Abstract
The objective of this study was to investigate the preventive effects and mechanisms of genistein (GEN) on production performance and metabolic disorders in broilers under chronic heat stress (HS). A total of 120 male 3-wk-old Ross broilers were randomly assigned to 5 groups: a thermoneutral zone (TN) group maintained at normal temperature (21°C ± 1°C daily), an HS group subjected to cyclic high temperature (32°C ± 1°C for 8 h daily), and 3 groups exposed to HS with varying doses of GEN (50, 100, or 150 mg/kg diet). The experimental period lasted for 3 wk. Here, HS led to a decline in growth performance parameters and hormone secretion disorders (P < 0.05), which were improved by 100 and 150 mg/kg GEN treatment (P < 0.05). Moreover, the HS-induced increases in the liver index (P < 0.01) and abdominal fat rate (P < 0.05) were attenuated by 150 mg/kg GEN (P < 0.05). The HS-induced excessive lipid accumulation in the liver and serum (P < 0.01) was ameliorated after 100 and 150 mg/kg GEN treatment (P < 0.05). Furthermore, the HS-induced decreases in lipolysis-related mRNA levels and increases in lipid synthesis-related mRNA levels in the liver (P < 0.01) were effectively blunted after 100 and 150 mg/kg GEN treatment (P < 0.05). Importantly, the HS-stimulated hepatic mitochondrial energetic dysfunction and decreases in the mRNA or protein levels of peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α), nuclear respiratory factor 1, and mitochondrial transcription factor A in the liver were ameliorated by 150 mg/kg GEN (P < 0.05). Moreover, 50 to 150 mg/kg GEN treatment resulted in a significant increase in the mRNA or protein levels of G protein-coupled estrogen receptor (GPR30), AMP-activated protein kinase (AMPK) α1, phosphorylated AMPKα, and phosphorylated acetyl-CoA carboxylase α. Collectively, GEN alleviated metabolic disorders and hepatic mitochondrial energetic dysfunction under HS, possibly through the activation of GPR30-AMPM-PGC-1α pathways. These data provide a sufficient basis for GEN as an additive to alleviate HS in broilers.
Key words: genistein, heat stress, lipid metabolism disorder, G protein-coupled estrogen receptor, broiler chicken
INTRODUCTION
With benefits of a relatively low price and high protein content in chicken products, broiler chickens have become an important source of meat products. In recent decades, the gradual warming of the global climate has increased the intensity of high temperatures, prolonged the duration of high temperatures, and led to frequent high-temperature phenomena. Broiler chickens exhibit sensitivity to high environmental temperatures in summer due to their special physiological structure and function, such as lack of sweat glands and vigorous metabolism (Zhang et al., 2017; Ringseis and Eder, 2022). Heat stress (HS) promotes the reactive oxygen species (ROS) generation in mitochondria, thereby disrupting the balance of oxidation and antioxidant systems. This leads to protein, lipid, and DNA damage and ultimately results in mitochondrial energetic dysfunction and lipid metabolism disorders (Yao et al., 2023). In addition, HS also leads to the homeostasis imbalance of endocrine hormones and abnormal metabolism of various nutrients. Thus, results in many problems such as decreased animal production performance, increased fat deposition, and mortality, which cause enormous economic losses to the broiler breeding industry (Lu et al., 2017). Therefore, the implementation of affordable and safe physiological regulators to mitigate the negative impacts of HS will exert an important action in fostering the sustainable growth of broiler farming.
Genistein (GEN) is the most effective active ingredient in isoflavones. It has the “dual biological effects” of estrogen-like and antiestrogen and plays a variety of potential functions, such as regulating lipid metabolism, antioxidation, and immune function (Gao et al., 2021; Ullah et al., 2022; Ding et al., 2023). As an estrogen analog, GEN has been shown to play a variety of beneficial roles mainly by activating estrogen receptors (ERs), including G protein-coupled estrogen receptor (GPR30), ERβ, and ERα (Xiao et al., 2019; Vásquez-Reyes et al., 2022). In recent research, we observed that treating broiler chickens with GEN suppressed lipid accumulation through the activation of ERβ-AMP-activated protein kinase (AMPK)-peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α) pathways (Jiang et al., 2021, 2023). Moreover, GEN exerted a positive regulatory effect on biomarkers, including the levels of heat shock protein 70, lactate dehydrogenase, and creatine kinase, in broilers under continuous summer stress, which implies that GEN might be a potential additive that improves the adverse impacts of chronic HS on broilers (Kamboh et al., 2013). However, the regulatory roles of GEN in lipid metabolism and growth performance in broilers exposed to chronic HS have not yet been fully elucidated. Therefore, conducting a comprehensive investigation into the regulatory effects and underlying mechanisms of GEN on metabolic disorders stimulated by chronic HS in broiler chickens can serve as a foundation for considering GEN as a potential additive to prevent the negative impacts of chronic HS on broilers in summer.
Hence, the present study mainly investigates the regulatory roles and mechanisms of GEN in lipid metabolism and growth performance in broilers subjected to HS. Our findings indicated that GEN supplementation alleviates the chronic HS-stimulated decrease in growth performance in broilers. Moreover, the HS-induced lipid metabolism disorder and mitochondrial energetic dysfunction were significantly relieved by GEN treatment. Mechanistically, GEN activates the GPR30-AMPK-PGC-1α pathway and then exerts a potential beneficial effect. The data obtained from this study contribute to establishing a solid theoretical foundation for considering GEN as a potential additive to mitigate the negative effects of HS in broilers.
MATERIALS AND METHODS
All experimental procedures involving broilers were performed in accordance with the guidelines of the Care and Use of Laboratory Animals Centre of the Nanjing Agricultural University (NJAU.No20220602118).
Broiler Chicken Feeding and Grouping
A total of 120 one-day-old male Ross broiler chickens were procured from a poultry farm (Nanjing, China). The broilers were housed in the chicken breeding center of Nanjing Agricultural University under conditions of a 12:12 h light/dark cycle from the 1st day to the 20th day and 23:1 light/dark cycle from the 21st day to the 41st day and relatively constant temperature and humidity. Three broiler chickens were raised in each cage (60 cm × 50 cm × 50 cm) equipped with a papillary automatic water dispenser. From the 1st day to the 20th day, broilers were fed the starter diet, and the nutritional composition is shown in Table 1. Then, based on similar body weights, the chickens were randomly assigned to 5 groups. These groups included a thermoneutral zone (TN) group maintained at normal temperature, an HS group subjected to cyclic high temperature, and 3 groups exposed to HS with varying doses of GEN; each group consisting of a total of 24 chickens in 8 cages. During the experimental period, the birds in the TN group were provided a finisher diet and housed in a room with a normal temperature of 21°C ± 1°C daily. The nutritional composition of the finisher diet is listed in Table 1. The birds in the HS group and HS + GEN groups were provided a finisher diet containing GEN at the indicated dose (0, 50, 100, or 150 mg/kg diet) and housed in a room with the temperature maintained at 32°C ± 1°C from 9:00 to 17:00 (8 h) and 21°C ± 1°C from 17:00 to 9:00 (16 h) daily. The test lasted for 21 d. The chickens were fasted for 12 h before slaughter, and 1 chicken was randomly selected and euthanized from each cage. The pectoral muscle, leg muscle, abdominal fat, and liver tissue were collected and weighed for analysis of carcass characteristics, abdominal fat rate, and liver index. Blood samples from the vein under the wing were collected and centrifuged at 3,000 rpm for 10 min to isolate serum samples.
Table 1.
The composition and nutrient levels of the experimental diets.
| Item | Starter | Finisher |
|---|---|---|
| Ingredient (g/kg) | ||
| Corn | 526 | 574 |
| Wheat bran | 20 | 40 |
| Soybean meal | 311 | 270 |
| Fish meal | 60 | 30 |
| Calcium phosphate | 10 | 15 |
| Limestone | 12 | 12 |
| DL-Methionine | 3 | 1 |
| Vitamin–mineral premix1 | 5 | 5 |
| Rapeseed oil | 50 | 50 |
| Salt | 3 | 3 |
| Calculated nutrient composition (%) | ||
| ME2 (Mcal/kg) | 3.10 | 3.14 |
| Crude protein | 22.52 | 19.74 |
| Lysine | 1.19 | 1.08 |
| Methionine + cystine | 0.93 | 0.71 |
| Calcium | 1.00 | 0.90 |
| Total phosphorus | 0.80 | 0.76 |
| Available phosphorus | 0.47 | 0.39 |
The vitamin–mineral premix supplied the following per kilogram of diet: vitamin A, 1,500 IU; vitamin D3, 200 IU; vitamin E, 10 mg; vitamin K3, 0.5 mg; thiamine, 1.8 mg; riboflavin, 3.6 mg; D-pantothenic acid, 10 mg; folic acid, 0.55 mg; pyridoxine, 3.5 mg; niacin, 35 mg; cobalamin, 0.01 mg; biotin, 0.15 mg; Fe, 80 mg; Cu, 8 mg; Mn, 60 mg; Zn, 40 mg; I, 0.35 mg; and Se, 0.15 mg.
ME = metabolizable energy.
Oil Red O Staining Analysis
The analysis of hepatic lipid droplet deposition in broilers used the Oil Red O staining method. A previously described protocol was followed to perform Oil Red O staining of the liver tissue (Li et al., 2021). In brief, liver samples from broiler chickens were rapidly fixed in 4% paraformaldehyde. Staining with Oil Red O was conducted on liver sections embedded in paraffin and optimal cutting temperature (OCT) compound. Imaging of all sections was performed using a light microscope (Nikon, Japan), and the images were subsequently quantified using Image-Pro Plus 6.0 software.
Hepatic Lipid Analysis
The nonesterified fatty acid (NEFA, Cat. A042-2-1), total cholesterol (TC, Cat. A111-1-1), and triglyceride (TG, Cat. A110-1-1) levels in the livers of broiler chickens were measured by commercial kits obtained from Nanjing Jiancheng Biotechnology Institution (Nanjing, China). Briefly, the liver sample, weighing approximately 100 mg, was collected from broilers and placed in 900 μL of PBS. Following homogenization in an ice bath, the homogenized sample was then centrifuged at 12,000 rpm for 10 min to obtain the supernatant. For TC analysis, blank, cholesterol standard, and sample supernatant were mixed with the working fluid provided from the commercial kit. After incubation at 37°C for 10 min, the absorbance was detected at 500 nm with an enzyme-labeled instrument (Bio-Rad, Hercules, CA). For TG analysis, blank, glycerin standard, and sample supernatant were mixed with the working fluid provided from the commercial kit. After incubation at 37°C for 10 min, the absorbance was detected at 510 nm with an enzyme-labeled instrument (Bio-Rad, Hercules, CA). For NEFA analysis, blank, standard, and sample supernatant were mixed with working fluid 1 provided from the commercial kit. After incubation at 37°C for 5 min, the absorbance was detected at 546 nm with an enzyme-labeled instrument (Bio-Rad, Hercules, CA). Then, the mixed liquid was mixed with working fluid 2 provided from the commercial kit. After incubation at 37°C for 5 min, the absorbance was detected at 546 nm with an enzyme-labeled instrument (Bio-Rad, Hercules, CA). The concentrations of NEFA, TC, and TG were calculated using methods provided by commercial kits.
Serum Lipids and Hormones Analysis
The levels of serum lipids, including high-density lipoprotein cholesterol (HDL-C, Cat. A112-1-1), TC, TG, and low-density lipoprotein cholesterol (LDL-C, Cat. A113-1-1), were determined by commercial kits obtained from Nanjing Jiancheng Biotechnology Institution (Nanjing, China). The levels of serum hormones, including insulin (INS, Cat. HB030-Ch), triiodothyronine (T3, Cat. HB079-Ch), corticosterone (CORT, Cat. HB098-Ch), and thyroxine (T4, Cat. HB137-Ch), were determined by ELISA kits procured from Shanghai HengYuan Biological Technology Co., Ltd. (Shanghai, China). The measurements were performed following the manufacturer's instructions.
ATP Content Analysis
The liver sample, weighing approximately 100 mg, was collected from broilers and placed in 900 μL of PBS. Following homogenization in an ice bath, the homogenized sample was then centrifuged at 12,000 rpm for 10 min to obtain the supernatant. The level of adenosine 5′-triphosphate (ATP, Cat. A095-1-1) in the liver was detected by commercial assay kits obtained from Nanjing Jiancheng Biotechnology Institution (Nanjing, China). The measurements were performed following the manufacturer's instructions.
Analysis of Enzyme Activity Related to Energy Metabolism
The liver sample, weighing approximately 100 mg, was collected from broilers and placed in 900 μL of PBS. Following homogenization in an ice bath, the homogenized sample was then centrifuged at 12,000 rpm for 10 min to obtain the supernatant. The activities of enzymes related to energy metabolism, including malate dehydrogenase (MDH, Cat. A021-2-1), complex I, complex V, and succinate dehydrogenase (SDH, Cat. A022-1-1), were detected by commercial kits following the manufacturer's instructions. The assay kits for MDH and SDH were provided by the Nanjing Jiancheng Biotechnology Institution (Nanjing, China), and the assay kits for complex V and complex I were provided by Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China).
Extraction of RNA and Quantitative Real-Time PCR
Total RNA extraction of liver samples from chickens was performed using TRIzol reagent. A reverse transcription kit was used to reverse transcribe 1,000 ng of total RNA into cDNA. For quantitative real-time PCR (RT-qPCR), the SYBR Green PCR Master Mix Kit was utilized. The 2−ΔΔCT method was employed to calculate and normalize the mRNA expression levels of the target gene using β-actin as the reference. The primer sequences for G protein-coupled estrogen receptor (GPR30), adipose triglyceride lipase (ATGL), fatty acid synthetase (FASN), acetyl-CoA carboxylase (ACC), AMP-activated protein kinase (AMPK) α1, ATP-citrate lyase (ACLY), mitochondrial transcription factor A (TFAM), carnitine palmitoyltransferase1 (CPT-1), peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α), sterol regulatory element binding protein 1c (SREBP-1c), nuclear respiratory factor-1 (NRF-1), and β-actin were synthesized by Gen-Script Biotechnology Co., Ltd. (Nanjing, China) and are listed in Table 2. The TRIzol reagent, reverse transcription kit, and SYBR Green PCR Master Mix Kit were provided by Vazyme Biotech Co., Ltd. (Nanjing, China).
Table 2.
Primer sequences of targeted genes and β-actin.
| Gene | GenBank no. | Primer sequences (5′–3′) | Orientation | Product size (bp) |
|---|---|---|---|---|
| β-actin | L08165 | TGCGTGACATCAAGGAGAAG TGCCAGGGTACATTGTGGTA |
Forward Reverse |
300 |
| GPR30 | NM_001162405 | ATCAGAGCACCAACAAT TTTCCTACAAAGCCAAT |
Forward Reverse |
84 |
| AMPKα1 | NM_001039603 | CAAGTAGTGTCTCGCACGGT GACTGATAGCTGGTCCCACG |
Forward Reverse |
133 |
| ACLY | AJ851548 | CACCCAGAGGTGGATGTTCT GTTGCAGGCCCAATGTTAGT |
Forward Reverse |
188 |
| ACC | J03541 | GTTGTGGTTGGCAGAGCAAG GCACCAAACTTGAGCACCTG |
Forward Reverse |
284 |
| FASN | NM_205155 | TGAAGGACCTTATCGCATTGC GCATGGGAAGCATTTTGTTGT |
Forward Reverse |
96 |
| SREBP-1c | AY029224 | GTCGGCGATCCTGAGGAA CTCTTCTGCACGGCCATCTT |
Forward Reverse |
105 |
| CPT-1 | AY675193 | GGGTTGCCCTTATCGTCACA TACAACATGGGCTTCCGTCC |
Forward Reverse |
151 |
| ATGL | EU240627.2 | TTGCGTGGAGTGAGATATGTTG GGTAGAGGTTGCGAAGGTTGA |
Forward Reverse |
187 |
| PGC-1α | NM001006457 | TCGTGGAGCAATAAAGCG AGGAGGGTCATCGTTCGT |
Forward Reverse |
102 |
| NRF-1 | NM001030646 | CGTCCGAAGTGATGTGC CAGGGTTACTGAAGGTTTGT |
Forward Reverse |
215 |
| TFAM | NM_204100.1 | GTGAAAGCCTGGCGAAACTG CACAGCTCAGGTTACACCGT |
Forward Reverse |
229 |
Protein Extraction and Western Blotting
The liver sample, weighing approximately 100 mg, was collected from broilers and placed in 1 mL RIPA lysis buffer containing 1% PMSF and then homogenized. Subsequently, the sample was centrifuged at 12,000 rpm for 10 min to obtain the supernatant. The protein concentration of the samples was measured using a BCA protein detection kit (Beyotime Biotechnology Co., Ltd., Shanghai, China). A total of 30 μg of protein was separated by SDS polyacrylamide gel electrophoresis and electrotransferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). The PVDF membranes containing protein samples were blocked with 5% nonfat milk for 2 h at room temperature and then incubated overnight at 4°C with primary antibodies recognizing PGC-1α (Proteintech Group, Wuhan, China), AMPKα, ACCα, p-AMPKα, p-ACCα, and tubulin β (Cell Signaling Technology, Danvers, MA). After washing with TBST for approximately 50 min, the blots were incubated with secondary horseradish peroxidase-conjugated antibody (Proteintech Group, Wuhan, China) for approximately 2 h. The enhanced chemiluminescence (ECL) system was utilized to observe and detect the protein bands.
Statistical Analysis
The results are presented as the means ± standard errors (SE), and statistical analysis was performed using 1-way analysis of variance (ANOVA) followed by Tukey's post hoc test for data meeting homogeneity of variance or with Tamhane's T2 analysis for data of heteroscedasticity. SPSS 20.0 for Windows was used for the statistical analyses, and a significance level of P < 0.05 was considered statistically significant.
RESULTS
GEN Improves Growth Performance in Broilers Stimulated by HS
The measured impact of GEN on the growth performance of chickens under chronic HS is presented in Table 3. The final body weight (BW) at 42 d, average daily gain (ADG), and average daily feed intake (ADFI) were markedly reduced, but the feed conversion ratio (FCR) was significantly elevated in the HS group in comparison with the TN group (P < 0.01). However, 50 to 150 mg/kg GEN treatment observably increased the final BW and ADG compared with the HS group (P < 0.05). Furthermore, the ADFI was markedly enhanced, and the FCR was remarkably decreased in the GEN groups at doses of 100 to 150 mg/kg compared with the HS group (P < 0.05).
Table 3.
Effect of genistein on growth performance of broiler chickens from 21 to 42 d of age.
| Item | TN | HS + GEN (mg/kg diet) |
|||
|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | ||
| Body weight (g) 21 d | 704.59 ± 9.06 | 703.47 ± 8.74 | 708.91 ± 23.91 | 695.19 ± 12.35 | 704.88 ± 14.33 |
| Body weight (g) 42 d | 2088.88 ± 43.87 | 1720.38 ± 45.06## | 1882.38 ± 54.36* | 1931.88 ± 32.16** | 1928.63 ± 37.72** |
| ADG (g) | 69.21 ± 2.06 | 50.85 ± 2.25## | 58.67 ± 2.99* | 61.83 ± 2.05** | 61.19 ± 1.74** |
| ADFI (g) | 144.04 ± 2.21 | 127.33 ± 1.75## | 132.88 ± 1.81 | 136.04 ± 1.46* | 137.65 ± 4.37** |
| FCR | 2.09 ± 0.05 | 2.54 ± 0.12## | 2.30 ± 0.11 | 2.21 ± 0.07* | 2.26 ± 0.10* |
Data are presented as means ± SE. Each treated group represents 8 chickens at the age of 42 d, ##P < 0.01, compared with the TN group; *P < 0.05 and **P < 0.01, compared with the HS group.
ADFI, average daily feed intake; ADG, average daily gain; FCR, feed conversion ratio; GEN, genistein; HS, heat stress; TN, thermoneutral zone.
The Regulatory Actions of GEN on the Abdominal Fat Rate, Liver Index, and Carcass Characteristics in Broilers Stimulated by Chronic HS
The measured impacts of GEN on the abdominal fat rate, liver index, and carcass characteristics of broiler chickens under chronic HS are presented in Table 4. No significant differences were found in leg muscle rate and pectoral muscle rate among all groups (P > 0.05). The liver index, which reflects the relative size of the liver, was significantly increased in the HS group compared to the TN group (P < 0.01). Conversely, supplementation with GEN at a dose of 150 mg/kg led to a significant reduction in the liver index compared to the HS group (P < 0.05). Similarly, the abdominal fat rate, representing the amount of fat accumulated in the abdominal area, was significantly higher in the HS group than in the TN group (P < 0.05). However, GEN treatment at doses of 100 and 150 mg/kg resulted in a significant decrease in the abdominal fat rate in the comparison with the HS group (P < 0.05).
Table 4.
Effect of genistein on the carcass characteristics, liver index, and abdominal fat rate in broiler chickens under heat stress.
| Item | TN | HS + GEN (mg/kg diet) |
|||
|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | ||
| Pectoral muscle rate (%) | 14.17 ± 0.85 | 14.70 ± 0.87 | 14.13 ± 1.22 | 13.96 ± 1.52 | 14.50 ± 0.74 |
| Leg muscle rate (%) | 16.67 ± 0.62 | 17.60 ± 0.64 | 17.29 ± 0.83 | 17.14 ± 0.23 | 16.71 ± 0.33 |
| Liver index (%) | 2.19 ± 0.07 | 2.54 ± 0.09## | 2.36 ± 0.12 | 2.34 ± 0.06 | 2.29 ± 0.58* |
| Abdominal fat rate (%) | 1.62 ± 0.11 | 2.04 ± 0.11# | 1.74 ± 0.12 | 1.68 ± 0.06* | 1.69 ± 0.13* |
Data are presented as means ± SE. #P < 0.05 and ##P < 0.01, compared with the TN group; *P < 0.05, compared with the HS group.
GEN, genistein; HS, heat stress; TN, thermoneutral zone.
Impacts of GEN on Lipid Levels in the Livers of Broilers Stimulated by Chronic HS
The hepatic fat droplet accumulation in the livers of broilers was analyzed by Oil Red O staining and we observed that chickens subjected to chronic HS exhibited a marked elevation in the accumulation of fat droplets in comparison with the TN group (P < 0.01) (Figure 1A and B). Nevertheless, the accumulation of fat droplets in the livers of broilers treated with 50 to 150 mg/kg GEN was lower than that in the livers of broilers (P < 0.01) (Figure 1A and B). Moreover, the hepatic TG, TC, and NEFA levels were markedly increased in broilers treated with HS compared to those in the TN group (P < 0.01) (Figure 1C–E). However, broilers fed 50 to 150 mg/kg GEN exhibited significant reductions in the contents of hepatic TG (P < 0.05) and NEFA (P < 0.01) compared to those in the HS group (Figure 1C–E). Furthermore, the hepatic TC content in broilers fed 100 to 150 mg/kg GEN was significantly lower than that in the HS group (P < 0.01) (Figure 1C–E).
Figure 1.
Effect of GEN on the hepatic lipid accumulation in broiler chickens under heat stress. (A) Oil Red O staining in the liver of broilers; (B) Quantitative analysis of (A); (C) Hepatic triglyceride (TG) content; (D) Hepatic total cholesterol (TC) content; (E) Hepatic nonesterified fatty acids (NEFA) content. Data are expressed as means ± SE (n = 5–8). ##P < 0.01 compared to the TN group; *P < 0.05 and **P < 0.01 compared to the HS group.
Impacts of GEN on Serum Lipid and Hormone Levels in Chronic HS-Stimulated Broilers
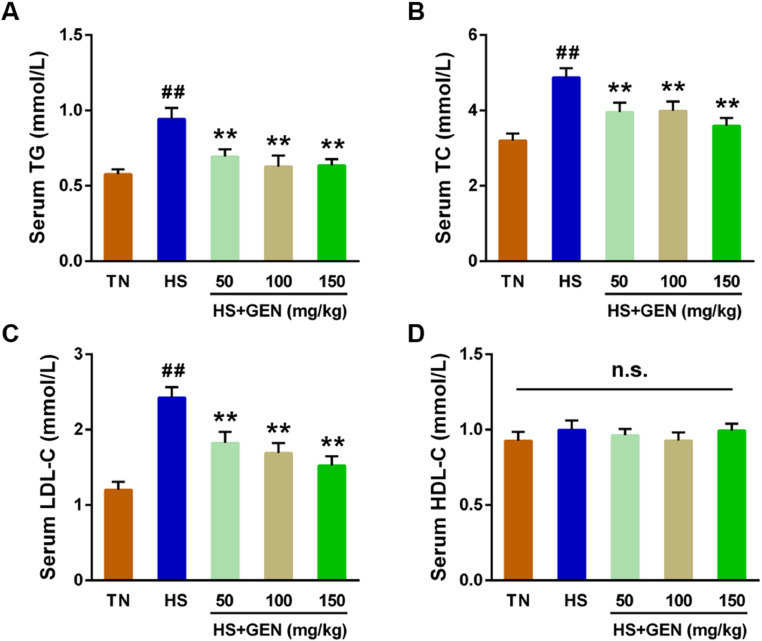
As shown in Figure 2, broilers under chronic HS displayed elevated concentrations of serum TG, TC, and LDL-C compared to those in the TN group (P < 0.01). However, treatment with 50 to 150 mg/kg GEN effectively mitigated the HS-stimulated increases in serum TG, TC, and LDL-C levels (P < 0.01) (Figure 2). Furthermore, the broilers stimulated with chronic HS displayed higher INS, T4, and CORT levels (P < 0.01) and lower T3 levels (P < 0.05) in serum than in the TN group (Figure 3). Nevertheless, supplementation with 50 to 150 mg/kg GEN significantly ameliorated HS-induced increases in serum CORT (P < 0.05), T4 (P < 0.05), and INS (P < 0.01) levels (Figure 3). Additionally, broilers fed 100 to 150 mg/kg GEN exhibited higher T3 levels in serum than the HS group (P < 0.05) (Figure 3).
Figure 2.
Effect of GEN on the serum lipid levels in heat stress-induced broiler chickens. (A) Serum triglyceride (TG) level; (B) Serum total cholesterol (TC) level; (C) Serum low-density lipoprotein cholesterol (LDL-C) level; (D) Serum high-density lipoprotein cholesterol (HDL-C) level. Data are expressed as means ± SE (n = 8). ##P < 0.01 compared to the TN group; **P < 0.01 compared to the HS group; n.s., no significance.
Figure 3.
Effect of GEN on the serum hormone levels in chronic heat stress-induced broiler chickens. (A) Serum corticosterone (CORT) level; (B) Serum T3 level; (C) Serum T4 level; (D) Serum insulin (INS) level. Data are expressed as means ± SE (n = 7–8). #P < 0.05 and ##P < 0.01 compared to the TN group; *P < 0.05 and **P < 0.01 compared to the HS group.
Impacts of GEN on the mRNA Expression of Factors Involved in Lipid Metabolism in Chronic HS-Challenged Broilers
As shown in Figure 4A–D, the gene levels of ACC, FASN, ACLY, and SREBP-1c were generally higher in the livers of chickens challenged by HS than in chickens in the TN group (P < 0.01). Nevertheless, the ACLY gene level was memorably decreased by treatment with 100 to 150 mg/kg GEN in the diet (P < 0.01), and treatment with 50 to 150 mg/kg GEN largely suppressed ACC (P < 0.05), FASN (P < 0.05), and SREBP-1c (P < 0.01) gene levels compared to those in the HS group (Figure 4A–D). In addition, in the comparisons with the TN group, the broiler chickens under chronic HS displayed lower hepatic ATGL and CPT-1 gene levels (P < 0.01) (Figure 4E and F). However, treatment with 50 to 150 mg/kg GEN dramatically ameliorated chronic HS-induced decreases in ATGL (P < 0.05) and CPT-1 (P < 0.01) gene levels in the livers of broilers (Figure 4E and F).
Figure 4.
Effect of GEN on the mRNA levels of lipid metabolism-related factors in the livers of broiler chickens under chronic heat stress. (A) Relative ATP-citrate lyase (ACLY) mRNA expression level; (B) Relative acetyl-CoA carboxylase (ACC) mRNA expression level; (C) Relative fatty acid synthetase (FASN) mRNA expression level; (D) Relative sterol regulatory element binding protein 1c (SREBP-1c) mRNA expression level; (E) Relative adipose triglyceride lipase (ATGL) mRNA expression level; (F) Relative carnitine palmitoyltransferase I (CPT-1) mRNA expression level. Data are expressed as means ± SE (n = 8). ##P < 0.01 compared to the TN group; *P < 0.05 and **P < 0.01 compared to the HS group.
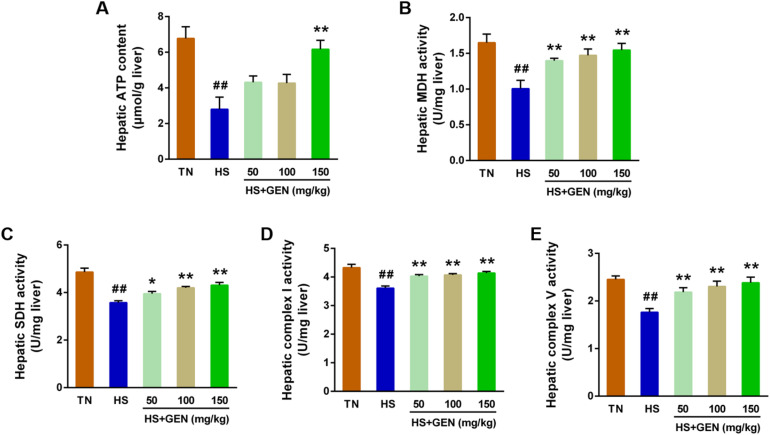
GEN Improves Energy Metabolism and Mitochondrial Biogenesis in Chronic HS-Induced Broilers
As shown in Figure 5, compared to those in the TN group, the ATP content and activities of MDH, SDH, complex I, and complex V in the livers of the chickens subjected to chronic HS were decreased (P < 0.01). However, chickens fed 150 mg/kg GEN exhibited higher ATP content (P < 0.01), and those fed 50 to 150 mg/kg GEN displayed higher activities of MDH (P < 0.01), SDH (P < 0.05), complex I (P < 0.01), and complex V (P < 0.01) in the liver than the HS group (Figure 5). As shown in Figure 6, the mRNA or protein levels of TFAM, NRF-1, and PGC-1α in the liver were lower in the HS-treated groups than in the TN group (P < 0.01), and these levels were markedly increased by treatment with 50 to 150 mg/kg GEN (P < 0.05).
Figure 5.
Effect of GEN on the energy metabolism in the livers of broiler chickens under chronic heat stress. (A) Hepatic ATP content; (B) Hepatic malate dehydrogenase (MDH) activity; (C) Hepatic succinate dehydrogenase (SDH) activity; (D) Hepatic complex I activity; (E) Hepatic complex V activity (n = 8). Data are expressed as means ± SE. ##P < 0.01 compared to the TN group; *P < 0.05 and **P < 0.01 compared to the HS group.
Figure 6.
Effect of GEN on the expression levels of mitochondrial biogenesis-related factors in the livers of broiler chickens under chronic heat stress. (A) Relative peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α) mRNA expression level; (B) Immunoblot of PGC-1α; (C) Quantitative analysis of (B); (D) Relative nuclear respiratory factor-1 (NRF-1) mRNA expression level; (E) Relative mitochondrial transcription factor A (TFAM) mRNA expression level. Data are expressed as means ± SE (n = 3–8). ##P < 0.01 compared to the TN group; *P < 0.05 and **P < 0.01 compared to the HS group.
GEN Enhances the Expression of Factors Involved in the GPR30/AMPK/PGC-1α Pathways in Chronic HS-Challenged Broilers
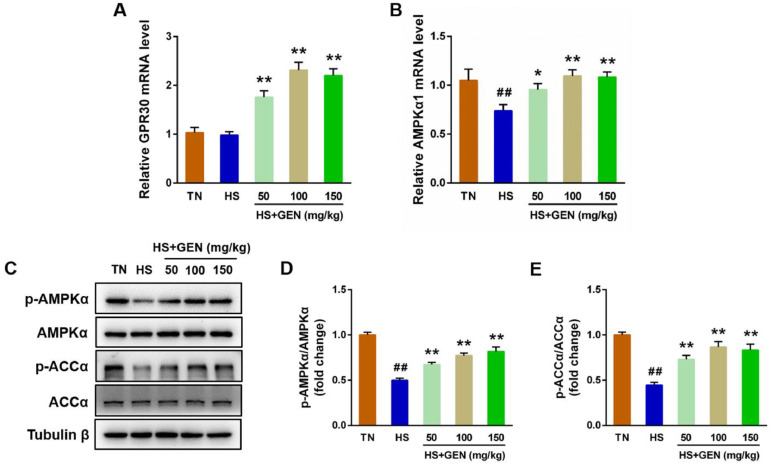
As shown in Figure 7A, the hepatic GPR30 mRNA expression levels were increased in the groups treated with GEN at doses of 50 to 150 mg/kg compared to the TN group (P < 0.01). Additionally, hepatic AMPKα1 mRNA levels were notably lower in the chronic HS group than in the TN group (P < 0.01) (Figure 7B). In contrast, GEN administration at doses of 50 to 150 mg/kg in the diet of broilers resulted in a marked increase in the mRNA expression levels of AMPKα1 compared to the HS group (P < 0.05) (Figure 7B). Compared with the TN treatment, chronic HS resulted in a marked inhibition in the protein levels of phosphorylated ACCα and AMPKα (P < 0.01). Conversely, broilers supplemented with 50 to 150 mg/kg GEN exhibited higher phosphorylated ACCα and AMPKα protein levels than broilers in the HS group (P < 0.01) (Figure 7C–E).
Figure 7.
Effect of GEN on the expression levels of mitochondrial biogenesis-related factors in the livers of broiler chickens under chronic heat stress. (A) Relative G protein-coupled estrogen receptor (GPR30) mRNA expression level; (B) Relative AMP-activated protein kinase (AMPK) α1 mRNA expression level; (C) Immunoblot of p-AMPKα, AMPK, acetyl-CoA carboxylase α (ACCα), and p-ACCα; (D) Quantitative analysis of p-AMPKα/AMPKα; (E) Quantitative analysis of p-ACCα/ACCα. Data are expressed as means ± SE (n = 3–8). ##P < 0.01 compared to the TN group; *P < 0.05 and **P < 0.01 compared to the HS group.
DISCUSSION
In animal breeding, livestock and poultry maintain a balance between the dissipation and production of heat through the regulation of metabolism, which maintains a relatively stable temperature. However, continuous high ambient temperatures will lead to a series of nonspecific defense responses, such as increased body temperature, decreased food intake, and inhibited growth performance. In the present research, we observed that broilers subjected to chronic HS exhibited a decrease in ADG, ADFI, and final BW. These findings align with previous studies showing that a long-term thermal environment leads to a decline in broiler growth performance (Kikusato et al., 2021). These data provide some evidence for the successful implementation of the chronic HS model for broilers. The principal finding of this study is that the administration of GEN effectively alleviated the negative impact of chronic HS on the productive performance of broilers. Furthermore, treatment with GEN effectively mitigated the disruption of lipid metabolism and mitochondrial energetic dysfunction induced by chronic HS, possibly by activating the GPR30/AMPK/PGC-1α pathways.
Accumulated evidence has indicated that chronic HS has significant adverse effects on the growth performance of chickens. It has been reported that if the ambient temperature is higher than the appropriate temperature, the feed intake of broilers will decrease by approximately 2.2% for every 1°C increase in temperature (Cooper and Washburn, 1998). The present research indicated that the detrimental effects of chronic HS on ADFI, ADG, and final BW in broilers were substantially improved with the administration of GEN. It is well known that the decrease in growth performance caused by chronic HS is closely related to the decline in food intake. These findings align with previous studies that have suggested that GEN supplementation improves growth performance in chickens and fish (Rasouli and Jahanian, 2015; Lv et al., 2019; Torno et al., 2019). In addition, dietary GEN also enhanced offspring growth performance in broilers (Lv et al., 2018a). Overall, dietary GEN supplementation improved growth performance, which might be related to the increase in ADFI in broilers under chronic HS. The adverse effects of HS on slaughter performance have been widely reported (Ain Baziz et al., 1996). Then, the slaughtering performance was analyzed in this study, and no significant differences were found in pectoral muscle rate and leg muscle rate after treatment with HS or GEN, which suggested that HS or GEN treatment had no effect on slaughtering performance. We speculated that the difference may be caused by the different durations of HS.
Several previous studies indicated that chronic HS significantly increased fat synthesis and deposition in chickens (Ain Baziz et al., 1996; Lu et al., 2019). In this research, we found a significant increase in the abdominal fat rate in the HS group compared to the TN group. However, dietary GEN remarkably alleviated the increase in abdominal fat rate stimulated by chronic HS in broiler chickens, which aligns with our recent report demonstrating the reduction in abdominal fat accumulation in broilers after GEN treatment (Jiang et al., 2021). In poultry, fat synthesis primarily occurs in the liver, and the fat is subsequently transported to adipose tissue via the transport of apolipoproteins in the blood. In the present research, we found that HS treatment led to increases in lipids levels in the liver and serum. These findings suggested that more lipids are synthesized in the liver and then transported to the blood after chronic HS treatment. However, GEN supplementation attenuated the increases in the liver and serum lipid contents caused by HS in broilers. Our findings suggested that GEN treatment reduces hepatic lipid accumulation and serum lipid content in chronic HS-challenged chickens, which is consistent with the reduction in the HS-induced increase in abdominal fat accumulation by GEN in broilers.
HS treatment has been shown to interfere with the neuroendocrine system of broilers through the hypothalamus-pituitary-thyroid axis, leading to increased secretion of CORT (Quinteiro-Filho et al., 2012). CORT has been recognized as a crucial hormone in regulating various physiological functions under stress stimulation conditions. In this study, GEN treatment significantly inhibited the chronic HS-stimulated increase in CORT levels, suggesting that GEN alleviates the endocrine disorder induced by chronic HS in broilers. Elevated plasma CORT levels have been associated with increased insulin (INS) levels and insulin resistance in chickens (Lin et al., 2006). INS, as a hypoglycemic hormone, inhibits fat decomposition and promotes fat synthesis (Norton et al., 2022). In the present study, GEN treatment alleviated chronic HS-regulated increases in serum INS levels, partially explaining the inhibitory effect of GEN on chronic HS-induced lipid overaccumulation in broilers. Thyroid hormones, including T3 and T4, play crucial roles in metabolic regulation, promoting material and energy metabolism in animals (Sinha et al., 2018). In this study, chronic HS elevated serum T4 contents but reduced serum T3 levels in broiler chickens. These effects were blunted by GEN treatment. These data imply that the chronic HS-regulated disorder of thyroid hormone secretion was improved by GEN.
Under normal conditions, the synthesis and decomposition of lipids usually maintain a dynamic balance, which is essential for maintaining stable metabolic activities in the body. However, this balance will be destroyed if lipid synthesis is enhanced or decomposition is weakened, which is regulated by the activity of enzymes related to fat metabolism. In the present research, we found a marked upregulation in the mRNA level of SREBP-1c following treatment with chronic HS. These data are in line with previous research that has shown elevated expression of SREBP-1c in broilers exposed to chronic HS (Lu et al., 2019). SREBP-1c, a pivotal nuclear transcription factor, plays a crucial role in the regulation of fat synthesis by influencing the expression of key factors involved in this process, such as ACC and FASN (Zeng et al., 2022). Our results revealed a significant upregulation in the gene expression of FASN, ACC, and ACLY in the livers of broilers exposed to chronic HS. This suggested that chronic HS promotes lipid synthesis by upregulating the expression levels of lipid synthesis-related factors. Notably, treatment with GEN effectively suppressed the chronic HS-challenged enhancements in the gene expression of lipid synthesis-related factors in the livers of broilers. Numerous studies have previously demonstrated that GEN possesses the ability to inhibit fatty acid synthesis in poultry and rodents (Lv et al., 2018b; Pummoung et al., 2020). Furthermore, our recent investigations have provided evidence of the ability of GEN to inhibit fatty acid synthesis both in primary chicken hepatocytes and broilers (Jiang et al., 2021, 2023). This finding implied that dietary GEN reduces fatty acid synthesis in broiler chickens under conditions of chronic HS. Additionally, our findings indicated that chickens stimulated by chronic HS displayed lower mRNA levels of CPT-1 and ATGL in the liver. The reduced expression of ATGL, the most significant lipolytic enzyme, results in the accumulation of TG in adipocytes and the liver, leading to obesity and other metabolic complications (Quiroga and Lehner, 2018; Fang et al., 2022). CPT-1 is a rate-limiting enzyme involved in fatty acid oxidation and catalyzes the transportation of fatty acids from the cytoplasm to the mitochondrial matrix for β-oxidation (Schlaepfer and Joshi, 2020). Notably, our study demonstrated that GEN treatment significantly improved the decrease in ATGL and CPT-1 mRNA levels stimulated by chronic HS in the livers of broilers. These data suggest that treatment with GEN promotes fatty acid catabolism in broilers exposed to chronic HS. In conclusion, GEN supplementation alleviates lipid metabolism disorders stimulated by chronic HS by enhancing fatty acid catabolism and inhibiting fatty acid anabolism in broilers.
Fatty acid metabolism is closely related to mitochondrial function due to the catabolism of fatty acids is carried out in mitochondria. Numerous studies have demonstrated that HS can induce mitochondrial structural abnormalities and then cause disorders of energy metabolism and lipid metabolism (Akbarian et al., 2016; Ouyang et al., 2022). In this study, we observed that the ATP content and activities of MDH, SDH, complex I, and complex V were largely reduced in broilers exposed to chronic HS, and these values were increased by GEN treatment. Mitochondrial biogenesis is essential in repairing the mitochondrial structure and maintaining its function, and this process is mainly regulated by PGC-1α, which is the key transcription-activating factor (Qian et al., 2019). Activation of PGC-1α can promote mitochondrial biosynthesis by activating the NRF1/TFAM pathway (Cardanho-Ramos and Morais, 2021). Previous studies reported that HS treatment induces mitochondrial energetic dysfunction related to the inhibition of PGC-1α/NRF1/TFAM pathways (Patton et al., 2018). Our previous research reported that GEN enhanced the PGC-1α pathway in broilers (Jiang et al., 2021). However, the effect of GEN on HS-induced mitochondrial energetic dysfunction has not been reported. Our data found that GEN ameliorated the reductions in the gene or protein levels of PGC-1α, TFAM, and NRF1 in the livers of broilers stimulated by chronic HS. This suggested that the protective roles of GEN on HS-challenged mitochondrial energetic dysfunction may be achieved by activating the PGC-1α/NRF1/TFAM signaling pathways.
GEN, as an estrogen analog, can bind to and activate classical and nonclassical ERs, exerting its biological effects. GPR30 is a nonclassical ER that can interact with GEN and mediate its rapid effects (Vásquez-Reyes et al., 2022). In our study, we observed that treatment with GEN significantly increased GPR30 mRNA levels in broiler chickens under chronic HS, indicating the activation of GPR30 by GEN. Activation of GPR30 can regulate lipid metabolism and mitochondrial function by activating multiple downstream pathways. In addition, our recent study indicated that activated GPR30 can activate AMPK and exert an anti-NAFLD effect (Li et al., 2021). AMPK, as a critical regulator of energy perception and regulation, plays a vital role in modulating mitochondrial function, lipid metabolism, oxidative stress, and other physiological activities (Herzig and Shaw, 2018). In our study, GEN treatment effectively reversed the inhibition of phosphorylated AMPKα and ACCα protein levels stimulated by chronic HS, suggesting activation of the AMPK pathway by GEN in chickens under HS. The activated AMPK pathway can not only alleviate mitochondrial energetic dysfunction through PGC-1α but also regulate the expression of lipid metabolism-related factors to reduce lipid deposition. Hence, we hypothesized that GEN improves disorders of lipid metabolism and mitochondrial energetic dysfunction by activating GPR30/AMPK pathways in broilers under chronic HS.
In summary, our data indicated that treatment with GEN dramatically improved the growth performance of broilers under chronic HS. Additionally, GEN can prevent excessive lipid deposition by regulating the expression of lipid metabolism-related factors and improving mitochondrial function through the activation of PGC-1α/NRF1/TFAM pathways in broiler chickens under chronic HS. Mechanistically, the potential beneficial effect of GEN may be attributed to the activation of the GPR30-AMPK pathway. The present research provides a solid foundation for the use of GEN as a supplement for mitigating chronic HS in broiler chickens.
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (No. 32202758), Natural Science Foundation of Jiangsu Province (No. BK20210400), Fundamental Research Funds for the Central Universities (No. KYQN2023006), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
DISCLOSURES
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “Genistein alleviates chronic heat stress-induced lipid metabolism disorder and mitochondrial energetic dysfunction by activating the GPR30-AMPK-PGC-1α signaling pathways in the livers of broiler chickens.”
REFERENCES
- Ain Baziz H., Geraert P.A., Padilha J.C., Guillaumin S. Chronic heat exposure enhances fat deposition and modifies muscle and fat partition in broiler carcasses. Poult. Sci. 1996;75:505–513. doi: 10.3382/ps.0750505. [DOI] [PubMed] [Google Scholar]
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardanho-Ramos C., Morais V.A. Mitochondrial biogenesis in neurons: how and where. Int. J. Mol. Sci. 2021;22:13059. doi: 10.3390/ijms222313059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M.A., Washburn K.W. The relationships of body temperature to weight gain, feed consumption, and feed utilization in broilers under heat stress. Poult. Sci. 1998;77:237–242. doi: 10.1093/ps/77.2.237. [DOI] [PubMed] [Google Scholar]
- Ding Q., Pi A., Hao L., Xu T., Zhu Q., Shu L., Yu X., Wang W., Si C., Li S. Genistein protects against acetaldehyde-induced oxidative stress and hepatocyte injury in chronic alcohol-fed mice. J. Agric. Food Chem. 2023;71:1930–1943. doi: 10.1021/acs.jafc.2c05747. [DOI] [PubMed] [Google Scholar]
- Fang C., Pan J., Qu N., Lei Y., Han J., Zhang J., Han D. The AMPK pathway in fatty liver disease. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.970292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Liu S., Tan L., Ding C., Fan W., Gao Z., Li M., Tang Z., Wu Y., Xu L., Yan L., Luo Y., Song S. Estrogen receptor α regulates metabolic-associated fatty liver disease by targeting NLRP3-GSDMD axis-mediated hepatocyte pyroptosis. J. Agric. Food Chem. 2021;69:14544–14556. doi: 10.1021/acs.jafc.1c05400. [DOI] [PubMed] [Google Scholar]
- Herzig S., Shaw R.J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Wang H., Yang Y., Yao Y., Ma H. Genistein activated SIRT1-AMPK signaling pathway mediated by ERβ-FOXO1-Nampt to reduce fat accumulation in chicken hepatocytes. Life Sci. 2023;312 doi: 10.1016/j.lfs.2022.121259. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Yang Z., Zhang H., Yao Y., Ma H. Genistein activated adenosine 5′-monophosphate-activated protein kinase-sirtuin1/peroxisome proliferator-activated receptor γ coactivator-1α pathway potentially through adiponectin and estrogen receptor β signaling to suppress fat deposition in broiler chickens. Poult. Sci. 2021;100:246–255. doi: 10.1016/j.psj.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboh A.A., Hang S.Q., Bakhetgul M., Zhu W.Y. Effects of genistein and hesperidin on biomarkers of heat stress in broilers under persistent summer stress. Poult. Sci. 2013;92:2411–2418. doi: 10.3382/ps.2012-02960. [DOI] [PubMed] [Google Scholar]
- Kikusato M., Xue G., Pastor A., Niewold T.A., Toyomizu M. Effects of plant-derived isoquinoline alkaloids on growth performance and intestinal function of broiler chickens under heat stress. Poult. Sci. 2021;100:957–963. doi: 10.1016/j.psj.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wang H., Yao Y., Cao J., Jiang Z., Yan W., Chu X., Li Q., Lu M., Ma H. The sex steroid precursor dehydroepiandrosterone prevents nonalcoholic steatohepatitis by activating the AMPK pathway mediated by GPR30. Redox Biol. 2021;48 doi: 10.1016/j.redox.2021.102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Sui S.J., Jiao H.C., Buyse J., Decuypere E. Impaired development of broiler chickens by stress mimicked by corticosterone exposure. Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 2006;143:400–405. doi: 10.1016/j.cbpa.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Lv Z., Fan H., Zhang B., Xing K., Guo Y. Dietary genistein supplementation for breeders and their offspring improves the growth performance and immune function of broilers. Sci. Rep. 2018;8:5161. doi: 10.1038/s41598-018-23530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z., Xing K., Li G., Liu D., Guo Y. Dietary genistein alleviates lipid metabolism disorder and inflammatory response in laying hens with fatty liver syndrome. Front. Physiol. 2018;9:1493. doi: 10.3389/fphys.2018.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z.P., Yan S.J., Li G., Liu D., Guo Y.M. Genistein improves the reproductive performance and bone status of breeder hens during the late egg-laying period. Poult. Sci. 2019;98:7022–7029. doi: 10.3382/ps/pez367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., He X.F., Ma B.B., Zhang L., Li J.L., Jiang Y., Zhou G.H., Gao F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019;98:3695–3704. doi: 10.3382/ps/pez056. [DOI] [PubMed] [Google Scholar]
- Norton L., Shannon C., Gastaldelli A., DeFronzo R.A. Insulin: the master regulator of glucose metabolism. Metab. Clin. Exp. 2022;129 doi: 10.1016/j.metabol.2022.155142. [DOI] [PubMed] [Google Scholar]
- Ouyang J., Zhou H., Li Q., Zheng J., Chen C., Guo S., You J., Li G. Tryptophan alleviates acute heat stress-induced impairment of antioxidant status and mitochondrial function in broilers. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.863156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton M.G., Gillum T.L., Szymanski M.C., Gould L.M., Lauterbach C.J., Vaughan R.A., Kuennen M.R. Heat acclimation increases mitochondrial respiration capacity of C2C12 myotubes and protects against LPS-mediated energy deficit. Cell Stress Chaperones. 2018;23:871–883. doi: 10.1007/s12192-018-0894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pummoung S., Werawatganon D., Chayanupatkul M., Klaikeaw N., Siriviriyakul P. Genistein modulated lipid metabolism, hepatic PPARγ, and adiponectin expression in bilateral ovariectomized rats with nonalcoholic steatohepatitis (NASH) Antioxidants. 2020;10:24. doi: 10.3390/antiox10010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Li X., Shi Z., Bai X., Xia Y., Zheng Y., Xu D., Chen F., You Y., Fang J., Hu Z., Zhou Q., Lu Z. KDM3A senses oxygen availability to regulate PGC-1α-mediated mitochondrial biogenesis. Mol. Cell. 2019;76:885–895. doi: 10.1016/j.molcel.2019.09.019. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Gomes A.V., Pinheiro M.L., Ribeiro A., Ferraz-de-Paula V., Astolfi-Ferreira C.S., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. 2012;41:421–427. doi: 10.1080/03079457.2012.709315. [DOI] [PubMed] [Google Scholar]
- Quiroga A.D., Lehner R. Pharmacological intervention of liver triacylglycerol lipolysis: the good, the bad and the ugly. Biochem. Pharmacol. 2018;155:233–241. doi: 10.1016/j.bcp.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Rasouli E., Jahanian R. Improved performance and immunological responses as the result of dietary genistein supplementation of broiler chicks. Animal. 2015;9:1473–1480. doi: 10.1017/S1751731115000853. [DOI] [PubMed] [Google Scholar]
- Ringseis R., Eder K. Heat stress in pigs and broilers: role of gut dysbiosis in the impairment of the gut-liver axis and restoration of these effects by probiotics, prebiotics and synbiotics. J. Anim. Sci. Biotechnol. 2022;13:126. doi: 10.1186/s40104-022-00783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer I.R., Joshi M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology. 2020;161:bqz046. doi: 10.1210/endocr/bqz046. [DOI] [PubMed] [Google Scholar]
- Sinha R.A., Singh B.K., Yen P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018;14:259–269. doi: 10.1038/nrendo.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torno C., Staats S., de Pascual-Teresa S., Rimbach G., Schulz C. Effects of resveratrol and genistein on growth, nutrient utilization and fatty acid composition of rainbow trout. Animal. 2019;13:933–940. doi: 10.1017/S1751731118002458. [DOI] [PubMed] [Google Scholar]
- Ullah T.R., Balka K.R., Ambrose R.L., Pépin G., Wilce M.C.J., Wilce J.A., Thomas B.J., De Nardo D., Williams B.R.G., Gantier M.P. Genistein targets STING-driven antiviral responses. mBio. 2022;13 doi: 10.1128/mbio.02064-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez-Reyes S., Vargas-Castillo A., Noriega L.G., Velázquez-Villegas L.A., Pérez B., Sánchez-Tapia M., Ordaz G., Suárez-Monroy R., Ulloa-Aguirre A., Offner H., Torres N., Tovar A.R. Genistein stimulation of white adipose tissue thermogenesis is partially dependent on GPR30 in mice. Mol. Nutr. Food Res. 2022;66 doi: 10.1002/mnfr.202100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y.Q., Shao D., Tong H.B., Shi S.R. Genistein increases progesterone secretion by elevating related enzymes in chicken granulosa cells. Poult. Sci. 2019;98:1911–1917. doi: 10.3382/ps/pey411. [DOI] [PubMed] [Google Scholar]
- Yao X., Zhu J., Li L., Yang B., Chen B., Bao E., Zhang X. Hsp90 protected chicken primary myocardial cells from heat-stress injury by inhibiting oxidative stress and calcium overload in mitochondria. Biochem. Pharmacol. 2023;209 doi: 10.1016/j.bcp.2023.115434. [DOI] [PubMed] [Google Scholar]
- Zeng H., Qin H., Liao M., Zheng E., Luo X., Xiao A., Li Y., Chen L., Wei L., Zhao L., Ruan X.Z., Yang P., Chen Y. CD36 promotes de novo lipogenesis in hepatocytes through INSIG2-dependent SREBP1 processing. Mol. Metab. 2022;57 doi: 10.1016/j.molmet.2021.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhao X.H., Yang L., Chen X.Y., Jiang R.S., Jin S.H., Geng Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266. [DOI] [PubMed] [Google Scholar]