Abstract
Background
Biotin-Thiamine-Responsive Basal Ganglia Disease (BTBGD) is a treatable neurometabolic condition associated with pathogenic variants in the SLC19A3 gene. The classical childhood-onset phenotype presents at a mean age of 4 years, ranging from birth to 12 years. These patients present with subacute encephalopathy, dysarthria, dysphagia, dystonia, external ophthalmoplegia, seizures, quadriparesis, and even death. Chronically, an MRI brain reveals atrophy and necrosis of the basal ganglia.
Case report
A 16-year-old girl presented in the context of pneumonia with gradual-onset, slowly progressive neurological symptoms. These initial symptoms self-resolved, without treatment with biotin or thiamine, though she had persistent concerns with her writing and memory. MRI brain noted bilateral abnormal signals in the basal ganglia, involving the head and body of the caudate nuclei and the putamen. Whole-exome sequencing (WES) revealed homozygosity for a likely pathogenic variant in the SLC19A3 gene, c.517A > G (p.N173D). Her residual neurological symptoms resolved with biotin and thiamine treatment, with the exception of ongoing memory concerns.
Conclusion
We describe a patient presenting with an atypical form of the classical childhood-onset phenotype of BTBGD. Our case emphasizes that BTBGD is a condition that should be considered as a potential diagnosis in all children, including older children, presenting with the new onset of even minor neurological deficits in the context of illness. It highlights the importance of brain MRI and WES in identifying patients with atypical presentations.
Keywords: Biotin-Thiamine-Responsive Basal Ganglia Disease (BTBGD), Neurometabolic, Neurogenetic, Leukoencephalopathy, Inborn errors of metabolism (IEM)
1. Case
Our patient was first seen in our Metabolic Genetics clinic as a 16-year-old girl. She was referred in the context of being previously very healthy, with no clinical concerns until 6 months prior to her appointment. Her pregnancy, delivery, neonatal, and early childhood histories were reviewed and were unremarkable for any notable clinical concerns. She was born following an uncomplicated pregnancy, at term, via repeat cesarean section. The delivery was uncomplicated, and her newborn screening was negative. Her past medical history was unremarkable for any overnight hospitalizations, concussions, seizures including febrile seizures, or intracranial infections. She had no history of developmental delays or regressions. She was a straight-A student. She was the product of a non-consanguineous union between healthy parents of Indian descent, with a healthy elder sibling and a healthy younger sibling. A review of her family history was negative for any concerns for developmental delays, significant childhood illnesses, early or unexplained deaths, neurological concerns, or known or suspected genetic diagnoses.
She first came to medical attention when she presented to her primary care physician with symptoms of a lower respiratory tract infection and was diagnosed with pneumonia. She was treated with a 5-day course of oral antibiotics. On the 6th day of her illness, she had an episode of emesis after having breakfast. She had no associated abdominal pain, diarrhea, or headache. She went to school that day and was able to perform well, but noticed visual blurriness exacerbated by movement. She then developed a dizzy sensation towards the end of the school day. She had no syncope, altered level of consciousness, motor or sensory dysfunction of the limbs, or changes in speech pattern. The following day, her dizziness subsided, but she had increasing episodes of visual blurriness. These were particularly on the right side, and episodic, when she walked or read. She was able to complete her activities of daily living well, despite the blurriness. There was no associated visual loss or diplopia. These episodes ceased entirely after approximately 2 weeks. Following these, the patient noticed a heaviness of her eyelids nearing the end of the day, with an associated difficulty opening her eyes. She had no associated diplopia, bulbar or oro-motor symptoms, focal weakness, generalized weakness, or fatiguability. These symptoms also resolved after approximately 2 weeks. Along with the aforementioned symptoms, the patient had increased fatigue, difficulty focusing and concentrating at school, and a mild headache behind her eyes. She missed her morning classes during this period, as she felt she needed extra sleep.
Following the cessation of the above-noted symptoms, she noticed the onset of restlessness in her legs when sitting for prolonged periods of time, and when trying to fall asleep. Associated with this, she had a painful heaviness or pressure from her thighs to the entirety of the soles of her feet, circumferentially. For the first 48 h after she first noticed these symptoms, she had paresthesia over the soles of her feet, at times up to the level of her knees bilaterally, more so on the right side than the left. She also had paresthesia over the entirety of the palmar aspect of her hands. The restlessness of her legs and the paresthesia improved after about a month. She reported no associated dysarthria, tremor, dystonia, encephalopathy, or cognitive regression.
As part of her initial work-up, the patient had magnetic resonance imaging (MRI) done of her brain. The imaging, performed approximately two months after her initial presentation at a community hospital, revealed bilateral, symmetric basal ganglia infarcts involving the caudate body, caudate head, and the anterior and posterior portions of the putamen. Little to no diffusion restriction was seen. It was felt that the basal ganglia infarcts appeared more subacute to chronic, versus acute, in nature. Upon her referral to a neurologist shortly after her MRI was done, she was found to have an unremarkable physical examination, with no focal motor or sensory deficits, no extrapyramidal signs, and no systemic symptoms or signs to suggest a specific etiology for her presentation. An extensive work-up was arranged, which was unremarkable. This included plasma amino acids indicating only a mildly elevated glutamine level, with normal ammonium, vitamin B12 level, and transaminases. Normal lactate of 1.0 mmol/L (reference 0.6–2.4 mmol/L) was noted, as well as normal uric acid, copper, caeruloplasmin, and urine organic acids.
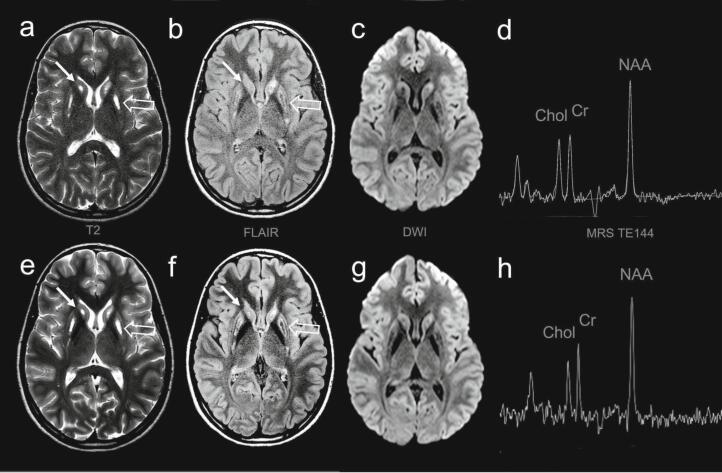
A repeat MRI brain (Fig. 1) was urgently arranged at The Hospital for Sick Children and was performed approximately one month after the initial study. There was, again, a note made of bilateral abnormal signal in the basal ganglia, predominantly involving the head and body of the caudate nuclei and putamen. Some central necrosis or cystic change was also noted. It was suspected that there was bilateral volume loss of the putamen. The differential diagnosis was felt to include metabolic etiologies (including mitochondrial disorders), toxic exposures, and remote injuries or insults, including previous infections that could predominantly affect the basal ganglia (including mycoplasma and influenza A).
Fig. 1.
MRI brain performed 2 months after the initial presentation (a–d) and subsequent MRI brain 1 year after diagnosis and treatment (e–h).
MRI of the brain performed 2 months following the onset of symptoms (a–d) revealed abnormal signal of the head and body of the caudate nuclei (arrows) and the putamen (open arrows) on T2 (a) and FLAIR (b) axial images. There was a subtly increased signal in these regions on DWI (c), with the ADC maps (not shown) suggesting T2 shine through (subacute to chronic) rather than acute diffusion restriction. There was no lactate shown on intermediate TE (d) or short TE single-voxel MRS (not shown). Sequential MRI performed 1 year after diagnosis and treatment (e–h) showed no change in T2 (e), FLAIR (f), or DWI (g) signal. There was no change in spectroscopy (h).
When the patient was seen in our clinic, she reported difficulties with her memory. She had difficulty with the recollection of names and explained that her difficulties with recall were making her homework take longer to complete. Despite these subjective deficits, the patient had no change in her overall academic performance, continuing to excel. The other symptoms she reported were that her writing was not as neat as it was prior to her illness and that it was more laborious than previously and sometimes painful. The patient was seen virtually due to the COVID-19 pandemic. While this did not allow for a physical examination to be performed, her history of writing difficulties was felt to be suspicious for dystonia. To better elucidate the underlying etiology of her persistent neurological deficits and MRI brain findings, clinical whole-exome sequencing (WES) was arranged. There was a significant delay in the patient having this testing done.
WES revealed homozygosity for a missense variant in exon 2 of the SLC19A3 gene (OMIM 606152), c.517A > G (p.N173D), inherited from her carrier parents. It was classified as likely pathogenic based on in silico analysis predicting a deleterious effect on protein structure/function, lack of observation in large population cohorts, and lack of observation in homozygous state in controls. Additionally, it was described in a homozygous state in a patient with a very similar adolescent-onset phenotype of BTBGD in the literature [1]. Our patient's asymptomatic younger sibling was found to be a carrier for the familial variant. Her asymptomatic elder sibling was referred to their local genetics clinic for testing and was reportedly unaffected. Our patient's clinical presentation and MRI findings were felt to be suggestive of (though atypical for) the metabolic disorder associated with pathogenic variants in SLC19A3, Biotin-Thiamine-Responsive Basal Ganglia Disease (BTBGD). Her genetic testing results were thus felt to be explanatory of her clinical presentation.
As there was a delay in the testing being done, her diagnosis was made approximately a year and a half after her initial presentation. At this time, she was prescribed supplementation with biotin (20,000 μg daily, or a dose of approximately 0.4 mg/kg/day), and thiamine (600 mg daily, or a dose of approximately 12 mg/kg/day). It was explained to the patient that initiation of the biotin and thiamine supplementation could improve her persistent neurological symptoms, and that this would be a lifelong treatment. An emergency care plan was provided to the patient describing the treatment with increased and intravenous doses of thiamine during acute decompensations.
Interestingly, at the time of her follow-up visit to review her genetic testing results, the patient reported having recently been infected with COVID-19 (SARS-CoV-2). She reported mild symptoms, coughing, fatigue, headaches, and nasal congestion. She also had a history of mild encephalopathy, being somewhat disoriented for a few days during her illness. She had no associated dystonia, ataxia, seizures, ocular symptoms, facial palsy, or bulbar symptoms during this time.
Outside of that period of illness, the patient reported improvements in her previously noted handwriting concerns, but not in her previously noted memory deficits. Despite the deficits persisting, she reported her academic performance was unchanged. She also reported a recurrence of nausea once or twice per week, with associated morning emesis and headaches on those days. Her physical examination at that time was unremarkable.
At her follow-up visit a month after initiation of treatment, as expected, the patient reported resolution of the aforementioned residual symptoms. These had resolved within a few days of initiation of the biotin and thiamine. She reported a fine, low-amplitude, low-velocity tremor in her hands for a few days just prior to starting the supplements, and paresthesias in her fingers. These symptoms also resolved within a few days of biotin and thiamine initiation and did not recur thereafter. She still reported that her memory has not quite recovered completely. Her physical examination, once again, was unremarkable. A repeat MRI brain performed a year following diagnosis and treatment showed stable symmetric signal alteration in the basal ganglia predominantly involving the caudate and putamen with volume loss and areas of cystic change/necrosis. There were no acute diffusion abnormalities or new areas of signal change (Fig. 1).
2. Discussion & conclusion
BTBGD is a treatable neurometabolic condition associated with pathogenic variants in the autosomal-recessive SLC19A3 gene [2], as discovered by Zeng et al. This gene encodes for the thiamine transporter 2 (hTHTR2), through which thiamine enters the cytosol and is converted into thiamine pyrophosphate (its active form) by thiamine pyrophosphokinase 1 [3]. This condition was first described by Ozand et al., and at this time, there have been more than 100 patients reported worldwide. Most of these patients have been reported in Saudi Arabia [3]. This condition is treated with the chronic and acute supplementation of biotin and thiamine. Administration early in the disease course results in complete clinical improvement, within days [2].
The clinical phenotype of this disorder is heterogenous, with 3 main phenotypes differing in their age of onset. The first is the classical childhood-onset phenotype, with a mean age of presentation of 4 years, but ranging from birth to 12 years of age [4]. These patients are known to present with a subacute encephalopathy, with associated dysarthria and dysphagia, Occasionally, they present with a supranuclear facial nerve palsy, external ophthalmoplegia, and progression to cogwheel rigidity, dystonia, seizures, quadriparesis, and sometimes even death. MRI of the brain in these patients shows, in acute crises, increased T2 signal with swelling in the basal ganglia (caudate and putamen), and diffuse swelling of the cortical and subcortical white matter and infratentorial brain. Chronically, brain MRI reveals atrophy and necrosis of the basal ganglia (caudate and putamen) [2]. The second major phenotype is an early-infantile, Leigh-like presentation, or an atypical infantile spasms-type presentation [3,5]. The third major phenotype is adult-onset, Wernicke's-like encephalopathy. This has been described in 2 adult male patients from Japan [6].
Wang et al. reviewed a total of 159 patients with BTBGD, in 40 reports published worldwide. They found that out of the 146 not excluded from their analysis, 101 (69.2%) were diagnosed with the classical, childhood-onset phenotype, 41 (28.1%) with more severe phenotypes including the early-infantile, Leigh-like presentation, and the atypical infantile spasms-type presentation, and 2 patients (1.4%) were diagnosed with the adult-onset, Wernicke's-like encephalopathy. Additionally, 1 patient was noted to be asymptomatic, and 1 to be presenting with a non-specific developmental delay [5].
The classical, childhood-onset phenotype of BTBGD has a mean onset of 4 years of age, with an age of onset ranging from birth to age 12 years [5]. In this case report, we describe the initial onset of clinical symptoms in a 16-year-old, with her first presentation occurring at a much later age than the majority of patients sharing her clinical phenotype. Phenotypically, while she fits best in the classical childhood-onset category of patients, she presented with relatively mild, slowly progressive neurological symptoms. Interestingly, her acute-phase symptoms self-resolved, rather than acutely worsening, despite a delay in diagnosis and treatment.
Maney et al. recently described a case of BTBGD diagnosed very early in a 2-month-old infant [8]. Our case emphasizes that BTBGD is a condition that can present with a milder, later-onset phenotype, further adding to the understanding of the disease spectrum.
Finally, our case adds to the knowledge of how this disorder may present in the context of COVID-19 infections. Al-Anezi et al. recently described BTBGD presenting for the first time in a patient with an acute COVID-19 infection. Their report described a progressive, acute encephalopathy in a 2-year-old child with a severe acute COVID-19 infection [7]. Our patient presented with relatively minor infectious symptoms in the context of her acute COVID-19 infection, and experienced relatively minor symptoms of subacute encephalopathy.
Since BTBGD is a treatable disorder with excellent treatment outcomes [2], it is not a diagnosis that clinicians can afford to miss. It should be considered as a potential diagnosis in all children, including older children, presenting with new-onset neurological deficits in the context of illness and basal ganglia disease on brain MRI. Our case also emphasizes that this diagnosis should be considered in patients with relatively minor neurological deficits, rather than the classically described presentation with subacute encephalopathy and associated movement disorder and seizures [3]. Finally, our case highlights the importance of MRI of the brain and WES in identifying atypical presentations of neurometabolic conditions like BTBGD in patients with persistent mild neurological complaints.
Notes on patient consent
Written, informed consent was obtained from the patient for publication of this case report, and any accompanying images.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Fassone E., Wedatilake Y., DeVile C.J., Chong W.K., Carr L.J., Rahman S. Treatable Leigh-like encephalopathy presenting in adolescence. BMJ Case Rep. 2013 Oct 7;(2013) doi: 10.1136/bcr-2013-200838. PMID: 24099834; PMCID: PMC3822156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabarki B., Al-Hashem A., Alfadhel M. In: GeneReviews® [Internet] Adam M.P., Ardinger H.H., Pagon R.A., et al., editors. University of Washington, Seattle; Seattle (WA): 1993–2022. Biotin-thiamine-responsive basal ganglia disease. 2013 Nov 21 [Updated 2020 Aug 20] Available from: https://www.ncbi.nlm.nih.gov/books/NBK169615/ [Google Scholar]
- 3.Alfadhel M., Tabarki B. SLC19A3 gene defects sorting the phenotype and acronyms: review. Neuropediatrics. 2018 Apr;49(2):83–92. doi: 10.1055/s-0037-1607191. Epub 2017 Sep 29. PMID: 28962040. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Wang J., Han X., Liu Z., Ma Y., Chen G., Zhang H., Sun D., Xu R., Liu Y., Zhang Y., Wen Y., Bao X., Chen Q., Fang F. Report of the largest Chinese cohort with SLC19A3 gene defect and literature review. Front. Genet. 2021 Jul 1;12 doi: 10.3389/fgene.2021.683255. PMID: 34276785; PMCID: PMC8281341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada K., Miura K., Hara K., Suzuki M., Nakanishi K., Kumagai T., Ishihara N., Yamada Y., Kuwano R., Tsuji S., Wakamatsu N. A wide spectrum of clinical and brain MRI findings in patients with SLC19A3 mutations. BMC Med. Genet. 2010 Dec 22;11:171. doi: 10.1186/1471-2350-11-171. PMID: 21176162; PMCID: PMC3022826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kono S., Miyajima H., Yoshida K., Togawa A., Shirakawa K., Suzuki H. Mutations in a thiamine-transporter gene and Wernicke’s-like encephalopathy. N. Engl. J. Med. 2009 Apr 23;360(17):1792–1794. doi: 10.1056/NEJMc0809100. (PMID: 19387023) [DOI] [PubMed] [Google Scholar]
- 7.Al-Anezi A., Sotirova-Koulli V., Shalaby O., Ibrahim A., Abdulmotagalli N., Youssef R., Hossam El-Din M. Biotin-thiamine responsive basal ganglia disease in the era of COVID-19 outbreak diagnosis not to be missed: a case report. Brain and Development. 2021 Dec 22 doi: 10.1016/j.braindev.2021.12.003. S0387-7604(21)00231-X. (Epub ahead of print. PMID: 34953623; PMCID: PMC8696467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maney K., Pizoly C., Russ J.B. Child neurology: infantile biotin thiamine responsive basal ganglia disease: case report and brief review. Neurology. 2023 Apr 25;100(17):836–839. doi: 10.1212/WNL.0000000000206832. (Epub 2023 Jan 19) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.