Abstract
Dengue virus requires the presence of an unidentified cellular receptor on the surface of the host cell. By using a recently published affinity chromatography approach, an 84-kDa molecule, identified as heat shock protein 90 (HSP90) by matrix-assisted laser desorption ionization-time of flight mass spectrometry, was isolated from neuroblastoma and U937 cells. Based on the ability of HSP90 (84 kDa) to interact with HSP70 (74 kDa) on the surface of monocytes during lipopolysaccharide (LPS) signaling and evidence that LPS inhibits dengue virus infection, the presence of HSP70 was demonstrated in affinity chromatography eluates and by pull-down experiments. Infection inhibition assays support the conclusion that HSP90 and HSP70 participate in dengue virus entry as a receptor complex in human cell lines as well as in monocytes/macrophages. Additionally, our results indicate that both HSPs are associated with membrane microdomains (lipid rafts) in response to dengue virus infection. Moreover, methyl-β-cyclodextrin, a raft-disrupting drug, inhibits dengue virus infection, supporting the idea that cholesterol-rich membrane fractions are important in dengue virus entry.
Dengue (DEN) virus, the most important arthropod-borne human pathogen, represents a serious public health threat. DEN virus is transmitted to humans by the bite of the domestic mosquito, Aedes aegypti, and circulates in nature as four distinct serological types (DEN-1 to -4). DEN virus has been recognized in over 100 countries, and 2.5 billion people live in areas where DEN virus is endemic (16). The clinical manifestations of DEN virus infection range in severity from a simple self-limited febrile illness known as dengue fever to a hemorrhagic fever (DHF) and potentially fatal hemorrhagic shock syndrome. Each year, more than 50 million cases of dengue fever and several hundred thousand cases of DHF occur. During the past 8 years the incidence of dengue has grown in areas of endemicity, particularly in the American region. A specific treatment or vaccine is not yet available.
DEN virus is an enveloped virus that belongs to the Flaviviridae family. Mature virions are icosahedral, 50 nm in diameter, and contain a single-strand and positive-polarity RNA as genome of about 10.7 kb (21). The DEN virus genome encodes three structural proteins (envelope glycoprotein, E; membrane, M; and capsid, C) and seven nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5). E protein is the major structural protein exposed on the surface of the particle, and it arrays in homodimers, parallel to viral surface. Recently, the structure of DEN virus E protein has been determined by X-ray crystallography (24). Each monomer consists of three domains: the structurally central amino-terminal domain I that organizes the structure; the dimerization domain II that contains the hydrophobic fusion peptide essential for virus-cell fusion; and finally the carboxy-terminal immunoglobulin (Ig)-like domain III, which has been proposed to function as the binding site for cellular receptors (11, 22).
The first step in DEN virus infection requires interaction between the E protein and the cellular receptor present on the surface of the host cell. This interaction initiates a chain of dynamic events that enable viral entry into the cell. Although monocytes and macrophages have been thought to be the major target cells in DEN virus infection and are responsible for the dissemination of the virus after its initial entry, the primary cell that supports DEN virus replication in severe cases remains unknown. In vitro, DEN virus can infect and replicate in a wide range of cells of different origins, and two types of molecules have been involved as DEN virus receptors. The first type of molecule is a glycosa-aminoglycan, specifically, heparan sulfate. Heparan sulfate has been reported to bind DEN virus E protein (9) or the native virion on the cell surface (13); however, it has been suggested that these molecules serve primarily as initial attachment factors, concentrating virus particles at the cell surface for subsequent interaction with other receptor molecules (4). The second type of molecules are proteins with different molecular masses that have been described as putative DEN virus receptors in several cell lines. Among them, a 45-kDa glycoprotein in C6/36 cells (33), a 74-kDa protein present in Vero cells (23), two proteins of approximately 40 to 45 kDa and 70 to 75 kDa from a B-cell line and a myelomonocytic cell line (6), a 105-kDa protein in erythroleukemia cells (32), and proteins of 29 and 43 kDa from an endothelial cell line (44) have been described. In monocytes/macrophages, membrane proteins of 27, 45, 67, and 87 kDa were described also as putative receptors for DEN virus by using an affinity chromatography approach (26). Thus, it is possible that the interaction between DEN virus and its cellular receptor(s) is a multistep process in itself, and multiple attachment receptors may be sequentially used for DEN virus to gain entry.
One of the proteins identified so far that mediates DEN virus infection is a C-type lectin present in monocyte-derived dendritic cells (27, 39), named dendritic cell-specific ICAM 3-grabbing nonintegrin (or CD209), that is also important in human immunodeficiency virus (HIV) (12), cytomegalovirus (17), Ebola (1), and hepatitis C infections. This interaction occurs through high-mannose N-linked glycans present in the viral envelope glycoproteins, and at least for HIV the interaction with dendritic cell-specific ICAM 3-grabbing nonintegrin does not induce conformational changes in HIV envelope glycoprotein (3). Additionally, another two proteins have been identified as specific serotype receptors in HepG2 cells: GRP-78 for DEN-2 (19) and a 37-kDa/67-kDa high-affinity laminin receptor for DEN virus (40).
On the other hand, it has been shown that the entry of DEN virus into monocytes/macrophages can be blocked by bacterial lipopolysaccharide (LPS), probably because LPS can interact with a certain cellular binding structure that is important for the attachment of DEN virus particles to membrane receptor(s) on the target cells (10). This membrane receptor has not been identified yet.
In an effort to isolate the molecules that comprise the DEN virus receptor complex, we recently designed an affinity chromatography approach that uses a recombinant DEN virus E protein as bait. Using this method, we could isolate a protein from C6/36 cells that has been previously described as a putative DEN virus receptor (31).
In the present report, an 84-kDa molecule was isolated from neuroblastoma and U937 cells. This protein interacts with DEN virus and was identified as the heat shock protein of 90 kDa (HSP90) by matrix-assisted laser desorption ionization-time of flight MALDI-TOF mass spectrometry. The presence of HSP90 on the surface of monocytes was demonstrated by using a monoclonal antibody against HSP90. Based on the ability of HSP90 (84 kDa) to interact with HSP70 (74 kDa) on the cell surface during LPS cell signaling and the evidence reported that a 70- to 75-kDa protein binds DEN virus, the presence of HSP70 was analyzed by affinity chromatography and pull-down experiments. Infection inhibition assays support the conclusion that HSP90 and HSP70 are part of a receptor complex required for DEN virus entry in neuroblastoma and monocytic cell lines as well as in human monocytes/macrophages. Moreover, our results indicate that the DEN virus receptor complex is associated with membrane microdomains (lipid rafts), supporting the idea that the cholesterol-rich membrane fraction is important in DEN virus entry. This is the first report that identifies a group of molecules involved as receptors for DEN virus in human monocytes/macrophages. The interaction between DEN virus with its cellular receptor complex is likely to extend our incomplete understanding of the host cell response and pathogenesis of DEN virus infection.
MATERIALS AND METHODS
Reagents and antibodies.
All fine chemicals were purchased from Sigma. Anti-HSP90 monoclonal antibody used in Western blot assays was obtained from Stressgen (Canada). Anti-HSP90 polyclonal serum used in infection inhibition assays was obtained by inoculating mice with recombinant HSP90α protein. Anti-HSP70 monoclonal antibody was purchased from Affinity Bioreagents. Anti-CD14 monoclonal antibody was obtained from Zymed, and anti-caveolin-1 monoclonal antibody was obtained from HyClone. The plasmid pQH6HSPα (28) containing the complete sequence of the human HSP90α and an amino-terminal histidine tag (kindly donated by Takayuki K. Nemoto, Nagasaki University) was amplified and expressed in bacteria, and the recombinant protein was purified by immobilized metal affinity chromatography. The recombinant protein was subsequently dialyzed against phosphate-buffered saline (PBS) and quantified.
Cells and virus.
U937 cells (a human monocytic cell line derived from a patient with generalized histiocytic lymphoma) were grown at 37°C in RPMI medium supplemented with 10% fetal calf serum, antibiotics (penicillin and streptomycin), fungizone (Gibco), 5.95 μg of HEPES per ml, and 600 μg of glutamine per ml (pH 7). Differentiation into macrophage-like cells was induced by using 120 mM phorbol myristate acetate (Sigma) for 2 days (29). SK-SY-5Y cells (human neuroblastoma cell line) were grown at 37°C in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% neonate calf serum, 5,000 U of penicillin, and 5 μg of streptomycin.
Monocyte-derived macrophages were obtained from peripheral blood human buffy coats from healthy donors without previous flavivirus infections. Leukocyte-enriched plasma was centrifuged over Ficoll-Hypaque (Pharmacia). Mononuclear cells were washed, resuspended in RPMI medium supplemented with 10% fetal calf serum, antibiotics (penicillin and streptomycin), 5.95 μg of HEPES per ml, and 2 mM l-glutamine, and plated onto plastic petri dishes (Costar). After 2 h of incubation at 37°C in a 5% CO2 atmosphere, nonadherent cells were removed. Adherent cells were further incubated for 7 days to allow monocytes to differentiate into macrophages. Adherent cell monolayers (2 × 105 cells/well) were subcultured in 24-well plates in RPMI medium.
DEN-2 strain 16681, was generously provided by Richard Kinney from the Centers for Disease Control and Prevention, Fort Collins, Colo. Virus was propagated in suckling mice brain and in C6/36 cells.
Preparation of total cell protein extract.
Neuroblastoma, U937 cells, and monocytes/macrophages were pelleted at 840 × g for 10 min and washed three times with PBS. The cell pellet was then resuspended in RSB-NP-40 (1.5 mM MgCl2, 10 mM Tris-HCl, 10 mM NaCl, and 1% Nonidet P-40) in the presence of protease inhibitor cocktail (2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 2 mM benzonidine, 5 μg of aprotinin per ml, 5 μg of pepstatin per ml, 5 μg of leupeptin per ml, and 5 μg of chymostatin per ml) (MiniComplete; Roche). Nuclei and debris were removed by centrifugation at 12,000 × g for 15 min at 4°C.
To obtain biotinylated membrane proteins, neuroblastoma and U937 cells were pelleted and washed as above; 100 μg of biotin sulfosuccinimidyl esther (Sulfo-NHS-biotin; Sigma) in 0.1 ml of PBS was added to approximately 106 cells, and cells were incubated in the dark for 1 h at room temperature. Cell viability was verified by trypan blue staining before and after cell biotinylation. Total protein extracts were obtained as above. The amount of protein was determined by using the Bradford method (7).
Purification of DEN virus receptor complex.
DEN virus receptor complex molecules present in neuroblastoma and U937 cells were purified by affinity chromatography as previously described (31). Briefly, 2 mg of total protein cell extract from both cell lines was passed over an affinity column, which consisted of full-length DEN-4 recombinant E protein immobilized by its amino-terminal His tag to a Ni-nitrilotriacetic acid (NiNTA) resin (QIAGEN). After extensive washing with interaction buffer (50 mM NaH2PO4, 200 mM NaCl, 1% Triton X-100 [pH 8]) the DEN virus receptor molecules were eluted with high-ionic strength buffer (50 mM NaH2PO4, 500 mM NaCl [pH 8]). The eluted molecules were concentrated in a Centricon 10 centrifuge microconcentrator (Amicon, Beverly, Mass.) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue. The 84-kDa band was excised; after in-gel digestion with trypsin, the peptides were analyzed by MALDI-TOF mass spectrometry, and the protein was identified at the Protein Core Lab Facility at Columbia University, New York, N.Y.
VOPBA.
To verify that the isolated proteins are involved in virus binding, a virus overlay protein binding assay (VOPBA) was performed. Briefly, total proteins from neuroblastoma cells or elution fraction were separated by SDS-10%PAGE and transferred to nitrocellulose membranes as described elsewhere (41). After overnight renaturalization of transferred proteins with 4% bovine serum albumin (BSA) in PBS at 4°C, the membranes were blocked overnight at 4°C with low-fat milk and washed three times with PBS. Afterwards, membranes were incubated with 2 × 104 PFU of DEN-2 in PBS-220 mM NaCl-5% low-fat milk for 5 h at 37°C. Then, membranes were washed once for 5 min with PBS, 1% skim milk, and 220 mM NaCl and three times with PBS. The membrane was then incubated with a mouse polyclonal anti-DEN antibody diluted 1:500 in PBS for 3 h at room temperature and with a goat polyclonal anti-mouse IgG antibody conjugated to peroxidase diluted 1:30,000 (Bio-Rad) in PBS for 1 h at room temperature. After extensive washing with PBS-Tween, the antigen was visualized by chemiluminescence by using a Femto-Mol detection system (Pierce) according to the manufacturer's instructions
His tag pull-down assay.
His tag recombinant E protein pull-down assays were employed to purify DEN virus receptor complex and associated proteins in human monocytes. Thirty micrograms of total protein extract from peripheral human monocytes previously preabsorbed with NiNTA Sepharose 4B beads was brought to a final volume of 1 ml with interaction buffer, mixed with 10 μg of recombinant E protein coupled to NiNTA resin or NiNTA alone as control, and rotated end over end for 2 h at 4°C. The beads were then collected by centrifugation for 2 min at 500 × g at 4°C and washed five times with 500 μl of interaction buffer. DEN virus receptor complex and interactors were eluted from the beads by a 10-min incubation at room temperature in 20 μl of elution buffer as before. Pull-down eluates were analyzed by SDS-PAGE and immunoblotting with anti-HSP90 (Stress-gene) and anti-HSP70 antibodies (Chemicon) as described.
Infection inhibition assay.
A total of 104 PFU of DEN-2 were incubated with increasing concentrations (from 100 to 500 ng) of recombinant HSP90 protein prior infection. Neuroblastoma cells were infected at a multiplicity of infection (MOI) of 1 PFU/cell at 37°C for 1 h, and later cells were washed three times with culture medium. Then, fresh medium was added to infected cells, and infection was allowed to proceed for 48 h at 37°C. After infection, cells were frozen until a plaque assay was performed.
In addition, 106 10-day-old monocytes/macrophages, plated in 24-well plates, were preincubated with culture medium containing 50 μg of human gamma globulin per ml at 4°C for 30 min to block Fc receptors. Afterwards, the cells were treated with increasing concentrations of the anti-HSP90 monoclonal antibody or anti-HSP70 monoclonal antibody diluted in the same culture medium for 1 h at 4°C. Then cells were infected at an MOI of 0.1 or 1 PFU/ml with DEN-2 diluted in culture medium for 1 h at 4°C. After cells were washed three times with fresh medium, infection was allowed to proceed for 48 h at 37°C, as mentioned above.
DEN virus titration.
DEN virus titers were determined by plaque assay as the cytopathic effect on confluent monolayers of BHK-21 cells cultured in MEM-supplemented with 10% fetal calf serum. Virus was obtained from the infected cells after freezing and thawing. When the adherent BHK-21 cells reached 80 to 90% confluence, aliquots of cell cryolysates from DEN virus-infected neuroblastoma cells and monocytes/macrophages were inoculated at 10-fold serial dilutions between 10−1 to 10−6. After 4 h of viral adsorption, the BHK-21 cell monolayers were overlaid with MEM containing 3% carboximethil-cellulose (Sigma), 0.5% fetal calf serum, and 2 mM l-glutamine. The cultures were incubated at 37°C for 6 days and then counted for plaque formation after fixation with 10% formalin and staining with 0.5% naphtol-blue-black (Sigma).
Indirect immunofluorescence assay.
Subconfluent (85%) monocytes/macrophages were plated in eight-microwell tissue culture chambers (Lab-Tek), and indirect immunofluorescence assays on nonpermeabilized cells were performed as described previously (23) with the following modifications: the anti-HSP90 and anti-HSP70 antibodies were diluted 1:50 in PBS with 10% human Ig and a fluorescein isothiocyanate-coupled goat anti-mouse antibody was used at a dilution of 1:175 (Zymed). As a control an anti-actin monoclonal antibody diluted 1:50 in PBS was used.
Isolation of lipid rafts.
U937 cells (5 × 106) were lysed in 1 ml of MEB buffer (150 mM NaCl, 20 mM MES [pH 6.5]) containing 1% Triton X-100 and protease inhibitor cocktail (2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 2 mM benzonidine, 5 μg of aprotinin per ml, 5 μg of pepstatin per ml, 5 μg leupeptin per ml, and 5 μg of chymostatin per ml) (MiniComplete; Roche) for 1 h on ice. The cells were mixed with an equal volume of 90% sucrose in MEB buffer and placed at the bottom of a centrifuge tube. The samples were overlaid with 5.5 ml of 30% sucrose and 4.5 ml of 5% sucrose in MEB buffer and centrifuged at 200,000 × g for 6 h. Fractions of 600 μl were gently removed from the top of the gradient and saved at 4°C for SDS-PAGE and immunoblotting. For isolation of lipid rafts following recombinant E protein stimulation, U937 cells were stimulated with 500 μg of recombinant E protein for 2 h at 37°C. For Western blot assays, equal portions of each fraction were analyzed by SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, Calif.) for 30 min at 16 V in the presence of transfer buffer (48 mM Tris, 39 mM glycine, and 20% [vol/vol] methanol) in a semidry electroblotting system (Bio-Rad). After transfer, membranes were blocked for 1 h at 37°C and probed with the appropriate dilution of primary antibody for 2 h at 37°C, followed by washing with PBS-Tween. Membranes were incubated with horseradish peroxidase conjugated to either goat anti-mouse IgG (1:50,000) or rabbit anti-goat IgG (1:50,000) for 1 h at 37°C. After extensive washing with PBS-Tween, the antigen was visualized by chemiluminescence by using a Femto-Mol detection system according to the manufacturer's instructions.
RESULTS
Isolation of DEN virus receptor complex by affinity chromatography.
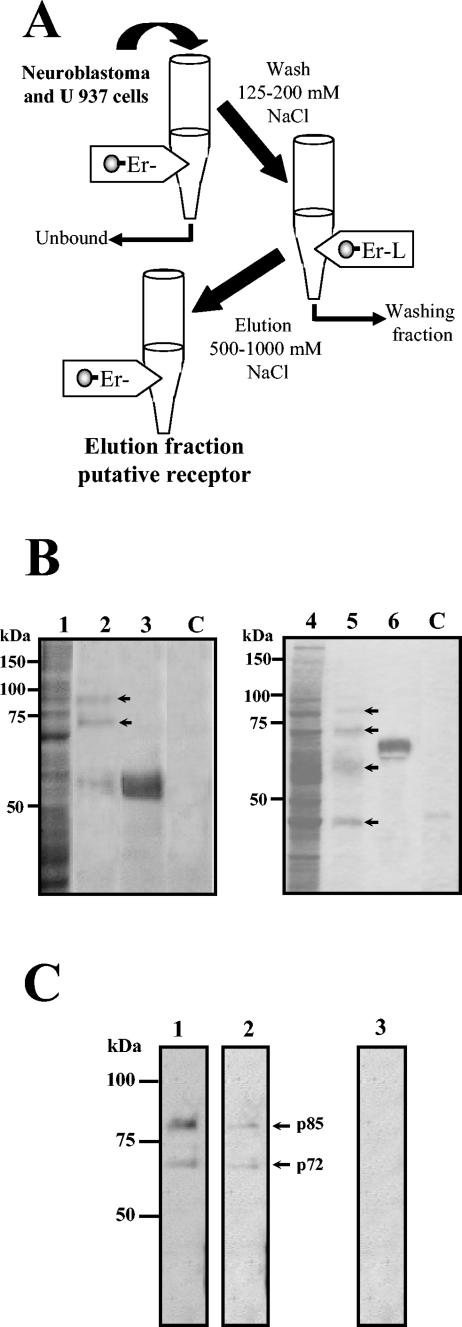
The isolation of DEN virus putative receptor proteins was performed by affinity chromatography by using a recombinant E protein as a ligand (31). Neuroblastoma and U 937 cell lysates were passed over an E protein column. After extensive washing with up to 200 mM NaCl buffer, the proteins that specifically interacted with E protein were eluted with a high-ionic-strength buffer (see Materials and Methods) (Fig. 1A). The eluted molecules were concentrated and analyzed by SDS-10%PAGE. After staining with Coomassie blue, two main bands with molecular masses of 84 and 100 kDa and a low amount of the E protein (60 kDa) were visualized in the elution fraction from neuroblastoma cells, and five main bands of 45, 60, 75, 84, and 100 kDa were visualized in the elution fraction from U937 cells (Fig. 1B, lanes 2 and 5, respectively). The presence of the E protein attached to the column after interaction with cell extracts was corroborated (Fig. 1B, lanes 3 and 6). Cell lysates passed through a NiNTA resin column alone revealed no protein band (Fig. 1B, lanes C), which suggests that the E protein binding was specific. To validate this result, a VOPBA was performed. The native DEN-2 virus recognized two proteins with similar molecular masses to the ones observed by affinity chromatography in neuroblastoma total cell extract and in the elution fraction (Fig. 1C, lanes 1 and 2, respectively). As a control, in the absence of DEN-2 virus, the anti-DEN virus antibody was unable to reveal any protein band (Fig. 1C, lane 3).
FIG.1.
Affinity chromatography with DEN virus E recombinant protein used as bait. (A) Schematic representation of the procedure for affinity isolation of putative DEN virus receptor with recombinant E protein as a ligand. Total proteins from neuroblastoma (SK-SY-5Y) and U937 cells were passed through the affinity chromatography column. After washing with 125 to 200 mM NaCl buffer, elution was accomplished by using a high-salt buffer (500 and 1,000 mM NaCl). (B) Isolation of cell proteins with affinity to DEN virus E protein. Total proteins from SK-SY-5Y (lanes 1 to 3) and U937 cells (lanes 4 to 6) were passed through an affinity column with recombinant E protein coupled to NiNTA-agarose. Ten microliters of the NiNTA-agarose coupled to the recombinant E protein (lanes 3 and 6), 10 μl of the protein fraction not bound to the affinity column (lanes 1 and 4), 50 μl of the elution fractions (lanes 2 and 5) and, as a control, 50 μl of the elution fractions obtained in the absence of the recombinant E protein (lanes C) were separated by SDS-10% PAGE and stained with Coomassie blue. (C) VOPBA assay of the elution fraction. Elution fraction containing the proteins with affinity to DEN virus E protein from SK-SY-5Y (lane 1) and U937 cells (lane 2) were separated by SDS-10% PAGE and transferred to nitrocellulose membranes. Membranes were incubated without (lane 3) or with 2 × 104 PFU of DEN-2 (lanes 1 and 2) and later with a mouse polyclonal anti-E antibody and with a second antibody, goat anti-mouse IgG coupled to alkaline phosphatase. Color was developed with 5-bromo-4-chloro-3 indolyl phosphate toluidinium and nitroblue tetrazolium chloride. Molecular mass markers are indicated on the left. Arrows indicate migration of eluted proteins as well as the 72- and 85-kDa proteins revealed for DEN virus in both cell lines.
To demonstrate that the molecules isolated by using the E recombinant column were located on the surface of the cells, a biotinylation of surface proteins from neuroblastoma and U937 cells was performed. After biotin labeling, cells were washed, and unincorporated biotin was quenched. Afterwards, a total cell extract was prepared (Fig. 2A, B, and C, lanes 1) and passed through the column. The 72- and 85-kDa proteins obtained in the elution fraction from both cell lines were revealed with streptavidin-peroxidase (Fig. 2A and B, lane 2), indicating that they are located on the surface of neuroblastoma and U937 cells. An additional band of 60 kDa was revealed with streptavidin-peroxidase only in U937 cells (Fig. 2B, lane 2). As a control, when a total extract from surface biotinylated cells was passed through a column without E protein, none of the aforementioned protein bands was observed (Fig. 2C, lane 2).
FIG. 2.
Surface localization of the 72- and 85-kDa proteins from SK-SY-5Y and U937 cells. Surface biotinylated proteins from SK-SY-5Y (A and C) and U937 cells (B) were passed through the column with (A and B) or without (C) recombinant E protein coupled to NiNTA-agarose. Ten microliters of total biotinylated proteins (lanes 1) and 50 μl of the elution fraction (lanes 2) were separated by SDS-10% PAGE, transferred to nitrocellulose membrane, and revealed with streptavidin-peroxidase. Molecular mass markers are indicated on the left. Arrows indicate migration of eluted 72- and 85-kDa proteins as well as a 60-kDa protein present only in U937 cells.
Identification of HSP90 and HSP70 in elution fractions from neuroblastoma and U937 cell extracts.
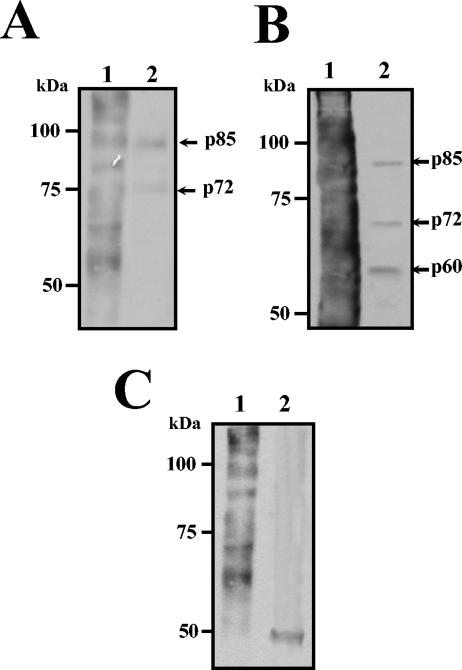
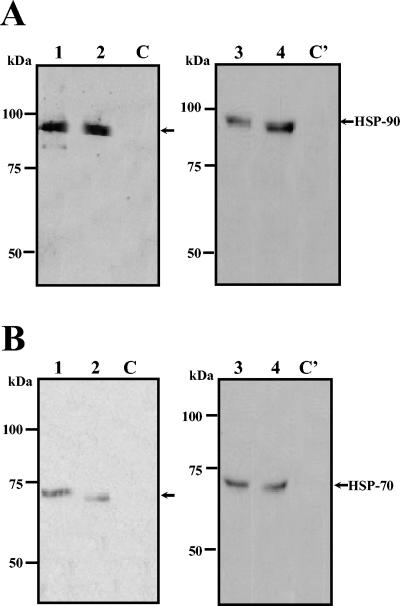
To identify the 84-kDa protein, the protein band in the gel was excised, and, after alkylating and in-gel digestion with trypsin, the peptide mass map was analyzed by mass spectrometry. This mass spectrum was compared with protein databases, and the protein was identified as HSP90α. To further corroborate this identification, transferred proteins obtained in elution fractions from neuroblastoma and U937 cell lysates were probed with an anti-HSP90 monoclonal antibody followed with the corresponding horseradish peroxidase-conjugated secondary antibody. The specific monoclonal anti-HSP90 antibody recognized the 84-kDa protein in the elution fraction obtained from neuroblastoma and U937 cells (Fig. 3A, lanes 2 and 4, respectively). As expected, HSP90 was also detected in total cell extract from both cell lines (Fig. 3A, lanes 1 and 3), while this protein was not revealed when the extracts were passed through the column in the absence of E protein (Fig. 3A, lanes C).
FIG. 3.
HSP90 and HSP70 are eluted from the affinity chromatography with DEN virus recombinant E protein. Thirty micrograms of total protein extract from SK-SY-5Y (lanes 1) and U937 cells (lanes 3), 50 μl of eluted fraction from SK-SY-5Y (lanes 2) and U937 cells (lanes 4) obtained in the presence of recombinant E protein, or 50 μl of eluted fraction from SK-SY-5Y (lanes C) and U937 cells (lanes C') obtained in the absence of recombinant E protein was separated by SDS-10% PAGE, transferred to nitrocellulose membrane, and incubated with monoclonal antibodies against HSP90 (A) or anti-HSP70 (B). Then membranes were incubated with a second antibody goat anti-mouse IgG coupled to peroxidase. Reactions were developed by chemiluminescence. Molecular mass markers are indicated on the left. Arrows indicate migration of HSP90 and HSP70.
Since it has been reported that LPS inhibits DEN virus infection in peripheral human monocytes through the blockade of virus entry (10) and HSP90 is one of the surface molecules involved in the CD14-independent LPS receptor cluster (42), the presence of other proteins of this cluster was looked for in our elution fractions. One of the proteins present in the CD14-independent LPS receptor cluster is HSP70 (72 kDa). To determine whether the protein with molecular mass of 72 to 74 kDa was HSP70, a Western blot assay was performed. Two different monoclonal antibodies directed against HSP70 recognized the 72-kDa band present in the elution fractions from both neuroblastoma and U937 cells lysates (Fig. 3B, lanes 2 and 4). As expected, this protein was also detected in total cell extract from both cell lines (Fig. 3B, lanes 1 and 3), while it was not revealed when the extracts were passed through the column in the absence of E protein (Fig. 3B, lanes C).
Identification of HSP70 and HSP90 in His tag pull-down eluates from human peripheral monocytes.
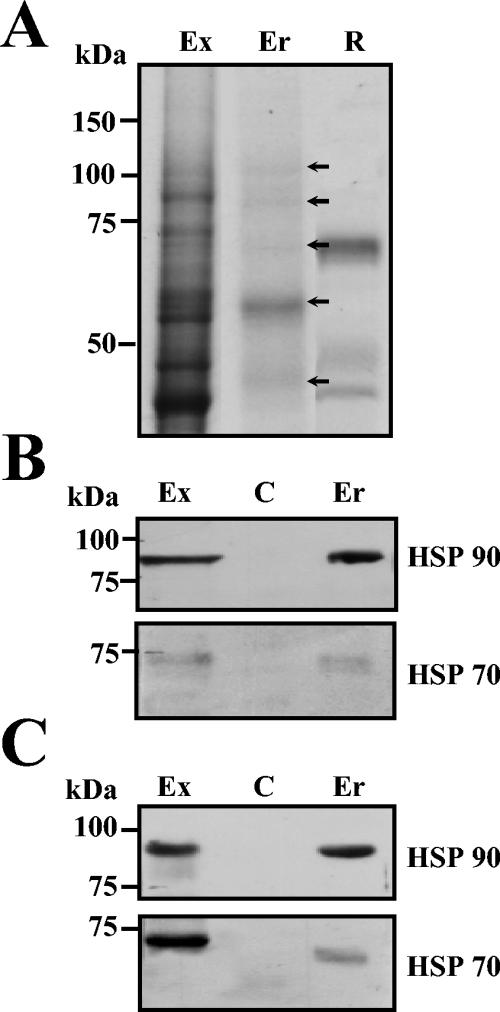
To verify the interaction between E protein and HSP70 and HSP90 from U937 cells lysates, the E protein was used as bait in the His tag pull-down assays. The protein band pattern pulled down in E protein complexes was like the one observed in the elution fraction from the affinity column (data not shown). The anti-HSP90 as well as anti-HSP70 monoclonal antibodies recognized the 84- and 72-kDa proteins in E protein pulled-down eluates from U937 cells (Fig. 4B, lane Er), while no protein bands were observed when the resin was incubated with the lysates in the absence of E recombinant protein (Fig. 4B, lane C). As expected, the presence of both HSP70 and HSP90 in total extract from U937 cells was also observed. (Fig. 4B, lane Ex).
FIG. 4.
Isolation of DEN virus receptor complex from SK-SY-5Y and peripheral blood monocytes by pull-down assay. (A) Isolation of cell proteins with affinity to DEN virus E protein. Total proteins from human peripheral blood monocytes/macrophages (lane Ex) were incubated with NiNTA-agarose coupled with the recombinant E protein (lane R). Pulled down eluates (lane Er) were separated by SDS-10% PAGE and stained with Coomassie blue. Arrows indicate migration of the pulled-down eluted proteins. (B and C) Total proteins (lanes Ex) from U937 cells (B) and peripheral blood monocytes (C) were incubated with NiNTA-agarose alone (lane C) or coupled with the recombinant E protein (lane Er). Pulled down eluates were separated by SDS-10% PAGE, transferred to nitrocellulose membrane, and incubated with anti-HSP90 (upper panels) or with anti-HSP70 antibodies (lower panels). Western blots were developed by chemiluminescence. Molecular mass markers are indicated on the left.
During pathogenesis of DEN virus infection, several tissues become infected. Peripheral monocytes/macrophages have been proposed to be preferred targets for DEN virus replication in vivo, and, therefore, the interaction of DEN virus with these cells is a relevant issue (29).
To determine the interaction between E protein and HSP70 and HSP90 from human peripheral blood monocytes, a His tag pull-down assay was performed. The protein band pattern pulled down by E protein, when interacted with cellular extracts from human peripheral monocytes/macrophages (Fig. 4A, lane Er), was much like the one observed in the elution fraction of U 937 cells (compare Fig. 1B, lane 5, with Fig. 4A, lane Er). Anti-HSP90 and anti-HSP70 monoclonal antibodies also recognized the 84- and 72-kDa proteins in E protein pulled-down eluates from monocytes/macrophages (Fig. 4C, lane Er). The presence of both HSP70 and HSP90 in total extract from monocytes/macrophages was also detected (Fig. 4C, lane Ex). These results strongly suggest that HSP90 and HSP70 from human blood monocytes interact with DEN virus E protein as seen with U937 and neuroblastoma cell lines.
HSP90 and HSP70 are present on the surface of peripheral blood monocytes/macrophages.
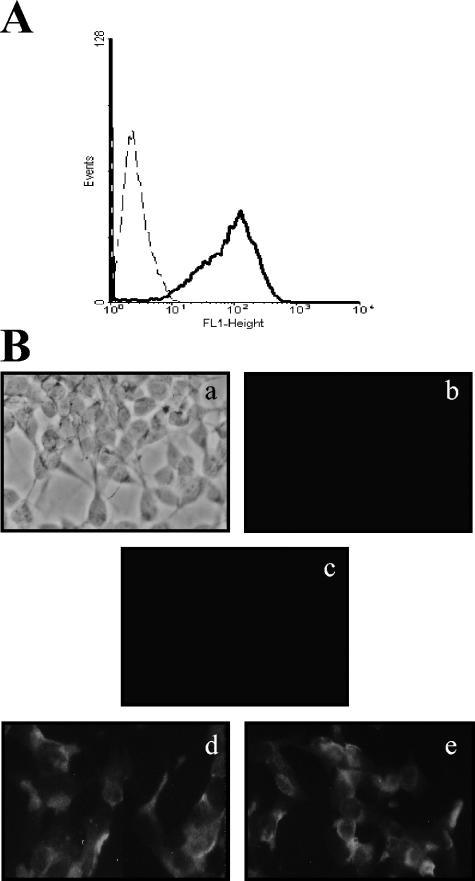
Although it has been previously reported that HSP90 and HSP70 are located on the surface of human monocytes (8, 42), the presence of both HSPs was corroborated by indirect immunofluorescence in primary cultures of CD14-positive cells, a typical monocyte marker (Fig. 5A). Anti-HSP90 and anti-HSP70 antibodies were able to detect both proteins on the surface of nonpermeabilized peripheral blood monocytes/macrophages (Fig. 5B, d and e, respectively). As expected, anti-actin antibody does not detect any molecule on the surface of nonpermeabilized monocytes as well as the isotype control (Fig. 5B, b and c, respectively).
FIG. 5.
Localization of HSP90 and HSP70 on the surface of peripheral blood monocytes. (A) Analysis of CD14 surface expression in 10-day-old peripheral blood monocytes/macrophages (solid line) and the control antibody of isotype IgG (dashed line) by flow cytometry. (B) Nonpermeabilized 10-day-old monocytes/macrophages were incubated with anti-actin (b), anti-HSP70 (d), and anti-HSP90 (e), or a control antibody (c), followed by staining with a fluorescein isothiocyanate-conjugated goat anti-mouse IgG (a to e). A phase contrast image is shown in panel a.
Recombinant HSP90α protein and an anti-HSP90α monoclonal antibody inhibit DEN virus infection in neuroblastoma cells.
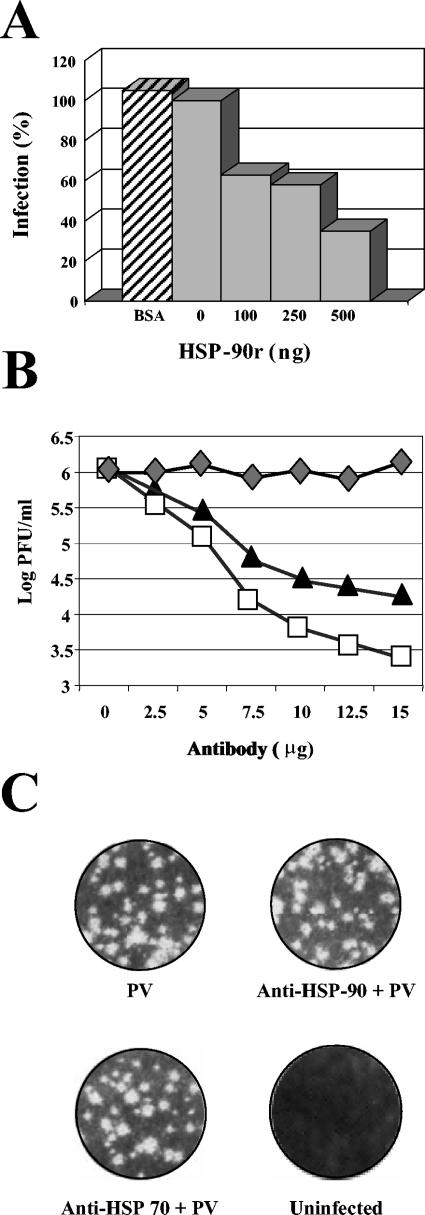
The importance of HSP90 in DEN virus infection was analyzed by using the recombinant HSP90α as competitor in an infection inhibition assay. DEN-2 virus was incubated with increasing concentrations of the recombinant HSP90 before its interaction with neuroblastoma cells. Infection was permitted to progress for 48 h, and the amount of DEN virus produced was evaluated by plaque assays. Recombinant HSP90 was able to block DEN virus infection in a dose-dependent manner. At a concentration of 500 ng/ml, recombinant HSP90 inhibited the infectivity of the viruses up to 60% (Fig. 6A). In contrast, BSA had no effect on DEN virus infectivity (Fig. 6A). Partial inhibition in DEN virus infection is in accordance with the hypothesis that a single molecule does not comprise the DEN virus receptor.
FIG. 6.
Infection inhibition assays by recombinant HSP90 protein and by anti-HSP90 and anti-HSP70 antibodies in neuroblastoma cells. (A) DEN-2 was preincubated with different concentrations of recombinant HSP90 (HSP-90r) protein or with BSA, as a negative control, for 1 h at 37°C prior to the incubation with SK-SY-5Y cells. Culture supernatants were harvested after 48 h of infection and assayed to determine the infectious virus titer. Each experimental point is pre-sented as the mean of results obtained from three separate experiments. (B) Infection inhibition assays by anti-HSP90 and anti-HSP70 antibodies in SK-SY-5Y cells. Neuroblastoma cells were preincubated with different concentrations of an unrelated antibody (mouse polyclonal serum; diamond) or with anti-HSP90 (square) and anti-HSP70 (triangle) antibodies for 1 h at 37°C. Subsequently, cells were infected with DEN-2 at an MOI of 5. Culture supernatants were harvested after 48 h of infection and assayed to determine the infectious virus titer. Each experimental point is presented as the mean of results obtained from three separate experiments. (C) Poliovirus (PV) infection inhibition assay by anti-HSP90 or anti-HSP70 antibodies in neuroblastoma cells. Cells were preincubated with anti-HSP90 and anti-HSP70 antibodies for 1 h at 37°C. Subsequently, cells were infected with poliovirus at an MOI of 10. Culture supernatants were harvested after 24 h of infection, and a plaque infectivity assay was performed on HeLa cells as reported (20).
As an alternative approach to evaluate the importance of HSP90 and HSP70 in DEN virus infection, we used anti-HSP90 and anti-HSP70 antibodies in an infection inhibition assay. The anti-HSP90 and anti-HSP70 antibodies blocked the infectivity of DEN virus in neuroblastoma cells in a dose-dependent manner up to 2 logs in infectious virus yields evaluated by plaque assay (Fig. 6B) This reduction in virus yields was observed at an MOI of 0.1 as well as at an MOI of 1 (Fig. 6B), suggesting that HSP90 and HSP70 are involved in DEN virus entry.
To demonstrate the specificity of both HSPs as receptors for DEN virus, we performed a poliovirus infection inhibition assay by using both anti-HSP90 and HSP70 antibodies in neuroblastoma cells. As expected, neither HSP90 nor HSP70 antibodies were able to inhibit poliovirus infection (Fig. 6C)
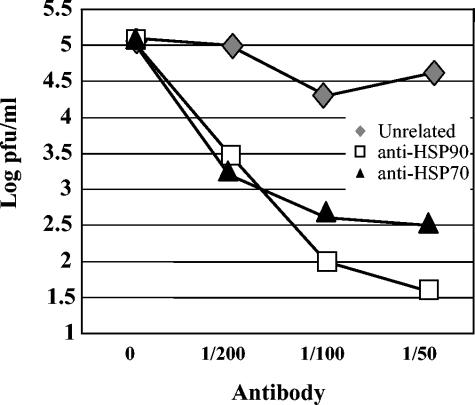
DEN virus infection in human peripheral monocytes/macrophages is inhibited with monoclonal antibodies anti-HSP90 or anti-HSP70.
To evaluate the role of HSP90 and HSP70 in DEN virus entry in monocytes/macrophages, 14-day-old isolated human monocytes/macrophages obtained from healthy donors, negative for DEN virus or any other flavivirus infection, were treated with anti-HSP90 or with anti-HSP70 monoclonal antibodies before DEN virus infection. As shown in Fig. 7, the pretreatment resulted in up to a 3 log reduction in infectious virus yields in the 48-h culture supernatant assayed to determine infectious virus titer. This last result is in agreement with the cooperative, multistep theory in DEN virus entry.
FIG. 7.
Infection inhibition assays by anti-HSP90 and anti-HSP70 antibodies in peripheral blood monocytes/macrophages. Monocytes/macrophages were preincubated with different concentrations of an unrelated antibody (mouse polyclonal serum; diamond) or with anti-HSP90 (square) and anti-HSP70 (triangle) antibodies for 1 h at 37°C. Subsequently, cells were infected with DEN-2 at an MOI of 5. Culture supernatants were harvested after 48 h of infection and assayed to determine the infectious virus titer. Each experimental point is presented as the mean of results obtained from three separate experiments.
Following Er protein adsorption, HSP70 and HSP90 concentrate in lipid rafts.
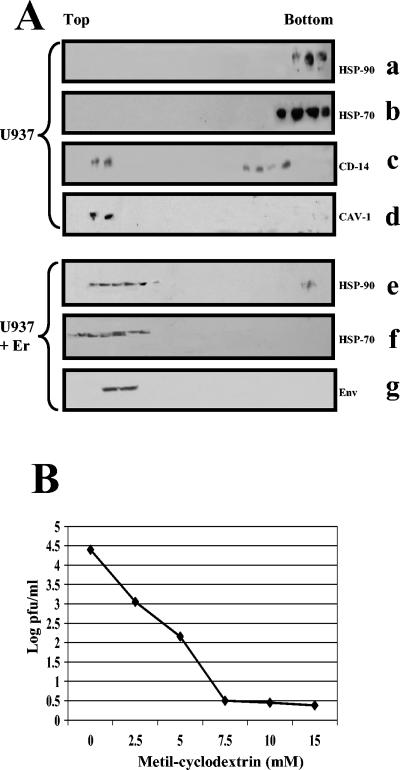
Although their existence has been debated, the presence of specific microdomains in cellular membranes is a largely accepted concept. Imaging these microdomains as floating islands in the membrane, Simons and Ikonen (35) describe them as lipid rafts. These structures in the cellular membrane are characterized by detergent insolubility, light density, and enrichment for cholesterol, glycosphingolipids, and glycosylphosphatidylinositol (GPI)-linked proteins (30). The fundamental principle by which rafts exert their function is by concentrating specific membrane proteins in microdomains. To determine whether HSP90 and HSP70 as receptor molecules are microdomain-associated proteins, from U 937 cells, a monocytic cell line, we isolated lipid rafts on the basis of their insolubility in cold Triton X-100 and low-buoyant density on sucrose gradients immediately after E recombinant protein incubation (see Materials and Methods). CD14, a GPI-linked protein and, hence, raft associated, and caveolin-1, a typical raft marker, were found to migrate near the top of the sucrose gradient, fractions 2 to 4, pointing the relative position of the microdomain (insoluble), while both HSPs migrate in nonraft (soluble) fractions (Fig. 8A, upper panel). In the presence of E protein, HSP90 and HSP70 clearly migrated in the raft fraction. Interestingly, we also found that E protein is located in the same fraction as its cellular receptor complex (Fig. 8A, lower panel).
FIG. 8.
Detergent-resistant membrane fraction localization and methyl-β-cyclodextrin treatment. (A) U937 cells were incubated in the absence (a, b, c, and d) or in the presence (e, f, and g) of E recombinant protein for 1 h at 37°C and lysed with 1% Triton X-100 and subjected to flotation on a discontinuous sucrose gradient. Gradient was fractionated from the top to the bottom as indicated. Equal volumes from each fraction were analyzed by SDS-10% PAGE followed by immunoblotting with anti-HSP90 (a and e), anti-HSP70 (b and f), anti-CD14 (c), anticaveolin (d), and anti-DEN virus E protein (g). (B) Infection inhibition induced by methyl-β-cyclodextrin treatment. Human peripheral blood monocytes were treated with increased concentrations of methyl-β-cyclodextrin for 1 h at 37°C prior to DEN-2 infection at an MOI of 5. Culture supernatants were harvested after 48 h of infection and assayed to determine the infectious virus titer. Each experimental point is presented as the mean of results obtained from three separate experiments.
To investigate the significance of lipid raft integrity for DEN virus entry, we tested the ability of DEN virus to infect human peripheral monocytes/macrophages that had been treated with a lipid raft disrupter, such as methyl-cyclodextrin (MCD), an agent that depletes the cholesterol from the cells. Monocytes were treated with increasing concentrations (from 2.5 to 15 mM) of MCD and then infected with DEN virus. MCD treatment inhibits DEN virus infection in a dose-dependent manner (Fig. 8B), suggesting that raft integrity is important in DEN virus infection by clustering its receptor complex.
DISCUSSION
Viruses must deliver their nucleic acids across a cellular membrane into the cytoplasm of their target cell to cause infection (34, 36). The strategy employed by enveloped viruses, such as DEN virus, is relatively simple; the major protein in the viral membrane, E protein, recognizes its cellular receptor on the cell surface, the virion is internalized to an endosome, and a reduced pH in the endosome causes conformational changes in E protein, resulting in viral membrane fusion with the endocytic vesicle membrane and release of viral nucleocapsid into the cytoplasm. This mechanism is simple but not completely understood at present. The carboxy-terminal domain in E protein, particularly the lateral face of domain III, has been proposed as the binding site for cellular receptors, and it has been implicated as a molecular determinant of virulence (11, 22)
Even though monocytes and macrophages have been described as the major target cells in DEN virus infection, the primary cell that supports DEN virus replication in severe cases remains unknown.
DEN virus entry in monocytes, particularly during secondary infections, has been thought to occur through an antibody-dependent enhancement mechanism, due to the presence of heterotypic subneutralizing antibodies raised in a previous infection (21). Such antibodies are suggested to mediate DEN virus entry in Fc receptor-harboring cells. However, DEN virus entry into monocytes, as in other cells, does not occur via a phagocytic event, and antibody-dependent enhancement is not observed in all Fc -receptor-harboring cells. Thus, it is possible that antibody attracts virus to a cell surface by getting entry through a primary cell receptor.
The cellular receptors that mediate the attachment and entry of DEN virus have been only partially characterized, and, to date, the definitive identification of the molecules required for these processes in host cells has not been achieved. Although several proteins have been reported as candidate protein receptors, their definite identification has not been successful so far. Putative receptors for the four serotypes of DEN virus in different cell types include proteins of 23, 40 to 45, 70 to 75, and 80 to 100 kDa; however, their physiological relevance remains unclear.
As an alternative approach to isolate proteins that can be specifically recognized by E protein, we designed an affinity column where E recombinant protein was attached by its amino-terminal His tag, allowing the carboxy-terminal region to interact with cellular proteins. This method was used to isolate molecules previously reported as putative DEN virus receptors (31).
In the present work, we isolated a group of proteins from neuroblastoma and U937 cell lines with affinity to DEN virus E protein. Both cell lines are highly susceptible to DEN virus and are relevant models for natural infection. Human neuroblastoma cells have been used as a model for DEN virus neural infection as has been described previously by Jan et al. (18). In this type of cells the virus triggers apoptosis and activation of NF-κB. During dengue outbreaks, an increased number of cases of encephalitis have been reported, beginning early in the course of illness mainly among the complicated cases. Although DEN virus is not a classic neurotropic virus like other flaviviruses, direct involvement of the brain during DEN virus infection has been demonstrated (2). As a result of the pathophysiology of DEN virus, encephalitis has gained increased attention (37).
The other human cell line, the promonocytic U937 cells, can be differentiated in macrophages by phorbol myristate acetate. This cell line has frequently been used as a model to study macrophage function and can support DEN virus replication in vitro (29). As noted above, monocytes and macrophages have long been recognized as major targets of DEN virus replication in the human host. DEN virus-infected human monocytes have been revealed to be potent sources of vasoactive cytokines such as tumor necrosis factor alpha and interleukin-1β. Monocytes also produce several vasoactive mediators, any of which could have powerful effects on endothelial cell physiology. Because most of the pathological changes associated with DHF are essentially hemostatic and vasoactive, it is suspected that virus-infected blood monocytes orchestrate many of these effects (14).
The affinity chromatography approach allowed us to isolate the HSP of 84 kDa. The HSPs have numerous functions, such as protein folding and peptide presentation to the immune system. HSP90 is distinguished from other chaperones since most of its known substrates are signal transduction proteins, whose instability is intrinsic to their action as molecular switches. Even though HSP90 and another HSP of 72 kDa (HSP70) mainly function as cytoplasmic chaperones, several studies suggest that they exist on the cell surface. To demonstrate this fact, indirect immunofluorescence assays using anti-HSP90 and anti-HSP70 monoclonal antibodies were performed and detected their presence on the surface of human monocytes/macrophages. As a cell surface receptor, HSP90 has been implicated as a mediator of the LPS-like effects of taxol (8), a plant-derived antitumor agent, in human macrophages. Furthermore, HSP90 and HSP70 have been identified as CD14-independent cell surface functional receptors for LPS in human monocytes/macrophages (42). Interestingly, it has been reported that DEN virus infection in human monocytes is inhibited by bacterial LPS (9). Our results offer an explanation for this finding. When monocytes are incubated with LPS prior to DEN virus infection, HSP90 and HSP70 are clustered around CD14, preventing them from interacting with DEN.
Infection inhibition assays in human monocytes/macrophages support the fact that HSP90 and HSP70 are part of a receptor complex required for DEN virus entry. It is important to notice that the inhibition induced by HSP90 and HSP70 antibodies is the highest one reported for a specific antibody for DEN virus. Other viruses, including rotavirus (15, 45) and coxsackievirus A9 (43), have been reported to use surface HSPs as cell receptors. Specifically, Hsc70 has been described as a member of a receptor complex for rotavirus, and glucose-regulated protein 78 (GRP78) is a member of a receptor complex in coxsackievirus A9.
In a recent report (19), the stress protein GRP78 was also described as a member of the receptor complex for DEN-2 in HepG2, a hepatic cell line. In this report authors identified by MALDI-TOF a protein that migrates in SDS-PAGE at an equivalent position to the major virus-binding band (90 kDa) observed by VOPBA. Since in SDS-PAGE several proteins are present in a protein band, it is possible that GRP78 is not the molecule that interacts with the viral particle. Supporting this fact, authors reported that preincubation of HepG2 cells with an anti-GRP78 antibody directed against its amino-terminal region prior to DEN-2 infection inhibited viral infection up to 60%. Since HSPs contain several conserved sequences, specifically in their amino termini, it is possible that this antibody had cross-reacted with HSP70 or even with HSP90 during the infection inhibition assay.
Additionally, Modis et al. (25) recently reported the structure of DEN virus E protein after membrane fusion, and a model to explain the conformational changes in E protein required to promote membrane fusion has been proposed. According to this model, after the cell receptor binds E protein through domain III and subsequent endosomal uptake, the protein rearranges to assemble a trimer, which is the structure that leads to membrane fusion. In this transition to a trimer, domain III shifts and rotates, folding the carboxy terminus back toward the fusion peptide. This domain is the one that undergoes the most significant displacement in the dimer-to-trimer transition. HSP90 and HSP70 as chaperones might participate in this proposed transition.
On the other hand, recent studies have shown that the plasma membrane is discontinuous, containing numerous microdomains that are essential for cellular functions. These lipid microdomains, also known as lipid rafts, are characterized by detergent insolubility, light density, and enrichment for cholesterol, glycosphingolipids, and GPI-linked proteins. Cholesterol has a strong promoting effect on membrane binding and trimerization of DEN virus E protein (38). Our results indicate that the DEN virus receptor complex is associated with membrane microdomains. Additionally, when a raft-disrupting drug was used, a significant inhibition in DEN virus infection was observed, indicating that rafts are a site for virus entry and thus have a profound impact on virus pathogenicity. Since monocytes/macrophages were treated with methyl-cyclodextrin only for 30 min before viral adsorption, it is not possible that the drug inhibits DEN virus infection or replication by other mechanisms than disruption of lipid rafts. The role of lipid rafts in DEN virus infection is a very important aspect to be analyzed, because viral particles could be the stimulus required for cluster molecules, such as HSPs, on specific regions of the surface of the target cells. Then, viral interaction with DEN virus receptor complex may trigger a chain of events that could participate in DEN virus pathogenesis. Further experiments directed to analyze the intracellular signaling induced by DEN virus receptor interactions as well as the role of other molecules in this process are being performed in our laboratory.
This is the first report that identifies a group of molecules involved as receptors for DEN virus in human monocytes/macrophages. The interaction between DEN virus with its receptor complex will allow us to better understand host cell response and DEN virus pathogenesis.
Acknowledgments
We thank Salvador Chavarría for technical assistance. We also acknowledge Lorena Gutiérrez-Escolano and Rosa Martha Yocupicio-Monroy for their critical comments on the manuscript. We thank Carlos Arias for HSP90 and HSP70 antibodies and Takayuki Nemoto for the plasmid to obtain recombinant HSP90.
This work was supported by a grant from Consejo Nacional de Ciencia y Tecnología. Jorge Reyes-del Valle had a scholarship from Consejo Nacional de Ciencia y Tecnología.
REFERENCES
- 1.Alvarez, C. P., F. Lasala, J. Carrillo, O. Muñiz, A. L. Corbi, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angibaud, G., J. Luaute, M. Laille, and C. Gaultier. 2001. Brain involvement in Dengue fever. J. Clin. Neurosci. 8:63-65. [DOI] [PubMed] [Google Scholar]
- 3.Bashirova, A. A., T. B. Geijtenbeek, G. C. van Duijnhoven, C. F. van Vliet, M. Eilering, P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. Kewal-Ramani, Y. van Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belting, M. 2003. Heparan sulfate proteoglycans as a plasma membrane carrier. Trends Biochem. Sci. 28:145-151. [DOI] [PubMed] [Google Scholar]
- 5.Bethell, D. B., K. Flobbe, X. T. Cao, N. P. Day, T. P. Pham, W. A. Buurman, M. J. Cardosa, N. J. White, and D. Kwiatkowski. 1998. Pathophysiologic and prognostic role of cytokines in dengue hemorrhagic fever. J. Infect. Dis. 177:778-782. [DOI] [PubMed] [Google Scholar]
- 6.Bielefeldt-Ohmann, H. 1998. Analysis of antibody-independent binding of dengue virus and dengue virus envelope protein to human myelomonocytic cells and B lymphocytes. Virus Res. 57:63-79. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of micrograms quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Byrd, C. A., W. Bornmann, H. Erdjument-Bromage, P. Temps, N. Plavetich, N. Rosen, C. F. Nathan, and A. Ding. 1999. Heat shock protein 90 mediates macrophages activation by Taxol and bacterial lipopolysaccharide. Proc. Natl. Acad. Sci. USA 96:5645-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Y., T. Maguire, R. E. Hileman, J. R. Fromm, J. D. Esko, R. J. Linhardt, and R. M. Marks. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866-871. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Y. C., S. Y. Wang, and C. C. King. 1999. Bacterial lipopolysaccharide inhibits DEN infection of primary human monocytes/macrophages by blockade of virus entry via a CD14-dependent mechanism. J. Virol. 73:2650-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crill, W. D., and J. T. Roehring. 2001. Monoclonal antibodies that bind to domain III of DEN E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75:4002-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. Kewal-Ramani, and D. R. Littman. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T-cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 13.Germi, R., J. M. Crance, D. Garin, J. Guimet, L. Lortat-Jacob, R. W. H. Ruigrok, J. P. Zarski, and E. Drouet. 2002. Heparan sulfate-mediated binding of infectious dengue virus type 2 and yellow fever virus. Virology 292:162-168. [DOI] [PubMed] [Google Scholar]
- 14.Green, S., D. W. Vaughn, S. Kalayanarooj, S. Nimmannitya, S. Suntayakorn, A. Nisalak, R. Lew, B. L. Innis, I. Kurane, A. L. Rothman, and F. A. Ennis. 1999. Early immune activation in acute dengue is related to development of plasma leakage and disease severity. J. Infect. Dis. 179:755-762. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero, C. A., D. Bouyssounade, S. Zárate, P. Iša, T. López, R. Espinosa, P. Romero, E. Méndez, S. López, and C. F. Arias. 2002. Heat shock cognate protein 70 is involved in rotavirus cell entry. J. Virol. 76:4096-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzman, M. G., and G. Kouri. 2002. Dengue: an update. Lancet Infect. Dis. 2:33-42. [DOI] [PubMed] [Google Scholar]
- 17.Halary, F., A. Amara, H. Lortat-Jacob, M. Messerle, T. Delaunay, C. Houles, F. Fieschi, F. Arenzana-Seisdedos, J. F. Moreau, and J. Dechanet-Merville. 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17:653-664. [DOI] [PubMed] [Google Scholar]
- 18.Jan, J. T., B.-H. Chen, S.-H. Ma, C.-I. Liu, H.-P. Tsai, H.-C. Wu, S.-Y. Jiang, K.-D. Yang, and M.-F. Shaio. 2000. Potential dengue virus-triggered apoptotic pathway in human neuroblastoma cells: arachidonic acid, superoxide anion, and NF-κB are sequentially involved. J. Virol. 74:8680-8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jindadamrongwech, S., C. Thepparit, and D. R. Smith. 2004. Identification of GRP 78 (BiP) as a liver cell expressed receptor element for DEN serotype 2. Arch. Virol. 149:915-927. [DOI] [PubMed] [Google Scholar]
- 20.La Monica, N., J. W. Almond, and V. R. Racaniello. 1987. A mouse model for poliovirus neurovirulence identifies mutations that attenuate the virus for humans. J. Virol. 61:2917-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the virus and their replication, p. 991-1042. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 22.Mandl, C. W., S. L. Allison, H. Holzmann, T. Meixner, and F. X. Heinz. 2000. Attenuation of tick-borne encephalitis virus by structure-based site-specific mutagenesis of a putative flavivirus receptor binding site. J. Virol. 74:9601-9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-Barragán, J. J., and R. M. del Angel. 2001. Identification of a putative coeceptor on Vero cells that participates in dengue 4 virus infection. J. Virol. 75:7818-7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 100:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature (London) 427:313-319. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Altamirano, M. M. B., F. J. Sánchez-Garcia, and M. L. Muñoz. 2002. Non Fc receptor-mediated infection of human macrophages by dengue virus serotype 2. J. Gen. Virol. 83:1123-1130. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieschi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Després. 2003. Dendritic-cell-specific ICAM 3-grabbing-non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue virus. EMBO Rep. 4:723-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemoto, T., T. Ono, and K. Tanaka. 2001. Substrate-binding characteristics of proteins in the 90 kDa heat shock protein family. Biochem. J. 354:663-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Sullivan, M., and H. Killen. 1994. The differentiation state of monocytic cells affects their susceptibility to infection and the effects of infection by dengue virus. J. Gen. Virol. 75:2387-2392. [DOI] [PubMed] [Google Scholar]
- 30.Pike, L. 2003. Lipid rafts: bringing order into chaos. J. Lip. Res. 44:655-667. [DOI] [PubMed] [Google Scholar]
- 31.Reyes-del Valle, J., and R. M. del Angel. 2004. Isolation of putative dengue virus receptor molecules by affinity chromatography using a recombinant E protein ligand. J. Virol. Methods 116:95-102. [DOI] [PubMed] [Google Scholar]
- 32.Rothwell, S. W., R. Putnak, and V. F. La Russa. 1996. Dengue-2-virus infection of human bone marrow: characterization of dengue-2 antigen-positive stromal cells. Am. J. Trop. Med. Hyg. 54:503-510. [DOI] [PubMed] [Google Scholar]
- 33.Salas-Benito, J. S., and R. M. del Angel. 1997. Identification of two surface proteins from C6/36 cells that bind dengue type 4 virus. J. Virol. 71:7246-7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider-Schaulies, J. 2000. Cellular receptors for viruses: links to tropism and pathogenesis. J. Gen. Virol. 81:1413-1429. [DOI] [PubMed] [Google Scholar]
- 35.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature (London) 387:569-573. [DOI] [PubMed] [Google Scholar]
- 36.Smith, A. E., and A. Helenius. 2004. How viruses enter animal cells. Science 304:237-242. [DOI] [PubMed] [Google Scholar]
- 37.Solomon, T., M. D. Mguyen, D. W. Vaughn, R. Kneen, L. T. T. Thao, B. Raengsakulrach, H. T. Loan, N. Day, J. Farrar, K. Myint, M. J. Warrel, W. S. James, A. Nisalak, and N. White. 2000. Neurological manifestation of Dengue infection. Lancet 355:1053-1059. [DOI] [PubMed] [Google Scholar]
- 38.Stiazny, K., C. Koessl, and F. X. Heinz. 2003. Involvement of lipids in different steps of the flavivirus fusion mechanism. J. Virol. 77:7856-7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tassaneetrithep, B., T. H. Burguess, A. Granelli-Piperno, C. Trumpfheller, J. Finke, S. Wellington, M. A. Eller, K. Pattanapanyasat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thepparit, C., and D. R. Smith. 2004. Serotype-specific entry of dengue virus into liver cells: identification of the 37-kilodalton/67 kilodalton high-affinity laminin receptor as dengue virus serotype 1 receptor. J. Virol. 78:12647-12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Towbin, H. T., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Triantafilou, K., M. Triantafilou, and R. L. Dedrick. 2001. A CD14-independent LPS receptor cluster. Nat. Immunol. 2:338-345. [DOI] [PubMed] [Google Scholar]
- 43.Triantafilou, K., and M. Triantafilou. 2003. Lipid raft microdomains: key sites for coxsackievirus A9 infectious cycle. Virology 317:128-135. [DOI] [PubMed] [Google Scholar]
- 44.Wei, H. Y., L. F. Jiang, D. Y. Fang, and H. Y. Guo. 2003. Dengue virus type 2 infects human endothelial cells through binding of the viral envelope glycoprotein to cell surface polypeptides. J. Gen. Virol. 84:3095-3098. [DOI] [PubMed] [Google Scholar]
- 45.Zárate, S., M. A. Cuadras, R. Espinosa, P. Romero, K. O. Juárez, M. Camacho-Nuez, C. F. Arias, and S. López. 2003. Interaction of rotaviruses with Hsc70 during cell entry is mediated by VP5. J. Virol. 77:7254-7260. [DOI] [PMC free article] [PubMed] [Google Scholar]