Abstract
Immunotherapy has ignited hope to cure paediatric solid tumours that resist traditional therapies. Among the most promising methods is adoptive cell therapy (ACT). Particularly, ACT using T cells equipped with chimeric antigen receptors (CARs) has moved into the spotlight in clinical studies. However, the efficacy of ACT is challenged by ACT-intrinsic factors, like lack of activation or T cell exhaustion, as well as immune evasion strategies of paediatric solid tumours, such as their highly immunosuppressive microenvironment. Novel strategies, including ACT using innate-like lymphocytes, innovative cell engineering techniques, and ACT combination therapies, are being developed and will be crucial to overcome these challenges. Here, we discuss the main classes of ACT for the treatment of paediatric extracranial solid tumours, reflect on the available preclinical and clinical evidence supporting promising strategies, and address the challenges that ACT is still facing. Ultimately, we highlight state-of-the-art developments and opportunities for new therapeutic options, which hold great potential for improving outcomes in this challenging patient population.
Keywords: Paediatric tumour, Adoptive cell therapy, CAR-T cells, NK cells, NKT cells, γδ T cells, TCR-T cells
Highlights
-
•
Different paediatric solid tumours share targetable common antigens.
-
•
Expanded and/or engineered αβ T, γδ T, NK, and NKT cells are explored ACT sources.
-
•
Pronounced immunosuppression hinders ACT efficacy.
-
•
New engineering techniques and combination therapies could bypass immunosuppression.
-
•
A disparity exists between target identification and their clinical implementation.
1. Introduction
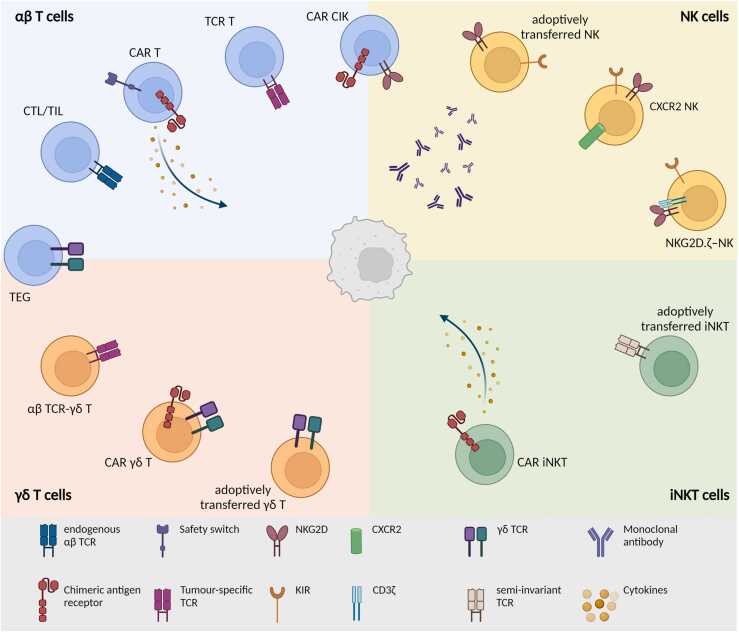
Immunotherapy is revolutionising the field of adult oncology, leading to improved survival in some poor-prognosis tumours [1]. It also holds great promises for paediatric solid tumour treatment, where immune cell infiltration is associated with a favourable prognosis [2], [3], [4]. However, a significant clinical benefit of immunotherapy is not yet achieved. The exception is the anti-GD2 monoclonal antibody (mAb) for high-risk neuroblastoma, integrated in the standard of care, which has considerably improved patient outcome [5], [6]. Although antibody-based therapies are the most explored approach for immunotherapy [7], their efficacy is still poor in childhood cancers [8], [9], [10]. Cellular immunotherapies offer a highly personalised therapeutic option with the benefit of a long-term immune protection and may provide an option for patients who still develop disease progression upon conventional care. To enhance tumour recognition and effector function of immune cells, ACT uses patient- or donor-derived cells that are activated, expanded ex vivo, and reinfused into the patient, with or without prior engineering. αβ T cells are the most explored cell subset for paediatric solid tumour ACT. Other, more innate/innate-like subsets, such as natural killer (NK), invariant NKT (iNKT), and γδ T cells, have also been used (Fig. 1).
Fig. 1.
Summary of the ACT strategies currently used for the treatment of paediatric extracranial solid tumours: conventional αβ T cells, NK, iNKT, and γδ T cells are currently evaluated as an adoptive cell strategy for different paediatric solid tumours. Briefly, αβ T cells can be (1) expanded and reinfused in the patient (CTL/TIL); (2) engineered to express a CAR, also in combination with safety switches and cytokine expression; (3) engineered with a tumour-specific TCR; and (4) engineered with a CAR and trained to express NK-cell markers. NK cells can be (1) adoptively transferred in the patients, often in combination with tumour-targeting mAb; (2) modified to express homing receptors; or (3) CAR-like constructs. iNKT cells are either (1) adoptively transferred or (2) engineered with a CAR, also in combination with cytokine production. Lastly, γδ T cells are currently (1) adoptively transferred, engineered (2) with a CAR, or (3) with an αβ TCR, and (4) their γδ TCR could be transduced in αβ T cells to generate the so-called TEG cells. CAR, chimeric antigen receptors; CIK, cytokine-induced killer; CTL, cytotoxic T lymphocytes; iNKT, invariant NKT; NK, natural killer; TIL, tumour-infiltrating lymphocytes.
In paediatric oncology, ACT has proven remarkably successful in treating haematological malignancies [11], [12] but still meets challenges in solid tumours [13], [14]. The limited success is related to (1) an immunosuppressive tumour microenvironment (TME), characterised by suppressive immune cells such as regulatory T cells, tumour-associated macrophages (TAM), myeloid-derived suppressor cells (MDSC), and their production of immunosuppressive mediators [15], [16]; (2) the paucity of tumour neoantigens and/or an impaired tumour antigen presentation [17], [18]; (3) a low mutational burden [19], [20]; and (4) poor trafficking to the tumour site [21], [22].
In this review, we discuss the current progress in ACT for paediatric extracranial solid tumours. We address the different immune cell subsets currently used for ACT, the state-of-the-art approaches used to overcome challenges, and future perspectives to improve ACT efficacy while maintaining safety.
2. Conventional αβ T cells
2.1. αβ T cells isolated from tumour (TILs)
One widely explored ACT uses tumour-infiltrating lymphocytes (TILs) isolated from patients’ tumour. Patients possess T cell clones that could potentially recognise tumour antigens, but their amount is limited, and they fail to be activated because of the immunosuppressive TME [23]. To reactivate them, isolated T cells are expanded ex vivo through rapid expansion protocols, tested for tumour reactivity, and reinfused into patients [23]. However, this strategy holds some limitations, including limited expansion of the cells after reinfusion and the reliance on MHC/HLA expression for antigen recognition, expressed at low levels in many paediatric solid tumours [18].
TIL therapy was pioneered in advanced melanoma where it demonstrated striking efficacy, resulting in long-lasting cure in some patients [23], [24]. It has also demonstrated its efficacy for adult sarcoma (NCT04052334, NCT03725605, NCT03935893) and new studies are recruiting young adults in their patient cohort (NCT03449108). TILs have been efficiently isolated and expanded from neuroblastoma (the most common extracranial solid tumour in children [25]) in the presence of high-dose IL-2 [26], but evidence for TIL therapy efficacy in other paediatric solid tumours is limited. Since TIL efficacy in these tumours may be hampered by the low neoantigen and MHC-1 expression, their efficacy could be improved by transduction with a CAR (CAR-TILs). This strategy could combine the natural tropism of the TILs to the tumour with enhanced tumour reactivity [26].
2.2. Antigen-specific αβ T cells isolated from peripheral blood
Tumour-reactive cytotoxic T lymphocytes (CTLs) can also be isolated from the patients’ peripheral blood. CTL therapy for paediatric extracranial solid tumours has been so far used to target Epstein–Barr virus (EBV)-infected carcinoma cells, cancer/testis antigens (CTAs), and other solid tumour antigens, which we will discuss here.
2.2.1. EBV
CTLs have been used for the treatment of EBV-associated paediatric nasopharyngeal carcinoma and are currently evaluated in different clinical trials (Table 1). Data from two independent EBV-CTL clinical trials (NCT00078546, NCT00609219) suggest a benefit, with 2/7 evaluable paediatric patients achieving complete responses (CR), 2/7 partial responses (PR), 2/7 progressive disease (PD), and 1/7 no response with no/limited toxicity. Another trial (NCT01498484) reported a mean overall response through 12 months of 64%. Of note, these clinical benefits are more pronounced in patients with local disease [27], [28], [29], [30], [31]. Due to their success in trials, EBV-CTLs are already suggested for clinical implementation for refractory/relapsed disease forms [32].
Table 1.
Summary of clinical trials using CTL/TIL for paediatric extracranial solid tumours (updated June 2023).
| NCT | Compound | Indication | Age group | Sponsor | Status | Phase | Results |
|---|---|---|---|---|---|---|---|
| NCT00953420 | Carboplatin and docetaxel + EBV-CTL | Nasopharyngeal carcinoma | 10 years and older | Baylor College of Medicine | Completed | 2 | Not available |
| NCT00609219 | EBV-CTL | Head and neck cancer | Children, adults, older adults | Baylor College of Medicine | Completed | 1 | Five out of 10 patients <18 years. Of these five: one CR, two PR, one no response, one NA. No/limited toxicity. |
| NCT01498484 | EBV-CTL | EBV-induced lymphomas, EBV-associated malignancies | Children, adults, older adults | Atara Biotherapeutics | Completed | 2 | Mixed ORR at 65.3 months depending on trial arm (from 14.3% to 68%). OR through 12 months ∼64% (mean of all arms). Limited serious adverse events. |
| NCT00078546 | EBV-CTL + anti-CD45 Mab | Nasopharyngeal cancer and EBV infections | Children, adults, older adults | Baylor College of Medicine | Completed | 1 | Three out of eight patients <18 years. Of these three: one CR >24 months and two PD. |
| NCT01447056 | LMP CTL | Nasopharyngeal carcinoma and others | Children, adults, older adults | Baylor College of Medicine | Completed | 1 | Not available |
| NCT00516087 | LMP1- and LMP2-specific CTLs | Nasopharyngeal carcinoma | Children, adults, older adults | Baylor College of Medicine | Completed | 1 | Not available |
| NCT02287311 | MABEL (EBV-CTL) | Nasopharyngeal carcinoma and others | Children, adults, older adults | Baylor College of Medicine | Recruiting | 1 | Not available |
| NCT02822495 | Tabelecleucel (EBV-CTL) | Nasopharyngeal carcinoma, EBV infections, EBV+ associated lymphoma, others |
Children, adults, older adults | Atara Biotherapeutics | Temporarily not available | NA | Not available |
| NCT04554914 | Tabelecleucel (EBV-CTL) | EBV-associated diseases | Children, adults, older adults | Atara Biotherapeutics | Recruiting | 2 | Not available |
| NCT02065362 | TGF-beta resistant EBV-CTL | Nasopharyngeal carcinoma | Children, adults, older adults | Baylor College of Medicine | Active, not recruiting | 1 | Not available |
| NCT03449108 | TILs (LN-145 or LN-145-S1) | Sarcomas and others | 16–70 years | M.D. Anderson Cancer Center | Recruiting | 2 | Not available |
| NCT02239861 | Tumour-associated antigen (TAA)-CTLs | Rhabdomyosarcoma | 2–80 years | Baylor College of Medicine | Completed | 1 | Not available |
| NCT02789228 | TAA-CTLs | Ewing sarcoma, Wilms tumour, neuroblastoma, rhabdomyosarcoma, soft tissue sarcomas, osteosarcoma, adenocarcinoma, and oesophageal carcinoma and renal cell carcinoma. | 6 months–60 years | Children's National Research Institute | Active, not recruiting | 1 | 11 out of 15 therapy responders. No disease progression in six out of 11 responders. No DLT and no infusion-related adverse events. |
See the ‘Clinical trial search’ section in supplementary methods for more information regarding search terms.
CR, complete responses; CTL, cytotoxic T lymphocytes; DLT, dose limiting toxicity; EBV, Epstein–Barr virus; OR, overall response; ORR, objective response rate; PD, progressive disease; PR, partial responses; TIL, tumour-infiltrating lymphocytes.
2.2.2. CTAs and other solid tumour antigens
CTAs are a large family of antigens that are only expressed during embryonic development and in adult male germ cells but are (re-)expressed by several malignancies, which makes them attractive targets for ACT [33]. PRAME, NY-ESO-1, MAGE, and SSX are CTAs that could be suitable immunotherapy targets for paediatric solid malignancies, especially for neuroblastoma and sarcomas [34], [35], [36], [37]. Additional immunotherapy targetable non-CTA antigens are WT1, a typical antigen for Wilms tumours [38], [39] and survivin, an antiapoptotic protein expressed by many malignancies [25].
PRAME-specific CTL killing activity was increased in vitro with sarcoma and neuroblastoma cell lines, by combining them with 5-aza-2′-deoxycytidine (DAC) administration [40]. DAC leads to DNA demethylation and reactivation of MHC gene expression and, consequentially, to increased antigen presentation on tumour cells [40]. CTL activity against PRAME, WT1, and survivin is being evaluated in an ongoing phase I clinical trial for relapsed/refractory solid tumours (NCT02789228). To date, this trial showed safety and efficacy, with 11/15 patients responding to the therapy but still undergoing PD, and 6/11 responders experiencing no disease progression at a median of 13.9 months [41]. Another clinical trial is currently evaluating the efficacy of PRAME, NY-ESO-1, MAGE-A4, survivin, and SSX-CTL for the treatment of rhabdomyosarcoma (RMS) (NCT02239861), with no data posted yet.
CTL and TIL therapies represent a valuable tool for the treatment of these malignancies, also given the ease of cell manufacturing compared to other T cell engineering techniques and limited side effects. However, the presence of tumour-reactive T cells is limited in paediatric solid tumours [42], due to often low/absent MHC expression [18] and low mutational burden [19]. Strategies to increase tumoral MHC expression by pharmaceutical intervention are currently being explored and could greatly benefit future CTL/TIL therapies [43].
2.3. TCR-engineered T cells (TCR-T)
Both CTL and TIL therapies rely on naturally occurring T cell populations in the cancer patients, which are not always sufficiently present. This can be circumvented by artificially generating T cells with tumour-specific TCRs but will not overcome the problem of absent MHC. Still, with novel strategies to enhance tumoral MHC expression [44] and novel neoantigens being identified [45], [46], TCR-T remain a valuable option. Crucial for TCR-T therapy is the selection of highly tumour-specific antigens to balance maximum tumour killing with minimal toxicities.
2.3.1. Cancer testis antigens
As already mentioned, CTAs are attractive targets for ACT. NY-ESO-1 TCR-T cells have been exploited in clinical trials for the treatment of paediatric and adult synovial cell sarcomas [47]. In one trial, 4/6 adult patients treated with NY-ESO-1-engineered T cells + IL-2, reported significant clinical responses with one patient achieving 18-months PR (NCT00670748) [48]. However, in a trial in paediatric and adult patients with synovial sarcoma, CR was observed only in 1/44 patients and PR in 14/44 patients (NCT01343043). This trial suggested that efficacy was dependent on persistent stem-cell memory (TSCM) and central memory (TCM) T cell subsets in patients (NCT01343043) [49]. TSCM can replenish the TCM pool, which can quickly convert into effector cells upon encountering NY-ESO-1 antigen, favouring rapid tumour cell clearance [50], [51]. Preclinically, T cells targeting NY-ESO-1 have demonstrated their efficacy in neuroblastoma models, controlling both localised and disseminated tumour progression [52].
Other CTAs suitable for neuroblastoma and sarcoma are PRAME and MAGE-A [34], [35], [36], [37]. MAGE-A TCR-T are currently being evaluated only in a clinical trial for adult malignancies, such as synovial sarcoma (NCT03132922).
Despite the attractiveness of CTAs for ACT, current trials targeting NY-ESO-1 for paediatric solid tumours have yet to demonstrate a clear therapeutic benefit (Table 2). Studies exploiting other CTAs or combination therapies with TME-targeting compounds represent some possibilities to increase efficacy.
Table 2.
Summary of clinical trials using TCR-engineered αβT cells for paediatric extracranial solid tumours (updated June 2023).
| NCT | Compound | Indication | Age group | Sponsor | Status | Phase | Results |
|---|---|---|---|---|---|---|---|
| NCT03462316 | NY-ESO-1 TCR | Sarcomas | 14–70 years | Sun Yat-sen University | Active, not recruiting | 1 | Not available |
| NCT02775292 | NY-ESO-1 TCR + vaccine therapy + nivolumab | Adult and childhood solid neoplasms | 16 years and older | Jonsson Comprehensive Cancer Center | Completed | 1 | Not available |
| NCT02650986 | NY-ESO-1 TCR/dnTGFbetaRII transgenic T cells | Synovial sarcoma and others | 12 years and older | Roswell Park Cancer Institute | Active, not recruiting | 1, 2 | Only one patient <18 years old. Only toxicity results available. High-grade CRS in four out of 15 patients. |
| NCT01343043 | NY-ESO-1-TCR | Neoplasms | 4 years and older | GlaxoSmithKline | Completed | 1 | CR in one out of 44 patients. 14 PR, 16 SD, and three PD. High-grade CRS in five out of 50 patients. |
| NCT02457650 | NY-ESO-1-TCR | Neuroblastoma, synovial sarcoma, others | 1 year and older | Shenzhen Second People’s Hospital | Unknown | 1 | Data available for four adult patients. One PR, one SD, and two NR. Grade 1–3 adverse events |
| NCT03240861 | NY-ESO-1-TCR | Sarcoma, other NY-ESO-1+ malignancies | 16 years and older | Jonsson Comprehensive Cancer Center | Recruiting | 1 | Not available |
| NCT02869217 | NY-ESO-TCR | Synovial sarcoma and others | 16 years and older | University Health Network, Toronto | Active, not recruiting | 1 | Two PR, five SD, one PD, and one pending. CRS 1/2 in five out of nine patients. |
See the ‘Clinical trial search’ section in supplementary methods for more information regarding search terms.
CR, complete responses; CRS, cytokine release syndrome; NR, non-responder; PD, progressive disease; PR, partial responses; SD, stable disease.
2.3.2. Other antigens
Preclinical data suggest that pappalysin-1 (PAPPA) and six-transmembrane epithelial antigen of prostate-1 (STEAP1) are potential targets for paediatric Ewing sarcomas. Ewing sarcoma xenograft models treated with either PAPPA [53] or STEAP1 TCR-T cells [54] exhibited a significant reduction in tumour growth. For paediatric nasopharyngeal carcinoma, TCR-T can be engineered against the EBV antigens latent membrane protein 1 and 2 (LMP1/2). Such EBV-LMP2 TCR-T lysed LMP2+ target cells [55] and controlled tumour growth in mice bearing LMP2+ human epithelial tumours [56]. EBV TCR-T are currently evaluated in clinical trials for adult nasopharyngeal carcinomas with no results posted yet (NCT03648697, NCT03925896, NCT04509726).
Overall, TCR-T represent a promising tool for the treatment of paediatric extracranial solid tumours, but further studies are needed to explore more targets and to potentiate current cell products. Furthermore, low-MHC expression limits the usage of TCR-T and contributes to the popularity of CAR-engineered αβ T cells (CAR-T), which are currently the most investigated ACT product for paediatric extracranial solid malignancies.
2.4. CAR-engineered αβ T cells (CAR-T)
CAR-T recognise tumour surface antigens MHC-independently, which circumvents MHC restriction but limits the number of available targets to surface molecules. First-generation CAR-T are engineered with a receptor composed of an extracellular antibody-derived antigen-recognising part (single-chain variable fragment [scFv]), a transmembrane portion, and an intracellular part composed of a CD3ζ domain responsible for signal transduction [57]. They displayed limited persistence because of a lack of costimulation [58]. To improve this, second- and third-generation CAR-T have been designed with one or more costimulatory domains like 4-1BB, CD28, and OX-40 [59]. Lastly, new and advanced design strategies and combinatorial approaches have been implemented to further improve CAR-T efficacy, persistence, proliferation, and safety, such as the transduction with cytokines and the addition of safety switches [60], [61]. These innovative strategies also try to overcome the pronounced immunosuppressive TME present in many paediatric solid tumorous, which can impair CAR-T antitumour activity.
2.4.1. First-generation CAR-T
The first CAR-T tested in a clinical trial for paediatric solid tumours were EBV-specific CTLs expressing a GD2-CAR in neuroblastoma patients (NCT00085930) [62], [63]. GD2 is a diganglioside widely expressed in a variety of paediatric tumours [64], [65], [66]. In this trial, 3/11 patients with active disease at the time of CAR-T infusion achieved CR, 1/11 a PR, and 7/11 exhibited either stable or PD. Most importantly, patient survival was strongly associated with CAR-T persistence in the circulation [62], [63].
First-generation CAR-T against cell-adhesion molecule L1CAM (CD171), associated with the poor prognosis of several solid tumours [67], were evaluated in a pilot study for neuroblastoma patients. In total, 1/6 patients experienced prolonged survival of 4.5 years after the first infusion; 1/6 achieved CR after additional chemotherapy; 1/6 displayed a PR with a survival of 1.5 years after the first infusion; and 3/6 suffered PD [68].
First-generation CAR-T against the foetal acetylcholine receptor, whose expression is increased in residual cells of chemotherapy-treated RMS patients, could lysate antigen-expressing tumour cells in vitro [69], but has not yet been evaluated in vivo or in patients.
In conclusion, first-generation CAR-T demonstrated a proof-of-concept in patients and paved the way for studies that focused on improving CAR-T persistence and efficacy by the addition of costimulatory domains, leading to the advent of second-generation CAR-T.
2.4.2. Second-generation CAR-T
Many of the studies investigating second-generation CAR-T sought to identify the costimulatory domain that was most beneficial for CAR-T activation, persistence, and resistance to exhaustion. Second-generation CAR-T have been generated against a wide range of target proteins on paediatric tumours, including GD2, B7-H3, L1CAM, human epidermal growth factor receptor 2 (HER2), glypican 2 and 3 (GPC2, GPC3). Some of these CAR-T are already implemented in clinical trials (Table 3).
Table 3.
Summary of clinical trials using CAR-engineered αβT cells for paediatric extracranial solid tumours (updated June 2023).
| NCT | Compound | Indication | Age group | Sponsor | Status | Phase | Results |
|---|---|---|---|---|---|---|---|
| NCT02919046 | GD2 CAR-T | R/R neuroblastoma | 1–14 years | Sinobioway Cell Therapy Co., Ltd., China | Unknown | NA | Not available |
| NCT02765243 | 4SCAR-GD2 | R/R neuroblastoma | 1–14 years | Zhujiang Hospital, China | Suspended | 1 | Tumour regression in 2/34 patients. After 1 year, 38% patients SD, 15% PR, 47% disease progression. No grade 3, 4 CRS. |
| NCT02992210 | Solid tumour | 1–65 years | Shenzhen Geno-Immune Medical Institute | Unknown | 1,2 | ||
| NCT03373097 | GD2-CART01, containing suicide gene | R/R neuroblastoma, sarcomas, other GD2+ tumours | 1–25 years | Bambino Gesù Hospital and Research Institute, Italy | Recruiting | 1,2 | 9/27 CR, 8/27 PR, 5/27 SD, 5/27 no response. 60% 3-year overall survival and 36% event-free survival. 1/27 grade 3 CRS. |
| NCT04539366 | GD2 CAR-T | R/R neuroblastoma, osteosarcoma | 0–35 years | National Cancer Institute (NCI), USA | Recruiting | 1 | Not available |
| NCT04637503 | GD2, PSMA, and CD276 CAR-T cells | Neuroblastoma | 1–65 years | Shenzhen Geno-Immune Medical Institute, China | Recruiting | 1,2 | Not available |
| NCT02311621 | CD171-specific CAR-T cells expressing EGFRt (second-generation T cells) | R/R neuroblastoma | 18 months–26 years | Seattle Children's Hospital, USA | Active, not recruiting | 1 | Not available |
| CD171-specific CAR-T cells expressing EGFRt (third-generation T cells) | |||||||
| CD171-specific CAR-T cells expressing EGFRt (long spacer second-generation T cells) | |||||||
| NCT02761915 | 1RG-CART | R/R neuroblastoma | 1 year and older | Cancer Research UK | Completed | 1 | No CR. Antitumour activity in three out of six patients associated with grade 1–3 CRS. |
| NCT04432649 | Fourth generation CD276-specific CAR | Solid tumours | 1–75 years | Shenzhen Geno-Immune Medical Institute | Recruiting | 1, 2 | Not available |
| NCT04897321 | B7-H3 CAR-T cells | R/R paediatric solid tumours | 0–21 years | St. Jude Children's Research Hospital, USA | Recruiting | 1 | Not available |
| NCT03635632 | C7R-GD2.CART cells | Neuroblastoma, sarcomas, and others | 1–74 years | Baylor College of Medicine, USA | Recruiting | 1 | Not available |
| NCT04864821 | CD276 CAR-T | Osteosarcoma, neuroblastoma, and others | 1–70 years | PersonGen BioTherapeutics (Suzhou) Co., Ltd., China | Unknown | early 1 | Not available |
| NCT00085930 | EBV-specific CTLs expressing GD-2 CAR | Neuroblastoma | Up to 21 years | Baylor College of Medicine | Active, not recruiting | 1 | CR in three out of 11 patients, one PR. Grade 1–3 localised pain in three patients. |
| NCT01460901 | GD2 CAR-modified tri-virus CTL | Neuroblastoma | 18 months–17 years | Children's Mercy Hospital Kansas City | Completed | 1 | Non-complete response and death of disease in all three patients. No short-term toxicity. |
| NCT02107963 | GD2 CAR-T | Sarcomas, neuroblastoma, melanoma | 1–35 years | National Cancer Institute (NCI), USA | Completed | 1 | Not available |
| NCT02932956 | GPC3 CAR-T cells | Liver cancer | 1–21 years | Baylor College of Medicine | Active, not recruiting | 1 | Not available |
| NCT00902044 | HER2 CAR | Sarcoma | Children, adults, older adults | Baylor College of Medicine | Active, not recruiting | 1 | No CR. OS of 10.3 months. No toxicity. |
| NCT04995003 | HER2 CAR-T cells in combination with checkpoint blockade | Sarcoma | 1–25 years | Baylor College of Medicine | Recruiting | 1 | Not available |
| NCT03721068 | iC9.GD2.CAR.IL-15 T-cells | Neuroblastoma, Osteosarcoma | 18 months and older | UNC Lineberger Comprehensive Cancer Center, USA | Recruiting | 1 | Not available |
| NCT03721068 | iC9.GD2.CAR.IL-15 T-cells | Neuroblastoma and osteosarcoma | 18 months and older | UNC Lineberger Comprehensive Cancer Center | Recruiting | 1 | Not available |
| NCT01953900 | iC9-GD2-CAR-VZV-CTLs | Osteosarcoma, neuroblastoma | Children, adults, older adults | Baylor College of Medicine, USA | Active, not recruiting | 1 | Not available |
| NCT01822652 | iC9-GD2-CD28-OX40 (iC9-GD2) T cells | Neuroblastoma | Children, adults, older adults | Baylor College of Medicine, USA | Active, not recruiting | 1 | Out of 11 patients: two CR, one grade 1 CRS, two grade 3/4 fever and neutropenia, low grade neurotoxicity. |
| NCT04715191 | IL-15 and IL-21 GPC3 CAR-T cells | Liver cancer, rhabdomyosarcoma, malignant rhabdoid tumours, and others | 1–21 years | Baylor College of Medicine | Not yet recruiting | 1 | Not available |
| NCT04377932 | IL-15 GPC3 CAR-T cells | Liver cancer, rhabdomyosarcoma, malignant rhabdoid tumours, and others | 1–21 years | Baylor College of Medicine | Recruiting | 1 | Not available |
| NCT04483778 | Second-generation 4-1BBζ B7-H3-EGFRt-DHFR | R/R paediatric solid tumours | 0–26 years | Seattle Children’s Hospital, USA | Recruiting | 1 | Not available |
| NCT03618381 | Second-generation 4-1BBζ EGFR806-EGFRt | R/R paediatric solid tumours | 1–30 years | Seattle Children’s Hospital, USA | Recruiting | 1 | Not available |
| NCT00889954 | TGFBeta resistant HER2/EBV-CTLs | HER2 positive malignancies | 3 years and older | Baylor College of Medicine | Completed | 1 | Not available |
See the ‘Clinical trial search’ section in supplementary methods for more information regarding search terms.
CAR, chimeric antigen receptors; CR, complete responses; CRS, cytokine release syndrome; CTL, cytotoxic T lymphocytes; EGFRt, epidermal growth factor receptor; GPC3, glypican 3; HER2, human epidermal growth factor receptor 2; OS, overall survival; PR, partial responses; SD, stable disease.
2.4.2.1. GD2
Preclinically, 4-1BB GD2 CAR-T exerted their antitumour activity in vitro and in a xenograft model, where tumour growth was suppressed in 5/6 mice [70]. Clinically, GD2-CD28 CAR-T were implemented in a phase I clinical trial for relapsed/refractory neuroblastoma tumours. In total, 3/6 patients receiving >108/m2 CAR-T showed antitumour activity at the primary and at metastatic site, but no CR was reported and 2/3 responding patients died within 5 months after the first CAR-T infusion due to PD [71]. In the other six patients receiving lower cell doses, CAR-T failed to efficiently expand and was below the detection threshold. Tumour reactivity was associated with grade 1–3 cytokine release syndrome (CRS) and toxicity linked to systemic immune activation (NCT02761915) [71].
2.4.2.2. B7-H3
Second-generation CAR-T have also been developed against B7-H3, a ubiquitous immune checkpoint molecule highly expressed by many paediatric solid tumours [72], [73], [74]. B7-H3 CAR could promote tumour regression in in vivo models of osteosarcoma, Ewing sarcoma [75], and neuroblastoma [76]. In the first study, MGA271 antibody-based B7-H3 CAR-T mediated complete regression of osteosarcoma and Ewing sarcoma xenografts [75]. MGA271-based CAR-T cells only respond to high-antigen-density tumour cells and not to low antigen densities, limiting their on-target off-tumour toxicity [75]. In neuroblastoma, mice receiving the highest 376.96 antibody-based CAR-T dose remained in remission for more than 90 days [76]. Second-generation CD28 B7-H3 CAR-T coexpressing 41BBL are currently being assessed in a phase I clinical trial for paediatric solid tumours (NCT04897321) [77]. Importantly, this second-generation CAR +/−41BBL was superior in terms of in vitro effector function and in vivo antitumour activity to its third-generation counterpart [77]. This underlines that there is no simple relationship between the number of endodomains and CAR-T effector function and the importance of individually testing different endodomain combinations for each CAR-T to find the optimal design.
2.4.2.3. L1CAM
4-1BB L1CAM CAR-T displayed a potent antitumour activity in vitro and an immunocompromised mouse model, and acceptable safety profile in non-human primates [78]. These cells are now under evaluation, together with CD28:4-1BB third-generation CAR-T, in a phase I clinical trial for neuroblastoma (NCT02311621) [78]. Of note, preclinical data show that CD28 versus 4-1BB costimulation in L1CAM CAR-T results in better expansion and tumour control in mouse models of neuroblastoma [79]. These results are in line with recent studies that show CD28 costimulation as better suited for low antigen densities, which are more common in solid versus haematological malignancies [80].
2.4.2.4. HER2
HER2 is expressed at low levels by sarcoma tumours and correlates with poor clinical outcome [81]. Despite its low expression, second-generation CD28 HER2 CAR-T efficiently eliminated tumour cells in vitro and in an osteosarcoma xenograft model [82], [83]. Moreover, in a metastatic mouse model, 8/10 animals treated with HER2 CAR-T achieved complete regression of metastases [82]. In a phase I clinical trial, among 19 patients with HER2+ sarcoma tumours, CD28 HER2 CAR-T persisted at least 6 weeks in 7/9 patients but with no post-infusion expansion. Treatment was associated with a median overall survival (OS) of 10.3 months without toxicities. However, no CR was observed and 13/19 patients underwent PD, suggesting the need of improving CAR-T expansion (NCT00902044) [84].
2.4.2.5. GPC2 and GPC3
GPC2 and GPC3 are oncofoetal proteins that can be upregulated by solid tumours [85]. GPC2 is highly expressed by neuroblastoma tumours [86]. GPC2 CAR-T were switched from 4-1BB [87] to CD28 costimulation (also in combination with c-Jun overexpression) [80] to tune the cells for low antigen density. Survival of high-tumour burden mice treated with CD28 GPC2 CAR-T was increased by ∼20 days compared to 4-1BB GPC2 CAR-treated mice and by ∼30 days compared to control mice [80]. GPC3 is highly expressed in hepatocellular carcinoma. Second-generation 4-1BB GPC3 CAR-T displayed a high proliferative potential, a Th-1 cytokine profile, and a strong antitumour activity in xenograft models of hepatocellular carcinoma and malignant rhabdoid tumours [88]. These cells are under evaluation in a phase I clinical trial with no results posted yet (NCT02932956). Of note, there is evidence that soluble GPC3 could hamper GPC3 CAR-T activity, representing a possible limitation to the success of this cell therapy [89].
2.4.3. Third-generation CAR-T
Although promising, trials with second-generation CAR-T still fail to achieve optimal tumour control. Studies are therefore moving towards third-generation CAR-T that comprise multiple costimulatory domains to improve CAR-T function.
In a preclinical model, neuroblastoma-targeting CD28:OX-40 GD2 CAR-T were superior in terms of proliferation, cytokine release, and effector functions to their first- and second-generation counterparts [90]. They have been evaluated in a clinical trial for neuroblastoma tumours (NCT01822652), in which only 2/11 patients achieved CR, 4/11 patients were alive with disease at the time of results publication, and 5/11 died of disease [91]. Very recent data of a phase I/II clinical trial (NCT03373097) show extremely encouraging results in treating neuroblastoma patients with CD28:4-1BB GD2 CAR-T. In this trial, 9/27 neuroblastoma patients had a CR after one CAR-T infusion, 8/27 had a PR, 5/27 stable disease (SD), and 5/27 no response with a 3-year OS of 60% and event-free survival (EFS) of 36%. Grade 3 CRS occurred only in one patient, and CAR-T persistence was reported up to 30 months after infusion in 26/27 patients [92].
For other targets, third-generation CD28:4-1BB GPC3 CAR-T have also been developed, and they abolished tumour growth in patient-derived hepatocellular carcinoma xenografts [93].
2.4.4. Next-generation CAR-T
With next-generation CAR-T, we refer to CAR-T designs that go beyond the manipulation of costimulatory domains and make use of complex engineering techniques to enhance T cell persistence, functionality, and infiltration, while reducing therapy-associated toxicities. These innovative strategies are also focusing on the fine-tuning of immunomodulatory stimuli present in the TME to reduce signals, which could dampen the CAR-T response, thereby increasing their antitumour potential.
2.4.4.1. Cytokine-transduced next-generation CAR-T
CAR-T can be transduced with cassettes that lead to the transcription of cytokines upon receptor signalling. These CAR-T, also referred to as T cells redirected for antigen-unrestricted cytokine-initiated killing, can be transduced with cytokines sustaining T cell proliferation. GD2 CAR-T coexpressing IL-15 are currently evaluated in a phase I clinical trial for neuroblastoma and osteosarcoma (NCT03721068). IL-15-transduced GD2 CAR-T displayed a stem-cell-like phenotype, a decreased expression of inhibitory receptors, and better persistence in a metastatic neuroblastoma xenograft model, compared to the classical GD2 CAR-T [94]. Moreover, IL-15 has been expressed in GPC3 CAR-T, together with IL-21, which resulted in better proliferation, reduced apoptosis, increased central and stem-cell memory phenotypes, and improved antitumour activity in hepatocellular carcinoma-bearing mice [95]. These cells are currently under evaluation in two phase I clinical trials for paediatric solid tumours (NCT04377932, NCT04715191). GPC3 CAR-T have also been engineered to coexpress IL-7, achieving an increased CAR-T persistence and antitumour activity [96]. Of note, the GPC3 CAR-T used in this study contained an intracellular CD3ε domain, instead of the classical CD3ζ, resulting in lower production of cytokines, which could be associated with a lower possibility of developing CRS, one of the major toxicities in CAR-T-treated patients [96]. In a preclinical neuroblastoma and melanoma model, GD2 CAR-T were engineered to coexpress IL-7 and CCR2b, the receptor for chemokine CCL2, produced by various tumours, exhibiting a stronger antitumour activity and tumour infiltration compared to the second-generation counterpart [97]. IL-18 and IL-12 are also important cytokines for sustained CAR-T persistence. IL-18-engineered GD2 CAR-T showed enhanced activation and production of pro-inflammatory cytokines when co-cultured with GD2-expressing cells [98]. Interestingly, IL-18 also recruited monocytes and NK cells, contributing to the antitumour activity [98]. IL-18 and IL-12-secreting GD2 CAR-T can be further improved by the addition of a modified NFAT promoter in the transgene, which results in locoregional cytokine release upon CAR signalling and a consequential diminished systemic toxicity. NFAT-modified IL-18+IL-15 GD2 CAR-T display increased cytotoxicity, activation, and monocyte recruitment in vitro [99]. Lastly, CAR-T could also be engineered for indirect expression of cytokine receptors. GD2 CAR-T transduced with p40, a subunit of the IL-23 receptor, showed superior antitumour activity and safety compared to IL-18 or IL-15-engineered cells in a neuroblastoma xenograft model [100].
Overall, these results point out the importance of exploring new approaches of CAR-T engineering to support and improve CAR-T functionality beyond the addition of costimulatory domains, for example, by the implementation of additional cargos.
2.4.4.2. Safety of next-generation CAR-T
One critical issue with CAR-T is treatment-associated toxicities, of which the most reported are CRS, neurotoxicity, and on-target off-tumour toxicities [101]. New designs implementing safety switches to turn off transiently or stably CAR-T activity have been developed. GD2 (NCT01822652, NCT02765243, NCT02992210) and B7-H3 (NCT04432649) CAR-T, in clinical trials for neuroblastoma and B7-H3+ solid tumours, respectively, contain an inducible caspase 9 (iCas9), which is activated upon administration of the drug AP1903, leading to CAR-T apoptosis [102], [103]. Overall, results from a trial with iCas9 GD2 CAR-T showed very limited toxicities but modest efficacy independent from the iCas9 cassette (tumour regression in 2/34 treated patients, Table 3) [102], [104]. Alternatively, truncated forms of epidermal growth factor receptor (EGFRt) or HER2t can be recognised by the antibodies cetuximab and trastuzumab leading to T cell apoptosis [105], [106]. These are used in ongoing B7-H3 (NCT04483778) and EGFR (NCT03618381) CAR-T trials for children with recurrent/refractory solid tumours. Inducing CAR-T ablation with these strategies also leads to an abrogation of the antitumour response [107]. Thus, new approaches to transiently turn off CAR-T activity have evolved. Preclinical studies on GD2-CAR-T with transient CAR expression, achieved by electroporation instead of viral transduction, demonstrated reduced CAR-T persistence and toxicities [108], [109]. However, this temporal CAR expression results in poorer tumour infiltration and the need for repetitive CAR-T administrations [108], [109]. Another way to improve CAR-T specificity is represented by split-signalling AND logic-gate CAR-T, which was recently applied to target ALK, the second most common mutated gene in neuroblastoma tumours [110], [111], [112]. T cells were engineered to coexpress a first-generation ALK-targeting CAR and a B7-H3 CAR bearing only the costimulatory receptor but lacking CD3ζ. Only when both receptors were engaged, full T cell activation was induced, granting specificity of the design [112]. SynNotch CAR-T can also mitigate on-target-off-tumour toxicity. In these cells, expression of the CAR is dependent on the engagement of a SynNotch receptor, which extracellularly bears a tumour-specific scFv and intracellularly has a site that can be cleaved, releasing a transcription factor that can activate the CAR transcription [113]. The need for simultaneous recognition of two antigens increases CAR-T tumour specificity. In preclinical studies for neuroblastoma tumours, these cells have been generated targeting B7-H3 upon GD2 binding [114]. Mice treated with three doses of these CAR-T showed an increased 40-day survival compared to control or single-infused mice and no sign of neurotoxicity.
2.4.5. Combination strategies
ACTs have been also combined with small-molecule inhibitors to improve killing and circumvent the resistance that arises with single-agent therapies.
GD2 CAR-T have been combined with MEK inhibitors or doxorubicin, displaying a synergistic effect in an in vivo neuroblastoma model and in vitro with osteosarcoma cell lines [115], [116]. Despite the ability of MEK inhibitors to synergise in vivo with CAR-T, MEK inhibition in vitro resulted in suppression of GD2 CAR-T killing [115]. Combining L1CAM CAR-T with lysine demethylase 1A inhibitors boosted antigen-independent FAS/FAS-ligand-mediated killing and improved therapy efficacy in vitro [117]. Subpharmacological doses of sorafenib, an inhibitor of kinases involved in tumour proliferation and angiogenesis, synergised with GPC3 CAR-T in a hepatocellular carcinoma mouse model [118]. The synergistic effect was explained by a combined apoptotic activity on tumour cells and an indirect effect of sorafenib modulating macrophage activity, leading to increased IL-12 secretion and, consequentially, better CAR-T activation [118].
2.4.5.1. Overcoming the immunosuppressive TME
It has become clear that improving CAR-T efficacy should be coupled with strategies that can manage the immunosuppressive TME of paediatric solid tumours. These tumours exploit many tactics to avoid immune cell recognition, including (1) downregulation of MHC molecules, hindering antigen presentation and abrogating TCR-mediated killing; (2) expression of immune checkpoint molecules, including the well-known PD-L1; (3) recruitment of immunosuppressive cell populations, such as MDSCs, an immature myeloid cell population with the ability to suppress immune responses; (4) secretion of immunosuppressive soluble factors; and (5) displaying an immunosuppressive metabolic profile. Both smarter CAR-T designs and combinatorial strategies are needed to overcome these immunosuppressive features [119], [120], [121].
To address the issue of immune checkpoint molecule expression, GD2 CAR-T therapy has been coupled with programmed cell death protein 1 (PD-1) blockade [122]. In a phase I clinical trial for neuroblastoma tumours treated with GD2 CAR-T + anti-PD-1 either without or after lymphodepletion, only 2/11 patients achieved CR. PD-1 inhibition showed no further enhancement of CAR-T expansion and persistence and no clear clinical benefit (NCT01822652) [91].
MDSCs have been shown to impair GD2 CAR-T efficacy in neuroblastoma and sarcoma [123], [124]. In a sarcoma xenograft model, GD2 CAR-T therapy combined with MDSC depletion using all-trans retinoic acid resulted in an increased antitumour effect and better survival [124].
Arginine is an essential amino acid for T cell proliferation, which is depleted in the TME due to the high consumption by tumour cells. To improve their metabolic fitness in an arginine-low environment, GD2 CAR-T were engineered to express arginine resynthesis pathway enzymes that can restore arginine supply, resulting in increased CAR-T proliferation and enhanced tumour control [125].
2.4.6. Other strategies to improve CAR-T therapy
In addition to next-generation CAR-T and combinatorial strategies, other elaborated designs are currently under evaluation to tailor CAR-T therapy for the paediatric TME, while reducing CAR-T-associated toxicities.
An alternative solution to regular CAR-T therapy is represented by CAR cytokine-induced killer (CAR-CIK) cells. CAR-CIK are derived from polyclonal T cell populations that, upon specific culturing, acquire the expression of several NK cell surface markers but retain the expression of their endogenous TCR [126], [127]. These cells are considered as potent as CAR-T but present limited toxicity due to their slow division rate, higher susceptibility to apoptosis, and persistent IFN-γ expression [128]. They have been explored also in allogeneic settings thanks to their limited graft-versus-host effect. CAR-CIK cells targeting chondroitin sulphate proteoglycan 4 (CSPG4) [129] and erythroblastic oncogene B 2 (ERBB2) [130] have been generated for the treatment of soft tissue sarcomas and RMS, respectively. CSPG4 CAR-CIK-treated mice with low tumour burden experienced a delay in tumour growth up to 11 days compared to control mice [129], while ERBB2 CAR-CIK treatment could increase survival of ∼20 days compared to untreated mice, but with no significant difference compared to WT CIK-treated mice [130].
To broaden the spectrum of antigens for CAR-T cells beyond surface molecules, so-called peptide-centric or TCR-mimicking CAR-T have been generated that can recognise antigens presented on MHC molecules, allowing CAR-T cells to recognise also intracellular oncoproteins. Peptide-centric CAR-T have been generated targeting PHOX2B, a transcription factor expressed at high levels in neuroblastoma [131], achieving complete regression of the tumour in xenograft models [132]. These innovative cells represent a powerful means to boost the efficacy of ACTs for paediatric solid tumours, but they still rely on MHC expression.
Overall, CAR-T therapy is the most studied ACT strategy for paediatric solid tumours because of their MHC independence. However, current αβ T cell-based ACT products have the common disadvantage of not being applicable as off-the-shelf products due to the risk of graft-versus-host disease (GVHD) and can therefore only be generated from autologous sources. To reduce the GVHD risk, genome-edited αβ T cell-based products are currently under development: CAR insertion and simultaneous ablation of T cell receptor alpha constant and β-2 microglobulin may allow to establish ‘off-the-shelf’ CAR-T cells [133].
3. NK cells
3.1. NK-based immunotherapy
NK cells exert a pivotal role in immunosurveillance by killing of malignant cells and by recruitment of other immune cells [134]. In oncological patients, NK cells are often impaired, mainly due to the immunosuppressive TME, and their tumour infiltration is limited [135], [136], [137], [138]. For example, osteosarcoma patients have low circulating NK cell numbers, but functionality and IFN-y production are retained [139].
NK function and cytotoxicity rely on a fine-tuned balance of inhibitory and stimulatory receptor engagement [140]. NK cells can directly kill target cells by antibody-dependent cell cytotoxicity (ADCC), which is mediated by immunoglobulins binding surface antigens on tumours and activating receptors CD16A (FcγRIIIA) or CD32c (FcγRIIC) on NK cells, leading to NK cell degranulation, cytokine secretion, and tumour cell lysis [141]. Another important mechanism of NK cell-target recognition relies on the ‘missing self-response’ [142], where the absence of MHC-I triggers NK cell activation and killing due to reduced binding of MHC-I molecules to the inhibitory killer immunoglobulin-like receptors (KIR). Paediatric tumours express low levels of MHC-I [18] and are therefore ideal targets for NK cell therapy. In addition, the use of haploidentical NK cells benefits of their alloreactivity induced by the mismatch between KIR receptors on donor cells and their ligands on recipient cells, resulting in a significant NK-versus-tumour effect [143]. Their ability to selectively recognise cancerous cells together with the lack of GVHD makes NK cells a promising ACT candidate, even as an allogeneic product [144], [145], [146].
Two ACT strategies exploiting NK cells have been employed in paediatric solid tumours: 1) haploidentical haematopoietic stem-cell transplantation (HSCT) to permanently establish the donor NK cells, and 2) transient expansion and adoptive transfer of NK cells from a haploidentical donor.
3.2. NK-enriched HSCT
Haploidentical T and B cell-depleted transplants offer a unique opportunity for mismatching KIRs on donors’ NK cells against HLA molecules present on the tumour, enhancing the graft versus tumour (GVT) effect [146], [147]. Of note, the risk of GVHD must be taken in consideration and balanced during haploidentical HSCT, as the transplant might be non-fully depleted in T cells. While there are numerous studies observing the benefits of KIR mismatch in leukaemias [144], [148], clinical and preclinical studies in solid tumours have lagged behind. Nevertheless, some studies showed increased susceptibility to allogenic NK-mediated osteosarcoma killing when the KIR-HLA-I incompatibility was maximised [149]. The feasibility of NK-enriched haploidentical HSCT to increase GVT was tested in an early clinical trial for relapsed solid cancers in children and young adults (NCT01804634). The procedure was effective and safe, with an OS rate of 88% at 6 months, 56% at 12 months, and 21% at 2 years. This study also underlined the need to optimise immune reconstitution after transplant to decrease morbidity and mortality in future trials [150]. A phase II trial (NCT02100891) is currently ongoing on solid tumours, including Ewing sarcoma, neuroblastoma, RMS, and osteosarcoma, consisting of a low-intensity chemotherapy and radiation therapy, followed by haploidentical HSCT and donor-derived NK infusions (Table 4).
Table 4.
Summary of clinical trials using NK cells for paediatric extracranial solid tumours (updated June 2023).
| NCT | Compound | Indication | Age group | Sponsor | Status | Phase | Results |
|---|---|---|---|---|---|---|---|
| NCT02508038 | Haploidentical SCT + zoledronate | Neuroblastoma, osteosarcoma, Ewing sarcoma, rhabdomyosarcoma (RMS), leukaemia, lymphoma | 7 months–21 years | University of Wisconsin, Madison | Recruiting | 1 | Not available |
| NCT03420963 | Cord blood-derived expanded allogeneic NK cells + cyclophosphamide, etoposide | Refractory neoplasms | 12 months–40 years | M.D. Anderson Cancer Center | Recruiting | 1 | Not available |
| NCT04214730 | Donor NK cell infusion and chemotherapy | Solid tumours | 10–90 years | Yantai Yuhuangding Hospital | Recruiting | NA | Not available |
| NCT00640796 | Haploidentical NK cells + IL-2+ cyclophosphamide, fludarabine, mesna | Relapsed/refractory haematological malignancies, Ewing sarcoma family of tumours, and RMS | Children, adults | St. Jude Children’s Research Hospital | Completed | 1 | Not available |
| NCT00698009 | Haploidentical NK cells + Il2+ fludarabine, cyclophosphamide, mesna | Neuroblastoma | Children, adults, older adults | M.D. Anderson Cancer Center | Not active, terminated (slow accrual) | 2 | Not available |
| NCT02409576 | Haploidentical NK cells infusions + IL2 | Ewing sarcoma, RMS | Children, adults, older adults | National University Hospital, Singapore | Unknown | 1, 2 | Not available |
| NCT02100891 | Haploidentical SCT + donor NK cells infusions | Ewing sarcoma, neuroblastoma, RMS, osteosarcoma, CNS tumours | Children, adults, older adults | Medical College of Wisconsin | Active, not recruiting | 2 | 6-months DCR is 72%, 1 and 2 year OS are 64% and 40%, respectively, and PFS is 29% and 22%. 4/15 patients developed acute or chronic GVHD. |
| NCT00582816 | Haploidentical SCT and donor NK cells infusions | Leukaemia, solid tumours | 6 months–25 years | University of Wisconsin, Madison | Terminated (toxicity) | 1,2 | Not available |
| NCT01875601 | NK cell infusion + rhIL15 | Sarcoma, neuroblastoma, others | 2–29 years | National Cancer Institute | Completed | 1 | Not available |
| NCT00877110 | Haploidentical NK cells + anti-GD2 m3F8 | Neuroblastoma bone marrow, sympathetic nervous system | Children, adults, older adults | Memorial Sloan Kettering Cancer Center | Completed | 1 | 10 CR/PR (29%), 17 NR (47%), 8 (23%) progressive disease. No GVHD. |
| NCT01857934 | Autologous SCT and haploidentical NK cells + IL2+ anti-GD2 hu14.18K322A | Neuroblastoma | Up to 18 years | St. Jude Children’s Research Hospital | Active, not recruiting | 2 | 60 out of 64 PRs or better, including 40 patients with mCRs. Only 31 patients received additional NK cell infusions. |
| NCT02130869 | Autologous SCT haploidentical NK cells + IL2+ G-CSF + anti-GD2 hu14.18K322A + melphalan + busulfan | Relapsed/refractory neuroblastoma, lymphoma, high-risk solid tumour | Up to 21 years | St. Jude Children’s Research Hospital | Completed | 1 | Not available |
| NCT02650648 | Haploidentical NK cells + anti-GD2 hu3F8 (naxitamab) + IL-2 | High-risk neuroblastoma | Children, adults, older adults | Memorial Sloan Kettering Cancer Center | Active, not recruiting | 1 | Not available |
| NCT01576692 | Haploidentical NK cells + anti-GD2 hu14.18K322A | Neuroblastoma | Up to 21 years | St. Jude Children’s Research Hospital | Completed | 1 | Response rate 61.5% (four CR, one very good PR, three PR), five SD. One patient with unacceptable toxicity and four patients discontinued treatment because of adverse events. |
| NCT02573896 | Autologous NK cells + anti-GD2 ch14.18/CHO (dinutuximab) + lenalidomide | Neuroblastoma | Up to 30 years | New Approaches to Neuroblastoma Therapy Consortium | Active, not recruiting | 1 | Not available |
| NCT03209869 | Haploidentical NK infusions + immunecytokine hu14.18-IL2 + GM-CSF | Neuroblastoma, osteosarcoma | 7 months–21 years | University of Wisconsin, Madison | Not active, withdrawn (limited resources due to COVID-19) | 1 | Not available |
| NCT03242603 | Haploidentical NK cells + anti-GD2 ch14.18/CHO (dinutuximab) | Recurrent neuroblastoma | 6 months–25 years | National University Hospital, Singapore | Unknown | 1, 2 | Not available |
| NCT04211675 | Allogenic TGFβi NK cell + anti-GD2 ch14.18/CHO (dinutuximab) + irinotecan, temozolomide, sargramostim | Neuroblastoma | Up to 29 years | Nationwide Children’s Hospital | Recruiting | 1, 2 | Not available |
See the ‘Clinical trial search’ section in supplementary methods for more information regarding search terms.
CNS, central nervous system; CR, complete responses; GVHD, graft versus host disease; mCR, metastatic complete response; NK, natural killer; NR, non-responder; OS, overall survival; PFS, progression-free survival; PR, partial responses; SCT, stem-cell transplantation; SD, stable disease.
3.3. Adoptive NK-cell therapy
Adoptive allogenic NK infusions are a valid option for replacing the patient’s NK cells, often poorly functional, while also providing a strong GVT effect with the KIR-HLA mismatch.
It has proven to be safe and well tolerated mainly due to a lack of GVHD and treatment-related toxicity [145]. The efficacy of allogenic NK infusions was first demonstrated by successful transfer and expansion of haploidentical NK cells in AML patients, where 5/19 patients with poor prognosis achieved CR [145]. Further feasibility proofs were confirmed in other studies on solid childhood cancers [151], [152].
3.3.1. NK cell transfer coupled with mAb
NK cells are major players involved in the efficacy of mAb-based therapies thanks to their ability to induce ADCC [141]. Among paediatric solid tumours, anti-GD2 antibody therapy with dinutuximab (human-mouse chimeric 14.18) and naxitamab (humanised 3F8) has shown improved EFS, OS, and substantial antineoplastic activity against neuroblastoma [153], [154], [155]. To boost the antitumour effect, mAb has been combined with the adoptive transfer of ex vivo-expanded NK cells. Expanded activated NK cells in combination with dinutuximab increased cytotoxicity against neuroblastoma in vitro and in a mouse model, compared with mAb or NK cells adoptive monotherapy [156].
In a phase I trial, 13 patients with recurrent/refractory neuroblastoma were treated with haploidentical NK cells, chemotherapy, GM-CSF, IL-2, and a humanised version of dinutuximab (hu14.18K322A) [157]. Although all patients were heavily pretreated, the response rate was 61.5% (four CR, one very good PR, and three PR), five patients had a SD, and OS was 77% at 1 year [158]. In a phase I trial with haploidentical NK-cells in combination with murine 3F8 in high-risk neuroblastoma patients [159], 10/35 patients (29%) reached CR or PR, OS was 30.7 months, and no GVHD was detected. Since most patients received other treatments after the trial, survival could not be solely attributed to the trial treatment. Moreover, NK cells were undetectable 14 days post-infusion, which leaves it unclear if the effect was NK-mediated. A follow-up phase I trial involving 3F8 with superior ADCC activity (NCT02650648) is currently active.
These findings show that NK cells can be safely combined with anti-GD2 mAb, showing antineuroblastoma effects.
3.3.2. Adoptive transfer of genetically engineered NK cells
Genetic manipulation of NK cells could improve antitumour effects, homing, and in vivo persistence. NK cells migratory capacity can be enhanced by increasing the expression of the chemokine receptor CXCR2 binding to CXCL3, CXCL5, and CXCL8, which are expressed by a variety of solid tumours [160]. Viral transduction of CXCR2 on primary NK cells augmented tumour migration and killing [161]. The expression of IL-15, an important activating cytokine for NK cells, could also be incorporated to enhance the in vivo expansion and persistence of NK cells [162].
NK cells can also be modified to express CARs. The use of NK cells as an engineering platform can yield some advantages over T cells, as they can be produced from an allogenic source, providing the option of an ‘off-the-shelf’ therapy [163]. Moreover, CAR-T-related toxicities can be overcome thanks to the short lifespan of CAR-NK cells [164]. Although there are still few studies in paediatric oncology, the use of CAR-NK cells targeting CD19 has been proven safe and effective in trials in adult patients with CLL [165], and two pilot studies (NCT00995137 and NCT01974479) have been approved for the treatment of ALL.
As previously stated, one of the main challenges for immunotherapy in solid tumours is the highly immunosuppressive microenvironment. NK cells engineered to express activating receptor NKG2D fused with the cytotoxic ζ chain of T cells (NKG2D.ζ-NK cells) showed improved cytotoxicity in vitro and were able to alter the TME in favour of an antitumour response, eliminating suppressive MDSCs in a xenograft TME model [166]. NKG2D.ζ-NK cells could even secrete pro-inflammatory cytokines and chemokines to recruit and activate co-delivered CAR-T that would be otherwise suppressed [167].
In conclusion, while NK cells offer a wide range of advantages and their safety and efficacy in cancer treatment have been proven, additional work is required to enable robust tumour infiltration and improve patient survival.
4. iNKT cells
4.1. iNKT cells in paediatric antitumour immunity
iNKT cells are T cells that lay in-between innate and adaptive immunity, expressing both, classical T and NK cell markers [168], [169], [170]. They owe their name to their semi-invariant TCR [171], [172] recognising lipids presented in the context of the CD1d molecule, a non-polymorphic MHC molecule [173], [174]. Because of their MHC independency, these cells represent a valid alternative to classical αβ T cell therapy for paediatric tumours [18].
High levels of tumour-infiltrating iNKT cells have been associated with good prognosis in a variety of solid tumours, including neuroblastoma [175], [176], where iNKT cell infiltration seems to be negatively regulated by the oncogene MYCN, overexpressed in aggressive tumour forms [177], [178]. Hepatoblastoma, RMS, and neuroblastoma patients display a frequency of circulating iNKT cells similar to those of healthy controls, and the cells retain their ability to expand in vitro upon stimulation with synthetic α-galactosylceramide, the prototypic iNKT cell antigen [179], suggesting their strong potential as ACT against paediatric extracranial solid tumours. However, iNKT cells constitute only around 0.01–0.1% of peripheral blood mononuclear cells, limiting their use as ACT products. Among paediatric extracranial solid tumours, iNKT cells have been so far only used for the treatment of neuroblastoma, leaving a gap in their implementation for other malignancies.
4.1.1. CAR-engineered iNKT cells
For neuroblastoma, GD2 CAR-iNKT cells demonstrated an efficient cytotoxicity in an in vitro model [180]. Upon target recognition, iNKT cells have the peculiar feature of producing both Th1 and Th2 cytokines. Comparing 4-1BB versus CD28 costimulation, the 4-1BB endodomain favoured a Th1 polarisation, leading to increased production of IFN-γ and GM-CSF and reduction of IL-4 and IL-10 [180]. Third-generation 4-1BB:CD28 GD2 CAR-iNKT cells displayed an enhanced persistence in a neuroblastoma xenograft model, where they could control tumour growth without inducing GVHD [180].
iNKT cells have also been coengineered with GD2-CAR + IL-15. IL-15 is (1) a key cytokine for iNKT cell development and homoeostasis [181], [182], (2) protective towards iNKT cell inhibition mediated by TAM [183], and (3) low IL-15 or iNKT cell presence within the neuroblastoma TME is associated with poor patient outcome [184]. CD28 GD2/IL15 CAR iNKT cells displayed enhanced persistence, antitumour activity, and tumour localisation in neuroblastoma mouse xenografts, as well as a reduced exhaustion profile in vitro [185]. These cells are currently evaluated in a phase I trial for relapsed/refractory neuroblastoma tumours (NCT03294954), and preliminary data showed a good safety profile but only 2/12 PR and 1/12 CR, which correlated with high CAR-iNKT cell expansion and regression of the bone metastatic lesions (Table 5) [186], [187].
Table 5.
Summary of clinical trials using iNKT cells for paediatric extracranial solid tumours (updated June 2023).
| NCT | Compound | Indication | Age group | Sponsor | Status | Phase | Results |
|---|---|---|---|---|---|---|---|
| NCT03294954 | GD2 IL15 CAR-NKT | Neuroblastoma | 1–21 years | Baylor College of Medicine | Active, not recruiting | 1 | No dose-limiting toxicities observed. 1/12 grade 2 CRS. Regression of metastatic lesions and CR in 1/12 patients, PR in 2/12 patients. |
See the ‘Clinical trial search’ section in supplementary methods for more information regarding search terms.
CAR, chimeric antigen receptors; CR, complete responses; CRS, cytokine release syndrome; NKT, invariant NKT; PR, partial responses.
4.1.2. ACTs with expanded iNKT cells
iNKT cells can also be isolated from the peripheral blood, expanded ex vivo, and reinfused in patients [188]. iNKT cells also provide maturation stimuli to dendritic cells and monocytes [189], [190], leading to IL-12 secretion and NK cell activation [191]. In an in vitro neuroblastoma model, iNKT cell infusion was combined with anti-GD2 antibodies, enhancing NK cell ADCC activity towards GD2+ tumour cells, providing optimal tumour killing [192].
In conclusion, the therapeutic potential of iNKT cells for paediatric solid tumours remains widely unexplored, offering several possibilities for the development of new allogeneic ACT strategies to improve patient survival, while decreasing GVHD-related toxicities.
5. γδ T cells
γδ T cells are T lymphocytes that contain a TCRγ and TCRδ chain and constitute around 1–5% of total T cells in peripheral blood. Combinations of the different TCRγ and δ chains form different γδ T cell types [193]. Vδ1-containing T cells are abundant in healthy epithelia and in tumour infiltrates [194], whereas the Vγ9Vδ2 subtype is rich in the peripheral blood [195]. Since mouse γδ TCRs are not homologous to human, preclinical studies are restrained to xenografts in immunodeficient mice or non-human primate models [196].
γδ T cells combine innate-like rapid responses and clonal expansion with pleiotropic effector functions. They can be activated in an HLA-unrestricted fashion through their TCR by non-peptide antigens, such as bisphosphonate isopentenyl-5-pyrophosphate (IPP), that could result from malignant transformation [197]. The synthetic phosphoantigen zoledronic acid was shown to selectively activate TCRVγ9Vδ2+ lymphocytes in clinical trials, due to the accumulation of intracellular IPP [198]. When activated, they induce apoptosis by releasing granzymes and perforins [199]. Soon after activation, γδ T cells acquire the ability to process and present antigens on MHC molecules to conventional CD4+ and CD8+ T cells, helping to initiate peptide-specific αβT cell responses [200].
5.1. γδ T cells in cancer immunotherapy
γδ T cell-based immunotherapy harbours two strategies: in vivo stimulation of γδ T cells through systemic administration of aminobisphosphonates and IL-2, or ex vivo expansion of γδ T cells for adoptive transfer. In vivo expansion of γδ T cells offers a relatively inexpensive and straightforward delivery option, while the ex vivo expansion allows control and optimisation of the effector cell population. Adoptive transfer of Vγ9+Vδ2+ T cells has been tested clinically in various solid tumours [201], and several groups have reported methods to expand also Vδ1+ T cells [202], [203], [204], [205].
In neuroblastoma, Vδ1+ as well as Vδ1−Vδ2− γδ T cell subsets show consistent higher innate tumour toxicity compared to Vδ2+ [206], partly due to the production of soluble NKG2D ligands sMICA, sMICB, and ULPB1-6 by neuroblastoma cells, which can block the NKG2D receptor on γδ T cells [207], [208].
The cytotoxicity of activated and expanded Vγ9Vδ2+ γδ T cells was enhanced by combination with mAbs in vitro [209], [210], [211] and in vivo [212]. A phase I trial combining Dinutuximab and ex vivo-expanded allogeneic γδ T cells in relapsed/refractory neuroblastoma (NCT05400603) has recently started (Table 6).
Table 6.
Summary of clinical trials using γδ T cells for paediatric extracranial solid tumours (updated June 2023).
| NCT | Compound | Indication | Age group | Sponsor | Status | Phase | Results |
|---|---|---|---|---|---|---|---|
| NCT05400603 | Expanded γδ T cells + anti-GD2 ch14.18/CHO + temozolomide, irinotecan, and zoledronate | Neuroblastoma | 12 months–16 years | Emory University | Recruiting | 1 | Not available |
See the ‘Clinical trial search’ section in supplementary methods for more information regarding search terms.
5.2. Combining αβ and γδ TCR engineering
Novel strategies, both by transferring αβTCRs into γδ T cells and by transferring γδ TCRs into αβT, have been explored. The first offers the advantage of redirecting γδ T cell immunity against specific antigen targets and has been tested in vitro against viral [213] and melanoma tumour targets [214]. However, αβTCR gene transfer carries inherent limitations, such as the restriction to particular HLA types [215]. One way to overcome MHC restriction is the transfer of γδ TCRs into αβ T cells, resulting in T cells engineered with defined γδ TCRs (TEGs) [216]. TEG002 consists of αβT cells engineered to express the tumour-specific Vγ9Vδ2 TCR and showed higher cytotoxicity against neuroblastoma organoids than untransduced αβ T cells, while also surviving longer than endogenous γδ T cells [217]. TEG002 was superior to T cells expressing either an αβ-TCR or a γδ-TCR alone, and effectively recognised and killed 50% of the tested organoids [218]. A phase I trial (NCT04688853) investigating the safety of TEG002 in multiple myeloma patients is ongoing and will possibly lead to further clinical investigation in paediatric solid tumours.
5.3. γδ T cells engineering
Γδ T cells have also been engineered to express a CAR to acquire additional antigen-specific cytotoxicity and proliferative response, while retaining their innate anti-cancer properties. First-generation GD2 CAR (14. G2aζ) in Vγ9+Vδ2+ T cells showed increased antigen-specific tumour reactivity, which was reflected in IFN-γ production and CD69 expression upon co-culture with the GD2+ neuroblastoma LAN-1 cells line [219].
γδT-cell responses can be tuned by modulating the level of stimulation. Vδ2+ γδ T cells have been engineered to coexpress their endogenous Vγ9Vδ2 TCR and a chimeric costimulatory receptor composed of an extracellular GD2-binding scFv and an intracellular DAP10 costimulatory domain [220]. The intracellular DAP10 domain is derived from NKG2D, an important activating receptor for γδ T cells, which paediatric tumours tend to evade [207], [208]. This provides additional costimulation to the γδ TCR, restoring Vγ9Vδ2+ T cell cytotoxicity and cytokine production in the presence of transformed cells [220].
6. Conclusions and future perspectives
In the past years, much effort has gone into the improvement of ACTs for paediatric extracranial solid malignancies. Many of the ongoing clinical trials are providing new insights on how to ameliorate ACTs for better efficacy and lower toxicities, with some of these trials achieving incredibly positive results [92]. ACT strategies currently exploit expanded and/or engineered conventional αβ T cells, NK, γδ T, and iNKT cells. αβ CAR-T are so far the most explored strategy because of their MHC independence, which makes them appealing for low MHC-expressing paediatric extracranial solid tumours. However, they are limited to the recognition of surface antigens. Strategies, such as CTL/TILs and TCR-αβ T cells recognising intracellular antigens, are also being explored and interest is increasing in off-the-shelf products using innate and innate-like cells that could be engineered from allogeneic sources without the risk of GVHD.
It has become clear that paediatric solid tumours are complex entities that cannot be eradicated using single-agent therapies. The pronounced immunosuppressive microenvironment and low-MHC expression are exemplary obstacles to ACT [44], [221], [222]. In this review, we have discussed novel approaches using complex engineering strategies or combination therapies that could outsmart the tumours.
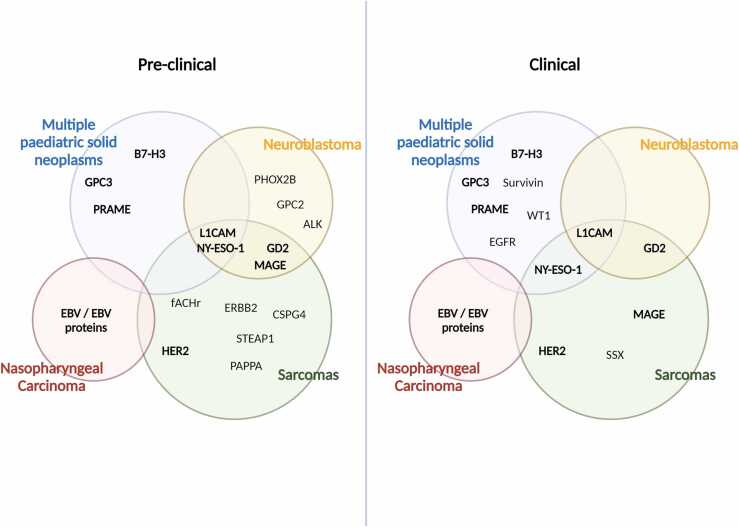
Future studies will have to address the current gap between bench and bedside to aid clinical implementation of newly developed ACTs for paediatric solid malignancies. While the field is expanding for some tumour types, such as neuroblastoma and sarcomas, more effort is needed for the clinical translation to rare paediatric kidney and liver tumours. As many of these cancers share common antigens, this could serve as a means for a broader clinical implementation (Fig. 2). Currently, a big disparity exists between the great preclinical effort of identifying new targets and the subsequent implementation of novel therapies into clinical practice (Fig. 2). Better preclinical models and an agreement on efficacy thresholds for clinical translation will be crucial to make new therapies accessible for paediatric patients. It will become essential to identify optimal patient selection criteria and monitor treatment responses during therapy using sequential tumour biopsies to enable for personalised therapies.
Fig. 2.
Overview of adoptive cell therapy (ACT) targets currently exploited in preclinical (left) and clinical (right) practice for paediatric extracranial solid tumours. Some tumours, such as neuroblastoma and sarcomas, are over-represented in the scenario of ACTs for paediatric solid malignancies. Of note, some targets, such as the CTAs NY-ESO, PRAME, and MAGE, are shared among several of these malignancies, making them ideal for more translational approaches. In bold are represented targets that are currently being investigated both at a preclinical and clinical level. GPC2, glypican 2; fACHr, fetal acetylcholine receptor; ERBB2, erythroblastic oncogene B 2; CSPG, chondroitin sulphate proteoglycan 4; STEAP1, six-transmembrane epithelial antigen of prostate-1; EGFR, epidermal growth factor receptor.
In conclusion, while the field of ACT in paediatric extracranial solid tumours is rapidly evolving, there is a need to develop more advanced strategies tailored to solid tumour environments to improve therapy efficacy and work towards curative interventions.
Funding
This work has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement, No. 956285 (VAGABOND). This work is part of the research programme Vernieuwingsimpuls Vidi (Combining targeted compounds in neuroblastoma tumours; is two better than one?) with project number 91716482 and Veni (Release the beast: Boosting CAR-T cell immunotherapy for neuroblastoma) with project number 09150162010022, which is partly financed by the Dutch Research Council (NWO) and ZonMW. In addition, this project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 716079 Predict, from grant H2020-iPC-826121, and from Villa Joep (Joining forces to activate T cell immunity against High-Risk Neuroblastoma).
CRediT authorship contribution statement
Elisa Zappa: Study conceptualization and design, Data curation and quality control, Manuscript writing and editing. Alice Vitali: Study conceptualization and design, Data curation and quality control, Manuscript writing and editing. Kathleen Anders: Data quality control, Manuscript editing and review. Jan J. Molenaar: Data quality control and manuscript review. Judith Wienke: Study conceptualization, Data quality control, Manuscript editing and review. Annette Künkele: Study conceptualization, Data quality control, Manuscript editing and review.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejca.2023.113347.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Galluzzi L., Chan T.A., Kroemer G., Wolchok J.D., López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10:1–15. doi: 10.1126/scitranslmed.aat7807. [DOI] [PubMed] [Google Scholar]
- 2.Lauder I., Aherne W. The significance of lymphocytic infiltration in neuroblastoma. Br J Cancer. 1972;26:321–330. doi: 10.1038/bjc.1972.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin R.F., Beckwith J.B. Lymphoid infiltrates in neuroblastomas: their occurrence and prognostic significance. J Pediatr Surg. 1968;3:161–164. doi: 10.1016/0022-3468(68)91005-1. [DOI] [PubMed] [Google Scholar]
- 4.Liu P., Xiao Q., Zhou B., Dai Z., Kang Y. Prognostic significance of programmed death ligand 1 expression and tumor-infiltrating lymphocytes in axial osteosarcoma. World Neurosurg. 2019;129:e240–e254. doi: 10.1016/j.wneu.2019.05.121. [DOI] [PubMed] [Google Scholar]
- 5.Cheung N.K.V., Cheung I.Y., Kushner B.H., Ostrovnaya I., Chamberlain E., Kramer K., et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte- macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu A.L., Gilman A.L., Ozkaynak M.F., London W.B., Kreissman S.G., Chen H.X., et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey D.L., Cheung N.V. Immunotherapy of pediatric solid tumors: treatments at a crossroads, with an emphasis on antibodies. Cancer Immunol Res. 2020;8:161–166. doi: 10.1158/2326-6066.CIR-19-0692.Immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebb D., Meyers P., Grier H., Bernstein M., Gorlick R., Lipshultz S.E., et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the children’s oncology group. J Clin Oncol. 2012;30:2545–2551. doi: 10.1200/JCO.2011.37.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merchant M.S., Geller J.I., Baird K., Chou A.J., Galli S., Charles A., et al. Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. J Clin Oncol. 2012;30:4141–4147. doi: 10.1200/JCO.2012.44.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp L.M., Malempati S., Krailo M., Gao Y., Buxton A., Brenda J., et al. Phase 2 trial of the GPNMB-targeted antibody-drug conjugate, glembatumumab vedotin (CDX-011) in recurrent osteosarcoma AOST1521: a report from the Children’s Oncology Group (COG) Eur J Cancer. 2020;121:177–183. doi: 10.1016/j.ejca.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullard A. FDA approves first CAR T therapy. Nat Rev Drug Discov. 2017;16:669. doi: 10.1038/nrd.2017.196. [DOI] [PubMed] [Google Scholar]
- 12.Lu J., Jiang G. The journey of CAR-T therapy in hematological malignancies. Mol Cancer. 2022;21:1–15. doi: 10.1186/s12943-022-01663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu W.L., Hua Z.C. Chimeric Antigen Receptor T-cell (CAR T) therapy for hematologic and solid malignancies: efficacy and safety-a systematic review with meta-analysis. Cancers (Basel) 2019;11(1):47. doi: 10.3390/cancers11010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marofi F., Motavalli R., Safonov V.A., Thangavelu L., Yumashev A.V., Alexander M., et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. 2021;12:1–16. doi: 10.1186/s13287-020-02128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan Q., Zhang H., Zheng J., Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer. 2020;6:605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348(80):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 18.Haworth K.B., Leddon J.L., Chen C.-Y., Horwitz E.M., Mackall C.L., Cripe T.P. Going back to class I: MHC and immunotherapies for childhood cancer. Pediatr Blood Cancer. 2015;61:515–525. doi: 10.1002/pbc.25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grobner S.N., Worst B.C., Weischenfeldt J., Buchhalter I., Kleinheinz K., Rudneva V.A., et al. The landscape of genomic alterations across childhood cancers. Nature. 2018;555:321–327. doi: 10.1038/nature25480. [DOI] [PubMed] [Google Scholar]
- 20.Campbell B.B., Light N., Fabrizio D., Zatzman M., Fuligni F., de Borja R., et al. Comprehensive analysis of hypermutation in human cancer. Cell. 2017;171:1042–1056.e10. doi: 10.1016/j.cell.2017.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Woude L.L., Gorris M.A.J., Halilovic A., Figdor C.G., de Vries I.J.M. Migrating into the tumor: a roadmap for T cells. Trends Cancer. 2017;3:797–808. doi: 10.1016/j.trecan.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Lanitis E., Dangaj D., Irving M., Coukos G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann Oncol. 2017;28:xii18–xii32. doi: 10.1093/annonc/mdx238. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin Cancer Res. 2011;83:1–11. doi: 10.1016/j.earlhumdev.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohaan M.W., Borch T.H., van den Berg J.H., Met Ö., Kessels R., Geukes Foppen M.H., et al. Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N Engl J Med. 2022;387:2113–2125. doi: 10.1056/nejmoa2210233. [DOI] [PubMed] [Google Scholar]
- 25.Coughlin C.M., Fleming M.D., Carroll R.G., Pawel B.R., Hogarty M.D., Shan X., et al. Immunosurveillance and survivin-specific T-cell immunity in children with high-risk neuroblastoma. J Clin Oncol. 2006;24:5725–5734. doi: 10.1200/JCO.2005.05.3314. [DOI] [PubMed] [Google Scholar]
- 26.Olle Hurtado M., Wolbert J., Fisher J., Flutter B., Stafford S., Barton J., et al. Tumor infiltrating lymphocytes expanded from pediatric neuroblastoma display heterogeneity of phenotype and function. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua D., Huang J., Zheng B., Lau S.Y., Luk W., Kwong D.L.W., et al. Adoptive transfer of autologous Epstein-Barr virus-specific cytotoxic T cells for nasopharyngeal carcinoma. Int J Cancer. 2001;94:73–80. doi: 10.1002/ijc.1430. [DOI] [PubMed] [Google Scholar]
- 28.Comoli P., Pedrazzoli P., Maccario R., Basso S., Carminati O., Labirio M., et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23:8942–8949. doi: 10.1200/JCO.2005.02.6195. [DOI] [PubMed] [Google Scholar]
- 29.Straathof K.C.M., Bollard C.M., Popat U., Huls M.H., Lopez T., Morriss M.C., et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus-specific T lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 30.Louis C.U., Straathof K., Bollard C.M., Ennamuri S., Gerken C., Lopez T.T., et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother. 2010;33:983–990. doi: 10.1097/CJI.0b013e3181f3cbf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J., Fogg M., Wirth L.J., Daley H., Ritz J., Posner M.R., et al. Epstein-Barr virus-specific adoptive immunotherapy for recurrent, metastatic nasopharyngeal carcinoma. Cancer. 2017;123:2642–2650. doi: 10.1002/cncr.30541. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Ami T., Kontny U., Surun A., Brecht I.B., Almaraz R.L., Dragomir M., et al. Nasopharyngeal carcinoma in children and adolescents: the EXPeRT/PARTNER diagnostic and therapeutic recommendations. Pediatr Blood Cancer. 2021;68:1–11. doi: 10.1002/pbc.29018. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y.T., Scanlan M.J., Sahin U., Türeci Ö., Gure A.O., Tsang S., et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luk S.J., van der Steen D.M., Hagedoorn R.S., Jordanova E.S., Schilham M.W., Bovée J.V.M.G., et al. PRAME and HLA Class I expression patterns make synovial sarcoma a suitable target for PRAME specific T-cell receptor gene therapy. Oncoimmunology. 2018;7:1–11. doi: 10.1080/2162402X.2018.1507600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakimoto T., Matsumine A., Kageyama S., Asanuma K., Matsubara T., Nakamura T., et al. Immunohistochemical expression and clinicopathological assessment of the cancer testis antigens NY-ESO-1 and MAGE-A4 in high-grade soft-tissue sarcoma. Oncol Lett. 2019;17:3937–3943. doi: 10.3892/ol.2019.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao L., Dunham K., Lucas K. MAGE-A1, MAGE-A3, and NY-ESO-1 can be upregulated on neuroblastoma cells to facilitate cytotoxic T lymphocyte-mediated tumor cell killing. Cancer Immunol Immunother. 2011;60:1299–1307. doi: 10.1007/s00262-011-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan R.A., Chinnasamy N., Abate-daga D.D., Gros A., Robbins F., Zheng Z., et al. Cancer regression and neurologic toxicity following anti-MAGE- A3 TCR gene therapy. J Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi X.W., Zhang F., Wu H., Liu J.L., Zong B.G., Xu C., et al. Wilms’ tumor 1 (WT1) expression and prognosis in solid cancer patients: a systematic review and meta-analysis. Sci Rep. 2015;5:11–15. doi: 10.1038/srep08924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakata J., Nakajima H., Hayashibara H., Imafuku K., Morimoto S., Fujiki F., et al. Extremely strong infiltration of WT1-specific CTLs into mouse tumor by the combination vaccine with WT1-specific CTL and helper peptides. Oncotarget. 2018;9:36029–36038. doi: 10.18632/oncotarget.26338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan M., Himoudi N., Basu B.P., Wallace R., Poon E., Adams S., et al. Increased PRAME antigen-specific killing of malignant cell lines by low avidity CTL clones, following treatment with 5-aza-2′-deoxycytidine. Cancer Immunol Immunother. 2011;60:1243–1255. doi: 10.1007/s00262-011-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hont A.B., Cruz C.R., Ulrey R., O’Brien B., Stanojevic M., Datar A., et al. Immunotherapy of relapsed and refractory solid tumors with ex vivo expanded multi-tumor associated antigen specific cytotoxic T lymphocytes: a phase I study. J Clin Oncol. 2019;37:2349–2359. doi: 10.1200/JCO.19.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prigione I., Corrias V., Airoldi I., Raffaghello L., Morandi F., Bocca P., et al. Immunogenicity of human neuroblastoma. 2004;80:69–80. doi: 10.1196/annals.1322.008. [DOI] [PubMed] [Google Scholar]
- 43.Cornel A.M., Dunnebach E., Hofman D.A., Das S., Sengupta S., Van Den Ham F., et al. Epigenetic modulation of neuroblastoma enhances T cell and NK cell immunogenicity by inducing a tumor-cell lineage switch. J Immunother Cancer. 2022;10(12) doi: 10.1136/jitc-2022-005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornel A.M., Mimpen I.L., Nierkens S. MHC class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers (Basel) 2020;12:1–33. doi: 10.3390/cancers12071760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang T.C., Carter R.A., Li Y., Li Y., Wang H., Edmonson M.N., et al. The neoepitope landscape in pediatric cancers. Genome Med. 2017;9:1–12. doi: 10.1186/s13073-017-0468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Shi T., Song X., Liu B., Wei J. Gene fusion neoantigens: emerging targets for cancer immunotherapy. Cancer Lett. 2021;506:45–54. doi: 10.1016/j.canlet.2021.02.023. [DOI] [PubMed] [Google Scholar]
- 47.Lai J., Robbins P.F., Raffeld M., Aung P.P., Tsokos M., Miettinen M.M., et al. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod Pathol. 2012;25:854–858. doi: 10.1038/modpathol.2012.31.NY-ESO-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robbins P.F., Morgan R.A., Feldman S.A., Yang J.C., Sherry R.M., Dudley M.E., et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’angelo S.P., Melchiori L., Merchant M.S., Bernstein D., Glod J., Kaplan R., et al. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1c259T cells in synovial sarcoma. Cancer Discov. 2018;8:944–957. doi: 10.1158/2159-8290.CD-17-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gattinoni L., Lugli E., Ji Y., Pos Z., Paulos C.M., Quigley M.F., et al. A human memory T cell subset with stem cell – like properties. Nat Med. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerlach C., van Heijst J.W.J., Schumacher T.N.M. The descent of memory T cells. Ann NY Acad Sci. 2011;1217:139–153. doi: 10.1111/j.1749-6632.2010.05830.x. [DOI] [PubMed] [Google Scholar]
- 52.Singh N., Kulikovskaya I., Barrett D.M., Binder-Scholl G., Jakobsen B., Martinez D., et al. T cells targeting NY-ESO-1 demonstrate efficacy against disseminated neuroblastoma. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2015.1040216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirschner A., Thiede M., Grünewald T.G.P., Alba Rubio R., Richter G.H.S., Kirchner T., et al. Pappalysin-1 T cell receptor transgenic allo-restricted T cells kill Ewing sarcoma in vitro and in vivo. Oncoimmunology. 2017;6(2):12. doi: 10.1080/2162402X.2016.1273301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schober S.J., Thiede M., Gassmann H., Prexler C., Xue B., Schirmer D., et al. MHC Class I-restricted TCR-transgenic CD4+ T cells against STEAP1 mediate local tumor control of ewing sarcoma in vivo. Cells. 2020;9:1–16. doi: 10.3390/cells9071581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huisman W., Gille I., van der Maarel L.E., Hageman L., Morton L.T., de Jong R.C.M., et al. Identification of functional HLA-A*01:01–restricted Epstein-Barr latent membrane protein 2–specific T-cell receptors. J Infect Dis. 2022;226(5):833–842. doi: 10.1093/infdis/jiaa512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng Y., Parsonage G., Zhuang X., Machado L.R., James C.H., Salman A., et al. Human Leukocyte Antigen (HLA) A*1101-restricted Epstein-Barr virus–specific t-cell receptor gene transfer to target nasopharyngeal carcinoma. Cancer Immunol Res. 2015;3:1138–1147. doi: 10.1158/2326-6066.CIR-14-0203-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krause A., Guo H.F., Latouche J.B., Tan C., Cheung N.K.V., Sadelain M. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J Exp Med. 1998;188:619–626. doi: 10.1084/jem.188.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125:3392–3400. doi: 10.1172/JCI80010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gargett T., Brown M.P. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T-cells. Front Pharmacol. 2014;5:1–7. doi: 10.3389/fphar.2014.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartmann J., Schüßler-Lenz M., Bondanza A., Buchholz C.J. Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9:1183–1197. doi: 10.15252/emmm.201607485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pule M.A., Savoldo B., Myers G.D., Rossig C., Russell H.V., Dotti G., et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Louis C.U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G.D., et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schulz G., Cheresh D.A., Varki N.M., Staffilano L.K., Reisfeld R.A., Yu A. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984;44:5914–5920. [PubMed] [Google Scholar]
- 65.Roth M., Linkowski M., Tarim J., Piperdi S., Sowers R., Geller D., et al. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer. 2014;120:548–554. doi: 10.1002/cncr.28461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kailayangiri S., Altvater B., Meltzer J., Pscherer S., Luecke A., Dierkes C., et al. The ganglioside antigen GD2 is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br J Cancer. 2012;106:1123–1133. doi: 10.1038/bjc.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altevogt P., Doberstein K., Fogel M. L1CAM in human cancer. Int J Cancer. 2016;138:1565–1576. doi: 10.1002/ijc.29658. [DOI] [PubMed] [Google Scholar]
- 68.Park J.R., DiGiusto D.L., Slovak M., Wright C., Naranjo A., Wagner J., et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 69.Gattenlöhner S., Marx A., Markfort B., Pscherer S., Landmeier S., Juergens H., et al. Rhabdomyosarcoma lysis by T cells expressing a human autoantibody-based chimeric receptor targeting the fetal acetylcholine receptor. Cancer Res. 2006;66:24–28. doi: 10.1158/0008-5472.CAN-05-0542. [DOI] [PubMed] [Google Scholar]
- 70.Prapa M., Caldrer S., Spano C., Bestagno M., Golinelli G., Grisendi G., et al. A novel anti-GD2/4-1BB chimeric antigen receptor triggers neuroblastoma cell killing. Oncotarget. 2015;6:24884–24894. doi: 10.18632/oncotarget.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Straathof K., Flutter B., Wallace R., Jain N., Loka T., Depani S., et al. Antitumor activity without on-target off-tumor toxicity of GD2–chimeric antigen receptor T cells in patients with neuroblastoma. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abd6169. [DOI] [PubMed] [Google Scholar]
- 72.Castriconi R., Dondero A., Augugliaro R., Cantoni C., Carnemolla B., Sementa A.R., et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101:12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L., Zhang Q., Chen W., Shan B., Ding Y., Zhang G., et al. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One. 2013;8:4–11. doi: 10.1371/journal.pone.0070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orentas R.J., Yang J.J., Wen X., Wei J.S., Mackall C.L., Khan J. Identification of cell surface proteins as potential immunotherapy targets in 12 pediatric cancers. Front Oncol. 2012;2:1–16. doi: 10.3389/fonc.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Majzner R.G., Theruvath J.L., Nellan A., Heitzeneder S., Cui Y., Mount C.W., et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25:2560–2574. doi: 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Du H., Hirabayashi K., Ahn S., Kren N.P., Montgomery S.A., Wang X., et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell. 2019;35:221–237.e8. doi: 10.1016/j.ccell.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen P., Okeke E., Clay M., Haydar D., Justice J., O’Reilly C., et al. Route of 41BB/41BBL costimulation determines effector function of B7-H3-CAR.CD28ζ T cells. Mol Ther Oncolytics. 2020;18:202–214. doi: 10.1016/j.omto.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Künkele A., Taraseviciute A., Finn L.S., Johnson A.J., Berger C., Finney O., et al. Preclinical assessment of CD171-directed CAR T-cell adoptive therapy for childhood neuroblastoma: CE7 epitope target safety and product manufacturing feasibility. Clin Cancer Res. 2017;23:466–477. doi: 10.1158/1078-0432.CCR-16-0354. [DOI] [PubMed] [Google Scholar]
- 79.Textor A., Grunewald L., Anders K., Klaus A., Schwiebert S., Winkler A., et al. Cd28 co-stimulus achieves superior car t cell effector function against solid tumors than 4-1bb co-stimulus. Cancers (Basel) 2021;13:1–17. doi: 10.3390/cancers13051050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heitzeneder S., Bosse K.R., Zhu Z., Zhelev D., Majzner R.G., Radosevich M.T., et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell. 2022;40(1):53–69. doi: 10.1016/j.ccell.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gilbertson R.J. ERBB2 in pediatric cancer: innocent until proven guilty. Oncologist. 2005;10:508–517. doi: 10.1634/theoncologist.10-7-508. [DOI] [PubMed] [Google Scholar]
- 82.Ahmed N., Salsman V.S., Yvon E., Louis C.U., Perlaky L., Wels W.S., et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther. 2009;17:1779–1787. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rainusso N., Brawley V.S., Ghazi A., Hicks M.J., Gottschalk S., Rosen J.M., et al. Immunotherapy targeting HER2 with genetically modified T cells eliminates tumor-initiating cells in osteosarcoma. Cancer Gene Ther. 2012;19:212–217. doi: 10.1038/cgt.2011.83. [DOI] [PubMed] [Google Scholar]
- 84.Ahmed N., Brawley V.S., Hegde M., Robertson C., Ghazi A., Gerken C., et al. Human epidermal growth factor receptor 2 (HER2) - specific chimeric antigen receptor - modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li N., Gao W., Zhang Y.-F., Ho M. Glypicans as cancer therapeutic targets. Trends Cancer. 2018;4:741–754. doi: 10.1016/j.trecan.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bosse K.R., Raman P., Zhu Z., Lane M., Martinez D., Heitzeneder S., et al. Identification of GPC2 as an oncoprotein and candidate immunotherapeutic target in high-risk neuroblastoma. Cancer Cell. 2017;32:295–309. doi: 10.1016/j.ccell.2017.08.003. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li N., Torres M.B., Spetz M.R., Wang R., Peng L., Tian M., et al. CAR T cells targeting tumor-associated exons of glypican 2 regress neuroblastoma in mice. Cell Reports Med. 2021;2 doi: 10.1016/j.xcrm.2021.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li W., Guo L., Rathi P., Marinova E., Gao X., Wu M.F., et al. Redirecting T cells to glypican-3 with 4-1BB zeta chimeric antigen receptors results in Th1 polarization and potent antitumor activity. Hum Gene Ther. 2017;28:437–448. doi: 10.1089/hum.2016.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun L., Gao F., Gao Z., Ao L., Li N., Ma S., et al. Shed antigen-induced blocking effect on CAR-T cells targeting Glypican-3 in Hepatocellular Carcinoma. J Immunother Cancer. 2021;9:1–14. doi: 10.1136/jitc-2020-001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pulè M.A., Straathof K.C., Dotti G., Heslop H.E., Rooney C.M., Brenner M.K. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 91.Heczey A., Louis C.U., Savoldo B., Dakhova O., Durett A., Grilley B., et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther. 2017;25:2214–2224. doi: 10.1016/j.ymthe.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Del Bufalo F., De Angelis B., Caruana I., Del Baldo G., De Ioris M.A., Serra A., et al. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N Engl J Med. 2023;388(14):1284–1295. doi: 10.1056/NEJMoa2210859. [DOI] [PubMed] [Google Scholar]
- 93.Jiang Z., Jiang X., Chen S., Lai Y., Wei X., Li B., et al. Anti-GPC3-CAR T cells suppress the growth of tumor cells in patient-derived xenografts of hepatocellular carcinoma. Front Immunol. 2017;7:1–10. doi: 10.3389/fimmu.2016.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y., Sun C., Landoni E., Metelitsa L., Dotti G., Savoldo B. Eradication of neuroblastoma by T cells redirected with an optimized GD2-specific chimeric antigen receptor and interleukin-15. Clin Cancer Res. 2019;25:2915–2924. doi: 10.1158/1078-0432.CCR-18-1811. [DOI] [PubMed] [Google Scholar]
- 95.Batra S.A., Rathi P., Guo L., Courtney A.N., Fleurence J., Balzeau J., et al. Glypican-3-specific CAR T cells coexpressing IL15 and IL21 have superior expansion and antitumor activity against hepatocellular carcinoma. Cancer Immunol Res. 2020;8:309–320. doi: 10.1158/2326-6066.CIR-19-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun Y., Dong Y., Sun R., Liu Y., Wang Y., Luo H., et al. Chimeric anti-GPC3 sFv-CD3ε receptor-modified T cells with IL7 co-expression for the treatment of solid tumors. Mol Ther Oncolytics. 2022;25:160–173. doi: 10.1016/j.omto.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li G., Zhang Q., Han Z., Zhu Y., Shen H., Liu Z., et al. IL-7 and CCR2b co-expression-mediated enhanced CAR-T survival and infiltration in solid tumors. Front Oncol. 2021;11:1–9. doi: 10.3389/fonc.2021.734593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Glienke W., Dragon A.C., Zimmermann K., Martyniszyn-Eiben A., Mertens M., Abken H., et al. GMP-compliant manufacturing of TRUCKs: CAR T cells targeting GD2 and releasing inducible IL-18. Front Immunol. 2022;13:1–17. doi: 10.3389/fimmu.2022.839783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zimmermann K., Kuehle J., Dragon A.C., Galla M., Kloth C., Rudek L.S., et al. Design and characterization of an “all-in-one” lentiviral vector system combining constitutive anti-gd2 car expression and inducible cytokines. Cancers (Basel) 2020;12 doi: 10.3390/cancers12020375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma X., Shou P., Smith C., Chen Y., Du H., Sun C., et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat Biotechnol. 2020;38:448–459. doi: 10.1038/s41587-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brudno J.N., Kochenderfer J.N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu L., Huang L., Lin D., Lai X., Wu L., Liao X., et al. GD2-specific chimeric antigen receptor-modified T cells for the treatment of refractory and/or recurrent neuroblastoma in pediatric patients. J Cancer Res Clin Oncol. 2021;148(10):2643–2652. doi: 10.1007/s00432-021-03839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu X., Zhao W., Yue Z., Qin M., Jin M., Chang L.J., et al. 4SCAR-GD2-modified T-cell therapy in neuroblastoma with MYCN amplification: a case report with over 4-year follow-up data. Pediatr Investig. 2020;4:55–58. doi: 10.1002/ped4.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang L., Ma X., Liu Y.-C., Zhao W., Yu L., Qin M., et al. Chimeric antigen receptor 4SCAR-GD2-modified T cells targeting high-risk and recurrent neuroblastoma: a phase II multi-center trial in China. Blood. 2017;130:3335. doi: 10.1182/blood.V130.Suppl_1.3335.3335. [DOI] [Google Scholar]
- 105.Wang X., Chang W.C., Wong C.L.W., Colcher D., Sherman M., Ostberg J.R., et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Summers C., Grier A., Gardner R., Delaney C., Jensen M.C. Multiplexed engineering of CD19CAR T cells for post-transplant consolidative immunotherapy. Blood. 2016;128 doi: 10.1182/blood.v128.22.1159.1159. 1159–1159. [DOI] [Google Scholar]
- 107.Jones B.S., Lamb L.S., Goldman F., Di Stasi A. Improving the safety of cell therapy products by suicide gene transfer. Front Pharmacol. 2014;5:254. doi: 10.3389/fphar.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh N., Liu X., Hulitt J., Jiang S., June C.H., Grupp S.A., et al. Nature of tumor control by permanently and transiently modified GD2 chimeric antigen receptor T cells in xenograft models of neuroblastoma. Cancer Immunol Res. 2014;2:1059–1070. doi: 10.1158/2326-6066.CIR-14-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rajan T.S., Gugliandolo A., Bramanti P., Mazzon E. In vitro-transcribed mrna chimeric antigen receptor T cell (IVT mrna car T) therapy in hematologic and solid tumor management: a preclinical update. Int J Mol Sci. 2020;21:1–13. doi: 10.3390/ijms21186514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duijkers F.A.M., Gaal J., Meijerink J.P.P., Admiraal P., Pieters R., De Krijger R.R., et al. High anaplastic lymphoma kinase immunohistochemical staining in neuroblastoma and ganglioneuroblastoma is an independent predictor of poor outcome. Am J Pathol. 2012;180:1223–1231. doi: 10.1016/j.ajpath.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 111.Trigg R.M., Turner S.D. ALK in neuroblastoma: biological and therapeutic implications. Cancers (Basel) 2018;10 doi: 10.3390/cancers10040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Halliwell E., Vitali A., Muller H., Alonso-Ferrero M., Barisa M., Gavriil A., et al. Targeting of low ALK antigen density neuroblastoma using AND logic-gate engineered CAR-T cells. Cytotherapy. 2022;25:46–58. doi: 10.1016/j.jcyt.2022.10.007. [DOI] [PubMed] [Google Scholar]
- 113.Morsut L., Roybal K.T., Xiong X., Gordley R.M., Coyle S.M., Thomson M., et al. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell. 2016;164:780–791. doi: 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moghimi B., Muthugounder S., Jambon S., Tibbetts R., Hung L., Bassiri H., et al. Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat Commun. 2021;12:1–15. doi: 10.1038/s41467-020-20785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tomida A., Yagyu S., Nakamura K., Kubo H., Yamashima K., Nakazawa Y., et al. Inhibition of MEK pathway enhances the antitumor efficacy of chimeric antigen receptor T cells against neuroblastoma. Cancer Sci. 2021;112:4026–4036. doi: 10.1111/cas.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chulanetra M., Morchang A., Sayour E., Eldjerou L., Milner R., Lagmay J., et al. GD2 chimeric antigen receptor modified T cells in synergy with sub-toxic level of doxorubicin targeting osteosarcomas. Am J Cancer Res. 2020;10:674–687. [PMC free article] [PubMed] [Google Scholar]
- 117.Sulejmani O., Grunewald L., Andersch L., Schwiebert S., Klaus A., Winkler A., et al. Inhibiting lysine demethylase 1a improves l1cam-specific car T cell therapy by unleashing antigen-independent killing via the FAS-FASL axis. Cancers (Basel) 2021;13:1–18. doi: 10.3390/cancers13215489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu X., Luo H., Shi B., Di S., Sun R., Su J., et al. Combined antitumor effects of sorafenib and GPC3-CAR T cells in mouse models of hepatocellular carcinoma. Mol Ther. 2019;27:1483–1494. doi: 10.1016/j.ymthe.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wienke J., Dierselhuis M.P., Tytgat G.A.M., Künkele A., Nierkens S., Molenaar J.J. The immune landscape of neuroblastoma: challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur J Cancer. 2021;144:123–150. doi: 10.1016/j.ejca.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 120.Koumarianou A., Duran-Moreno J. The sarcoma immune landscape: emerging challenges, prognostic significance and prospective impact for immunotherapy approaches. Cancers (Basel) 2021;13:1–16. doi: 10.3390/cancers13030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sherif S., Roelands J., Mifsud W., Ahmed E.I., Raynaud C.M., Rinchai D., et al. The immune landscape of solid pediatric tumors. J Exp Clin Cancer Res. 2022;41:1–18. doi: 10.1186/s13046-022-02397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gargett T., Yu W., Dotti G., Yvon E.S., Christo S.N., Hayball J.D., et al. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther. 2016;24:1135–1149. doi: 10.1038/mt.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tumino N., Weber G., Besi F., Del Bufalo F., Bertaina V., Paci P., et al. Polymorphonuclear myeloid-derived suppressor cells impair the anti-tumor efficacy of GD2.CAR T-cells in patients with neuroblastoma. J Hematol Oncol. 2021;14:1–7. doi: 10.1186/s13045-021-01193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Long A.H., Highfill S.L., Cui Y., Smith J.P., Walker A.J., Ramakrishna S., et al. Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunol Res. 2016;4:869–880. doi: 10.1158/2326-6066.CIR-15-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fultang L., Booth S., Yogev O., da Costa B.M., Tubb V., Panetti S., et al. Metabolic engineering against the arginine microenvironment enhances CAR-T cell proliferation and therapeutic activity. Blood. 2020;136:1155–1160. doi: 10.1182/blood.2019004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Introna M., Correnti F. Innovative clinical perspectives for cik cells in cancer patients. Int J Mol Sci. 2018;19(2):358. doi: 10.3390/ijms19020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pievani A., Borleri G., Pende D., Moretta L., Rambaldi A., Golay J., et al. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118:3301–3310. doi: 10.1182/blood-2011-02-336321. [DOI] [PubMed] [Google Scholar]
- 128.Nishimura R., Baker J., Beilhack A., Zeiser R., Olson J.A., Sega E.I., et al. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood. 2008;112:2563–2574. doi: 10.1182/blood-2007-06-092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leuci V., Donini C., Grignani G., Rotolo R., Mesiano G., Fiorino E., et al. CSPG4-specific CAR. CIK lymphocytes as a novel therapy for the treatment of multiple soft-tissue sarcoma histotypes. Clin Cancer Res. 2020;26:6321–6334. doi: 10.1158/1078-0432.CCR-20-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Merker M., Wagner J., Kreyenberg H., Heim C., Moser L.M., Wels W.S., et al. ERBB2-CAR-engineered cytokine-induced killer cells exhibit both CAR-mediated and innate immunity against high-risk rhabdomyosarcoma. Front Immunol. 2020;11:1–13. doi: 10.3389/fimmu.2020.581468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dharia N.V., Kugener G., Guenther L.M., Clare F., Durbin A.D., Hong A.L., et al. A first-generation pediatric cancer dependency map. Nat Genet. 2021;53:529–538. doi: 10.1038/s41588-021-00819-w.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yarmarkovich M., Marshall Q.F., Warrington J.M., Premaratne R., Farrel A., Groff D., et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature. 2021;599:477–484. doi: 10.1038/s41586-021-04061-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 133.Razeghian E., Nasution M.K.M., Rahman H.S., Gardanova Z.R., Abdelbasset W.K., Aravindhan S., et al. A deep insight into CRISPR/Cas9 application in CAR-T cell-based tumor immunotherapies. Stem Cell Res Ther. 2021;12(1):17. doi: 10.1186/s13287-021-02510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fauriat C., Long E.O., Ljunggren H., Bryceson Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Immunobiology. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang W., Zhao Z., Li F. Natural killer cell dysfunction in cancer and new strategies to utilize NK cell potential for cancer immunotherapy. Mol Immunol. 2022;144:58–70. doi: 10.1016/j.molimm.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 136.Bi J., Tian Z. NK cell exhaustion. Front Immunol. 2017;8:760. doi: 10.3389/fimmu.2017.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Balch C.M., Tilden A.B., Dougherty P.A., Cloud G.A. Depressed levels of granular lymphocytes with natural killer (NK) cell function in 247 cancer patients. Ann Surg. 1983;198:192–199. doi: 10.1097/00000658-198308000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guillerey C., Smyth M.J. NK cells and cancer immunoediting. Curr Top Microbiol Immunol. 2016;395:115–145. doi: 10.1007/82_2015_446. [DOI] [PubMed] [Google Scholar]
- 139.Buddingh E.P., Schilham M.W., Ruslan S.E.N., Berghuis D., Szuhai K., Suurmond J., et al. Chemotherapy-resistant osteosarcoma is highly susceptible to IL-15-activated allogeneic and autologous NK cells. Cancer Immunol Immunother. 2011;60:575–586. doi: 10.1007/s00262-010-0965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morvan M.G., Lanier L.L. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16:7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 141.Wang W., Erbe A.K., Hank J.A., Morris Z.S., Sondel P.M. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ljunggren H.-G., Kaerre K. In search of the missing self MHC molecules and NK cell recognition. Encycl Genet Genomics, Proteomics Informatics. 2008;11(7):237–244. doi: 10.1007/978-1-4020-6754-9_10515. 1225–1225. [DOI] [PubMed] [Google Scholar]
- 143.Handgretinger R., Lang P., André M.C. Exploitation of natural killer cells for the treatment of acute leukemia. Blood. 2016;127:3341–3349. doi: 10.1182/blood-2015-12-629055. [DOI] [PubMed] [Google Scholar]
- 144.Rubnitz J.E., Inaba H., Kang G., Gan K., Hartford C., Triplett B.M., et al. Natural killer cell therapy in children with relapsed leukemia. Pediatr Blood Cancer. 2015;62:1468–1472. doi: 10.1002/pbc.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Miller J.S., Soignier Y., Panoskaltsis-Mortari A., McNearney S.A., Yun G.H., Fautsch S.K., et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 146.Ruggeri L., Capanni M., Urbani E., Perruccio K., Shlomchik W.D., Tosti A., et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(80):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 147.Yu S., Huang F., Wang Y., Xu Y., Yang T., Fan Z., et al. Haploidentical transplantation might have superior graft-versus-leukemia effect than HLA-matched sibling transplantation for high-risk acute myeloid leukemia in first complete remission: a prospective multicentre cohort study. Leukemia. 2020;34:1433–1443. doi: 10.1038/s41375-019-0686-3. [DOI] [PubMed] [Google Scholar]
- 148.Meazza R., Falco M., Loiacono F., Canevali P., Chiesa M.D., Bertaina A., et al. Phenotypic and functional characterization of NK cells in αβT-cell and B-cell depleted haplo-HSCT to cure pediatric patients with acute leukemia. Cancers (Basel) 2020;12:1–21. doi: 10.3390/cancers12082187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Delgado D., Webster D.E., DeSantes K.B., Durkin E.T., Shaaban A.F. KIR receptor-ligand incompatibility predicts killing of osteosarcoma cell lines by allogeneic NK cells. Pediatr Blood Cancer. 2010;23:1–7. doi: 10.1002/pbc.22665.KIR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Llosa N.J., Cooke K.R., Chen A.R., Gamper C.J., Klein O.R., Zambidis E.T., et al. Reduced-intensity haploidentical bone marrow transplantation with post-transplant cyclophosphamide for solid tumors in pediatric and young adult patients. Biol Blood Marrow Transplant. 2017;23:2127–2136. doi: 10.1016/j.bbmt.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang F., Sun X.F., Li Y.Q., et al. Safety of in vitro amplified HLA-haploidentical donor immune cell infusions for childhood malignancies. Chin J Cancer. 2013;32(12):661–666. doi: 10.5732/cjc.012.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rubnitz J.E., Inaba H., Ribeiro R.C., Pounds S., Rooney B., Bell T., et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yu A.L., Gilman A.L., Ozkaynak M.F., Naranjo A., Diccianni M.B., Gan J., et al. Long-term follow-up of a phase III study of ch14.18 (dinutuximab) + cytokine immunotherapy in children with high-risk neuroblastoma: COG study ANBL0032. Clin Cancer Res. 2021;27:2179–2189. doi: 10.1158/1078-0432.CCR-20-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park J.A., Cheung N.K.V. Targets and antibody formats for immunotherapy of neuroblastoma. J Clin Oncol. 2020;38:1836–1848. doi: 10.1200/JCO.19.01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kushner B.H., Cheung I.Y., Modak S., Basu E.M., Roberts S.S., Cheung N.K. Humanized 3F8 anti-GD2 monoclonal antibody dosing with granulocyte-macrophage colony-stimulating factor in patients with resistant neuroblastoma: a phase 1 clinical trial. JAMA Oncol. 2018;4:1729–1735. doi: 10.1001/jamaoncol.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barry W.E., Jackson J.R., Asuelime G.E., Wu H.-W., Sun J., Wan Z., et al. Activated natural killer cells in combination with anti-GD2 antibody dinutuximab improve survival of mice after surgical resection of primary neuroblastoma. Clin Cancer Res. 2019;25:325–333. doi: 10.1158/1078-0432.CCR-18-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Navid F., Sondel P.M., Barfield R., Shulkin B.L., Kaufman R.A., Allay J.A., et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14. 18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. 2014;32(14):1445–1452. doi: 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Federico S.M., Mccarville M.B., Shulkin B.L., Sondel P.M., Hank J.A., Hutson P., et al. A pilot trial of humanized anti-GD2 monoclonal antibody (hu14. 18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res. 2017;23:6441–6449. doi: 10.1158/1078-0432.CCR-17-0379.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Modak, Luduec S, Le J, Cheung IY, Goldman DA, Ostrovnaya I, et al. Adoptive immunotherapy with haploidentical natural killer cells and anti-GD2 monoclonal antibody m3F8 for resistant neuroblastoma: results of a phase I study. Oncoimmunology 2018;7(8):1–10. e1461305. 10.1080/2162402X.2018.1461305. [DOI] [PMC free article] [PubMed]
- 160.Mestas J., Burdick M.D., Reckamp K., Figlin R.A., Strieter R.M., Mestas J., et al. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J Immunol. 2005;175:5351–5357. doi: 10.4049/jimmunol.175.8.5351. [DOI] [PubMed] [Google Scholar]
- 161.Kremer V., Ligtenberg M., Zendehdel R., Seitz C., Duivenvoorden A., Wennerberg E., et al. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J Immunother Cancer. 2017;5:1–14. doi: 10.1186/s40425-017-0275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu E., Tong Y., Dotti G., Shaim H., Savoldo B., Mukherjee M., et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32:520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382:545–553. doi: 10.1056/nejmoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:1–4. doi: 10.4161/onci.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu Y., Tian Z., Wei H. Developmental and functional control of natural killer cells by cytokines. Front Immunol. 2017;8:930. doi: 10.3389/fimmu.2017.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Parihar R., Rivas C., Huynh M., Omer B., Lapteva N., Metelitsa L.S., et al. NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol Res. 2019;7:363–375. doi: 10.1158/2326-6066.CIR-18-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moon E.K., Wang L.-C., Dolfi D.V., Wilson C.B., Ranganathan R., Sun J., et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor–transduced human T cells in solid tumors. Clin Cancer Res. 2014;20:4262–4273. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bendelac A., Killeen N., Littman D.R., Schwartz R.H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263(80):1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 169.Gadola S.D., Dulphy N., Salio M., Cerundolo V. Vα24-JαQ-independent, CD1d-restricted recognition of α-galactosylceramide by human CD4+ and CD8αβ+ T lymphocytes. J Immunol. 2002;168:5514–5520. doi: 10.4049/jimmunol.168.11.5514. [DOI] [PubMed] [Google Scholar]
- 170.Lanier L.L., Chang C., Phillips J.H. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol. 1994;153:2417–2428. doi: 10.4049/jimmunol.153.6.2417. [DOI] [PubMed] [Google Scholar]
- 171.Dellabona P., Padovan E., Casorati G., Brockhaus M., Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8-T cells. J Exp Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lantz O., Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8-T cells in mice and humans. J Exp Med. 1994;180:1077–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., et al. CD1d-restricted and TCR-mediated activation of V(α)14 NKT cells by glycosylceramides. Science. 1997;278(80):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 175.Hishiki T., Mise N., Harada K., Ihara F., Takami M., Saito T., et al. Invariant natural killer T infiltration in neuroblastoma with favorable outcome. Pediatr Surg Int. 2018;34:195–201. doi: 10.1007/s00383-017-4189-x. [DOI] [PubMed] [Google Scholar]
- 176.Song L., Asgharzadeh S., Salo J., Engell K., Wu H., Sposto R., et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Metelitsa L.S., Wu H.-W., Wang H., Yang Y., Warsi Z., Asgharzadeh S., et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–1221. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Song L., Ara T., Wu H.-W., Woo C.-W., Reynolds C.P., Seeger R.C., et al. Oncogene MYCN regulates localization of NKT cells to the site of disease in neuroblastoma. J Clin Invest. 2007;117:2702–2712. doi: 10.1172/JCI30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hishiki T., Mise N., Harada K., Ihara F., Takami M., Saito T., et al. Frequency and proliferative response of circulating invariant natural killer T cells in pediatric patients with malignant solid tumors. Pediatr Surg Int. 2018;34:169–176. doi: 10.1007/s00383-017-4185-1. [DOI] [PubMed] [Google Scholar]
- 180.Heczey A., Liu D., Tian G., Courtney A.N., Wei J., Marinova E., et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124:2824–2833. doi: 10.1182/blood-2013-11-541235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Matsuda J.L., Gapin L., Sidobre S., Kieper W.C., Tan J.T., Ceredig R., et al. Homeostasis of Vα14i NKT cells. Nat Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 182.Baev D.V., Peng X.H., Song L., Barnhart J.R., Crooks G.M., Weinberg K.I., et al. Distinct homeostatic requirements of CD4+ and CD4- subsets of Vα24-invariant natural killer T cells in humans. Blood. 2004;104:4150–4156. doi: 10.1182/blood-2004-04-1629. [DOI] [PubMed] [Google Scholar]
- 183.Liu D., Song L., Wei J., Courtney A.N., Gao X., Marinova E., et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest. 2012;122:2221–2233. doi: 10.1172/JCI59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liao Y.M., Hung T.H., Tung J.K., Yu J., Hsu Y.L., Hung J.T., et al. Low expression of il-15 and nkt in tumor microenvironment predicts poor outcome of mycn-non-amplified neuroblastoma. J Pers Med. 2021;11:1–14. doi: 10.3390/jpm11020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xu X., Huang W., Heczey A., Liu D., Guo L., Wood M., et al. NKT cells coexpressing a GD2-specific chimeric antigen receptor and IL15 show enhanced in vivo persistence and antitumor activity against neuroblastoma. Clin Cancer Res. 2019;25:7126–7138. doi: 10.1158/1078-0432.CCR-19-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Heczey A., Courtney A.N., Montalbano A., Robinson S., Liu K., Li M., et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. 2020;26:1686–1690. doi: 10.1038/s41591-020-1074-2. [DOI] [PubMed] [Google Scholar]
- 187.Heczey A., Xu X., Courtney A.N., Tian G., Barragan G.A., Guo L., et al. Anti-GD2 CAR-NKT cells in relapsed or refractory neuroblastoma: updated phase 1 trial interim results. Nat Med. 2023;29(6):1379–1388. doi: 10.1038/s41591-023-02363-y. [DOI] [PubMed] [Google Scholar]
- 188.Ngai H., Tian G., Courtney A.N., Ravari S.B., Guo L., Liu B., et al. IL-21 selectively protects CD62L+ NKT cells and enhances their effector functions for adoptive immunotherapy. J Immunol. 2018;201:678–687. doi: 10.4049/jimmunol.1800429.IL-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kitamura H., Iwakabe K., Yahata T., Nishimura S.I., Ohta A., Ohmi Y., et al. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–1127. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fujii S.I., Liu K., Smith C., Bonito A.J., Steinman R.M. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Carnaud C., Lee D., Donnars O., Park S.H., Beavis A., Koezuka Y., et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 192.Mise N., Takami M., Suzuki A., Kamata T., Harada K., Hishiki T., et al. Antibody-dependent cellular cytotoxicity toward neuroblastoma enhanced by activated invariant natural killer T cells. Cancer Sci. 2016;107:233–241. doi: 10.1111/cas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhao Y., Niu C., Cui J. Gamma-delta (γδ) T cells: friend or foe in cancer development. J Transl Med. 2018;16(1):3. doi: 10.1186/s12967-017-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Silva-santos B., Serre K., Norell H. γδ T cells in cancer. Nat Rev Immunol. 2015;15(11):683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 195.De Rosa S.C., Andrus J.P., Perfetto S.P., Mantovani J.J., Herzenberg L.A., Herzenberg L.A., et al. Ontogeny of γδ T cells in humans. J Immunol. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 196.Joalland N., Scotet E. Emerging challenges of preclinical models of anti-tumor immunotherapeutic strategies utilizing Vγ9Vδ2 T Cells. Front Immunol. 2020;11:992. doi: 10.3389/fimmu.2020.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kabelitz D. γδ T-cells cross-talk between innate and adaptive immunity. Cell Mol Life Sci. 2011;68:2331–2333. doi: 10.1007/s00018-011-0696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dieli F., Gebbia N., Poccia F., Caccamo N., Montesano C., Fulfaro F., et al. Induction of gd T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 199.Dieli F., Troye-Blomberg M., Ivanyi J., Fournié J.J., Krensky A.M., Bonneville M., et al. Granulysin-dependent killing of intracellular and extracellular mycobacterium tuberculosis by V-γ9/Vδ2 T lymphocytes. J Infect Dis. 2001;184:1082–1085. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 200.Brandes M., Willmann K., Bioley G., Levy N., Eberl M., Luo M., et al. Cross-presenting human gd T cells induce robust CD8+ ab T cell responses. Proc Natl Acad Sci U S A. 2009;106:2307–2312. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fisher J.P.H., Heuijerjans J., Yan M., Gustafsson K., Anderson J. γδ T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology. 2014;3(1) doi: 10.4161/onci.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Landin A.M., Cox C., Yu B., Bejanyan N., Davila M., Kelley L. Expansion and enrichment of gamma-delta (γδ) t cells from apheresed human product. J Vis Exp. 2021 doi: 10.3791/62622. 175. [DOI] [PubMed] [Google Scholar]
- 203.Tan W.K., Tay J.C., Zeng J., Zheng M., Wang S. Expansion of gamma delta T cells - a short review on bisphosphonate and K562-based methods. J Immunol Sci. 2018;2:6–12. doi: 10.29245/2578-3009/2018/3.1133. [DOI] [Google Scholar]
- 204.Ferry G.M., Agbuduwe C., Forrester M., Dunlop S., Chester K., Fisher J., et al. A simple and robust single-step method for CAR-Vδ1 γδT cell expansion and transduction for cancer immunotherapy. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.863155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Correia D.V., Fogli M., Hudspeth K., Gomes Da Silva M., Mavilio D., Silva-Santos B. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood. 2011;118:992–1001. doi: 10.1182/blood-2011-02-339135. [DOI] [PubMed] [Google Scholar]
- 206.Fisher J.P.H., Yan M., Heuijerjans J., Carter L., Abolhassani A., Frosch J., et al. Neuroblastoma killing properties of Vδ2 and Vδ2-negative γδT cells following expansion by artificial antigen-presenting cells. Clin Cancer Res. 2014;20:5720–5732. doi: 10.1158/1078-0432.CCR-13-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Raffaghello L., Prigione I., Airoldi I., Camoriano M., Morandi F., Bocca P., et al. Mechanisms of immune evasion of human neuroblastoma. Cancer Lett. 2005;228:155–161. doi: 10.1016/j.canlet.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 208.Raffaghello L., Prigione I., Airoldi I., Camoriano M., Levreri I., Gambini C., et al. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia. 2004;6:558–568. doi: 10.1593/neo.04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Himoudi N., Morgenstern D.A., Yan M., Vernay B., Saraiva L., Wu Y., et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol. 2012;188:1708–1716. doi: 10.4049/jimmunol.1102654. [DOI] [PubMed] [Google Scholar]
- 210.Gertner-Dardenne J., Bonnafous C., Bezombes C., Capietto A.H., Scaglione V., Ingoure S., et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–4884. doi: 10.1182/blood-2008-08-172296. [DOI] [PubMed] [Google Scholar]
- 211.Tokuyama H., Hagi T., Mattarollo S.R., Morley J., Wang Q., Fai-So H., et al. Vγ9Vδ2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs - rituximab and trastuzumab. Int J Cancer. 2008;122:2526–2534. doi: 10.1002/ijc.23365. [DOI] [PubMed] [Google Scholar]
- 212.Fisher J.P.H., Flutter B., Wesemann F., Frosch J., Rossig C., Gustafsson K., et al. Effective combination treatment of GD2-expressing neuroblastoma and Ewing’s sarcomausing anti-GD2 ch14.18/CHO antibody with Vγ9Vδ2+γδT cells. Oncoimmunology. 2016;5(1):9. doi: 10.1080/2162402X.2015.1025194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dörrie J., Krug C., Hofmann C., Müller I., Wellner V., Knippertz I., et al. Human adenovirus-specific γ/δ and CD8+ T cells generated by T-cell receptor transfection to treat adenovirus infection after allogeneic stem cell transplantation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Harrer D.C., Simon B., Fujii S., Shimizu K., Uslu U., Schuler G., et al. RNA-transfection of γ/δ T cells with a chimeric antigen receptor or an α/β T-cell receptor: a safer alternative to genetically engineered α/β T cells for the immunotherapy of melanoma. BMC Cancer. 2017;17 doi: 10.1186/s12885-017-3539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Beatty G.L., Gladney W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Mil Med. 2006;171:844–848. doi: 10.7205/MILMED.171.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Marcu-Malina V., Heijhuurs S., Van Buuren M., Hartkamp L., Strand S., Sebestyen Z., et al. Redirecting αβT cells against cancer cells by transfer of a broadly tumor-reactive γδT-cell receptor. Blood. 2011;118:50–59. doi: 10.1182/blood-2010-12-325993. [DOI] [PubMed] [Google Scholar]
- 217.Straetemans T., Gründer C., Heijhuurs S., Hol S., Slaper-Cortenbach I., Bönig H., et al. Untouched GMP-ready purified engineered immune cells to treat cancer. Clin Cancer Res. 2015;21:3957–3968. doi: 10.1158/1078-0432.CCR-14-2860. [DOI] [PubMed] [Google Scholar]
- 218.Strijker J.G.M., Pscheid R., Drent E., van der Hoek J.J.F., Koopmans B., Ober K., et al. αβ-T cells engineered to express γδ-T cell receptors can kill neuroblastoma organoids independent of MHC-I expression. J Pers Med. 2021;11(9):923. doi: 10.3390/jpm11090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rischer M., Pscherer S., Duwe S., Vormoor J., Jurgens H., Rossig C. Human γδ T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br J Haematol. 2004;126:583–592. doi: 10.1111/j.1365-2141.2004.05077.x. [DOI] [PubMed] [Google Scholar]
- 220.Fisher J., Abramowski P., Dilrukshi N., Don W., Flutter B., Capsomidis A., et al. Avoidance of on-target off-tumor activation using a co-stimulation-only chimeric antigen receptor. Mol Ther. 2017;25:1234–1247. doi: 10.1016/j.ymthe.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lauss M., Donia M., Harbst K., Andersen R., Mitra S., Rosengren F., et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun. 2017;8:1–11. doi: 10.1038/s41467-017-01460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Khong H.T., Wang Q.J., Rosenberg S.A. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother. 2004;27:184–190. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material