Abstract
Purpose
Chronic obstructive pulmonary disease (COPD) is a progressive disease resulting in a range of symptoms including breathlessness. “Symptom burden” describes the severity and impact of multiple symptoms in an individual and is best quantified using validated symptom instruments but is not routinely measured in clinical practice. Therefore, we wanted to assess overall symptom burden in patients with moderate-to-severe COPD and find associated independent predictors.
Patients and methods
A single-centre cross-sectional study of patients with COPD who attended the Westmead Breathlessness Service between March 2017 and May 2022 was conducted. We obtained baseline demographic data, lung function, assessed quality of life (CAT), anxiety/depression (HADS), and measured symptom burden (CMSAS). We compared variables between men and women using unpaired t tests or Mann–Whitney tests for continuous variables, and Fisher’s exact tests for categorical variables. We used multiple regression to look for independent predictors of overall symptom burden. Data were analysed using Stata/IC 15.1.
Results
Eighty-nine patients with COPD, mean age 72.6 years, 55% male, mean FEV1 32% predicted, reported an average of 8.9 symptoms including 6.9 physical and 1.6 psychological symptoms. The most common physical symptoms were shortness of breath (100%) and lack of energy (80%), and the most common psychological symptoms were worrying (65%) and feeling anxious (61%). Median CMSAS total score was higher in women than men (1.34 versus 1.04, respectively; p=0.03) with more women experiencing nervousness (p=0.011) and anxiety (p=0.005). Female sex (p=0.003), HADS-Anxiety (p=0.0001), and HADS-Depression (p=0.0001) were independently associated with total CMSAS score in a multiple linear regression model and explained 63% of total CMSAS variability.
Conclusion
Very high physical and psychological symptom burden exists among patients with severe COPD. Anxiety, depression, and female sex were independently associated with increasing symptom burden. Identifying and understanding sex differences for COPD symptoms, and interventions targeting anxiety and depression may help to reduce overall symptom burden within this population.
Keywords: dyspnoea, anxiety, depression, respiratory, gender/sex
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung disease characterised by chronic respiratory symptoms such as dyspnoea, cough, and sputum production due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that causes persistent, often progressive, airflow obstruction.1 Patients with COPD often experience symptoms despite maximal medical management including ongoing dyspnoea, cough, dry mouth, fatigue, pain, trouble sleeping, depression, and anxiety.2–6 The impact of multiple symptoms within an individual can be conceptualised by the term “symptom burden” which may be defined as “the sum of the severity and impact of symptoms reported by a significant proportion of patients with a given disease or treatment”7 and is best quantified by validated symptom instruments. Symptom burden is a significant problem in patients with severe COPD as it has been comparable to those with cancer.8–10 Given that symptoms are specific to the individual, aspects of life including living situation, disease severity, comorbidities, quality of life, and sex/gender may have an impact on symptom burden. Therefore, understanding the epidemiology of symptoms in specific populations serves to enable development of strategies to ameliorate distress and reduce suffering.11
The Westmead Breathlessness Service (WBS) undertakes a comprehensive assessment of patients with moderate to very severe COPD which includes symptom burden, and provides multidisciplinary, non-pharmacological management of breathlessness in patients who remain troubled by breathlessness despite receiving appropriate therapy from their usual care providers. A protocol outlining the model of care has been recently published.12 To be able to address the problem of symptom burden in this population, it is necessary to quantify the extent of the burden and identify factors that are predictive of symptom burden. Therefore, we aimed to, firstly, describe the physical and psychological symptom burden for patients attending the WBS and, secondly, identify factors that are independently associated with symptom burden.
Methods
Study Design and Setting
We performed a cross-sectional study of all patients with a diagnosis of COPD who were enrolled in a randomised controlled trial (RCT) of a non-pharmacological integrated care intervention to reduce the impact of breathlessness through the WBS12 who underwent an initial assessment between March 2017 and May 2022 [The Australian New Zealand Clinical Trial Registry ACTRN12617000499381]. The RCT study was conducted at a single site (Westmead Hospital) after approval from Western Sydney Local Health District Human Research Ethics Committee (WSLHD HREC), informed written consent was obtained for each participant, and the RCT was conducted in accordance with the Declaration of Helsinki. For this study, analysis of baseline data prior to intervention was also approved by WSLHD HREC (2023–01 QA). Individual deidentified participant baseline data in this study will be made available for five years after manuscript publication in Excel spreadsheet form upon request via email.
Inclusion criteria for referral to the WBS required all three of the following criteria:
a diagnosis of moderate to very severe COPD (ie, FEV1/FVC ratio ≤0.7 and FEV1% predicted ≤60%),
severe breathlessness (modified Medical Research Council [mMRC] breathlessness scale ≥2), and
being willing and able to actively participate in own care.
Participants who were assessed as being unable to participate in interventions to address breathlessness were excluded from the WBS (see12 for the full list of exclusion criteria).
Participants were referred by respiratory specialists, general practitioners, nurses, and allied health staff in Western Sydney and were assessed for suitability for WBS intervention by staff prior to enrolment.
Assessments
Demographic Data
We obtained demographic data at the initial multidisciplinary assessment clinic which included age, sex, body-mass index (BMI), smoking status, comorbidities, pulmonary rehabilitation completion in the last 12 months, and social situation (eg, living arrangements, country of birth).
Lung Function
We obtained standardised measurements of lung function via spirometry (MicroLab 36-ML3500 MK80STK, CareFusion) and recorded forced expiratory volume in the first second (FEV1), FEV1 as percent predicted (FEV1%), forced vital capacity (FVC), and FVC as percent predicted (FVC%). Where possible, participants performed two standardised six-minute walk tests (6MWT) at the initial assessment, and the best distance was recorded.
Symptom Burden Assessment
Symptom burden was assessed using the Condensed Memorial Symptom Assessment Scale (CMSAS) which is a brief, well-validated scale that assesses the ‘bothersomeness’ of eleven common physical symptoms on a 5-point Likert scale and the frequency of three psychological symptoms on a 4-point Likert scale.13 Three scores are calculated: CMSAS PHYS assesses overall physical symptom bothersomeness, CMSAS PSYCH assesses psychological symptom frequency, and CMSAS total contains all 14 symptoms. Symptoms which are scored in the upper half of the scale are regarded as more severe.14 We used a modification of the CMSAS which has been used in other studies related to COPD,15,16 adding in two additional physical symptoms (cough and sputum) and one additional psychological symptom (feeling anxious) as previously described,16 given that these symptoms are common in patients with COPD. To preserve the integrity of the CMSAS scores, these additional symptoms were not included in the calculation of any scores.
Other Questionnaires
Overall COPD-specific quality of life was assessed using the COPD Assessment Test (CAT) which is a patient-completed questionnaire assessing the impact of eight COPD symptoms (each scoring 0–5; total score 40) on health status with higher scores representing more severe impact of COPD.17 We used the Hospital Anxiety and Depression Scale (HADS), a 14-item scale, to assess anxiety and depression.18
Statistical Analysis
We described demographic and baseline assessment data as either mean (standard deviation), median (interquartile range), or number (% of total). We compared variables between men and women using unpaired t tests or Mann–Whitney tests for continuous variables, and Fisher’s exact tests for categorical variables. To look for significant predictors of CMSAS total in our population, we performed univariable linear regressions using CMSAS total and CMSAS PHYS as the dependent variable and the following independent variables: age, gender, country of birth, indigenous status, level of education, living arrangements, previous pulmonary rehabilitation, smoking status, FEV1 (% predicted), FVC (% predicted), 6-minute walk distance (6MWD), CAT, HADS-Anxiety (HADS-A), HADS-Depression (HADS-D), and mMRC (2 or 3 versus 4). All independent variables with univariable p<0.20 were included in a backward elimination multiple regression model. Variables with p<0.05 were included in the final regression model. We assessed for: (1) potential confounders by testing for interactions; (2) collinearity by examining variance inflation factors; and (3) the assumptions of multiple regression by examining residual plots. Data were analysed using Stata/IC 15.1 (StataCorp, TX, USA).
Results
Data were available on 89 patients with COPD, mean age 72.6 (7.7) years, 55% male with an average smoking history of 61.9 pack-years. As a requirement for entry to the breathlessness clinic was a MMRC score ≥2, 52% of patients had MMRC 2 or 3 while 48% had MMRC of 4. Most lived with a carer (70%) and 30% of patients had completed pulmonary rehabilitation in the previous 12 months. Mean FEV1 was 32% predicted consistent with severe COPD. The average CAT score was elevated at 23.2 and median HADS-A was 8 while HADS-D was 6. Other details of subjects are presented in Table 1. In our population, women were younger on average than men (mean age 70.6 versus 74.3 years, respectively), but there was no significant difference in lung function measured as % predicted, BMI, or six-minute walk distance (Table 1). More men had a history of ischaemic heart disease (51% of men compared with 25% of women; p=0.017), but more women had a history of depression (42.5% of women compared with 14.3% of men; p=0.004). There was no difference in the prevalence of chronic asthma, hypertension, cardiac failure, diabetes mellitus, or vascular disease (Table 1).
Table 1.
Summary of Demographics and Clinical Characteristics of Patients
| Total | Male | Female | p | ||||
|---|---|---|---|---|---|---|---|
| n | n | n | |||||
| Demographic factors | |||||||
| Age (years) | 89 | 72.6 (7.7) | 49 | 74.3 (6.5) | 40 | 70.6 (8.6) | 0.02 |
| Born in Australia | 89 | 75 (84.3%) | 49 | 37 (75.5%) | 40 | 38 (95%) | 0.017 |
| Living situation | 89 | 49 | 40 | 0.28 | |||
| Alone | 24 (27.0%) | 10 (20.4%) | 14 (35%) | ||||
| With carer | 62 (69.6%) | 37 (75.5%) | 25 (62.5%) | ||||
| Aged care facility | 3 (3.4%) | 2 (4.1%) | 1 (2.5%) | ||||
| Indigenous status | 89 | 2 (2.3%) | 49 | 0 (0%) | 40 | 2 (5%) | 0.20 |
| Previous pulmonary rehabilitation last year | 89 | 27 (30.3%) | 49 | 16 (32.7%) | 40 | 11 (27.5%) | 0.65 |
| Level of education | 89 | 49 | 40 | 0.94 | |||
| Up to high school | 77 (86.5%) | 42 (85.7%) | 35 (87.5%) | ||||
| Beyond high school | 12 (13.5%) | 7 (14.3%) | 5 (12.5%) | ||||
| Current smoker | 89 | 18 (20.2%) | 49 | 8 (16.3%) | 40 | 10 (25%) | 0.43 |
| Pack-years | 61.9 (38.3) | 67.3 (37.8) | 55.4 (38.4) | 0.15 | |||
| Comorbidities | 89 | 49 | 40 | ||||
| Chronic asthma | 11 (12.3%) | 6 (12.2%) | 5 (12.5%) | 1.0 | |||
| Coronary artery disease | 35 (39.3%) | 25 (51%) | 10 (25%) | 0.017 | |||
| Hypertension | 50 (56.2%) | 30 (61.2%) | 20 (50%) | 0.39 | |||
| Cardiac failure | 15 (16.9%) | 11 (22.4%) | 4 (10%) | 0.16 | |||
| Diabetes mellitus | 23 (25.8%) | 14 (28.6%) | 9 (22.5%) | 0.63 | |||
| Vascular disease | 14 (15.7%) | 7 (14.3%) | 7 (17.5%) | 0.77 | |||
| Depression | 24 (27.0%) | 7 (14.3%) | 17 (42.5%) | 0.004 | |||
| Inhaler medications | 89 | 49 | 40 | 0.37 | |||
| LAMA | 2 (2.2%) | 2 (4.1%) | 0 (0%) | ||||
| LAMA/LABA | 5 (5.6%) | 4 (8.2%) | 1 (2.5%) | ||||
| LABA/ICS | 3 (3.4%) | 1 (2%) | 2 (5%) | ||||
| LAMA/LABA/ICS | 79 (88.8%) | 42 (85.7%) | 37 (92.5%) | ||||
| Clinical measurements | |||||||
| FEV1 (litres) | 76 | 0.83 (0.51–1.23) | 42 | 0.83 (0.57–1.39) | 34 | 0.69 (0.48–1.17) | 0.056 |
| FEV1 (% predicted) | 76 | 32 (22–49) | 42 | 31 (19–48) | 34 | 32 (23–54) | 0.12 |
| FVC (litres) | 76 | 2.2 (1.29–3.46) | 42 | 2.49 (1.57–3.69) | 34 | 1.82 (1.16–2.65) | <0.0001 |
| FVC (% predicted) | 76 | 68 (44–92) | 42 | 67 (48–94) | 34 | 69 (43–89) | 0.83 |
| BMI (kg/m2) | 89 | 25.1 (18.1–36.9) | 49 | 24.7 (18.5–36.4) | 40 | 26.2 (17.4–39.6) | 0.94 |
| 6MWD (metres) | 65 | 318.5 (98.8) | 35 | 311.1 (93.7) | 30 | 327.1 (105.4) | 0.52 |
| Questionnaires | |||||||
| CAT | 89 | 23.2 (6.1) | 49 | 22.4 (6.2) | 40 | 24.1 (5.8) | 0.19 |
| HADS-A | 89 | 8 (2–15) | 49 | 7 (1–15) | 40 | 8 (2.5–14.5) | 0.17 |
| HADS-D | 89 | 6 (2–12) | 49 | 7 (2–12) | 40 | 6 (2–11) | 0.22 |
| mMRC | 89 | 49 | 40 | 0.42 | |||
| 2 | 14 (15.7%) | 10 (20.4%) | 4 (10%) | ||||
| 3 | 32 (36.0%) | 17 (34.7%) | 15 (37.5%) | ||||
| 4 | 43 (48.3%) | 22 (44.9%) | 21 (52.5%) | ||||
| CMSAS physical symptoms | 89 | 49 | 40 | ||||
| Shortness of breath | 3.2 (1.6–4) | 3.2 (1.6–4) | 3.2 (2–4) | 0.30 | |||
| Dry mouth | 1.6 (0–4) | 1.6 (0–3.2) | 2 (0–4) | 0.54 | |||
| Difficulty concentrating | 0 (0–2.4) | 0 (0–2.4) | 0 (0–2.8) | 0.56 | |||
| Difficulty sleeping | 0 (0–4) | 0.8 (0–3.2) | 0 (0–4) | 0.98 | |||
| Feeling drowsy | 0.8 (0–3.2) | 0.8 (0–2.4) | 1.6 (0–3.2) | 0.12 | |||
| Pain | 0.8 (0–4) | 0 (0–3.2) | 1.6 (0–4) | 0.02 | |||
| Lack of energy | 2.4 (0–4) | 2.4 (0–4) | 2.4 (0–4) | 0.87 | |||
| Lack of appetite | 0 (0–3.2) | 0 (0–2.4) | 0 (0–3.6) | 0.39 | |||
| Weight loss | 0 (0–1.6) | 0 (0–1.6) | 0 (0–0.8) | 0.37 | |||
| Constipation | 0 (0–1.6) | 0 (0–1.6) | 0 (0–2) | 0.70 | |||
| Nausea | 0 (0–1.6) | 0 (0–0) | 0 (0–2) | 0.04 | |||
| CMSAS physical total | 1.16 (0.65–2.16) | 1.12 (0.65–1.96) | 1.2 (0.62–2.22) | 0.15 | |||
| CMSAS psychological symptoms | 89 | 49 | 40 | ||||
| Worrying | 2 (0–3) | 2 (0–3) | 2 (0–4) | 0.008 | |||
| Feeling sad | 1 (0–2) | 0 (0–3) | 1.5 (0–3.5) | 0.63 | |||
| CMSAS Psychological total | 1.33 (0–3) | 0.67 (0–2.67) | 2 (0–3.17) | 0.014 | |||
| CMSAS total | 89 | 1.14 (0.63–2) | 49 | 1.04 (0.57–1.93) | 40 | 1.34 (0.8–2.34) | 0.03 |
| CMSAS modified symptoms | 89 | 49 | 40 | ||||
| Cough | 1.6 (0–4) | 1.6 (0–4) | 1.6 (0–4) | 0.64 | |||
| Sputum | 1.6 (0–4) | 1.6 (0–4) | 1.6 (0–4) | 0.53 | |||
| Feeling nervous | 0 (0–3) | 0 (0–3) | 2 (0–4) | 0.011 | |||
| Feeling anxious | 2 (0–4) | 1 (0–3) | 2 (0–4) | 0.005 | |||
Notes: Data are shown as mean (SD) or median (10th and 90th percentiles) for continuous variables, and n (%) for categorical variables of total subjects, males, and females.
Abbreviations: LAMA, long-acting muscarinic antagonist; LABA, long-acting beta-agonists; ICS, inhaled corticosteroids; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; BMI, body-mass index; 6MWD, six-minute walk distance; CAT, COPD assessment test; HADS-A, Hospital Anxiety and Depression Scale – Anxiety; HADS-D, Hospital Anxiety and Depression Scale – Depression; mMRC, modified Medical Research Council Dyspnoea Scale; CMSAS, Condensed Memorial Symptom Assessment Scale.
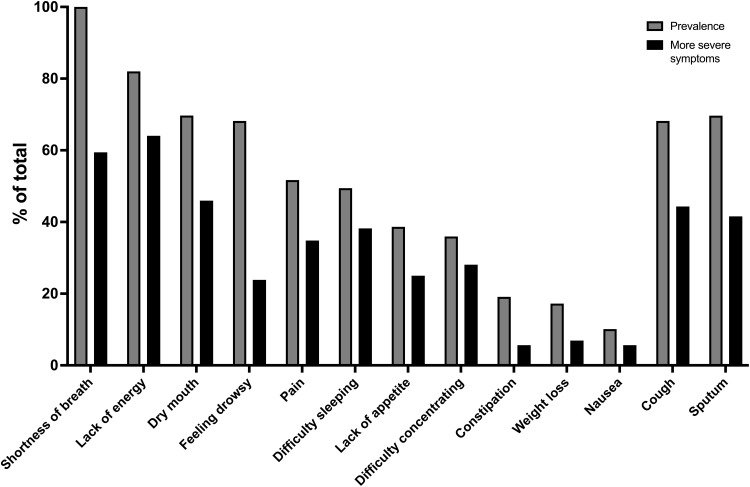
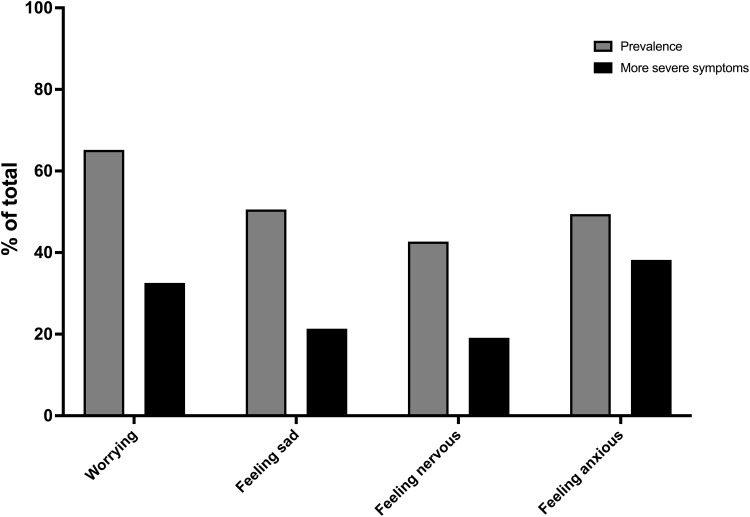
The overall symptom burden was high in our population. Patients reported an average of 8.9 symptoms (SD 2.8) including 6.9 (SD 2.5) CMSAS physical symptoms and 1.6 (SD 1.1) CMSAS psychological symptoms (Table 1). Highly prevalent physical symptoms (Figure 1) included shortness of breath (100%), lack of energy (80%), dry mouth (70%), sputum (70%), and cough and drowsiness (both 67%). Over 50% of patients reported more severe shortness of breath and lack of energy that caused “quite a bit” or “very much” distress. Prevalent reported psychological symptoms (Figure 2) included worrying (65%), feeling anxious (61%), and feeling sad (51%), and nearly 40% of patients reported feeling anxious “frequently” or “almost constantly”. The median CMSAS total score was 1.14, the CMSAS PHYS score was 1.16, and the CMSAS PSYCH score was 1.33 (Table 1).
Figure 1.
Prevalence of reported physical symptoms and more severe symptoms (% of total patients).
Note: “More severe symptoms” defined as symptoms which bothered or distressed patients quite a bit or very much in the past 7 days.
Figure 2.
Prevalence of reported psychological symptoms and more severe symptoms (% of total patients).
Note: “More severe symptoms” defined as symptoms which occurred frequently or almost constantly in the past 7 days.
Of the physical symptoms, women reported being more bothered by pain (p=0.02) and nausea (p=0.04) than men but there was no significant sex difference in the CMSAS PHYS score (p=0.15; Table 1). Women had a higher CMSAS PSYCH total than men (p=0.014) with worrying (p=0.008) and feeling nervous (p=0.011) occurring more frequently than in men, but there was no difference in the frequency of feeling sad (p=0.63). The median CMSAS total score was higher in women compared with men (1.34 versus 1.04, respectively; p=0.03; Table 1). There was no sex difference in the CMSAS modified symptoms of the bothersomeness of cough or sputum (both p>0.53), but women experienced feeling nervous (p=0.011) and anxious (p=0.005) more frequently than men.
Univariable regression showed significant positive relationships between CMSAS total and female sex, being born in Australia, CAT score, HADS-A, HADS-D, and mMRC (all univariable p<0.05; Table 2). Weaker associations were found for lower level of education (high school completion or less; p=0.096) and 6MWD (p=0.053). Using the backward elimination method, the final multiple linear regression model established that female sex (p=0.003), HADS-A (p=0.0001), and HADS-D (p=0.0001) were very strongly positively associated with the CMSAS total score and explained 63% of the variability of the CMSAS total score (F3,85=48.12, p=0.0001; R2=0.63). Females had on average a higher CMSAS total score than males by 0.24 units after adjusting for HADS-A and HADS-D (95% CI 0.082 to 0.399 units). For a unit increase in HADS-A, CMSAS total increased by 0.062 units after adjusting for sex and HADS-D (95% CI 0.039 to 0.085 units), and for each unit increase in HADS-D, CMSAS total increased by 0.056 units after adjustment for HADS-A and sex (95% CI 0.025 to 0.083 units). CMSAS total can be summarised as follows: CMSAS total (units)=0.057+(0.24×sex)+(0.062×HADS-A)+(0.056×HADS-D), where sex is coded 0 for males and 1 for females. There was no evidence of collinearity and examination of residuals confirmed the assumptions of linearity in the final model.
Table 2.
Regression Diagnostic Data with CMSAS Total as the Dependent Variable
| Unadjusted (Univariable) | Adjusted (Multivariable) | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (β) | SE | 95% CI | p | Coefficient (β) | SE | 95% CI | p | |
| Age | −0.007 | 0.008 | −0.023 to 0.009 | 0.37 | ||||
| Sex | ||||||||
| Male | Reference | |||||||
| Female | 0.272 | 0.120 | 0.033 to 0.511 | 0.026 | 0.240 | 0.08 | 0.082 to 0.399 | 0.003 |
| Country of birth | ||||||||
| Australia | Reference | |||||||
| Not Australia | −0.476 | 0.16 | −0.796 to −0.156 | 0.004 | ||||
| Indigenous status | ||||||||
| No | Reference | |||||||
| Yes | 0.037 | 0.415 | −0.79 to 0.86 | 0.93 | ||||
| Level of education | ||||||||
| Up to high school | Reference | |||||||
| Beyond high school | −0.30 | 0.18 | −0.65 to 0.054 | 0.096 | ||||
| Living arrangements | ||||||||
| Alone | Reference | |||||||
| With carer or ACF | 0.018 | 0.14 | −0.26 to 0.29 | 0.98 | ||||
| Previous PR last year | ||||||||
| No | Reference | |||||||
| Yes | −0.01 | 0.13 | −0.28 to 0.26 | 0.94 | ||||
| Smoking status | ||||||||
| Ex-smoker | reference | |||||||
| Current smoker | 0.135 | 0.152 | −0.168 to 0.438 | 0.38 | ||||
| FEV1 (% predicted) | 0.0003 | 0.0056 | −0.011 to 0.011 | 0.96 | ||||
| FVC (% predicted) | −0.0002 | 0.0035 | −0.007 to 0.007 | 0.96 | ||||
| BMI (kg/m2) | 0.0043 | 0.008 | -0.011 to 0.02 | 0.59 | ||||
| 6MWD (metres) | −0.0013 | 0.007 | −0.003 to 0.0002 | 0.053 | ||||
| CAT | 0.049 | 0.009 | 0.031 to 0.066 | 0.0001 | ||||
| HADS-A | 0.096 | 0.009 | 0.077 to 0.115 | 0.0001 | 0.062 | 0.012 | 0.039 to 0.085 | 0.0001 |
| HADS-D | 0.099 | 0.013 | 0.074 to 0.125 | 0.0001 | 0.056 | 0.014 | 0.028 to 0.083 | 0.0001 |
| mMRC | ||||||||
| 2 or 3 | Reference | |||||||
| 4 | 0.29 | 0.119 | 0.056 to 0.529 | 0.016 | ||||
Abbreviations: ACF, aged care facility; PR, pulmonary rehabilitation; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; BMI, body-mass index; 6MWD, six-minute walk distance; CAT, COPD assessment test; HADS-A, Hospital Anxiety and Depression Scale – Anxiety; HADS-D, Hospital Anxiety and Depression Scale – Depression; mMRC, modified Medical Research Council Dyspnoea Scale; CMSAS, Condensed Memorial Symptom Assessment Scale; SE, standard error; 95% CI, 95% confidence interval.
Multiple linear regression using CMSAS PHYS as the dependent variable demonstrated that CAT (p=0.023) and HADS-A and HADS-D (both p=0.001) were significantly positively related in a multivariable model (Table 3); however, the effect of sex was no longer significant.
Table 3.
Regression Diagnostic Data with CMSAS Physical as the Dependent Variable
| Unadjusted (Univariable) | Adjusted (Multivariable) | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (β) | SE | 95% CI | p | Coefficient (β) | SE | 95% CI | p | |
| Age | −0.005 | 0.007 | −0.020 to 0.010 | 0.54 | ||||
| Sex | ||||||||
| Male | Reference | |||||||
| Female | 0.183 | 0.114 | −0.044 to 0.411 | 0.11 | ||||
| Country of birth | ||||||||
| Australia | Reference | |||||||
| Not Australia | −0.387 | 0.153 | −0.691 to −0.083 | 0.013 | ||||
| Indigenous status | ||||||||
| No | Reference | |||||||
| Yes | 0.129 | 0.389 | −0.645 to 0.037 | 0.74 | ||||
| Level of education | ||||||||
| Up to high school | Reference | |||||||
| Beyond high school | −0.293 | 0.166 | −0.623 to 0.054 | 0.081 | ||||
| Living arrangements | ||||||||
| Alone | Reference | |||||||
| With carer or ACF | 0.026 | 0.13 | −0.233 to 0.284 | 0.84 | ||||
| Previous PR | ||||||||
| No | Reference | |||||||
| Yes | 0.034 | 0.126 | −0.216 to 0.283 | 0.78 | ||||
| Smoking status | ||||||||
| Ex-smoker | Reference | |||||||
| Current smoker | 0.148 | 0.143 | −0.136 to 0.431 | 0.30 | ||||
| FEV1 (% predicted) | 0.001 | 0.005 | −0.009 to 0.012 | 0.82 | ||||
| FVC (% predicted) | −0.002 | 0.003 | −0.009 to 0.004 | 0.43 | ||||
| 6MWD (metres) | −0.0016 | 0.007 | −0.003 to −0.0003 | 0.018 | ||||
| CAT | 0.045 | 0.008 | 0.029 to 0.062 | 0.0001 | 0.018 | 0.008 | 0.003 to 0.034 | 0.023 |
| HADS-A | 0.077 | 0.102 | 0.057 to 0.097 | 0.0001 | 0.041 | 0.012 | 0.016 to 0.065 | 0.001 |
| HADS-D | 0.091 | 0.012 | 0.067 to 0.116 | 0.0001 | 0.046 | 0.015 | 0.016 to 0.076 | 0.001 |
| mMRC | ||||||||
| 2 or 3 | Reference | |||||||
| 4 | 0.240 | 0.087 | 0.067 to 0.414 | 0.007 | ||||
Abbreviations: ACF, aged care facility; PR, pulmonary rehabilitation; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; 6MWD, six-minute walk distance; CAT, COPD assessment test; HADS-A, Hospital Anxiety and Depression Scale – Anxiety; HADS-D, Hospital Anxiety and Depression Scale – Depression; mMRC, modified Medical Research Council Dyspnoea Scale; CMSAS, Condensed Memorial Symptom Assessment Scale; SE, standard error; 95% CI, 95% confidence interval.
Discussion
Although other studies have looked at symptom burden in patients with COPD using a multi-dimensional symptom burden assessment tool,2–4,19–21 to our knowledge our study is the first to look at independent predictors of overall symptom burden including the influence of anxiety, depression, and sex. We have demonstrated the high prevalence of multiple physical and psychological symptoms that contribute to overall symptom burden in a group of stable breathless outpatients with COPD. Some of these symptoms may be directly attributable to the pathophysiology of COPD including shortness of breath, cough, and sputum; however, we highlight other symptoms that may be troubling for these patients which may not necessarily be assessed in the setting of an outpatient consultation such as lack of energy, pain, feeling drowsy, difficulty sleeping, dry mouth, worrying, and feeling sad, nervous, and anxious. Overall symptom burden is high with an average of 8.9 symptoms, and more than 50% of patients experiencing more severe shortness of breath and lack of energy.
Our results show that women experience a greater overall symptom burden than men with a CMSAS total score which was almost 30% higher despite similar FEV1 (as % predicted), breathlessness (as per mMRC values), and anxiety or depression at the time of assessment (as per HADS results). The increase in symptom burden in women was mainly driven by being more bothered by pain or nausea, and being more frequently worried or nervous. Of concern, the psychological symptom burden that women experienced was approximately triple that experienced by men as shown by the CMSAS Psych scores (men=0.67; women=2.0; Table 1). The symptoms that are more troublesome for women than men are consistent with the “emotional symptom burden cluster” which includes worrying, feeling nervous, and pain, more than the “gastrointestinal symptom burden cluster” (ie weight loss, constipation, nausea) or the “unwellness symptom burden cluster” (ie feeling drowsy, lack of appetite, difficulty swallowing, or shortness of breath).22
Understanding the Complex Interplay Between Symptoms, Influencing Factors, and Outcomes
The theory of unpleasant symptoms highlights the relationship between a symptom that an individual may be experiencing, influencing factors that cause or modify the symptom, and the effect that the symptom may have on the individual,23 and the complex interactions between influencing factors, multiple symptoms, and the subsequent influence of their impacts on performance on the original influencing factors and the symptoms themselves.24 Symptoms may be described in terms of duration, quality, and intensity but how much distress they cause (ie bothersomeness) is the aspect that has been shown to contribute most to quality of life.24 Influencing factors on COPD symptoms include: (1) physiologic factors such as degree of airflow obstruction, the presence of chronic bronchitis, malnutrition and weight loss, and gender; (2) psychologic state such as mental state and the reaction to illness state; and (3) situational factors such as the presence or absence of social supports, employment, and social isolation. In turn, the impact of the symptom burden on patients with COPD may determine their functional status, cognitive functioning, and physical performance;24 therefore a comprehensive assessment of contributors to symptom burden apart from physiologic factors may help identify areas to improve not only quality of life but also overall performance status.
Use of CMSAS Over MSAS in COPD Assessment
The Memorial Symptom Assessment Scale (MSAS) has only been validated in advanced cancer populations14 and consists of 32 items, but has been used to describe symptom burden in patients with COPD2–4,19–21 although one study made a minor adjustment by replacing the symptom “hair loss” with “weight gain” to be more relevant to COPD.2 However, we utilised the shorter 14-item CMSAS as it has been shown to demonstrate approximately equivalent quality of life information in cancer patients and only takes 2–4 minutes to complete,13 making it a more practical assessment tool in patients with COPD who are acutely unwell15 and also in the clinical consultation setting.25 The MSAS and CMSAS directly ask about the distress or bothersomeness of 11 physical symptoms and frequency of psychological symptoms in the last 7 days; these aspects differs from the COPD assessment test and St George’s Respiratory Questionnaire which mostly assess the impact of physical symptoms on patients’ lives. Therefore, the CMSAS may be a useful tool to quickly identify symptoms that may be most distressing to the patient with COPD and therefore impacting the most on their quality of life.
Sex/Gender Influences on Symptom Burden
Patients with advanced COPD have been shown to have distress greater than the severity of their symptoms,26 which implies that the associated emotional burden is greater for the patient than the actual physiological impact of the symptom.10 For female patients with COPD, whether the increased frequency of feeling worried or nervous compared to males related specifically to their COPD disease, or other influences such as the increased underlying prevalence of depression or social pressures of the female responsibility in the household and their interactions with COPD is not able to be determined from our data and further qualitative research is required.
Several recent review articles have highlighted the increasing awareness and importance of sex and gender differences in lung diseases27–29 including COPD.30 Previous studies have demonstrated that women with COPD have greater levels of anxiety, depression, dyspnoea, and symptom-related quality of life than men for the same or lesser degree of lung impairment.31,32 Therefore, as anxiety and depression are more prevalent among women with COPD, one may conclude that sex/gender is a confounder of the increased symptom burden that patients with COPD bear. However, our data show that female sex is independently associated with increased symptom burden even after adjusting for anxiety and depression, which may therefore relate to sex differences (ie biological differences between males and females including physiological, hormonal, or functional differences) or gender differences (ie social factors that include cultural, behavioural, or individual self-identity that are defined by a society or culture).29
Anxiety and Depression Influences on Symptom Burden
Anxiety and depression are important comorbidities in COPD33 and are strongly associated with poorer quality of life and health status, more than spirometric values.34 Patients with chronic medical illnesses including COPD report more symptoms if they have comorbid depression or anxiety than those without when disease severity is controlled.35 We have demonstrated independent linear relationships for both anxiety and depression and total symptom burden as measured by the CMSAS, demonstrating that a reduction in either or both may result in improvement in overall symptoms. Although pharmacological interventions may be considered for anxiety and depression in patients with COPD to alleviate symptom burden, there is inconclusive evidence of their benefit at this stage and further trials are needed.36,37 On the other hand, meta-analysis data from pulmonary rehabilitation trials have shown a moderate improvement in anxiety symptoms and a large improvement in depression symptoms,38 highlighting the importance of this intervention in patients with COPD. Recent data from a moderate to severe COPD cohort referred to a pulmonary rehabilitation programme demonstrated that one in three patients had both anxiety and depression and that the pulmonary rehabilitation programme resulted in a larger improvement in quality of life, and reduction in dyspnoea and stress than those with only anxiety or depression alone, or with neither.39 The significant benefits on symptom burden from pulmonary rehabilitation may be due to its effect on multiple areas of the unpleasant symptom theory24 including improvement in physiology (eg exercise capacity, muscle function), psychological factors (eg social support through the programme, interactions with healthcare professionals), and situational factors (eg less isolation, increased independence) but which components of the rehabilitation programme provide these benefits remain unclear.40
Finally, a systematic review and meta-analysis of holistic services for patients with breathlessness due to a diverse variety of advanced disease has shown a reduction in distress due to breathlessness and a significant reduction in depression but not anxiety.41 Whether a novel non-pharmacological breathlessness service like the WBS specifically for patients with COPD may reduce anxiety, depression, and overall symptom burden in addition to breathlessness is not yet known and further RCT data are required.
Conclusion
Patients attending the Westmead Breathlessness Service with moderate to severe COPD reported a wide range of non-respiratory symptoms beyond the classic COPD symptoms of breathlessness, cough, and sputum and there is a high symptom burden within this population. Although having similar baseline demographics, female patients with COPD reported the bothersomeness of pain and nausea, and frequency of psychological symptoms more often than males. There is a need to further identify and understand sex differences for COPD symptoms and to study interventions that reduce anxiety and depression to see if they can significantly reduce overall symptom burden in this population.
Acknowledgments
The authors thank Ms Jan Gesling for assistance in data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Venkatesan P. GOLD COPD report: 2023 update. Lancet Respir Med. 2023;11(1):18. doi: 10.1016/S2213-2600(22)00494-5 [DOI] [PubMed] [Google Scholar]
- 2.Christensen VL, Holm AM, Cooper B, et al. Differences in Symptom Burden Among Patients With Moderate, Severe, or Very Severe Chronic Obstructive Pulmonary Disease. J Pain Symptom Manage. 2016;51(5):849–859. doi: 10.1016/j.jpainsymman.2015.12.324 [DOI] [PubMed] [Google Scholar]
- 3.Melhem O, Savage E, Al Hmaimat N, et al. Symptom burden and functional performance in patients with chronic obstructive pulmonary disease. Appl Nurs Res. 2021;62:151510. doi: 10.1016/j.apnr.2021.151510 [DOI] [PubMed] [Google Scholar]
- 4.Melhem O, Savage E, Lehane E. Symptom burden in patients with chronic obstructive pulmonary disease. Appl Nurs Res. 2021;57:151389. doi: 10.1016/j.apnr.2020.151389 [DOI] [PubMed] [Google Scholar]
- 5.Mihaltan F, Adir Y, Antczak A, et al. Importance of the relationship between symptoms and self-reported physical activity level in stable COPD based on the results from the SPACE study. Respir Res. 2019;20(1):89. doi: 10.1186/s12931-019-1053-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18(1):67. doi: 10.1186/s12931-017-0548-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleeland CS. Symptom Burden: multiple Symptoms and Their Impact as Patient-Reported Outcomes. JNCI Monographs. 2007;2007(37):16–21. doi: 10.1093/jncimonographs/lgm005 [DOI] [PubMed] [Google Scholar]
- 8.Habraken JM, ter Riet G, Gore JM, et al. Health-related quality of life in end-stage COPD and lung cancer patients. J Pain Symptom Manage. 2009;37(6):973–981. doi: 10.1016/j.jpainsymman.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 9.Walke LM, Gallo WT, Tinetti ME, et al. The burden of symptoms among community-dwelling older persons with advanced chronic disease. Arch Intern Med. 2004;164(21):2321–2324. doi: 10.1001/archinte.164.21.2321 [DOI] [PubMed] [Google Scholar]
- 10.Joshi M, Joshi A, Bartter T. Symptom burden in chronic obstructive pulmonary disease and cancer. Curr Opin Pulm Med. 2012;18(2):97–103. doi: 10.1097/MCP.0b013e32834fa84c [DOI] [PubMed] [Google Scholar]
- 11.Emanuel L. Relief of suffering is the business of every discipline. Arch Intern Med. 2006;166(2):149–150. doi: 10.1001/archinte.166.2.149 [DOI] [PubMed] [Google Scholar]
- 12.Smith TA, Roberts MM, Cho JG, et al. Protocol for a Single-Blind, Randomized, Parallel-Group Study of a Nonpharmacological Integrated Care Intervention to Reduce the Impact of Breathlessness in Patients with Chronic Obstructive Pulmonary Disease. Palliat Med Rep. 2020;1(1):296–306. doi: 10.1089/pmr.2020.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang VT, Hwang SS, Kasimis B, et al. Shorter symptom assessment instruments: the Condensed Memorial Symptom Assessment Scale (CMSAS). Cancer Invest. 2004;22(4):526–536. doi: 10.1081/CNV-200026487 [DOI] [PubMed] [Google Scholar]
- 14.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326–1336. doi: 10.1016/0959-8049(94)90182-1 [DOI] [PubMed] [Google Scholar]
- 15.Smith TA, Ingham JM, Jenkins CR. Respiratory Failure, Noninvasive Ventilation, and Symptom Burden: an Observational Study. J Pain Symptom Manage. 2019;57(2):282–289 e1. doi: 10.1016/j.jpainsymman.2018.10.505 [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan M, Swami V, Roberts M, Cho J, Wheatley J, Smith T. Symptom Burden for Inpatients with Acute Exacerbations of COPD. Respirology. 2019;24(1):127. doi: 10.1111/resp.13437 [DOI] [PubMed] [Google Scholar]
- 17.Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 19.Eckerblad J, Tödt K, Jakobsson P, et al. Symptom burden in stable COPD patients with moderate or severe airflow limitation. Heart Lung. 2014;43(4):351–357. doi: 10.1016/j.hrtlng.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 20.Jablonski A, Gift A, Cook KE. Symptom assessment of patients with chronic obstructive pulmonary disease. West J Nurs Res. 2007;29(7):845–863. doi: 10.1177/0193945906296547 [DOI] [PubMed] [Google Scholar]
- 21.Park SK, Stotts NA, Douglas MK, et al. Symptoms and functional performance in Korean immigrants with asthma or chronic obstructive pulmonary disease. Heart Lung. 2012;41(3):226–237. doi: 10.1016/j.hrtlng.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 22.Kenne Sarenmalm E, Browall M, Gaston-Johansson F. Symptom Burden Clusters: a Challenge for Targeted Symptom Management. A Longitudinal Study Examining Symptom Burden Clusters in Breast Cancer. J Pain Symptom Manage. 2014;47(4):731–741. doi: 10.1016/j.jpainsymman.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 23.Lenz ER, Suppe F, Gift AG, et al. Collaborative development of middle-range nursing theories: toward a theory of unpleasant symptoms. ANS Adv Nurs Sci. 1995;17(3):1–13. doi: 10.1097/00012272-199503000-00003 [DOI] [PubMed] [Google Scholar]
- 24.Lenz ER, Pugh LC, Milligan RA, et al. The middle-range theory of unpleasant symptoms: an update. ANS Adv Nurs Sci. 1997;19(3):14–27. doi: 10.1097/00012272-199703000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Fasolino T, O’Hara S. Assessing SPACES in Patients with Chronic Obstructive Pulmonary Disease Helps Identify Unmet Needs. J Palliat Med. 2023;26(1):149–152. doi: 10.1089/jpm.2022.0178 [DOI] [PubMed] [Google Scholar]
- 26.Blinderman CD, Homel P, Billings JA, et al. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage. 2009;38(1):115–123. doi: 10.1016/j.jpainsymman.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 27.Sodhi A, Pisani M, Glassberg MK, et al. Sex and Gender in Lung Disease and Sleep Disorders: a State-of-The-Art Review. Chest. 2022;162(3):647–658. doi: 10.1016/j.chest.2022.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somayaji R, Chalmers JD. Just breathe: a review of sex and gender in chronic lung disease. Eur Respir Rev. 2022;31(163):210111. doi: 10.1183/16000617.0111-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silveyra P, Fuentes N, Rodriguez Bauza DE. Sex and Gender Differences in Lung Disease. Adv Exp Med Biol. 2021;1304:227–258. doi: 10.1007/978-3-030-68748-9_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gut-Gobert C, Cavaillès A, Dixmier A, et al. Women and COPD: do we need more evidence? Eur Respir Rev. 2019;28(151):180055. doi: 10.1183/16000617.0055-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Marco F, Verga M, Reggente M, et al. Anxiety and depression in COPD patients: the roles of gender and disease severity. Respir Med. 2006;100(10):1767–1774. doi: 10.1016/j.rmed.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 32.Raherison C, Tillie-Leblond I, Prudhomme A, et al. Clinical characteristics and quality of life in women with COPD: an observational study. BMC Womens Health. 2014;14(1):31. doi: 10.1186/1472-6874-14-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Diagnosis, Management and Prevention of COPD. 2023 Report. Available from: www.goldcopd.org. Accessed March 24, 2023.
- 34.Tsiligianni I, Kocks J, Tzanakis N, et al. Factors that influence disease-specific quality of life or health status in patients with COPD: a review and meta-analysis of Pearson correlations. Prim Care Respir J. 2011;20(3):257–268. doi: 10.4104/pcrj.2011.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry. 2007;29(2):147–155. doi: 10.1016/j.genhosppsych.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 36.Usmani ZA, Carson KV, Cheng JN, et al. Pharmacological interventions for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;11:Cd008483. [DOI] [PubMed] [Google Scholar]
- 37.Pumar MI, Gray CR, Walsh JR, et al. Anxiety and depression-Important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615–1631. doi: 10.3978/j.issn.2072-1439.2014.09.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon CS, Waller JW, Cook RM, et al. Effect of Pulmonary Rehabilitation on Symptoms of Anxiety and Depression in COPD: a Systematic Review and Meta-Analysis. Chest. 2019;156(1):80–91. doi: 10.1016/j.chest.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 39.Yohannes AM, Casaburi R, Dryden S, et al. The effectiveness of pulmonary rehabilitation on chronic obstructive pulmonary disease patients with concurrent presence of comorbid depression and anxiety. Respir Med. 2022;197:106850. doi: 10.1016/j.rmed.2022.106850 [DOI] [PubMed] [Google Scholar]
- 40.Coventry PA. Does pulmonary rehabilitation reduce anxiety and depression in chronic obstructive pulmonary disease? Curr Opin Pulm Med. 2009;15(2):143–149. doi: 10.1097/MCP.0b013e3283218318 [DOI] [PubMed] [Google Scholar]
- 41.Brighton LJ, Miller S, Farquhar M, et al. Holistic services for people with advanced disease and chronic breathlessness: a systematic review and meta-analysis. Thorax. 2019;74(3):270–281. doi: 10.1136/thoraxjnl-2018-211589 [DOI] [PMC free article] [PubMed] [Google Scholar]