Abstract
Purpose
Scleritis is an extremely painful and potentially blinding inflammation of the sclera with unknown pathogenesis and unpredictable course. To gain insight in its disease process and identify biomarker candidates, we performed extensive proteomics in serum and tear fluid.
Design
Prospective multicenter cohort study.
Participants
A total of 121 patients with noninfectious scleritis (of which 39 active cases), 30 healthy controls, and 23 disease controls (uveitis and rheumatoid arthritis) were enrolled in the Netherlands from 2020 to 2022.
Methods
Serum, tear fluid of both eyes, and clinical data were gathered. The level of 368 inflammatory proteins was measured using proximity extension assays. Results were validated in an independent cohort of 15 patients with scleritis, and using addressable laser bead immunoassay, or enzyme-linked immunoassays. In addition, we studied an extended panel of matrix metalloproteinases in tear fluid of necrotizing scleritis with addressable laser bead immunoassay.
Main Outcome Measures
Statistically significant differences in the level of inflammatory proteins between patients with scleritis and control groups.
Results
Proteomics revealed 18 significantly upregulated or downregulated serum proteins in active scleritis cases compared with all control groups in both the discovery cohort and the validation cohort. The most upregulated protein was nuclear migration protein nudC (NudC; P = 0.0032), a protein involved in neurogenesis. The other significant hits included proteins involved in T-cell activation, apoptosis, epithelial barrier maintenance, and angiogenesis. Our tear fluid analysis showed matrix metalloproteinase 9 (MMP9) to be upregulated in the tear fluid of patients with scleral necrosis.
Conclusions
The results of our proteomics analysis suggest a role for neurogenesis, T-cell activation, disruption of epithelial barrier, and angiogenesis in the pathogenesis of scleritis, and highlight MMP9 and NudC as biomarkers with potential clinical relevance.
Funding Disclosure(s)
The authors have no proprietary or commercial interest in any materials discussed in this article.
Keywords: Biomarkers, Proteomics, Scleritis, Serum, Tear fluid
Scleritis is a severe and extremely painful inflammation of the sclera, frequently leading to vision-threatening complications.1,2 The etiology of scleritis is complex, up to 50% of cases are associated with systemic autoimmune diseases, including rheumatoid arthritis (RA).3,4 Less frequently, the putative cause of scleritis may be attributed to local or systemic infection, trauma, specific drugs, irradiation, or malignancy.4 To control the noninfectious inflammation, long-term treatment with systemic immunosuppressive agents is commonly required.5
The frequent association with systemic autoimmune diseases and the beneficial response to immunosuppressive treatment indicates a crucial role for the immune system in the pathogenesis of noninfectious scleritis. Both the innate and the adaptive immune system have been postulated to be involved in the pathogenesis of scleritis. In small case series, increased levels of the proinflammatory cytokines interleukin (IL)-1β, IL-22, tumor necrosis factor alfa (TNF-α), and specific proteolytic enzymes, matrix metalloproteinase 3 (MMP3) and MMP9, were reported in blood and/or tear fluid samples of patients with active scleritis.6, 7, 8, 9, 10, 11, 12 In addition, signs of vasculitis, angiogenesis and lymphangiogenesis, and fibrosis have been reported in sclera affected by scleritis.1,13, 14, 15, 16
However, so far it remains unclear which are the proteins, including cytokines and proteases, of importance in scleritis and its complications. Comprehensive research on biomarkers in scleritis is rare, probably because of the relative scarcity of scleritis and thus a limited availability of patients’ biological samples.8,10,11,17,18 The lack of biomarkers in scleritis complicates its clinical management, and it is yet impossible to identify patients at risk for complications or to predict individual treatment response.
To gain more insight in the disease process and identify biomarker candidates, we performed extensive serum and tear fluid proteomics in a large multicenter cohort of patients with active scleritis and healthy and disease controls in the Netherlands.
Methods
Discovery Cohort
We conducted a multicenter cohort study within the Netherlands (Erasmus Medical Center, Amsterdam University Medical Center, University Medical Center Groningen, Utrecht University Medical Center, and Maastricht University Medical Center) from 2020 to 2022. We included 39 patients with active scleritis and 82 patients with scleritis in a quiet phase of the disease, as well as 3 control groups: healthy individuals (n = 30), ocular disease controls (specifically, HLA-B27 positive anterior uveitis and birdshot chorioretinopathy [n = 11]), and systemic autoimmune disease controls (patients with RA and without any history of eye involvement and/or dry eyes [n = 12]) (Fig 1). A subset of the included patients with scleritis was measured twice, first at an active state and secondly at the moment scleritis had become inactive in 1 to 12 months after (temporary) treatment (n = 11). The study was approved by the Ethics Committee of the Erasmus MC (MEC-2019-0777), and all local ethics committees from the collaborating medical centers. All patients gave written informed consent to participate in the study. The research was performed according to the Tenets of the Declaration of Helsinki.
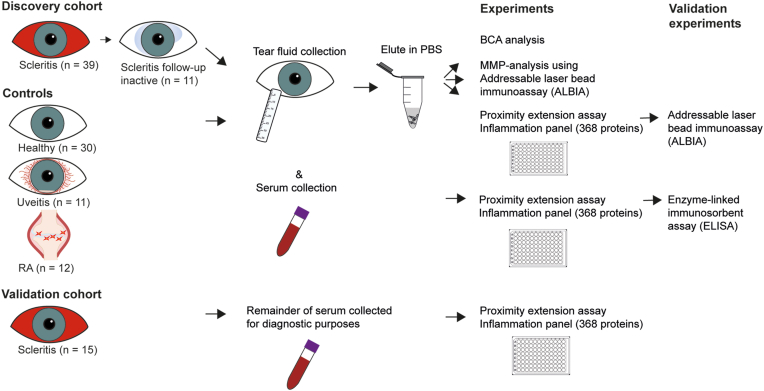
Figure 1.
Methodological procedure of sample collection and analysis. The discovery cohort was collected from university medical centers in the Netherlands from 2020 to 2022. Thirty-nine patients with active scleritis, whereof 11 were also in an inactive state, were collected. In addition, 30 healthy controls and 23 disease controls were collected. Tear fluid collected using Schirmer strips was first eluted in phosphate buffered saline (PBS) buffer and consequently evaluated for total protein measurement, a hypotheses bases analysis into matrix metalloproteinases (MMPs) using addressable laser bead assay, and for 368 inflammatory proteins using proximity extension assays (PEAs). To validate tear fluid PEA analysis, a selection of most differentially expressed proteins was tested with addressable laser bead immunoassays (ALBIA). A validation cohort (serum) was collected and consisted of 15 patients with active scleritis, who visited the department of ophthalmology at the Erasmus Medical Center between 2013 and 2021. The remainder of serum collected for diagnostic purposes was stored for this study. Stored serum of both the discovery as the validation cohort was analyzed for 368 inflammatory proteins using PEA. A selection of differentially expressed proteins in scleritis versus controls was validated using enzyme-linked immunosorbent assay (ELISA). BCA = bicinchoninic acid; RA = rheumatoid arthritis.
The diagnoses of noninfectious scleritis and specific uveitis entities were made by an experienced ophthalmologist. Signs and symptoms specific for scleritis were deep scleral redness, persistent redness after phenylephrine drop administration, and/or T-sign/scleral thickening/subscleral fluid on ultrasound B-scan, and/or signs of scleritis on magnetic resonance imaging. Not all symptoms were required simultaneously for a diagnosis of scleritis; for example, in posterior scleritis redness is not required. Active scleritis was defined by the presence of symptoms at the time of sampling (first attack or recurrence with or without treatment). Inactive scleritis was defined by the absence of active scleritis symptoms regardless of treatment. Scleritis location was defined according to the classification by Watson and Hayreh into anterior, posterior, sclerouveitis, and panscleritis.4 The subtype of scleritis was defined as diffuse, nodular, and necrotizing scleritis. Scleral necrosis was defined as scleral thinning noted at any time of the disease course by split lamp examination. All included patients (scleritis and uveitis) underwent full ophthalmic evaluation and work-up examination for scleritis or uveitis according to national uveitis guidelines, which included analysis of erythrocyte sedimentation rate, C-reactive protein, HLA-B27, antinuclear antibodies (including anti-ENA and anti-dsDNA), antineutrophil cytoplasmic antibodies (ANCA) (including specific PR3-cANCA and MPO-pANCA), anti-citrullinated peptide antibodies, syphilis serology, and interferon-gamma release assay (QuantiFERON-TB Gold In-Tube). Radiologic chest imaging was also performed.19 According to the clinical manifestations, additional examinations and referrals to appropriate subspecialists were performed for a tailored approach.
Validation Cohort
We additionally collected serum samples from an additional 15 patients with active scleritis, who visited the ophthalmology department at the Erasmus MC, Rotterdam, the Netherlands, between 2013 and 2021. These were mainly patients who presented before the start of the multicenter study, or during, but because of technical reasons were not included. All patients underwent diagnostic tests as stated previously, and the remainder of serum was used for this purpose. Sample collection and storage conditions were identical to the samples of the discovery cohort and indicated here after. The local Medical Ethics Committee (Erasmus MC, MEC-2012-016) has reviewed and approved this research protocol.
MMP Analysis
To assess the putative role of MMPs in the development of scleral necrosis, we analyzed tear fluid samples of patients with active scleritis complicated by necrosis (n = 8 patients; n = 10 eyes). In 4 of these patients with scleral necrosis (6 eyes), an additional tear fluid sample was collected during inactive disease state. Patients with active scleritis without necrosis (n = 12), patients with inactive scleritis with scleral necrosis (n = 12), patients with inactive scleritis without necrosis (n = 12), healthy controls (n = 12), and uveitis controls (n = 10) were selected from the original cohort as controls. The inflammation panel in the proximity extension assay (PEA) analysis (Olink Proteomics) included only MMP1 and MMP10, whereas MMPs that were previously found to be upregulated in tear fluid of patients with scleritis were MMP3 and MMP9.6,8,20 Therefore, we have performed an additional analysis described below to investigate the level of 13 MMPs and proteases in the tear fluid of patients with scleritis. The detectability was calculated by dividing the number of samples with values higher than the lower limit of detection by the total number of samples tested.
Sample and Data Collection
We collected serum and tear fluid from all participants, except for one uveitis patient of whom only serum was obtained. Tear fluid samples were obtained using Schirmer strips, according to following protocol. The head of the Schirmer strip (TrueBlue Optics) was placed behind the patient’s lower eyelid and thereafter the patient had their eyes closed for 5 minutes to allow the tear fluid to be absorbed by the strip. The samples were taken without local anesthesia from both the right eye (oculus dexter) and the left eye (oculus sinister) under comparable conditions. Tear-filled strips and serum samples were frozen at −80° C before further processing. For proteomics analysis, tear fluid from the eye with active scleritis was taken; in case both eyes were active at the moment of inclusion, a random selection was made, except for the MMP analysis wherein both eyes were analyzed. Clinical data of included patients were collected from medical charts and included demographics, associated systemic autoimmune diseases (if present), and use of systemic immunosuppressive treatment. In addition, clinical ocular data were gathered, including activity of scleritis, presence of scleral necrosis, and other ocular complications. Characteristics of scleritis include the onset and duration of disease and the laterality, location, and subtype of scleritis.
Tear Fluid Sample Preparation
The collected Schirmer strips were cut into small pieces of approximately 1 mm and placed in a 4° C precooled 1.5 ml Eppendorf Tube. The strip pieces were submerged in a precooled extraction buffer (phosphate buffered saline pH 7.4 with Complete Mini Protease Inhibitor [Sigma-Aldrich]). The samples were incubated on a thermomixer for 1.5 hours at 4° C and 900 rpm. Thereafter, the elution with the extracted proteins was transferred to a 2 ml Eppendorf Tube. The strip pieces were transferred to a 0.5 ml Eppendorf Tube with a syringe needle-punctured hole at the tip. The 0.5 ml Eppendorf Tube was placed in the Eppendorf with the elution and centrifuged at 13,000 rpm for 1 minute at 4° C to obtain all the eluted tear fluid. After centrifugation, the strip pieces were discarded and the obtained elutes were stored at −80° C until further processing.21,22 Total protein concentration measurements were performed using a bicinchoninic acid assay according to manufacturer’s instructions (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific).
PEA Technology
Serum (both the discovery and validation cohort) and tear fluid samples were tested for the presence of 368 inflammatory proteins using PEA technology (Inflammation Panel, Olink Explore 3072, Olink Proteomics). A full list of included proteins is available in Table S1 (available at www.ophthalmologyscience.org). In PEA, matched pairs of specific antibodies both carrying a unique, complementary DNA tag will bind to the respective target protein. The DNA tags of both bound antibodies are able to hybridize when brought in proximity. The hybridized tags are extended to an amplicon and subsequently detected and quantified using quantitative PCR. The number of quantitative PCR cycles is related to the expression of the protein in the tear sample, shown in log base-2 normalized protein expression values.23
Enzyme-Linked Immunosorbent Assay
To validate the findings of serum PEA, 2 proteins that were upregulated in the discovery as well as the validation cohort were additionally tested using enzyme-linked immunosorbent assay (ELISA) in the discovery cohort. Human nuclear migration protein nudC (NudC) was chosen because this protein showed the largest difference between scleritis and controls, and human BH3-interacting domain death agonist was chosen because of differential expression and availability by a reputable ELISA producer. The BH3-interacting domain death agonist ELISA (Invitrogen, Thermo Fischer Scientific) was performed according to manufacturer’s instructions. Briefly, all reagents and samples were brought to room temperature (RT). A total of 100 μl of standards and diluted samples (1:2) were added to the antibody coated 96-wells plates and incubated over night at 4° C with gentle shaking. After washing 4 times using the Bio-Plex Pro wash station (Bio-Rad Laboratories, Inc) 100 μl of biotin conjugated secondary antibody was added and incubated for 1 hour at RT. Afterward, the wash step was repeated, and 100 μl of streptavidin-horseradish peroxidase solution was added and incubated for 45 minutes at RT. Tetramethylbenzidine substrate was added after a wash step, which was incubated for 30 minutes at RT. After addition of 50 μl of stop solution the absorbance was red at 450 nm using a microplate reader (BioTek, BioTek Instruments, Inc). In addition, the NudC ELISA (Lifespan Biosciences) was performed using manufacturer’s instructions. Briefly, the previously stated protocol was followed with samples diluted 1:50, using an incubation times of 1 hour for sample incubation, 30 minutes for the streptavidin-horseradish peroxidase solution, and 15 minutes tetramethylbenzidine substrate. All incubation steps were executed at 37° C.
Addressable Laser Bead Immunoassay
For validation purposes of the tear fluid PEA, we performed addressable laser bead immunoassay using a Luminex Panel Kit from R&D Systems including the proteins that were most differentially expressed (not significant) between scleritis and controls and were available in one panel (i.e., IL-18, C-C motif chemokine ligand 25 [CCL25]/thymus expressed cytokine, transforming growth factor alfa, IL-1β, CCL3/macrophage inflammatory protein-1 alfa, CCL4/macrophage inflammatory protein-1 beta, epidermal growth factor, cluster of differentiation 40 [CD40], IL-6, hepatocyte growth factor [HGF], and CCL21.
For the hypothesis-based analysis into MMPs, we used a second Luminex Panel Kit from R&D Systems including a disintegrin and metalloproteinase with thrombospondin motifs 13, Neurosin, MMP3, MMP8, MMP12, Proteinase 3, U-Plasminogen activator, Cathepsin S, MMP2, MMP7, MMP9, MMP13, and Serpin A12. The assays were performed according to the manufacturer’s instructions. In both assays, all thawed tear fluid samples were vortexed immediately before the 1:2 dilution. The standards and diluted samples were loaded into the wells of the microplates, and the diluted microparticle cocktail was added. The microplate was then incubated for 2 hours at RT on a microtiter plate shaker at 800 rpm. The Bio-Plex Pro wash station (Bio-Rad Laboratories, Inc) was used to wash the wells 3 times. Afterward, a diluted biotin-labeled antibody cocktail was added to each well and incubated for 1 hour at RT on a microplate shaker at 800 rpm. The wash step was repeated, after which diluted Streptavidin-PE was added to each well which incubated for 30 minutes at RT on the shaker set at 800 rpm. After another wash step the plate was incubated for 2 minutes on the shaker at 800 rpm and the mean fluorescence intensity was measured directly using a MAGPIX System (Luminex Corporation).
Data Analysis
Proteins detected with PEA in more than 25% of all samples were included in the data analysis. This cut-off value resulted in the inclusion of 346 of 368 (94%) and 316 of 368 (86%) proteins, from serum and tear fluid samples, respectively. To test for statistical significance in the level of inflammatory proteins between groups a Mann–Whitney U or Kruskal–Wallis test was used with multiple testing correction using the Benjamini–Hochberg procedure, also known as false discovery rate correction. A Pearson chi-square test, Fischer exact test, Student t test, one-way ANOVA with Tukey’s post hoc analysis, or a paired samples t test were applied for further analysis, which are specified in all figures and tables. Pathway analysis was conducted with the R package “pathfindR” using the Kyoto Encyclopedia of Genes and Genomes, Reactome, and BioCarta en Gene Ontology databases.24 Multivariate logistic regression analyses were used to evaluate associations between high levels of specific inflammatory proteins and clinical characteristics of scleritis. Cut-off values based on Youden’s indices were used for dichotomous distribution of the relevant serum proteins. Statistical analysis was performed using R (v4.2.2, R Core Team 2021) and IBM SPSS Statistics 27. Descriptive statistics are shown as median ± interquartile range. A false discovery rate-adjusted P value < 0.05 was considered statistically significant.
Results
Baseline Patient Characteristics
In the discovery cohort we included 39 patients with active scleritis, with a median age at onset of scleritis of 52 ± 18 years and 74% female patients (Table 2). The majority had bilateral disease (54%) and an idiopathic origin of scleritis (74%). Complications of scleritis (such as scleral necrosis, cystoid macular edema, or choroidal effusion_ were common (59%). A total of 30 of 39 patients (77%) used systemic treatment at the moment of inclusion, whereof 26% used biologicals or antimetabolite treatment. In the validation cohort 15 patients with active scleritis were included, with similar baseline characteristics compared with the discovery cohort (Table 2). The 30 healthy controls had a median age of 55 ± 17 years, which was similar to the 11 uveitis controls (57 ± 25 years) and 12 RA controls (60 ± 29 years). The RA control group had a more pronounced female dominance (92%).
Table 2.
Baseline Characteristics of Included Patients and Controls, Presented as Median ± Standard Deviation Unless Stated Otherwise
| Characteristics | Scleritis Discovery Cohort (n = 39) | Scleritis Validation Cohort (n = 15) | Healthy Controls (n = 30) | Uveitis Controls (n = 11)∗ | RA Controls (n = 12) | P Value |
|---|---|---|---|---|---|---|
| Age at inclusion, yrs | 53 ± 21 | 55 ± 20 | 55 ± 17 | 57 ± 25 | 60 ± 29 | 0.89 |
| Male, n (%) | 10 (26) | 7 (47) | 15 (50) | 7 (64) | 1 (8) | 0.014 |
| Scleritis patients | ||||||
| Age onset scleritis, yrs | 52 ± 18 | 47 ± 22 | 0.52 | |||
| Duration scleritis, yrs | 2 ± 5 | 2 ± 5 | 0.59 | |||
| Bilateral disease, n (%) | 21 (54) | 5 (33) | 0.23 | |||
| Etiology, n (%) | 0.21 | |||||
| Systemic disease† | 10 (26) | 5 (33) | ||||
| Idiopathic | 29 (74) | 9 (60) | ||||
| Location scleritis, n (%) | 0.44 | |||||
| Anterior | 18 (46) | 4 (27) | ||||
| Posterior | 4 (10) | 1 (7) | ||||
| Sclero-uveitis | 12 (31) | 6 (40) | ||||
| Panscleritis | 5 (13) | 4 (27) | ||||
| Subtype scleritis, n (%) | 0.32 | |||||
| Diffuse | 21 (60) | 10 (83) | ||||
| Nodular | 9 (26) | 1 (8) | ||||
| Necrotizing | 5 (14) | 1 (8) | ||||
| Complications, n (%)‡ | 23 (59) | 8 (57)§ | 0.57 | |||
| Systemic treatment at inclusion, n (%)‖ | 30 (77) | 9 (60) | 0.31 | |||
| NSAIDs/CS < 3 mos | 14 (36) | 3 (20) | 0.37 | |||
| DMARDs/CS > 3 mos | 17 (44) | 5 (33) | 0.46 | |||
| Biologicals/cytostatics | 10 (26) | 3 (20) | 0.46 | |||
A Pearson chi-square test was used for categorical data, whereas a one-way analysis of variance or Student t test was used for continuous data.
CS = corticosteroids; DMARDs = disease-modifying antirheumatic drugs; NSAIDs = nonsteroidal anti-inflammatory drugs; RA = rheumatoid arthritis.
In one patient with uveitis, no tear fluid was collected.
In the discovery cohort, out of 11 patients with systemic disease, 6 had RA, 2 had Crohn’s disease, 1 had relapsing polychondritis, 1 had granulomatosis with polyangiitis, 1 had arthritis psoriatica, and 1 had sarcoidosis. In the validation cohort, out of 4 patients with systemic disease, 2 had relapsing polychondritis, 1 had GCA, and 1 had sarcoidosis.
Including scleral necrosis (n = 8; 20%), cataract (n = 8; 20%), cystoid macular edema and/or papillitis (n = 10; 25%), choroidal effusion/detachment/folds (n = 8; 20%), serous retinal detachment (n = 4; 10%); peripheral ulcerative keratitis (n = 3; 8%), diplopia (n = 1; 3%), synechiae (n = 1; 3%), ocular hypertension (n = 1; 3%), and enucleation (n = 1; 3%).
For 1 patient in the validation cohort, data on complications was unknown.
From 30 patients using systemic treatment at inclusion, 8 used treatment for <1 months, 5 for <3 months, and 17 for >3 months before inclusion.
Serum Inflammatory Proteins in Scleritis
Out of 368 proteins from the inflammation panel, 346 were present in at least 25% of serum samples. Excluded proteins are shown in Table S3 (available at www.ophthalmologyscience.org). A supervised clustering heatmap of all inflammatory proteins in scleritis and control samples is shown in Figure 2A. Upregulation of a subset of inflammatory proteins is seen in scleritis versus control groups. Figure S3 (available at www.ophthalmologyscience.org) shows the unsupervised clustering heatmap of 346 inflammatory proteins, wherein scleritis samples generally clustered separately from the controls, although some heterogeneity is present. Within patients with scleritis, no clear clustering was seen based on etiology, location, or subtype.
Figure 2.
Serum inflammatory proteins in active scleritis versus controls. A, Supervised clustering heatmap of 346 inflammatory serum proteins in active scleritis (n = 39) vs. healthy controls (n = 30), uveitis controls (n = 11), and rheumatoid arthritis (RA) controls (n = 12) of the discovery cohort. Upregulation of a subset of inflammatory proteins is seen in scleritis versus control groups. No clear clustering was seen regarding scleritis etiology, location, or subtype. However, we do notice center differences. B, Venn diagram of significant upregulated or downregulated serum proteins in the discovery scleritis cohort (n = 39) versus the validation scleritis cohort (n = 15). Eighteen proteins were found to be significantly upregulated or downregulated in the discovery as well as in the validation cohort. C, Pathway analysis of 18 significantly upregulated or downregulated serum proteins in scleritis found in the discovery as well as in the validation cohort shows multiple proteins have functions in apoptosis and the mitogen-activated protein kinase (MAPK) signaling pathway. Pathway analysis was performed using the pathfindR package in R. AUMC = Amsterdam University Medical Center; EMC = Erasmus Medical Center; MUMC = Maastricht University Medical Center; NA = not applicable; UMCG = University Medical Center Groningen; UMCU = Utrecht University Medical Center.
In serum samples of the discovery cohort, we observed 75 significantly upregulated or downregulated proteins in scleritis, compared with all control groups. Figure 2B shows that 18 of these proteins were also significantly upregulated or downregulated in our validation cohort. Table 4 lists the aforementioned 18 proteins, with the most upregulated protein in scleritis being NudC (P = 0.0032), a protein with a role in neurogenesis. Pathway analysis reveals that multiple significantly upregulated or downregulated proteins have functions in apoptosis, as well as in the mitogen-activated protein kinase pathway (Fig 2C). Also, levels of proteins involved in T-cell activation, epithelial barrier maintenance, and angiogenesis were found to be significantly altered in scleritis. Individual dot plots of significantly upregulated or downregulated proteins are shown in Figures 4A–F. To further validate our proteomics results, we evaluated specific proteins using commercially available ELISAs in the discovery cohort versus control groups (Fig S5, available at www.ophthalmologyscience.org). For example, upregulation of the BH3-interacting domain death agonist protein in scleritis (156 ± 197 ng/ml; n = 39) compared with healthy controls (49 ± 124 ng/ml; n = 30; P = 0.004) was confirmed by ELISA (Fig S5A, available at www.ophthalmologyscience.org). Also, a trend toward higher NudC levels was seen in scleritis (2.1 ± 7.9 ng/ml; n = 39) compared with healthy controls (0.04 ± 0.2 ng/ml; n = 30; P = 0.59). Interestingly, RA controls had significantly higher NudC levels (8.8 ± 12.8 ng/ml) than scleritis and other control groups (n = 39; n = 30; n = 11; all P < 0.05) (Fig S5B, available at www.ophthalmologyscience.org).
Table 4.
Significantly Upregulated or Downregulated Serum Proteins in Scleritis versus Healthy and Disease Controls Overlapping in the Discovery and Validation Cohort
| Protein | Protein Name | Fold Change | Punadjusted | Padjusted | Protein Function |
|---|---|---|---|---|---|
| NudC | Nuclear migration protein nudC | 529.8 | 0.0006 | 0.0032 | Plays a role in neurogenesis and neuronal migration. Necessary for correct formation of mitotic spindles and chromosome separation during mitosis. |
| HPCAL1 | Hippocalcin like protein 1 | 11.3 | 8.98.10–6 | 0.0001 | May be involved in the calcium-dependent regulation of rhodopsin phosphorylation. |
| PLA2G4A | Cytosolic phospholipase A2 | 7.3 | 9.32.10–6 | 0.0001 | Positive regulation of T-helper 1 type immune response. |
| ACTN4 | Alfa-actinin-4 | 6.0 | 3.47.10–7 | < 0.0001 | F-actin cross-linking protein which is thought to anchor actin to a variety of intracellular structures. Involved in tight junction assembly in epithelial cells. |
| HSPA1A | Heat shock 70 kDa protein 1A | 4.0 | 1.89.10–8 | < 0.0001 | Molecular chaperone implicated in a wide variety of cellular processes, including protecting the proteome from stress, protein refolding, and degradation. Essential FOXP3 downregulation in regulatory T cells. |
| EIF4G1 | Eukaryotic translation initiation factor 4 gamma 1 | 3.1 | 3.14.10–6 | 0.0001 | Component of the protein complex eIF4F, which is involved in regulation of protein synthesis initiation. |
| GLOD4 | Glyoxalase domain-containing protein 4 | 2.8 | 4.45.10–7 | < 0.0001 | Little is known about GLOD4, the glyoxalase gene family has broad roles in metabolism. |
| CCL7 | CC motif chemokine ligand 7 | 2.6 | 0.0024 | 0.0094 | Chemotactic factor that attracts monocytes, eosinophils, natural killer cells and activated T lymphocytes. Influence on diapedesis and extravasation of leukocytes. Induces the release of gelatinase-B. |
| CLEC4A | C-type lectin domain family 4 member A | 2.3 | 0.0017 | 0.0069 | C-type lectin receptor, involved in regulating immune reactivity. Once triggered by antigen, results in cross-priming of CD8+ T cells. |
| OSM | Oncostatin M | 2.0 | 1.92.10–7 | < 0.0001 | Pleiotropic cytokine that belongs to the interleukin 6 group of cytokines. |
| BID | BH3 interacting domain death agonist | 1.9 | 1.47.10–6 | < 0.0001 | Induces caspases and apoptosis. Counters the protective effect of BCL2. |
| FKBP1B | Peptidyl-prolyl cis-trans isomerase FKBP1B | 1.7 | 3.80.10–6 | 0.0001 | Has the potential to contribute to the immunosuppressive and toxic effects of FK506 and rapamycin. |
| F2R/PAR1 | Proteinase-activated receptor 1/coagulation factor II (thrombin) receptor | 1.7 | 0.0012 | 0.0053 | Involved in the regulation of thrombotic response. Described to mediate interplay between inflammation, coagulation and vascular development. Involved in maintenance of endothelial barrier integrity. |
| HGF | Hepatocyte growth factor | 1.7 | 4.10.10–6 | 0.0001 | Regulate cell growth, cell motility, and morphogenesis in numerous cell and tissue types. Acts as a multi-functional cytokine on cells of mainly epithelial origin. Also plays a role in angiogenesis and tissue regeneration. |
| RAB6A | Ras-related protein Rab-6A | 1.6 | 1.39.10–5 | 0.0002 | Its main function is the regulation of protein transport from the Golgi complex to the endoplasmic reticulum. |
| CD40 | Cluster of differentiation 40 | 1.6 | 0.0006 | 0.0031 | Type I transmembrane protein found on antigen-presenting cells which is required for their activation. Binding of CD40L on Th cells activated antigen-presenting cells. |
| MAPK9 | Mitogen-activated protein kinase 9 | 1.5 | 7.06.10–5 | 0.0005 | Serine/threonine-protein kinase involved in various processes such as cell proliferation, differentiation, migration, transformation, and apoptosis, activated by proinflammatory cytokines. Required for polarized differentiation of T-helper cells into Th1 cells. Also plays an important role in the osmotic stress-induced epithelial tight-junctions disruption. |
| GMPR | GMP reductase 1 | 0.6 | 0.013 | 0.0402 | Enzyme that catalyzes NADPH-dependent reductive deamination of guanosine monophosphate into inosinic acid. Suppressor of melanoma invasion. |
Seventeen proteins are upregulated, and 1 protein is downregulated both in the discovery as well as the validation cohort in scleritis cases versus healthy and disease controls.
Figure 4.
Dot plots of individual serum proteins, which are upregulated in scleritis compared with controls. A-F, Nuclear migration protein nudC (NudC), hippocalcin like protein 1 (HPCAL1), cytosolic phospholipase A2 (PLA2G4A), alpha-actinin-4 (ACTN4), heat shock 70 kDa protein 1A (HSPA1A), and BH3-interacting domain death agonist (BID) were significantly upregulated in scleritis compared to control groups. NPX = Normalized Protein eXpression; RA = rheumatoid arthritis.
In a multivariate logistic regression analysis adjusted for age, sex, and the presence of systemic disease, we observed that 2 proteins were significantly associated with bilateral disease, namely HGF with an odds ratio of 8.5 (95% confidence interval: 1.6–66.2; P = 0.021), and oncostatin M with an odds ratio of 8.8 (95% confidence interval: 1.8–62.4; P = 0.041) (Table S5A, B, available at www.ophthalmologyscience.org). No additional associations between the significant serum proteins and clinical characteristics of scleritis were found. Furthermore, no differences were observed in the level of upregulated or downregulated inflammatory serum proteins between samples collected during active and inactive disease state (n = 11). In Table S6 (available at www.ophthalmologyscience.org), we elaborate on the baseline characteristics of patients with scleritis from all inclusion centers (Erasmus Medical Center, Amsterdam University Medical Center, Utrecht University Medical Center, University Medical Center Groningen, and Maastricht University Medical Center). Variances were seen in etiology, although not significant, as well as in the frequency of systemic disease, which was higher in the Erasmus Medical Center (50%) and Maastricht University Medical Center (29%) compared with the Amsterdam University Medical Center (0%) and Utrecht University Medical Center (0%).
Tear Fluid Inflammatory Proteins in Scleritis
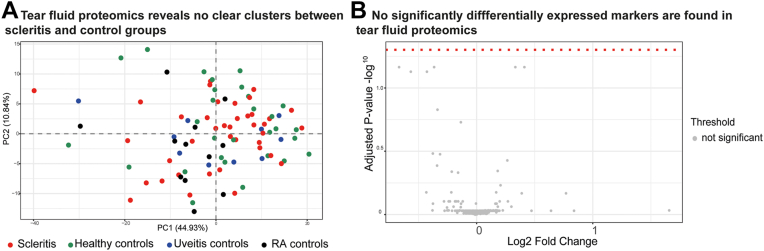
Characteristics of the tear fluid Schirmer strips and tear fluid eluates (migration length and total protein concentration) are shown in Table S7 (available at www.ophthalmologyscience.org). No significant differences were found in the migration length. The total protein concentration in tear fluid was slightly lower in the RA controls (342 ± 130 μg/ml) compared with patients with scleritis (808 ± 582 μg/ml; P = 0.04) and healthy controls (874 ± 519; P = 0.02). Principal component analysis of the tear fluid PEA results did not show differential clustering of samples when scleritis cases were compared with the control groups (Fig 6A). No significantly upregulated or downregulated inflammatory proteins were found in scleritis tear fluid compared with all controls (Fig 6B). Table S8 (available at www.ophthalmologyscience.org) shows a list of the 10 proteins with the lowest P value (P < 0.34). A subset of tear fluid proteins (IL-18, CCL25, transforming growth factor alfa, IL-1β, CCL3, and CCL4) was also investigated in the discovery cohort using addressable laser bead immunoassay, confirming the results by PEA analysis (Figs S7A–F, available at www.ophthalmologyscience.org).
Figure 6.
Tear fluid proteomics reveals no clear cluster or significantly differentially expressed markers in scleritis (n = 39) versus all control groups (n = 52). A, Principal component analysis of 316 inflammatory tear fluid proteins within scleritis and controls tear fluid samples, showing no evident clustering. B, Volcano plot of tear fluid inflammatory proteins in scleritis compared with all control groups shows no significantly different proteins in scleritis tear fluid compared with all control groups. A Mann–Whitney U test was used to compare each protein between scleritis and all other controls, and a false discovery rate correction was applied to correct for multiple testing. RA = rheumatoid arthritis.
MMPs and Other Proteases in Tear Fluid
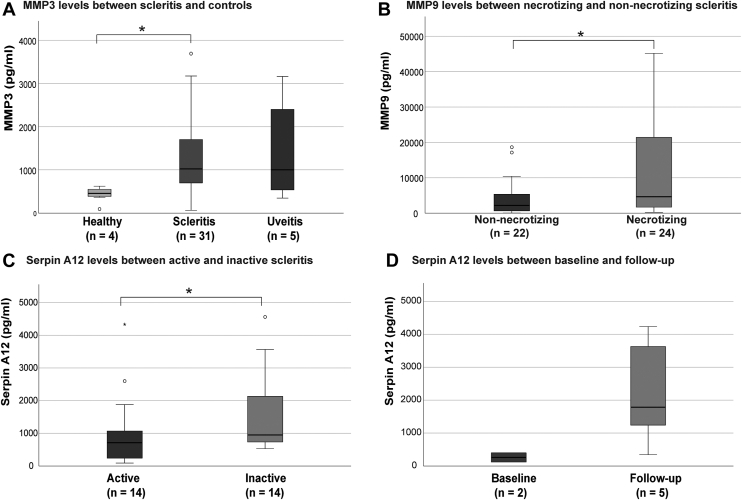
Baseline characteristics of this discovery cohort selection are shown in Table S9 (available at www.ophthalmologyscience.org). A total of 4 of 13 addressable laser bead immunoassay tested proteins were not detectable (detectability < 1.2%; Fig S8, available at www.ophthalmologyscience.org). Matrix metalloproteinase 3 (MMP3) levels were increased in tear fluid samples of patients with scleritis compared with healthy controls (P = 0.044) but not uveitis controls (P = 0.96) (Fig 9A). Matrix metalloproteinase 9 (MMP9) levels were significantly increased in tear fluid of necrotizing patients with scleritis compared with nonnecrotizing controls (4640 ± 19876; n = 24 vs. 2187 ± 5470 pg/ml; n = 22; P = 0.024) (Fig 9B). No differences in MMP3 or MMP9 levels were observed between active and inactive scleritis. Serpin A12, a protease inhibitor was significantly decreased in active versus inactive scleritis samples (713 ± 679; n = 14; vs. 1232 ± 1721 pg/ml; n = 14; P = 0.04) (Fig 9C). Repeated analysis in a subset of patients measured at baseline (active) and follow-up (inactive) revealed a trend toward increasing levels of Serpin A12 at follow-up (inactive; n = 2; 1241 ± 3283 pg/ml) versus baseline (active; n = 5; 259 ± 280 pg/ml) (Fig 9D).
Figure 9.
Analysis of matrix metalloproteinases and other proteases in tear fluid of necrotizing scleritis. A, The protein concentration of matrix metalloproteinase 3 (MMP3) in patients with scleritis was significantly higher compared with healthy controls (1022 ± 1031; n = 31 vs. 458 ± 198 pg/ml; n = 4; P = 0.044), however, not compared with uveitis controls (1002 ± 2246; n = 5; P = 0.96). Three outliers (> 4000 pg/ml) were present within scleritis samples. B, No significant difference was found in MMP3 levels between necrotizing and nonnecrotizing scleritis (862 ± 2791; n = 15 vs. 1178 ± 703 pg/ml; n = 18; P = 0.69; not shown). Matrix metalloproteinase 9 (MMP9) levels were significantly higher in necrotizing vs. nonnecrotizing scleritis tear samples (4640 ± 19876; n = 22 vs. 2187 ± 5470 pg/ml; n = 24; P = 0.024). C, The level of MMP3 and MMP9 were similar between active scleritis tear samples and inactive scleritis tear samples (1040 ± 1579; n = 16 vs. 1221 ± 2844 pg/ml; n = 17; P = 0.48 and median 2402 ± 5564; n = 22 vs. 7952 ± 15 359 pg/ml; n = 24; P = 0.43 for respectively MMP3 and MMP9; not shown). The level of the protease inhibitor Serpin A12 was significantly reduced in active vs. inactive scleritis tear samples (713 ± 679; n = 14 vs. 1232 ± 1721 pg/ml; n = 14; P = 0.04). D, In a continuous analysis between active scleritis at baseline and during inactive follow-up no significant differences were noted, however, a trend toward an increase in Serpin A12 could be observed (259 ± 280; n = 2 vs. 1241 ± 3283 pg/ml; n = 5). Median ± interquartile range is shown for concentrations of MMP3, MMP9, and Serpin A12. Log conversion of MMP3, MMP9, and Serpin A12 was performed for statistical analysis with normally distributed data. A Student t test was used in A–C, whereas the number of samples precluded statistical analysis in D.
Discussion
This study demonstrates that the serum protein NudC, and other serum proteins involved in T-cell activation, epithelial barrier maintenance, apoptosis, and angiogenesis represent potential biomarkers for noninfectious scleritis. Furthermore, we observed upregulated MMP9 levels in the tear fluid of patients with scleritis complicated by necrosis.
The scarcity of reports on biomarkers in noninfectious scleritis results in a lack of insight in the disease course, outcome, and individual treatment response of patients. The only clinical parameters known to be predictive for a worse outcome of scleritis are associated systemic disease and bilateral involvement.5,25 The presence of ANCA and anticitrullinated peptide antibodies has been associated with severe scleritis; however, there is a clear overlap with the occurrence of associated systemic disease.17,18,26 Increased levels of IL-1β, TNF-α, IL-22, MMP9, and filaggrin-2 were previously found in blood, tear fluid and/or scleral tissue of patients with active scleritis, but whether these inflammatory proteins are clinically relevant is not known.8,10,11,22
Our serum analyses revealed 18 proteins to be significantly upregulated or downregulated in scleritis compared with controls. The most upregulated protein was NudC, a protein involved in cell cycle progression, neuronal migration, platelet production, ciliogenesis, and the inflammatory response.27 The role of NudC in the inflammatory response lies in the regulation of the proinflammatory phospholipid platelet activating factor; however, the exact function in vivo is largely unknown.28 This protein was not previously linked to systemic or ocular inflammatory diseases. Interestingly, increased NudC levels were also found in patients with RA in the ELISA, which might indicate an association with scleritis development, be a result of similarities in disease pathogenesis, or may be due to a lower test specificity.
Further, proteins involved in T cells activation and regulation were found to be upregulated (cytosolic phospholipase A2 (PLA2G4A), heat shock protein 1A (HSPA1A), CCL7, cluster of differentiation 40, and mitogen-activated protein kinase 9) indicating a hypothetical role for T-cell activity in scleritis. CD4+ and CD8+ T cells were previously identified in affected scleral tissue, and Th17 cells were found to be increased in scleritis in human peripheral blood mononuclear cells.1,13,22,29, 30, 31 However, further functional studies need to investigate the nature of the T-cell response in scleritis. In addition, proteins involved in epithelial barrier maintenance, such as alfa-actinin-4 (ACTN4), proteinase-activated receptor 1 (F2R), and mitogen-activated protein kinase 9, were elevated in the serum of patients with scleritis. Recently, multiple other proteins involved in epithelial barrier function were found to be upregulated in scleral tissue affected by scleritis.22 Lastly, proteins involved in angiogenesis (F2R and HGF) were enriched. In RA and other systemic immune mediated diseases, neovascularization has a clear pathogenic role.32 In scleritis, multiple signs of angiogenesis and lymphangiogenesis or neovascularization were documented previously. However, whether this process is pathogenic and should be therapeutically targeted is yet unclear.16,22
Interleukin-1β was previously reported to be enriched in serum of scleritis.10 However, this was not confirmed in our results nor in the study of Sainz-de-la-Maza et al,11 devaluating the importance of this proinflammatory marker. Interleukin-22 was found to be significantly upregulated in a comprehensive study by Sainz de la Maza et al; unfortunately, IL-22 was not included in the inflammatory protein panel. We did evaluate the IL-22 receptor subunit α1 (IL-22RA1), which was not significantly different in the serum of patients with scleritis compared with controls. The role of IL-22 in the pathogenesis of scleritis is, therefore, subject for further studies. In addition, whether upregulated proteins might have clinical value as a biomarker needs to be evaluated in follow-up studies. Although we observed that HGF and oncostatin M were significantly associated with bilateral disease, our study was not designed to assess any predictive value.
The studies reporting on biomarkers in scleritis infrequently include tear fluid. Tear fluid is a potential source for biomarker research, given its minimally invasive collection.33, 34, 35, 36, 37 In scleritis, the lacrimal gland is not actively involved in the inflammatory process, except for individual cases. Still, we hypothesized that proteins involved in the local inflammatory process in scleritis may diffuse into the tear film.38 Unfortunately, our extensive analysis of 368 inflammatory proteins using PEA technology in tear fluid did not unravel novel biomarker candidates specific for scleritis. Our methods of tear fluid collection and processing using Schirmer strips and elution are well accepted and used before.21,34,36,39,40 Also, sufficient total protein concentration was found within study groups. Possible explanations for the lack of differences are multiple and might involve decreased diffusion or increased dilution of proteins involved in the local inflammation in the tear fluid in scleritis. The substantial differences in migration length and total protein concentration between individuals could also preclude statistically significant differences. Finally, using this large-scale protein discovery assay, proteins with small but clinically relevant differences might be overlooked.
In tear fluid, MMP9 and TNF-α were previously reported to be increased in necrotizing scleritis by Seo et al.8 However, in our study, levels of TNF-α in tear fluid or serum did not differ between patients with scleritis compared with controls, which is in accordance with Sainz-de-la-Maza et al,11 who included only serum. In our extended study of tear fluid, MMP9 was upregulated in patients with scleral necrosis compared with those without necrosis, confirming earlier findings.8 Matrix metalloproteinase 9, also called gelatinase-B, is a member of the gelatinases and is physiologically active in connective tissue turnover, wound healing, and angiogenesis by cleaving types IV, V, VII, IX, and XI collagen, also present in scleral tissue.22,41, 42, 43, 44, 45, 46 Matrix metalloproteinase 9 in tear fluid could reflect the enhanced production of MMP by scleral fibroblasts, as was seen in an in vitro study by Di Girolamo et al6 and might play a role in the initiation of necrosis. However, it could also be a protein that is released in case of necrosis. Further, blockage of MMP9 was proposed as a potential treatment strategy for RA and constitutes a potential option in the treatment of scleral necrosis.47 We additionally observed an increased level of MMP3 in tear fluid from patients with scleritis, confirming previous reports describing expression of MMP3 in diseased scleral tissue.20 However, in the current study, increased tear fluid levels of MMP3 were also noted in patients with uveitis and thus might not be specific for scleritis.43,48 Serpin A12 was found to be decreased in tear fluid of active versus inactive scleritis. It is a serine protease inhibitor with antidiabetic and antiatherogenic properties, and it is thought to inhibit vascular inflammation.49,50 We hypothesize that it might have a regulatory role in scleritis and could possibly be identified as biomarker for disease activity. No other types of MMPs that are believed to be important in cleaving collagen in RA were found to be increased in scleritis in our study.51 However, we have not evaluated the levels of the inhibitor of MMP9 (TIMP), as well as membrane-type MMP (MMP14), which was identified previously as a master regulator of extracellular matrix in RA.44,52
One of the limitations of our study is the use of systemic medication by the majority of included patients. Inclusion of treatment-naïve patients remains a challenge because most patients referred to a tertiary clinical center are already on treatment. Additionally, we observed differences between the inflammatory serum proteome of patients with scleritis from different centers within the Netherlands, which we could not completely explain. Scleritis is a heterogeneous disease, and sample numbers from different centers are low. However, an influence of sampling conditions, although differences were kept to a minimum, could not be ruled out. Therefore, our independent validation cohort is valuable to increase significance of candidate biomarkers identified. Lastly, associations of significantly upregulated or downregulated proteins with clinical parameters of scleritis should be further evaluated in follow-up studies, preferably with more patients and a continuous analysis to assess their value as a definite biomarker in the clinical setting. Still, we have performed extensive proteomics in the largest cohort of patients with active scleritis so far, employing relevant disease controls to exclude inflammatory markers that are not specific for scleritis.
In conclusion, our study reveals that NudC, MMP9, and other inflammatory proteins are upregulated in serum or tear fluid of patients with noninfectious scleritis. Our results indicate that T-cell activation, apoptosis, and angiogenesis are involved in the pathogenesis of scleritis, which might have consequences for treatment strategies.
Acknowledgments
The authors thank Ina Hidding-Bruintjes, Angelique van Veen, Sanela Kuc, and all other staff from the Erasmus Medical Center, Amsterdam University Medical Center, Utrecht University Medical Center, University Medical Center Groningen, and Maastricht University Medical Center who have contributed to patient inclusion and sample collection. The authors also thank Amber Schotting for contributing to the elution procedure of the tear fluid Schirmer strips and the MMP analysis. Further, special thanks to Nicole Nachtzaam for performing the addressable laser bead immunoassay analysis, and Sanae Boukhrissi for performing the ELISA analysis.
Manuscript no. XOPS-D-23-00172R1.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The authors have no proprietary or commercial interest in any materials discussed in this article.
Supported by the Lijf en Leven foundation under grant (number L&L/mon/19-007), this funding organization had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. The study was approved by the Ethics Committee of the Erasmus MC (MEC-2019-0777), and all local ethics committees from the collaborating medical centers. All patients gave written informed consent to participate in the study. The research was performed according to the tenets of the Declaration of Helsinki.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Vergouwen, Kolijn, de Hoog, de Boer, Los, Gijs, Erckens, de Jong, Rothova, Ten Berge, Schreurs
Data collection: Vergouwen, de Hoog, de Boer, Los, Gijs, Erckens, de Jong, Rothova, Ten Berge, Schreurs
Analysis and interpretation: Vergouwen, Kolijn, de Hoog, de Boer, Los, Gijs, Erckens, de Jong, Rothova, Ten Berge, Schreurs
Obtained funding: N/A
Overall responsibility: Vergouwen, Kolijn, de Hoog, de Boer, Los, Gijs, Erckens, de Jong, Rothova, Ten Berge, Schreurs
Supplementary Data
References
- 1.Wakefield D., Di Girolamo N., Thurau S., et al. Scleritis: immunopathogenesis and molecular basis for therapy. Prog Retin Eye Res. 2013;35:44–62. doi: 10.1016/j.preteyeres.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Sainz de la Maza M., Molina N., Gonzalez-Gonzalez L.A., et al. Scleritis therapy. Ophthalmology. 2012;119:51–58. doi: 10.1016/j.ophtha.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 3.Sainz de la Maza M., Molina N., Gonzalez-Gonzalez L.A., et al. Clinical characteristics of a large cohort of patients with scleritis and episcleritis. Ophthalmology. 2012;119:43–50. doi: 10.1016/j.ophtha.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Watson P.G., Hayreh S.S. Scleritis and episcleritis. Br J Ophthalmol. 1976;60:163–191. doi: 10.1136/bjo.60.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wieringa W.G., Wieringa J.E., ten Dam-van Loon N.H., Los L.I. Visual outcome, treatment results, and prognostic factors in patients with scleritis. Ophthalmology. 2013;120:379–386. doi: 10.1016/j.ophtha.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Di Girolamo N., Lloyd A., McCluskey P., et al. Increased expression of matrix metalloproteinases in vivo in scleritis tissue and in vitro in cultured human scleral fibroblasts. Am J Pathol. 1997;150:653–666. [PMC free article] [PubMed] [Google Scholar]
- 7.Di Girolamo N., Visvanathan K., Lloyd A., Wakefield D. Expression of TNF-α by human plasma cells in chronic inflammation. J Leukoc Biol. 1997;61:667–678. doi: 10.1002/jlb.61.6.667. [DOI] [PubMed] [Google Scholar]
- 8.Seo K.Y., Lee H.K., Kim E.K., Lee J.H. Expression of tumor necrosis factor alpha and matrix metalloproteinase-9 in surgically induced necrotizing scleritis. Ophthalmic Res. 2006;38:66–70. doi: 10.1159/000090010. [DOI] [PubMed] [Google Scholar]
- 9.Wakefield D., Di Girolamo N., Thurau S., et al. Scleritis: challenges in immunopathogenesis and treatment. Discov Med. 2013;16:153–157. [PubMed] [Google Scholar]
- 10.Palexas G.N., Puren A., Savage N., Welsh N.H. Serum interleukin (IL-1β) in patients with diffuse scleritis. Scand J Immunol. 1992;36:171–172. doi: 10.1111/j.1365-3083.1992.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 11.Sainz-de-la-Maza M., Molins B., Mesquida M., et al. Interleukin-22 serum levels are elevated in active scleritis. Acta Ophthalmol. 2016;94:e395–e399. doi: 10.1111/aos.13005. [DOI] [PubMed] [Google Scholar]
- 12.Vergouwen D.P.C., Rothova A., Berge J.C.T., et al. Current insights in the pathogenesis of scleritis. Exp Eye Res. 2020;197 doi: 10.1016/j.exer.2020.108078. [DOI] [PubMed] [Google Scholar]
- 13.Rao N.A., Marak G.E., Hidayat A.A. Necrotizing scleritis. A clinico-pathologic study of 41 cases. Ophthalmology. 1985;92:1542–1549. [PubMed] [Google Scholar]
- 14.Watson P.G., Young R.D. Scleral structure, organisation and disease. A review. Exp Eye Res. 2004;78:609–623. doi: 10.1016/s0014-4835(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 15.Watson P., Romano A. The impact of new methods of investigation and treatment on the understanding of the pathology of scleral inflammation. Eye (Lond) 2014;28:915–930. doi: 10.1038/eye.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishio Y., Taniguchi H., Takeda A., Hori J. Immunopathological analysis of a mouse model of arthritis-associated scleritis and implications for molecular targeted therapy for severe scleritis. Int J Mol Sci. 2021;23:341. doi: 10.3390/ijms23010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang L.T., Lim L.L., Vaillant B., et al. Antineutrophil cytoplasmic antibody-associated active scleritis. Arch Ophthalmol. 2008;126:651–655. doi: 10.1001/archopht.126.5.651. [DOI] [PubMed] [Google Scholar]
- 18.de Sousa J.M., Trevisani V.F.M., Modolo R.P., et al. Comparative study of ophthalmological and serological manifestations and the therapeutic response of patients with isolated scleritis and scleritis associated with systemic diseases. Arq Bras Oftalmol. 2011;74:405–409. doi: 10.1590/s0004-27492011000600004. [DOI] [PubMed] [Google Scholar]
- 19.Gezelschap N.O. Richtlijn uveitis. Uveitis Screening: Nederlands Oogheelkundig Gezelschap. 2015 [Google Scholar]
- 20.Di Girolamo N., Tedla N., Lloyd A., Wakefield D. Expression of matrix metalloproteinases by human plasma cells and B lymphocytes. Eur J Immunol. 1998;28:1773–1784. doi: 10.1002/(SICI)1521-4141(199806)28:06<1773::AID-IMMU1773>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Gijs M., Veugen J.M.J., Wolffs P.F.G., et al. In-depth investigation of conjunctival swabs and tear fluid of symptomatic COVID-19 patients, an observational cohort study. Transl Vis Sci Technol. 2021;10:32. doi: 10.1167/tvst.10.12.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vergouwen D.P.C., Ten Berge J.C., Guzel C., et al. Scleral proteome in noninfectious scleritis unravels upregulation of filaggrin-2 and signs of neovascularization. Invest Ophthalmol Vis Sci. 2023;64:27. doi: 10.1167/iovs.64.3.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solier C., Langen H. Antibody-based proteomics and biomarker research – current status and limitations. Proteomics. 2014;14:774–783. doi: 10.1002/pmic.201300334. [DOI] [PubMed] [Google Scholar]
- 24.Ulgen E., Ozisik O., Sezerman O.U. pathfindR: an R package for comprehensive identification of enriched pathways in omics data through active subnetworks. Front Genet. 2019;10:858. doi: 10.3389/fgene.2019.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kempen J.H., Pistilli M., Begum H., et al. Remission of non-infectious anterior scleritis: incidence and predictive factors. Am J Ophthalmol. 2021;223:377–395. doi: 10.1016/j.ajo.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Vergouwen D.P.C., Ten Berge J.C., Boukhrissi S., et al. Clinical relevance of autoantibodies and inflammatory parameters in non-infectious scleritis. Ocul Immunol Inflamm. 2022;30:1859–1865. doi: 10.1080/09273948.2021.1966050. [DOI] [PubMed] [Google Scholar]
- 27.Fu Q., Wang W., Zhou T., Yang Y. Emerging roles of NudC family: from molecular regulation to clinical implications. Sci China Life Sci. 2016;59:455–462. doi: 10.1007/s11427-016-5029-2. [DOI] [PubMed] [Google Scholar]
- 28.Riera J., Rodríguez R., Carcedo M.T., et al. Isolation and characterization of nudC from mouse macrophages, a gene implicated in the inflammatory response through the regulation of PAF-AH(I) activity. FEBS Lett. 2007;581:3057–3062. doi: 10.1016/j.febslet.2007.05.065. [DOI] [PubMed] [Google Scholar]
- 29.Riono W.P., Hidayat A.A., Rao N.A. Scleritis: a clinicopathologic study of 55 cases. Ophthalmology. 1999;106:1328–1333. doi: 10.1016/S0161-6420(99)00719-8. [DOI] [PubMed] [Google Scholar]
- 30.Usui Y., Parikh J., Goto H., Rao N.A. Immunopathology of necrotising scleritis. Br J Ophthalmol. 2008;92:417–419. doi: 10.1136/bjo.2007.126425. [DOI] [PubMed] [Google Scholar]
- 31.Amadi-Obi A., Yu C.R., Liu X., et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 32.Elshabrawy H.A., Chen Z., Volin M.V., et al. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis. 2015;18:433–448. doi: 10.1007/s10456-015-9477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nättinen J., Aapola U., Nukareddy P., Uusitalo H. Looking deeper into ocular surface health: an introduction to clinical tear proteomics analysis. Acta Ophthalmol. 2022;100:486–498. doi: 10.1111/aos.15059. [DOI] [PubMed] [Google Scholar]
- 34.Azkargorta M., Soria J., Acera A., et al. Human tear proteomics and peptidomics in ophthalmology: toward the translation of proteomic biomarkers into clinical practice. J Proteomics. 2017;150:359–367. doi: 10.1016/j.jprot.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Jones V.S., Wu J., Zhu S.W., Huang R.P. Application of multiplex immunoassay technology to investigations of ocular disease. Expert Rev Mol Med. 2016;18:e15. doi: 10.1017/erm.2016.15. [DOI] [PubMed] [Google Scholar]
- 36.Posa A., Bräuer L., Schicht M., et al. Schirmer strip vs. capillary tube method: non-invasive methods of obtaining proteins from tear fluid. Ann Anat. 2013;195:137–142. doi: 10.1016/j.aanat.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Nättinen J., Aapola U., Jylhä A., et al. Comparison of capillary and Schirmer strip tear fluid sampling methods using SWATH-MS proteomics approach. Transl Vis Sci Technol. 2020;9:16. doi: 10.1167/tvst.9.3.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulus Y.M., Cockerham K.P., Cockerham G.C., Gratzinger D. IgG4-positive sclerosing orbital inflammation involving the conjunctiva: a case report. Ocul Immunol Inflamm. 2012;20:375–377. doi: 10.3109/09273948.2012.709574. [DOI] [PubMed] [Google Scholar]
- 39.Gijs M., Arumugam S., van de Sande N., et al. Pre-analytical sample handling effects on tear fluid protein levels. Sci Rep. 2023;13:1317. doi: 10.1038/s41598-023-28363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vergouwen D.P.C., Schotting A.J., Endermann T., et al. Evaluation of pre-processing methods for tear fluid proteomics using proximity extension assays. Sci Rep. 2023;13:4433. doi: 10.1038/s41598-023-31227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matrisian L.M. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 42.Okada Y., Gonoji Y., Naka K., et al. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J Biol Chem. 1992;267:21712–21719. [PubMed] [Google Scholar]
- 43.Rose B.J., Kooyman D.L. A tale of two joints: the role of matrix metalloproteases in cartilage biology. Dis Markers. 2016;2016 doi: 10.1155/2016/4895050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mondal S., Adhikari N., Banerjee S., et al. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: a minireview. Eur J Med Chem. 2020;194 doi: 10.1016/j.ejmech.2020.112260. [DOI] [PubMed] [Google Scholar]
- 45.Mohanty V., Subbannayya Y., Najar M.A., et al. Proteomics and visual health research: proteome of the human sclera using high-resolution mass spectrometry. Omics. 2019;23:98–110. doi: 10.1089/omi.2018.0185. [DOI] [PubMed] [Google Scholar]
- 46.Zhang P., Karani R., Turner R.L., et al. The proteome of normal human retrobulbar optic nerve and sclera. Proteomics. 2016;16:2592–2596. doi: 10.1002/pmic.201600229. [DOI] [PubMed] [Google Scholar]
- 47.Chen S.J., Lin G.J., Chen J.W., et al. Immunopathogenic mechanisms and novel immune-modulated therapies in rheumatoid arthritis. Int J Mol Sci. 2019;20:1332. doi: 10.3390/ijms20061332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato T., Yamano Y., Tomaru U., et al. Serum level of soluble triggering receptor expressed on myeloid cells-1 as a biomarker of disease activity in relapsing polychondritis. Mod Rheumatol. 2014;24:129–136. doi: 10.3109/14397595.2013.852854. [DOI] [PubMed] [Google Scholar]
- 49.Phalitakul S., Okada M., Hara Y., Yamawaki H. Vaspin prevents TNF-α-induced intracellular adhesion molecule-1 via inhibiting reactive oxygen species-dependent NF-κB and PKCθ activation in cultured rat vascular smooth muscle cells. Pharmacol Res. 2011;64:493–500. doi: 10.1016/j.phrs.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Jung C.H., Lee M.J., Kang Y.M., et al. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Diabetol. 2014;13:41. doi: 10.1186/1475-2840-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burrage P.S., Mix K.S., Brinckerhoff C.E. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 52.Sabeh F., Fox D., Weiss S.J. Membrane-type I matrix metalloproteinase-dependent regulation of rheumatoid arthritis synoviocyte function. J Immunol. 2010;184:6396–6406. doi: 10.4049/jimmunol.0904068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.