Abstract
Bacillus subtilis cell wall-bound protein CWBP33 is encoded by lytE, a gene expressed during the exponential growth phase. Sequence analysis of LytE, a 33-kDa protein, reveals two domains. The N-terminal domain contains a threefold-repeated motif common to several peptidoglycan binding proteins, while the C-terminal domain, probably carrying the catalytic activity, has homology with certain exoproteins. Zymographs unambiguously reveal that the absence of CWBP33, due to inactivation of lytE, is accompanied by the loss of a lytic activity. In lytE mutants, the cell autolysis rate is significantly decreased, although autolysis of corresponding, purified cell walls does not seem to be affected.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of proteins contained in cell wall preparations has revealed that 12 major proteins, the so-called cell wall-bound proteins (CWBP), are associated with the cell wall of exponentially growing cells (23). Four of the CWBP, attached by electrostatic forces, are peptidoglycan (PG) hydrolases (9, 20). They comprise LytC, an N-acetylmuramyl-l-alanine amidase, and LytD (21), an N-acetylglucosaminidase, corresponding to CWBP49 (16) and CWBP90 (18), respectively. The absence of any effect of lytC and lytD inactivation on cell growth (18) prompted us to search for novel PG hydrolases. We report the identification and characterization of the gene lytE, whose product, a 33-kDa polypeptide endowed with lytic activity, is involved in cell autolysis.
Identification of the DNA sequence encoding CWBP35 and its analysis.
CWBP were separated by SDS-PAGE and transferred onto a membrane, and their N-terminal amino acid sequences were determined (17). QSIKVKKGDTL, the sequence corresponding to CWBP33, matched perfectly residues 26 to 36 of a putative protein encoded by a gene called papQ (GenBank accession no. U38819), subsequently renamed lytE (see below).
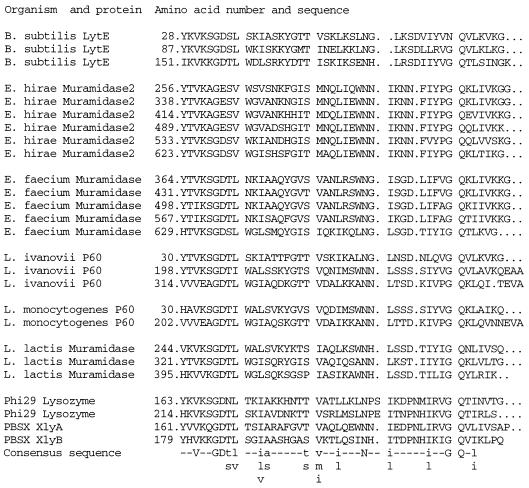
The N-terminal sequence of the mature LytE protein starts with amino acid 26. The first 25 residues, corresponding to a signal peptide, according to von Heijne (26), were cleaved. Amino acid sequence similarity analyses of LytE (1, 8, 19) revealed two domains. The N-terminal domain contains three repeats of a 44-amino-acid motif (Fig. 1). It has been proposed (12) that this motif found in a series of cell wall lytic enzymes is required for their binding to PG. The C-terminal domain of LytE has sequence homology with several classes of proteins that are found outside the cytoplasmic membrane (Fig. 2) and, surprisingly, are endowed with rather different enzymatic activities, such as endopeptidase (Bacillus sphaericus) or amylase (Clostridium acetobutylicum). None of these motifs were similar to those characteristic of the main cell wall hydrolases of Bacillus subtilis, i.e., LytC (14) and LytD (18), suggesting that LytE is a novel type of PG hydrolase. The sequence homologies between the C-terminal domains of LytE and a well-characterized γ-d-Glu-ld-(meso)-diaminopimelate peptidase of B. sphaericus (11) suggests that LytE may also encode such a peptidase. In addition, LytE has a cysteine residue in a well-conserved motif which may be compatible with an endopeptidase activity, since it has been proposed that the B. sphaericus peptidase is a cysteine enzyme (3).
FIG. 1.
N-terminal domain of LytE. Repeats and their sequence homology to the following cell wall-associated proteins are shown: muramidase-2 of Enterococcus hirae (6), muramidase of Enterococcus faecium (2), invasion-associated protein P60 of Listeria ivanovii and Listeria monocytogenes (4), muramidase of Lactococcus lactis (5), B. subtilis phage φ29 lysozyme (25), and amidases of the B. subtilis defective phage PBSX (7, 15). The consensus sequence is presented at the bottom of the figure (uppercase letters represent amino acids conserved in all examined proteins; lowercase letters represent conserved amino acids belonging to the same family).
FIG. 2.
C-terminal domain of LytE. Its homology to the following exoproteins is shown: invasion-associated protein P60 of Listeria ivanovii and Listeria monocytogenes (4), amylase of Clostridium acetobutylicum (24), P54 of Enterococcus faecium (10), NlpC of Escherichia coli (13), and d-Glu-ld-(meso)-diaminopimelate endopeptidase of B. sphaericus (13). The consensus sequence is presented at the bottom of the figure (uppercase letters represent amino acids conserved in all examined proteins; lowercase letters represent conserved amino acids belonging to the same family).
lytE regulation.
While a sequence corresponding to the consensus −10 domain of a ςA promoter is apparent 70-bp upstream of the start codon, there is no satisfactory match with the corresponding −35 consensus sequence. Unambiguous evidence that lytE is transcribed by a vegetative phase promoter are (i) the presence of LytE in native cell walls obtained from exponentially growing cells (Fig. 3) and (ii) the expression of a lytE-lacZ transcriptional fusion during vegetative growth (data not shown). Since cell wall preparations of parent strains and of sigD- and sinR-bearing mutants contained comparable amounts of CWBP33 (data not shown), it follows that the alternate factor ςD does not play a role in lytE transcription. Moreover, transfer of the lytE-lacZ fusion into a sinR background did not affect lytE expression (data not shown).
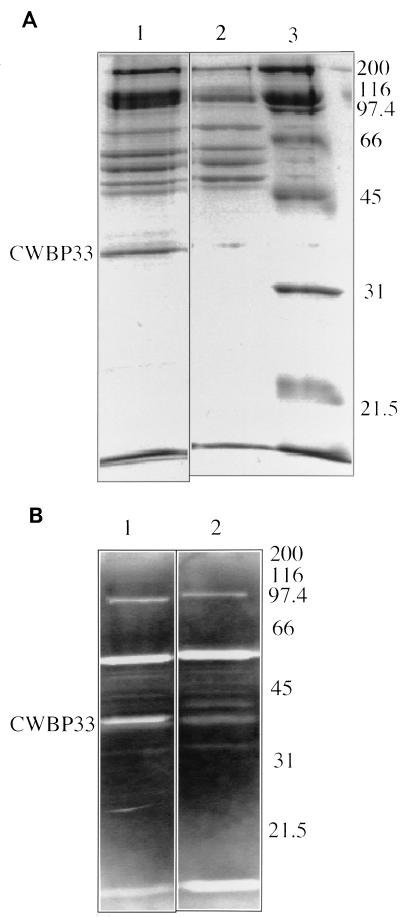
FIG. 3.
(A) SDS-PAGE of CWBP extracted from L16601 lyt+ (lane 1) and L16638 lytE (lane 2). Standard Mr proteins (Bio-Rad broad range) are also shown (lane 3). (B) Results of renaturation gel assay performed with CWBP extracted from L16601 (lane 1) and L16638 lytE (lane 2). Pictures of the gels were digitally recorded with a charge-coupled device camera (Vilbert Lourmat, Marme la Vallée, France) and processed by computer. Molecular weights of standard proteins are shown on the right (in thousands).
lytE mutant behavior.
To construct strain L16638 carrying a ΔlytE::Cam, a cat cassette was inserted between the ClaI and PstI restriction sites (bases 644 to 1184 of the sequence of GenBank accession no. U38819) of a plasmid whose insert contains the whole sequenced region. The resulting plasmid was used to transform strain L16601 to chloramphenicol resistance (17). The motility, growth rate, chain separation, and sporulation in the MTOa or Schaeffer media (17, 22) did not differ between the lytE-deficient and the parent strains. Analysis of native cell walls of strain L16638 lytE by SDS-PAGE revealed the absence of CWBP33, confirming that lytE does indeed encode this protein (Fig. 3A). As expected, the renaturation gel assay (9) of strain L16638 did not reveal the clearing zone corresponding to the CWBP33 lytic activity (Fig. 3B). LytE may correspond to CwlF, a 35-kDa protein (20), or to autolysin A3, a 34-kDa protein (9). However, methods for extracting the CwlF and autolysin A3 differ from that used for CWBP33, making comparison difficult. Incidentally, the weak clearing zone corresponding to a protein with a slightly higher Mr (Fig. 3B) may be due to either CwlF or to autolysin A3.
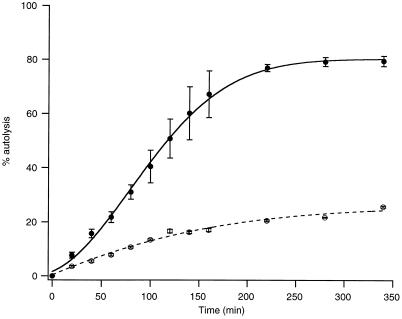
Native cell walls of strains L16601 lyt+ and L16638 lytE exhibited similar autolysis rates (data not shown), suggesting that the contribution of LytE to global cell wall lytic activity is marginal, a behavior characteristic of LytD, the glucosaminidase (18). However, autolysis of whole cells (17) of the lytE-deficient mutant was considerably decreased and delayed as compared to that of the parent strain (Fig. 4), a behavior resembling that of a strain deficient in LytC, the main vegetative amidase (16, 21). However, while lytC-deficient mutants are characterized by a very long delay in their cell wall turnover kinetics (16), no such delay was observed in lytE-bearing strains (data not shown). We hypothesize that LytE may be located at some specific points in the cell wall and, through its action, could create points suitable for the action of the LytC amidase; this, in turn, could rapidly perforate the cell wall and accelerate the release of the cytoplasmic material. In the absence of LytE, the main vegetative amidase, LytC, most likely located at the outer wall surface, would have to gradually degrade the entire surface of the cell wall, leading to delayed autolysis. This interpretation is compatible with our observation that the absence of LytE has no significant effect on the autolysis of isolated cell wall preparations.
FIG. 4.
Cell autolysis. The symbols along the solid line (L16601) and the broken line (L16638 lytE) represent average values from three different experiments. The vertical line for each point corresponds to the standard deviation. Results were normalized to nephelometric density (ND) at time 0 (ND0), i.e., percent lysis at time t = (ND0 − ND at time t)/ND0.
Acknowledgments
The expertise of Arthur Moir (University of Sheffield, Sheffield, United Kingdom), who performed the protein sequencing, is gratefully acknowledged.
This work was supported by grant 31-42322.94 from the Fonds National Suisse de la Recherche Scientifique (to D.K.) and by Public Health Service grant GM43577 from the National Institutes of Health (to P.P.).
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Beliveau C, Potvin C, Trudel J, Asselin A, Bellemare G. Cloning, sequencing, and expression in Escherichia coli of a Streptococcus faecalis autolysin. J Bacteriol. 1991;173:5619–5623. doi: 10.1128/jb.173.18.5619-5623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourgogne T, Vacheron M J, Guinand M, Michel G. Purification and partial characterization of the gamma-d-glutamyl-l-di-amino acid endopeptidase II from Bacillus sphaericus. Int J Biochem. 1992;24:471–476. doi: 10.1016/0020-711x(92)90041-x. [DOI] [PubMed] [Google Scholar]
- 4.Bubert A, Kohler S, Goebel W. The homologous and heterologous regions within the iap gene allow genus- and species-specific identification of Listeria spp. by polymerase chain reaction. Appl Environ Microbiol. 1992;58:2625–2632. doi: 10.1128/aem.58.8.2625-2632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buist G, Kok J, Leenhouts K J, Dabrowska M, Venema G, Haandrikman A J. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J Bacteriol. 1995;177:1554–1563. doi: 10.1128/jb.177.6.1554-1563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu C P, Kariyama R, Daneo-Moore L, Shockman G D. Cloning and sequence analysis of the muramidase-2 gene from Enterococcus hirae. J Bacteriol. 1992;174:1619–1625. doi: 10.1128/jb.174.5.1619-1625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Silva, E. Unpublished data.
- 8.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster S J. Analysis of the autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J Bacteriol. 1992;174:464–470. doi: 10.1128/jb.174.2.464-470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furst P, Mosch H U, Solioz M. A protein of unusual composition from Enterococcus faecium. Nucleic Acids Res. 1989;17:6724. doi: 10.1093/nar/17.16.6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hourdou M L, Duez C, Joris B, Vacheron M J, Guinand M, Michel G, Ghuysen J M. Cloning and nucleotide sequence of the gene encoding the γ-d-glutamyl-l-diamino acid endopeptidase II of Bacillus sphaericus. FEMS Microbiol Lett. 1992;91:165–170. doi: 10.1111/j.1574-6968.1992.tb05203.x. [DOI] [PubMed] [Google Scholar]
- 12.Joris B, Englebert S, Chu C P, Kariyama R, Daneo-Moore L, Shockman G D, Ghuysen J M. Modular design of the Enterococcus hirae muramidase-2 and Streptococcus faecalis autolysin. FEMS Microbiol Lett. 1992;70:257–264. doi: 10.1016/0378-1097(92)90707-u. [DOI] [PubMed] [Google Scholar]
- 13.Kadner, R. J. 1989. Swissprot accession no. P23898.
- 14.Lazarevic V, Margot P, Soldo B, Karamata D. Sequencing and analysis of the Bacillus subtilis lytRABC divergon: a regulatory unit encompassing the structural genes of the N-acetylmuramoyl-l-alanine amidase and its modifier. J Gen Microbiol. 1992;138:1949–1961. doi: 10.1099/00221287-138-9-1949. [DOI] [PubMed] [Google Scholar]
- 15.Longchamp P F, Mauël C, Karamata D. Lytic enzymes associated with defective prophages of Bacillus subtilis: sequencing and characterization of the region comprising the N-acetylmuramoyl-l-alanine amidase gene of prophage PBSX. Microbiology. 1994;140:1855–1867. doi: 10.1099/13500872-140-8-1855. [DOI] [PubMed] [Google Scholar]
- 16.Margot P, Karamata D. Identification of the structural genes for N-acetylmuramoyl-l-alanine amidase and its modifier in Bacillus subtilis 168—inactivation of these genes by insertional mutagenesis has no effect on growth or cell separation. Mol Gen Genet. 1992;232:359–366. doi: 10.1007/BF00266238. [DOI] [PubMed] [Google Scholar]
- 17.Margot P, Karamata D. The wprA gene of Bacillus subtilis 168, expressed during exponential growth, encodes a cell-wall-associated protease. Microbiology. 1996;142:3437–3444. doi: 10.1099/13500872-142-12-3437. [DOI] [PubMed] [Google Scholar]
- 18.Margot P, Mauël C, Karamata D. The gene of the N-acetylglucosaminidase, a Bacillus subtilis 168 cell wall hydrolase not involved in vegetative cell autolysis. Mol Microbiol. 1994;12:535–545. doi: 10.1111/j.1365-2958.1994.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 19.Moszer I, Glaser P, Danchin A. SubtiList: a relational database for the Bacillus subtilis genome. Microbiology. 1995;141:261–268. doi: 10.1099/13500872-141-2-261. [DOI] [PubMed] [Google Scholar]
- 20.Rashid M H, Mori M, Sekiguchi J. Glucosaminidase of Bacillus subtilis: cloning, regulation, primary structure and biochemical characterization. Microbiology. 1995;141:2391–2404. doi: 10.1099/13500872-141-10-2391. [DOI] [PubMed] [Google Scholar]
- 21.Rogers H J, Perkins H R, Ward J B, editors. Microbial cell walls and membranes. London, United Kingdom: Chapman and Hall; 1980. [Google Scholar]
- 22.Schaeffer P, Ionesco H, Ryter A, Ballassa G. La sporulation de Bacillus subtilis: étude génétique et physiologique. Colloq Int Cent Natl Rech Sci. 1965;124:553–563. [Google Scholar]
- 23.Studer R E. Caractérisation de la paroi native de Bacillus subtilis et étude des protéines qui lui sont associées. Ph.D. thesis. Lausanne, Switzerland: Université de Lausanne; 1988. [Google Scholar]
- 24.Verhasselt, J., J. Vanderleyden, and K. Van Leuven. 1993. Swissprot accession no. Q45834.
- 25.Vlcek C, Paces V. Nucleotide sequence of the late region of Bacillus phage phi 29 completes the 19,285-bp sequence of phi 29 genome. Comparison with the homologous sequence of phage PZA. Gene. 1986;46:215–225. doi: 10.1016/0378-1119(86)90406-3. [DOI] [PubMed] [Google Scholar]
- 26.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;12:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]