Abstract
Pseudomonas aeruginosa (P. aeruginosa) is a gram-negative, opportunistic bacteria commonly found in wounds and in lungs of immunocompromised patients. These bacteria commonly form biofilms which encapsulate the bacteria, making it difficult for antibiotics or immune cells to reach the bacterial cells. We previously reported that Lipoxin A4 (LxA4), a Specialized Pro-resolving Mediator has direct effects on P. aeruginosa where it reduced biofilm formation and promoted ciprofloxacin antibiotic efficacy in a static biofilm-forming system. In the current studies, we examined the actions of LxA4 on established biofilms formed in a biofilm reactor under dynamic conditions with constant flow and shear stress. These conditions allow for biofilm growth with nutrient replenishment and for examination of bacteria within the biofilm structure. We show that LxA4 helped ciprofloxacin reduction of live/dead ratio of bacteria within the biofilm. THP-1 monocytes interacted with the biofilm to increase the number of viable bacteria within the biofilm as well as TNF-α production in the biofilm milieu, suggesting that monocyte interaction with bacterial biofilm exacerbates the inflammatory state. Pre-treatment of the THP-1 monocytes with LxA4 abolished the increase in biofilm bacteria and reduced TNF-α production. The effect of to decrease biofilm bacteria was associated with increased LxA4-induced monocyte adherence to biofilm but not increased bacteria killing suggesting that the mechanism for the reduced biofilm bacteria was due to LxA4 mediated increase in adherence to biofilm. These results suggest that LxA4 can help antibiotic efficacy and promote monocyte activity against established P. aeruginosa biofilm formed under hydrodynamic conditions.
Keywords: bacteria, infection, virulence, ciprofloxacin, THP-1 cells
INTRODUCTION
Pseudomonas aeruginosa (P. aeruginosa) is a gram-negative opportunistic bacterium commonly found in wound infections1, 2. This bacterium is hard to eradicate because of its ability to attain antibiotic resistance and to form biofilms. Indeed, P. aeruginosa biofilms are present in burns, open wounds, and lungs of immunocompromised patients3, 4. Bacterial virulence including biofilm formation is regulated through a population density mechanism known as quorum sensing5–7. When a threshold population density is reached, activation of quorum sensing signaling mechanisms lead to increased expression of many virulence genes, including genes involved in biofilm formation, exotoxin release and antibiotic resistance6, 8, 9.
The biofilm matrix that surrounds and encapsulates the bacterial cells is made of extracellular polymeric substances (EPS) consisting of expelled proteins, nuclear material such as extracellular DNA (eDNA), lipids, exopolysaccharides, and cellular debris to form an intricate network2, 10–12. Bacterial virulence is increased because of the reduced ability of antibiotics or immune cells to reach the bacteria within the biofilm matrix. Current treatment strategies for P. aeruginosa infections are antibiotics or combination antibiotics, which are mainly aimed at killing planktonic bacteria or inhibiting biofilm formation. Much less work has been focused on strategies to reduce established biofilm. Therefore, there is a clear need for treatment strategies that target formed/established biofilm. In addition, most of the work has been done in static biofilms, and less in biofilms grown under hydrodynamic conditions of flow and shear stress. This is important as biofilms are not formed under static conditions in the human body (e.g. urinary tract). In addition, shear stress can increase the adhesion time of bacteria when forming biofilms13 and flow can influence the transport rates of oxygen to the biofilm14. Therefore, we believe that is imperative to extend studies into biofilms grown under hydrodynamic conditions15.
In addition to being a barrier against leukocytes, P. aeruginosa biofilms impair neutrophil mobility out of the biofilm, increase neutrophil degranulation, and increase respiratory burst16, 17. These changes provide an inflammatory environment to the host and overall impair the host’s ability to clear the infection. Blood monocytes incubated with biofilm-associated bacteria induced an increase in monocyte production of TNF-α and IL-617,18. Along the same lines, addition of peripheral blood mononuclear cells to P. aeruginosa biofilm caused a profound inflammatory response with increased cytokine production and bacteria in the surrounding environment19. These reports strongly suggest that P. aeruginosa biofilm not only impairs host defense mechanisms but also promotes potentially injurious leukocyte mediated inflammation. There are, however, no reports on the effect(s) of monocytes on bacteria viability within the biofilm structure, nor are there any investigations on whether monocyte contact with the biofilm affects biofilm-associated bacteria viability.
Specialized Pro-Resolving Mediators (SPMs) are a class of endogenously produced lipids which have been extensively studied in relation to the inflammation resolution process20–23. In inflammation or infection, these lipid compounds are produced through enzymatic transcellular biosynthesis between different cell types (monocytes, neutrophils, platelets, epithelial cells, and endothelial cells). Lipoxins are converted from arachidonic acid, D-series resolvins from docosahexaenoic acid, and E-series resolvins from eicosapentaenoic acid. With respect to inflammation resolution, Lipoxin A4 (LxA4) was shown to reduce neutrophil activation, increase monocyte/macrophage recruitment, and decrease inflammatory cytokine production in animal models of infection and/or sepsis24–28. Studies from our lab have shown that LxA4 increases phagocytic ability of neutrophils in septic mice, helping decrease blood bacteria load26, while other studies have reported that LxA4 promoted non-phlogistic macrophage efferocytosis of apoptotic neutrophils29, 30. Almost all reports of SPMs such as LxA4 have focused on the actions of the SPM on host responses and signaling pathways. On the other hand, we have shown that LxA4 has direct effects on P. aeruginosa virulence by inhibiting the quorum sensing receptor LasR and inhibiting pyocyanin (an exotoxin) release25. In addition, we reported that LxA4 directly inhibited P. aeruginosa biofilm formation and enhanced ciprofloxacin killing efficacy on biofilm-associated bacteria31. These studies were performed on biofilms grown in static conditions and did not examine the effects of LxA4 on leukocyte action on established biofilms.
In the studies presented, we investigated the actions of LxA4 on established P. aeruginosa biofilms grown under constant flow and shear stress conditions. Potential synergistic action of LxA4 on ciprofloxacin antibiotic activity in established biofilm was examined. Importantly, we investigated the effects of LxA4 on monocyte interactions with established biofilm. We show that LxA4 has a modest effect of enhancing ciprofloxacin killing activity within established biofilm. Addition of THP-1 monocytes to P. aeruginosa biofilms significantly increased bacteria number within the biofilm structure. Pre-treatment of these monocytes with LxA4 increased monocyte adherence to the biofilm and completely abolished the deleterious effect of the monocytes. These results suggest that LxA4 has direct effects on Pseudomonas aeruginosa established biofilm to enhance antibiotic efficacy and aids monocyte activity against biofilm-encased bacteria.
MATERIALS AND METHODS
LxA4 Synthesis
LxA4 was prepared by total organic synthesis by Dr. Bernd Spur and Dr. Ana Rodriguez. Purity of the compounds was measured by HPLC-Mass Spectrometry and was determined to be > 98%. LxA4 was diluted in saline that was bubbled with argon to displace oxygen and used as previously published25.
Established Biofilm Formation
P. aeruginosa ATCC 27853™ (American Type Culture Collection, Manassas, VA, USA) was streaked on a tryptic soy agar plate (TSA; Ward’s Scientific, Rochester, NY, USA) and incubated overnight at 37°C. From the streaked plate a swab was added to a flask with 100 mL TSB (3 g/L) and incubated for 24 h at 35°C with shaking (150 rpm). To make the biofilm, we used the CBR-90 Standard CDC Bioreactor (Biosurface Technologies, Bozeman, MT). 1 mL of the liquid culture suspension was used to inoculate the biofilm reactor in “batch mode” – adding the inoculum to 300 mL TSB (3 g/L), then setting the stir plate to 120 rpm and 25°C for 24 h. Colony-forming unit (CFU) plating was also done with the inoculum to gain an accurate bacterial culture concentration. Following “batch mode”, “flow mode” was initiated by connecting fresh TSB (300mg/L) to the reactor via a peristaltic pump, operating at a flow rate of 11.5 mL/min, for 24 h. Waste media was collected such that the volume in the reactor was consistent across batch and flow modes. To collect the established biofilms, the reactor was drained, and the rods pulled out from the lid to remove the polycarbonate disks containing biofilm. The disks were transferred to 24-well plates containing 1 mL of M63 minimal media (Amresco, Cleveland, OH, USA). We chose this media to align these studies with our previous work with LxA4 and antibiotics in static biofilm31.
Antibiotic and LxA4 Studies
Biofilms were treated with saline vehicle, LxA4 (10 nM), ciprofloxacin (1 μg/mL)(3 μM), or both and incubated for 24 h at 37°C. Additionally, some biofilms were not treated and instead stained and imaged to serve as t = 0 h controls. After 24 h, some biofilms from each treatment were stained and imaged to serve as t = 24 h time points, while in other biofilms media were removed, fresh media added, 2nd treatments were given and biofilms incubated another 24 h at 37°C. to serve as t = 48 h time points.
% Live, Live/Dead Ratio and Biomass Area Quantitation
Quantitation was performed using LIVE/DEAD BacLight Bacterial Viability Kit (Invitrogen, Carlsbad, CA, USA) which utilizes SYTO9 and propidium iodide. Media was removed from the wells containing the biofilm disks, and 1 mL of staining solution was added to biofilms for 15 min. The staining solution was removed, and the biofilms were washed in saline. Imaging was performed using a Keyence BZX-700 fluorescent microscope using an inverted petri dish to ensure the biofilms were facing the lens. Tiff image files from the microscope were processed in ImageJ. The images were converted to 16-bit grayscale images and the color channels were split. Each of the red and green channels were selected in turn and the color threshold adjusted such that the background noise was not included in the image. Setting the threshold converted the images to binary, with the background white and the fluorescence black. The black pixels were counted to quantify the fluorescence by way of percentage of area covered. The green channel (SYTO9 stain) reflected all bacteria and biofilm components; the red channel (PI) reflected only damaged or dead bacteria and their cellular debris. Therefore, green – red = live bacteria per unit area; (green – red) / red = live/dead ratio. Image processing and analysis for biofilm area was done as described above, except using only the green channel as it represented all area covered by biofilm biomass. It should be noted that The BacLight Live/dead (Bacterial viability) kit does not significantly stain eukaryotic cells and can be used to examine bacterial viability in the presence of mammalian cells32.
Experiments with THP-1 Monocytes
THP-1 monocytes ATCC TIB-202™ (American Type Culture Collection, Manassas, VA, USA) were cultured and maintained in RPMI 1640 with L-glutamine (Corning, Manassas, VA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Corning, Manassas, VA, USA), 0.05 mM 2-mercaptoethanol (VWR, Solon, OH, USA), 100 U/mL penicillin G (VWR Life Science, Radnor, PA, USA), and 100 μg/mL streptomycin (VWR, Solon, OH, USA) at 37°C with 5% CO2. NucSpot 488 stain (VWR, Solon, OH, USA) was used to count live cells using the Countess 2 Automated Cell Counter (Thermo Fisher Scientific, Waltham, MA, USA). Passages were seeded with 4×105 cells in two-thirds fresh complete media and one-third spent complete media from the previous culture.
For experiment usage, cells were pelleted and resuspended at 3×105 cells/mL in serum-free, antibiotic-free media. LxA4 was diluted in argon-bubbled saline, added to THP-1 monocytes at either 1 nM or 10 nM concentrations, and incubated at 37°C in 5% CO2 for 2 h. Monocytes were stained with CellBrite Blue Cytoplasmic Membrane Dye (Biotium, Inc., Fremont, CA, USA) according to manufacturer’s instructions and incubated for 30 min at 37°C in 5% CO2 before washing. Cells were washed three times in serum-free RPMI. Media was pipetted from the wells containing the biofilm-coated disks, and 1 mL of LxA4 pre-treated THP-1 monocytes, control monocytes, or vehicle RPMI was added. Biofilms were co-incubated with monocytes at 37°C in 5% CO2 for 24 h. After incubation, biofilms were washed with RPMI before being stained with BacLight and imaged.
Monocyte Adhesion
Image processing and analysis for THP-1 monocyte adhesion to the biofilm was done as described above with the exception that the blue channel (CellBrite Blue) was kept, and it was the only fluorescent channel used as it represented all area covered by monocytes.
Z-Stacking for 3D Plots and Biomass
Using the same images that produced the biofilm percent area with THP-1 monocytes, 3D surface plots and biomass quantification was done using ImageJ and the plug-in Comstat233–35. Each z plane image was split into its color channels, keeping only the green channel. It was then converted to an 8-bit grayscale image, and all the z planes were merged into a stack. In Comstat2, the color threshold was adjusted such that the background noise was not included in the quantification. Comstat2 then scanned through the z planes using the threshold as the cutoff to determine the presence or absence of biofilm, and it output biomass in three dimensions (μm3/μm2). The image stacks were also used with ImageJ’s built-in plug-in Interactive 3D Surface Plot to visualize the biomass quantified by Comstat2.
Supernatant Collection and Cytokine Measurements
After 6 h and 24 h co-incubation of the monocytes with the biofilms, media was collected from wells. Samples were centrifuged for 5 min at 4°C and 500 g. Supernatants were sterile filtered at 0.2 μm and stored at −70°C until analyses. ELISA kits were used to perform analysis of TNF-α (Invitrogen, Carlsbad, CA, USA) and IL-8 (RayBiotech, Peachtree Corners, GA, USA) cytokine levels following manufacturer’s instructions.
Bacteria clearance assays
Monocytes were cultured in 96-well plates and were subsequently differentiated into macrophages with treatment of 100 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, St. Louis, MO, USA) for 48 h. After differentiation, the media was aspirated, and the cells were washed with saline three times. Serum-free and pen/strep- free media was replenished. Prior to differentiation with PMA or following differentiation with PMA. THP-1 monocytes/macrophages were preincubated with vehicle (sterile saline) or LxA4 at 1 nM or 10 nM for 2.5 h in 37°C, 5% CO2. They were subsequently incubated with P. aeruginosa at Multiplicity of Infections (MOIs) of 15:1 and 5:1 for 1 h in 37°C, 5% CO2. Following this incubation, supernatants were collected to assay for bacterial clearance. Macrophage bacterial clearance of P. aeruginosa was assessed by measurement of bacterial colony forming units (CFUs) following plating of supernatants on TSA plates and overnight incubation in 37°C. CFUs were then counted by operators blind to the treatment groups.
Statistical Analyses
All analyses were performed using GraphPad Prism. P < 0.05 was taken as significant. All data was expressed as mean ± s.e.m. All data were subjected to one-way ANOVA. A post hoc Sidak’s test was then used to test for significance. When the data was not normally distributed, we used the Kruskal Wallis ANOVA test. We also used a paired t-test when analyzing the changes in TNF-α levels in control samples and its LxA4 treated counterpart.
RESULTS
LxA4 with ciprofloxacin reduced live/dead bacteria ratio in established biofilm
P. aeruginosa biofilm was grown for 48 h on polycarbonate disks under dynamic flow conditions using a biofilm reactor. The disks were then removed from the biofilm reactor and placed in 24-well plates containing M63 minimal media. The disk-containing wells were divided into 4 groups: controls (+M63), LxA4 (10 nM), ciprofloxacin (1 μg/mL) and ciprofloxacin (1 μg/mL)(3 μM) + LxA4 (10 nM). After 24 h, some disks were removed for staining and imaging. In other wells, treatments were administered again. At the end of 48 h, disks were removed for staining and imaging. The results show that at 24 h after treatment, there was an increase in live bacteria within the biofilm when both ciprofloxacin and LxA4 were given together (Figure 1a). At the same time point there was a strong tendency for both LxA4 and ciprofloxacin individually to decrease live/dead bacteria ratio, suggesting that the initial effects of these two compounds was to kill biofilm-associated bacteria (Figure 1b). At 48 h after the biofilms had received a 2nd dose of treatments, both ciprofloxacin and ciprofloxacin + LxA4 treated groups significantly reduced the number of live bacteria (Figure 1a) but only the ciprofloxacin + LxA4 group significantly lowered the live/dead ratio (Figure 1b). These results suggest that LxA4 helped the bacteria killing efficiency of ciprofloxacin. Apart from quantitating the relative number of live cells within the biofilm matrix, we also quantitated the area of biofilm on the individual disks (Figure 1c). When comparing the area of biofilm to controls, neither ciprofloxacin nor LxA4 alone affected the biofilm area. Use of ciprofloxacin and LxA4 together however, significantly increased the biofilm area compared to controls. Taken together with the live/dead data, the increase in biofilm area may be due to an accumulation of dead bacteria and its associated debris.
Figure 1.
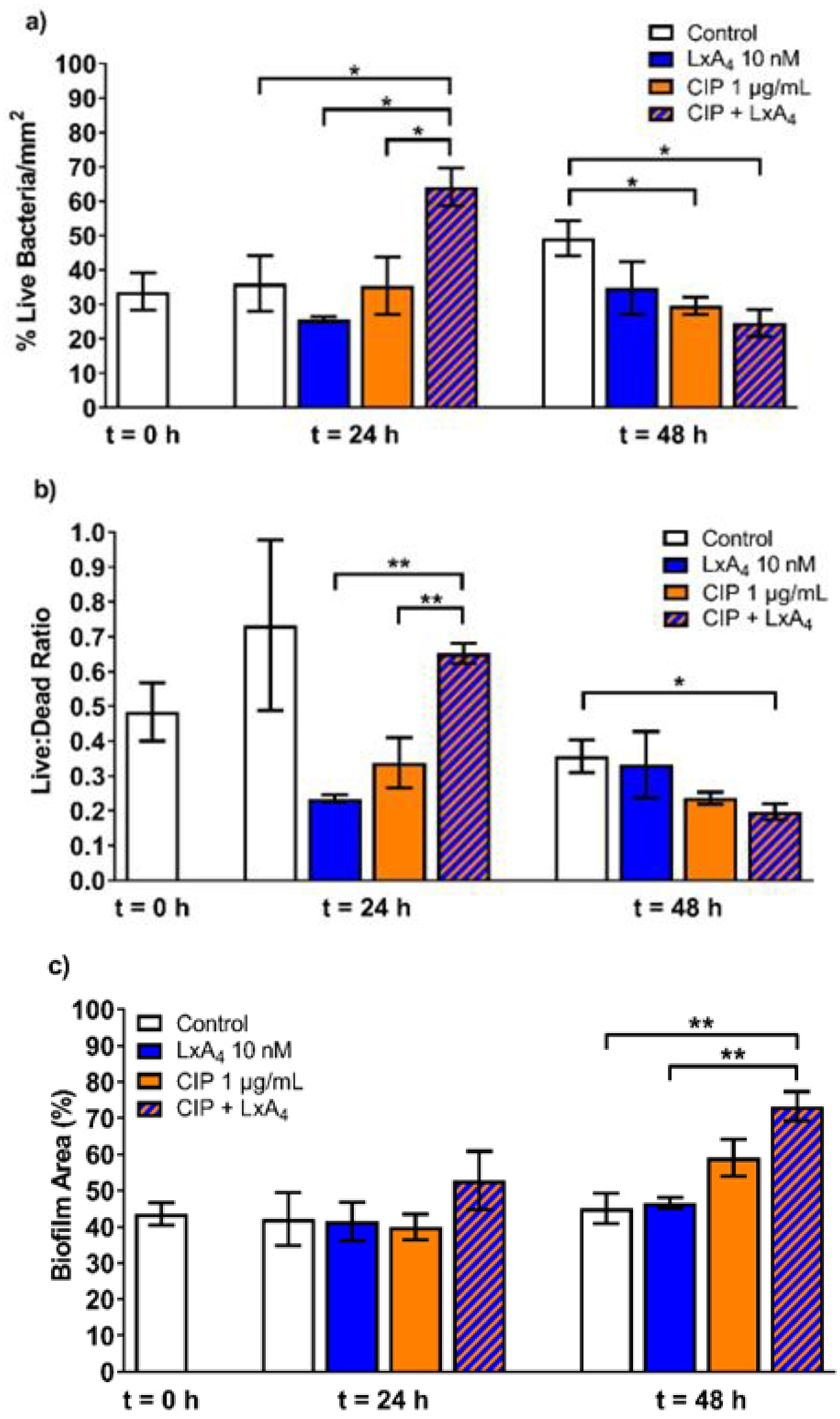
LxA4 combined with ciprofloxacin acted on established biofilms to reduce the proportion of live bacteria with 2-hit treatments but increased biofilm area. After growing P. aeruginosa biofilms in a dynamic system for 48 h, treatments of LxA4, ciprofloxacin, or both were given at t = 0 h and t = 24 h. Biofilms and bacteria were stained and imaged at t = 0 h, 24 h, and 48 h. (a) After one dose, the combined treatment showed a transient increase in the live bacteria. After a second dose, the combined treatment significantly reduced the live bacteria associated with the biofilm. (b) Similarly, after one dose of the combined LxA4 + ciprofloxacin treatment, there was an increase in the live/dead ratio. After 2 doses, only the combined treatment reduced the live/dead ratio (c) After two doses, LxA4 and ciprofloxacin combined treatment significantly increased the area covered by biofilm. This increase in biofilm area may be due to the increase in the proportion of dead bacteria. Data are mean ± s.e.m. * p < 0.05, ** p < 0.01. n = 3 – 5.
LxA4 abolished monocyte-induced rise in live bacteria within biofilm
In our next set of experiments, we wished to investigate the effects of LxA4 on the ability of monocytes to kill bacteria within the biofilm matrix. In these studies, P. aeruginosa biofilms were grown as detailed above. After 48 h of growth in the biofilm reactor, disks were transferred to 24-well plates. THP-1 monocytes in serum-free RPMI, which had been incubated with either saline vehicle or LxA4 (1, 10 nM) for 2 h and stained with CellBrite Blue, were then transferred to wells containing biofilm disks. Biofilm disks were incubated with THP-1 cells (3 × 105 cells/well) for 1 h or 24 h. At the end of these periods, live/dead stain was applied to biofilms to quantify the live and dead biofilm-associated bacteria and biofilm area. At 1h, biofilms incubated with control THP-1 monocytes showed little change in live biofilm-associated bacteria compared to control biofilms without THP-1 monocytes (Figure 2, Figure 3a). At this time point, there was a small increase in live/dead ratio when THP-1 cells were pre-incubated with LxA4 (10 nM) compared to biofilm incubated with untreated THP-1 cells (Figure 3b). At 24 h however, control THP-1 monocytes significantly increased the number of live bacteria as well as the live/dead ratio within the biofilm (Figures 3a and 3b). Monocytes pre-incubated with LxA4 (10 nM) completely abolished this increase (Figure 2 and Figures 3a, 3b). Indeed, in biofilms with LxA4 treated THP-1 cells, the number of live bacteria (Figure 3a) and the live/dead ratio (Figure 3b) were not significantly different from that of control biofilms without exposure to THP-1 monocytes. These results firstly suggest that any change in the early time point with LxA4 pre-incubated monocytes was transient and secondly, that the major effects of LxA4 on monocytes in this model was to abolish the increase in live bacteria in biofilm when monocytes encounter P. aeruginosa biofilm. Control THP-1 monocytes did not alter the biofilm area at 1 h, but THP-1 monocytes which were pre-incubated with LxA4 (10 nM) reduced the biofilm area at this time point (Figure 3c). These changes were transient, as at 24 h there were no changes in any of the groups. Taken together with the effects of LxA4 pre-treated monocytes on live bacteria (Figures 3a and 3b), the results suggest that at 1 h, the LxA4 treated monocytes actively attack the biofilm, exposing and perhaps stimulating the bacteria which are no longer encased in biofilm. Then these monocytes kill these “exposed” biofilm-associated bacteria.
Figure 2.
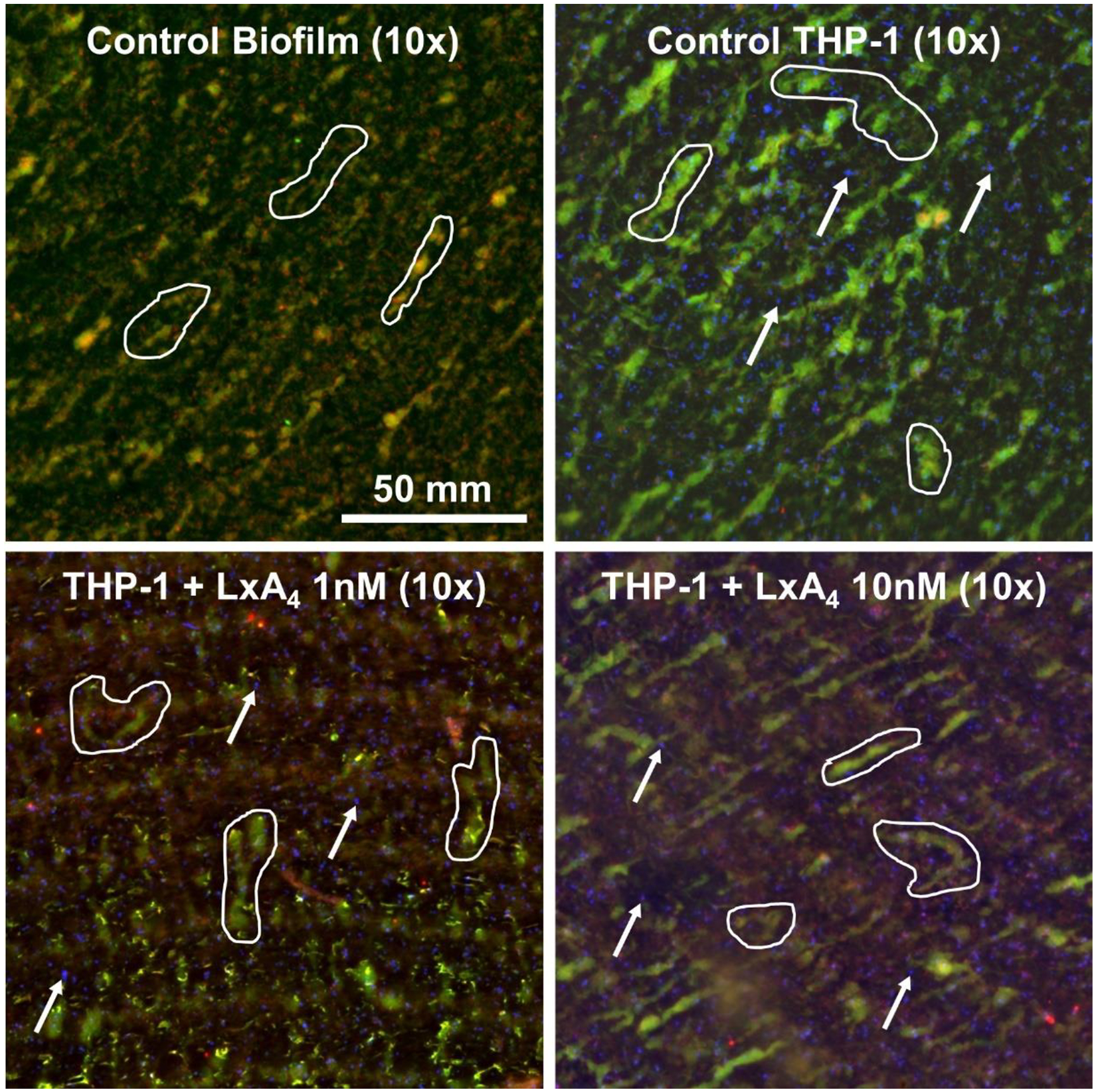
After growing P. aeruginosa biofilms under dynamic conditions for 48 h, THP-1 monocytes pre-treated with LxA4 or vehicle were co-incubated with the biofilms for 1 h or 24 h. Biofilms were stained and imaged. Representative images at t = 24 h demonstrating changes in live:dead ratios. All biological material stains with SYTO9 (green); damaged or dead material stains with PI (red); THP-1 monocytes were stained with CellBrite Blue (blue). Outlines indicate biofilm tendrils extending from the biofilm surface. Arrows indicate THP-1 monocytes. Compared to untreated control biofilm, biofilm co-incubated with untreated THP-1 monocytes showed increased biofilm-associated bacteria. LxA4 pre-treatment abolished the increase in biofilm-associated bacteria caused by THP-1 monocyte interactions.
Figure 3.
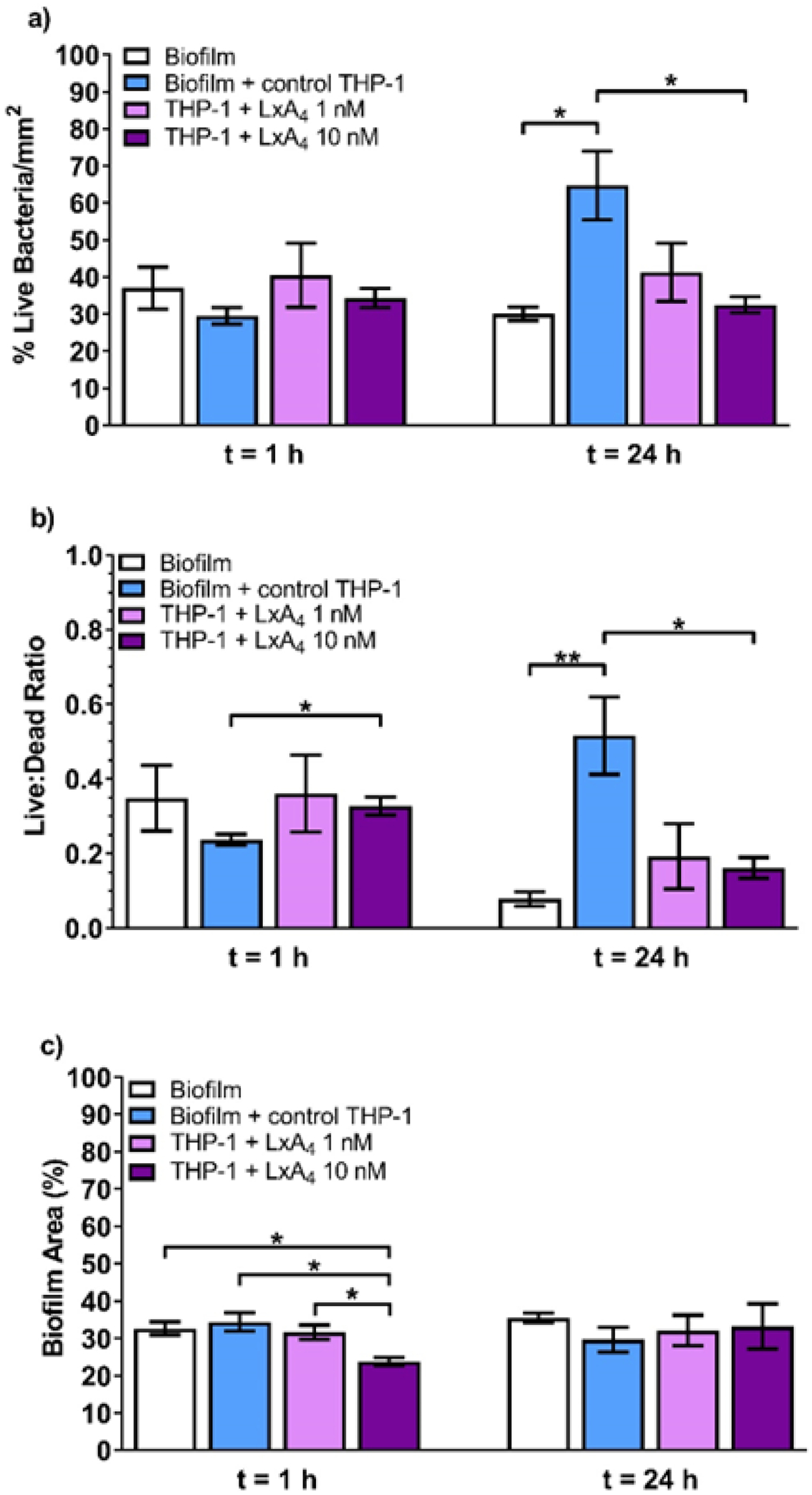
LxA4 pre-treatment abolished the increase in biofilm-associated bacteria caused by THP-1 monocyte interactions. After growing P. aeruginosa biofilms under dynamic conditions for 48 h, THP-1 monocytes pre-treated with LxA4 or vehicle were co-incubated with the biofilms for 1 h or 24 h. (a) At 24 h, untreated THP-1 monocytes caused a significant increase in the amount of live bacteria per unit area, but 10 nM LxA4 pre-treatment eradicated this effect. (b) 10 nM LxA4 pre-treatment initially caused an increase in the proportion of live bacteria associated with the biofilm. At 24 h, untreated THP-1 monocytes caused a significant increase in the proportion of biofilm-associated live bacteria, but 10 nM LxA4 pre-treatment abolished this effect. (c) 10 nM LxA4 pre-treatment initially reduced biofilm area, but this effect was transient and did not persist by 24 h. Data are mean ± s.e.m. * p < 0.05, ** p < 0.01. n = 4 – 5.
Three-dimensional structural changes in biofilm biomass after monocyte addition to biofilm
To illustrate some of the changes that occur to the structure of the P. aeruginosa biofilm after addition of THP-1 monocytes, we show 3D images that represent the changes that occurred in the biofilm biomass after 1 h (Figure 4, top). The figure shows that the biofilm matrix is a complex series of peaks. A decrease in the biofilm mass reduced the height of the peaks and thus the overall biomass of the biofilm bacteria at 1 h (Figure 4 bottom). It is important to note that the major structural three-dimensional changes are reflected in the two-dimensional measurements of biofilm area (Figure 3c).
Figure 4.
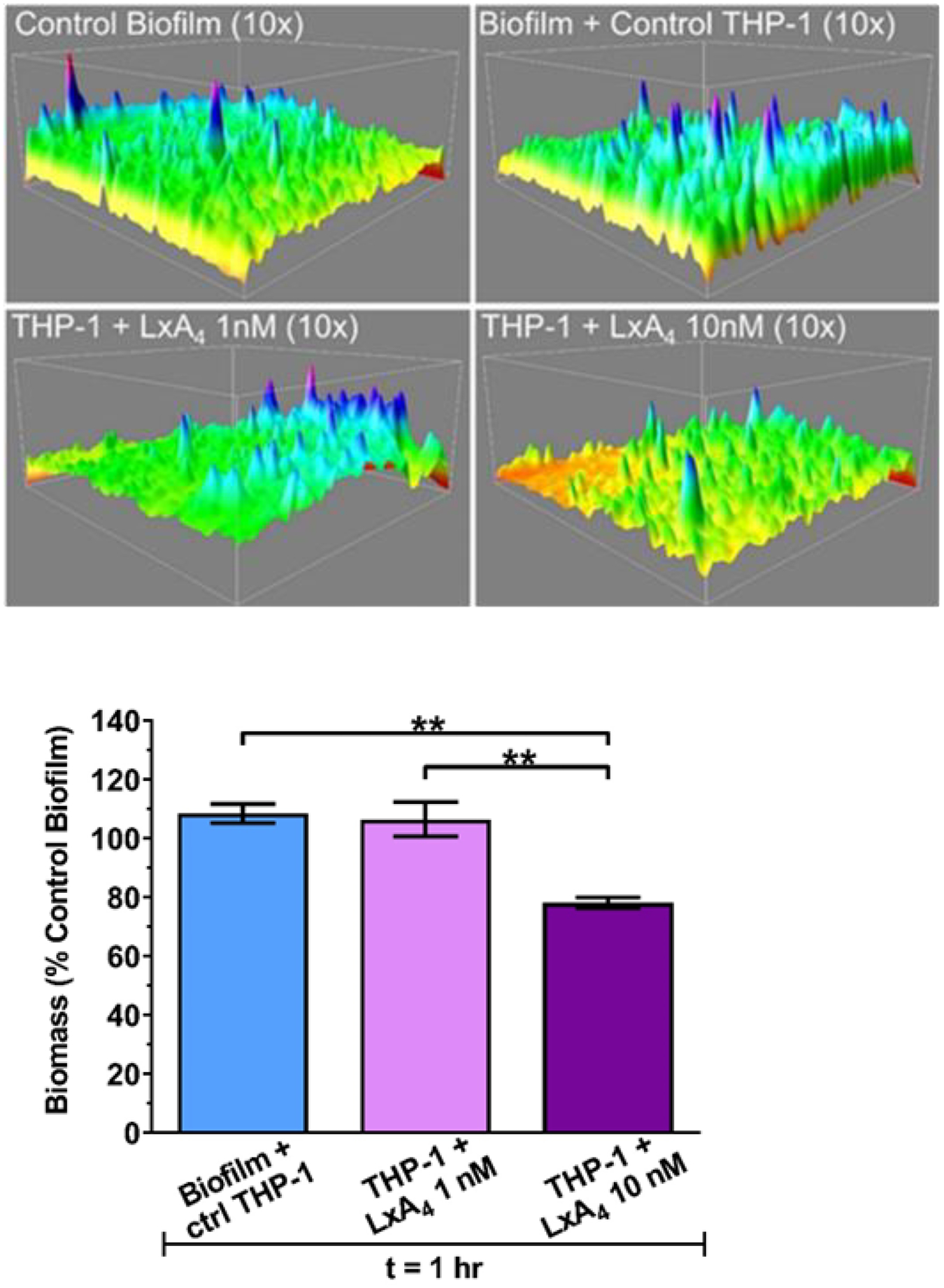
(Top) Representative 3D surface plots of established biofilm after co-incubation with LxA4 pre-treated THP-1 monocytes. Plots were constructed using z-plane stacked images with ImageJ and Comstat2. Plots are t = 1 h. (Bottom) LxA4 pre-treated THP-1 monocytes decrease P. aeruginosa established biofilm biomass. Biomass was quantified from z-plane stacked images used to quantify biofilm area. This reduction in biomass (three-dimensional) mirrors the reduction in biofilm area (two-dimensional) as seen in Figure 3c. Data are mean ± s.e.m. percent of control biofilm. * p < 0.05, ** p < 0.01. n = 3.
LxA4 increased monocyte adhesion to bacterial biofilm
To try to understand the mechanism of how LxA4 treated THP-1 monocytes may decrease the live bacterial cells within the P. aeruginosa biofilms, we repeated these studies to quantify the extent by which THP-1 monocytes adhere to the biofilms. The studies showed that LxA4 increased adherence of THP-1 monocytes to the biofilm compared to vehicle treated control monocytes (Figure 5). To evaluate if the increased adherence is a non-specific effect of LxA4 on THP-1 monocytes or if there were specific qualities in biofilm that would make the LxA4 treated monocytes more adherent, we examined the effects of LxA4 on THP-1 monocyte adherence to the biofilm reactor polycarbonate disks without biofilm. There was virtually no monocyte adherence to polycarbonate disks without biofilm, with only 0.345 ± 0.036% area coverage with control THP-1 cells. LxA4 (10 nM) pre-treatment increased adherence to 1.495 ± 0.15% area coverage (P < 0.05; n = 3 independent experiments). Taken together, the results suggest that LxA4 activated the THP-1 monocytes to increase overall adherence and that the increased adherence was not a result of biofilm-monocyte interaction.
Figure 5.
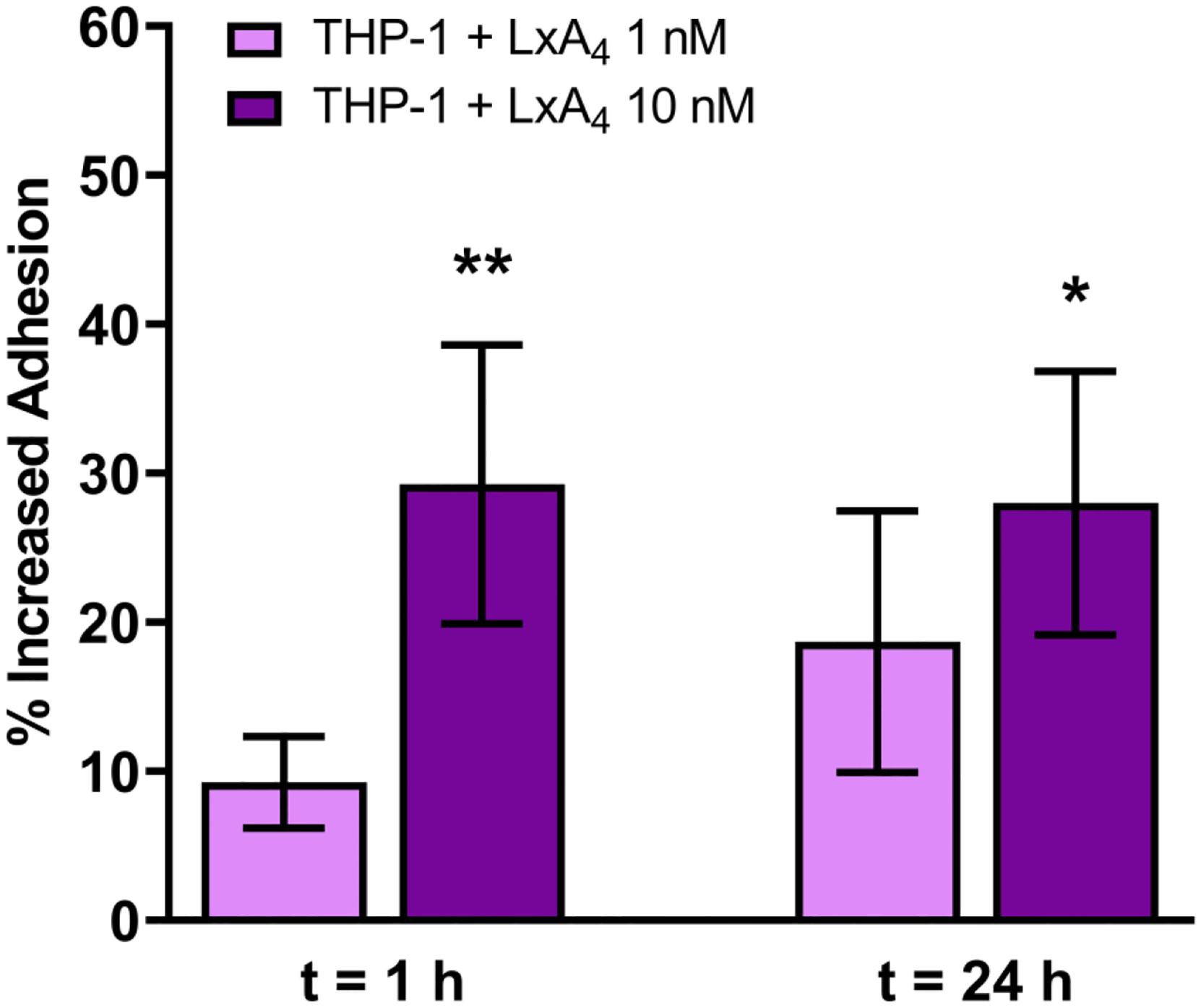
After growing P. aeruginosa biofilms in a dynamic system for 48 h, Cell Brite blue stained THP-1 monocytes pre-treated with LxA4 or vehicle were co-incubated with the biofilms for 1 h or 24 h. Biofilms were then imaged. Pre-treatment with LxA4 (10 nM) significantly increased adhesion of monocytes to biofilms. Data are mean ± s.e.m. percent change from control, adjusted to zero. * p < 0.05. n = 3 – 4.
LxA4 did not affect bacteria clearance by THP-1 macrophages
In these studies, THP-1 monocytes were incubated with LxA4 (1, 10 nM) or vehicle saline for 2.5 h before addition of different concentrations of P. aeruginosa (5 MOI and 15 MOI) for 1 h. We also performed studies where LxA4 or vehicle was added after THP-1 differentiation to macrophages before addition of P. aeruginosa for 1 h. At the end of this period, the supernatants were taken, serially diluted, and plated on TSA plates overnight at 37°C before colonies were counted. The results showed that the concentrations of LxA4 given did not affect the ability of the THP-1 cells to clear bacteria (Figures 6a and 6b).
Figure 6.
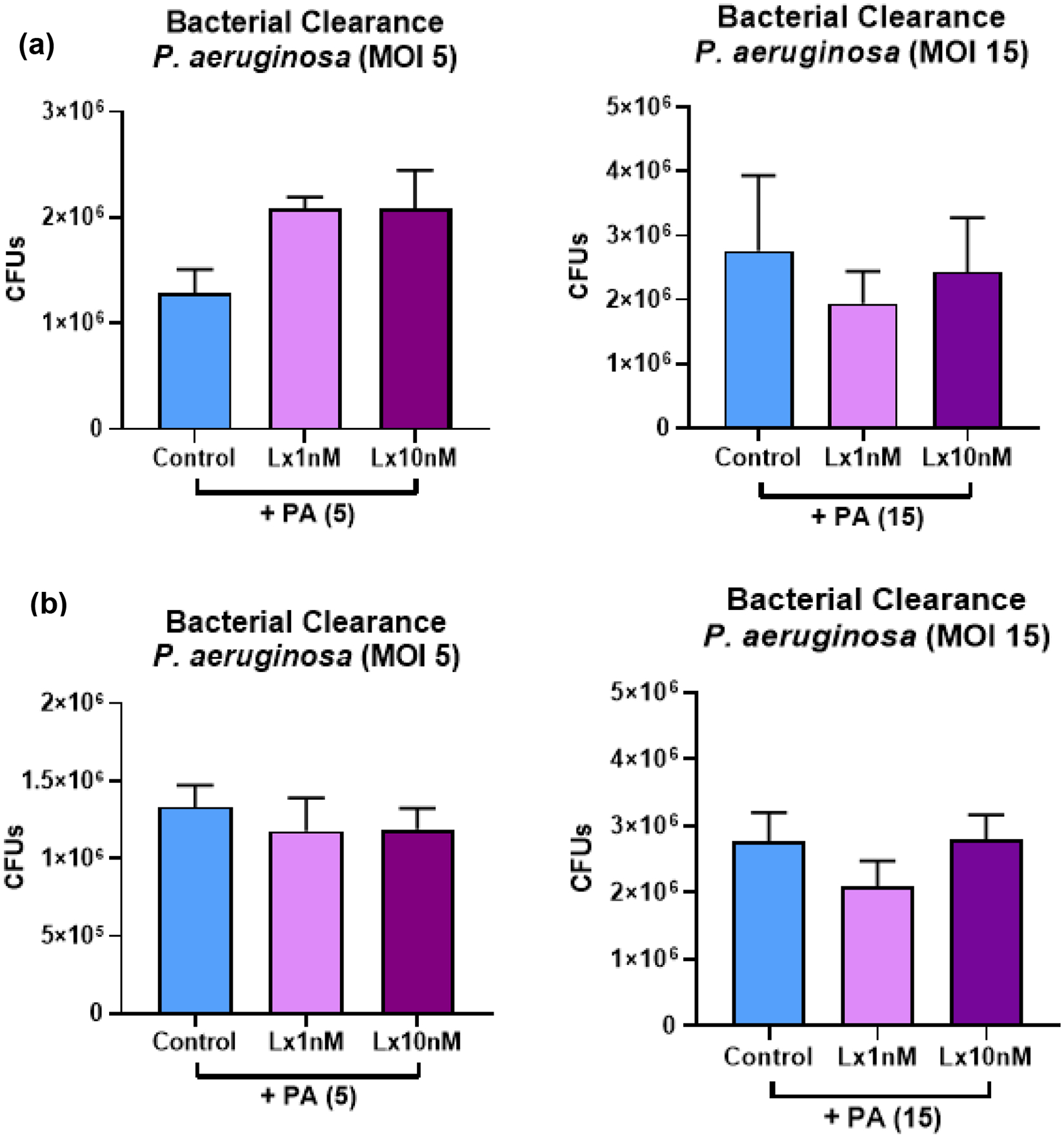
(a) THP-1 monocytes were pre-treated with LxA4 or vehicle before being differentiated into macrophages with the addition of PMA. P. aeruginosa at two multiplicities of infection (5 and 15) were then added. After 1h, the supernatants were taken and plated. Results show that LxA4 did not affect the bacteira clearance ability of THP-1 macrophages when given before differentiation (b) THP-1 monocytes were differentiated into macrophages with PMA. LxA4 or vehicle was then added for 2.5 h before addition of P. aeruginosa for 1 h. Results show that LxA4 did not affect the bacteira clearance ability of THP-1 macrophages when given after differentiation. Data are mean ± s.e.m. for n = 3 in all groups.
LxA4 reduced inflammation in the monocyte-biofilm environment
To quantify the inflammatory status within the milieu, we measured cytokine levels in the supernatant at different time points after addition of THP-1 monocytes which had been treated with vehicle saline or LxA4. It should be noted that THP-1 cells not incubated with biofilm did not produce measurable amounts of TNF-α (data not shown). At 6 h after THP-1 addition, there was no significant difference in TNF-α in the supernatants of biofilms which had been co-incubated with monocytes pre-treated with LxA4 as compared to control monocytes (Figure 7a). At 24 h however, there was an overall decrease in TNF-α in all groups. We did not find a difference amongst groups, but we found that in all experiments we performed, supernatants from LxA4 (10 nM) treated monocytes were lower than the control counterparts (Figure 7a). Using a paired t-test, we found that the LxA4 significantly reduced TNF-α production in the supernatants. With respect to IL-8, there was no significant difference in groups although there was a tendency for LxA4 (10 nM) treated monocytes to increase the production of IL-8 at the 6 h time point (Figure 7b). Taken together the results suggest that LxA4 could reduce TNF-α production in the THP-1 biofilm environment.
Figure 7.
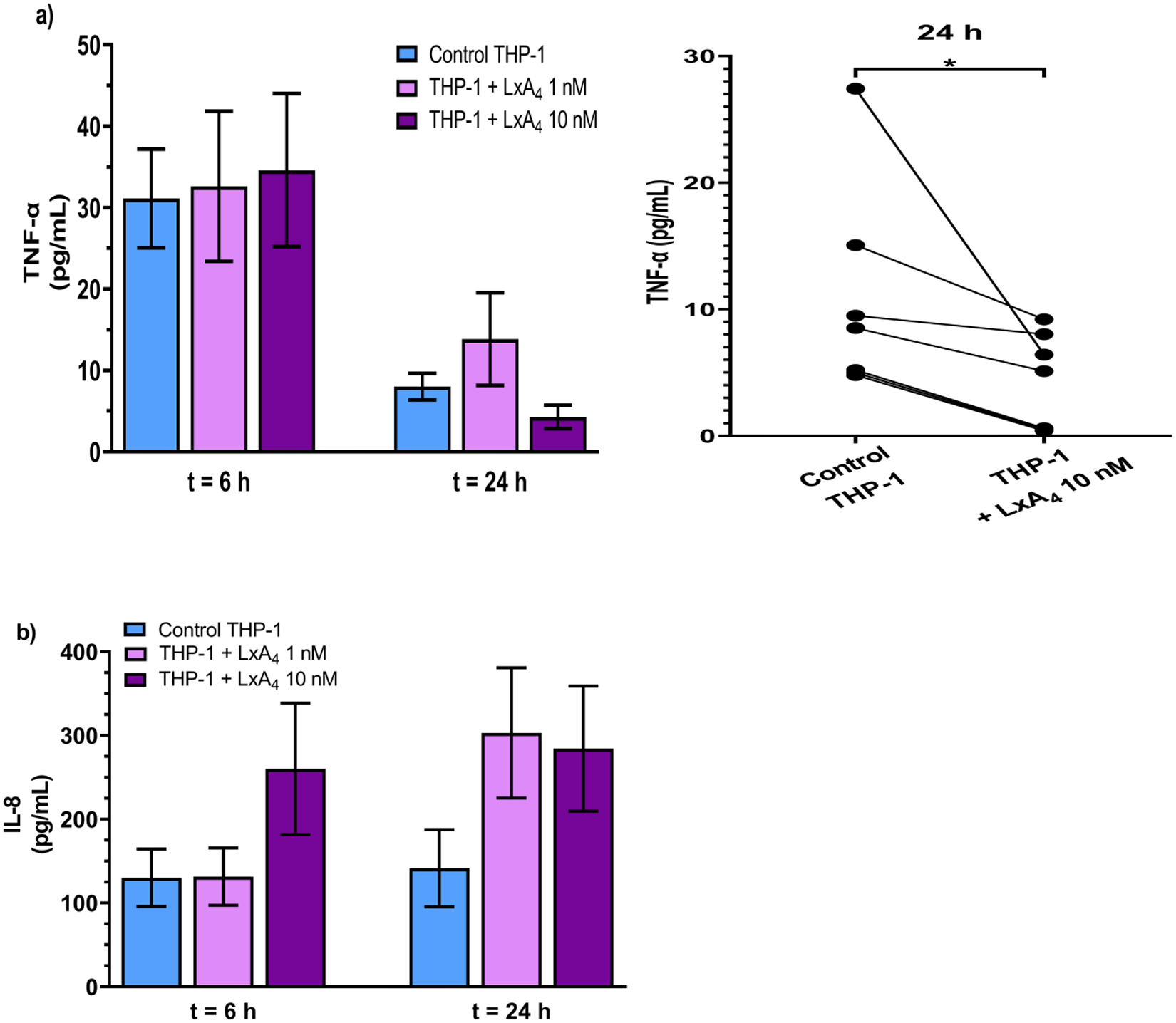
THP-1 monocytes pre-treated with LxA4 were co-incubated with established biofilms for 6 h or 24 h before supernatants were collected and cytokines were quantified using ELISA. (a) LxA4 pre-treatment did not significantly affect TNF-α production at 6 h, but by 24 h, 10 nM LxA4 pre-treatment caused a decrease in TNF-α. When examined as paired before-after data, 10 nM LxA4 pre-treatment significantly decreased TNF-α production in every experiment. (b) 10 nM LxA4 pre-treatment caused a short-term (transient) increase in IL-8 production. Data are mean ± s.e.m. * p < 0.05. n = 7 – 9.
DISCUSSION
P. aeruginosa infections are a major health problem because the infections can be difficult to eliminate. Persistent infection can lead to chronic inflammation and tissue injury36, 37. Part of the virulence of P. aeruginosa is their capacity to form biofilms, which increase the bacteria’s ability to evade antibiotics and host defense mechanisms. This study investigated the direct effects of LxA4 on established biofilm and importantly we also examined the actions of LxA4 on monocyte interaction with the biofilm matrix. In these studies, the P. aeruginosa biofilm was grown under hydrodynamic conditions in a biofilm reactor rather than in static conditions. The biofilm was grown under conditions of constant flow and shear stress38. These conditions are closer to the growth conditions observed in vivo. After growing biofilm in these conditions, we used staining and imaging techniques to examine the effects of LxA4 on P. aeruginosa biofilm matrix. We found that ciprofloxacin alone could significantly reduce live bacteria within the biofilm (Figure 1a). When we examined the live/dead ratio, only LxA4 together with ciprofloxacin, was able to reduce the proportion of live bacteria within the P. aeruginosa biofilm (Figure 1b). In addition, we show that interaction of monocytes with biofilm resulted in a significant increase in live bacteria (and live/dead ratio) within the biofilm after 24 h (Figures 3a and 3b). Treatment of monocytes with LxA4 before co-incubation with the biofilm abolished the increase in live bacteria (and live/dead ratio) within the biofilm (Figures 3a and 3b). The LxA4-mediated decrease in live bacteria was associated with a reduction in TNF-α. We also show that LxA4 increased monocyte adherence to the biofilm, providing evidence that LxA4-treated monocytes decrease biofilm-associated live bacteria by a mechanism that involves direct contact.
Most research has focused on preventing P. aeruginosa biofilm formation and significantly less on reducing established biofilm and very little on the interaction of immune cells with the established biofilm Research has focused on three groups of compounds: (i) combination antibiotics, (ii) antimicrobial peptides (AMPs), and (iii) quorum sensing inhibitors39–41. We have previously shown that LxA4 is a quorum sensing inhibitor through inhibiting the LasR signaling system25. We have also shown that LxA4 can inhibit the expression of virulence genes essential in biofilm formation, and LxA4 increased the efficacy of ciprofloxacin to reduce metabolically active bacteria within the biofilm31. In this previous report, we found that the small beneficial effect of LxA4 in combination with ciprofloxacin in a static biofilm environment only occurred at a low concentration of ciprofloxacin. In our current study, we show that in biofilm that is pre-formed with constant media flow and shear stress, ciprofloxacin was able to reduce live biofilm-associated bacteria when applied twice. Interestingly however, when we quantitated the live/dead ratio, only the ciprofloxacin + LxA4 treatment significantly reduced the live/dead ratio of biofilm-associated bacteria. The results suggest that LxA4 addition to ciprofloxacin helped the killing efficiency of ciprofloxacin. The mechanism for this effect is not known but may be through the inhibition of QS signaling which alters ongoing biofilm formation and allows ciprofloxacin to penetrate the biofilm matrix more readily. Additionally, and interestingly, this regimen of LxA4 and ciprofloxacin given to these biofilms increased the biofilm area. As biofilm comprises proteins and cell components such as exopolysaccharides and nucleic acids, we reasoned that increased cell death would result in an increased biofilm area. This may be due to the reduction in biofilm-associated bacteria being relatively rapid, and the dead cellular material was not degraded fast enough such that the added dead cellular material increased the biofilm area. This important phenomenon warrants further investigation as it may be a mechanism for antibiotic resistance. One possible mechanism postulated for ciprofloxacin resistance in biofilm is due to a subpopulation switch to a persister phenotype, which contributes to a failure in biofilm removal42, 43.
Blood monocytes and tissue macrophages are important components of the immune system that is critical for pathogen recognition and clearance44. SPMs such as LxA4 and resolvins have been shown to induce infection resolution in several models of infection26, 45–54. In these models of infection, the SPMs were reported to decrease bacteria load, increase macrophage recruitment, and reduce neutrophil migration. This generally decreased the inflammatory response and helped the host reach homeostasis. LxA4 has been shown to increase monocyte migration and adherence to laminin-coated plastic plates55. In addition, LxA4 has been reported by several investigators to increase macrophage phagocytosis of bacteria or apoptotic neutrophils30, 56. The mechanism for this increased action is thought to be through a direct effect on macrophage redistribution of IIA and cdc4257. The effects of LxA4 on monocytes’ antimicrobial activity on bacterial biofilm has not been investigated. When peripheral blood mononuclear cells were added to P. aeruginosa biofilm, there was an unexpected rise in biofilm-associated bacteria19. It was reasoned that soluble factor(s) released from the interaction of mononuclear cells with bacterial biofilms led to this increase in biofilm-associated bacteria. Results from our studies are consistent with this report but importantly, we demonstrate that LxA4-treated monocytes completely abolished the rise in live biofilm bacteria, suggesting that LxA4-treated monocytes have increased ability to attack live bacteria within the biofilm. In these studies, we also measured TNF-α and IL-8 levels in the surrounding biofilm/monocyte media in order to measure the inflammatory environment. Our results showed that at 24 h, LxA4-treated monocytes had reduced live bacteria as well as live/dead ratio within the biofilm, and that TNF-α levels were reduced compared to the vehicle saline treated monocyte–biofilm group. LxA4-treated monocytes however did not alter IL-8 levels compared to control monocytes, suggesting that monocyte interaction with biofilm induces a certain level of chemokine secretion that is not affected by either LxA4 pre-treatment or reduction in viable biofilm bacteria. The results suggest that LxA4 increased the activation of the monocytes to produce TNF-α when interacting with P. aeruginosa biofilm. The mechanism for this increased activation may be due to a modification in LPS structure in the biofilm-associated bacteria which enhanced cytokine production from monocytes18. LxA4 has been reported to decrease tissue TLR/NF-κB signaling in lungs after paraquat injury58, LPS stimulated ligament cells28, and in peritoneal macrophages after cecal ligation and puncture sepsis26. Aspirin-triggered LxA4 reduced LPS-induced acute kidney injury and LPS-induced inflammation in microglial cells via down regulation of NF-κB59. The cellular signaling has not been fully investigated in the conditions of bacterial activation as opposed to LPS.
It should be noted that we used the THP-1 cell line and not human peripheral blood mononuclear cells or blood monocytes. This limitation however is minimal because the results obtained using untreated THP-1 cells are identical to that obtained with PBMCs16 with regard to the increase in live bacteria after addition. THP-1 cells have been used extensively as an in vitro model of monocytes/macrophages60. Indeed, THP-1 differentiated macrophages have been used to investigate aspirin-triggered lipoxin mediated phagocytosis of bacteria56 and to elucidate the mechanism by which LxA4 increases THP-1 differentiated macrophage phagocytosis of apoptotic neutrophils57. The major differences between THP-1 cells and PBMCs or primary human monocytes is that THP-1 cells produce less cytokines after stimulation with LPS61. This limitation is minimal because our objective was to measure the overall relative effect of monocytes (with and without LxA4 pretreatment) on the inflammatory environment after addition of cells to biofilm and not any absolute immunomodulatory effects of LxA4. Importantly, similar to the aforementioned report19, there was an increase in cytokines in the biofilm milieu with the addition of THP-1 monocytes. This increase was modulated by LxA4.
Our results showing that LxA4-treated monocytes have increased adhesion to biofilm suggests that part of the mechanism of LxA4-treated monocytes’ ability to reduce viable biofilm bacteria is through increased adhesion. Monocytes adhere to basement membrane proteins such as laminin, elastin and fibronectin62. It has previously been shown that LxA4 can increase monocyte adherence to laminin coated plates55. Importantly, monocyte adherence is an initial step towards differentiation to macrophages63. The results imply that LxA4-activated monocytes have increased ability to adhere to biofilm matrix components. To the best of our knowledge, this is the first report that any compound could increase monocyte adherence to bacterial biofilm. In addition, we showed that LxA4 did not increase the bacteria clearance ability of THP-1 macrophages (Figure 6b). These results are consistent with a previous report showing that aspirin-triggered LxA4 had no effect on the phagocytic ability of macrophages when given before addition of bacteria but could increase phagocytosis if given at the same time56. These results differ from that of a report showing that LxA4 pre-treatment increases macrophage phagocytosis of apoptotic neutrophils29. The reason for these differences may be due to the action of LxA4 to facilitate inflammation resolution before its antimicrobial action. The results suggest that the mechanism by which LxA4 reduced live bacteria within the biofilm is mainly via an increase in the number of adherent THP-1 cells and not through an increase in LxA4 mediated bacteria killing/clearance. Under the conditions of our studies, the increase in adhesion correlated to biofilm biomass reduction at 1 h (Figure 3c, Figure 4). This exposes the bacteria, which are no longer protected by the biofilm matrix, then the monocytes appear to kill the newly exposed bacteria by 24 h.
In summary, our studies suggest that LxA4 can work with ciprofloxacin to reduce viable bacteria in an established biofilm of P. aeruginosa grown under hydrodynamic conditions. Importantly, we show that monocytes induced a rise in live biofilm bacteria and that this effect was abolished by LxA4 pre-treatment of the monocytes. This action of LxA4 was associated with an ability to increase monocyte adherence to biofilm. Together with the decrease in live bacteria, LxA4-treated monocytes also reduced the inflammatory environment around the biofilm. The results suggest that LxA4 can be used as an adjunct together with ciprofloxacin to treat chronic P. aeruginosa infections due to its direct actions with the antibiotic, and also because of its effects to reduce the deleterious monocyte-biofilm interactions.
Acknowledgments
This work was supported by an NIH grant (AI128202) to KY.
Footnotes
Disclosures
The authors declare no conflicts of interest.
Data sharing statement
The data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
- 1.Thi MTT, Wibowo D, Rehm BHA. Pseudomonas aeruginosa Biofilms. Int J Mol Sci 2020; 21: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 2013; 67: 159–173. [DOI] [PubMed] [Google Scholar]
- 3.Raizman R, Little W, Smith AC. Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-Of-Care Fluorescence Imaing. Diagnostics (Basel) 2021; 11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munguia J, Nizet V. Pharmacological Targeting of the Host-Pathogen Interaction: Alternatives to Classical Antibiotics to Combat Drug-Resistant Superbugs. Trends Pharmacol Sci 2017; 38: 473–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 2012; 76: 46–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci U S A 2013; 110: 17981–17986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015; 6: 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumas JL, van Delden C, Perron K, Kohler T. Analysis of antibiotic resistance gene expression in Pseudomonas aeruginosa by quantitative real-time-PCR. FEMS Microbiol Lett 2006; 254: 217–225. [DOI] [PubMed] [Google Scholar]
- 9.Kalia M, Yadav VK, Singh PK, Sharma D, Pandey H, Narvi SS, Agarwal V. Effect of Cinnamon Oil on Quorum Sensing-Controlled Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa. PLoS One 2015; 10: e0135495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branda SS, Vik S, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol 2005; 13: 20–26. [DOI] [PubMed] [Google Scholar]
- 11.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998; 280: 295–298. [DOI] [PubMed] [Google Scholar]
- 12.Strempel N, Neidig A, Nusser M, Geffers R, Vieillard J, Lesouhaitier O, Brenner-Weiss G, Overhage J. Human host defense peptide LL-37 stimulates virulence factor production and adaptive resistance in Pseudomonas aeruginosa. PLoS One 2013; 8: e82240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecuyer S, Rusconi R, Shen Y, Forsyth A, Vlamakis H, Kolter R, Stone HA. Shear stress increases the residence time of adhesion of Pseudomonas aeruginosa. Biophys J 2011; 100: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes LC, Teixeira-Santos R, Romeu MJ, Mergulhão FJ. (2022) Bacterial Adhesion and Biofilm Formation: Hydrodynamics Effects. In Urinary Stents: Current State and Future Perspectives (Soria F, Rako D, and de Graaf P, eds) pp. 225–243, Springer International Publishing, Cham [Google Scholar]
- 15.Pereira MO, Kuehn M, Wuertz S, Neu T, Melo LF. Effect of flow regime on the architecture of a Pseudomonas fluorescens biofilm. Biotechnol Bioeng 2002; 78: 164–171. [DOI] [PubMed] [Google Scholar]
- 16.Jesaitis AJ, Franklin MJ, Berglund D, Sasaki M, Lord CI, Bleazard JB, Duffy JE, Beyenal H, Lewandowski Z. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol 2003; 171: 4329–4339. [DOI] [PubMed] [Google Scholar]
- 17.Walker TS, Tomlin KL, Worthen GS, Poch KR, Lieber JG, Saavedra MT, Fessler MB, Malcolm KC, Vasil ML, Nick JA. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun 2005; 73: 3693–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciornei CD, Novikov A, Beloin C, Fitting C, Caroff M, Ghigo JM, Cavaillon JM, Adib-Conquy M. Biofilm-forming Pseudomonas aeruginosa bacteria undergo lipopolysaccharide structural modifications and induce enhanced inflammatory cytokine response in human monocytes. Innate Immun 2010; 16: 288–301. [DOI] [PubMed] [Google Scholar]
- 19.Kaya E, Grassi L, Benedetti A, Maisetta G, Pileggi C, Di Luca M, Batoni G, Esin S. In vitro Interaction of Pseudomonas aeruginosa Biofilms With Human Peripheral Blood Mononuclear Cells. Front Cell Infect Microbiol 2020; 10: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol 2016; 16: 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem 2020; 64: 443–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014; 510: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 2018; 128: 2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, Bauman JG, Subramanyam B, Perez HD, Parkinson JF, Serhan CN. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol 2004; 143: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu B, Capilato J, Pham MP, Walker J, Spur B, Rodriguez A, Perez LJ, Yin K. Lipoxin A4 augments host defense in sepsis and reduces Pseudomonas aeruginosa virulence through quorum sensing inhibition. FASEB J 2016; 30: 2400–2410. [DOI] [PubMed] [Google Scholar]
- 26.Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, Yin K. Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock 2011; 36: 410–416. [DOI] [PubMed] [Google Scholar]
- 27.Borgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O’Sullivan TP, Guiry PJ, Goldschmeding R, Higgins DF, Godson C. Lipoxin A(4) and benzo-lipoxin A(4) attenuate experimental renal fibrosis. FASEB J 2011; 25: 2967–2979. [DOI] [PubMed] [Google Scholar]
- 28.Ali M, Yang F, Jansen JA, Walboomers XF. Lipoxin suppresses inflammation via the TLR4/MyD88/NF-kappaB pathway in periodontal ligament cells. Oral Dis 2020; 26: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol 2000; 164: 1663–1667. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR, Godson C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol 2002; 13: 2497–2507. [DOI] [PubMed] [Google Scholar]
- 31.Thornton JM, Walker JM, Sundarasivarao PYK, Spur BW, Rodriguez A, Yin K. Lipoxin A4 promotes reduction and antibiotic efficacy against Pseudomonas aeruginosa biofilm. Prostaglandins Other Lipid Mediat 2021; 152: 106505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson MB, Criss AK. Fluorescence microscopy methods for determining the viability of bacteria in association with mammalian cells. J Vis Exp 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heydorn A, Ersboll BK, Vorregaard M. (2015) Comstat 2 (homepage). [Google Scholar]
- 34.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology (Reading) 2000; 146 (Pt 10): 2395–2407. [DOI] [PubMed] [Google Scholar]
- 35.Vorregaard M (2008) Comstat2 - a modern 3D image analysis environment for biofilms, in Informatics and Mathematical Modelling. Technical University of Denmark: Kongens Lyngby, Denmark [Google Scholar]
- 36.Yum HK, Park IN, Shin BM, Choi SJ. Recurrent Pseudomonas aeruginosa Infection in Chronic Lung Diseases: Relapse or Reinfection? Tuberc Respir Dis (Seoul) 2014; 77: 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant SS, Hung DT. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence 2013; 4: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azeredo J, Azevedo NF, Briandet R, Cerca N, Coenye T, Costa AR, Desvaux M, Di Bonaventura G, Hebraud M, Jaglic Z, Kacaniova M, Knochel S, Lourenco A, Mergulhao F, Meyer RL, Nychas G, Simoes M, Tresse O, Sternberg C. Critical review on biofilm methods. Crit Rev Microbiol 2017; 43: 313–351. [DOI] [PubMed] [Google Scholar]
- 39.Leon-Buitimea A, Garza-Cardenas CR, Garza-Cervantes JA, Lerma-Escalera JA, Morones-Ramirez JR. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front Microbiol 2020; 11: 1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terreni M, Taccani M, Pregnolato M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021; 26: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remy B, Mion S, Plener L, Elias M, Chabriere E, Daude D. Interference in Bacterial Quorum Sensing: A Biopharmaceutical Perspective. Front Pharmacol 2018; 9: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis K Persister cells, dormancy and infectious disease. Nat Rev Microbiol 2007; 5: 48–56. [DOI] [PubMed] [Google Scholar]
- 43.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 2001; 9: 34–39. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140: 805–820. [DOI] [PubMed] [Google Scholar]
- 45.Sundarasivarao PYK, Walker JM, Rodriguez A, Spur BW, Yin K. Resolvin D2 induces anti-microbial mechanisms in a model of infectious peritonitis and secondary lung infection. Front Immunol 2022; 13: 1011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker JM, Sundarasivarao PYK, Thornton JM, Sochacki K, Rodriguez A, Spur BW, Acharya NK, Yin K. Resolvin D2 promotes host defense in a 2 - hit model of sepsis with secondary lung infection. Prostaglandins Other Lipid Mediat 2022; 159: 106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med 2015; 212: 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009; 461: 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Purvis GSD, Collotta D, Al Zoubi S, Sugimoto MA, Cacace A, Martin L, Colas RA, Collino M, Dalli J, Thiemermann C. RvE1 Attenuates Polymicrobial Sepsis-Induced Cardiac Dysfunction and Enhances Bacterial Clearance. Front Immunol 2020; 11: 2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haas-Stapleton EJ, Lu Y, Hong S, Arita M, Favoreto S, Nigam S, Serhan CN, Agabian N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS One 2007; 2: e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol 2010; 184: 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horta AL, Williams T, Han B, Ma Y, Menezes APJ, Tu V, Talvani A, Weiss LM, Huang H. Resolvin D1 Administration Is Beneficial in Trypanosoma cruzi Infection. Infect Immun 2020; 88: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isopi E, Mattoscio D, Codagnone M, Mari VC, Lamolinara A, Patruno S, D’Aurora M, Cianci E, Nespoli A, Franchi S, Gatta V, Dubourdeau M, Moretti P, Di Sabatino M, Iezzi M, Romano M, Recchiuti A. Resolvin D1 Reduces Lung Infection and Inflammation Activating Resolution in Cystic Fibrosis. Front Immunol 2020; 11: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu X, Peng X, Lin J, Zhang Y, He H, Zhao G. Lipoxin A4 activates ALX/FPR2 to attenuate inflammation in Aspergillus fumigatus keratitis. Int Immunopharmacol 2021; 96: 107785. [DOI] [PubMed] [Google Scholar]
- 55.Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med 1996; 183: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prescott D, McKay DM. Aspirin-triggered lipoxin enhances macrophage phagocytosis of bacteria while inhibiting inflammatory cytokine production. Am J Physiol Gastrointest Liver Physiol 2011; 301: G487–497. [DOI] [PubMed] [Google Scholar]
- 57.Reville K, Crean JK, Vivers S, Dransfield I, Godson C. Lipoxin A4 redistributes myosin IIA and Cdc42 in macrophages: implications for phagocytosis of apoptotic leukocytes. J Immunol 2006; 176: 1878–1888. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Wang N, Ma Z, Wang Y, Yuan Y, Zhong Z, Hong Y, Zhao M. Lipoxin A4 protects against paraquat-induced acute lung injury by inhibiting the TLR4/MyD88-mediated activation of the NF-kappaB and PI3K/AKT pathways. Int J Mol Med 2021; 47: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang YP, Wu Y, Li LY, Zheng J, Liu RG, Zhou JP, Yuan SY, Shang Y, Yao SL. Aspirin-triggered lipoxin A4 attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-kappaB and MAPKs in BV-2 microglial cells. J Neuroinflammation 2011; 8: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol 2014; 23: 37–45. [DOI] [PubMed] [Google Scholar]
- 61.Schildberger A, Rossmanith E, Eichhorn T, Strassl K, Weber V. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediators Inflamm 2013; 2013: 697972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tobias JW, Bern MM, Netland PA, Zetter BR. Monocyte adhesion to subendothelial components. Blood 1987; 69: 1265–1268. [PubMed] [Google Scholar]
- 63.Nielsen MC, Andersen MN, Moller HJ. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology 2020; 159: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article will be made available by the authors, without undue reservation.


