Abstract
The role of dendritic cells (DC) in the initiation of immune responses against foot-and-mouth disease virus (FMDV) is poorly understood. We analyzed the innate response of freshly isolated swine skin DC to the virus and show a rapid induction of beta interferon (IFN-β) mRNA but not IFN-α mRNA. However, these DC secreted both IFN-α and IFN-β proteins in response to live virus but not killed virus. Furthermore, the surface expression of swine major histocompatibility complex class II (SLA II) or CD80/CD86 molecules and antigen processing functions were not affected by FMDV exposure. Given the demonstrated sensitivity of FMDV to IFN-α/β, there was no productive or nonproductive infection of these cells. Finally, freshly isolated skin DC constitutively expressed intracellular IFN-α protein in the absence of stimulation, with no detectable secretion of the cytokine until virus exposure. In situ analysis of these DC showed that these cells express and store IFN-α in uninfected animals. This is the first demonstration of the constitutive expression of IFN-α in resident, tissue-derived DC and indicates that skin DC can play an important role in the innate immune response of swine to viral infections.
Foot-and-mouth disease virus (FMDV) causes a highly contagious vesicular disease in cloven-hoofed animals and is an economically important pathogen for the livestock industry. The devastation that can be caused by an FMD outbreak was recently demonstrated by the 2001 outbreak in the United Kingdom (18, 19, 26). That epidemic and the continuing report of outbreaks of this disease in other countries underscore the need for the development of more efficient vaccines and treatments that safely prevent FMDV dissemination.
Relatively little is known about the immune response to the virus in susceptible species, especially the early stages of the immune response. The virus targets epidermal cells expressing cellular receptors, which are all integrins (6, 22, 39), spreading from the initial site of infection via the blood. Levels of viremia peak between 2 and 3 days after infection, and there is a rapid development of antibodies correlating with viral clearance. Consistent with this, the presence of neutralizing antibodies correlates with protection against reinfection and is believed to be the important effector function in vaccinated animals (31, 33, 52). However, the role of innate and cellular immune responses in the protection induced by vaccines is not understood.
Recent studies have shown that alpha/beta interferon (IFN-α/β) may have an important role in the outcome of FMDV infection (10, 11, 63). Cells that are normally permissive for FMDV infection become resistant to productive virus replication upon stimulation with either IFN-α or IFN-β (10, 63). Similarly, in vivo inoculation of a recombinant adenovirus expressing the porcine IFN-α gene induced rapid protection against FMDV infection in swine (10) and significantly reduced the pathogenesis of FMDV in cattle (63). In vitro studies have shown that FMDV has evolved a mechanism to inhibit IFN-α/β responses (10) by blocking cap-dependent cellular protein synthesis as a result of the cleavage of the eukaryotic translation initiation factor eIF-4G mediated by the viral leader proteinase (14, 34, 43, 44, 48). In addition, our previous studies demonstrated that FMDV induces a transient lymphopenia and negatively modulates peripheral T-cell responses (3). These data suggest that FMDV evades both innate and adaptive immune responses during the critical acute phase of infection, when maximal virus shedding is observed.
Previous studies suggested that epidermal dendritic cells (DC) of swine (20) and cattle (13) may be susceptible to FMDV infection and that DC have a potential role in the establishment of FMDV infection in these species. Epidermal DC have been shown to be highly phagocytic, and upon antigen exposure, they migrate to the lymph nodes, where they induce primary T-cell responses (2). In previous studies, we characterized a cell population isolated from porcine skin explants by its migratory and low-density properties (4, 20). We demonstrated that this highly enriched population has all the phenotypic and functional characteristics of activated DC. Specifically, this DC population was shown to induce primary allogeneic T-cell responses in vitro and could be identified by the consistent coexpression of CD1 and SWC3a markers (4). These cells express high densities of SLA-II and CD80/CD86 and can process and present antigens.
The pathogenesis of FMDV infection has been studied by the use of attenuated leaderless virus variants derived from the A12 strain (LLA12). These variants lack the leader protease, which plays a key role in down-regulating host protein synthesis (10, 43). With LLA12-infected animals, no systemic spread of virus, no disseminated lesions, and viral clearance prior to the appearance of neutralizing antibodies were demonstrated (8). These results suggest a potential role of innate immunity in clearing infection in FMDV-infected animals. Given that the primary sites of FMDV infection are the skin and mucosal surfaces, understanding the role of DC in these tissues in the outcome of FMDV infection is critical for the development of strategies to prevent and control this important disease.
Studies with human and mouse systems have demonstrated the important role of DC in linking the innate and adaptive immune responses (42), and IFN-α plays a key role in this function (23, 24, 27). We initiated studies designed to understand the role of epidermal DC in innate immunity and the initiation of adaptive immune responses to FMDV infection in swine and analyzed the interaction of FDMV with skin-derived DC, testing opposing hypotheses. Specifically, either DC are infected by FMDV and mediate immune pathology by blocking proinflammatory responses and interfering with T-cell immunity or DC respond to virus exposure by initiating innate and adaptive immune responses and blocking virus spread. We determined that the exposure of skin DC to virulent or attenuated FMDV showed no evidence of infection and had no effect on the phagocytic activity or expression of SLA-II and the costimulatory molecules CD80/CD86 by DC. Furthermore, DC had vigorous IFN-α and β responses to an in vitro FMDV exposure, making these cells refractory to FMDV replication, a result which was due not only to a rapid induction of IFN-β expression but also to constitutive expression of IFN-α.
MATERIALS AND METHODS
Animals.
The animals used for this study were conventionally reared pigs obtained from a commercial farm that was free of specific pathogens. Animals were housed in the biocontainment level 3 isolation facilities of Plum Island Animal Disease Center and were cared for according to the procedures of the Institutional Animal Use and Care Committee. The pigs were maintained for several weeks or few months prior to euthanasia, at which time they were approximately 5 to 6 months old and completely healthy, with no signs of any skin disease condition.
Viruses.
The viruses used for this study comprised the following strains of FMDV: A24 (49), O Taiwan 1997 (17, 61), infectious clone A12 (A12-IC) (47), attenuated leaderless strain LLA12 (43), O-Manisa (53), O1 Campos (51), and the attenuated heparin-binding O1 Campos variant vCRM4 (51). Virus stocks used for in vitro exposure to DC were prepared by propagation on BHK-21 cells. The infectious FMDV strains A24, O Taiwan, O1 Campos, and A12-IC were purified by sucrose gradient centrifugation. The LLA12, O Manisa, and vCRM4 strains were used as culture fluid supernatants. Inactivation of purified viruses was done by exposure to a germicidal UV lamp.
Medium and cells.
Skin-derived DC were isolated based on their migratory and low-density properties as previously described (4). Briefly, DC that migrated out of skin explants after overnight culture were purified by density centrifugation, washed, and suspended in a culture medium consisting of RPMI-1640 supplemented with antibiotics, l-glutamine, and 10% fetal bovine serum. The percentage of DC based on costaining for CD1 and SWC3a (pSIRP, CD172) in the low-density cell population was in the range of 70 to 85%, consistent with our previous report (4). Pulmonary alveolar macrophages (>95% CD1− SWC3a+) and peripheral blood mononuclear cells were collected from the same swine as described elsewhere (5).
In vitro FMDV exposure and sample collection.
To determine the effect of FMDV exposure on DC function, we cultured isolated cells in suspension at 106 cells/ml and exposed them to the indicated FMDV strain at a multiplicity of infection (MOI) of 1 for 1 h (virus adsorption), after which they were washed and resuspended in complete medium. Cells from the first time point, 0 h, were immediately centrifuged at 400 × g for 10 min and then processed as described below for cell staining, RNA isolation, or isolation of live virus. Later samples were harvested at the indicated time points, centrifuged at 400 × g for 10 min, and processed as indicated. Culture supernatants were collected in separate tubes for analyses of cytokine secretion. Cells were immediately analyzed by flow cytometry for costimulatory surface molecules, mounted on microscope slides by use of a Cytospin3 centrifuge (Shandon Lipshaw, Pittsburgh, Pa.) and fixed with acetone for immunostaining, or collected in TRIZOL reagent (Invitrogen, Carlsbad, Calif.) for RNA isolation. All supernatant, cytospin, and TRIZOL samples were stored at −70°C until analysis.
Analysis of virus replication in DC.
To determine whether skin DC support FMDV replication, we incubated cells for 1 h with the virus at an MOI of 2 at 37°C in a 5% CO2 incubator. After virus adsorption, the cells were washed and subjected to a brief mild acid treatment to remove the virus inoculum. This treatment was performed by incubating cells with morpholineethanesulfonic acid (MES), pH 5.5, on ice for 1 min, after which the cells were washed, resuspended in medium, and either collected immediately at time zero or cultured in fresh complete medium for 3 or 24 h. Culture supernatants were collected and analyzed for virus progeny by plaque titration on BHK cells. Cells were collected in TRIZOL for RNA isolation and an analysis of viral RNA levels by real-time PCR as previously described (41, 46). Virus replication was also assessed by immunoprecipitation analysis with a bovine polyclonal anti-FMDV serum which reacts with all structural and nonstructural proteins of FMDV, as previously described (31). Briefly, cells were exposed to virus for 2 h, starved for 30 min (incubated in medium lacking methionine), and then incubated overnight in medium containing reduced methionine and 35S-labeled methionine. Cell lysates were prepared and immunoprecipitated with the polyclonal bovine antiserum, and samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography.
Flow cytometry analysis.
To determine the effect of FMDV on the expression of costimulatory molecules on DC, we stained cells with the 05h2-18-1 (Pharmingen, San Diego, Calif.) or hCTLA-4-mFc (Ancell, Bayport, Minn.) monoclonal antibody to detect the expression of the SLA-II and CD80/86 markers, respectively. Background levels of fluorescence were determined by staining with the corresponding isotype controls. Flow cytometry analysis was performed by use of a FACSCalibur flow cytometer with Cell Quest software (Becton Dickinson, San Jose, Calif.). Data are expressed as mean fluorescent intensities (MFI) after subtraction of the background levels.
Phagocytosis and antigen-processing analysis.
The effect of FMDV on the phagocytic and antigen-processing functions of DC was tested by analyzing the uptake and processing of DQ-ovalbumin (Molecular Probes Inc., Eugene, Oreg.) as previously described (4). Briefly, cells were incubated in the presence or absence of the virus at an MOI of 1 for 2 h at 37°C prior to the assay. The cells were then chilled on ice and exposed to DQ-ovalbumin, after which a set of samples were transferred to 37°C and incubated for 2 h. Background fluorescence was determined from samples that were incubated on ice for 2 h in the presence of DQ-ovalbumin. After the 2-h incubation, the samples were washed, fixed with 2% formaldehyde, and analyzed by flow cytometry. The specific MFI for each sample was calculated by subtracting the background MFI of the corresponding 4°C samples from the MFI of the 37°C samples. Data are expressed as arbitrary units relative to the specific MFI of medium control (MC) samples at 37°C. To assess whether FMDV exposure had a significant effect on the uptake and processing of DQ-ovalbumin or on costimulatory molecule expression, we subjected the data to analysis of variance.
Real-time PCR analysis.
The isolation of total cellular RNA was performed according to the recommendations of the TRIZOL reagent manufacturer (Invitrogen). Prior to cDNA synthesis, approximately 1 μg of total RNA was treated with DNase (Sigma, St. Louis, Mo.) as directed by the manufacturer. The synthesis of cDNA was performed with 100 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen) in 25-μl reaction mixtures containing 5 μg of random hexamers/ml, a 600 nM concentration of each deoxynucleoside triphosphate, and 1 U of RNase inhibitor/μl by standard procedures.
The primers and TaqMan minor groove binding probes were designed with Primer Express software, version 1.5 (Applied Biosystems, Foster City, Calif.), based on sequences available in GenBank and following the criteria recommended by Applied Biosystems. In addition, when possible, primers for cytokine analysis were selected to amplify a DNA segment to which the probe annealed with sequences comprising two exons. The exon-intron boundaries were determined based on available sequence information or were predicted based on alignments with related genomic cytokine sequences available for humans or other species. Primers and probes for FMDV RNA analysis were developed for a pen-side diagnostic assay of the conserved 5′ untranslated region, which allows the quantitative measure of viral RNAs for various FMDV serotypes, as previously described (41, 46). Using this assay, we could detect 1 to 100 viral genomes in a clinical sample (9). Primers were purchased from Invitrogen, and 6-carboxyfluorescein-labeled TaqMan minor groove binding probes were purchased from Applied Biosystems. Table 1 shows the sequences of the primers and probes used for this study, the accession numbers from which the sequences were derived, and the optimized concentrations of primers and probes used for real-time PCR analysis.
TABLE 1.
Primers and probes used for real-time PCR analysis of porcine genes
| Primer or probe namea | Sequence (5′-3′) | Concentration (nM) | Accession no. |
|---|---|---|---|
| IFN-beta-11F | AGTGCATCCTCCAAATCGCT | 100 | S41178 |
| IFN-beta-69R | GCTCATGGAAAGAGCTGTGGT | 100 | S41178 |
| IFN beta-32T | TCCTGATGTGTTTCTC | 200 | S41178 |
| IFN-alpha-236F | TGGTGCATGAGATGCTCCA | 100 | M28623 |
| IFN-alpha-290R | GCCGAGCCCTCTGTGCT | 100 | M28623 |
| IFN-alpha-256T | CAGACCTTCCAGCTCT | 300 | M28623 |
| TNF apha-354F | AACCCTCTGGCCCAAGGA | 100 | X57321 |
| TNF apha-410R | GGCGACGGGCTTATCTGA | 100 | X57321 |
| TNF apha-373T | TCAGATCATCGTCTCAAAC | 200 | X57321 |
| 18S rRNA-178F | GCATTCGTATTGCGCCG | 50 | AF102857 |
| 18S rRNA-228R | CCGTCTTGCGCCGGT | 50 | AF102857 |
| 18S rRNA-196T | CAAGAATTTCACCTCTA | 150 | AF102857 |
F, forward primer; R, reverse primer; T, Taqman minor binding groove FAM-labeled probe.
TaqMan real-time PCRs were performed by use of the ABI Prism 7700 sequence detection system (Applied Biosystems). Approximately 10 ng of cDNA (RNA equivalent) per 25-μl reaction was tested in duplicate or triplicate, with optimized concentrations of primers and probe and with TaqMan universal PCR master mix (Applied Biosystems), according to the standard protocol recommended by the manufacturer. The level of cytokine mRNA expression was determined by the comparative cycle threshold (CT) method (User bulletin no. 2, Applied Biosystems), with the 18S rRNA gene used as an endogenous reference to normalize the data for each sample. The relative mRNA expression for each cytokine tested in the virus-stimulated cells was expressed in arbitrary units relative to the levels obtained in the unstimulated samples.
To determine the copy numbers of transcripts per nanogram of total cellular RNA, we prepared standards for the quantitation of IFN-α and IFN-β. The RNA standards were prepared by in vitro transcription, with plasmids that were cloned to contain the complete coding sequences of the porcine IFNs (pIFNs) used as templates. The plasmid containing pIFN-α, KSpIFNA7.6, was digested with BamHI and transcribed with T3 polymerase. The plasmid containing IFN-β (PCI.porB1.4) was also digested with BamHI and was transcribed with T7 polymerase. In vitro transcription was performed according to protocols established by the Megascript kit manufacturer (Ambion, Austin, Tex.). The in vitro-transcribed RNAs were purified, and 50 ng was used to generate cDNA. The numbers of RNA molecules were calculated based on the nucleotide sequences of the transcripts. Tenfold serial dilutions of cDNA, corresponding to 106 to 1 molecules, were included in each plate as standards to calculate the number of IFN mRNA copies present in each sample tested in the same assay. The quantity of the total cellular RNA was determined based on a standard curve obtained for 18S rRNA by the use of 10-fold serial dilutions (corresponding to 106 to 1 fg) of a standard total cellular RNA which were included in each assay plate. The efficiency of amplification was similar for all of the cytokine genes included in this study, as determined by a ≤0.1 difference in the slopes of the curves obtained. Data for IFNs were expressed as numbers of mRNA transcripts per nanogram of total cellular RNA.
Analysis of IFN-α and -β secretion.
The secretion of IFN-α and -β by DC was evaluated by determining the biological activity of the IFNs and by measuring the levels of protein in the culture supernatants. The biological activity of IFNs was determined by a 50% plaque reduction of A12-IC in IBRS-2 cells (10, 37). The levels of IFN proteins were measured by enzyme-linked immunosorbent assays (ELISAs).
Levels of IFN-α in DC culture supernatants were determined by a solid sandwich ELISA (15, 28, 56), as optimized by Moraes et al. (37) and used routinely in our laboratory. Briefly, the monoclonal antibodies K9 and F17 (PBL Biomedical Laboratories, Piscataway, N.J.), both of the immunoglobulin G1 (IgG1) isotype (28, 56), were used as antigen capture and detecting antibodies, respectively. The K9 antibody was used at 1 μg/ml. The F17 antibody was labeled with biotin and used at a dilution of 1:1,000. Data are expressed as picograms of secreted cytokine per milliliter, calculated based on standard curves prepared with serial dilutions of recombinant porcine IFN-α (PBL Biomedical Laboratories), as reported by us (37) and others (59).
IFN-β secretion by DC was determined by an antigen capture ELISA developed in our laboratory (unpublished data). Briefly, antipeptide antibodies to the N and C termini of porcine IFN-β were prepared and purified from sera collected from immunized rabbits by the manufacturer (Zymed Laboratories). The anti-N-pIFN-β antibody was used as the capture antibody at 2 μg/ml. The anti-C-pIFN-β antibody was labeled with biotin and used as the detecting antibody at 1 μg/ml. The levels of IFN-β in the DC supernatants are expressed in arbitrary units based on a standard curve prepared by use of a recombinant porcine IFN-β protein expressed in our laboratory by a replication-defective adenovirus (human Ad5) expressing IFN-β in IBRS-2 cells (38). The biological activity of this recombinant protein preparation was determined by the plaque reduction assay described above.
Confocal microscopy.
For confirmation of the expression of IFN-α by skin DC, frozen sections of fresh and cultured skin explants and cytospin preparations of freshly isolated and cultured DC were prepared, fixed in cold acetone, and stored at −70°C until analysis. Cytospin preparations from FMDV-exposed DC cultures were prepared from cells treated for the last 4 to 5 h of culture with Golgi-Plug (Pharmingen, San Diego, Calif.).
The samples were rehydrated and blocked in a phosphate-buffered saline solution containing 0.1% sodium azide, 0.2% bovine serum albumin, and 10% porcine serum for 20 min at room temperature. Prepared samples were then doubly stained with an anti-IFN-α (F17 [IgG1]) monoclonal antibody (28) and an anti-porcine CD1 (76-7-4 [IgG2a]) or anti-SWC3 (now CD-172; 74-22-12 [IgG2b]) monoclonal antibody as previously described (59). Briefly, samples were incubated with 5 μg of the F17 antibody/ml, washed, and then stained with 10 μg of fluorescein isothiocyanate-labeled anti-mouse IgG1/ml. The samples were thoroughly washed and incubated with either the biotin-labeled anti-CD1 or phycoerythrin-labeled anti-SWC3 antibody. For the detection of CD1 expression, samples were further stained with allophycocyanin-labeled streptavidin (Molecular Probes). All antibodies and conjugates were diluted in blocking buffer, and incubations were continued for 1 h at 37°C. After the last wash with blocking buffer containing 0.02% Tween 20, the samples were air dried and mounted with mounting medium (KPL). All samples were analyzed in an inverted Leica confocal laser scanning microscope.
The specificity of staining was determined in preliminary experiments by the use of IBRS2 cells infected with either of the recombinant adenoviruses expressing porcine IFN-α or IFN-β or noninfected control cells. Only the cells that had been infected with the IFN-α-expressing adenovirus stained positively with the F17 antibody. In addition, isotype-matched mouse IgG1 was also used to determine the background staining of the cells. The specificity of CD1 staining was confirmed by the lack of staining of alveolar macrophages with the anti-CD1 antibody and by the minimum background fluorescence obtained in the DC with a biotin-labeled mouse IgG2a isotype to match the anti-CD1 antibody.
RESULTS
DC are refractory to FMDV infection.
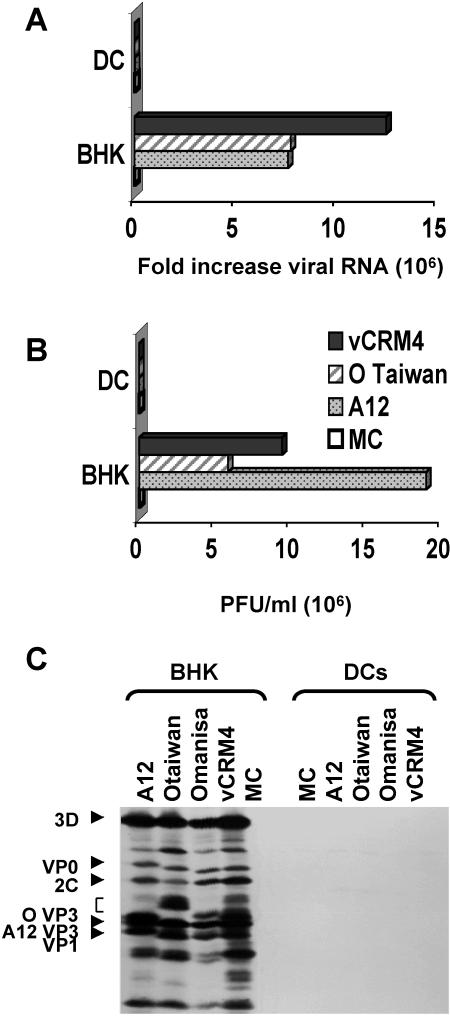
Previous studies had indicated that porcine skin DC may be susceptible to FMDV infection and that they might play a role in viral spread (20). To further explore this issue, we investigated whether DC support viral replication and yield viable virus progeny. Thus, viral replication was determined by real-time PCR and by a plaque assay after the exposure of cells to various FMDV strains. The strains tested included vCRM4, which infects cells through heparin sulfate (39, 51) and bypasses the normal requirement for the expression of appropriate integrins, the FMDV receptors, on target cells. After 1 h of virus adsorption at 37°C, significant cell-associated viral RNA was detected in the virus-exposed DC, ranging from 1 to 3 log10 units relative to control cells (no virus during absorption period). Since nonadsorbed virus was removed by a mild acid treatment prior to analysis, these results indicated that FMDV can bind to DC and be internalized. However, no significant increase in viral RNA levels was detected relative to the virus input levels after an additional 3 h of incubation (Fig. 1A). Similarly, viral progeny were not detected in the FMDV-exposed DC culture supernatants after overnight culture (Fig. 1B) relative to baby hamster kidney (BHK) cells, which support viral replication and are used for virus propagation. This was not a result of species differences between swine and hamsters, as O'Donnell et al. (40) and Knowles et al. (25) have shown that swine and bovine keratinocytes harvested from the tongue can support the replication of these and other viral isolates with the same kinetics as BHK cells. To further confirm that DCs did not support viral replication, we immunoprecipitated all potential viral proteins from radioactively labeled and lysed cells. We did not detect any specific viral proteins in the FMDV-exposed DC, whereas all structural and nonstructural proteins could be detected in infected BHK cells (Fig. 1C).
FIG. 1.
Analysis of FMDV replication in DC. DC were infected with FMDV at an MOI of 1 and cultured in complete medium for 0, 3, or 20 h. Samples of RNA were collected 0 and 3 h after virus adsorption. Culture supernatants were collected 0 and 24 h after adsorption for FMDV plaque titration. The permissive BHK cell line was infected under the same conditions as the positive control for virus replication. (A) Viral RNA levels measured by real-time PCR, with 18S rRNA as an internal control. The data are expressed as fold increases in the levels of FMDV RNA over the levels obtained 0 h after virus adsorption. (B) Standard virus plaque titrations performed with BHK cell monolayers. The data are expressed in PFU per milliliter of culture supernatant. The data shown here are averages of two different experiments. (C) Immunoprecipitation analysis of FMDV proteins in BHK cells and DC.
FMDV does not alter the expression of costimulatory molecules or antigen uptake and processing by DC.
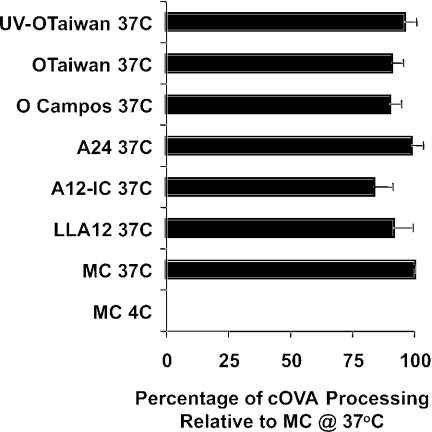
To determine whether the immunosuppressive effect of FMDV reported in previous studies (3) is mediated through DC, we analyzed the effect of FMDV on the antigen-presenting functions of skin-derived DC. Isolated DC were exposed to virulent, attenuated, or UV-inactivated strains of FMDV in vitro and then analyzed for antigen uptake and processing and for the level of expression of costimulatory molecules. Consistent with our previous findings (4), freshly isolated low-density skin-derived DC from all animals tested coexpressed the CD1 and SWC3a cell surface antigens. The purity of this population ranged from 70 to 85%, based on this staining and on morphological and ultrastructural characteristics. The percentage of cells expressing SLA-II and the CD80/86 molecules ranged from 74 to 80%, which was consistent with the percentage of cells that coexpressed the CD1 and SWC3a antigens. These cells had the ability to uptake and process the soluble antigen DQ-ovalbumin, which is temperature dependent, as demonstrated by flow cytometry analysis. Although the levels of antigen uptake and processing by DC, based on the MFI, were variable among animals and lower than those observed for alveolar macrophages or blood-derived monocytes from the same animals, the levels of antigen processing were consistent regardless of exposure to virulent, attenuated, or killed virus. Exposure of the DC to FMDV did not affect antigen uptake and processing relative to uninfected control cells (P > 0.05) (Fig. 2). In addition, FMDV exposure had no effect on the expression of swine major histocompatibility complex class II (SLA-II) or the costimulatory molecules CD80/86, which were assessed over time after virus exposure by flow cytometry. No significant differences were observed in the percentages of positive cells for either SLA-II or CD80/86 or in the levels of expression of these antigens in the virus-exposed DC (P > 0.05) (data not shown).
FIG. 2.
Effect of FMDV on the ability of DC to uptake and process antigens. DC were incubated with viruses for 2 h and tested for the uptake and processing of self-quenched, fluorescently labeled ovalbumin (DQ-OVA). Green fluorescence is only detected after the protein is ingested and degraded by DC. Each MFI, after subtracting the background fluorescence, is expressed (as a percentage) relative to that of the no-virus control cells (MC).
Innate cytokine responses of DC to FMDV.
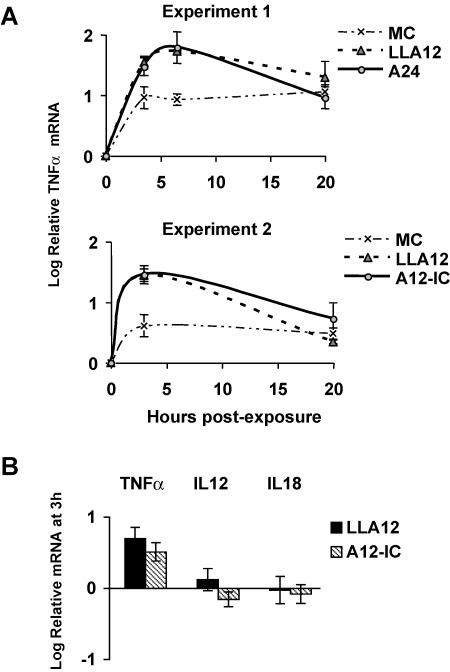
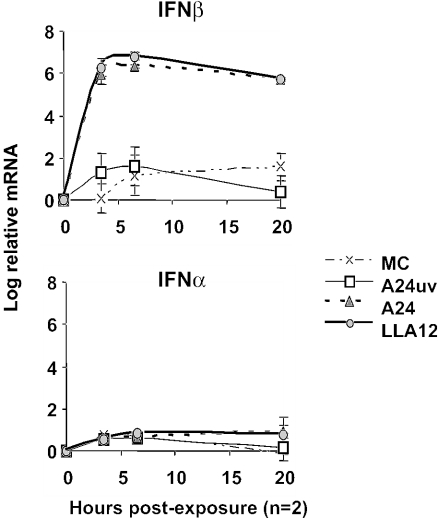
To determine whether DC elicit an innate cytokine response to FMDV, we measured the expression of inflammatory and anti-inflammatory cytokine mRNAs by real-time PCR analysis. We first measured relative mRNA levels in freshly isolated DC based on the CT for each cytokine normalized to the signal from the 18S rRNA gene. The effects of virus exposure on the levels of mRNA expression over time relative to those in freshly isolated cells were then determined. Freshly isolated DC were found to express no or barely detectable levels of IFN-β and interleukin-12 (IL-12) p35, low levels of IFN-α and IL-12 p40, and higher and variable levels of tumor necrosis factor alpha (TNF-α), IL-1α, IL-1β, IL-10, and transforming growth factor beta. After infection with the virulent FMDV strain A24 (49) or A12-IC (47) or the attenuated virus of the same serotype lacking the leader protease, LLA12 (8, 43), there was no significant increase in mRNA levels of proinflammatory (IL-1β and IL-1α) or anti-inflammatory (IL-10 and transforming growth factor beta) cytokines or in the apoptosis-related gene products FAS and caspase-3 (data not shown). The effects on IL-18 and IL-12 mRNA levels were variable among pigs (Fig. 3B), whereas TNF-α was most consistently increased at low levels in DC exposed to either the pathogenic or the attenuated, leaderless FMDV virus (Fig. 3A and B). In contrast, there was a significant upregulation of IFN-β mRNA levels in DC exposed to FMDV (P < 0.0001). This was true regardless of whether the pathogenic A24 or attenuated, leaderless LLA12 live virus was used. However, this effect was dependent on live virus, since UV-inactivated A24 did not induce an IFN-β mRNA response (Fig. 4, top). IFN-β mRNA expression peaked between 4 and 6 h after exposure, and high mRNA levels were maintained after overnight culture. The IFN-α mRNA levels in the FMDV-exposed DC, however, were only slightly altered relative to the no-virus control cells (Fig. 4, bottom).
FIG. 3.
Real-time PCR analysis of cytokine mRNA expression by DC in response to in vitro FMDV exposure. (A) Kinetic analysis of TNF-α mRNA expression by DC in response to FMDV exposure for two independent experiments. (B) Real-time PCR analysis to confirm the expression of proinflammatory cytokine mRNAs. Whole RNAs were isolated, and mRNA determinations were done by real-time PCR. The analysis was internally controlled by measuring 18S rRNA levels. Cytokine mRNA levels for each treatment were calculated relative to those at 0 h for the kinetic analysis shown in panel A or relative to those in MC at 3 h for the data shown in panel B. Data are expressed as log10 mRNA levels.
FIG. 4.
Kinetic analysis of IFN-α/β mRNA induction by real-time PCR. Isolated, skin-derived DC were incubated in the presence of infectious FMDV, either live or UV-inactivated strain A24, an attenuated derivative of the A12 strain lacking the leader protease (LLA12), or a medium control (MC). The analysis was internally controlled by measuring 18S rRNA levels. The cytokine mRNA levels for each treatment are expressed as log10 mRNA levels. Data are means ± standard errors (SE) of two experiments.
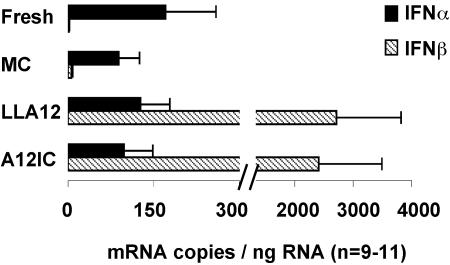
Finally, we developed an absolute quantitation assay by real-time PCR analysis, with in vitro-generated porcine IFN-α and IFN-β RNAs as standards, to determine the numbers of mRNA copies of IFN-α and IFN-β in each sample relative to input cellular RNAs. With this assay, we confirmed that freshly isolated DC express low levels of IFN-α mRNA. However, the number of mRNA copies for this cytokine was not significantly altered (n = 9 for MC, A12, and LLA12; P > 0.05) by exposure to either the attenuated (LLA12) or the pathogenic (A12-IC) FMDV virus (Fig. 5). In contrast, IFN-β mRNA was not detected in the freshly isolated cells and in the cultured control cells (MC). After virus exposure, significantly (P < 0.05) higher levels were detected within 3 h (Fig. 5). The IFN-β mRNA levels induced by LLA12 tended to be higher than those induced by A12-IC in DC isolated from all animals tested, but they were not significantly different (P > 0.05).
FIG. 5.
Absolute quantitative analysis of IFN-α and IFN-β mRNA expression. RNAs from freshly isolated DC (fresh) or DC cultured for 3 h in the absence (MC) or presence of the A12-IC or LLA12 virus were analyzed by real-time PCR. The total cellular RNA levels used for each sample were measured and expressed in nanograms based on a standard curve included in each assay. The number of mRNA copies for each cytokine was calculated based on a standard curve prepared for each gene and included in each assay. Data are reported as means (n = 11) ± SE.
Expression and secretion of IFN-α and -β by DC exposed to FMDV.
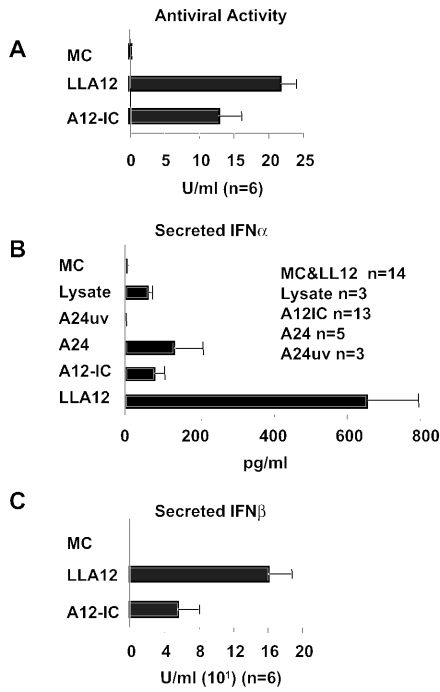
To further investigate the expression of IFN-α and -β in response to FMDV, we analyzed skin-derived DC for the capacity to secrete biologically active IFN-α and -β proteins. Thus, culture supernatants were analyzed for the presence of antiviral activity. To remove any remaining input virus and to test only for acid-resistant IFN-α/β, we treated samples at pH 2 followed by neutralization and measured the antiviral activity by determining the reduction in the number of A12-IC plaques in the porcine epithelial cell line IBRS2 (12). Interestingly, we found that exposure to the pathogenic A12-IC virus did not completely eliminate the ability of DC to synthesize and secrete IFN-α/β, despite the reported viral suppression of host protein synthesis (14). However, the attenuated virus induced significantly (P < 0.05) higher antiviral activity by DC than the pathogenic virus (Fig. 6A), while no antiviral activity was detected in the medium control samples.
FIG. 6.
Analysis of IFN-α and -β secretion by DC. Isolated, skin-derived DC were incubated in the presence of infectious FMDV strain A12-IC, an attenuated derivative of the A12 strain lacking the leader protease (LLA12), live strain A24, UV-inactivated strain A24 (A24uv), or a medium control (MC). Culture supernatants were collected after 20 h of in vitro virus exposure and analyzed for IFN-α and -β secretion. (A) Biological activities were determined by a 50% plaque reduction assay of the A12-IC virus in IBRS-2 cells stimulated with acid-treated supernatants. Activities are expressed as means (n = 6) + SE. The protein concentrations of IFN-α (B) and IFN-β (C) in the culture supernatants were measured by antigen capture ELISAs. Mean levels of IFN-α protein are expressed in picograms per milliliter, and mean levels of IFN-β protein (n = 6) are expressed in relative units per milliliter.
Despite the fact that IFN-α mRNA expression was not upregulated after exposure to FMDV, secreted IFN-α protein was readily detected by an antigen capture ELISA (37). Detectable levels of IFN-α were present in supernatants of DC exposed to either a pathogenic or attenuated virus. Consistent with the effect of the viral leader protein, higher IFN-α levels were detected in the LLA12-exposed DC culture supernatants than in A12-IC-exposed DC culture supernatants (Fig. 6B). To detect porcine IFN-β, we analyzed these samples by a semiquantitative antigen capture ELISA with reagents developed in our laboratory. A standard curve was generated for a recombinant porcine IFN-β protein expressed in a human replication-defective adenovirus (38). Units were determined based on the biological activity in the plaque reduction assay for antiviral activity. We confirmed that DC express and secrete the IFN-β protein after exposure to both the attenuated LLA12 virus and (although at lower levels) the pathogenic A12-IC virus (Fig. 6C).
Constitutive expression of IFN-α by skin DC.
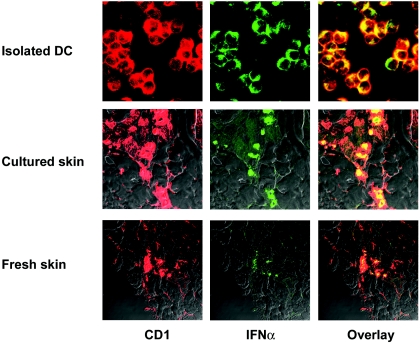
To determine whether the constitutive expression of IFN-α mRNA in skin DC corresponded to constitutively expressed IFN-α protein, we analyzed the expression of IFN-α by immunocytochemistry. Double staining with an antibody to the DC marker S100β (4, 20, 50) and an anti-IFN-α antibody confirmed that DC constitutively express the IFN-α protein in the absence of virus exposure (data not shown). These preliminary results showed that a large proportion of the S100β-positive cells were also positive for IFN-α. To confirm these findings, we also analyzed freshly isolated skin-derived DC for the coexpression of CD1 or SWC3 (CD172), markers of porcine DC (4), and IFN-α by confocal microscopy. As shown in Fig. 7, approximately 50 to 90% of the CD1-positive cells isolated from the skin of six different animals were positive for IFN-α. These images are representative of at least three experiments and show that these isolated DC constitutively expressed intracellular IFN-α protein, which was not secreted until FMDV exposure (Fig. 6).
FIG. 7.
Confocal microscopy analysis of IFN-α protein expression by DC. Staining was performed to detect the expression of CD1 (red) and IFN-α (green) by isolated DC or in situ DC in skin sections. Skin layers were collected from healthy pigs. Frozen sections were prepared from samples collected immediately after the euthanasia of pigs (fresh skin) or after overnight culture (cultured skin) for DC isolation and were fixed in acetone. The data shown are representative of six different experiments.
To determine whether the expression of IFN-α by DC was induced during overnight culture of the skin, we also stained frozen skin sections prepared from skin layers collected immediately after euthanasia and compared these to skin layers that were cultured overnight for DC isolation. Confocal microscopy revealed that CD1-positive cells in freshly collected skin as well as in cultured skin express IFN-α protein (Fig. 7). Histological analyses of skin sections from the same animals used for the study showed that the skin analyzed was normal, with no evidence of any pathological condition. In addition, the animals used for these studies were specific-pathogen-free animals who were completely healthy with no signs of disease and had been maintained in isolation for several weeks to 3 to 4 months. Consequently, these data strongly indicate that IFN-α is constitutively expressed by a population of porcine dendritic cells of the skin. Importantly, these data provide an explanation for the lack of productive replication of FMDV in the skin-derived DC found in this study.
DISCUSSION
The study of DC biology continues to expand our knowledge of the innate and adaptive immune responses to pathogens. Understanding early immune responses to FMDV is critical for the development of rapidly acting vaccines and antiviral agents for use in response to disease outbreaks. Here we describe an important characteristic of porcine DC of the skin, the constitutive expression of IFN-α. For the first time, our data show that isolated DC not only constitutively express IFN-α mRNA but also express the IFN-α protein and likely store it in the cytoplasm. Alternatively, these cells may secrete IFN-α and consume it as an autocrine factor which is therefore not free in the supernatant. Virus stimulation, then, would either induce an increase in protein production and secretion or a downregulation of IFN-α receptor expression, releasing the protein into the supernatant. In either case, the constitutive expression of IFN-α protein is a characteristic of both the migratory DC isolated from the skin of swine and DC analyzed in situ.
Although the constitutive expression of IFN-α by normal unstimulated skin DC is a unique finding, these data are consistent with other recent studies showing an important role of constitutively produced IFN-α in DC maturation. Such reports make it clear that IFN-α is not only involved in but also required for DC maturation and activation processes. These include the migration of the DC that normally occur in the skin in vivo (30) and the spontaneous differentiation and maturation of cultured human blood (30) or splenic DC progenitors, which are mediated by autocrine IFN-α expression (36).
We previously reported (4) that DC derived from the skin of swine have the phenotypic, ultrastructural, and functional characteristics of mature and partially activated DC, which was further established in the present study. In this study, we confirmed the expression of the important costimulatory molecules SLA-II and CD80/86 and the ability of the cells to uptake and process antigens. In addition, we found that these DC constitutively express variable mRNA levels for proinflammatory and anti-inflammatory cytokines. It has been previously demonstrated both in vitro and in vivo that human and mouse DC mature during the migration process through the skin (45, 57, 58, 62) and that IFN-α increases the level of migration of human split-skin-derived DC (30). Since most of the skin DC expressing IFN-α were located in the dermis, we hypothesize that DC are stimulated to express IFN-α by the migration process that naturally occurs in vivo, which is maintained in the cultured skin of swine. An analysis of healthy unstimulated skin from adult humans did not reveal a constitutive expression of IFN-α (7, 29). Whether this finding is restricted to swine skin DC requires analyses of other species of interest. However, the constitutive expression and cytoplasmic storage of cytokines are not without precedent. It is well established that macrophages express and store cytokines such as IL-1β and IL-18 (54, 55) in the cytoplasm. The molecular mechanisms regulating the secretion of the early, proinflammatory cytokines are just starting to be understood (35). Given the increasing evidence of the important role of IFN-α and in the natural differentiation, maturation, activation (27, 30, 36), and homeostasis (32, 60) of DC, it is possible that IFN-α is also involved in the homeostatic steady state, migration, and/or maturation of skin DC.
In addition, this study investigated the interaction of FMDV with skin DC in order to understand the potential role of these cells in pathogenesis and immunity. Our data show that the skin DC of swine are not susceptible to FMDV infection. The inability of the virus to productively replicate in the isolated skin DC of swine may be explained by the state of maturation and/or activation of the cells discussed above. Previous reports of other viral infections in DC, such as measles virus infections, described infections of Langerhans cells and an inhibition of cell function (21). The data from our study indicate that FMDV exposure does not inhibit the function of DC, as virus exposure did not significantly alter the levels of the costimulatory molecules or the antigen uptake and processing capability. In addition, we showed that the mRNA levels for various pro- and anti-inflammatory cytokines or of apoptosis-related genes of DC were also unaffected. However, our data also show that DC are still responsive to FMDV exposure, as clearly demonstrated by a rapid and strong activation of the IFN-β gene, as determined by an upregulation of mRNA expression. Since only infectious live virus induced the IFN-β mRNA response, some level of viral RNA replication may be required to provide the double-stranded RNA stimulatory signal for either Toll-like receptor 3 (1)- or protein kinase R (16, 64)-mediated IFN-β induction. Further studies are required to determine the molecular mechanism of FMDV-mediated induction of IFN-β mRNA.
The IFN-α and -β response of DC to FMDV exposure was not limited to mRNA expression, as we also demonstrated that bioactive IFN-α and -β proteins were secreted upon virus exposure. IFN-α and -β secretion was detected only after overnight culture, suggesting that some level of protein synthesis was required. FMDV replicates in target cells by inhibiting protein synthesis and therefore blocks IFN-α/β protein expression (12). Since FMDV is highly sensitive to the action of IFN-α/β (10-12, 37), the constitutive expression of IFN-α and its secretion upon FMDV exposure by DC further explain the inability of pathogenic FMDV strains to inhibit the activation of the IFN-β gene. Furthermore, the synthesis and secretion of IFN-β protein were also unaffected, rendering the virus unable to productively replicate in these cells. Therefore, our data do not support the hypothesis that DC derived from the skin of swine have an important role in viral propagation and spread, as previously suggested (20). The discrepancy of our data with that report can be explained by the methodologies used. The previous report showed that 10% of the isolated cells were positive for FMDV antigens, as determined by immunocytochemistry. These DC, which were isolated by migration from the skin, could uptake and process antigens (4) and would stain positively for FMDV antigens after overnight culture. The data we present in this study also show that skin DC can bind and internalize FMDV in the absence of any detectable productive or nonproductive infection. By measuring the increase in viral RNA by real-time PCR and determining the de novo viral protein synthesis and production of virus progeny, we clearly demonstrated that DC derived from the skin of swine do not support the productive replication of FMDV. Rather, the present study provides evidence that swine DC derived from skin play an important role in the innate response to FMDV infection.
In vivo, FMDV has developed the capacity to rapidly replicate in susceptible cells such as keratinocytes (40). This allows the virus to propagate in large numbers and to disseminate to a new host before the innate and adaptive immune responses can clear the virus. We have previously shown that inoculation with a recombinant adenovirus expressing porcine IFN-α prevents and controls the disease (10, 11, 37). Our understanding of the role of skin DC now offers a biological explanation for the effects of IFN-α on FMDV infections of livestock. Furthermore, our finding of the constitutive expression of IFN-α by the resident DC of swine may serve as a natural model for studying the regulation of DC maturation and migration by IFN-α and the role of skin DC in the early, innate immune response of skin-associated lymphoid tissues to pathogens.
Acknowledgments
We greatly appreciate the excellent technical assistance provided by Mike LaRocco, Amy Kozer, and Mary Jo Mc Donald. We also appreciate the valuable discussions with Barry Baxt and Luis Rodriguez and thank David Woodland for a critical reading of the manuscript.
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Bautista, E. M., G. S. Ferman, and W. T. Golde. 2003. Induction of lymphopenia and inhibition of T cell function during acute infection of swine with foot and mouth disease virus (FMDV). Vet. Immunol. Immunopathol. 92:61-73. [DOI] [PubMed] [Google Scholar]
- 4.Bautista, E. M., D. Gregg, and W. T. Golde. 2002. Characterization and functional analysis of skin-derived dendritic cells from swine without a requirement for in vitro propagation. Vet. Immunol. Immunopathol. 88:131-148. [DOI] [PubMed] [Google Scholar]
- 5.Bautista, E. M., and T. W. Molitor. 1997. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 10:83-94. [DOI] [PubMed] [Google Scholar]
- 6.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin αVβ3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 69:2664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomberg, S., M. L. Eloranta, B. Cederblad, K. Nordlin, G. V. Alm, and L. Ronnblom. 2001. Presence of cutaneous interferon-α producing cells in patients with systemic lupus erythematosus. Lupus 10:484-490. [DOI] [PubMed] [Google Scholar]
- 8.Brown, C. C., M. E. Piccone, P. W. Mason, T. S. McKenna, and M. J. Grubman. 1996. Pathogenesis of wild-type and leaderless foot-and-mouth disease virus in cattle. J. Virol. 70:5638-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callahan, J. D., F. Brown, F. A. Osorio, J. H. Sur, E. Kramer, G. W. Long, J. Lubroth, S. J. Ellis, K. S. Shoulars, K. L. Gaffney, D. L. Rock, and W. M. Nelson. 2002. Use of a portable real-time reverse transcriptase-polymerase chain reaction assay for rapid detection of foot-and-mouth disease virus. J. Am. Vet. Med. Assoc. 220:1636-1642. [DOI] [PubMed] [Google Scholar]
- 10.Chinsangaram, J., M. Koster, and M. J. Grubman. 2001. Inhibition of L-deleted foot-and-mouth disease virus replication by alpha/beta interferon involves double-stranded RNA-dependent protein kinase. J. Virol. 75:5498-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinsangaram, J., M. P. Moraes, M. Koster, and M. J. Grubman. 2003. Novel viral disease control strategy: adenovirus expressing alpha interferon rapidly protects swine from foot-and-mouth disease. J. Virol. 77:1621-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinsangaram, J., M. E. Piccone, and M. J. Grubman. 1999. Ability of foot-and-mouth disease virus to form plaques in cell culture is associated with suppression of alpha/beta interferon. J. Virol. 73:9891-9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David, D., Y. Stram, H. Yadin, Z. Trainin, and Y. Becker. 1995. Foot and mouth disease virus replication in bovine skin Langerhans cells under in vitro conditions detected by RT-PCR. Virus Genes 10:5-13. [DOI] [PubMed] [Google Scholar]
- 14.Devaney, M. A., V. N. Vakharia, R. E. Lloyd, E. Ehrenfeld, and M. J. Grubman. 1988. Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J. Virol. 62:4407-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz de Arce, H., K. Artursson, R. L'Haridon, A. Perers, C. La Bonnardiere, and G. V. Alm. 1992. A sensitive immunoassay for porcine interferon-α. Vet. Immunol. Immunopathol. 30:319-327. [DOI] [PubMed] [Google Scholar]
- 16.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 17.Dunn, C. S., and A. I. Donaldson. 1997. Natural adaptation to pigs of a Taiwanese isolate of foot-and-mouth disease virus. Vet. Rec. 141:174-175. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson, N. M., C. A. Donnelly, and R. M. Anderson. 2001. The foot-and-mouth epidemic in Great Britain: pattern of spread and impact of interventions. Science 292:1155-1160. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson, N. M., C. A. Donnelly, and R. M. Anderson. 2001. Transmission intensity and impact of control policies on the foot and mouth epidemic in Great Britain. Nature 413:542-548. [DOI] [PubMed] [Google Scholar]
- 20.Gregg, D. A., D. H. Schlafer, and C. A. Mebus. 1995. African swine fever virus infection of skin-derived dendritic cells in vitro causes interference with subsequent foot-and-mouth disease virus infection. J. Vet. Diagn. Investig. 7:44-51. [DOI] [PubMed] [Google Scholar]
- 21.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson, T., W. Blakemore, J. W. Newman, N. J. Knowles, A. P. Mould, M. J. Humphries, and A. M. King. 2000. Foot-and-mouth disease virus is a ligand for the high-affinity binding conformation of integrin α5β1: influence of the leucine residue within the RGDL motif on selectivity of integrin binding. J. Gen. Virol. 81:1383-1391. [DOI] [PubMed] [Google Scholar]
- 23.Kadowaki, N., S. Antonenko, J. Y. Lau, and Y. J. Liu. 2000. Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadowaki, N., and Y. J. Liu. 2002. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum. Immunol. 63:1126-1132. [DOI] [PubMed] [Google Scholar]
- 25.Knowles, N. J., P. R. Davies, T. Henry, V. O'Donnell, J. M. Pacheco, and P. W. Mason. 2001. Emergence in Asia of foot-and-mouth disease viruses with altered host range: characterization of alterations in the 3A protein. J. Virol. 75:1551-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knowles, N. J., A. R. Samuel, P. R. Davies, R. P. Kitching, and A. I. Donaldson. 2001. Outbreak of foot-and-mouth disease virus serotype O in the UK caused by a pandemic strain. Vet. Rec. 148:258-259. [PubMed] [Google Scholar]
- 27.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 28.L'Haridon, R. M., P. Bourget, F. Lefevre, and C. La Bonnardiere. 1991. Production of an hybridoma library to recombinant porcine alpha I interferon: a very sensitive assay (ISBBA) allows the detection of a large number of clones. Hybridoma 10:35-47. [DOI] [PubMed] [Google Scholar]
- 29.Livden, J. K., R. Nilsen, J. R. Bjerke, and R. Matre. 1989. In situ localization of interferons in psoriatic lesions. Arch. Dermatol. Res. 281:392-397. [DOI] [PubMed] [Google Scholar]
- 30.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 31.Mayr, G. A., J. Chinsangaram, and M. J. Grubman. 1999. Development of replication-defective adenovirus serotype 5 containing the capsid and 3C protease coding regions of foot-and-mouth disease virus as a vaccine candidate. Virology 263:496-506. [DOI] [PubMed] [Google Scholar]
- 32.McCarty, M. F., D. Bielenberg, C. Donawho, C. D. Bucana, and I. J. Fidler. 2002. Evidence for the causal role of endogenous interferon-α/β in the regulation of angiogenesis, tumorigenicity, and metastasis of cutaneous neoplasms. Clin. Exp. Metastasis 19:609-615. [DOI] [PubMed] [Google Scholar]
- 33.McCullough, K. C., and R. N. Butcher. 1991. Function of idiotypic networks in vivo: immunisation with idiotype-bearing (Id1) antibody induces further production of Id1 antibody. Immunol. Lett. 27:9-12. [DOI] [PubMed] [Google Scholar]
- 34.Medina, M., E. Domingo, J. K. Brangwyn, and G. J. Belsham. 1993. The two species of the foot-and-mouth disease virus leader protein, expressed individually, exhibit the same activities. Virology 194:355-359. [DOI] [PubMed] [Google Scholar]
- 35.Mehta, V. B., J. Hart, and M. D. Wewers. 2001. ATP-stimulated release of interleukin (IL)-1β and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J. Biol. Chem. 276:3820-3826. [DOI] [PubMed] [Google Scholar]
- 36.Montoya, M., G. Schiavoni, F. Mattei, I. Gresser, F. Belardelli, P. Borrow, and D. F. Tough. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99:3263-3271. [DOI] [PubMed] [Google Scholar]
- 37.Moraes, M. P., J. Chinsangaram, M. C. Brum, and M. J. Grubman. 2003. Immediate protection of swine from foot-and-mouth disease: a combination of adenoviruses expressing interferon alpha and a foot-and-mouth disease virus subunit vaccine. Vaccine 22:268-279. [DOI] [PubMed] [Google Scholar]
- 38.Moraes, M. P., G. A. Mayr, and M. J. Grubman. 2001. pAd5-Blue: direct ligation system for engineering recombinant adenovirus constructs. BioTechniques 31:1050, 1052, 1054-1056. [DOI] [PubMed] [Google Scholar]
- 39.Neff, S., D. Sa-Carvalho, E. Rieder, P. W. Mason, S. D. Blystone, E. J. Brown, and B. Baxt. 1998. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αvβ3 as its receptor. J. Virol. 72:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Donnell, V. K., J. M. Pacheco, T. M. Henry, and P. W. Mason. 2001. Subcellular distribution of the foot-and-mouth disease virus 3A protein in cells infected with viruses encoding wild-type and bovine-attenuated forms of 3A. Virology 287:151-162. [DOI] [PubMed] [Google Scholar]
- 41.Oleksiewicz, M. B., A. I. Donaldson, and S. Alexandersen. 2001. Development of a novel real-time RT-PCR assay for quantitation of foot-and-mouth disease virus in diverse porcine tissues. J. Virol. Methods 92:23-35. [DOI] [PubMed] [Google Scholar]
- 42.Palucka, K., and J. Banchereau. 1999. Dendritic cells: a link between innate and adaptive immunity. J. Clin. Immunol. 19:12-25. [DOI] [PubMed] [Google Scholar]
- 43.Piccone, M. E., E. Rieder, P. W. Mason, and M. J. Grubman. 1995. The foot-and-mouth disease virus leader proteinase gene is not required for viral replication. J. Virol. 69:5376-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piccone, M. E., M. Zellner, T. F. Kumosinski, P. W. Mason, and M. J. Grubman. 1995. Identification of the active-site residues of the L proteinase of foot-and-mouth disease virus. J. Virol. 69:4950-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rambukkana, A., J. D. Bos, D. Irik, W. J. Menko, M. L. Kapsenberg, and P. K. Das. 1995. In situ behavior of human Langerhans cells in skin organ culture. Lab. Investig. 73:521-531. [PubMed] [Google Scholar]
- 46.Reid, S. M., N. P. Ferris, G. H. Hutchings, Z. Zhang, G. J. Belsham, and S. Alexandersen. 2002. Detection of all seven serotypes of foot-and-mouth disease virus by real-time, fluorogenic reverse transcription polymerase chain reaction assay. J. Virol. Methods 105:67-80. [DOI] [PubMed] [Google Scholar]
- 47.Rieder, E., T. Bunch, F. Brown, and P. W. Mason. 1993. Genetically engineered foot-and-mouth disease viruses with poly(C) tracts of two nucleotides are virulent in mice. J. Virol. 67:5139-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts, L. O., R. A. Seamons, and G. J. Belsham. 1998. Recognition of picornavirus internal ribosome entry sites within cells; influence of cellular and viral proteins. RNA 4:520-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robson, K. J., J. R. Crowther, A. M. King, and F. Brown. 1979. Comparative biochemical and serological analysis of five isolates of a single serotype of foot-and-mouth disease virus. J. Gen. Virol. 45:579-590. [DOI] [PubMed] [Google Scholar]
- 50.Rowden, G., S. Boudreau, and H. Higley. 1985. Langerhans cells and extra-epidermal dendritic cells. An investigation in laboratory animals and man with immunomorphological methods. Scand. J. Immunol. 21:471-478. [DOI] [PubMed] [Google Scholar]
- 51.Sa-Carvalho, D., E. Rieder, B. Baxt, R. Rodarte, A. Tanuri, and P. W. Mason. 1997. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J. Virol. 71:5115-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saiz, M., J. I. Nunez, M. A. Jimenez-Clavero, E. Baranowski, and F. Sobrino. 2002. Foot-and-mouth disease virus: biology and prospects for disease control. Microbes Infect. 4:1183-1192. [DOI] [PubMed] [Google Scholar]
- 53.Samuel, A. R., E. J. Ouldridge, A. E. Arrowsmith, R. P. Kitching, and N. J. Knowles. 1990. Antigenic analysis of serotype O foot-and-mouth disease virus isolates from the Middle East, 1981 to 1988. Vaccine 8:390-396. [DOI] [PubMed] [Google Scholar]
- 54.Siders, W. M., J. C. Klimovitz, and S. B. Mizel. 1993. Characterization of the structural requirements and cell type specificity of IL-1 alpha and IL-1 beta secretion. J. Biol. Chem. 268:22170-22174. [PubMed] [Google Scholar]
- 55.Siders, W. M., and S. B. Mizel. 1995. Interleukin-1β secretion. A possible multistep process that is regulated in a cell type-specific manner. J. Biol. Chem. 270:16258-16264. [DOI] [PubMed] [Google Scholar]
- 56.Splichal, I., M. Bonneau, and B. Charley. 1994. Ontogeny of interferon alpha secreting cells in the porcine fetal hematopoietic organs. Immunol. Lett. 43:203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoitzner, P., K. Pfaller, H. Stossel, and N. Romani. 2002. A close-up view of migrating Langerhans cells in the skin. J. Investig. Dermatol. 118:117-125. [DOI] [PubMed] [Google Scholar]
- 58.Stosel, H., F. Koch, and N. Romani. 1997. Maturation and migration of murine dendritic cells in situ. Observations in a skin organ culture model. Adv. Exp. Med. Biol. 417:311-315. [DOI] [PubMed] [Google Scholar]
- 59.Summerfield, A., L. Guzylack-Piriou, A. Schaub, C. P. Carrasco, V. Tache, B. Charley, and K. C. McCullough. 2003. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology 110:440-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taki, S. 2002. Type I interferons and autoimmunity: lessons from the clinic and from IRF-2-deficient mice. Cytokine Growth Factor Rev. 13:379-391. [DOI] [PubMed] [Google Scholar]
- 61.Tsai, C. P., C. H. Pan, M. Y. Liu, Y. L. Lin, C. M. Chen, T. S. Huang, I. C. Cheng, M. H. Jong, and P. C. Yang. 2000. Molecular epidemiological studies on foot-and-mouth disease type O Taiwan viruses from the 1997 epidemic. Vet. Microbiol. 74:207-216. [DOI] [PubMed] [Google Scholar]
- 62.Weinlich, G., M. Heine, H. Stossel, M. Zanella, P. Stoitzner, U. Ortner, J. Smolle, F. Koch, N. T. Sepp, G. Schuler, and N. Romani. 1998. Entry into afferent lymphatics and maturation in situ of migrating murine cutaneous dendritic cells. J. Investig. Dermatol. 110:441-448. [DOI] [PubMed] [Google Scholar]
- 63.Wu, Q., M. C. Brum, L. Caron, M. Koster, and M. J. Grubman. 2003. Adenovirus-mediated type I interferon expression delays and reduces disease signs in cattle challenged with foot-and-mouth disease virus. J. Interferon Cytokine Res. 23:359-368. [DOI] [PubMed] [Google Scholar]
- 64.Zinn, K., A. Keller, L. A. Whittemore, and T. Maniatis. 1988. 2-Aminopurine selectively inhibits the induction of β-interferon, c-fos, and c-myc gene expression. Science 240:210-213. [DOI] [PubMed] [Google Scholar]