Abstract
The cornerstone treatment of hyperphenylalaninemia (HPA) and phenylketonuria (PKU) is a lifelong low-protein diet with phenylalanine (Phe) free L-amino acid supplements. However, the PKU diet has significant shortcomings, and there is a clinically unmet need for new therapeutics to improve patient outcomes. CDX-6114 is a modified phenylalanine ammonia-lyase (PAL) enzyme obtained by a mutation in the Anabaena variabilis PAL sequence. CodeEvolver® protein engineering technology has been applied to improve the degradation resistance of the enzyme. In our first phase I trial, 19 patients were given a single oral dose of CDX-6114 at 7.5 g, 2.5 g, 0.7 g, or placebo in a cross-over design. After an overnight fast, patients received a standardised breakfast of 20 g of protein, thus exceeding the dietary recommendations for a single meal in patients with PKU. Plasma levels of Phe and cinnamic acid (CA) were measured over a 5-h period following CDX-6114 dosing. During the development of CDX-6114, a stability assessment using reverse-phase high-performance liquid chromatography (HPLC) assay revealed two peaks. The second peak was identified as CA. It was not previously known that as part of the mechanism of action, the CA remained associated with the protein following the conversion of Phe. Thus, recalculating the historical PAL enzyme amounts in CDX-6114 bulk substance was necessary. An updated extinction coefficient was achieved by applying a correction factor of 0.771 to previously reported doses. Postprandial plasma levels of Phe increased in all dose cohorts over time between 10% and 30% from baseline, although the actual peak of Phe levels was not achieved within the 5-h observation. When accounting for the interquartile ranges, these concentrations were similar to the placebo. As plasma levels of Phe were no longer a reliable marker for pharmacodynamics, the consistently detectable amount of CA seen in all patients who received CDX-6114 provided proof of the enzymatic activity of CDX-6114 in metabolising gastrointestinal Phe. Peak levels of CA were seen shortly after CDX-6114 intake, with a rapid decline, and remained low compared with the plasma Phe levels. This pattern indicates a short half-life, possibly due to the liquid formulation or the inability to withstand the lower pH in the human stomach compared with animal models in earlier studies. This was the first trial in patients with PKU to establish the safety and tolerability of CDX-6114. A single dose of CDX-6114 was safe and well tolerated, with no serious adverse events or presence of anti-drug antibodies detected. Efficacy will be explored in future trials using an optimised formulation.
Keywords: Phenylketonuria, CDX-6114, Enzyme, Cinnamic acid, Phenylalanine hydroxylase
Graphical abstract
Highlights
-
•
CDX-6114: A modified version of a phenylalanine ammonia-lyase (PAL) enzyme.
-
•
CDX-6114 removes Phe from protein in the gastrointestinal tract before systemic absorption.
-
•
PAL contains both Phe and cinnamic acid (CA), which should be accounted for.
-
•
The historical extinction coefficient did not account for the presence of CA, requiring a dose correction factor of 0.771.
-
•
CDX-6114: Safe and well tolerated in patients with PKU.
1. Introduction
Hyperphenylalaninemia (HPA) and phenylketonuria (PKU) are autosomal recessive inborn errors of phenylalanine metabolism resulting from mutations in the phenylalanine hydroxylase (PAH) (EC 1.14.16.1) gene [1,2] or more rarely elevated levels of phenylalanine is also seen due to defects in the genes of the tetrahydrobiopterin (BH4) biosynthesis and recycling pathway [3], BH4 is a co-factor required for PAH activity. The global prevalence of PKU is estimated to be 1:23,930 live births. It is considered an orphan drug population, with the global phenotype distribution of classic PKU (62%), mild PKU (22%), and mild HPA (16%) [4]. PAH catalyses the conversion of phenylalanine (Phe) to tyrosine (Tyr), and deficiency of this enzyme results in elevated levels of systemic Phe, most severely affecting the brain. Without treatment, this toxic exposure can lead to progressive mental disability (most notably relating to cognition and executive functioning), seizures, depression, movement disorders, poor growth, and a variety of other debilitating neurological, behavioural, and psychological outcomes [[5], [6], [7], [8]].
Management of PKU varies widely internationally and between clinics within the same healthcare system [9]. The cornerstone treatment for PKU is a lifelong low-protein diet, avoiding meat, eggs, and dairy products, with a controlled intake of other foods such as potatoes and cereals. People with PKU are supplemented with glycomacropeptide-based or Phe-free L-amino acid supplements and, where available, low-protein versions of everyday foods, such as rice and pasta, to ensure a balanced diet. Blood Phe levels decrease during the day and should be monitored in the morning after fasting, aiming for a level of <600 μmol/l, in accordance with European guidelines for adults [10]. Target levels are age and geography dependant. However, it is well-accepted by patients and clinicians alike that the PKU diet has significant shortcomings. Adhering to a low Phe diet during adolescence and adulthood puts a high burden on patients and their families [11]. A study conducted in the UK found that 17% of children under ten years of age had above-target blood Phe levels, which rose to over 75% by 20 years of age [12]. There is an unmet clinical need for individualised PKU management practices and new therapeutics to optimise well-being and cognitive and neuropsychological outcomes. Sapropterin and pegvaliase are approved drugs for the treatment of PKU, but they are not suitable or available for all PKU patients [13,14]. More information on their indications, risks and benefits can be found in the scientific literature. SYNB1618 was a first-generation oral PAL delivered in a microbiome, which was in clinical trial at the start of this study [15]. However, it has since been replaced by another generation molecule due to a lack of efficacy [16].
CDX-6114 is a modified version of a phenylalanine ammonia-lyase (PAL) enzyme obtained by a mutation in the Anabaena variabilis PAL sequence (AvPAL). CDX-6114 is produced by fermentation of a recombinant Escherichia coli (E. coli) W3110 strain, a derivative of E. coli K12, carrying a modified gene coding for CDX-6114. The molecular weight of the tetramer is approximately 247 kDa, while the monomer is approximately 62 kDa. In order to improve the degradation resistance of the naturally occurring AvPAL, Codexis applied its CodeEvolver® protein engineering technology using sequential rounds of directed mutagenesis and recombination. In vitro studies evaluating the pH stability of CDX-6114 demonstrated that CDX-6114 shows improved stability compared with the wild-type AvPAL enzyme over a pH range of 3–6 (internal report).
CDX-6114 converts Phe in the gastrointestinal tract to produce CA and ammonia and performs its action by a process called spontaneous elimination reaction, postulating that the mode of action is the same as described in plants [17]. Ammonia is endogenously produced, degraded, and excreted in the urine. Humans do not metabolise Phe to CA, making CA a PAL-dependent metabolite [18]. Phe is the only substrate that CDX-6114 degrades to produce CA; therefore, CA can be measured in plasma and used as an indicator of enzyme activity. CA is rapidly metabolised, predominantly to hippuric acid, excreted in the urine, and is generally recognised as safe (GRAS) [19].
CA also occurs naturally in our diet resulting from ingesting many fruits and vegetables, such as wheat, barley, cabbage, spinach, broccoli, bananas, and citrus fruits and is the major ingredient in cinnamon oil [20]. The US regulatory status of CA includes approval by the FDA under the category of Synthetic Flavouring Substances and Adjuvants [21] and has GRAS status as a flavour ingredient based on a GRAS assessment by an expert panel of the Flavor and Extract Manufacturers Association (FEMA) [19]. CA is listed in the European Union Food Improvement Agents database of flavouring substances [22]. Thus, the degradation products from CDX-6114 metabolism are considered safe.
CDX-6114 has the potential to be of benefit to patients with either PKU or HPA by removing Phe from protein in the gastrointestinal tract before it is absorbed, thereby maintaining a lower Phe level. This could act as an oral enzyme substitution therapy (OST) with the mode of action entirely limited to the gastrointestinal tract with no significant systemic absorption. The primary outcome of this study was to determine the safety of CDX-6114. The secondary outcomes were to determine whether CDX-6114 could reduce plasma Phe levels in patients with PKU.
2. Materials and methods
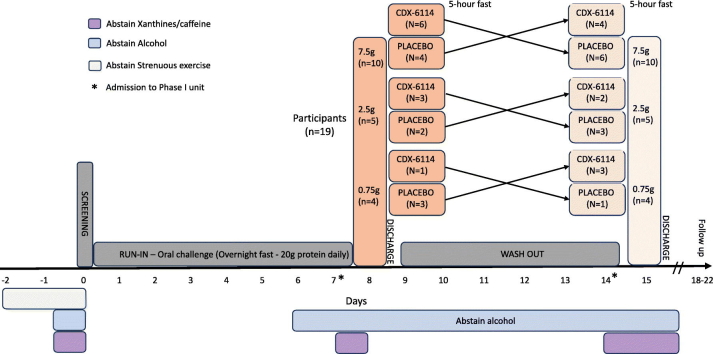
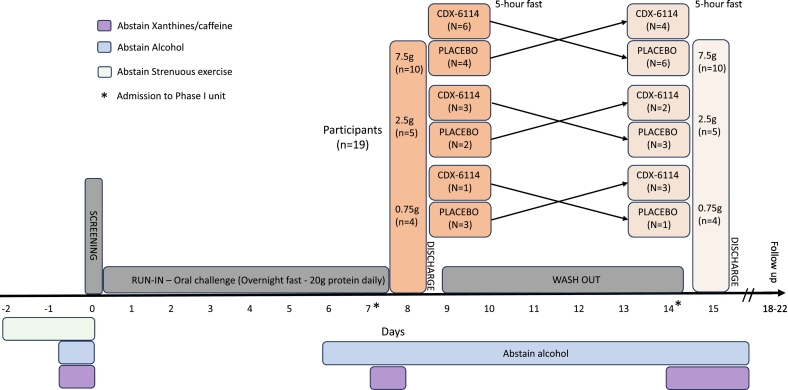
A phase I, randomised, double-blind, placebo-controlled, cross-over study was carried out to evaluate the safety, tolerability, pharmacodynamics, and pharmacokinetics of CDX-6114 in patients with PKU (NCT04085666). This study was conducted between August 2019 and June 2020 across six clinical centres in Australia and Germany, in accordance with the ethical principles that have their origin in the Declaration of Helsinki and was consistent with GCP, ICH Guidelines, and all applicable country-specific laws and regulations, including, without limitation, data privacy laws. The study documentation was approved in both countries, including all amendments to the protocol. Inclusion criteria were: adults aged 18–65 with a diagnosis of PKU by historical blood Phe concentration > 1200 μmol/l (at any time) or a genetic diagnosis of PKU; males and females, with a body mass index (BMI) between 18.0 and 35.0 kg/m2, weighing at least 50 kg and < 120 kg; a blood Phe <1200 μmol/l at screening; and patients who, in the opinion of the investigator, were in good health and capable of following dietary instructions to maintain stable protein intake throughout the study and were able to give informed consent. Patients were excluded from participation if they were using sapropterin, pegylated phenylalanine ammonia-lyase (pegvaliase), SYNB1618, or any Phe-lowering drug within four weeks prior to screening. Patients with any current or chronic history of gastrointestinal illness or conditions interfering with normal gastrointestinal anatomy or motility, evidence or history of clinically significant haematologic, renal endocrine, pulmonary, cardiovascular, hepatic, psychiatric, neurologic, or allergic disease at the time of screening were also excluded. Informed consent was obtained for all screened subjects.
Patients were randomised in a 1:1 ratio to receive a single oral dose of CDX-6114 at 7.5 g, 2.5 g, or 0.75 g or a single dose of a matching placebo in a cross-over design. A stable daily Phe intake with regular dietitian review was maintained during the 7-day run-in period before the first treatment and a washout period of 6 days between treatments. This oral food challenge comprised 20 g of protein, giving a protein load of around 1 g of Phe or the equivalent of about 20 protein exchanges, thus exceeding the usual dietary recommendations for a single meal protein content in patients with PKU. The higher protein load ensured a standard but adequate amount of Phe was given as a substrate to contribute to the Phe pool after an overnight fast, combined with sufficient calories. Patients were advised to avoid strenuous exercise during the 48 h prior to the screening day. In addition, the consumption of foods and beverages containing the following substances were prohibited:
-
•
Xanthines/caffeine – within 24 h prior to screening, 24 h prior to dosing on Day 1 up to discharge, 24 h prior to dosing on Day 8, and up to discharge from the phase I unit.
-
•
Alcohol – within 24 h prior to screening, for 48 h prior to dosing on Day 1 through to discharge from the phase I unit.
While confined in the phase I unit, except for the standard breakfast, on dosing days, and the 5-h post-dose fasting, patients continued their individually tailored Phe-stable diet (including a stable medical formula regimen if usually consumed). However, on Day 1 and Day 8, the protein intake following the 20 g at breakfast was kept as low as possible for the remainder of the day (e.g., a daily total of <25 g). Plasma phenylalanine levels were the pharmacodynamic marker in this study. All samples were collected in the phase I unit and transported via cold chain to a central reference laboratory, where they were analysed by liquid chromatography-tandem mass spectrometry (LS-MS/MS) by standard published methodology. Pharmacokinetics was measured by the absorption of a single dose of CDX-6114 into serum over a 5-h period following the standardised breakfast.
Patients were confined in the clinic for each treatment visit, with an overnight stay prior to treatment, and were discharged on the day of the treatment (Days 1 and 8) following all the in-clinic evaluations. Study visits included a screening visit within 28 days of enrolment and a follow-up visit 4–7 days after the second treatment visit (Fig. 1).
Fig. 1.
Study flow chart. Randomised cross-over trial.
Secondary outcomes were the safety and tolerability of a single oral dose of CDX-6114 assessed by the number and severity of adverse events, vital signs, physical examination, 12‑lead ECG, laboratory parameters, and development of anti-CDX-6114 antibodies. Blood samples for anti-CDX-6114 antibodies (ADAs) analysis were collected on Day 1, Day 8 (pre-dose), and at the end-of-study follow-up visit. Each sample was analysed using a validated method. The analysis was repeated if the first analysis yielded a positive result for ADAs. A positive result was recorded if both analyses yielded a positive result for ADAs.
The primary objective was to evaluate the pharmacodynamics of a single oral dose of CDX-6114 in patients with PKU. Following a standardised breakfast, plasma Phe and CA, peak Phe and CA concentrations, and Phe and CA area under the curve (AUC) were measured over a 5-h period following dosing. The peak and AUC of Phe and CA were analysed using a mixed effects model with treatment and period as fixed effects, patient as a random effect, and the period-specific baseline value as a covariate. The estimated treatment effect of CDX-6114 to placebo and the corresponding 95% confidence interval (CI) were calculated.
3. Results
A total of 34 patients were screened for eligibility, of whom 19 were randomised into the study, and 15 did not meet the inclusion criteria. Ten patients were randomised to receive CDX-6114, followed by a placebo, and nine received a placebo, followed by CDX-6114. Ten patients received 7.5 g of CDX-6114 (cohort 1), five patients received 2.5 g (cohort 2), and four patients received 0.75 g (cohort 3). All randomised patients completed the study. The mean age of patients was 33.4 (standard deviation [SD]: 11.8), 58% (11/19) were female, and all were of White race. The mean height, weight, and BMI at screening were 169.8 cm (SD: 10.9), 76.7 kg (SD: 10.1), and 26.8 kg/m2 (SD: 4.2), respectively. All patients had plasma Phe levels below 1200 μmol/l at screening and during the prespecified time points in the run-in period (Days −6, −4, −2) and washout period (Days 2, 4, 6). The median (Q1, Q3) phe at baseline was comparable between dosing groups; 613 μmol/l (448, 765) for the 7.5 g cohort, 613 μmol/l (292, 647) for the 2.5 g cohort, 591 μmol/l (563, 602) for the 0.75 g cohort, and 632 μmol/l (453, 705) for the pooled placebo group. Patient demographics are presented in Table 1.
Table 1.
Patient demographics.
| Cohort 1: 7.5 g (5.78 g^) N = 10 |
Cohort 2: 2.5 g (1.93 g^) N = 5 |
Cohort 3: 0.75 g (0.58 g^) N = 4 |
All Patients N = 19 |
||||
|---|---|---|---|---|---|---|---|
| CDX-6114/ Placebo (N = 6) |
Placebo/ CDX-6114 (N = 4) |
CDX-6114/ Placebo (N = 3) |
Placebo/ CDX-6114 (N = 2) |
CDX-6114/ Placebo (N = 1) |
Placebo/ CDX-6114 (N = 3) |
||
| Mean Age (years) (SD) | 41.5 (10.3) | 28.8 (8.9) | 30.7 (7.8) | 21.0 (2.8) | 26.0 | 37.0 (18.8) | 33.4 (11.8) |
| Sex; n (%) | |||||||
| Male | 2 (33.3) | 2 (50.0) | 2 (66.7) | 1 (50.0) | 1 (100.0) | 0 | 8 (42.1) |
| Female | 4 (66.7) | 2 (50.0) | 1 (33.3) | 1 (50.0) | 0 | 3 (100.0) | 11 (57.9) |
| Race; n (%)† | |||||||
| White | 6 (100) | 4 (100) | 3 (100) | 2 (100) | 1 (100) | 3 (100) | 19 (100) |
| Mean Height (cm) (SD) | 167.7 (9.2) | 172.0 (12.9) | 177.6 (5.7) | 167.6 (16.4) | 185.5 | 159.3 (6.0) | 169.8 (10.9) |
| Mean Weight (Kg) (SD) | 84.1 (10.1) | 74.3 (13.4) | 75.3 (9.8) | 70.6 (2.8) | 77.1 | 70.1 (4.0) | 76.7 (10.1) |
| Mean BMI (kg/m2) (SD) | 30.0 (3.5) | 25.1 (4.4) | 24.0 (3.8) | 25.6 (6.0) | 22.4 | 27.7 (3.3) | 26.8 (4.2) |
| Median Baseline Phe (μmol/l) (Q1, Q3) |
613 (448, 765) | 613 (292, 647) | 591 (563, 602) | 632 (453, 705) | |||
| Median Heart Rate (Beats/Min) (Q1, Q3) |
55 (52, 63) | 55 (53, 65) | 58.5 (53, 64.5) | 62 (56, 68) | |||
| Median Systolic BP (mmHg) (Q1, Q3) | 108 (102, 115) | 103 (101, 119) | 119 (109.5, 127.5) | 108 (104, 118) | |||
| Median Diastolic BP (mmHg)(Q1, Q3) | 65 (63, 70) | 62 (61, 68) | 70 (66.5, 74.5) | 70 (64, 78) | |||
Corrected dose.
All subjects were of White race.
3.1. Calculation of the correction factor
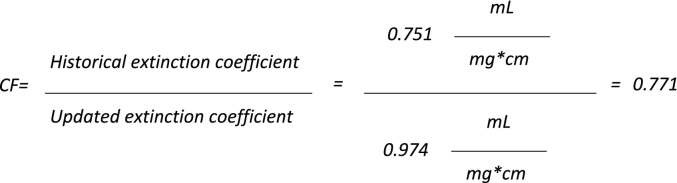
CDX-6114 protein concentration has historically been determined by ultraviolet (UV) spectrometry at 290 nm. During the development of the product, a reverse-phase HPLC assay was implemented to further evaluate product stability. The data generated in this method was expected to show one primary peak and associated impurities. Instead, the presence of two major peaks was observed. A literature search confirmed the enzyme's mechanism of action and enabled the second peak to be identified as CA [19]. It is believed that the presence of the CA may result from the conversion of excess Phe to CA during processing, at the end of fermentation, and prior to product purification. Free Phe and other amino acids are removed from the product during purification, as evidenced by the correct ratios of the amino acid versus the expected primary structure (amino acid sequence). However, it was not previously known that as part of the mechanism of action of the CDX-6114 enzyme, the CA product remained associated with the protein following the conversion of Phe. Thus, as CA absorbs strongly at 290 nm, its contribution to the total UV absorbance profile would yield an apparently higher concentration for the PAL enzyme. Since the historical extinction coefficient used for this UV spectrophotometric determination was based solely on the amino acid sequence for PAL, it does not factor in the contribution from CA. Discrepancies in protein concentration measurement and reporting were addressed through the determination of a modified extinction coefficient, taking into account the contribution of the signal from both the CA and the PAL enzyme. The determination of protein concentration by UV was determined using Beer's law; therefore, a correction factor was calculated using an extinction coefficient multiplied by the historical values. Recalculation of historical PAL enzyme amounts in CDX-6114 bulk drug substance using the updated extinction coefficient was achieved by applying a correction factor of 0.771 to all previously reported dosages (Fig. 2). The molecular mass of CA is 148.16; thus, depending on whether calculations are made using native/denatured, non-reduced/reduced, or expected/measured post-translational modifications, the correction factor will vary between 0.780 and 0.735.
Fig. 2.
The calculation to determine the correction factor is shown in the equation above:
3.2. Safety
Previous nonclinical safety and toxicity studies in rats and cynomolgus monkeys did not reveal any safety concerns, resulting in a no-observed adverse effect level (NOAEL) of CDX-6114 of 555.12 mg/kg/day. No serious adverse events (SAEs) were reported in this study, and no patients discontinued due to a treatment-emergent adverse event (TEAE). Overall, 70.0% (7/10) of patients in the 5.78 g cohort, 60.0% (3/5) in the 1.93 g cohort, none in the 0.58 g cohort, and 36.8% (7/19) in the pooled placebo cohort experienced at least one TEAE. The total number of TEAEs reported in each cohort was: ten in the 5781 g cohort, four in the 1.93 g cohort, none in the 0.58 g cohort, and seven in the pooled placebo cohort. Most TEAEs were in the system organ class of nervous system and gastrointestinal disorders and were of mild or moderate intensity. Treatment-related TEAEs were reported in the 5.51 g and the pooled placebo cohorts, which included headache (n = 2), drug withdrawal headache (n = 1, two episodes), dyskinesia (n = 1), nausea (n = 1) in the 5.51 g cohort; and headache (n = 1) and medical device site irritation (n = 1) in the pooled placebo cohort (Table 2).
Table 2.
Overview of Treatment-Emergent Adverse Events (Safety Population).
| Cohort 1: 5.78 g N = 10 |
Cohort 2: 1.93 g N = 5 |
Cohort 3: 0.58 g N = 4 |
Pooled Placebo N = 19 |
|||||
|---|---|---|---|---|---|---|---|---|
| Events | Patients (%) | Events | Patients (%) | Events | Patients (%) | Events | Patients (%) | |
| Total Number of TEAEs | 10 | 7 (70.0) | 4 | 3 (60.0) | 0 | 0 | 7 | 7 (36.8) |
| Total Number of SAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total Number of Treatment-related SAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TEAEs Leading to Discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severity1 | ||||||||
| Mild | 7 | 4 (40.0) | 4 | 3 (60.0) | 0 | 0 | 7 | 7 (36.8) |
| Moderate | 3 | 3 (30.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Relationship to Study Treatment | ||||||||
| Unrelated | 4 | 3 (30.0) | 4 | 3 (60.0) | 0 | 0 | 5 | 5 (26.3) |
| Related2 | 6 | 4 (40.0) | 0 | 0 | 0 | 0 | 2 | 2 (10.5) |
A treatment-emergent adverse event (TEAE) was defined as an adverse event that started on or after the start of the administration of study treatment. If adverse event dates and times were incomplete and it was unclear whether the adverse event was treatment-emergent, it was assumed to be treatment-emergent and assigned to both periods.
N = the number of participants in the analysis set receiving the treatment. (%) = participants/N*100.
If a participant experienced more than one TEAE, the participant was counted once at the most severe or most related event.
Related adverse events are those classified as possibly related, probably related, or definitely related. All related adverse events reported in the study were deemed “possibly” related.
There were no clinically meaningful changes from baseline in any physical examinations or laboratory parameters, including haematology, blood chemistry, coagulation, or urinalysis. Equally, the principal investigator deemed no changes in any vital sign parameters (temperature, pulse rate, respiratory rate, blood pressure, ECG) clinically meaningful or treatment-related. Anti-drug antibodies were not detected in any patient during the study.
3.3. Anti-CDX-6114 antibodies (ADAs)
No patients had a positive result for ADAs on either Day 1 or Day 8. Samples for the end-of-study visit were not analysed if Day 1 or Day 8 samples were negative.
3.4. Pharmacodynamics
The pharmacodynamics of CDX-6114 were calculated by postprandial plasma levels of Phe (μmol/l) over time, peak plasma levels, and AUC over a 5-h period following dosing and a standardised breakfast. The postprandial plasma Phe increased in all dose cohorts over time by between 10% and 30% from baseline. However, it appeared that the “true” peak plasma Phe was not reached within the 5-h observation period in any cohort, implying that Phe would be expected to increase further. The median peak Phe in the 5-h observational period for all CDX-6114 doses were considered similar to placebo due to the overlapping interquartile ranges.
The point estimate (95% CI) for the absolute difference in total Phe AUC(0-5h) (h*μmol/l) between CDX-6114 and placebo was −172.5 (−372.0, 27.0) for the 5.78 g cohort (p = 0.0811), −54.8 (−98.6, −11.1) for the 1.93 g cohort (p = 0.0294), and 240.0 (−289.5, 769.2) for the 0.58 g cohort (p = 0.1361) in the per-protocol pharmacodynamic analysis set (PP-PDAS). While a dose-proportionate nominal reduction in AUC(0-5h) versus placebo was observed for the 5.78 g and 1.93 g cohorts, the absolute magnitude of the difference (∼5% 5.78 g cohort, ∼2% 1.93 g cohort) in AUC(0-5h) was considered not clinically meaningful. Results for incremental AUC and net incremental AUC were overall consistent with the findings on total AUC across all three doses of CDX-6114. The highest postprandial CA concentrations were consistently observed with the 5.78 g dose of CDX-6114. However, no clear dose-response was observed between the 1.93 g and 0.58 g doses (Table 3).
Table 3.
Pharmacodynamics of CDX-6114.
| Pharmacodynamic Parameter, Median (Q1, Q3); |
Cohort 1: 5.78 g N = 10 |
Cohort 2: 1.93 g N = 5 |
Cohort 3: 0.58 g N = 4 |
|||
|---|---|---|---|---|---|---|
| CDX-6114 | Placebo | CDX-6114 | Placebo | CDX-6114 | Placebo | |
| AUC(0-5h) (h*μmol/l) | 3294 (2657, 4283) |
3913 (2958, 4374) |
3391 (1780, 3802) |
3858 (1244, 3949) |
3410 (3340.5, 3497) |
3105 (2800, 3301.5) |
| Incremental AUC(0-5h) (h*μmol/l) | 260 (220, 416) |
446 (321, 534) |
326 (319, 533) |
417 (364, 605) |
491 (400.5, 607.5) |
333 (232, 419) |
| Net Incremental AUC(0-5h) (h*μmol/l) | 253 (194, 416) |
446 (320, 525) |
326 (319, 533) |
416 (364, 605) |
491 (400.5, 607.5) |
333 (226, 418.5) |
| Time to Peak Measurement (h) | 1.5 (0.5, 5.0) | 4 (0.5, 5.0) | 4.0 (1.0, 5.0) | 5.0 (2.0, 5.0) | 1.8 (0.5, 3.0) | 4 (2.3, 4.5) |
| Peak Phe Concentration (μmol/l) | 702 (588, 895) |
853 (605, 926) † |
690 (374, 847) |
853 (605, 926) † |
711 (702, 744) |
853 (605, 926) † |
^ Corrected dose.
The peak Phe concentration is for the pooled placebo cohort (N = 19).
Overall, the plasma CA was low (single-digit μmol range), rapidly increased following CDX-6114 administration (with a peak generally within <30 min), and thereafter declined toward baseline levels within the first 2 h of the postprandial observation period. Plasma CA was generally not sustained over the 5-h assessment window.
3.5. Pharmacokinetics
The serum concentrations of CDX-6114 were below the level of quantitation (BLQ), 0.5 ng/ml, for all three dose cohorts at all time points, indicative of limited to no systemic absorption of CDX-6114 following oral administration of up to 5.78 g.
4. Discussion
Our study evaluated the pharmacodynamics, safety, tolerability, and pharmacokinetics of a single oral dose of CDX-6114 in patients with PKU. It also represents the first study of CDX-6114 for subsequent development activities in patients with PKU. Notably, the baseline Phe levels for the randomised patients were all within the target range (<600 μmol/l). On an individual patient level, overall, Phe levels were comparable between the two cross-over periods, thus supporting the validity of the pharmacodynamic data from the chosen cross-over design.
These results confirm that a single dose of CDX-6114 was safe and well tolerated at all three dosing regimens, showing an overall safety profile similar to the placebo. None of the patients experienced an SAE or discontinued the study.
The pharmacokinetic analysis found that systemic absorption of CDX-6114 after single dose administration was either absent or BLQ (0.5 ng/ml) for all three dose cohorts at all time points, up to 8 h post-dose. The BLQ was not considered to have a clinically relevant systemic pharmacological impact. Moreover, none of the patients in the study had a positive result for ADAs during the study.
The oral food challenge led to a numerical increase in postprandial plasma Phe levels of approximately 10–30% across individuals and during the 5-h postprandial observation period. CDX-6114 also includes Phe in its structure, calculated to be 25 mg/g of the enzyme, so an additional 145 mg, 48.5 mg, and 14.5 mg were added to the 5.78 g, 1.93 g, and 0.58 g cohorts, respectively. This affected the Phe load patients received in addition to the natural protein load of 20 g. A large inter-individual variability in postprandial plasma Phe following CDX-6114 administration may be due to variabilities in the rate of absorption or metabolism linked to the severity of the PAH mutation. The exact mechanism and impact on patients is unclear. Still, differences in the phenylalanine transporters, which may be linked to the PAH mutation or the effect of the microbiome, have been postulated [23]. Additionally, the peak plasma Phe was not reached within the chosen 5-h postprandial observation window, suggesting differences in its half-life between PKU patients and healthy volunteers [23].
These changes in plasma Phe after the food challenge and CDX-6114 administration showed a numerical reduction at the two higher doses (1.93 g and 5.78 g) compared with the matching placebo cohort, although not clinically meaningful. While disappointing, this finding was not surprising as the major component of Phe in the GI tract is protein-bound, and PAL requires free Phe for its activity, a substantially lower component of total GI Phe. Overall, no decrease in postprandial Phe was observed at the lowest dose (0.58 g).
An important finding from this study was a consistently detectable amount of CA across all patients who received CDX-6114 but not in the placebo group. In the absence of using plasma Phe as a reliable pharmacodynamics marker (for the reasons outlined above), the increased CA levels following CDX-6114 administration provided proof of CDX-6114's enzymatic activity in metabolising gastrointestinal Phe [24]. However, the total amount of Phe being metabolised must be considered low, as the peak levels of CA remained in a single-digit micromolar range, compared with the plasma Phe level increases in a 3-digit micromolar range. In addition, shortly after CDX-6114 intake, peak levels of CA were generally observed, with a subsequent rapid decline toward baseline levels.
This pattern is indicative that CDX-6114 has a short half-life, which may be due to the liquid formulation being applied in the study or the inability to withstand the lower pH encountered in the human stomach compared with the animal models used in earlier studies [25]. PAL sensitivity to low pH is well described [26], and multiple attempts have been made to protect it to optimise its function using proton pump inhibitors (PPIs) [27]. Unfortunately, the long-term use of PPIs is an increasing concern [28]. PPI use in the paediatric population is especially undesirable due to increasing concerns around their link to hyperactivity, anxiety, fractures, allergy, and predisposition to Clostridium difficile [[29], [30], [31], [32]].
Lessons learned from this study are invaluable for future studies. Several confounders in this study should be considered when analysing the data. Notably, there was insufficient control of an individual patient's Phe intake, their precise baseline Phe levels, and their tolerance to Phe. Secondly, the CDX-6114 dose was fixed irrespective of body weight. Thus, heavier patients given the lower dose would have a similar per kilogram dose to a lighter person on a higher dose. Providing detailed patient data and the course of Phe levels post-dosing with CDX-6114 compared with placebo would give a clearer picture of the true effect of this potentially important drug. All of which reinforces our current poor understanding of the effect of diet on individual patients.
An oral form of PAL would provide improved treatment possibilities for people with PKU, avoiding the requirement for injections and the occasional anaphylaxis seen with pegvaliase. Formulation improvements are in progress to optimise the Phe metabolising capacity of CDX-6114.
5. Conclusion
This study demonstrated that CDX-6114 is safe and well tolerated in patients with PKU at a single oral dose of up to 5.78 g. No systemic absorption of CDX-6114 or ADA formation was detected. While a modest trend in pharmacodynamic effects on Phe and CA was observed with the higher doses of CDX-6114 (5.78 g and 1.93 g), the overall magnitude of the impact was considered to be low (∼2–5% reduction in total systemic Phe levels) and thus not considered to be clinically meaningful. Moreover, postprandial Phe levels did not generally peak during the observation period of 5 h across all dosing cohorts, further impeding the overall interpretation of study results for clinical meaningfulness.
This was a single oral dose study to establish safety in the PKU patient population, and efficacy will be explored in future chronic exposure studies using an optimised formulation of CDX-6114.
Submission declaration and verification
The above work has not been previously published and is not under consideration for publication elsewhere. The publication is approved by all authors. If accepted, this manuscript will not be published elsewhere in the same form, in English, or any other language, including electronically, without the written consent of the copyright holder.
Funding of the research (Statement required)
This work was funded by Nestlé Health Science.
CRediT authorship contribution statement
Timothy Nicholas Fazio: Writing – review & editing. Tim Heise: Writing – review & editing. Anita Inwood: Writing – review & editing. Catherine Manolikos: Writing – review & editing. Yusof Rahman: Writing – review & editing. Hans-Juergen Woerle: Writing – review & editing. Louise Healy: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Timothy Fazio: Nothing to declare.
Tim Heise: declares institutional research grant from Nestlé Health Science.
Anita Inwood: Nothing to declare.
Catherine Manolikos: Nothing to declare.
Yusof Rahman: Nothing to declare.
Hans-Juergen Woerle: Employee of Nestlé Healthcare Science.
Louise Healy: Nothing to declare.
Christian J. Hendriksz: Employee of Nestlé Healthcare Science.
Acknowledgements
The authors wish to thank all the investigators, research staff, patients, and family members, for their valuable time and commitment in support of this study. The authors would also like to acknowledge Michael Heathman for all the PK/PD analyses, Drago Bratkovic, Kate Lefebure, Kate Billmore, Claire Rutledge, Claudia Büssenschütt, and Nina Carrasco-Schmitz for their contributions to the study. Medical writing support was provided by Heather L. Mason (Coufetery Comms). Financial support for this study was provided by Nestlé Health Science.
Data availability
The data that has been used is confidential.
References
- 1.van Spronsen F.J., Blau N., Harding C., Burlina A., Longo N., Bosch A.M. Phenylketonuria. Nat. Rev. Dis. Primers. 2021;7(1):36. doi: 10.1038/s41572-021-00267-0. PMC8591558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell J.J., Trakadis Y.J., Scriver C.R. Phenylalanine hydroxylase deficiency. Genet. Med. 2011;13(8):697–707. doi: 10.1097/GIM.0b013e3182141b48. [DOI] [PubMed] [Google Scholar]
- 3.Shintaku H. Disorders of tetrahydrobiopterin metabolism and their treatment. Curr. Drug Metab. 2002;3(2):123–131. doi: 10.2174/1389200024605145. [DOI] [PubMed] [Google Scholar]
- 4.Hillert A., Anikster Y., Belanger-Quintana A., Burlina A., Burton B.K., Carducci C., et al. The genetic landscape and epidemiology of phenylketonuria. Am. J. Hum. Genet. 2020;107(2):234–250. doi: 10.1016/j.ajhg.2020.06.006. PMC7413859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyle J.J., Fox A.M., Arthur M., Bynevelt M., Burnett J.R. Meta-analysis of neuropsychological symptoms of adolescents and adults with PKU. Neuropsychol. Rev. 2007;17(2):91–101. doi: 10.1007/s11065-007-9021-2. [DOI] [PubMed] [Google Scholar]
- 6.Burton B.K., Leviton L., Vespa H., Coon H., Longo N., Lundy B.D., et al. A diversified approach for PKU treatment: routine screening yields high incidence of psychiatric distress in phenylketonuria clinics. Mol. Genet. Metab. 2013;108(1):8–12. doi: 10.1016/j.ymgme.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Waisbren S.E., Noel K., Fahrbach K., Cella C., Frame D., Dorenbaum A., et al. Phenylalanine blood levels and clinical outcomes in phenylketonuria: a systematic literature review and meta-analysis. Mol. Genet. Metab. 2007;92(1–2):63–70. doi: 10.1016/j.ymgme.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Jurecki E.R., Cederbaum S., Kopesky J., Perry K., Rohr F., Sanchez-Valle A., et al. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol. Genet. Metab. 2017;120(3):190–197. doi: 10.1016/j.ymgme.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Enns G.M., Koch R., Brumm V., Blakely E., Suter R., Jurecki E. Suboptimal outcomes in patients with PKU treated early with diet alone: revisiting the evidence. Mol. Genet. Metab. 2010;101(2–3):99–109. doi: 10.1016/j.ymgme.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 10.van Wegberg A.M.J., MacDonald A., Ahring K., Bélanger-Quintana A., Blau N., Bosch A.M., et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet. J. Rare Dis. 2017;12(1):162. doi: 10.1186/s13023-017-0685-2. PMC5639803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas L., Olson A., Romani C. The impact of metabolic control on cognition, neurophysiology, and well-being in PKU: a systematic review and meta-analysis of the within-participant literature. Mol. Genet. Metab. 2023;138(1) doi: 10.1016/j.ymgme.2022.106969. 106969. [DOI] [PubMed] [Google Scholar]
- 12.Walter J.H., White F.J. Blood phenylalanine control in adolescents with phenylketonuria. Int. J. Adolesc. Med. Health. 2004;16(1):41–45. doi: 10.1515/ijamh.2004.16.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Hegge K.A., Horning K.K., Peitz G.J., Hegge K. Sapropterin: a new therapeutic agent for phenylketonuria. Ann. Pharmacother. 2009;43(9):1466–1473. doi: 10.1345/aph.1M050. [DOI] [PubMed] [Google Scholar]
- 14.Lowe T.B., DeLuca J., Arnold G.L. Similarities and differences in key diagnosis, treatment, and management approaches for PAH deficiency in the United States and Europe. Orphanet. J. Rare Dis. 2020;15(1):266. doi: 10.1186/s13023-020-01541-2. PMC7519570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puurunen M.K., Vockley J., Searle S.L., Sacharow S.J., Phillips J.A., 3rd, Denney W.S., et al. Publisher correction: safety and pharmacodynamics of an engineered E. coli Nissle for the treatment of phenylketonuria: a first-in-human phase 1/2a study. Nat. Metab. 2022;4(9):1214. doi: 10.1038/s42255-022-00635-4. [DOI] [PubMed] [Google Scholar]
- 16.Levy H.L., Sarkissian C.N., Scriver C.R. Phenylalanine ammonia lyase (PAL): from discovery to enzyme substitution therapy for phenylketonuria. Mol. Genet. Metab. 2018;124(4):223–229. doi: 10.1016/j.ymgme.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Koukol J., Conn E.E. The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J. Biol. Chem. 1961;236:2692–2698. [PubMed] [Google Scholar]
- 18.Sarkissian C.N., Gámez A. Phenylalanine ammonia lyase, enzyme substitution therapy for phenylketonuria, where are we now? Mol. Genet. Metab. 2005;86(Suppl. 1):S22–S26. doi: 10.1016/j.ymgme.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Adams T.B., Cohen S.M., Doull J., Feron V.J., Goodman J.I., Marnett L.J., et al. The FEMA GRAS assessment of cinnamyl derivatives used as flavor ingredients. Food Chem. Toxicol. 2004;42(2):157–185. doi: 10.1016/j.fct.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Anlar H.G., Bacanlı M., Çal T., Aydın S., Arı N., Ündeğer Bucurgat Ü., et al. Effects of cinnamic acid on complications of diabetes. Turk. J. Med. Sci. 2018;48(1):168–177. doi: 10.3906/sag-1708-8. [DOI] [PubMed] [Google Scholar]
- 21.FDA . In: CFR- Code of Federal Regulations Title 21. Synthetic Flavoring Substances and Adjuvants. SERVICES DOHAH, editor. 2023. [Google Scholar]
- 22.Commission Implementing Regulation (EU) No 872/2012 [Internet]. EUR-Lex. 2012. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0872 [cited 16 August 2023]. Available from:
- 23.Kaufman S. A model of human phenylalanine metabolism in normal subjects and in phenylketonuric patients. Proc. Natl. Acad. Sci. U. S. A. 1999;96(6):3160–3164. doi: 10.1073/pnas.96.6.3160. PMC15912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkissian C.N., Shao Z., Blain F., Peevers R., Su H., Heft R., et al. A different approach to treatment of phenylketonuria: phenylalanine degradation with recombinant phenylalanine ammonia lyase. Proc. Natl. Acad. Sci. U. S. A. 1999;96(5):2339–2344. doi: 10.1073/pnas.96.5.2339. PMC26785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatton G.B., Yadav V., Basit A.W., Merchant H.A. Animal farm: considerations in animal gastrointestinal physiology and relevance to drug delivery in humans. J. Pharm. Sci. 2015;104(9):2747–2776. doi: 10.1002/jps.24365. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert H.J., Tully M. Protection of phenylalanine ammonia-lyase from proteolytic attack. Biochem. Biophys. Res. Commun. 1985;131(2):557–563. doi: 10.1016/0006-291x(85)91272-0. [DOI] [PubMed] [Google Scholar]
- 27.Besada C., Hakami A., Pillai G., Yetsko K., Truong N., Little T., et al. Preformulation studies with phenylalanine Ammonia Lyase: essential prelude to a microcapsule formulation for the management of phenylketonuria. J. Pharm. Sci. 2022;111(7):1857–1867. doi: 10.1016/j.xphs.2022.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Nehra A.K., Alexander J.A., Loftus C.G., Nehra V. Proton pump inhibitors: review of emerging concerns. Mayo Clin. Proc. 2018;93(2):240–246. doi: 10.1016/j.mayocp.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Pasman E.A., Ong B., Witmer C.P., Nylund C.M. Proton pump inhibitors in children: the good, the bad, and the ugly. Curr Allergy Asthma Rep. 2020;20(8):39. doi: 10.1007/s11882-020-00926-4. [DOI] [PubMed] [Google Scholar]
- 30.De Bruyne P., Ito S. Toxicity of long-term use of proton pump inhibitors in children. Arch. Dis. Child. 2018;103(1):78–82. doi: 10.1136/archdischild-2017-314026. [DOI] [PubMed] [Google Scholar]
- 31.Dipasquale V., Cicala G., Spina E., Romano C. A narrative review on efficacy and safety of proton pump inhibitors in children. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.839972. 839972. PMC8866943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y.H., Wintzell V., Ludvigsson J.F., Svanström H., Pasternak B. Proton pump inhibitor use and risk of depression and anxiety in children: nationwide cohort study. Clin. Transl. Sci. 2022;15(5):1112–1122. doi: 10.1111/cts.13225. PMC9099128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.