Abstract
Deficiencies of lysosomal enzymes responsible for the degradation of glycosaminoglycans (GAG) cause pathologies commonly known as the mucopolysaccharidoses (MPS). Each type of MPS is caused by a deficiency in a specific GAG-degrading enzyme and is characterized by an accumulation of disease-specific GAG species. Previously, we have shown the potential of the beta-D-xyloside, odiparcil, as an oral GAG clearance therapy for Maroteaux-Lamy syndrome (MPS VI), an MPS characterized by an accumulation of chondroitin sulphate (CS) and dermatan sulphate (DS). This work suggested that odiparcil acts via diverting the synthesis of CS and DS into odiparcil-bound excretable GAG. Here, we investigated the effect of odiparcil on lysosomal abundance in fibroblasts from patients with MPS I and MPS VI. In MPS VI fibroblasts, odiparcil reduced the accumulation of a lysosomal-specific lysotracker dye. Interestingly, a reduction of the lysotracker dye was also observed in odiparcil-treated fibroblasts from patients with MPS I, a disorder characterized by an accumulation of DS and heparan sulphate (HS). Furthermore, odiparcil was shown to be effective in reducing CS, DS, and HS concentrations in liver and eye, as representative organs, in MPS VI and MPS I mice treated with 3 doses of odiparcil over 3 and 9 months, respectively. In conclusion, our data demonstrates odiparcil efficiently reduced lysosome abundance and tissue GAG concentrations in in vitro and in vivo models of MPS VI and MPS I and has potential as a treatment for these disorders.
Keywords: Mucopolysaccharidosis type VI, Mucopolysaccharidosis type I, Lysosome, Odiparcil, MPS VI therapy, MPS I therapy, GAG composition
1. Introduction
Mucopolysaccharidoses (MPS) are rare lysosomal storage diseases [[1], [2], [3]] resulting from deficiencies in the degradation of glycosaminoglycans (GAG) and exhibiting clinical symptoms with systemic character. Since GAG are important for tissue architecture, bones, muscles and connective tissues are most severely affected in patients with MPS [2,4]. Depending on the MPS disease, defects are seen in degradation of one or more GAG species, which may include chondroitin sulphate (CS), dermatan sulphate (DS), heparan sulphate (HS), keratan sulphate (KS) and hyaluronic acid [2]. Thus, MPS type I (MPS I, Hurler syndrome, Hurler-Scheie syndrome or Scheie syndrome) is caused by deficiency of alpha-L-iduronidase and affects the degradation of DS and HS [2,5], and MPS type VI (MPS VI or Maroteaux-Lamy syndrome) is caused by deficiency of arylsulphatase B and affects the degradation of CS and DS [2,6,7]. Although all MPS diseases have deficiency in the degradation of GAG and exhibit common symptoms, they have specificities both on a molecular level and in the clinical manifestations [2,8].
Lysosomes are the cellular organelles primarily affected in the MPS diseases. It has been established that their morphology changes and numbers increase due to insufficiencies in GAG degradation [2]. It has emerged that the engorgement of lysosomes is not the sole and only direct mediator of disease pathophysiology but a starting point for a pathogenic cascade leading to a dysregulation of numerous cellular processes [4,9,10]. Thus, GAG accumulation also affects other cellular organelles and intracellular processes (such as autophagy, mitochondrial function, apoptosis, vesicle trafficking [[11], [12], [13]]). Outside of the cells, GAGs are major pericellular and extracellular matrix components serving as a scaffold in tissue architecture [14,15]. They define tissue physical properties and contribute to signalling functions (as receptor or co-receptors and signalling modulators) [[16], [17], [18]]. Therefore, MPS results in a complex perturbation of cellular GAG accumulation, localisation and activity.
Changes in cellular and tissue GAG accumulation are reflected by increased GAG in urine and body fluids [[19], [20], [21]]. The increase in urinary GAG has been used as a diagnostic marker for MPS diseases [20,21]. Despite the fact that total GAG tissue deposition is observed and is used for MPS characterisation as well as for markers to evaluate treatments [22,23], surprisingly little quantitative information exists on specific GAG types in organs in relation to the different MPS (except some early reports e.g. [24]).
Currently, enzyme replacement therapy (ERT) via intravenous infusions, hematopoietic stem cells (HPSC) transplantation and various treatments for symptoms management are established therapeutic approaches for patients with MPS [25,26]. Gene therapy (GT) is also a promising approach to treat MPS [25,26] and, notably, there are two undergoing GT trials for MPS I [26]. However, because of the incomplete efficacy and/or associated risks of these therapies, there remains a significant medical need, justifying the research and development of new therapies for MPS [27]. Recently, we have shown the potential for an orally available treatment for MPS VI by a novel GAG clearance approach using odiparcil, a beta-D-xyloside, which competes with D-xylose in the synthesis of GAG, with mainly CS and DS affected [28]. In this way odiparcil diverts endogenous GAG synthesis to the production of soluble GAG which are secreted and eliminated from the organism via urine, and results in a net decrease in tissue GAG. Notably, odiparcil showed a broad distribution in tissues relevant for MPS such as bone, cartilage, cornea and heart [28]. In odiparcil-treated MPS VI mice, a reduction in the disease burden is demonstrated by a decrease in the total GAG concentration in liver and kidney, a reduction of cartilage thickening, and an improvement of corneal morphology [28,29]. Furthermore, an odiparcil clinical Phase 2a study (iMProveS) in adult patients with MPS VI demonstrated good safety and tolerability and improvements in pain, corneal clouding, cardiac, vascular, and respiratory function for the odiparcil-treated groups compared with the placebo-treated group [30].
Here, we investigated whether odiparcil treatment could have an effect on the lysosomal abundance and substrate accumulation in in vitro and in vivo models of MPS I and MPS VI. Lysosomes in fibroblast cells from MPS patients were visualized by lysotracker staining as previously shown [31]. Thus, reduction in the dye accumulation indicated reduction of lysosome number and size and demonstrated the efficacy of odiparcil treatment in fibroblasts from patients with CS and DS accumulation (MPS VI) and with DS and HS accumulation (MPS I). Further, in order to study the GAG species affected in MPS VI and MPS I pathology, we analysed the component GAG from the liver and eye of MPS VI and MPS I mouse models by ultra-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS). Liver and eye were selected as representative organs for component GAG analysis as a continuation of our previous studies where we showed that odiparcil treatment of MPS VI mice leads to reduction of total liver GAG [28] and leads to improvement of corneal morphology [29]. Thus, we demonstrated reduction of individual GAG types (CS, DS and HS) after odiparcil treatment. Taken together, this study demonstrates a direct effect of odiparcil on lysosomes in human fibroblasts from patients with MPS VI and MPS I and a direct effect on the GAG species accumulating in the liver and eye (as representative tissues) of the mouse models of MPS VI and MPS I.
2. Materials and methods
2.1. Materials
Odiparcil (chemical name 4-methyl-7-(5-thio-beta-D-xylopyranosyloxy)-2H-chromen-2-one) was synthetized either at Inventiva or at Dr. Reddy's Laboratories, India. All other chemicals were purchased from Sigma Aldrich unless otherwise indicated.
2.2. Cell culture and cells treatment
Primary skin fibroblasts from patients with MPS and controls were obtained from the NIGMS Human Genetic Cell Repository at the Coriell Institute for Medical Research. The following fibroblast cells were used: GM00034, GM00798, GM00887 and GM01254 were from patients with MPS I (clinically affected and biochemically characterized with IDUA deficiency by the supplier) and GM00031 and GM00799 as controls; GM03722, GM00538 and GM02572 were from patients with MPS VI (clinically affected and biochemically characterized with ARSB deficiency measured by the supplier) and GM003720 as a control. Cell culturing was done according to the conditions provided by the Coriell Institute. For evaluation of the effect of odiparcil, cells were seeded in 96 well imaging plates and treated with odiparcil (3 and 10 μM) added to the culture media 24 h after plating, with treatment continuing for 72 h. All experimental conditions were performed in triplicate, i.e., 3 wells/test condition.
2.3. Lysotracker labelling, imaging and fluorescence analysis
After odiparcil treatment as described above, culture media were removed. Each well was stained with 100 μL/well 100 nM Lysotracker-red DND-99 dye (Invitrogen) in medium at 37 °C for 60 min. After lysotracker incubation wells were washed twice with phosphate-buffered saline (PBS). Cells were fixed and nuclei stained by adding of 100 μL/well of 1 μg/mL Hoechst 33342 (Invitrogen) in 3.2% formaldehyde solution (made with PBS) for 30 min at ambient temperature. After incubation, wells were washed twice with PBS and stored at 4 °C until imaging. Image acquisition was done using ImageXpress Micro (Molecular Devices). In order to have a good representation for the levels of lysotracker from the lysosomal labelling, nine images were taken per well. Fluorescence levels were analysed using the MetaXpress' Transfluor application module for punctate staining (parameter <Cell: Pit integrated intensity >).
2.4. Animal studies
Male MPS I mice model [32] (Iduatm1.1Kmke allele homozygous, referred to as Idua−; note we observed visibly stronger MPS I appearance in the male mice) and male and female MPS VI mice model [28,33] [28,33] (Arsbm1J allele homozygous, referred to as Arsb−) and wildtype (WT) littermates were obtained from internal breeding of stock mice derived from The Jackson Laboratory (MPS I model: strain No: 017681; B6.129S-Iduatm1.1Kmke/J and MPS VI model: strain No: 005598; C57BL/6 J-Arsbm1J/GrsrJ). Genotyping and identification of respective mutations for Idua and Arsb were performed according to the protocol provided by The Jackson Laboratory. Treatment with odiparcil was done through the dietary route at the indicated concentration (1.5, 4.5 and 7.5 g odiparcil per kg chow diet). Full description of the animal odiparcil treatment studies was as previously published for early disease model [28]. However, the duration of treatment was different for the Idua− (9 months) and for the Arsb− (3 months).
2.5. Tissue GAG isolation, purification and component analysis
GAG isolation and purification from weighed eye and liver samples was done as described in [29]. CS, DS, and HS in the purified extracts were analysed according to the method described in [34] which uses LC-MS/MS for quantification of dimethylated uronic or iduronic acid acetylhexosamine and iduronic acid glucosamine dimers derived from the methanolysis of the GAGs.
2.6. Statistical method
Statistical analyses were performed with GraphPad Prism's one-way ANOVA with Dunnett's multiple comparison test using as a control Idua− or Arsb− non treated control values. A p value of ≤0.05 was considered significant.
3. Results and discussion
3.1. Enlarged lysosomes in MPS fibroblasts revealed by lysotracker staining and lysosomes number and size reduction following odiparcil treatment
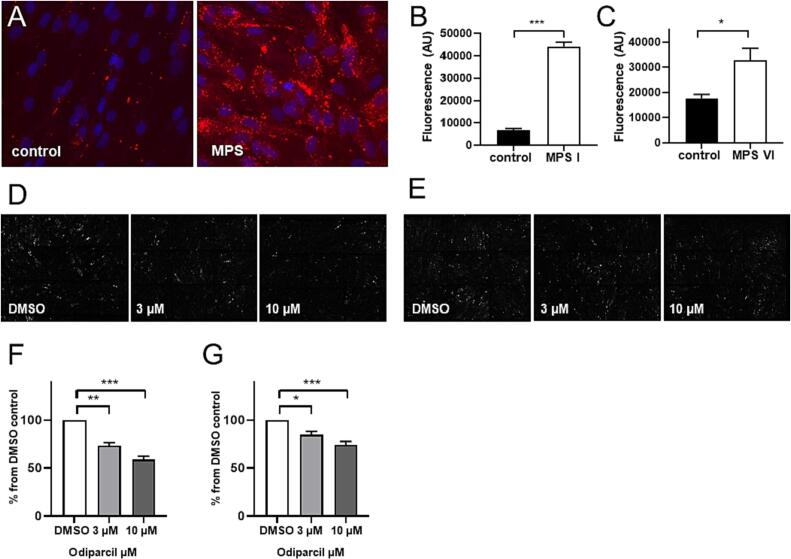
To address whether changes in lysosomal appearance can be detected with lysotracker dye, we incubated dermal fibroblasts from healthy controls and MPS I or MPS VI patients with the commonly used dye Lysotracker-red DND-99 [35] which has been previously shown to be suitable for lysosomal labelling in MPS fibroblasts [31]. Thus, lysosomes were defined as lysotracker positive structures. Microscopic examination showed a clear difference in the appearance of the lysosomes between healthy and MPS patient cells (Fig. 1A). In the MPS patient cells, lysotracker staining showed a higher number of bigger and brighter lysosomes (Fig. 1A). Quantification of the lysotracker fluorescence (Fig. 1B, C) demonstrated that MPS I and MPS VI cells have more and bigger lysosomes. Next, we examined the effect of odiparcil treatment on the lysotracker positive structures in the fibroblasts from MPS patients. Based on the mechanism of action of odiparcil engaging mainly the synthesis of CS/DS type of GAG [28] it was expected that odiparcil treatment would affect disease phenotype when CS/DS degradation was perturbed, i.e. in MPS VI. Accordingly, significant reduction in the detected lysotracker fluorescence was seen in MPS VI fibroblasts treated with odiparcil (representative images (Fig. 1D) and quantification of the total fluorescence (Fig. 1F)). Interestingly, in MPS I fibroblasts (in which degradation of both HS and DS is affected), treatment with odiparcil also led to a decrease of lysotracker labelling as seen in representative images (Fig. 1E) and after quantification of the total fluorescence (Fig. 1G). In addition, the lysosome reduction in both MPS VI and MPS I was dose dependent as seen with the two doses of odiparcil used (3 and 10 μM). Thus, odiparcil showed a positive effect in reducing the number and size of lysosomes (less accumulation of the lysotracker dye) not only in MPS VI but also in MPS I fibroblasts. This effect on the lysosomes in MPS fibroblasts treated with odiparcil validates the approach whereby diverting the production of endogenous GAG in the biosynthetic machinery (located in Golgi and endoplasmic reticulum [36,37]) a relief from the lysosomal engorgement is achieved. Provided that other cellular organelles [38,39] are affected in MPS and that odiparcil primarily acts in the biosynthetic pathway [28,40], it would be interesting to assess other cellular compartments after odiparcil treatment in MPS diseased cells.
Fig. 1.
Lysotracker visualization of lysosomes in MPS I and MPS VI fibroblasts and effect of odiparcil treatment.
A. Representative images of lysotracker labelling in healthy (control) and MPS I fibroblasts. B, C. Quantification of fluorescence of lysotracker in control and MPS I (B) or MPS VI (C) fibroblasts. D, E. Representative images of MPS VI (D) or MPS I (E) fibroblasts treated with vehicle (DMSO) or odiparcil. F, G. Quantification of fluorescence of lysotracker after odiparcil treatment in MPS VI (F) or MPS I (G) fibroblasts, normalized to the DMSO vehicle control. Data is presented as mean ± SEM. Groups were statistically analysed using a one-way ANOVA followed by Dunnett's test; * p < 0.05, ** p < 0.01, *** p < 0.001 compared to non-affected or DMSO controls (as indicated).
3.2. Odiparcil mediated reduction of component GAG (CS, DS and HS) in liver and eye of MPS VI and MPS I mouse models
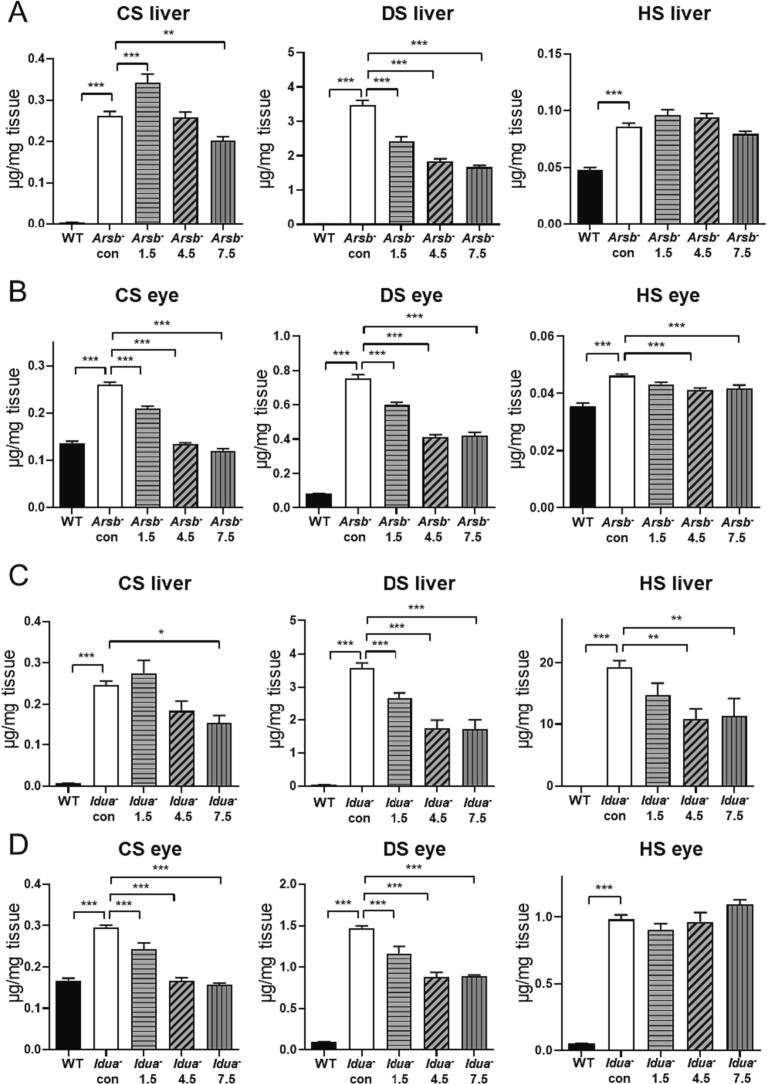
The effect of odiparcil on lysosomes in both MPS VI and MPS I fibroblasts prompted the question of how odiparcil treatment affected the individual GAG species within the MPS diseased cells. We were unable to obtain from patient primary fibroblasts a sufficient amount of GAG, required for the component analysis, and, therefore we extracted GAG from tissue homogenates of MPS I and MPS VI murine models. For the quantification of CS, DS and HS, UPLC-MS/MS analysis of the dimethylated dimers derived from the methanolysis of the GAG was used. Liver and eye were chosen as representative organs affected by the MPS disease. In the untreated Arsb- mice, CS and DS were dramatically increased compared with the wild type levels in both liver and eye (Fig. 2A,B), consistent with the MPS VI disease. As expected from the MPS VI pathology DS was the dominant species (3.5 μg/mg DS vs 0.26 μg/mg CS in liver, 0.76 μg/mg DS vs 0.26 μg/mg CS in the eye of Arsb- mice). The lower abundance in CS observed in comparison to DS might be related to a suggested bypass of the block of CS degradation by endoglycosidases [1]. Surprisingly in the liver and eyes from Arsb- mice, HS also showed a small but significant increase (0.086 μg/mg in Arsb- liver vs 0.048 μg/mg in control WT siblings, 0.046 μg/mg in Arsb- eye vs 0.035 μg/mg in WT). This increase of HS, degradation of which should not be affected in MPS VI, might be explained by secondary storage as previously seen for DS in Sanfilippo disease [41]. Based on the fact that odiparcil engages mainly in the synthesis of CS and DS [28], similar to the effect on the lysosomes, it was expected that odiparcil treatment will be effective in reducing CS and DS. Indeed, CS and DS were reduced in the liver and eye in MPS VI mice treated with odiparcil. The reduction of DS in both the liver and eye appeared to be dose dependent, with a possible saturation of the effect between the two high doses. CS in the eye also showed a dose-dependent reduction after odiparcil treatment. The effect on CS in the liver was more complex with a reduction detected only with the highest dose. Interestingly, a small but significant increase of liver CS was observed with the low dose of odiparcil. This might be explained by a possible effect specific only for odiparcil-bound CS, which might be trapped in the excess of the endogenous GAG (most probably extracellularly since odiparcil-bound GAG are readily secretable [28]). Notably, liver DS was decreased at all doses of odiparcil. Odiparcil treatment did not change significantly HS levels in the liver; however, with the high doses, a small but significant reduction in HS was seen in the eye. Thus, odiparcil treatment had a clear effect in reducing the dominant accumulating GAG species in MPS VI (DS) liver and also showed a significant reduction of CS, DS and HS in the eye.
Fig. 2.
GAG Quantification in Tissues from MPS VI and MPS I mice treated with odiparcil.
A, B. CS, DS and HS quantification in extracts from liver (A) and eye (B) of WT control, Arsb- control and Arsb- mice treated with the indicated dose of odiparcil (g odiparcil per kg diet); C, D. CS, DS and HS quantification in extracts from liver (C) and eye (D) of WT control, Idua- control and Idua- mice treated with the indicated dose of odiparcil (g odiparcil per kg diet); Data is presented as mean ± SEM. Groups were statistically analysed using a one-way ANOVA followed by Dunnett's test; ** p < 0.01, *** p < 0.001 compared to WT or Arsb−or Idua- controls (as indicated).
In liver and eye samples from MPS I Idua- mouse model, all three GAG types were detected (Fig. 2C, D). As expected from the MPS I pathology, DS and HS showed the highest abundance, with CS also significantly increased. Remarkably, in untreated Idua- liver and eye samples, CS and DS showed similar levels to those detected in untreated Arsb- samples. HS, however, was markedly increased in the liver and eye of untreated Idua- mice (liver: 19.3 μg/mg vs 0.052, and eye: 0.98 μg/mg vs 0.051 in Idua- vs control WT siblings, respectively). In comparison, HS was only mildly elevated in the liver and eye of untreated Arsb- mice (see above). Odiparcil treatment also led to a reduction in different GAG species from Idua- mice with a strong effect on DS and CS at the 2 high doses of odiparcil. Interestingly, HS was also reduced significantly but only in the liver. Notably, not only was the accumulation of CS and DS similar between the MPS VI and MPS I models, but additionally the odiparcil effect on CS and DS in MPS I mimicked the effect in MPS VI.
A comparison of the component GAG species showed not only the difference between the disease states, but it also demonstrated a different GAG burden in the organs, as exemplified by the liver and eye (e.g. HS was 20 μg/mg in liver vs 1 μg/mg in eye from untreated Idua- mice). This might reflect specificities of GAG tissue metabolism in different organs and be related to the disease pathophysiology. Similarly, the different efficacy of odiparcil treatment in reducing GAG concentrations in the different organs might be also explained by difference in GAG metabolism across the different organs. Previously, it was shown that odiparcil was efficacious in reducing CS/DS accumulation in MPS VI models as a result of its ability to stimulate the production and secretion of odiparcil primed CS/DS [28]. Here, we find that odiparcil was also efficacious in reducing HS accumulation, as seen in the eye of Arsb- mice and the liver of Idua- mice. Thus, it remains to be clarified whether this effect on HS is direct (by affecting directly the rate of HS synthesis in some cells types) or indirect (via some mechanism associated with the reduction of CS and DS).
The lysosomal accumulation of GAG and GAG fragments is recognized as a primary defect leading to the pathophysiology in MPS [2,42]; principally, excess amounts of GAG accumulate both intracellularly and in the extracellular matrix [9,43]. The GAG species (CS, DS and HS) analysed in this study are components of the total tissue GAG derived from both extracellular (e.g. matrix) and intracellular depositions. Thus, the methods used to isolate and quantify the GAG species in this study cannot discriminate between the GAG localised within biosynthetic organelles, with that located in the pericellular / extracellular space or in the degradation pathway (e.g. lysosomes). Even without knowing the precise localisation of GAG, the quantification of individual GAG species in tissue homogenates can be useful for the evaluation of treatment efficacy, as demonstrated in this study on the therapeutic potential of odiparcil. Odiparcil therapy provides a mechanism to interfere with the level of GAG entering from the biosynthetic pathway [28] and our data show that odiparcil is effective in reducing the three species of GAG analysed (CS, DS and HS). In addition to the specific cellular localisation, it remains to be clarified what effect odiparcil or other therapies have on the GAG chain size, disaccharide composition, level of protein glycosylation, and GAG functionality.
4. Conclusion
In this study we have further investigated the therapeutic potential of the orally available beta-D-xyloside odiparcil for treatment of MPS. In conclusion, we could differentiate MPS VI and MPS I patient fibroblasts from normal fibroblasts by using the common lysotracker dye and demonstrate the efficacy of odiparcil in reducing the lysosome engorgement in both diseases' fibroblasts. Furthermore, we quantified the CS, DS and HS concentrations in liver and eye tissue extracts from MPS VI, MPS I murine models and WT control. We also showed that odiparcil treatment was efficacious in reducing the component GAG in the selected organs in both MPS VI and MPS I, suggesting that odiparcil might be further investigated as a therapeutic (as a monotherapy or in a combination with other therapies such as ERT and GT) for a broader group of MPS, in addition to MPS VI.
Funding
Funding for this research was provided by Inventiva SA.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
PTB, IJ and PB are employees of Inventiva and receive financial compensations from Inventiva; EE and MT were employees of Inventiva while the study was conducted. HZ and SPY (Duke University) provided services on behalf of Inventiva. Odiparcil has been under development as a product for treatment of Mucopolysaccharidosis by Inventiva.
Acknowledments
This work was sponsored by Inventiva SA. We are thankful to the Inventiva animal facility personnel for their help in the mouse studies. We also thank Irena Konstantinova and Jean-François Mirjolet for the critical reading of the manuscript.
Contributor Information
Pierre Broqua, Email: pierre.broqua@inventivapharma.com.
Eugeni Entchev, Email: eugeni.entchev@inventivapharma.com.
Data availability
Data will be made available on request.
References
- 1.Neufeld E.F., Muenzer J. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver C., Beaudet A., Sly W., et al., editors. McGraw Hill; New York: 2001. The Mucopolysaccharidoses; pp. 3421–3452. [Google Scholar]
- 2.Alroy J., Lyons J.A. Lysosomal storage diseases. J. Inborn Error. Metabol. Screen. 2014;2 doi: 10.1177/2326409813517663. 232640981351766. [DOI] [Google Scholar]
- 3.Ferreira C.R., Gahl W.A. Lysosomal storage diseases. Transl. Sci. Rare Dis. 2017;2:1–71. doi: 10.3233/TRD-160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke L.A. Pathogenesis of skeletal and connective tissue involvement in the mucopolysaccharidoses: glycosaminoglycan storage is merely the instigator. Rheumatology. 2011;50:v13–v18. doi: 10.1093/rheumatology/ker395. [DOI] [PubMed] [Google Scholar]
- 5.Kubaski F., de Oliveira Poswar F., Michelin-Tirelli K., da Matte U.S., Horovitz D.D., Barth A.L., Baldo G., Vairo F., Giugliani R. Mucopolysaccharidosis Type I. Diagnostics (Basel) 2020;10:161. doi: 10.3390/diagnostics10030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valayannopoulos V., Nicely H., Harmatz P., Turbeville S. 2010. RMeviuewcopolysaccharidosis VI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harmatz P. Mucopolysaccharidosis VI pathophysiology diagnosis and treatment. Front. Biosci. 2017;22:385–406. doi: 10.2741/4490. [DOI] [PubMed] [Google Scholar]
- 8.Stapleton M., Arunkumar N., Kubaski F., Mason R.W., Tadao O., Tomatsu S. Clinical presentation and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2018;125:4–17. doi: 10.1016/j.ymgme.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Ballabio A., Gieselmann V. Lysosomal disorders: from storage to cellular damage. Biochim. Biophys. Acta. 2009;1793:684–696. doi: 10.1016/j.bbamcr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Fecarotta S., Tarallo A., Damiano C., Minopoli N., Parenti G. Pathogenesis of Mucopolysaccharidoses, an update. Int. J. Mol. Sci. 2020;21:2515. doi: 10.3390/ijms21072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonaro C.M., Haskins M.E., Schuchman E.H. Articular chondrocytes from animals with a dermatan sulfate storage disease undergo a high rate of apoptosis and release nitric oxide and inflammatory cytokines: a possible mechanism underlying degenerative joint disease in the Mucopolysaccharidoses. Lab. Investig. 2001;81:1319–1328. doi: 10.1038/labinvest.3780345. [DOI] [PubMed] [Google Scholar]
- 12.Tessitore A., Pirozzi M., Auricchio A. Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI. Pathogenetics. 2009;2:4. doi: 10.1186/1755-8417-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaffke L., Pierzynowska K., Cyske Z., Podlacha M., Węgrzyn G. Contribution of vesicle trafficking dysregulation to the pathomechanism of mucopolysaccharidosis. Biochem. Biophys. Res. Commun. 2023;665:107–117. doi: 10.1016/j.bbrc.2023.04.093. [DOI] [PubMed] [Google Scholar]
- 14.Mattson J.M., Turcotte R., Zhang Y. Glycosaminoglycans contribute to extracellular matrix fiber recruitment and arterial wall mechanics. Biomech. Model. Mechanobiol. 2017;16:213–225. doi: 10.1007/s10237-016-0811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva J.C., Carvalho M.S., Han X., Xia K., Mikael P.E., Cabral J.M.S., Ferreira F.C., Linhardt R.J. Compositional and structural analysis of glycosaminoglycans in cell-derived extracellular matrices. Glycoconj. J. 2019;36:141–154. doi: 10.1007/s10719-019-09858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarrazin S., Lamanna W.C., Esko J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011;3:a004952. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizumoto S., Yamada S., Sugahara K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015;34:35–42. doi: 10.1016/j.sbi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Minami K., Morimoto H., Morioka H., Imakiire A., Kinoshita M., Yamamoto R., Hirato T., Sonoda H. Pathogenic roles of Heparan sulfate and its use as a biomarker in Mucopolysaccharidoses. Int. J. Mol. Sci. 2022;23:11724. doi: 10.3390/ijms231911724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H., Wood T., Young S.P., Millington D.S. A straightforward, quantitative ultra-performance liquid chromatography-tandem mass spectrometric method for heparan sulfate, dermatan sulfate and chondroitin sulfate in urine: an improved clinical screening test for the mucopolysaccharidoses. Mol. Genet. Metab. 2015;114:123–128. doi: 10.1016/j.ymgme.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Khan S.A., Mason R.W., Giugliani R., Orii K., Fukao T., Suzuki Y., Yamaguchi S., Kobayashi H., Orii T., Tomatsu S. Glycosaminoglycans analysis in blood and urine of patients with mucopolysaccharidosis. Mol. Genet. Metab. 2018;125:44–52. doi: 10.1016/j.ymgme.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubaski F., de Oliveira Poswar F., Michelin-Tirelli K., Burin M.G., Rojas-Málaga D., Brusius-Facchin A.C., Leistner-Segal S., Giugliani R. Diagnosis of Mucopolysaccharidoses. Diagnostics (Basel) 2020;10:172. doi: 10.3390/diagnostics10030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia A.R., DaCosta J.M., Pan J., Muenzer J., Lamsa J.C. Preclinical dose ranging studies for enzyme replacement therapy with idursulfase in a knock-out mouse model of MPS II. Mol. Genet. Metab. 2007;91:183–190. doi: 10.1016/j.ymgme.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Ferla R., Claudiani P., Cotugno G., Saccone P., De Leonibus E., Auricchio A. Similar therapeutic efficacy between a single Administration of Gene Therapy and Multiple Administrations of recombinant enzyme in a mouse model of lysosomal storage disease. Hum. Gene Ther. 2014;25:609–618. doi: 10.1089/hum.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haskins M.E., Otis E.J., Hayden J.E., Jezyk P.F., Stramm L. Hepatic storage of Glycosaminoglycans in feline and canine models of Mucopolysaccharidoses I, VI, and VII. Vet. Pathol. 1992;29:112–119. doi: 10.1177/030098589202900203. [DOI] [PubMed] [Google Scholar]
- 25.Giugliani R., Federhen A., Vairo F., Vanzella C., Pasqualim G., Da Silva L.M.R., Giugliani L., De Boer A.P.K., De Souza C.F.M., Matte U., Baldo G. Emerging drugs for the treatment of mucopolysaccharidoses. Expert. Opin. Emerg. Drugs. 2016;21:9–26. doi: 10.1517/14728214.2016.1123690. [DOI] [PubMed] [Google Scholar]
- 26.Penon-Portmann M., Blair D.R., Harmatz P. Current and new therapies for mucopolysaccharidoses. Pediatr. Neonatol. 2023;64(Suppl. 1):S10–S17. doi: 10.1016/j.pedneo.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Grabowski G.A., Mistry P.K. Therapies for lysosomal storage diseases: principles, practice, and prospects for refinements based on evolving science. Mol. Genet. Metab. 2022;137:81–91. doi: 10.1016/j.ymgme.2022.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Entchev E., Jantzen I., Masson P., Bocart S., Bournique B., Luccarini J.-M., Bouchot A., Lacombe O., Junien J.-L., Broqua P., Tallandier M. Odiparcil, a potential glycosaminoglycans clearance therapy in mucopolysaccharidosis VI—evidence from in vitro and in vivo models. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Entchev E., Antonelli S., Mauro V., Cimbolini N., Jantzen I., Roussey A., Germain J.-M., Zhang H., Luccarrini J.-M., Lacombe O., Young S.P., Feraille L., Tallandier M. MPS VI associated ocular phenotypes in an MPS VI murine model and the therapeutic effects of odiparcil treatment. Mol. Genet. Metab. 2022;135:143–153. doi: 10.1016/j.ymgme.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Guffon N., Chowdary P., Teles E.L., Hughes D., Hennermann J.B., Huot-Marchand P., Faudot-Vernier E., Lacombe O., Fiquet A., Richard M.-P., Abitbol J.-L., Tallandier M., Hendriksz C.J. Oral treatment for mucopolysaccharidosis VI: outcomes of the first phase IIa study with odiparcil. J. Inherit. Metab. Dis. 2022;45:340–352. doi: 10.1002/jimd.12467. [DOI] [PubMed] [Google Scholar]
- 31.Xu M., Liu K., Swaroop M., Sun W., Dehdashti S.J., McKew J.C., Zheng W. A phenotypic compound screening assay for lysosomal storage diseases. SLAS Discov. 2014;19:168–175. doi: 10.1177/1087057113501197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D., Shukla C., Liu X., Schoeb T.R., Clarke L.A., Bedwell D.M., Keeling K.M. Characterization of an MPS I-H knock-in mouse that carries a nonsense mutation analogous to the human IDUA-W402X mutation. Mol. Genet. Metab. 2010;99:62–71. doi: 10.1016/j.ymgme.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtain M.M., Donahue L.R. A Mutation in the Arsb Gene; a Mouse Model that Resembles Maroteaux-Lamy Syndrome: MGI Direct Data Submission. 2009. http://www.informatics.jax.org/reference/J:149960
- 34.Zhang H., Young S.P., Auray-Blais C., Orchard P.J., Tolar J., Millington D.S. Analysis of glycosaminoglycans in cerebrospinal fluid from patients with mucopolysaccharidoses by isotope-dilution ultra-performance liquid chromatography-tandem mass spectrometry. Clin. Chem. 2011;57:1005–1012. doi: 10.1373/clinchem.2010.161141. [DOI] [PubMed] [Google Scholar]
- 35.Neun B.W., Stern S.T. Monitoring lysosomal activity in nanoparticle-treated cells. Methods Mol. Biol. 2011;697:207–212. doi: 10.1007/978-1-60327-198-1_22. [DOI] [PubMed] [Google Scholar]
- 36.Stanley P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011;3:a005199. doi: 10.1101/cshperspect.a005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikami T., Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta Gen. Subj. 2013;1830:4719–4733. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Gaffke L., Pierzynowska K., Rintz E., Cyske Z., Giecewicz I., Węgrzyn G. Gene expression-related changes in morphologies of organelles and cellular component Organization in Mucopolysaccharidoses. Int. J. Mol. Sci. 2021;22:2766. doi: 10.3390/ijms22052766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leal A.F., Benincore-Flórez E., Rintz E., Herreño-Pachón A.M., Celik B., Ago Y., Alméciga-Díaz C.J., Tomatsu S. Mucopolysaccharidoses: cellular consequences of Glycosaminoglycans accumulation and potential targets. Int. J. Mol. Sci. 2022;24:477. doi: 10.3390/ijms24010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toomey J.R., Abboud M.A., Valocik R.E., Koster P.F., Burns-Kurtis C.L., Pillarisetti K., Danoff T.M., Erhardt J.A. A comparison of the β-D-xyloside, odiparcil, to warfarin in a rat model of venous thrombosis. J. Thromb. Haemost. 2006;4:1989–1996. doi: 10.1111/j.1538-7836.2006.02064.x. [DOI] [PubMed] [Google Scholar]
- 41.Lamanna W.C., Lawrence R., Sarrazin S., Esko J.D. Secondary storage of dermatan sulfate in Sanfilippo disease. J. Biol. Chem. 2011;286:6955–6962. doi: 10.1074/jbc.M110.192062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saville J.T., McDermott B.K., Fuller M. Glycosaminoglycan fragments as a measure of disease burden in the mucopolysaccharidosis type I mouse. Mol. Genet. Metab. 2018;123:112–117. doi: 10.1016/j.ymgme.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Hampe C.S., Yund B.D., Orchard P.J., Lund T.C., Wesley J., McIvor R.S. Differences in MPS I and MPS II disease manifestations. Int. J. Mol. Sci. 2021;22:7888. doi: 10.3390/ijms22157888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.