Abstract
Objective—
To evaluate transduction efficiency of gene therapy for treatment of osteoarthritis in horses.
Sample—
Cartilage and synovial tissues were aseptically collected from the stifle joints of 3 Thoroughbreds; horses were 3, 7, and 12 years old and free from sepsis and long-term drug treatment and were euthanized for reasons unrelated to joint disease.
Procedures—
Gene transfer experiments were performed with 8 recombinant adeno-associated viral vector (rAAV) serotypes in monolayer-cultured equine chondrocytes, synovial cells, and mesenchymal stromal cells and in cartilage and synovial tissues.
Results—
Serotypes rAAV2/5 and rAAV2/2 yielded the highest transduction efficiency in cultured cells 6 days after transduction. Synovial cells and mesenchymal stromal cells were more readily transduced than were chondrocytes. Serotype rAAV2/6.2 yielded the highest rate of gene expression in both cartilage and synovial tissues at 6 days after inoculation. However, at 30 and 60 days after inoculation, gene expression of serotypes rAAV2/2 and rAAV2/5 surpassed that of rAAV2/6.2 and all other serotypes.
Conclusions and Clinical Relevance—
Maximally expressing serotypes changed between 6 and 30 days in tissues; however, the most efficient serotypes for transduction of joint cells over time were also the most efficient serotypes for transduction of joint tissues. In addition, the low transduction efficiency of articular cartilage tissue was paralleled by a low transduction efficiency of isolated chondrocytes. This suggested that the typically low transduction efficiency of articular cartilage may be attributable in part to the low transduction efficiency of the chondrocytes and not solely a result of the dense cartilage matrix. (Am J Vet Res 2012;73:1178–1185)
Osteoarthritis is the most common form of arthritis and is a major contributor to functional impairment and reduced independence in humans and other animals.1–3 Osteoarthritis is characterized by the progressive destruction of articular cartilage and concurrent proliferation of bone, cartilage, and connective tissue cells. This progressive destruction and proliferation response leads to destabilization and remodeling of the entire joint structure, which results in pain, inflammation, stiffness, and restriction of movement.4 In humans, up to 40% of those > 65 years of age may have clinical signs of osteoarthritis, and approximately 80% of this population have some radiographic evidence of osteoarthritis.5 Many osteoarthritis treatment strategies are targeted at reducing pain and physical disability and limiting structural deterioration in affected joints. In humans, treatment for osteoarthritis includes the use of analgesics, NSAIDs, or intra-articular injections of hyaluronan or corticosteroids for temporary relief of pain and inflammation. However, such treatments can be associated with numerous adverse effects, including gastric ulcers, impaired renal function, osteoporosis, and hypertension.6 In patients who fail to respond to medical treatments, joint replacement may eventually be required to provide patient comfort.7,8
Numerous strategies have been developed for cartilage repair with various degrees of success. To our knowledge, complete, functional permanent repair has never been reported in postnatal animals. Horses provide a unique opportunity for the study of osteoarthritis. Horses are an accessible species with considerable longevity, and osteoarthritis is a common and serious problem in horses.9,10 Degeneration of articular cartilage is a hallmark of osteoarthritis in horses, and development of osteoarthritis in horses is often associated with the stresses of racing and training. Signs of joint pain and loss of mobility are common causes of poor performance and early retirement of equine athletes. Some of the most popular treatments for osteoarthritis in horses are comparable with those used in human medicine, including NSAIDs, corticosteroids, and hyaluronan treatments. In contrast to treatments currently used in human medicine, injection of platelet-rich plasma and transplantation of stem cells are routinely used to treat orthopedic conditions in horses. Some horses can be maintained via a combination of therapeutic approaches, but osteoarthritis often signals the end of a horse’s competitive career. Similar to the situation in humans, most osteoarthritis treatments in horses are targeted at ameliorating the signs of the disease and are not curative.
Gene therapy is currently being investigated as an alternative approach to the treatment of arthritis.11,12 Osteoarthritis is an appropriate target for gene therapy because it is a common, poorly treatable condition and a leading cause of disability. Gene therapy can be used in vivo to modify the intra-articular environment of joints without disrupting the native architecture. Despite the promise of gene therapy, 3 major hurdles need to be overcome before these treatments can become clinically viable: the transient nature of transgene expression, characteristic subtherapeutic transduction efficiency, and frequent host responses toward the vector and transgene product.13,14
The specific vectors used for transgene delivery can influence all aspects of the safety and efficacy of any gene therapy. Adeno-associated virus is predominantly a nonintegrating vector; it rarely inserts its viral DNA into the chromosomal DNA of the host organism and, as such, is associated with a greatly reduced risk of causing insertional mutagenesis. If insertional mutagenesis occurs in a gene involved in cell replication or apoptosis, the insertion may compromise the cell’s viability or trigger the cell to replicate incessantly, which would lead to the development of a tumor. Adeno-associated virus is known to be less immunogenic than are other commonly used adenoviral vectors and can provide long-term expression in a variety of target organs, and novel rAAV capsids are available with improved transduction properties.15,16
Historically, strategies for gene delivery to articular joints have targeted synovial tissue, and most studies17,18 have indicated that transgenic expression persists for only 2 to 3 weeks. In many of these studies, most transduced cells are resident synovial fibroblasts. Although synovial fibroblasts are highly amenable to genetic modification with nonintegrating vector systems, they are also highly transient because of the physiologically normal turnover of cells in the synovium. The apparent half-life of synovial fibroblasts in a rat articular joint is < 1 month.19 Cell turnover increases in diseased joints.20 If most nonintegrating, vector-transduced cells in a target joint are transient, the major therapeutic effects also will be transient, with any long-term therapeutic effect dependent on the small remaining nontransient cell population. Targeting cell types other than synovial fibroblasts may provide a better opportunity for gene therapy of secreted proteins with long-term therapeutic effects. The purpose of the study reported here was to evaluate differences in transduction efficiency for various rAAV serotypes in equine synovial joint cells and tissues.
Materials and Methods
Samples—
Cartilage and synovial tissues were collected aseptically from the stifle joints, and bone marrow was collected aseptically from the sternum of 3 geldings that were 3, 7, and 12 years old. Samples were collected within 30 minutes after horses were euthanized. Horses were assessed to be free from sepsis and long-term drug treatment and were euthanized for reasons unrelated to joint disease. Stifle joints were separated from euthanized animals, skinned, and aseptically prepared prior to dissection. All tissues and cells were collected in accordance with protocols and procedures approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Cartilage and synovium—
Cartilage explant tissue was recovered from the articular surfaces of each stifle joint with a 6-mm biopsy punch.a The punch was pushed into the cartilage perpendicular to the articular surface until contact was made with the surface of the bone. The punch was then rotated and removed, and the resultant explant was removed from the bone with a scalpel bladeb via dissection parallel to the articular surface. Cartilage explants were trimmed to a thickness of approximately 100 μm to eliminate any mineralized tissue that might have affected subsequent culture or analysis procedures. Synovial explants were collected by use of iris dissection scissors and consisted of synovial, adipose, and fibrous capsular tissue. Synovial explants were further dissected into 6-mm3 pieces. The explants were placed in 50-mL centrifuge tubes with PBS solution (with Ca2+ and Mg2+) that contained a 2X antimicrobial solutionc and were maintained at room temperature (21°C) until collection of all tissues was completed.
When tissue collection was completed, cartilage and synovial tissue explants were placed in a 24-well plate (1 explant/well) and cultured in culture mediumd containing Dulbecco modified Eagle medium and a nutrient mixture (high glucose) with 10% fetal bovine serum and 1% antimicrobial solution in an incubator at 37°C with 5% CO2. An additional 50 μg of ascorbate-2-phosphate/mL was added to cartilage explant cultures. The 24-well plates were pretreated with polyhydroxyethylmethacrylate prior to long-term culture, as described elsewhere.21
Bone marrow—
Because of their potential for involvement in the repair of damaged articular joints, MSCs were included in the monolayer-cultured cell experiments. Bone marrow was collected aseptically from the sternum with an 11-gauge bone marrow biopsy needlee and a 60-mL sterile Luer-tip syringe that contained 10 mL of sterile acid-citrate-dextrose solution. After aspiration of the bone marrow, the aspirate was briefly mixed with the acid-citrate-dextrose solution, and the syringe then was placed on ice and transported to the laboratory for isolation of MSCs.
Cell culture—
Cells were isolated from explant tissue and bone marrow, as described elsewhere (chondrocytes,21 synovial cells,22 and MSCs23). Briefly, chondrocytes and synovial cells were isolated from explant tissues and MSCs were isolated from bone marrow aspirates; cells were plated at 3.3 X 103 cells/cm2 in a 150-mm tissue culture-treated dish and cultured with culture medium.d An additional 50 μg of ascorbate2-phosphate/mL was added to chondrocyte cultures. All cells were used at passage 3 for transduction assays. Nontransduced control cells and tissues were cultured and assayed in parallel with experimental groups.
Vector production—
Recombinant AAV2-cytomegalovirus-ffl and the rAAV2/1, 2/2, 2/5, 2/6.2, 2/7, 2/8, 2/9, and rh32.33 packaging constructs were generated, and vectorsf were produced by triple transfection and purified as described previously.24–27 Briefly, a cytomegalovirus-driven transgene cassette encoding the ffl flanked by AAV2-inverted terminal repeats was cotransfected with a packaging plasmid that encoded the rep and cap genes. Trans adenoviral helper function was provided by a third helper plasmid, pΔF6. Recombinant AAV purification was performed as described elsewhere.27 All vector preparations were separated on 3 sequential CsCl density gradients. Vector titers were assessed via a real-time PCR assayg with primers and a probe specific for the polyadenylation signal in the vector transgene cassette (bovine growth hormone [forward]: GCCAGCCATCTGTTGT; bovine growth hormone [reverse]: GGAGTGGCACCTTCCA; and bovine growth hormone [probe]: 6FAM-TCC CCC GTG CCT TCC TTG ACC-TAMRA).
Transduction of cells and tissue explants—
For monolayer-cultured cell experiments, 1.0 X 103 cells were plated directly in 96-well, opaque-walled assay platesh (day 0), and transduction was initiated the following day (day 1). Cells were transduced with rAAV at 1.0 X 101 GCs/cell to 1.0 X 105 GCs/cell in 100 μL of basal medium consisting of RPMI 1640 with no protein added. One hour after initiation of transduction, an additional 100 μL of serum-containing culture medium was added to each well. Two hours after initiation of transduction, cells were rinsed with PBS solution (with Ca2+ and Mg2+), and the wells were filled with 200 μL of culture mediumd and placed back into the incubator (37°C at 5% CO2).
In tissue explants, transduction was initiated on the same day as collection (day 0). Explants were removed from the polyhydroxyethylmethacrylate-coated 24-well plates and placed in a 48-well noncoated plate (1 explant/well). Tissue explants were transduced with rAAV at 3.0 X 101 GCs/estimated cell to 3.0 X 105 GCs/estimated cell in 100 μL of basal medium consisting of RPMI 1640 with no protein added. Cell numbers in tissue explants were estimated via a water-soluble tetrazolium-1 colorimetric assay of metabolic activityi on representative explant samples. The relationship between the resultant absorbance and the actual number of cells recovered after explant digestion was corrected on the basis of the efficiency of cell recovery from the tissue. One hour after initiation of transduction, an additional 100 μL of serum-containing culture medium was added to each well. Two hours after initiation of transduction, explants were rinsed with PBS solution (with Ca2+ and Mg2+), placed in polyhydroxyethylmethacrylate-coated 24-well plates filled with 500 μL of culture medium,d and placed back into the incubator (37°C at 5% CO2).
Transduction assays—
Eight rAAV serotypes were assessed for expression (bioluminescence) of a marker gene in monolayer-cultured chondrocytes, MSCs, and synovial cells and synovial and cartilage explant tissues, with ffl as the marker gene. Bioluminescence was measured 2 minutes after addition of 100 μL of d-luciferinj at 50 μg/mL in a bioluminescence imaging systemk and recorded as the number of photons emitted from a designated region of interest. Each vector-treated or control well in the 96-well assay plate encompassed a unique region of interest drawn over each well. Transduction efficiency was defined as BPC. Efficiencies for monolayer-cultured cells were recorded 6 days after initiation of transduction. The point of inflection on the transduction-efficiency curves was between 1.0 X 102 GCs/cell and 1.0 X 103 GCs/cell for most serotypes tested. Corrections were not made for cell growth over the 6-day culture period between vector inoculation (ie, initiation of transduction) and assessment of gene expression. During the 6-day period, nonconfluent, monolayer-cultured chondrocytes, synovial cells, and MSCs routinely multiplied (10-fold increase).
In tissue experiments, explants were of similar sizes but contained an unknown number of cells. To standardize the bioluminescence among explants, a water-soluble tetrazolium-1 assay was performed for all treated and control wells containing explants. Additional wells containing a known number of cells were assayed in the same plate to yield a standard curve of metabolic activity per cell for each plate assayed. Transduction efficiencies for tissue explants were measured for 60 days beginning 24 hours after transduction and again on days 2, 4, 6, 8, 10, 30, and 60. The lowest vector dose at which all tested serotypes generated a detectable bioluminescent signal by day 6 was 3.0 X 102 GCs/cell.
Statistical analysis—
Experimental data were analyzed with paired 2-tailed Student t tests (with unequal distribution of variance assumed) and χ2 analysis. Values of P < 0.05 were considered significant.
Results
Transduction of monolayer cell cultures—
In monolayer-cultured chondrocytes at 6 days after initiation of transduction, there were detectable differences in BPC in all rAAV serotypes tested at vector doses from 1.0 X 102 GCs/cell to 1.0 X 104 GCs/cell. When cultures were transduced at a lower concentration (1.0 X 101 GCs/cell), no serotype had a BPC greater than that for the nontransduced control cells. At a higher concentration (1.0 X 105 GCs/cell), differences among vectors were not significant because of a large increase in variation. At a concentration of 1.0 X 102 GCs/cell, the highest-ranking serotypes were clearly differentiated from the lowest-ranking serotypes on the basis of a considerably higher BPC.
Of the 8 serotypes tested at 1.0 X 102 GCs/cell, cells transfected with serotype rAAV2/5 had the greatest BPC at 6 days after inoculation in chondrocyte cultures. Serotype rAAV2/5 had a significantly higher BPC than did serotypes rAAV2/1 (P = 0.024) and rAAV2/2 (P = 0.017; Figure 1). Synovial cells and MSCs had patterns of transduction similar to those observed in chondrocytes, with serotypes rAAV2/2 and rAAV2/5 having efficient transduction at 1.0 X 102 GCs/cell. In synovial cells at 1.0 X 102 GCs/cell, rAAV2/2 was ranked higher than the second-ranked serotype rAAV2/5, but these values did not differ significantly. Serotypes rAAV2/2 (P = 0.029) and rAAV2/5 (P = 0.016) had a significantly higher BPC than did the third-ranked serotype, rAAV2/7. In MSCs at 1.0 X 102 GCs/cell, serotype rAAV2/5 was ranked higher, but was not significantly different from serotypes rAAV2/2 (P = 0.153), rAAV2/1 (P = 0.088), and rAAV2/8 (P = 0.091). Serotype rAAV2/5 had a significantly (P = 0.008) higher BPC than did serotype rAAV2/7. In most serotypes tested at 1.0 X 102 GCs/cell, synovial cells and MSCs were more readily transduced than were chondrocytes.
Figure 1—
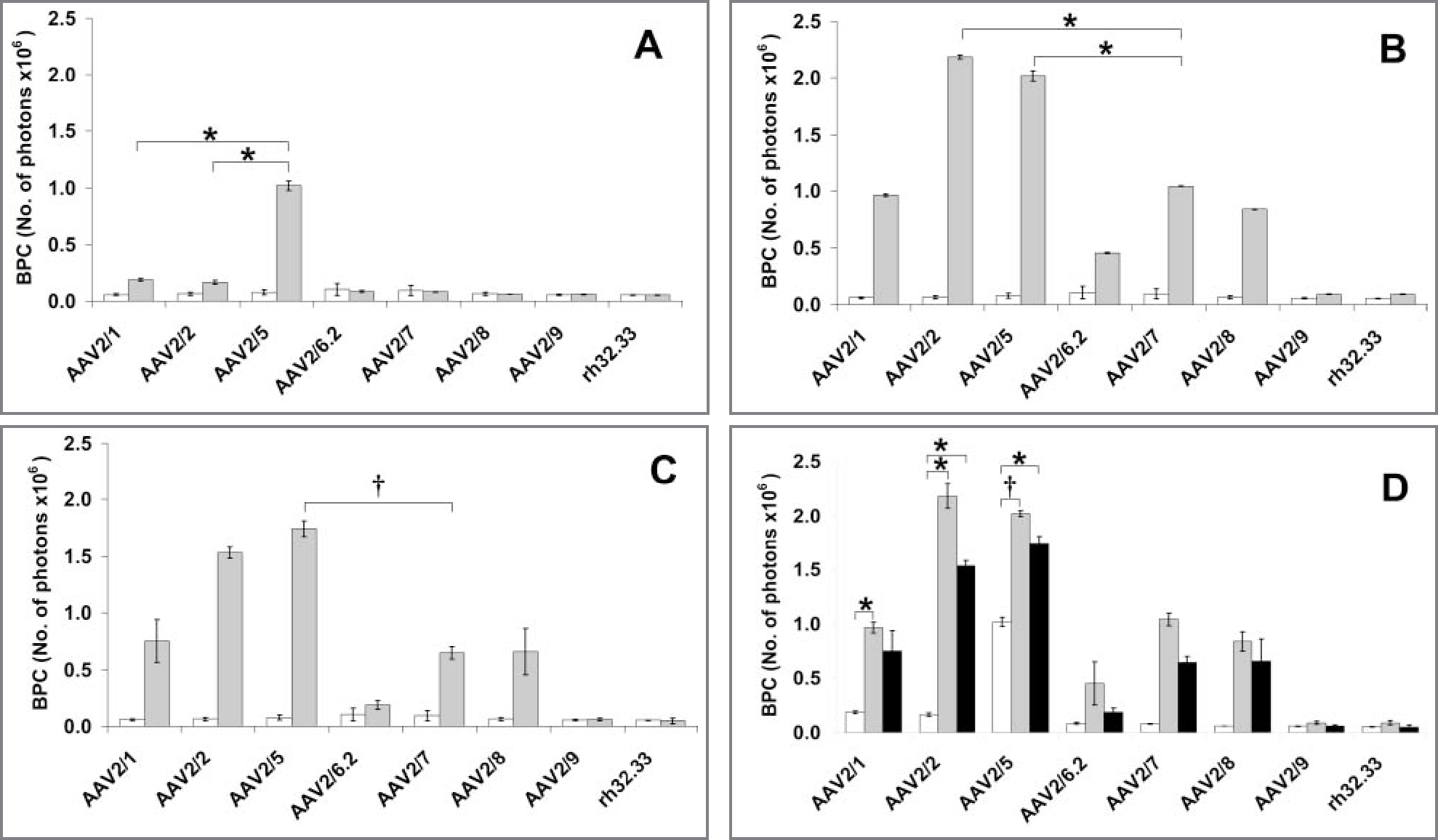
Mean ± SE results for serotype transduction in control and vector-inoculated monolayer-cultured cells. A—In control (white bars) and vector-inoculated (6 days after vector inoculation at 1.0 X 102 GCs/cell; gray bars) monolayer-cultured chondrocytes, serotype rAAV2/5 had the highest BPC, followed by serotypes rAAV2/1 and rAAV2/2. Results for serotype rAAV2/5 were significantly higher than results for serotypes rAAV2/1 (P = 0.024) and rAAV2/2 (P = 0.017). B—In control (white bars) and vector-inoculated (gray bars) monolayer-cultured synovial cells 6 days after vector inoculation, serotype rAAV2/2 had the highest BPC, followed by serotypes rAAV2/5 and rAAV2/7. At a concentration of 1.0 X 102 GCs/cell, serotypes rAAV2/2 (P = 0.029) and rAAV2/5 (P = 0.016) had values significantly higher than did serotype rAAV2/7. C—In control (white bars) and vector-inoculated (gray bars) MSCs at 6 days after inoculation, serotype rAAV2/5 had the highest BPC, followed by serotypes rAAV2/2 and rAAV2/1. At a concentration of 1.0 X 102 GCs/cell, serotype rAAV2/5 had values significantly (P = 0.008) higher than did serotype rAAV2/7. D—Transduction efficiency 6 days after vector inoculation at 1.0 X 102 GCs/cell was greatest in synovial cells (gray bars), followed by MSCs (black bars) and then chondrocytes (white bars). *†Values differ significantly (*P < 0.05; †P < 0.01).
Transduction of tissue explants—
In tissue explants at 6 days after inoculation, the lowest vector dose at which the tested serotypes commonly generated a detectable bioluminescent signal greater than that of control explants was 3.0 X 102 GCs/cell. Analysis of the BPC measurements 6 days after inoculation in cartilage explant tissue revealed that at 3.0 X 102 GCs/cell, serotype rAAV2/6.2 had the highest transduction efficiency, compared with that for rAAV2/2 (P = 0.040) and rAAV2/5 (P = 0.001; Figure 2). Vector rAAV2/6.2 inoculated into synovial explant tissue also had the highest transduction efficiency; it was significantly (P < 0.001) higher than that for rAAV2/5 and rAAV2/2. Vector rAAV2/6.2 had a transduction efficiency in synovial tissue explants that was significantly (P < 0.001) higher (3-fold as high) than the transduction efficiency for that vector when inoculated in cartilage explants.
Figure 2—
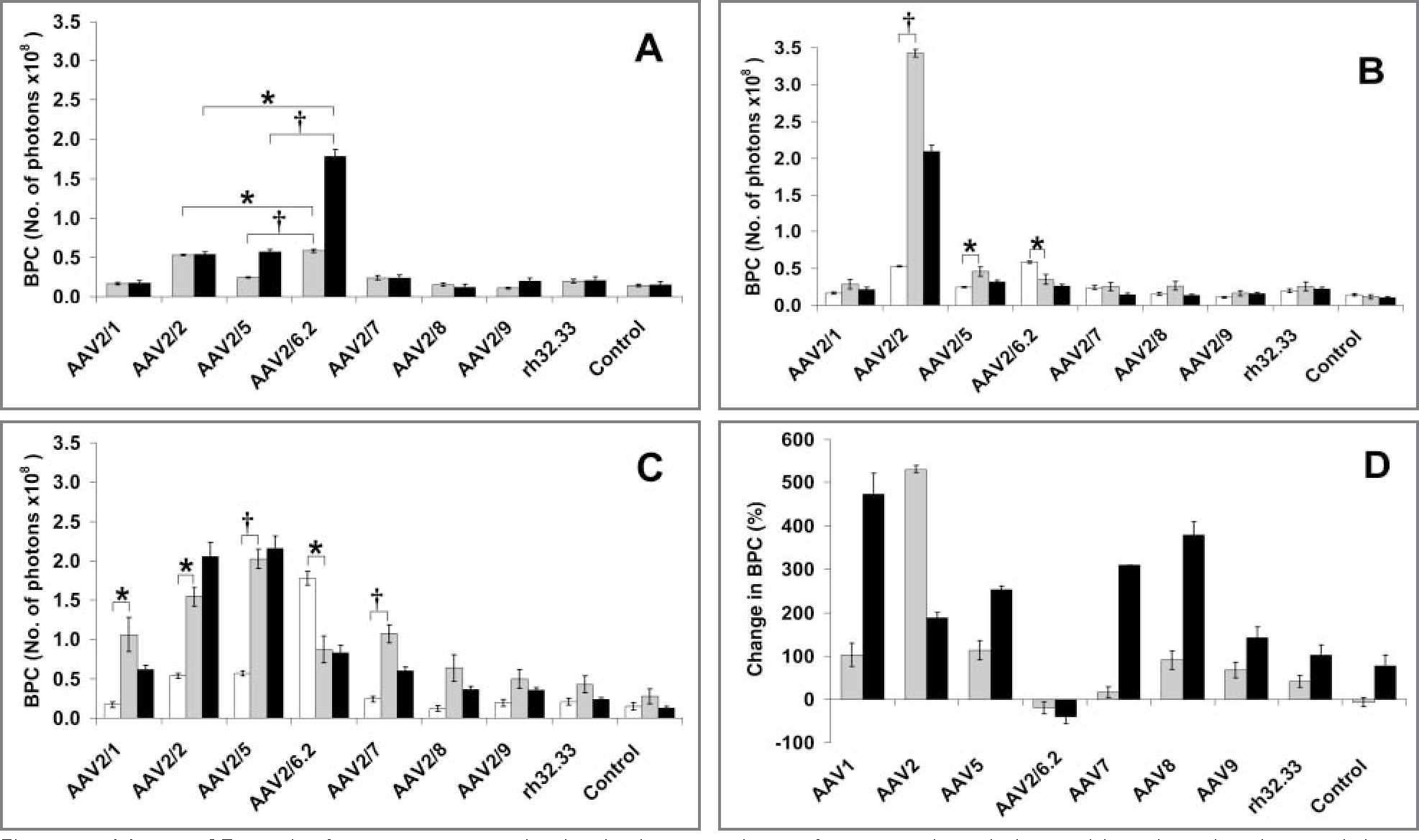
Mean ± SE results for serotype transduction in tissue explants after vector inoculation and in uninoculated control tissue explants. A—In cartilage tissue explants (gray bars) at 6 days after vector inoculation at 3.0 X 102 GCs/cell, serotype rAAV2/6.2 had a significantly (P = 0.040) higher BPC, compared with rAAV2/2 and all other serotypes tested. Serotype rAAV2/6.2 also had a significantly (P < 0.001) higher BPC in synovial tissue explants (black bars) at 6 days after inoculation, compared with that for serotypes rAAV2/5 and rAAV2/2. B—Cartilage tissue explants at 6 (white bars), 30 (gray bars), and 60 days (black bars) after vector inoculation at 3.0 X 102 GCs/cell. At day 30, serotype rAAV2/2 had the highest BPC, followed by serotypes rAAV2/5 and rAAV2/6.2, whereas at day 60, serotype rAAV2/2 had the highest BPC, followed by serotypes rAAV2/5 and rAAV2/6.2. C—Synovial tissue explants at 6 (white bars), 30 (gray bars), and 60 days (black bars) after vector inoculation. Notice that at 30 and 60 days, serotype rAAV2/5 had the highest BPC, followed by serotype rAAV2/2. D—All vector serotypes, except for serotype rAAV2/6.2, had an increase in BPC from day 6 to day 30 after inoculation in cartilage tissue explants (gray bars) and synovial tissue explants (black bars). See Figure 1 for remainder of key.
Transduction at extended time points—
When the vector-transduced tissue explants assayed at day 6 were again assayed at days 30 and 60, the hierarchy in transduction efficiency and BPC changed from that detected at day 6. In cartilage tissue explants, BPC measurements increased (> 6-fold increase) at 30 days after inoculation for serotype rAAV2/2, which had the highest BPC among transduced explants at day 30 and was significantly (P < 0.001) higher than the next highest serotypes, rAAV2/5 and rAAV2/6.2. This serotype relationship and the significant differences (P < 0.001) between rAAV2/2 and rAAV2/5 and rAAV2/6.2 were maintained through day 60 in cartilage explants. In synovial tissue explants, serotype rAAV2/5 had the highest BPC at 30 days after inoculation and was significantly (P = 0.029) higher than serotype rAAV2/2, which ranked second (Figure 2). At 60 days after inoculation in synovial tissue explants, values for BPC after inoculation with serotype rAAV2/5 were again higher than the values for the second-ranked serotype, rAAV2/2, but these values did not differ significantly (P = 0.668). At 60 days after inoculation, serotype rAAV2/6.2 was the third-ranked serotype (P < 0.001) in synovial tissue explants. In tissue explants, all vector serotypes but 1 had an increase in BPC from day 6 to day 30. The exception was serotype rAAV2/6.2, which was the only serotype that had a decrease in BPC from day 6 to day 30 and from day 6 to day 60.
Discussion
Although technological advances have been reported to increase the rate of onset of gene expression in AAV-transduced cells, cellular access and intracellular transport in joint tissues still appear to be the rate-limiting steps in an effective use of gene therapy for the treatment of synovial joints.28,29 Most transduced cells within vector-injected joints are of synovial or capsular origin.18,19 In addition to the inherent low transduction efficiency of chondrocytes embedded in chondral matrix, degraded proteoglycans in diseased tissues can competitively bind vectors, which further decreases transduction efficiency of cartilage. Chondrocytes in a monolayer culture are readily transduced, compared with transduction for chondrocytes embedded within the dense extracellular matrix of their native habitat. To efficiently target all tissue types in articular joints, we initially separated the tissue matrices from the cells we ultimately sought to transduce (ie, chondrocytes, synovial cells, and MSCs). In the experiments described here, we defined transduction efficiency as the greatest measure of expression of a marker gene at the lowest common concentration of vector. A vector concentration of 1.0 X 102 GCs/cell allowed the greatest difference in BPC among vector serotypes in all monolayer-cultured cell types tested. This dose was subsequently used to compare serotype transduction efficiency within and between cell types.
At the low dose of 1.0 X 102 GCs/cell, serotype rAAV2/5 was the dominant serotype in monolayer-cultured chondrocytes at day 6 after inoculation. Gene expression with serotypes rAAV2/2 and rAAV2/5 was closely matched in synovial cells and MSCs. However, synovial cells and MSCs were more readily transduced than were chondrocytes with most serotypes tested. Gene expression was consistently higher (≥ 2-fold as high) in synovial cells and MSCs than in chondrocytes.
Given that we used cells at passage 3, the vector transduction properties of these cells may have differed from properties in primary cells or cells within a native tissue matrix. To more closely mimic in vivo articular joint tissues, we transitioned from experiments in monolayer-cultured cells to experiments in synovial and cartilage explant tissues. Cartilage biopsy specimens obtained from the articular surface were used to mimic the cartilage surface to be transduced in vivo.30 In contrast to the relatively homogenous cell population of articular cartilage, the cells of the synovial tissue that lines the articular joint capsule are composed of a heterogeneous population from different origins, which are embedded in different matrices. To most closely emulate the in vivo state, we chose to include synovial, adipose, and fibrous capsular tissue in our samples of synovial tissue explants. Vector doses were initially estimated from metabolic assays of representative samples of tissue explants. These doses were further refined by selecting a dose that would induce measurable gene expression in amounts greater than those of control explants in most vectors tested.
At 6 days after inoculation, serotypes rAAV2/6.2, rAAV2/2, and rAAV2/5 were the 3 serotypes with the highest expression in both cartilage and synovium tissue explants. Serotype rAAV2/6.2 had higher (3-fold as high) gene expression in synovial explants, compared with that in cartilage explants, whereas the second- and third-ranking serotypes had almost identical gene expression in both synovial and cartilage tissue explants. Interestingly, the top-ranking serotype in tissue explants at day 6 after inoculation, rAAV2/6.2, ranked only fourth for expression in monolayer-cultured chondrocytes at day 6 after inoculation and was ranked sixth of the 8 serotypes tested for expression in synovial cells and MSCs.
Long-term monolayer culture of cells proved impractical because of the expansion of cell numbers and subsequent dilution of the nonintegrating rAAV genome. In contrast to monolayer-cultured cells, we cultured explant tissues on polyhydroxyethylmethacrylate-coated culture surfaces. The tissue explants did not attach to the culture surface and essentially remained intact and relatively undegraded, which enabled long-term culture. This provided the opportunity to assay the same cultures over an extended period. We initially reported gene expression of tissue explants at 6 days after inoculation to match the inoculation-to-assay time point in monolayer-cultured cells. These same explant tissues that were assayed at day 6 were again assayed at 30 and 60 days after initiation of transduction. At 30 days after initiation of transduction, all serotypes tested, except for serotype rAAV2/6.2, displayed increased gene expression. Serotype rAAV2/6.2 was the only vector serotype that had a decrease in gene expression at day 30 after inoculation, although the amount of gene expression displayed by serotype rAAV2/6.2 at days 30 and 60 after inoculation was similar to the amount of expression for other serotypes tested at these same time points.
Recombinant rAAV2/6.2 is an AAV serotype derived from AAV6, which in turn appears to be a hybrid between AAV1 and AAV2.31,32 Recombinant AAV2/6.2, similar to AAV6, uniquely binds heparin through an interaction that requires the presence of a lysine residue on the peaks surrounding the 3-fold axis of symmetry of the particle architecture.33,34 However, heparin or heparin sulfate proteoglycan does not mediate viral entry of AAV6.35 Serotype AAV6 also binds alpha-2,3 and alpha-2,6 N-linked sialic acids and uses this interaction to facilitate transduction. Serotype AAV1, a closely homologous clade member of AAV6, also has this property. In the present study, vector serotype rAAV2/1 did not have the enhanced gene transfer of rAAV2/6.2 at day 6 after inoculation, but rAAV2/1 had the greatest increase in gene expression (520%) from day 6 to day 30 in synovial tissue. The heparin affinity combined with the sialic acid interaction of serotype rAAV2/6.2 may have played a role in the early (day 6 after inoculation) transduction pattern observed in the present study. Interestingly, the top-performing serotype in cartilage tissue at 30 and 60 days was AAV2/2, which also binds heparin (albeit through a motif distinct from that of AAV6), and the top-performing serotype in synovial tissue at 30 and 60 days was AAV2/5, which uses sialic acid as a cell-surface uptake receptor.36–38
Because the expression for serotype rAAV2/6.2 was so high at day 6 after inoculation, this serotype may have outpaced the other serotypes in initiation of gene expression. Because expression of the ffl gene for serotypes rAAV2/2 and rAAV2/5 surpassed the gene expression for serotype rAAV2/6.2 at 30 and 60 days after inoculation, serotypes rAAV2/2 and rAAV2/5 may have had higher initial rates of transfection with delayed expression. Alternatively, these vector serotypes may have been maintained in the tissue matrix, which allowed a longer duration of time for infection of the resident cells. The superior performance of serotypes rAAV2/2 and rAAV2/5 in monolayer-cultured cells at day 6 after inoculation provides an argument against a delay in gene expression, although the matrix of each tissue affects the metabolism of its resident cells and could easily have influenced the dynamics of gene expression of the cells contained in each tissue. The relationship between cells and their surrounding matrix likely changed over the 60-day culture period, which would contribute to observed differences in gene expression at 30 and 60 days. Because cell number differed between explants in both tissue types at the time of transduction, some explants may have initially been exposed to more or fewer GCs per cell. In preliminary tissue-transduction experiments that used various GC concentrations per well, the absolute values for BPC varied with the number of GCs per cell, but the hierarchy in gene expression between serotypes remained constant, which supported our observations for results obtained with 3.0 X 102 GCs/cell.
Although the maximally expressing serotypes changed over time in transduced explant tissues, the top-ranked vector serotypes in tissue explants were also the top-ranked serotypes in monolayer-cultured cells. The only exception to this pattern was serotype rAAV2/6.2, which had moderate gene expression in cultured cells and explants at 30 and 60 days after inoculation but was the top-ranked serotype in explants at day 6 after inoculation. Over time, the most efficient rAAV serotypes selected for transduction of isolated equine articular joint cells were also the most efficient serotypes for transduction of articular joint tissues. In addition, the observed low transduction efficiency of articular cartilage tissue, compared with that of other joint tissues, was paralleled by a low transduction efficiency in isolated chondrocytes, compared with that in other isolated types of joint cells. This suggests that the typically low transduction efficiency of articular cartilage may be attributable in part to the low transduction efficiency of the chondrocytes and was not solely a result of the dense cartilage matrix. As was evident in monolayer-cultured chondrocytes, there was 1 exception to the low transduction efficiency of cartilage tissue explants. Cartilage explants transduced with vector serotype rAAV2/2 had a 6-fold increase in gene expression from days 6 to 30, which surpassed the expression for synovial tissue at day 30 (Figure 2).
Analysis of the results for the study reported here supports the potential use of multiple rAAV serotypes for effective transduction of equine synovial joint tissues. This can be relevant to therapeutic applications in subjects that have preexisting immunity to a particular serotype and therefore may be refractory to rAAV gene therapy. The use of multiple rAAV serotypes may also be a critical tool for protocols that require repeat vector administration over time and for which the availability of alternative vector serotypes may provide an answer for the vector-neutralizing antibodies induced by prior administration of an rAAV-based gene therapy.
Acknowledgments
Supported in part by Brushwood Stables; the James Foundation; the United States Equestrian Federation Equine Health Research Fund; the Vector Core of the Gene Therapy Program, University of Pennsylvania; and a grant to Dr. Wilson from the National Institutes of Health (grant No. 2-P30-DK047757-16).
The authors thank Drs. Marty Keough, Julie Johnstone, Peter Bell, and Sadik Kassim and Andrea Phillips for technical assistance.
Abbreviations
- AAV
Adeno-associated viral vector
- BPC
Bioluminescence per cell
- ffl
Firefly luciferase
- GC
Genome copy
- MSC
Mesenchymal stromal cell
- rAAV
Recombinant adeno-associated viral vector
Footnotes
Presented in part at the Annual Experimental Biology Conference, New Orleans, April 2009.
Sklar Instruments, West Chester, Pa.
Miltex Inc, York, Pa.
Gibco, Grand Island, NY.
DMEM/F12, Gibco, Grand Island, NY.
Jamshidi needle, Sherwood Medical Co, St Louis, Mo.
Vector Core Facility, Gene Therapy Program, University of Pennsylvania, Philadelphia, Pa.
Taqman, Applied Biosystems Inc, Foster City, Calif.
Costar, Corning Inc, Corning, NY.
Roche Diagnostics, Indianapolis, Ind.
Promega Corp, Madison, Wis.
IVIS Imaging System, Xenogen Corp, Alameda, Calif.
Contributor Information
Jeffrey B. Mason, Department of Clinical Studies, New Bolton Center, School of Veterinary Medicine, University of Pennsylvania, Kennett Square, PA 19348.
Luk H. Vandenberghe, Gene Therapy Program, Department of Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104.
Ru Xiao, Gene Therapy Program, Department of Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104.
James M. Wilson, Gene Therapy Program, Department of Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104.
Dean W. Richardson, Department of Clinical Studies, New Bolton Center, School of Veterinary Medicine, University of Pennsylvania, Kennett Square, PA 19348.
References
- 1.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage 2007;15:981–1000. [DOI] [PubMed] [Google Scholar]
- 3.Sutton S, Clutterbuck A, Harris P, et al. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet J 2009;179:10–24. [DOI] [PubMed] [Google Scholar]
- 4.Martel-Pelletier J, Alaaeddine N, Pelletier J-P. Cytokines and their role in the pathophysiology of osteoarthritis. Front Biosci 1999;4:D694–D703. [DOI] [PubMed] [Google Scholar]
- 5.Dawson J, Linsell L, Zondervan K, et al. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology 2004;43:497–504. [DOI] [PubMed] [Google Scholar]
- 6.Fraenkel L, Bogardus ST, Concato J, et al. Treatment options in knee osteoarthritis—the patient’s perspective. Arch Intern Med 2004;164:1299–1304. [DOI] [PubMed] [Google Scholar]
- 7.Walker-Bone K, Javaid K, Arden N, et al. Regular review—medical management of osteoarthritis. BMJ 2000;321:936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter DJ, Felson DT. Osteoarthritis. BMJ 2006;332:639B–642B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossdale PD, Hopes R, Digby NJW. Epidemiological study of wastage among racehorses. 1982 and 1983. Vet Rec 1985;116:66–69. [DOI] [PubMed] [Google Scholar]
- 10.Wilsher S, Allen WR, Wood JLN. Factors associated with failure of Thoroughbred horses to train and race. Equine Vet J 2006;38:113–118. [DOI] [PubMed] [Google Scholar]
- 11.Evans CH, Ghivizzani SC, Robbins PD. Progress and prospects: genetic treatments for disorders of bones and joints. Gene Ther 2009;16:944–952. [DOI] [PubMed] [Google Scholar]
- 12.Mease PJ, Wei N, Fudman EJ, et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 study. J Rheumatol 2010;37:692–703. [DOI] [PubMed] [Google Scholar]
- 13.Evans CH, Gouze E, Gouze JN, et al. Gene therapeutic approaches-transfer in vivo. Adv Drug Deliv Rev 2006;58:243–258. [DOI] [PubMed] [Google Scholar]
- 14.Saraf A, Mikos AG. Gene delivery strategies for cartilage tissue engineering. Adv Drug Deliv Rev 2006;58:592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenenbaum L, Lehtonen E, Monahan PE. Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr Gene Ther 2003;3:545–565. [DOI] [PubMed] [Google Scholar]
- 16.Cottard V, Valvason C, Falgarone G, et al. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J Clin Immunol 2004;24:162–169. [DOI] [PubMed] [Google Scholar]
- 17.Hung GL, Galealauri J, Mueller GM, et al. Suppression of intraarticular responses to interlukin-1 by transfer of the interlukin-1 receptor antagonist gene to synovium. Gene Ther 1994;1:64–69. [PubMed] [Google Scholar]
- 18.Khoury M, Bigey P, Louis-Plence P, et al. A comparative study on intra-articular versus systemic gene electrotransfer in experimental arthritis. J Gene Med 2006;8:1027–1036. [DOI] [PubMed] [Google Scholar]
- 19.Gouze E, Gouze JN, Palmer GD, et al. Transgene persistence and cell turnover in the diarthrodial joint: implications for gene therapy of chronic joint diseases. Mol Ther 2007;15:1114–1120. [DOI] [PubMed] [Google Scholar]
- 20.Pan RY, Xiao X, Chen SL, et al. Disease-inducible transgene expression from a recombinant adeno-associated virus vector in a rat arthritis model. J Virol 1999;73:3410–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novotny JE, Turka CM, Jeong C, et al. Biomechanical and magnetic resonance characteristics of a cartilage-like equivalent generated in a suspension culture. Tissue Eng 2006;12:2755–2764. [DOI] [PubMed] [Google Scholar]
- 22.Frean SP, Lees P. Effects of polysulfated glycosaminoglycan and hyaluronan on prostaglandin E2 production by cultured equine synoviocytes. Am J Vet Res 2000;61:499–505. [DOI] [PubMed] [Google Scholar]
- 23.Giovannini S, Brehm W, Mainil-Varlet P, et al. Multilineage differentiation potential of equine blood-derived fibroblast-like cells. Differentiation 2008;76:118–129. [DOI] [PubMed] [Google Scholar]
- 24.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998;72:2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auricchio A, Hildinger M, O’Connor E, et al. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther 2001;12:71–76. [DOI] [PubMed] [Google Scholar]
- 26.Hildinger M, Auricchio A, Gao G, et al. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol 2001;75:6199–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao GP, Alvira MR, Wang LL, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A 2002;99:11854–11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodrich LR, Choi VW, Carbone BAD, et al. Ex vivo serotype-specific transduction of equine joint tissue by self-complementary adeno-associated viral vectors. Hum Gene Ther 2009;20:1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santangelo KS, Baker SA, Nuovo G, et al. Detectable reporter gene expression following transduction of adenovirus and adeno-associated virus serotype 2 vectors within full-thickness osteoarthritic and unaffected canine cartilage in vitro and unaffected guinea pig cartilage in vivo. J Orthop Res 2010;28:149–155. [DOI] [PubMed] [Google Scholar]
- 30.Sumer EU, Schaller S, Sondergaard BC, et al. Application of biomarkers in the clinical development of new drugs for chondroprotection in destructive joint diseases: a review. Biomarkers 2006;11:485–506. [DOI] [PubMed] [Google Scholar]
- 31.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol 1998;72:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandenberghe LH, Breous E, Nam HJ, et al. Naturally occurring singleton residues in AAV capsid impact vector performance and illustrate structural constraints. Gene Ther 2009;16:1416–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu ZJ, Miller E, Agbandje-McKenna M, et al. Alpha 2,3 and alpha 2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol 2006;80:9093–9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandenberghe LH, Wilson JM. AAV as an immunogen. Curr Gene Ther 2007;7:325–333. [DOI] [PubMed] [Google Scholar]
- 35.Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol 2001;75:6615–6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kern A, Schmidt K, Leder C, et al. Identification of a heparinbinding motif on adeno-associated virus type 2 capsids. J Virol 2003;77:11072–11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 1998;72:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walters RW, Yi SMP, Keshavjee S, et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem 2001;276:20610–20616. [DOI] [PubMed] [Google Scholar]


