stock options. JB and RG are employees of Fulcrum Research Group, which conducted this research with funding from Sanofi. CT reports research funding from Sanofi to her institution for the AMETHIST trial.
Acknowledgments
The authors thank the patients and healthcare professionals who participated in this research and Laurie LaRusso, MS, ELS (Chestnut Medical Communications) for medical writing support funded by Sanofi.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2023.101014.
Appendix A. Supplementary data
Supplementary Material A. Patient & Caregiver Survey
Supplementary Material B. Patient & Caregiver Interview Discussion Guide.
Supplementary Material C. HCP Interview Discussion Guide
Data availability
Data will be made available on request.
References
- 1.Mahuran D.J. The biochemistry of HEXA and HEXB gene mutations causing GM2 gangliosidosis. Biochim. Biophys. Acta. 1991;1096(2):87–94. doi: 10.1016/0925-4439(91)90044-a. [DOI] [PubMed] [Google Scholar]
- 2.Mahuran D.J. The GM2 activator protein, its roles as a co-factor in GM2 hydrolysis and as a general glycolipid transport protein. Biochim. Biophys. Acta. 1998;1393(1):1–18. doi: 10.1016/s0005-2760(98)00057-5. [DOI] [PubMed] [Google Scholar]
- 3.Bisel B., Pavone F.S., Calamai M. GM1 and GM2 gangliosides: recent developments. Biomol. Concepts. 2014;5(1):87–93. doi: 10.1515/bmc-2013-0039. [DOI] [PubMed] [Google Scholar]
- 4.Mahuran D.J. Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim. Biophys. Acta. 1999;1455(2–3):105–138. doi: 10.1016/s0925-4439(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 5.Neudorfer O., Pastores G.M., Zeng B.J., Gianutsos J., Zaroff C.M., Kolodny E.H. Late-onset Tay-Sachs disease: phenotypic characterization and genotypic correlations in 21 affected patients. Genet. Med. 2005;7(2):119–123. doi: 10.1097/01.gim.0000154300.84107.75. [DOI] [PubMed] [Google Scholar]
- 6.Lyn N., Pulikottil-Jacob R., Rochmann C., Krupnick R., Gwaltney C., Stephens N., Kissell J., Cox G.F., Fischer T., Hamed A. Patient and caregiver perspectives on burden of disease manifestations in late-onset Tay-Sachs and Sandhoff diseases. Orphanet J. Rare Dis. 2020;15(1):92. doi: 10.1186/s13023-020-01354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toro C., Zainab M., Tifft C.J. The GM2 gangliosidoses: unlocking the mysteries of pathogenesis and treatment. Neurosci. Lett. 2021;764 doi: 10.1016/j.neulet.2021.136195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall P., Minnich S., Teigen C., Raymond K. Diagnosing lysosomal storage disorders: the GM2 gangliosidoses. Curr. Protoc. Hum. Genet. 2014;83 doi: 10.1002/0471142905.hg1716s83. (17 16 11-18) [DOI] [PubMed] [Google Scholar]
- 9.Zhang J., Chen H., Kornreich R., Yu C. Prenatal diagnosis of Tay-Sachs disease. Methods Mol. Biol. 2019;1885:233–250. doi: 10.1007/978-1-4939-8889-1_16. [DOI] [PubMed] [Google Scholar]
- 10.Kaback M., Desnick R. In: GeneReviews. Adam M., Ardinger H., Pagon R., Wallace S., editors. University of Washington; Seattle: 2011. Hexosaminidase A Deficiency. [Google Scholar]
- 11.Masingue M., Dufour L., Lenglet T., Saleille L., Goizet C., Ayrignac X., Ory-Magne F., Barth M., Lamari F., Mandia D., Caillaud C., Nadjar Y. Natural history of adult patients with GM2 Gangliosidosis. Ann. Neurol. 2020;87(4):609–617. doi: 10.1002/ana.25689. [DOI] [PubMed] [Google Scholar]
- 12.Cachon-Gonzalez M.B., Zaccariotto E., Cox T.M. Genetics and therapies for GM2 Gangliosidosis. Curr. Gene Ther. 2018;18(2):68–89. doi: 10.2174/1566523218666180404162622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey L.C., Ringel S.P., Filley C.M. The natural history of cognitive dysfunction in late-onset GM2 gangliosidosis. Arch. Neurol. 2005;62(6):989–994. doi: 10.1001/archneur.62.6.989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material A. Patient & Caregiver Survey
Supplementary Material B. Patient & Caregiver Interview Discussion Guide.
Supplementary Material C. HCP Interview Discussion Guide
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data will be made available on request.