Abstract
Objective:
[18F]Fluorodeoxyglucose positron emission tomography ([18F]FDG-PET) is a valuable method for detecting focal brain dysfunction associated with epilepsy. Evidence suggests that a progressive decrease in [18F]FDG uptake occurs in the epileptogenic cortex with an increase in the duration of epilepsy. In this study, our aim was to use statistical parametric mapping (SPM) to test the validity of this relationship in a retrospective study of patients with temporal lobe epilepsy (TLE).
Methods:
[18F]FDG-PET scans of 46 adult patients with pharmacoresistant unilateral TLE (25 RTLE and 21 LTLE) were subjected to SPM analysis.
Results:
Forty-six patients were diagnosed with nonlesional TLE, 16 of whom had hippocampal sclerosis (HS). The average duration of epilepsy was 17.4 ± 12.3 years (3−46 years), <5 years in 10 patients and ≥10 years in 30 patients. Visual analysis of [18F]FDG-PET scans revealed hypometabolism in the epileptogenic temporal cortex in 31 (67%) patients. After SPM analysis of all [18F]FDG-PET images, hypometabolism was unilateral and reported in lateral and mesial structures of the epileptogenic temporal cortex in addition to the ipsilateral fusiform and middle occipital gyrus. Subsequent analysis revealed that temporal lobe hypometabolism was present only in patients with longer epilepsy duration (≥10 years) in parahippocampal gyrus, uncus, and middle and superior temporal gyrus (P < 0.05 corrected). Epilepsy duration was inversely correlated with decreased glucose uptake in the inferior temporal gyrus, hippocampus, and parahippocampal gyrus of the epileptogenic temporal cortex (P < 0.05). Age at seizure onset did not affect the correlation between epilepsy duration and glucose uptake except in the inferior temporal gyrus (P < 0.05).
Conclusion:
Voxel-based mapping supports the assertion that glucose hypometabolism of the epileptogenic temporal lobe cortex and other neighboring cortical regions increases with longer epilepsy duration in TLE.
Keywords: Temporal lobe epilepsy, Positron emission tomography, Epilepsy duration, Statistical parametric mapping
1. Introduction
[18F]Fluorodeoxyglucose positron emission tomography ([18F]FDG-PET) scanning is a useful tool for evaluating baseline in vivo brain metabolism and localizing dysfunctional cortex in patients with focal epilepsy. Numerous studies indicate that hypometabolism is typically localized to the epileptogenic temporal cortex in TLE [1−5]. Moreover, [18F]FDG-PET hypometabolism can identify dysfunctional cortex even in the absence of obvious magnetic resonance imaging (MRI) abnormalities in patients with pharmacoresistant TLE [1,6−8]. Therefore, [18F]FDG-PET imaging has been universally accepted as a useful tool to localize the epileptogenic cortex as part of the presurgical evaluation for intractable epilepsy [5,6,9].
Visual interpretation of [18F]FDG-PET is routinely performed for clinical assessment in epilepsy practice. A number of quantitative methods have been introduced to analyze [18F]FDG-PET data and have added more detailed information to identify dysfunctional cortex. The application of quantitative methods to functional imaging data has demonstrated the topography and extension of hypometabolism within and often beyond the epileptogenic temporal cortex into other cortical and subcortical regions [4,7,10,11]. Therefore, [18F]FDG-PETquantification has been proposed as a complementary tool for localizing epileptogenic cortex when combined with the traditional analysis of [18F]FDG-PET data [2].
Statistical parametric mapping (SPM), as one of the quantitative methods used to analyze imaging data, is an effective, objective, and reliable method supplementing visual interpretation [12,13]. SPM provides a voxel-based analysis that generates a global analysis of whole-brain metabolism. Therefore, SPM has the potential to demonstrate metabolic changes missed by other quantitative methods that focus solely on regions of interest.
The present study used SPM to investigate the correlation between metabolic activity in the epileptogenic temporal cortex and duration of epilepsy. We hypothesize that hypometatolism increases with an increase in epilepsy duration independent of age at onset.
2. Methods
2.1. Subjects
Adult patients with refractory unilateral TLE were identified from our epilepsy surgery database (years 2004−2006). All patients had complex partial seizures. Video/EEG monitoring with scalp electrodes to identify and correlate seizure semiology with electrographic findings. Brain MRI, ictal/interictal single-photon emission tomography (SPECT), and resting state [18F]FDG-PET imaging were obtained as part of presurgical evaluation at our center. Inclusion criteria for this study included (1) age >17 years; (2) unilateral TLE diagnosed based on the findings of presurgical evaluation; and (3) available [18F]FDG-PET data for quantitative analysis. The patients were excluded when (1) ictal and interictal EEG findings remained inconclusive for the accurate diagnosis; (2) epilepsy surgery had been performed previously; (3) the patient had a coexisting progressive neurodegenerative disease or dementia; (4) brain MRI demonstrated significant cortical atrophy (>50% asymmetric enlargement of the ventricles compared with the contralateral site) and/or or the presence of encephalomalacia in one or both temporal cortices or extratemporal structures; and (5) TLE was identified with bilateral temporalictal onset.
2.2. Selection of subjects with TLE
Forty-nine cases were identified with a diagnosis of TLE. [18F]FDG-PET imaging data were not available for three patients. Mesial TLE (MTLE) was diagnosed on the basis of the clinical semiology of seizures including aura (i.e., epigastric rising sensation), oral and/or hand automatisms (ipsilateral to ictal onset), interictal EEG findings of temporal spikes and/or sharp waves, ictal onset in the unilateral temporal lobe (i.e., rhythmic theta pattern at ictal onset), and MRI findings indicating hippocampal sclerosis.
Neocortical TLE (NTLE) was diagnosed on the basis of (1) clinical semiology, such as experiential, sensory, autonomic, or visual aura; (2) interictal spikes located in the anterior or posterior temporal regions; (3) ictal EEG findings demonstrating focal polymorphic delta slowing, focal attenuation with fast activity, periodic lateralized sharp waves, or widespread spike discharges; and (4) normal or abnormal MRI findings other than mesial temporal sclerosis, such as cortical dysplasia, gliosis, vascular malformations, or no discernible MRI abnormality in temporal lobe structures.
Seizure variables recorded included age at onset of first afebrile seizure, seizure frequency, history of febrile seizures and secondarily generalized tonic−clonic seizures, gender, and clinical features of seizures including ictal dystonia, automatism, and aura. Type of epilepsy surgery and clinical outcome at the end of the 2-year follow-up period were reviewed. Length of education was also recorded.
2.3. PET scanning protocol: Image acquisition
An interictal [18F]FDG-PET scan was obtained for each patient following a bolus injection of 10 mCi [18F]FDG-PET after a 6-hour fast. [18F]FDG PET scans were acquired on a Siemens ECAT EXACT HR+ scanner (Siemens Medical Solutions, Malvern, PA, USA) with full-width half-maximum (FWHM) = 4 mm, 30 minutes after the injection. Attenuation correction was performed using an 8-minute transmission scan performed prior to initiating the [18F]FDG-PET scan. [18F]FDG-PET images were reconstructed using attenuation correction and filtered back projection with a Hann filter (0.40 cycles per pixel cutoff). All [18F]FDG PET scans were initially evaluated by visual inspection to exclude any scans of suboptimal image quality including those containing artifacts. All scans were of good technical quality and none was excluded based on the visual inspection.
2.4. Analysis of [18F]FDG-PET images: SPM analysis
SPM analysis (SPM2, Wellcome Department of Imaging Neuroscience, University College London, UK) was performed on a Dell workstation, using MATLAB 7.0 (Mathworks, Sherborn, MA, USA). Prior to statistical analysis, all images were spatially normalized to the Montreal Neurological Institute (MNI) space provided by the SPM2 software. In SPM, spatially normalized PET images were then count normalized to a global average activity of the entire brain according to the algorithms implemented in SPM, and the count normalized activity was assigned a value of 50 and a threshold of 0.8 for every subject.
Normalized images were smoothed with an isotropic Gaussian kernel with 16-mm FWHM. Smoothing increases the signal-to-noise ratio, which allows evaluation of subtle anatomic variations. The analysis resulted in a t statistic for each voxel (2 × 2 × 2 mm), which constituted the statistical parametric map, SPM{t}. The resulting SPM{t} was then transformed into a normal distribution, SPM{z}. Voxel height that reached significance at P < 0.05 (corrected) was considered significantly different, and clusters with more than 20 contiguous voxels (voxel extend threshold, ke) were considered to be of significant size [14,15]. The spatial coordinates of the significant voxels were used to identify the corresponding brain areas based on the probabilistic cytoarchitectonic maps described by Eickhoff et al. [16].
For purposes of statistical analysis, [18F]FDG-PET images of the patients with right-sided TLE (RTLE) and left-sided TLE (LTLE) were pooled to create a single group with epileptogenic focus on the same side. To accomplish this, the spatially normalized [18F]FDG-PET images were reoriented by “flipping” at the midsagittal plane to create a “mirror” image for each scan [15,17]. This allowed us to study a “single” group with epileptogenic cortex uniformly lateralized to the left. Thus, this group included the images of patients with epileptogenic cortex “lateralized to the left” after images of patients with RTLE were flipped and combined with the original images of patients with LTLE.
2.5. Statistical analysis
2.5.1. Analysis 1: Comparison of temporal lobes
A To identify regional metabolic changes in temporal cortex and asymmetry between the hemispheres, a paired t-test was applied from SPM procedural options. This analysis provided a comparison between all voxels of both hemispheres for each subject within the group. Two groups were required for this analysis. The first group included the unflipped original images of LTLE and flipped images of RTLE, which set the epileptogenic temporal cortex on the left. The second group represented a mirror image of the first group and comprised the flipped images of LTLE and unflipped (original) images of patients with RTLE, which set the epileptogenic temporal cortex on the right. Comparison was performed between these two groups and the resulting foci of significant difference were reported with a corrected P value of 0.05.
B. Patients were regrouped on the basis of epilepsy duration. Epilepsy duration was identified as “short” if less than 5 years and “long” if 10 years or longer. A paired t-test was applied to each group of patients independently to examine asymmetry in glucose uptake between the hemispheres within the group.
This division in epilepsy duration was chosen based on two earlier reports describing the importance of epilepsy duration in assessment of changes in metabolic activity in TLE. The first study described minimal or no metabolic changes within the first 4 years following the initial diagnosis of partial seizures [18]. The second study reported increasing hemispheric asymmetry between epileptogenic and contralateral temporal cortex with longer epilepsy duration ≥10 years [19].
2.5.2. Analysis 2: Correlation between epilepsy duration and glucose uptake
A. Multiple-subject correlation analysis was performed using SPM on 46 images. Epilepsy duration was entered as covariate. The regions reached significance, suggesting a correlation between decreasing glucose uptake and increasing epilepsy duration.
B. A separate SPM analysis was performed to examine the effect of epilepsy duration on temporal lobe metabolism in the MTLE and NTLE groups. This analysis was performed independently for MTLE and NTLE to identify the distribution of hypometabolic regions with increasing epilepsy duration.
C. Regression analysis was performed to examine the correlation between seizure variables including epilepsy duration and brain glucose uptake. Voxels of interest (VOIs) were found for the coordinates that reached significance at the end of first analysis (analysis 2A). VOI on SPM analysis indicates the amount of glucose in selected coordinates. Metabolic activity values were listed for each patient based on the anatomical coordinates and subjected to regression analysis to assess the impact of seizure variables on brain glucose uptake. The variables included in the regression analysis model were: (1) age at seizure onset, (2) gender, (3) history of febrile seizures, (4) history of secondarily generalized seizures, (5) monthly seizure frequency, and (6) years of education. Statistical analysis was performed using commercially available software (SPSS Version 11.0).
3. Results
3.1. Clinical and demographic features
Forty-six patients were identified as having unilateral TLE, and SPM analysis was performed on these [18F]FDG-PET images. The epileptogenic focus was lateralized to the right temporal cortex in 25 patients (RTLE) and left temporal cortex in 21 patients (LTLE). Only 16 patients (34%) were identified as having MTLE.
Age at seizure onset (first afebrile seizure) was 19.5 ± 13.8 (range: 1−61) years, epilepsy duration 17.4 ± 12.3 (range: 3−46) years, and age at the time of PET scanning 37.8 ± 11.1 (range: 13–57) years. Length of education was reported as 13.6 ± 2.2 (range: 10−20) years. All but six patients were on polytherapy with two or more antiepileptic drugs (AEDs). Ten (20.4%) patients had a history of febrile seizures. Complex partial seizures with secondary generalization were reported in 28 (60%); contralateral ictal limb dystonia in 22 (47%); an aura such as déjà vu, unusual sensation, tachycardia, epigastric discomfort, or tinnitus in 21 (68%); and automatisms (hand, oral−alimentary, gestural, or mixed) in 45 patients. Seizure frequency varied between 1 and 10 seizures per month. Clinical features of the patients are summarized with respect to epilepsy duration in Table 1. Video/EEG monitoring results suggested a neocortical origin in 30 patients (64%) and a mesial temporal origin in 16 (34%).
Table 1.
Clinical features of the patients with TLE.
| Duration <5 years (n = 10) | Duration ≥10 years (n = 30) | |
|---|---|---|
| Age (years) | 34.8±13.2a | 39.4±10.6 |
| Age at onset (years) | 29.4±13.9 | 14.8±11.6 |
| Epilepsy duration (years) | 3.15±1.2 | 24.1±10 |
| Gender, M/F | 4/6 | 17/13 |
| TLE, left/right | 6/4 | 14/16 |
| Neocortical/mesial TLE | 8/2 | 15/14 |
| Febrile seizure history | 0 | 9 |
| Secondarily generalized seizures | 7 | 17 |
| Hippocampal sclerosis | 0 | 11 |
Mean ± SD.
When the clinical features of patients with MTLE were compared with those of patients with NTLE, mean age at seizure onset was 12.8 years in MTLE and 22.8 in NTLE (P = 0.02). Epilepsy duration was slightly longer in MTLE, although the difference was not significant: 22.1 years in MTLE and 15.1 in NTLE (P = 0.07). Six patients (40%) with MTLE versus four patients (12.7%) with NTLE had a history of febrile seizures (P = 0.047).
All but three patients had interictal unilateral epileptiform discharges in the middle temporal and/or anterior temporal regions in MTLE and in the anterior or posterior temporal regions in NTLE. Four patients with TLE (3 MTLE and 1 NTLE) had interictal epileptiform discharges in bilateral temporal lobes.
Ictal EEG revealed that ictal onset lateralized to the epileptogenic temporal cortex in all patients. Rhythmic slow waves were described at a frequency of 2−5 Hz in the midtemporal regions in MTLE and anterior or posterior temporal regions in NTLE.
3.2. Surgery and outcome
Twenty-five patients underwent epilepsy surgery. Anterior and mesial temporal lobe resection was performed in 24 and selective amygdalohippocampectomy in 1 patient. Postsurgical outcome was evaluated at the end of a 2-year follow-up period according to Engel’s classification [20]. Favorable outcome was reported in 20 patients (Engel I and II) and unfavorable outcome in 5 (Engel III and IV).
3.3. Brain imaging findings
3.3.1. Brain MRI
TLE was identified as nonlesional in 45 patients (all but one), 16 (34%) of whom had hippocampal sclerosis. MRI results were unremarkable in 19 (41%) patients. Cortical dysplasia was reported in only two patients, subtle regional cortical atrophy in six, and focal enlargement of the hippocampus in one. In those with short epilepsy duration, MRI detected no structural abnormality except in one patient in whom increased T2 signal was reported in the hippocampus (Table 1).
3.3.2. [18F]FDG-PET imaging
All [18F]FDG-PET scans were subjected to a routine clinical interpretation of visual analysis by an experienced nuclear medicine physician. Glucose hypometabolism was reported as unilateral (lateralized to the epileptogenic temporal lobe) in 31 (67%) patients and bilateral in 6 (13%). Among 31 patients, hypometabolism was confined to the temporal cortex alone in 22 (70%) patients and extended to the extratemporal regions in 9 (30%). Hypometabolism was reported in the extratemporal cortex alone (ipsilateral frontal cortex) in one patient. [18F]FDG-PET scans revealed no definite abnormalities in 8 patients (17%).
Because this cohort comprised a mixed TLE population with neocortical or mesial origin, [18F]FDG-PET images were additionally reviewed on the basis of positive or negative MRI findings for HS. Hypometabolism in the epileptogenic temporal cortex was reported in 60% of the patients with HS compared with 30% of the patients without HS. Alternatively, extratemporal hypometabolism was reported more frequently in patients without HS than in patients with HS (30 and 13%, respectively).
3.4. Statistical analysis: [18F]FDG-PET
3.4.1. Interhemispheric asymmetry
A. Glucose hypometabolism was reported as unilateral and localized to the epileptogenic temporal cortex in all patients (Table 2). Hypometabolism was also present in the ipsilateral fusiform gyrus (P < 0.05 corrected). The results of this analysis suggest that there is hemispheric asymmetry between the normal and epileptogenic temporal cortex for each patient (Fig. 1, Table 2).
Table 2.
Temporal lobe regions where glucose metabolism was decreased: Paired t-test results of all patients.
| Area | Anatomical location | Side | Cluster Ke | Z | Coordinates |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Hippocampus | Left | 5091 | 5.76 | −32 | −30 | −14 | |
| BA 38 | Middle temporal gyrusa | Left | 5.65 | −40 | 12 | −40 | |
| BA 20 | Inferior temporal gyrusa | Left | 5.42 | −32 | −6 | −42 | |
| BA 38 | Superior temporal gyrusa | Left | 5.35 | −36 | 18 | −36 | |
| BA 36 | Parahippocampal gyrusa | Left | 5.04 | −30 | −24 | −24 | |
| BA 20 | Fusiform gyrusa | Left | 4.93 | −60 | −14 | −24 | |
| BA 36 | Uncusa | Left | 4.54 | −26 | −2 | −28 | |
| BA 39 | Middle temporal gyrusa | Left | 3.76 | −52 | −62 | 25 | |
| BA 19 | Middle occipital gyrusa | Left | 3.75 | −46 | −72 | −4 | |
| BA22 | Superior temporal gyrusa | Left | 251 | 4.23 | −60 | −44 | 18 |
| BA39 | Middle temporal gyrusa | Left | 3.62 | −52 | −62 | 25 | |
Note. Areas are the best estimates based on MNI space as coordinates [x, y, z] show the centroid of each cluster. The Z score gives the magnitude of statistical difference. Cluster size refers to the number of significant voxels.
P < 0.05 corrected.
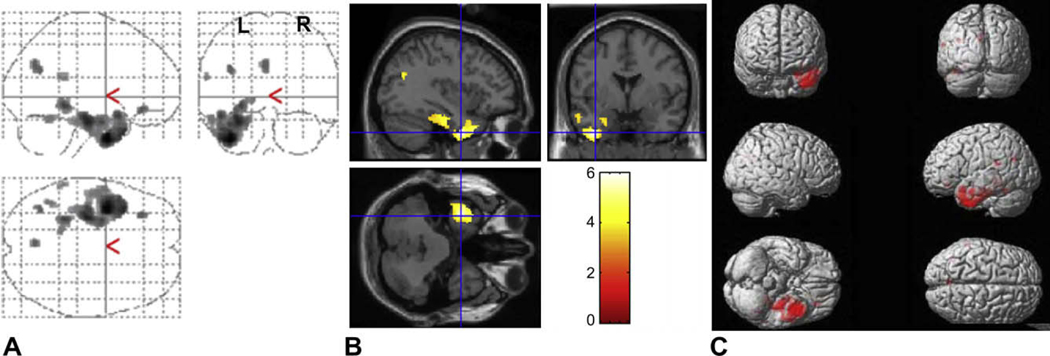
Fig. 1.
Statistical parametric map of paired t-test results used to examine interhemispheric asymmetry. (A) Glucose hypometabolism (shown in red) is present in the epileptogenic temporal cortex lateralized to the left (original images of LTLE and flipped images of RTLE combined). The number of voxels remained above the threshold, indicating decreased metabolic activity in the epileptogenic temporal cortex (left). (B) The SPM results are superimposed onto the realigned MRI data to demonstrate (left) temporal lobe hypometabolism at the coordinate level of [−36, 0, −40]. (C) The three-dimensional brain illustrates the distribution of glucose hypometabolism.
B. Paired t-tests were used to examine interhemispheric asymmetry in the subgroups of patients created on the basis of epilepsy duration (patients with a duration <5 years, short duration group; patients with a duration ≥ 10 years, long duration group). With long epilepsy duration, significant hypometabolism was reported in the middle temporal gyrus (Z = 4.63), superior temporal gyrus (Z = 4.12), parahippocampal gyrus (Z = 3.9), and uncus (Z = 3.37) of the epileptogenic temporal lobe ( Figs. 2A, B; Table 3). Additional hypometabolism was noted in the ipsilateral lingual gyrus (Z = 3.5) (Table 3). With short epilepsy duration, no significant hypometabolism was reported in either temporal cortex (Fig. 2C).
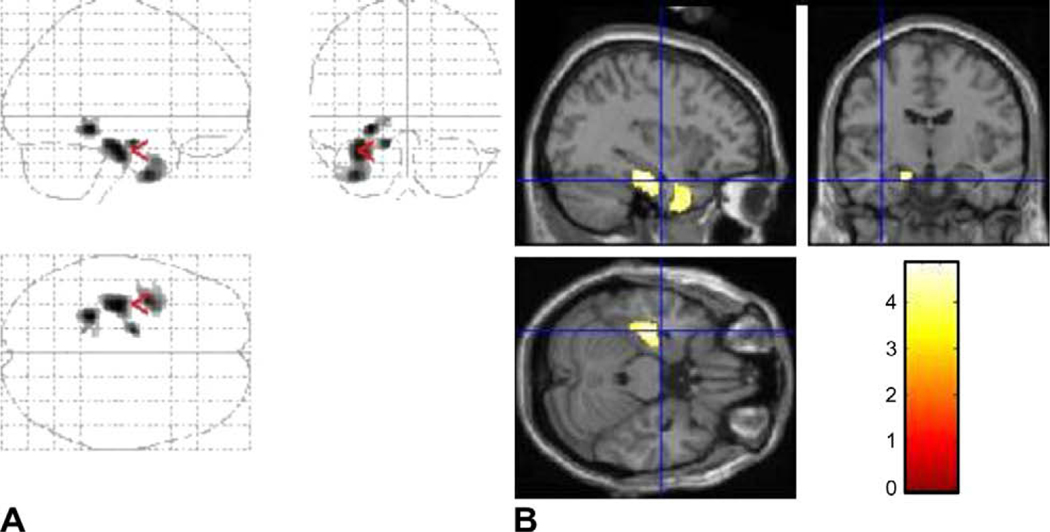
Fig. 2.
Statistical parametric map of paired t-test results used to examine interhemispheric asymmetry in [18F]FDG-PET images subgrouped on the basis of epilepsy duration. (A) Glucose hypometabolism (shown in red) is present in the epileptogenic temporal cortex lateralized to the left (original images of LTLE and flipped images of RTLE combined) in longer epilepsy duration (≥10 years). The number of voxels remained above the threshold, indicating decreased metabolic activity in the epileptogenic temporal cortex (lateralized to the left). (B) The SPM results are superimposed onto the realigned MRI data to demonstrate (left) temporal lobe hypometabolism at the coordinate level of [−36, −10, −24]. (C) Glucose hypometabolism was not present with short epilepsy duration (<5 years). The number of voxels remained below the threshold, indicating no evidence of decreased metabolic activity in the epileptogenic temporal cortex (lateralized to the left).
Table 3.
Brain regions where glucose hypometabolism is reported with long epilepsy duration (≥10 years): Paired t-test results.
| Area | Cluster Ke | Anatomical location | Side | Z | Coordinates |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| BA 34 | 395 | Parahippocampal gyrus a | Left | 3.9 | −14 | −8 | −18 |
| BA 20 | Uncusa | Left | 3.37 | −28 | −16 | −36 | |
| BA 20 | 338 | Middle temporal gyrusa | Left | 4.63 | −36 | 2 | −40 |
| BA 38 | Superior temporal gyrusa | Left | 4.12 | −34 | 8 | −32 | |
| BA 36 | 141 | Parahippocampal gyrusa | Left | 4.12 | −24 | −38 | −8 |
| BA 19 | Lingual gyrusa | Left | 3.5 | −14 | −44 | −2 | |
| BA 30 | Parahippocampal gyrusa | Left | 3.21 | −14 | −36 | −6 | |
Note. Areas are the best estimates on MNI space. Coordinates [x, y, z] show the centroid of each cluster. The Z score shows the magnitude of statistical difference. Cluster size is the number of significant voxels.
P < 0.05 corrected.
3.4.2. Epilepsy duration and temporal lobe hypometabolism
The results of SPM analysis performed to examine the correlation between epilepsy duration and temporal lobe glucose uptake are summarized in Table 4. [18F]FDG-PET data of 46 subjects demonstrated hypometabolism in the inferior temporal gyrus (Z = 4.02), fusiform gyrus (Z = 3.53), parahippocampal gyrus (Z = 3.52), and hippocampus (Z = 2.95) of the epileptogenic temporal lobe and in the ipsilateral thalamus (Z = 3.07) and ipsilateral posterior cingulate gyrus (Z = 3.13) (corrected P = 0.028).
Table 4.
Areas of hypometabolism with increasing epilepsy duration in unilateral TLE (n = 46).
| Area | Anatomical location | Side | K e | Z score | Coordinates |
P a | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| BA 20 | Inferior temporal gyrus | Left | 4032 | 4.02 | −60 | −26 | −24 | 0.028 |
| BA 20 | Fusiform gyrus | Left | 3.53 | −20 | −44 | −12 | ||
| BA 37 | Parahippocampal gyrus | Left | 3.52 | −24 | −36 | −8 | ||
| Hippocampus | Left | 2.95 | −22 | −34 | 0 | |||
| Thalamus | Left | 3.07 | −8 | −10 | 10 | |||
| BA 30 | Posterior cingulate cortex | Left | 2590 | 3.13 | −6 | −52 | 6 | |
| Middle cingulate cortex | Left | 3.10 | −4 | 10 | 40 | |||
Note. Areas are the best estimates on MNI space. Coordinates [x, y, z] show the centroid of each cluster. The Z score shows the magnitude of statistical difference. Cluster size is the number of significant voxels.
Corrected P value for cluster level.
In additional SPM analyses, increasing epilepsy duration correlated with hypometabolism in the parahippocampal gyrus (Z= 3.22), hippocampus (Z = 2.88), and ipsilateral thalamus (Z = 3.98) in MTLE (uncorrected P = 0.001) and in the inferior temporal gyrus (Z = 2.97) and fusiform gyrus (Z = 3.22) in NTLE (uncorrected P = 0.001) (Fig. 3).
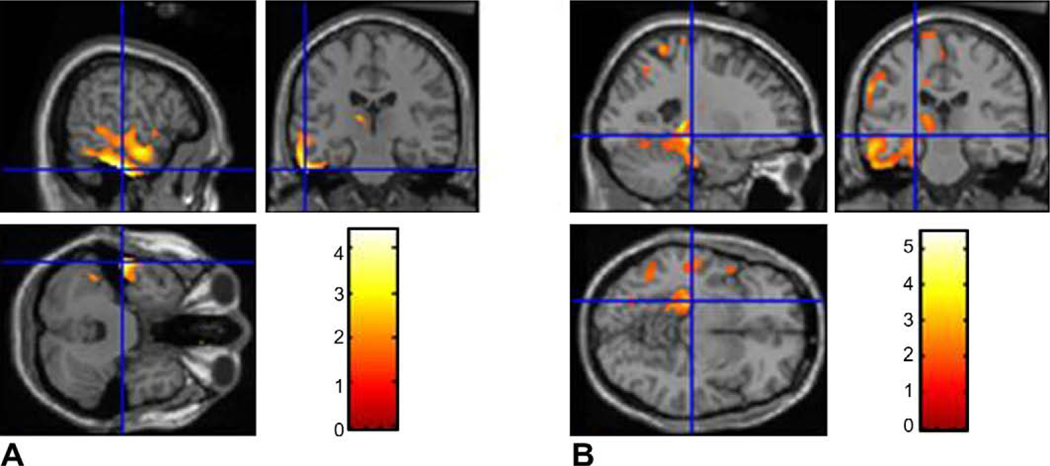
Fig. 3.
Statistical parametric map used to examine the correlation with epilepsy duration independently for MTLE and NTLE subgroups: glucose hypometabolism (shown in red) is seen in the epileptogenic temporal cortex with increasing epilepsy duration in patients with (A) NTLE and (B) MTLE. The number of voxels remained above the threshold, indicating decreased metabolic activity in the epileptogenic temporal cortex. The SPM results are superimposed onto the realigned MRI data to demonstrate temporal lobe hypometabolism.
Regression analysis was performed as described under Analysis 2C. Increasing epilepsy duration was inversely correlated with (decreasing) glucose uptake in the parahippocampal gyrus (r = −0.43), hippocampus (r = −0.43), and inferior temporal gyrus (r = −0.55) of the epileptogenic temporal cortex.
The analysis was repeated with the other clinical variables (age at seizure onset, history of secondarily generalized seizures, history of febrile seizure, age at the time of PET imaging, gender, and length of education). Age at seizure onset was the only clinical variable correlated with decreasing glucose uptake in the parahippocampal gyrus, hippocampus, and inferior temporal gyrus (P < 0.05). Subsequent stepwise regression including the age at onset in the statistical model revealed that epilepsy duration was correlated with lower glucose uptake only in the inferior temporal gyrus (P < 0.001) (Table 5).
Table 5.
Correlation between hypometabolism and epilepsy duration in TLE: results of regression analysis.
| Unadjusted R2 | β | P | Adjusteda R2 | β | P | |
|---|---|---|---|---|---|---|
| Parahippocampal gyrus | 0.191 | −0.298 | 0.002 | 0.254 | −0.171 | 0.135 |
| nferior temporal gyrus | 0.311 | −0.391 | <0.001 | 0.311 | −0.381 | 0.001 |
| Cingulate gyrus | 0.201 | −0.335 | 0.002 | 0.224 | −0.252 | 0.051 |
| Fusiform gyrus | 0.190 | −0.325 | 0.002 | 0.236 | −0.282 | 0.094 |
| Hippocampus | 0.189 | −0.43 | 0.003 | 0.22 | −0.307 | 0.079 |
Controlled by age at seizure onset.
On average, each additional year of epilepsy duration was associated with a 0.38-unit decrease in [18F]FDG uptake into the inferior temporal cortex.
We also studied whether impact of epilepsy duration on glucose uptake differs if seizure onset occurs at a younger age or during adulthood. No significant interaction was found between age at epilepsy onset and duration of epilepsy and [18F]FDG uptake (P = 0.89).
4. Discussion
The present study using voxel-based analysis of [18F]FDG metabolism in TLE demonstrates that: (1) glucose metabolism decreases in the epileptogenic temporal cortex as epilepsy duration increases; and (2) hypometabolism is not limited to the epileptogenic cortex and may extend to other neighboring cortical regions as duration increases.
Not surprisingly, age at seizure onset was also correlated with glucose uptake in this study. However, when we included age at onset in the analysis, glucose hypometabolism remained significant with increasing epilepsy duration only in the inferior temporal cortex. These findings imply that increasing epilepsy duration leads to a progressive decline in glucose metabolism in the inferior temporal cortex, but not in the mesial temporal structures in TLE.
A limited number of studies by other investigators have also evaluated the duration of epilepsy and its impact on cerebral glucose metabolism [10,19,21,22]. A similar correlation was described between the epilepsy duration and temporal lobe hypometabolism by Theodore et al. in the patients with TLE. Hippocampal sclerosis was described in 60% of their patients. [19]. These authors found that the inferior mesial temporal hypometabolism ipsilateral to the epileptic focus correlated with increasing epilepsy duration. Furthermore, the asymmetry in temporal lobe metabolism had increased over time, although the impact of age at seizure onset on temporal lobe hypometabolism was not reported [19]. Another study described the correlation between epilepsy duration and temporal lobe glucose hypometabolism in patients with MRI-negative TLE. These authors found that only ipsilateral thalamus hypometabolism was correlated with increasing epilepsy duration as controlled by age. Our patient sample appears to be similar to that reported by Benedik et al. with respect to the overwhelming number of patients with NTLE in the study cohort. These authors also included age at seizure onset in the statistical model as a potential confounder. The differences in the patient population and in the analytical methods used between the studies by others and our study may explain the slightly differing results of earlier studies.
The underlying pathophysiology of resting glucose hypometabolism is not fully understood in TLE. The role of focal structural abnormalities and changes in regional cerebral blood flow were initially believed to account for hypometabolism in the epileptogenic cortex [23,24]. Focal atrophy as a result of cell death and neuronal loss was therefore proposed to explain the pathophysiology of regional glucose hypometabolism [2,23,24]. On the contrary, the severity of hypometabolism was found to not necessarily correlate with the degree of hippocampal cell loss or gliosis and would precede the volume loss or anatomic changes seen in temporal lobe structures [4,25]. Bruehl and Witte reported that in the absence of structural abnormality, changes in deoxyglucose uptake did not directly correlate with neuronal excitation or inhibition; instead, deoxyglucose uptake was related to the overall strength of synaptic activity. Both strong excitations and strong inhibitions increase demand for glucose to maintain brain metabolism. Reduction of metabolism below normal values would suggest reduced synaptic activity and tonic hyperpolarization of the cells [26]. Therefore, deoxyglucose uptake reflects the overall strength of synaptic activity. Both strong excitation and strong inhibition would increase brain metabolism, whereas impaired inhibitory synaptic activity would result in decreasing energy requirements and, therefore, reduced glucose uptake [26−28].
In patients with medically refractory TLE, longer duration of epilepsy represents a dynamic course with frequent seizures, insufficient seizure control on treatment with multiple AEDs, and functional and/or anatomical changes over time in the temporal cortex [15,29−34]. Jokeit et al. reported a reduction in bilateral hippocampal volume as well as temporal lobe glucose metabolism as epilepsy duration increases [21]. The authors therefore proposed that longitudinal changes in glucose metabolism would indicate a progressive clinical course in TLE. Epilepsy duration was also found to be the most important predictor of long-term outcome in TLE [32]. More specifically, Yoon et al. described the role of longer epilepsy duration (>20 years) as a strong predictor of poor long-term prognosis following temporal lobe surgery [35]. The lower incidence of glucose hypometabolism associated with new-onset complex partial seizures supports the observation of the importance of epilepsy duration in glucose metabolism [8]. Moreover, the present study confirms that temporal lobe hypometabolism becomes regional and prominent in temporal cortex as epilepsy duration increases, whereas hypometabolism may not occur within the first 5 years of onset.
The timing of the development of temporal lobe hypometabolism remains uncertain. Interhemispheric asymmetry in temporal lobe glucose metabolism is demonstrated as the duration of epilepsy extends beyond 10 years [19]. We could not establish the onset of hypometabolic activity because of its retrospective nature and the small number of patients recruited for this study. We chose instead to examine the effect of duration in two dichotomous groups that represented short and long epilepsy duration. A long-term prospective study, perhaps for two decades, from the time of epilepsy onset forward, is necessary to achieve the goal of establishing the critical time at which the metabolic changes begin.
In this study we also observed extension of the hypometabolism to the fusiform gyrus and middle occipital gyrus ipsilateral to temporal cortex. Extension of hypometabolism beyond the epileptogenic cortex is not a novel finding [10,22,36]. In particular, extratemporal lobe hypometabolism has been reported in patients with neocortical or cryptogenic TLE [2,20,31,36−38]. Morphometric studies of the limbic network have shown that cortical thinning occurs in adjacent cortical structures. Riederer and his colleagues also reported network atrophy in patients with MTLE and NTLE. They reported atrophy of the temporal gray matter beyond the hippocampus of ipsilateral temporal cortex in patients with MTLE, as well as more widespread atrophy extending to the frontal, orbitofrontal cortex, neocortical temporal regions in patients with cryptogenic TLE. Quantitative analysis of the volumetric and metabolic imaging data on TLE suggests that the anatomical and functional integrity of neural circuitry between temporal and extratemporal structures changes with time in TLE [38,39]. These changes are possibly the result of (1) a secondary epileptogenic focus beyond the temporal cortex, (2) seizure propagation to the adjacent structures, or (3) atrophy of the neuronal network in the temporal cortex and adjacent structures.
Our study has a number of limitations. Our cohort represents a mixed population with epileptogenic zones involving either neocortical or mesial temporal structures. The frequency of hippocampal sclerosis was only 34%, and normal brain MRI findings were reported in 41% of the patients. These findings indicate that the number of patients with neocortical or cryptogenic TLE outweighs the TLE associated with hippocampal sclerosis in the present study, which is different than in the other series. Because of the unusual predominance of NTLE in this study group, the results should be interpreted accordingly. Therefore selection bias for patient referral to our tertiary care center cannot be excluded. The retrospective nature of this study also limits the retrieval of imaging and clinical data in detail. In addition, we were unable to recruit an age-matched control group to study differences in metabolic activity between normal and epileptogenic temporal cortex. Therefore, we applied an interhemispheric asymmetry model to examine glucose uptake in the ipsilateral and contralateral temporal lobe for each subject. This analytical model had been studied earlier and found to improve the specificity for correct localization of the epileptogenic focus [17,40]. However, differences between left and right temporal anatomy may also have an effect on the anatomical coordinates of the hypometabolic areas. PET and functional MRI data are often normalized to the templates provided by the Montreal Neurological Institute (MNI). The most widely used MNI template is a single subject template. This template, however, does not match the Talaraich brain in size and shape [41]. Moreover, transformation of multisubject data from the MNI space to Talaraich space may map to different points of Talaraich space and provide approximate Talaraich coordinates for reference. This discrepancy becomes an issue when the data are analyzed in MNI space but the results are reported using Talaraich space [42]. In this study, we preferred use of the cytoarchitectonic map for multisubject analysis [16]. This map provides stereotactic information on the location and variability of cortical areas in MNI reference space. These cortical areas are defined on the observer independent analysis of the cytoarchitecture in a sample of 10 human postmortem brains. However, its validity in temporal lobe structures requires future studies to confirm our results. Finally, this was a cross-sectional and not a longitudinal study of patients undergoing evaluation for epilepsy surgery. Therefore, the findings may not be generalizable to all patients with drug-resistant temporal lobe onset epilepsy.
In conclusion, voxel-based analysis demonstrates a dynamic rather than static process in brain glucose metabolism as a function of increasing epilepsy duration in patients with medically refractory TLE. Prospective studies are needed to replicate these results with a large TLE group. This approach will help to elucidate the time course of the metabolic, functional, and anatomical changes in TLE and their clinical implications in decision making on the timing of epilepsy surgery and clinical outcome of patients with TLE.
Supplementary Material
Acknowledgments
The authors thank Dr. J. Riviello for his review and valuable comments and Dr. Mary L. Chapieski and O’Brien E. Smith for their advice on statistical analysis.
Footnotes
Conflict of interest statement
The authors report no conflicts of interest.
Ethical approval
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.yebeh.2009.12.007.
References
- [1].Abou-Khalil BW, Siegel GJ, Sackellares JC, Gilman S, Hichwa R, Marshall R. Positron emission tomography studies of cerebral glucose metabolism in chronic partial epilepsy. Ann Neurol 1987;22:480–6. [DOI] [PubMed] [Google Scholar]
- [2].Hajek M, Antonini A, Leenders KL, Wieser HG. Mesiobasal versus lateral temporal lobe epilepsy: metabolic differences in the temporal lobe shown by interictal 18F-FDG positron emission tomography. Neurology 1993;43:79–86. [DOI] [PubMed] [Google Scholar]
- [3].Henry TR, Mazziotta JC, Engel J Jr, et al. Quantifying interictal metabolic activity in human temporal lobe epilepsy. J Cereb Blood Flow Metab 1990;10:748–57. [DOI] [PubMed] [Google Scholar]
- [4].Sackellares JC, Siegel GJ, Abou-Khalil BW, et al. Differences between lateral and mesial temporal metabolism interictally in epilepsy of mesial temporal origin. Neurology 1990;40:1420–6. [DOI] [PubMed] [Google Scholar]
- [5].Theodore WH, Brooks R, Sato S, et al. The role of positron emission tomography in the evaluation of seizure disorders. Ann Neurol 1984;15(Suppl.):S176–9. [DOI] [PubMed] [Google Scholar]
- [6].Engel J Jr, Henry TR, Risinger MW, et al. Presurgical evaluation for partial epilepsy: relative contributions of chronic depth-electrode recordings versus FDG-PET and scalp-sphenoidal ictal EEG. Neurology 1990;40:1670–7. [DOI] [PubMed] [Google Scholar]
- [7].Henry TR, Frey KA, Sackellares JC, et al. In vivo cerebral metabolism and central benzodiazepine-receptor binding in temporal lobe epilepsy. Neurology 1993;43:1998–2006. [DOI] [PubMed] [Google Scholar]
- [8].Matheja P, Kuwert T, Ludemann P, et al. Temporal hypometabolism at the onset of cryptogenic temporal lobe epilepsy. Eur J Nucl Med 2001;28:625–32. [DOI] [PubMed] [Google Scholar]
- [9].Ryvlin P, Philippon B, Cinotti L, Froment JC, Le Bars D, Mauguiere F. Functional neuroimaging strategy in temporal lobe epilepsy: a comparative study of 18FDG-PET and 99mTc-HMPAO-SPECT. Ann Neurol 1992;31:650–6. [DOI] [PubMed] [Google Scholar]
- [10].Benedek K, Juhasz C, Muzik O, Chugani DC, Chugani HT. Metabolic changes of subcortical structures in intractable focal epilepsy. Epilepsia 2004;45:1100–5. [DOI] [PubMed] [Google Scholar]
- [11].Sperling MR, Gur RC, Alavi A, et al. Subcortical metabolic alterations in partial epilepsy. Epilepsia 1990;31:145–55. [DOI] [PubMed] [Google Scholar]
- [12].Friston KJ. Commentary and opinion: II. Statistical parametric mapping: ontology and current issues. J Cereb Blood Flow Metab 1995;15:361–70. [DOI] [PubMed] [Google Scholar]
- [13].Swartz BE, Thomas K, Simpkins F, Kovalik E, Mandelkern MM. Rapid quantitative analysis of individual (18)FDG-PET scans. Clin Positron Imaging 1999;2:47–56. [DOI] [PubMed] [Google Scholar]
- [14].Friston KJ. Testing for anatomically specified regional effects. Hum Brain Mapp 1997;5:133–6. [DOI] [PubMed] [Google Scholar]
- [15].Chassoux F, Semah F, Bouilleret V, et al. Metabolic changes and electro-clinical patterns in mesio-temporal lobe epilepsy: a correlative study. Brain 2004;127:164–74. [DOI] [PubMed] [Google Scholar]
- [16].Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 2005;25:1325–35. [DOI] [PubMed] [Google Scholar]
- [17].Van Bogaert P, Massager N, Tugendhaft P, et al. Statistical parametric mapping of regional glucose metabolism in mesial temporal lobe epilepsy. NeuroImage 2000;12:129–38. [DOI] [PubMed] [Google Scholar]
- [18].Gaillard WD, Kopylev L, Weinstein S, et al. Low incidence of abnormal (18)FDG-PET in children with new-onset partial epilepsy: a prospective study. Neurology 2002;58:717–22. [DOI] [PubMed] [Google Scholar]
- [19].Theodore WH, Kelley K, Toczek MT, Gaillard WD. Epilepsy duration, febrile seizures, and cerebral glucose metabolism. Epilepsia 2004;45:276–9. [DOI] [PubMed] [Google Scholar]
- [20].Henry TR, Mazziotta JC, Engel J Jr. Interictal metabolic anatomy of mesial temporal lobe epilepsy. Arch Neurol 1993;50:582–9. [DOI] [PubMed] [Google Scholar]
- [21].Jokeit H, Ebner A, Arnold S, et al. Bilateral reductions of hippocampal volume, glucose metabolism, and Wada hemispheric memory performance are related to the duration of mesial temporal lobe epilepsy. J Neurol 1999;246:926–33. [DOI] [PubMed] [Google Scholar]
- [22].Rubin E, Dhawan V, Moeller JR, et al. Cerebral metabolic topography in unilateral temporal lobe epilepsy. Neurology 1995;45:2212–23. [DOI] [PubMed] [Google Scholar]
- [23].Gaillard WD, Fazilat S, White S, et al. Interictal metabolism and blood flow are uncoupled in temporal lobe cortex of patients with complex partial epilepsy. Neurology 1995;45:1841–7. [DOI] [PubMed] [Google Scholar]
- [24].Lee DS, Lee SK, Lee MC. Functional neuroimaging in epilepsy: FDG PET and ictal SPECT. J Kor Med Sci 2001;16:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Semah F, Baulac M, Hasboun D, et al. Is interictal temporal hypometabolism related to mesial temporal sclerosis? A positron emission tomography/ magnetic resonance imaging confrontation. Epilepsia 1995;36:447–56. [DOI] [PubMed] [Google Scholar]
- [26].Bruehl C, Witte OW. Cellular activity underlying altered brain metabolism during focal epileptic activity. Ann Neurol 1995;38:414–20. [DOI] [PubMed] [Google Scholar]
- [27].Koutroumanidis M, Binnie CD, Elwes RD, et al. Interictal regional slow activity in temporal lobe epilepsy correlates with lateral temporal hypometabolism as imaged with 18FDG PET: neurophysiological and metabolic implications. J Neurol Neurosurg Psychiatry 1998;65:170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Knowlton RC, Laxer KD, Klein G, et al. In vivo hippocampal glucose metabolism in mesial temporal lobe epilepsy. Neurology 2001;57:1184–90. [DOI] [PubMed] [Google Scholar]
- [29].Benedek K, Juhasz C, Chugani DC, Muzik O, Chugani HT. Longitudinal changes in cortical glucose hypometabolism in children with intractable epilepsy. J Child Neurol 2006;21:26–31. [DOI] [PubMed] [Google Scholar]
- [30].Leiderman DB, Albert P, Balish M, Bromfield E, Theodore WH. The dynamics of metabolic change following seizures as measured by positron emission tomography with fludeoxyglucose F 18. Arch Neurol 1994;51:932–6. [DOI] [PubMed] [Google Scholar]
- [31].Savic I, Altshuler L, Baxter L, Engel J Jr. Pattern of interictal hypometabolism in PET scans with fludeoxyglucose F 18 reflects prior seizure types in patients with mesial temporal lobe seizures. Arch Neurol 1997;54:129–36. [DOI] [PubMed] [Google Scholar]
- [32].Janszky J, Janszky I, Schulz R, et al. Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain 2005;128:395–404. [DOI] [PubMed] [Google Scholar]
- [33].Wieser HG. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2004;45:695–714. [DOI] [PubMed] [Google Scholar]
- [34].Theodore WH. PET: cerebral blood flow and glucose metabolism− pathophysiology and drug effects. Adv Neurol 2000;83:121–30. [PubMed] [Google Scholar]
- [35].Yoon HH, Kwon HL, Mattson RH, Spencer DD, Spencer SS. Long-term seizure outcome in patients initially seizure-free after resective epilepsy surgery. Neurology 2003;61:445–50. [DOI] [PubMed] [Google Scholar]
- [36].Jokeit H, Seitz RJ, Markowitsch HJ, Neumann N, Witte OW, Ebner A. Prefrontal asymmetric interictal glucose hypometabolism and cognitive impairment in patients with temporal lobe epilepsy. Brain 1997;120(Pt. 12):2283–94. [DOI] [PubMed] [Google Scholar]
- [37].Morgan VL, Gore JC, Abou-Khalil B. Cluster analysis detection of functional MRI activity in temporal lobe epilepsy. Epilepsy Res 2007;76:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Riederer F, Lanzenberger R, Kaya M, Prayer D, Serles W, Baumgartner C. Network atrophy in temporal lobe epilepsy: a voxel-based morphometry study. Neurology 2008;71:419–25. [DOI] [PubMed] [Google Scholar]
- [39].Bernhardt BC, Worsley KJ, Besson P, et al. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. NeuroImage 2008;42:515–24. [DOI] [PubMed] [Google Scholar]
- [40].Kim YK, Lee DS, Lee SK, et al. Differential features of metabolic abnormalities between medial and lateral temporal lobe epilepsy: quantitative analysis of (18)F-FDG PET using SPM. J Nucl Med 2003;44:1006–12. [PubMed] [Google Scholar]
- [41].Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci 2002;3:243–9. [DOI] [PubMed] [Google Scholar]
- [42].Chau W, McIntosh AR. The Talairach coordinate of a point in the MNI space. How to interpret it? NeuroImage 2005;25:408–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.