Abstract
The tagAB and tagDEF operons, which are adjacent and divergently transcribed, encode genes responsible for cell wall teichoic acid synthesis in Bacillus subtilis. The Bacillus data presented here suggest that PhoP and PhoR are required for direct repression of transcription of the two operons under phosphate starvation conditions but have no regulatory role under phosphate-replete conditions. These data identify for the first time that PhoP∼P has a negative role in Pho regulon gene regulation.
Teichoic acid, an essential anionic polymer containing polyglycerol or polyribitol phosphate, is the major cell wall component of Bacillus subtilis grown in phosphate-replete media (2, 17). Approximately 15% of cellular phosphorus is stored in the form of teichoic acid under these conditions (1). During phosphate starvation, however, the cell ceases to produce teichoic acid and replaces it with an anionic phosphate-free polymer, teichuronic acid (5, 13). As a result, the cell saves phosphorus for cellular metabolism and DNA synthesis. When phosphate becomes available again, the cell will restore synthesis of teichoic acid and stop production of teichuronic acid (reference 5; for reviews, see references 1 and 29).
The genes responsible for synthesis of teichoic acid and teichuronic acid have been cloned and analyzed (16, 27, 28). The genes which are involved in teichuronic acid synthesis, the tuaABCDEFGH operon, are repressed when phosphate is in excess and turned on under phosphate starvation conditions (14a, 28). Expression of this operon under phosphate-limiting conditions is directly activated by PhoP and PhoR, a pair of bacterial two-component regulatory proteins (24, 25). PhoP, the response regulator, binds to the tuaA promoter at B. subtilis Pho boxes, as it does to other Pho regulon promoters which are induced during phosphate starvation (14a). Divergently transcribed operons tagAB and tagDEF encode products which are directly involved in teichoic acid synthesis. The gene products of tagAB are poorly characterized, although assumptions about their functions have been made based on the similarity of the sequences of their products to those of other proteins (15, 16). The tagDEF operon, on the other hand, encodes CDP-glycerol pyrophosphorylase (20), polyglycerolphosphate glucosyltransferase (22), and polyglycerolphosphate glycerol phosphate transferase (21). Expression of the tagA and tagD operons is reduced when the cell is grown in low-phosphate medium (18).
It has recently been suggested that not all proteins whose synthesis is regulated in response to changing phosphate levels are members of the Pho regulon (6, 19). To understand if the tagA and tagD operons are repressed by PhoP and PhoR, we studied expression of the divergently transcribed operons in a phoP mutant and a phoR mutant. We also used gel shift and DNase I footprinting assays to determine if PhoP directly regulates expression of these genes. We conclude that PhoP∼P directly binds to and is essential for transcriptional repression of the tagAB and tagDEF operons but activates the tuaABCDEFGH operon (14a) and therefore regulates the switch of the two anionic polymers during phosphate starvation.
Cloning of the promoter region shared by tagAB and tagDEF operons.
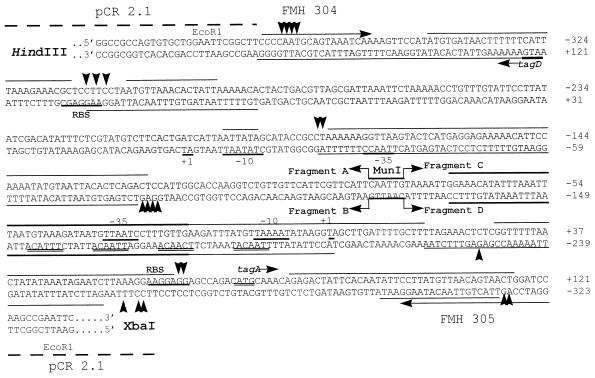
The divergently transcribed operons tagAB and tagDEF from B. subtilis 168 have been cloned and sequenced (16). The transcriptional start sites from tagA and tagD have been identified by primer extension analysis (15). Two primers, FMH304 (5′-354CCCCAATGCAGTAAATCAA372-3′) and FMH305 (5′-GGATCC845AGTTACTGTTAACATAAGGAA824-3′), which are located within the tagA and tagD genes, were used to amplify the promoter region shared by the two operons with B. subtilis JH642 (a derivative of B. subtilis 168) chromosomal DNA as the template (Fig. 1). The supercript numbers in the primers are the nucleotide numbers assigned by Mauël et al. (16). The 493-bp promoter fragment was cloned into pCR2.1 (Invitrogen) to construct pSE90. The cloned promoter fragment contains nucleotides (nt) −371 to +121 for tagA and nt −325 to +167 for tagD, relative to the transcriptional start site for each gene. The sequence of the insert was confirmed by DNA sequencing, and it is the same as that for B. subtilis 168. Figure 1 shows the sequence of the insert in pSE90 containing the common promoter region of the tagAB and tagDEF operons.
FIG. 1.
Sequence of the promoter region shared by tagAB and tagDEF operons. The 493-bp fragment containing promoters for both tagAB and tagDEF operons were cloned into pCR2.1. The junction of pCR2.1 and the promoter fragment is shown with the relevant restriction sites indicated above the sequence. The primers FMH304 and FMH305 within the coding region of tagD and tagA, respectively, are also indicated by arrows. The −10 regions, ribosomal binding sites, transcriptional start sites for ςA, and translational start sites for both tagA and tagD are labeled and underlined (14). Thin lines above or below the sequences, binding sites for PhoP∼P only; thick lines above or below the sequences, binding sites for both PhoP and PhoP∼P; arrowheads, hypersensitive sites. To number the nucleotides, the top strand (the coding strand for tagA) and the bottom strand (the coding strand for the tagD) are numbered separately, with transcriptional start sites of each gene indicated by +1. To facilitate the location of the protection area by PhoP (Fig. 4), the fragments A to D and the MunI restriction site are indicated by bent arrows and labeled. The four repeated TTAACA-like sequences located on the tagA noncoding strand upstream of −10 are also doubly underlined.
Expression of tagAB and tagDEF is repressed by PhoP and PhoR during phosphate starvation.
Mauël et al. (18) used a 399-bp intergenic region between tagA and tagD to make lacZ fusions of the two gene promoters and found that expression of B. subtilis 168 tagA and tagD is decreased in phosphate-limited culture. To examine if the lowered expression of the two promoters is dependent on the phoP and phoR genes, both tagA and tagD promoter-lacZ fusions were introduced into a phoP nonpolar deletion mutant (MH5600 [11]), a phoR deletion mutant (MH5124 [11]), and the parent strain (JH642) as follows. The promoter region shared by tagA and tagD was subcloned from pSE90 into pDH32 (26) in two different orientations to make tagA-lacZ (pSE91) and tagD-lacZ (pSE92) fusions. Single-copy promoter-lacZ fusion strains were obtained by integrating the promoter-lacZ fusions into the amy locus of each strain. The resultant strains containing the tagA-lacZ fusion in the parent, phoP, or phoR background were named MH5630, MH5634, and MH5632, respectively. The strains containing tagD-lacZ fusion in the parent, phoP, or phoR background were named MH5631, MH5635, and MH5633, respectively.
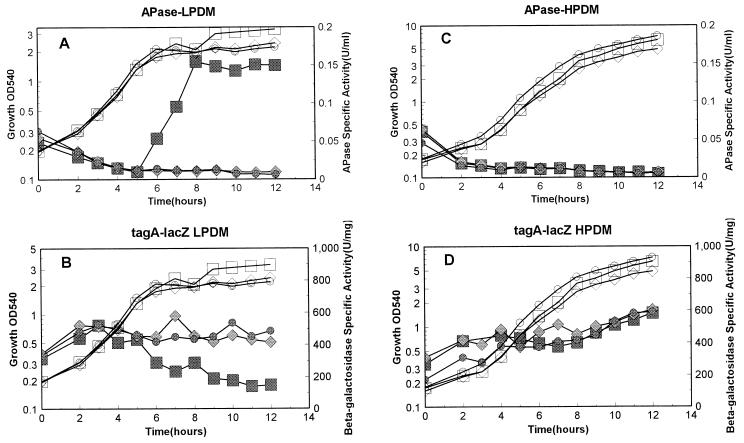
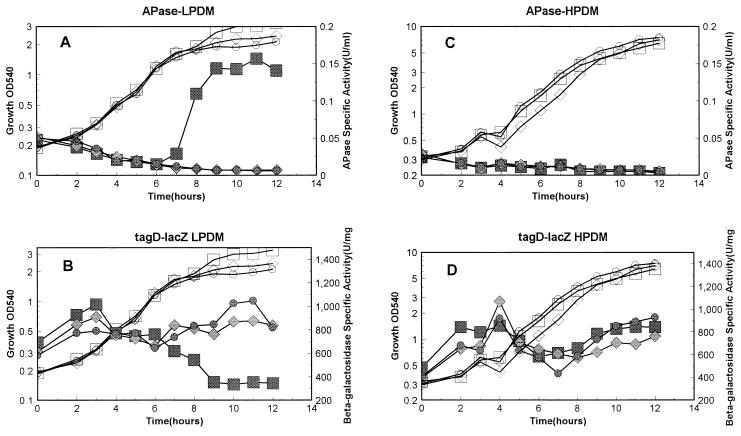
All six strains described above were grown in both high-phosphate defined medium (HPDM) containing 5 mM phosphate (23) and low-phosphate defined medium (LPDM) containing 0.42 mM phosphate (10). The promoter activities were detected under these conditions as described previously (10), and alkaline phosphatase (APase) activity was measured as a reporter for Pho regulon gene expression (10). Under high-phosphate conditions, transcription from tagA in the three strains was basically the same (Fig. 2D), as was that of the tagD promoter (Fig. 3D). There was no APase produced under these conditions, indicating no induction of the Pho response during phosphate-replete growth (Fig. 2C and 3C). These results strongly argue that neither PhoP nor PhoR plays a role in regulation of either of the two tag promoters under high-phosphate conditions. Phosphate is the limiting nutrient in LPDM, and cultures enter into stationary phase due to a phosphate deficiency. APases were induced in the parent strain but were not induced in either the phoP or the phoR strain (Fig. 2A and 3A). In LPDM, transcription from both the tagA and tagD promoters was dramatically decreased in the parent strain (Fig. 2B and Fig. 3B) at the same time that APase was induced in the same culture. In contrast, transcription from the tagA and tagD promoters continued during the whole growth period in either the phoP or the phoR strain (Fig. 2B and 3B). These results strongly suggest that both PhoP and PhoR are required for repression of transcription of these two promoters under phosphate starvation conditions. Transcription of tagD was two- to threefold higher than that of tagA, as reported by Maüel et al. (18). However, under our culture conditions, the decrease in the level of either tagA or tagD transcription in the parent strain during phosphate starvation was more dramatic (threefold) compared to the results from Maüel et al. (less than twofold) (18).
FIG. 2.
Promoter activity of tagA under phosphate starvation and phosphate-replete conditions. Strains containing the tagA-lacZ fusion in the parent strain or the phoP or phoR mutant strain were grown in LPDM (0.42 mM) or HPDM (5 mM) as described previously (10). A 12-h growth was monitored with respect to optical density at 540 nm, (OD540), APase activity, and β-galactosidase, which represents promoter activity. (A and B) APase and β-galactosidase activities, respectively, in LPDM; (C and D) APase and β-galactosidase activities, respectively, in HPDM. Open symbols, growth; filled symbols, APase or β-galactosidase activity. Strains included MH5630 (squares [parent]), MH5632 (diamonds [phoR]), and MH5634 (circles [phoP]).
FIG. 3.
Promoter activity of tagD under phosphate starvation and phosphate-replete conditions. Strains containing the tagD-lacZ fusion in the parent strain or the phoP or phoR mutant strain were grown in LPDM and HPDM as described in the legend to Fig. 2. Strains included MH5631 (squares [parent]), MH5633 (diamonds [phoR]), and MH5635 (circles [phoP]). OD540, optical density at 540 nm.
The level of tagA and tagD transcription changed little in either the phoP or the phoR mutant during phosphate starvation, corroborating the cell wall biochemical quantitation data (19). Under the same conditions, tuaA transcription is not turned on (14a), and no teichuronic acid synthesis is induced in these mutant strains (19). In order to survive, these mutants continue to synthesize teichoic acid for cell wall assembly, although the limiting phosphate reserve has to be used for this purpose. However, in the parent strain, the cell represses teichoic acid synthesis and switches to teichuronic acid synthesis during phosphate starvation to conserve the limited phosphorus sources (5). The activation of tuaA transcription (14a) and repression of tagA and tagD transcription require phoP and phoR.
Since the tagAB and tagDEF operons were regulated by PhoP and PhoR during phosphate starvation, these operons should be considered part of the Pho regulon. The definition of the Pho regulon genes, therefore, is broadened to include not only the genes which are activated by PhoP∼P, but genes which are repressed by PhoP∼P as well.
Both PhoP and PhoP∼P bind to the promoter region shared by tagA and tagD.
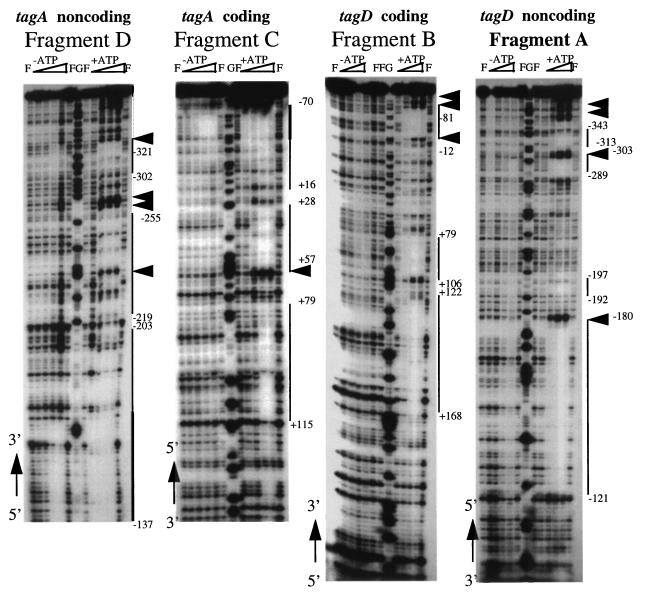
PhoP regulates the Pho regulon genes by binding to the target promoters (13a, 14, 14a). To determine if regulation of the tagA and tagD promoters by PhoP is through direct binding of PhoP to these promoters, we performed gel shift assays (data not shown) and DNase I footprinting assays using the promoter region shared by both tagA and tagD in pSE90. PhoP bound to multiple regions within these two promoters (data not shown). To obtain clearer footprinting data, the promoter region was dissected into two subregions by DNA digestion at the unique MunI restriction site, which is located between the −35 regions of the two promoters (Fig. 1). Figure 4 shows that PhoP∼P bound to multiple regions whereas unphosphorylated PhoP bound only to a region in which multiple TTAACA-like sequences were found (Fig. 1). It has been found that the DNA regions containing multiple TTAACA-like sequences (separated by about five nonconserved nucleotides) were bound by both unphosphosphorylated PhoP and PhoP∼P in all of the tested Pho promoters (13a, 14, 14a) and have been proposed to compose the B. subtilis PhoP core binding region (13a, 14a). Four TTAACA-like sequences in the tagA and tagD promoters were located in the region between −10 and −50 of the tagA promoter but on the noncoding strand. In contrast, in other Pho promoters which are activated by PhoP∼P, the TTAACA-like sequences were located at and upstream of the −22 region of the coding strand (12, 14a). The appearance of the PhoP core binding region on different strands may be important for the different role of PhoP∼P in the regulation of these promoters. PhoP∼P binding sites within the tagA and tagD promoters covered the −10 region, partially covered the ribosomal binding sites, and extended to the 5′ coding regions for each of the two genes. However, binding of PhoP∼P on tuaA, which is activated by PhoP∼P, is only located upstream of −10 (14a). PhoP∼P binding in the 5′ coding region may also be critical for the negative role of PhoP∼P in tagA and tagD regulation.
FIG. 4.
DNase I footprinting assays of the tagA and tagD promoters by PhoP and PhoP∼P. Plasmid pSE90 was digested with MunI (Fig. 1), end labeled with Klenow fragment, and digested with HindIII to obtain the fragment A showing nt −371 to −79 of the tagD noncoding strand. To get fragment B showing nt +168 to −124 of the tagD coding strand, pSE90 was digested with HindIII, end labeled with Klenow fragment, and digested with MunI. Fragment C showing nt −82 to +121 of the tagA coding strand was made by digesting pSE90 with XbaI, end labeling with Klenow fragment, and further digesting with MunI. Fragment D showing nt −121 to −323 of the tagA noncoding strand was made by digesting pSE90 with MunI, end labeling with Klenow fragment, and further digesting with XbaI. The ends were labeled in the presence of [α-32P]dCTP. The footprinting experiments were done as described previously (14). The amounts of PhoP added to each reaction mixture were 40 ng (55 nM), 200 ng (275 nM), 1 μg (1.4 μM), and 5 μg (6.9 μM) from left to right. To each reaction mixture, 0.7 μg of *PhoR, the cytoplasmic domain of PhoR (14), was added. +ATP, reaction mixtures containing ATP which were used for producing PhoP∼P; −ATP, reaction mixtures for unphosphorylated PhoP; F, lanes without PhoP; G, the G sequencing lane; thick lines, binding sites for both PhoP and PhoP∼P; thin lines, binding sites for PhoP∼P alone; arrowheads, hypersensitive sites. The numbers of the arrowheads do not reflect the numbers of hypersensitive sites shown in Fig. 1 because of space constraints due to clustering. To correlate the locations of the footprinting results with the sequences, the 5′ and 3′ ends of each sequence are labeled along with the sequencing gels.
Maüel et al. (16) found a sequence in the tagA and tagD promoter region which shows similarity with the Escherichia coli Pho box. A similar sequence has also been found in the phoA and phoB promoters. However, these sequences, at least for transcription of the phoA and phoB promoters, are not necessary (3, 13a).
A model for PhoP∼P regulation of cell wall synthesis.
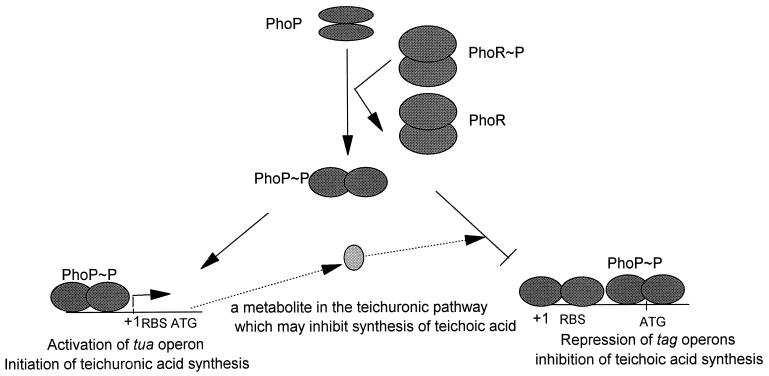
We have shown that PhoP is the substrate for PhoR, its cognate kinase, with respect to phosphorylation and dephosphorylation (14). It has been proposed that PhoP∼P is the active form for stimulation of Pho regulon gene transcription during phosphate starvation (7–9, 14a). In control of B. subtilis cell wall synthesis, we have shown that under phosphate starvation conditions PhoP is required for activation of the tuaABCDEFGH operon (14a) and simultaneous repression of the tagAB and tagDEF operons. Therefore, teichuronic acid is synthesized and teichoic acid synthesis is inhibited under these conditions (5, 13). In this switch process, we propose that PhoP∼P plays a key role in activation of tuaA and repression of tagA and tagD by binding to different regions of these promoters (14a). In the phoP or phoR mutant, transcription of genes for teichuronic acid synthesis is not initiated due to the lack of the activator, PhoP∼P, during phosphate starvation; However, the cell continues to transcribe the genes for teichoic acid synthesis to compensate for cell wall anionic polymers because repression from PhoP∼P does not exist. Continued synthesis of teichoic acid has also been observed in the mutants unable to synthesize teichuronic acid (gtaB) during phosphate limitation (19). The proposed explanation for this phenomenon was that a component of teichuronic acid synthesis is involved in repression of teichoic acid synthesis (Fig. 5). Any repression by a metabolite dependent on teichuronic synthesis must require PhoP and PhoR (14a), and this idea is supported by the observation that no repression of teichoic acid was detected in a phoP or phoR mutant under phosphate-limited conditions.
FIG. 5.
A model for teichoic acid and teichuronic acid synthesis regulated by PhoP∼P. Under phosphate starvation conditions, PhoP∼P phosphorylated by PhoR binds to both the tag operon promoters and the tua operon promoter. As a result, PhoP∼P binds to the −10 region and/or the ribosomal binding site and to the 5′ end of the coding region to repress transcription from the tag operons and simultaneously binds upstream of the −10 region of the tua operon to activate transcription. A component involved in teichuronic acid synthesis has also been suggested to repress teichoic acid synthesis during phosphate limitation (19), as indicated by the arrows with dashed lines. The number of the PhoP dimers in this model is not meant to reflect the physiological state. The number of PhoP dimers binding to any promoter is unknown.
When excess phosphate is provided, PhoP∼P is dephosphorylated by PhoR. In the absence of PhoP∼P, teichuronic acid synthesis is not induced and teichoic acid synthesis is relieved from repression. Thus, PhoP and PhoR do not play a role during phosphate-replete growth.
This is the first time that the negative role of PhoP∼P has been observed for Pho regulation. That the PhoP-PhoR two-component regulatory system participates in the regulation of B. subtilis cell wall anionic polymer synthesis distinguishes the B. subtilis Pho regulon from the well-studied E. coli Pho regulon. These data together with tuaA activation establish the importance of the Pho regulon in B. subtilis cell wall synthesis and perhaps in other gram-positive bacteria whose cell walls contain teichoic acid and teichuronic acid anionic polymers.
REFERENCES
- 1.Archibald A R, Hancock I C, Harwood C R. Cell wall structure, synthesis and turn over. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular biology. Washington, D.C: American Society for Microbiology; 1993. pp. 381–410. [Google Scholar]
- 2.Baddiley J. Structure, biosynthesis and function of teichoic acids. Accounts Chem Res. 1970;3:98–105. [Google Scholar]
- 3.Chesnut R S, Bookstein C, Hulett F M. Separate promoters direct expression of phoAIII, a member of the Bacillus subtilis alkaline phosphatase multigene family, during phosphate starvation and sporulation. Mol Microbiol. 1991;5:2181–2190. doi: 10.1111/j.1365-2958.1991.tb02148.x. [DOI] [PubMed] [Google Scholar]
- 4.Eder S, Shi L, Jensen K, Yamane K, Hulett F M. A Bacillus subtilis secreted phosphodiesterase/alkaline phosphatase is the product of a Pho regulon gene, phoD. Microbiology. 1996;142:2041–2047. doi: 10.1099/13500872-142-8-2041. [DOI] [PubMed] [Google Scholar]
- 5.Ellwood D C, Tempest D W. Control of teichoic acid and teichuronic acid biosyntheses in chemostat cultures of Bacillus subtilis var. niger. Biochem J. 1969;111:1–5. doi: 10.1042/bj1110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eymann C, Mach H, Harwood C R, Hecker M. Phosphate-starvation-inducible proteins in Bacillus subtilis: a two-dimensional gel electrophoresis study. Microbiology. 1996;142:3163–3170. doi: 10.1099/13500872-142-11-3163. [DOI] [PubMed] [Google Scholar]
- 7.Hulett F M. The signal transduction network for Pho regulation. Mol Microbiol. 1996;19:933–939. doi: 10.1046/j.1365-2958.1996.421953.x. [DOI] [PubMed] [Google Scholar]
- 8.Hulett F M. Complex phosphate regulation by sequential switches in Bacillus subtilis. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: American Society for Microbiology; 1995. pp. 289–302. [Google Scholar]
- 9.Hulett F M. Regulation of phosphorus metabolism. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 229–235. [Google Scholar]
- 10.Hulett F M, Bookstein C, Jensen K. Evidence for two structural genes for alkaline phosphatase in Bacillus subtilis. J Bacteriol. 1990;172:735–740. doi: 10.1128/jb.172.2.735-740.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulett F M, Lee J, Shi L, Sun G, Chesnut R, Sharkova E, Duggan M F, Kapp N. Sequential action of two-component genetic switches regulates the PHO regulon in Bacillus subtilis. J Bacteriol. 1994;176:1348–1358. doi: 10.1128/jb.176.5.1348-1358.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hulett F M, Sun G, Liu W. The Pho regulon of Bacillus subtilis is regulated by sequential action of two genetic switches. In: Torriani-Gorini A, Yogil E, Silver S, editors. Phosphate in microorganisms: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1994. pp. 50–54. [Google Scholar]
- 13.Lang W K, Glassey K, Archibald A R. Influence of phosphate supply on teichoic acid and teichuronic acid content of Bacillus subtilis cell walls. J Bacteriol. 1982;151:367–375. doi: 10.1128/jb.151.1.367-375.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Liu, W., et al. Submitted for publication.
- 14.Liu W, Hulett F M. Bacillus subtilis PhoP binds to the phoB tandem promoter exclusively within the phosphate starvation-inducible promoter. J Bacteriol. 1997;179:6302–6310. doi: 10.1128/jb.179.20.6302-6310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Liu, W., and F. M. Hulett. Submitted for publication.
- 15.Mauël C, Bauduret A, Chervet C, Beggah S, Karamata D. In Bacillus subtilis 168, teichoic acid of the cross-wall may be different from that of the cylinder: a hypothesis based on transcription analysis of tag genes. Microbiology. 1995;141:2379–2389. doi: 10.1099/13500872-141-10-2379. [DOI] [PubMed] [Google Scholar]
- 16.Mauël C, Young M, Karamata D. Genes concerned with synthesis of poly(glycerol phosphate), the essential teichoic acid in Bacillus subtilis strain 168, are organized in two divergent transcription units. J Gen Microbiol. 1991;137:929–941. doi: 10.1099/00221287-137-4-929. [DOI] [PubMed] [Google Scholar]
- 17.Mauël C, Young M, Margot P, Karamata D. The essential nature of teichoic acids in Bacillus subtilis as revealed by insertional mutagenesis. Mol Gen Genet. 1989;215:388–394. doi: 10.1007/BF00427034. [DOI] [PubMed] [Google Scholar]
- 18.Mauël C, Young M, Monsutti-Grecescu A, Marriott S A, Karamata D. Analysis of Bacillus subtilis tag gene expression using transcription fusions. Microbiology. 1994;140:2279–2288. doi: 10.1099/13500872-140-9-2279. [DOI] [PubMed] [Google Scholar]
- 19.Müller J P, An Z, Merad T, Hancock I C, Harwood C. Influence of Bacillus subtilis phoR on cell wall anionic polymers. Microbiology. 1997;143:947–956. doi: 10.1099/00221287-143-3-947. [DOI] [PubMed] [Google Scholar]
- 20.Pooley H M, Abellan F-X, Karamata D. A conditional-lethal mutant of Bacillus subtilis 168 with a thermosensitive glycerol-3-phosphate cytidylyltransferase, an enzyme specific for the synthesis of the major teichoic acid. J Gen Microbiol. 1991;137:921–928. doi: 10.1099/00221287-137-4-921. [DOI] [PubMed] [Google Scholar]
- 21.Pooley H M, Abellan F-X, Karamata D. CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase, which is involved in the synthesis of the major wall teichoic acid in Bacillus subtilis 168, is encoded by tagF (rodC) J Bacteriol. 1992;174:646–649. doi: 10.1128/jb.174.2.646-649.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pooley H M, Paschoud D, Karamata D. The gtaB marker in Bacillus subtilis 168 is associated with a deficiency in UDP glucose pyrophosphorylase. J Gen Microbiol. 1987;133:3481–3493. doi: 10.1099/00221287-133-12-3481. [DOI] [PubMed] [Google Scholar]
- 23.Qi Y, Kobayashi Y, Hulett F M. The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the Pho regulon. J Bacteriol. 1996;179:2534–2539. doi: 10.1128/jb.179.8.2534-2539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seki T, Yoshikawa H, Takahashi H, Saito H. Cloning and nucleotide sequence of phoP, the regulatory gene for alkaline phosphatase and phosphodiesterase in Bacillus subtilis. J Bacteriol. 1987;169:2913–2916. doi: 10.1128/jb.169.7.2913-2916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seki T, Yoshikawa H, Takahashi H, Saito H. Nucleotide sequence of the Bacillus subtilis phoR gene. J Bacteriol. 1988;170:5935–5938. doi: 10.1128/jb.170.12.5935-5938.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimotsu H, Henner D. Construction of a single copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene. 1986;43:85–94. doi: 10.1016/0378-1119(86)90011-9. [DOI] [PubMed] [Google Scholar]
- 27.Soldo B, Lavarevic V, Margot P, Karamata D. Sequencing and analysis of the divergon comprising gtaB, the structural gene of UDP-glucose pyrophosphorylase of Bacillus subtilis 168. J Gen Microbiol. 1993;139:3185–3195. doi: 10.1099/00221287-139-12-3185. [DOI] [PubMed] [Google Scholar]
- 28.Soldo B, Lazarevic V, Pagni M, Mauël C, Karamata D. Abstracts for the 8th International Conference on Bacilli. 1995. Transcriptional regulation of teichuronic acid gene expression; p. 77. Stanford, Calif. [Google Scholar]
- 29.Ward J B. Teichoic acid and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981;45:211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]