Abstract
Myxococcus xanthus tgl mutants lack social motility and type IV pili but can be transiently stimulated to swarm and to make pili by contacting tgl+ cells. The absence of pili in tgl mutants is shown not to be due to the absence of pilin. The rate of pilus elongation after Tgl stimulation is shown to be similar to the rate of pilus elongation in wild-type cells, using a new more rapid assay for stimulation.
Myxococcus xanthus has two genetic systems, called adventurous (A) and social (S) motility, which control its gliding motility (2, 4, 9, 22). Unlike A motility, S motility involves movements of cells which are close to each other, thus implying cell-cell interactions (9, 11). S motility absolutely requires polarly localized type IV pili, since no mutant lacking pili has S motility (10, 13, 18, 25). Homologous type IV pili in Pseudomonas aeruginosa and Neisseria gonorrhoeae have been shown to be involved in another type of surface translocation called twitching (6). Many of the pil gene products in M. xanthus, P. aeruginosa, and N. gonorrhoeae share sequence homologies with the type II, or general secretion, pathway found in enteric bacteria (15).
Mutants for a particular S motility gene, called tgl (for transient gliding), lack S motility and type IV pili, but these qualities can be phenotypically rescued by contact with tgl+ (donor) cells, a process called stimulation (9, 10). Stimulation does not involve a diffusable factor but instead requires physical contact between live cells. Formerly, to detect tgl stimulation, nonswarming (A− S−) recipient and donor strains have been used so that the phenotypically stimulated tgl recipients were visible as cells that swarmed out of the initial mixture. These stimulated cells have pili (pili+). If the stimulated cells are cultured, their offspring are S− and pili−; hence, Tgl stimulation is transient and only phenotypic (10). An analogous type of phenotypic rescue occurs for a curli (pili-like) mutant in Escherichia coli (3). However, unlike type IV pili (7), curlin subunits are secreted and then are nucleated and polymerized on the cell surface. In the A motility system, which does not involve pili, five groups of mutants lack A motility but can be stimulated for A motility by donor cells which have the corresponding wild-type allele. Stimulation is thus a general phenomenon and may play an important role in the swarming of M. xanthus.
The tgl gene product contains a signal peptidase II recognition sequence, suggesting that Tgl is a lipoprotein (16). Tgl appears to be localized to the periplasm, probably attached to the outer membrane, and to contain six tandem copies of the tetratrico peptide repeat (TPR) (17). TPR domains are thought to be important in protein-protein interactions (12). Thus, Tgl likely interacts with other proteins involved in pilus assembly. To find the molecular basis of Tgl stimulation, we sought a new assay that would allow stimulation to be monitored on a time scale of hours instead of days and that could use swarming as well as nonswarming strains.
Δtgl mutants express PilA.
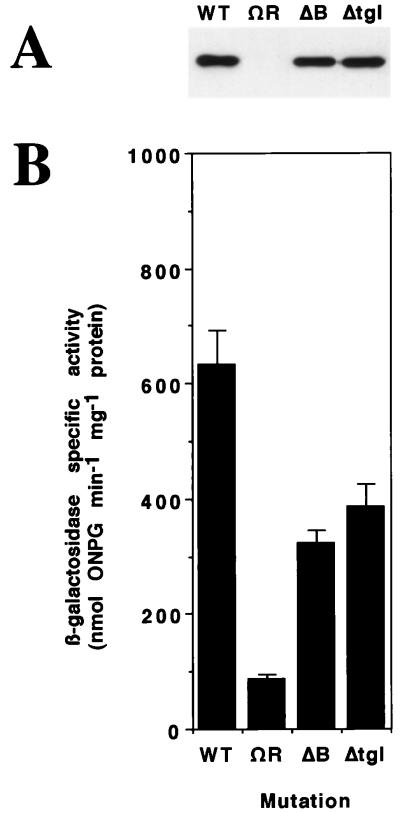
tgl mutants fail to make pili that can be detected by electron microscopy (10). Type IV pili are helical assemblies of pilin monomers, the pilA gene product (14). To determine whether the block in pilus biogenesis in tgl mutants was at the level of PilA expression or its assembly into pili, whole-cell extracts from a deletion (Δ) tgl mutant (16) were probed for expression of PilA with antibody to PilA (24) (see below). As shown in Fig. 1A, a Δtgl mutant expresses near-wild-type levels of PilA protein. In a complementary experiment, the expression of pilA was monitored with a pilA::lacZ transcriptional fusion (24). Transcription of the pilA::lacZ operon fusion was found to be similar in Δtgl and tgl+ cells (Fig. 1B). A ΔpilB strain, which also lacks pili and S motility (27), also expresses near-wild-type levels of PilA (Fig. 1). By contrast, expression of pilA was blocked in a ΔpilR mutant; PilR is known to be a transcriptional activator of pilA gene expression (24, 25). We conclude that the absence of pili in tgl mutants is due to a failure to assemble pilin monomer subunits into pili.
FIG. 1.
(A) Immunoblot of M. xanthus whole-cell protein probed with anti-PilA serum. Proteins were visualized by anti-rabbit IgG–peroxidase and chemiluminescence (Renaissance; NEN Life Science Products). Samples were prepared from strains containing null mutations of the pil genes indicated. Lanes (labeled with the mutation present in each strain): WT, DK1219 (S+) (9); ΩR, DK10414 (pilR-Ω3163, a Tn5 insertion) (24); ΔB, DK10416 (ΔpilB) (27); Δtgl, DK10413 (Δtgl) (16, 24). Protein from 5 × 106 cells was loaded in each lane. (B) β-galactosidase specific activity of the same strains as in panel A, into which a single copy of a pilA-lacZ transcriptional fusion has been introduced. The values are averages of four or more independent measurements; error bars indicate standard deviations. ONPG, o-nitrophenyl-β-d-galactopyranoside.
Stimulation assay for pili.
Since S motility absolutely depends on the production of pili, we reasoned that Tgl stimulation might be monitored by measuring the assembly of pili rather than the resultant swarming of S motile cells. Hence, Δtgl recipient cells were mixed with tgl+ ΔpilA donor cells (which also lack pili) and pili were measured at various times after mixing. To enumerate pili, the cells were grown in CTT medium (8) to a density of 80 to 120 Klett units, concentrated by centrifugation, washed, and resuspended in TPM buffer (10 mM Tris-HCl, 1 mM KPO4, and 8 mM MgSO4 [pH 7.6]) to a calculated density of 500 Klett units. The cells were then mixed at a ratio of 2 recipient cells to 1 donor cell, and four 20-μl samples were placed on one-half CTT agar plates (8). We observed that stimulation only occurs on a solid surface and not in liquid (10). Cells were harvested from the plates at various times and suspended in 0.4 ml of TPM buffer. Pili were sheared off cells by vortexing the cell suspension in microcentrifuge tubes (1.5 ml) for 2 min (1, 5). The cells were separated from the suspended pili by centrifugation at 14,000 rpm (16,000 × g) for 5 min at room temperature. The supernatant was collected, and MgCl2 was added to a final concentration of 100 mM to precipitate pilus fragments (1, 5). After ≥1 h of incubation on ice, which allowed the pilus fragments to form paracrystalline aggregates, the precipitate was collected by centrifugation for 15 min at 4°C, also at 14,000 rpm. The precipitate was resuspended in sample buffer, boiled for 5 min, and separated overnight by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (19). To detect PilA protein, the gels were blotted and the blots were probed with a 1:2,000 dilution of anti-PilA serum.
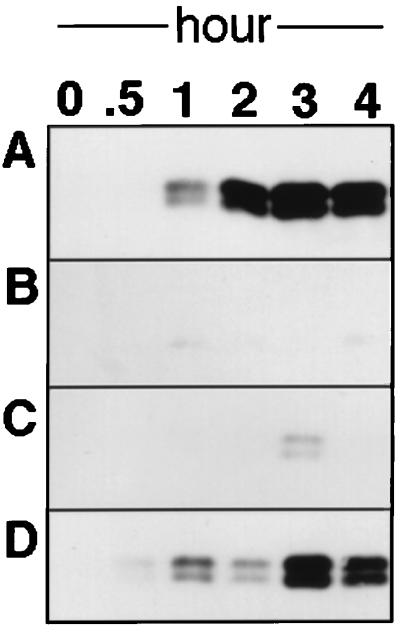
As shown in Fig. 2A, the Δtgl recipient cells produce pili that are long enough to become sensitive to shearing when they are mixed with tgl+ ΔpilA (DK10407) donor cells. It is important to note that the PilA protein which makes up these pili can have arisen only from the Δtgl cells, since they are pilA+ while the donor cells are ΔpilA. The ability of the PilA protein to assemble into pilus filaments was confirmed by electron microscopy. The PilA protein seen as a doublet in the gels of sheared samples (as in Fig. 2) may reflect incomplete reduction of disulfide bonds or, alternatively, a posttranslational modification of the protein (21). Stimulation is specific to tgl, as shown by the following: (i) when the same culture of recipient cells was mixed with an otherwise isogenic donor which lacks Tgl (Δtgl ΔpilA) no pilus bands were detected (Fig. 2B) and (ii) when a ΔpilB mutant was mixed with the same donor strain DK10407, which is pilB+ tgl+ ΔpilA, no pilB stimulation was observed (Fig. 2C). To date, tgl mutants are the only type of S motility mutants known which can be stimulated (4, 9, 20).
FIG. 2.
Immunoblot of sheared pilus samples probed with anti-PilA serum. Cells were incubated on one-half CTT agar plates (9) for the indicated times as described in the text, except that at 0 h cells were directly assayed in CTT medium. (A) DK10405 (Δtgl) (16) mixed with DK10407 (ΔpilA) (26); (B) DK10405 mixed with DK8600 (ΔpilA Δtgl); (C) DK10407 mixed with DK10416 (ΔpilB); (D) sheared DK1622 (wild type) (10) alone.
Time course of Tgl stimulation.
The speed of pilus elongation after stimulation of Δtgl cells was compared with the normal extension rate of pili in growing cells. Since wild-type cells constitutively express pili, wild-type (DK1622) cells were first sheared by vortexing for 3 min to remove their pili. Rosenbluh and Eisenbach have shown that sheared cells are able to regrow pili (18). In fact, as shown in figure 2D, the regrowth of pili on sheared wild-type cells was somewhat slower than pilus elongation after the stimulation of Δtgl mutants (Fig. 2A). More rapid growth of pili after stimulation may reflect the accumulation of PilA monomer units in Δtgl cells; wild-type cells may have to synthesize and process their pilin monomers (18, 23). These data argue that tgl mutants have all the necessary components for pilus biogenesis except for Tgl protein. The time required for stimulation is comparable to the rate of pilus extension during the growth of wild-type cells. Thus, it is possible that stimulation is involved in the control of swarming. It is hoped that the assay described here will permit the identification of proteins with which Tgl interacts and of the cellular and/or molecular basis of stimulation.
Acknowledgments
This work was supported by a grant from the National Science Foundation (MCB 9423182). D.W. was the recipient of an American Cancer Society postdoctoral fellowship (PF-4138).
REFERENCES
- 1.Alm R A, Mattick J S. Identification of a gene, pilV, required for type 4 fimbrial biogenesis in Pseudomonas aeruginosa, whose product possesses a pre-pilin-like leader sequence. Mol Microbiol. 1995;16:485–496. doi: 10.1111/j.1365-2958.1995.tb02413.x. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammar M, Bian Z, Normark S. Nucleator-dependent intercellular assembly of adhesive curli organelles in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:6562–6566. doi: 10.1073/pnas.93.13.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartzell P L, Youderian P. Genetics of gliding motility and development in Myxococcus xanthus. Arch Microbiol. 1995;164:309–323. doi: 10.1007/BF02529977. [DOI] [PubMed] [Google Scholar]
- 5.Heckels J E, Virji M. Separation and purification of surface components. In: Hancock I C, Poxton I R, editors. Bacterial cell surface techniques. Chichester, England: John Wiley & Sons; 1988. pp. 67–135. [Google Scholar]
- 6.Henrichsen J. Twitching motility. Annu Rev Microbiol. 1983;37:81–93. doi: 10.1146/annurev.mi.37.100183.000501. [DOI] [PubMed] [Google Scholar]
- 7.Hobbs M, Mattick J S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 8.Hodgkin J, Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci USA. 1977;74:2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol Gen Genet. 1979;171:177–191. [Google Scholar]
- 10.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser D, Crosby C. Cell movement and its coordination in swarms of Myxococcus xanthus. Cell Motil. 1983;3:227–245. [Google Scholar]
- 12.Lamb J R, Tugendreich S, Hieter P. Tetratrico peptide repeat interaction: to TPR or not to TPR. Trends Biochem Sci. 1995;20:257–258. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- 13.MacRae T H, McCurdy H D. Evidence for motility-related fimbriae in the gliding microorganism Myxococcus xanthus. Can J Microbiol. 1976;22:1589–1593. doi: 10.1139/m76-234. [DOI] [PubMed] [Google Scholar]
- 14.Parge H E, Forest K T, Hickey M J, Christensen D A, Gertzoff E D, Tainer J A. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 15.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Soto J P, Kaiser D. Identification and localization of the Tgl protein, which is required for Myxococcus xanthus social motility. J Bacteriol. 1997;179:4372–4381. doi: 10.1128/jb.179.13.4372-4381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Soto J P, Kaiser D. The tgl gene: social motility and stimulation in Myxococcus xanthus. J Bacteriol. 1997;179:4361–4371. doi: 10.1128/jb.179.13.4361-4371.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbluh A, Eisenbach M. Effect of mechanical removal of pili on gliding motility of Myxococcus xanthus. J Bacteriol. 1992;174:5406–5413. doi: 10.1128/jb.174.16.5406-5413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Sodergren E, Kaiser D. Insertions of Tn5 near genes that govern stimulatable cell motility in Myxococcus. J Mol Biol. 1983;167:295–310. doi: 10.1016/s0022-2836(83)80337-4. [DOI] [PubMed] [Google Scholar]
- 21.Virji M. Post-translational modifications of meningococcal pili. Identification of common substituents: glycans and α-glycerophosphate—a review. Gene. 1997;192:141–147. doi: 10.1016/s0378-1119(97)00082-6. [DOI] [PubMed] [Google Scholar]
- 22.Ward M J, Zusman D R. Regulation of directed motility in Myxococcus xanthus. Mol Microbiol. 1997;24:885–893. doi: 10.1046/j.1365-2958.1997.4261783.x. [DOI] [PubMed] [Google Scholar]
- 23.Womack B J, Gilmore D F, White D. Calcium requirement for gliding motility in myxobacteria. J Bacteriol. 1989;171:6093–6096. doi: 10.1128/jb.171.11.6093-6096.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S S, Kaiser D. Regulation of expression of the pilA gene of Myxococcus xanthus. J Bacteriol. 1997;179:7748–7758. doi: 10.1128/jb.179.24.7748-7758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu S S, Kaiser D. Genetic and functional evidence that type IV pili are required for social gliding motility in Myxococcus xanthus. Mol Microbiol. 1995;18:547–558. doi: 10.1111/j.1365-2958.1995.mmi_18030547.x. [DOI] [PubMed] [Google Scholar]
- 26.Wu S S, Kaiser D. Markerless deletion of pil genes in Myxococcus xanthus generated by counterselection with the Bacillus subtilis sacB gene. J Bacteriol. 1996;178:5817–5821. doi: 10.1128/jb.178.19.5817-5821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S S, Wu J, Kaiser D. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol Microbiol. 1997;23:109–121. doi: 10.1046/j.1365-2958.1997.1791550.x. [DOI] [PubMed] [Google Scholar]