Abstract
Objective:
to relate Frailty Syndrome and sarcopenia in older adults with and without type 2 diabetes mellitus and identify potential risk factors for frailty and sarcopenia.
Method:
this descriptive epidemiological study was conducted with 140 older adults in the municipality of Sinop, Mato Grosso, Brazil. The frailty phenotype was used for the assessment of Frailty Syndrome, and a physical assessment questionnaire with calf circumference measurement was used for the assessment of sarcopenia.
Results:
regarding Frailty Syndrome, a higher percentage was observed in older adults with type 2 diabetes mellitus compared to those without the disease (p = 0.00). Concerning the presence of sarcopenia, older adults with and without type 2 diabetes mellitus showed similar values, with no statistical significance (p = .74). Frailty Syndrome was associated with physical inactivity (95%CI: 3.29-56.55), age over 75 years (95%CI: 3.30- 27.82), low family income (95%CI: 1.80-50.98), and comorbidities (95%CI: 4.90-5.40). However, sarcopenia was associated with the presence of physical inactivity (95%CI: 1.26-10.44), low weight/ eutrophic (95%CI: 3.32-26.76), and malnutrition/nutritional risk (95%CI: 1.30-7.70) for older adults with and without type 2 diabetes mellitus.
Conclusion:
older adults with diabetes have a higher vulnerability to develop Frailty Syndrome, necessitating the adoption of preventive measures in primary healthcare.
Descriptors: Aged, Diabetes Mellitus , Mellitus, Frailty, Sarcopenia, Primary Health Care, Nursing
Abstract
Objetivo:
relacionar el Síndrome de la Fragilidad y la sarcopenia en ancianos con y sin diabetes mellitus tipo 2; e identificar los potenciales factores de riesgo para la fragilidad y sarcopenia.
Método:
estudio epidemiológico descriptivo realizado en 140 ancianos del municipio de Sinop, Mato Grosso, Brasil. Para evaluar el Síndrome de la Fragilidad se utilizó el fenotipo de fragilidad y para la evaluación de la sarcopenia el cuestionario de evaluación física con medida de la circunferencia de la pantorrilla.
Resultados:
en lo que se refiere al Síndrome de la Fragilidad se obtuvo un mayor porcentaje para ancianos con diabetes mellitus tipo 2, cuando comparados con aquellos sin la enfermedad (p = 0,00). En relación a la presencia de sarcopenia, los ancianos con y sin diabetes mellitus tipo 2 presentaron valores semejantes, sin significación estadística (p = 0,74). El Síndrome de la Fragilidad presentó asociación con: inactividad física (IC95%: 3,29-56,55); intervalo etario superior a 75 años (IC95%: 3,30-27,82); baja renta familiar (IC95%: 1,80-50,98); y, comorbilidades (IC95%: 4,9-5,4). Entre tanto, la sarcopenia fue asociada a la presencia de la inactividad física (IC95%: 1,26-10,44), al bajo peso/eutrófico (IC95%: 3,32- 26,76) y a la desnutrición/riesgo nutricional (IC95%: 1,30-7,70), en los ancianos con y sin diabetes mellitus tipo 2.
Conclusión:
los ancianos diabéticos tienen mayor vulnerabilidad para desarrollar el Síndrome de Fragilidad, lo que requiere la adopción de medidas preventivas en la atención primaria a la salud.
Descriptores: Anciano, Diabetes Mellitus, Fragilidad, Sarcopenia, Atencion Primaria de Salud, Enfermería
Abstract
Objetivo:
relacionar a Síndrome da Fragilidade e a sarcopenia em idosos com e sem diabetes mellitus tipo 2; e identificar os potenciais fatores de risco para fragilidade e sarcopenia.
Método:
estudo epidemiológico descritivo realizado com 140 idosos do município de Sinop, Mato Grosso, Brasil. Para a avaliação da Síndrome da Fragilidade, utilizou-se o fenótipo de fragilidade, e, para a avaliação da sarcopenia, o questionário de avaliação física com medida de circunferência da panturrilha.
Resultados:
quanto à Síndrome da Fragilidade, obteve-se maior percentual para idosos com diabetes mellitus tipo 2 quando comparado àqueles sem a doença (p = 0,00). Em relação à presença de sarcopenia, os idosos com e sem diabetes mellitus tipo 2 apresentaram valores semelhantes, sem significância estatística (p = 0,74). Síndrome da Fragilidade apresentou associação com inatividade física (IC95%: 3,29-56,55), faixa etária superior a 75 anos (IC95%: 3,30-27,82), baixa renda familiar (IC95%: 1,80-50,98) e comorbidades (IC95%: 4,9-5,4). Entretanto, a sarcopenia foi associada à presença da inatividade física (IC95%: 1,26-10,44), baixo peso/eutrófico (IC95%: 3,32-26,76) e desnutrição/risco nutricional (IC95%: 1,30-7,70) para os idosos com e sem diabetes mellitus tipo 2.
Conclusão:
os idosos diabéticos têm maior vulnerabilidade para desenvolver a Síndrome de Fragilidade, requerendo a adoção de medidas preventivas na atenção primária à saúde.
Descritores: Idoso, Diabetes Mellitus , Mellitus, Fragilidade, Sarcopenia, Atenção Primária à Saúde, Enfermagem
Highlights:
(1) Frailty and sarcopenia, important syndromes to be assessed in older adults.
(2) Older adults with T2DM have a higher vulnerability for the development of frailty.
(3) The nurse should implement measures to prevent frailty and sarcopenia.
Introduction
The increase in the number of older adults worldwide is not considered a phenomenon exclusive to developed nations but also applies to countries in the process of development, such as Brazil ( 1 ). In the 1990s, life expectancy in Brazil was estimated to be 66 years, however, it could reach 78 years by 2030 ( 2 ). Estimates indicate that by the year 2050, the growth in the number of older adults will continue at a rate of 3.2% per year, making them 30% of the entire population ( 2 ). Recognizing demographic growth as a relevant and current process for society is essential to understand the needs presented by older adults, as the aging process involves changes in human body functions ( 3 ).
This demographic transition that the country is undergoing contributes to the increased burden of non-communicable chronic health problems, including diabetes mellitus (DM), which stands out with high rates of comorbidities and mortality, particularly among older adults. Currently, there are 537 million adults worldwide (20 - 79 years of age), with 1 in 10 living with DM. It is estimated that by 2030, this number will increase to 643 million, and by 2045, it will reach 784 million ( 4 ).
With the aging of the population, various syndromes are triggered, such as Frailty Syndrome and sarcopenia. Physical Frailty Syndrome is defined as a “clinical condition in which there is an increased vulnerability of an individual to the development of dependence and/or increased mortality when exposed to a stressor” ( 5 ). The presence of Frailty Syndrome in individuals over 60 years of age indicates the need for increased attention from healthcare providers due to the vulnerability of older adults and their predisposition to functional and physical deficits ( 6 ). One study showed a relationship between frailty, age, gender, level of education, marital status, economic status, the presence of heart disease, and hypertension, highlighting the need for early screening in primary healthcare ( 7 ).
According to the European Working Group on Sarcopenia in Older People (EWGSOP), sarcopenia refers to low levels in the assessment of the parameters: muscle quantity and/or quality (measured by skeletal muscle mass), muscle strength (evaluated by handgrip strength), and physical performance (checked through the short physical performance battery or the individual’s gait speed) as an indicator of severity ( 8 ).
A review study showed that in an aging society, the association between sarcopenia or frailty and type 2 diabetes mellitus (T2DM) is an important issue because the skeletal muscles of patients with the disease have a different distribution of myofibers compared to individuals without the disease. Its development is progressive and chronic, allowing for the implementation of effective care strategies to delay the condition ( 9 ).
Therefore, older adults with T2DM may have a higher occurrence of Frailty Syndrome and sarcopenia, as these conditions are related to chronic diseases ( 10 ). The increase in older adults with sarcopenia in the diabetic population and its impact on quality of life affects psychosocial and physical health, making it a significant public health issue ( 11 ). International ( 12 ) and national ( 7 , 13 - 14 ) literature shows several studies on frailty and sarcopenia syndromes in older adults, although it is still insufficient for the diagnosis of DM.
Accordingly, this study aimed to: relate Frailty Syndrome and sarcopenia in older adults with and without type 2 diabetes mellitus and to identify potential risk factors for frailty and sarcopenia.
Method
Study type
Descriptive epidemiological study, using the STROBE Statement (Strengthening the Reporting of Observational studies in Epidemiology) as a study reporting guide ( 15 ).
Location and population
The study was conducted with older adults registered and attended at the Jardim Botânico Primary Health Unit (PHU) in the municipality of Sinop, Mato Grosso (MT), Brazil.
Period
Data collection was carried out from September 2019 to April 2020.
Selection criteria
At the Primary Health Unit there were 420 older adults receiving care, of which 78 (18.6%) had a confirmed diagnosis of T2DM in their medical records. The selection of this PHU was due to the profile of the population attended, which was mostly composed of older adults. All older adults registered at the Jardim Botânico PHU, living at home, with a diagnosis of T2DM, and without the disease, who were able to communicate, were included. Older adults were excluded if they: had severe shortness of breath or other acute symptoms during the evaluation; were unable to move; had recent amputations and/or fractures (within the last three months); had severe sequelae from a stroke, with eight (8) individuals excluded, totaling 70 ( Figure 1).
Figure 1 -. Sample selection criteria of the study.
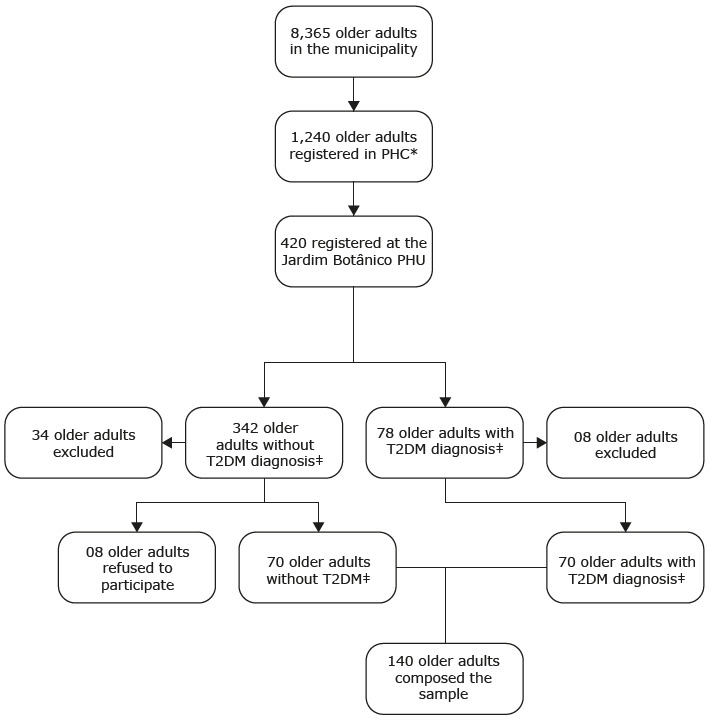
*PHC = Primary Health Center; †PHU = Primary Health Unit; ‡T2DM= Type 2 Diabetes mellitus
Sample definition
For the sample definition, the total number of older adults with a diagnosis of T2DM was considered ( n = 78). Five of them were unable to communicate, two were bedridden, and one was wheelchair-bound, resulting in 70 older adults eligible for the study. One participant without DM was selected for each diabetic older adult, matched for sex (randomly chosen from medical records) and approximate age (with a three-year variation, either older or younger). Accordingly, 140 older adults composed the sample, with 70 participants having T2DM and 70 without the disease. Participants aged 60 years or over were considered older adults ( 16 ).
Study variables
The sociodemographic and clinical independent variables included: sex, age, self-reported skin color, marital status, years of education, living arrangements, family income, waist and hip circumference (cm), weight (kg), height (cm), BMI (body mass index), years of education, and HbA1C (glycated hemoglobin) (%), number of comorbidities, and nutritional status.
The dependent variables were: Frailty Syndrome (non-frail, pre-frail, and frail); and sarcopenia (with sarcopenia and without sarcopenia). Confounding variables included: age; BMI; and nutritional assessment. The independent variables were: inactivity; education level; and family income.
Instruments used for data collection
To obtain sociodemographic and clinical data from the older adults, a questionnaire provided by the Research Center in Geriatrics and Gerontology of the University of São Paulo at Ribeirão Preto College of Nursing was used. This questionnaire included sociodemographic variables such as age, sex, marital status, living arrangements, education level, self-reported skin color, and income.
Frailty Syndrome was assessed using the frailty phenotype ( 17 ), which includes the criteria from the Cardiovascular Health Study: 1- Self-report of unintentional weight loss: This criterion was evaluated through the question: “In the past year, have you unintentionally (i.e., without diet or exercise) lost more than 4.5 kg?”; 2- Reduced physical strength: Evaluated by handgrip strength using a manual hydraulic dynamometer; 3- Self-report of exhaustion and/or fatigue: Two questions from the Brazilian version of the Center for Epidemiological Studies Depression Scale (CES-D) were used. 4- Gait speed: The test was administered to the older adult while seated in a 45 cm high chair, and they were instructed to stand up, walk a distance of 4.6 meters, return, and sit back down. 5- Decreased level of physical activity: Calculated based on the number of calories expended per week using the Minnesota Leisure Time Activity instrument, including activities like walking, household chores, and sports. Three levels of frailty were defined: frail if three (or more) criteria were met; pre-frail if 1 or 2 were met; and non-frail if there was no impairment ( 17 ).
Sarcopenia was assessed using an instrument that measures muscle function and calf circumference called the SARC F+CC ( 18 ). It is defined by evaluations of muscle strength, history of falls, ability to rise from a bed or chair, ability to climb stairs, and calf circumference. The sum of the points characterizes the older adult as either having no signs suggestive of sarcopenia (0 to 10 points) or having signs suggestive of sarcopenia (11 to 20 points).
Nutritional status was assessed using the Mini Nutritional Assessment (MNA) ( 19 ), which is divided into two parts: screening and global assessment. This assessment scores the older adult on three different nutritional indicators: 30.0 to 24.0, good nutritional status; 23.5 to 17.0, nutritional risk; and less than 17.0, malnourished ( 19 ). Glycated hemoglobin was assessed in the laboratory using the liquid chromatography method, considered the gold standard for this test.
Data collection
Throughout the data collection process, attention was given to quality control and standardization of the interviews and physical assessments. Continuous training of research assistants was conducted to ensure the internal validity of the data.
The team of interviewers consisted of one of the study authors and three students from the Federal University of Mato Grosso (UFTM), Sinop-MT Campus, enrolled in the Supervised Nursing Internship I course. During the training, each interviewer conducted a pilot assessment with five older adults randomly selected from the community (provided they met eligibility criteria) to address any questions or issues in the administration of questionnaires and procedures. These older adults were not part of the final sample.
Each older adult was personally invited to participate in the study, either in the waiting room of the PHC or through pre-scheduled phone contact. The selection of participants was performed randomly in the PHU reception waiting room as they sought healthcare, and by reviewing medical records to include 100% of older adults with a T2DM diagnosis. After interviewing the group of diabetic older adults, the search for participants without the disease was initiated in the medical records to match age and sex with the T2DM group.
Data treatment and analysis
Data were analyzed using the STATA program version 14.0. Descriptive statistical analysis was applied to both the older adults with T2DM and those without the disease. Results were presented through absolute frequency ( n) and relative percentage (%) for the categorical variables, and mean, median, standard deviation, minimum, and maximum values for the continuous variables. Fisher’s exact test, and the Chi-square test were used to identify the relationship between variables. Bivariate regression was applied to select the independent variables, followed by multivariate logistic regression. The goodness-of-fit of logistic regressions was assessed using the Hosmer-Lemeshow test ( 20 ) with the option of five intervals for data grouping. A significance level of 95% confidence intervals and a significance coefficient of 5% ( p ≤ 0.05) were adopted, meaning that statistical significance was considered when α = 5%.
Ethical considerations
The project was reviewed and approved by the Research Ethics Committee (CEP) in accordance with Resolution 466/2012 of the National Health Council, under authorization number 3.279.884.
Results
Regarding sociodemographic variables, among the 140 (100%) older adults with and without T2DM, the majority were female (55.7%), self-identified as having white skin color (57.1%), and were married (51.4%). In terms of age, there was a predominance of two age groups: 65 to 69 years (21.4%) and 80 years and over (21.4%). Most participants lived with a spouse (31.4%), had up to four years of education, and had a family income of three minimum wages (41.4%) ( Table 1).
Table 1 -. Characterization of older adults with and without type 2 diabetes mellitus, according to sociodemographic variables (n = 140). Sinop, MT, Brazil, 2020.
| Variable | Older adults with T2DM* | Older adults without T2DM* | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | Male | 31 | 44,28 | 31 | 44,28 |
| Female | 39 | 55,71 | 39 | 55,71 | |
| Age group (years) | 60 to 64 | 14 | 20,00 | 11 | 15,71 |
| 65 to 69 | 15 | 21,40 | 17 | 24,28 | |
| 70 to 74 | 14 | 20,00 | 15 | 21,40 | |
| 75 to 79 | 12 | 17,14 | 10 | 14,28 | |
| >80 | 15 | 21,40 | 17 | 24,28 | |
| Self-reported skin color | White | 40 | 57,14 | 47 | 67,14 |
| Brown | 24 | 34,29 | 21 | 30,00 | |
| Black | 4 | 5,71 | 5 | 7,14 | |
| Indigenous | 2 | 2,86 | 1 | 1,43 | |
| Marital status | Single | 3 | 4,29 | 7 | 10.00 |
| Married | 36 | 51,43 | 44 | 62,86 | |
| Divorced | 9 | 12,86 | 5 | 7,14 | |
| Separated | 2 | 2,86 | 1 | 1,43 | |
| Widowed | 20 | 28,57 | 13 | 18,57 | |
| Education | Up to 4 years of education | 52 | 74,28 | 44 | 62,85 |
| More than 4 years of education | 18 | 25,71 | 26 | 37,14 | |
| Living arrangements | Alone | 15 | 21,43 | 6 | 8,57 |
| Only with spouse | 22 | 31,43 | 30 | 42,86 | |
| Spouse and child(ren) | 11 | 15,71 | 9 | 12,86 | |
| Spouse, children, son-in-law or daughter-in-law | 2 | 2,86 | 5 | 7,14 | |
| Only with child(ren) | 8 | 11,43 | 3 | 4,29 | |
| Three-generation arrangements | 9 | 12.86 | 15 | 21,43 | |
| Intra-generational arrangements | 1 | 1,43 | 0 | 0 | |
| Only with grandchildren | 1 | 1,43 | 1 | 1,43 | |
| Non-family members | 1 | 1,43 | 1 | 1,43 | |
|
Family income in minimum
wages † in Brazilian Reais |
1 | 13 | 18,57 | 8 | 11,43 |
| 2 | 22 | 31,43 | 24 | 34,29 | |
| 3 | 29 | 41,43 | 30 | 42,86 | |
| 4 | 3 | 4,29 | 6 | 8,57 | |
| 5 | 2 | 2,86 | 2 | 2,86 | |
| Do not know | 1 | 1,43 | 0 | 0 | |
*T2DM = Type 2 diabetes mellitus; †Actual minimum wage (in Brazilian Reais): R$ 1.040,00, Brazil, 2020
Regarding the clinical variables, the older adults with T2DM had higher mean values of waist circumference, hip circumference, weight, BMI, glycated hemoglobin, and the number of comorbidities when compared to those without the disease. However, the height was greater in older adults without the disease.
Analysis using the Chi-square test showed that BMI, classified as normal/overweight, had statistical significance ( p ≤ .00) with the presence of T2DM, as did HbA1C (greater than 6.5%) ( p ≤ .00), and the number of comorbidities (more than 5) ( p ≤ .00). The other variables did not show statistical significance ( Table 2).
Table 2 -. Characterization of older adults with and without type 2 diabetes mellitus according to clinical variables (n = 140). Sinop, MT, Brazil, 2020.
| Older adults with T2DM* n = 70 | Older adults without T2DM* n = 70 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | Median | SD † | Min ‡ | Max § | Mean | Median | SD † | Min ‡ | Max § |
| WC || | 101.31 | 100.0 | 13.46 | 55.0 | 141.0 | 97.78 | 100.0 | 11.39 | 74.0 | 133.0 |
| HC ¶ | 106.07 | 104.0 | 10.39 | 80.0 | 143.0 | 103.31 | 99.0 | 8.79 | 88.0 | 126.0 |
| Weight | 73.96 | 71.2 | 15.22 | 40.0 | 131.0 | 72.44 | 71.0 | 17.95 | 45.0 | 168.0 |
| Height | 160.95 | 164.0 | 12.37 | 120.0 | 186.0 | 164.04 | 163.0 | 9.01 | 143.0 | 181.0 |
| BMI** | 28.04 | 27.3 | 5.38 | 16.0 | 48.7 | 26.33 | 26.9 | 4.61 | 19.3 | 38.0 |
| HbA1c †† | 6.80 | 5.8 | 1.40 | 3.9 | 11.9 | 5.80 | 5.8 | 0.40 | 4.7 | 7.3 |
| Comorbidities | 7.00 | 5.0 | 3.70 | 0.0 | 16.0 | 5.00 | 5.0 | 2.70 | 0.0 | 16.0 |
*T2DM = Type 2 diabetes mellitus; †SD = Standard deviation; ‡Min = Minimum; §Max = Maximum; ||WC = Waist Circumference; ¶HC = Hip Circumference; **BMI = Body Mass Index; ††HbA1c = Glycated Hemoglobin
In the Frailty Syndrome analysis, a higher percentage was observed in the older adults with T2DM (47; 67.1%) compared to those without the disease (29; 41.4%), with statistical significance ( p = 0.00). Regarding the presence of sarcopenia, the older adults with and without the disease presented similar values (21; 30.0% with T2DM and 22; 31.4% without the disease), with no statistical significance ( p = 0.85) according to the Chi-square test.
The sex variable did not show statistical significance considering the presence of frailty ( p = 0.28) and sarcopenia ( p = 0.57). However, the women presented a higher percentage of frailty (60; 42.2%) classified as pre-frail and frail, as well as a greater presence of sarcopenia compared to men. The 75 years and over age group showed statistical significance for both Frailty Syndrome ( p = 0.00) and sarcopenia ( p = 0.002). The majority of older adults in the 75 years and over age group, 78 (55.6%), were classified as pre-frail and frail. Regarding the presence of sarcopenia, the results were similar for both age groups.
Regarding nutritional status, the majority of the older adults, 78 (55.6%), were classified as pre-frail and frail and presented a normal nutritional assessment. Therefore, there was no association with the presence of frailty ( p = 0.69). However, an association was found between nutritional assessment and malnutrition and sarcopenia ( p = 0.00). It is important to highlight that all of the individuals with comorbidities presented some degree of frailty ( p = 0.00). However, the majority of older adults with comorbidities did not present sarcopenia ( p = 0.32) ( Table 3).
Table 3 -. Frailty and sarcopenia in older adults with and without T2DM*, by gender, age group, nutritional assessment, and comorbidities (n = 140). Sinop, MT, Brazil, 2020.
| Variables | Frailty n(%) | Sarcopenia n(%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Not frail | Pre-frail | Frail | p | Without sarcopenia | With sarcopenia | p | ||
| Sex | Male | 8 (5.7) | 19 (13.5) | 35 (25.0) | 0.28 | 43 (30.7) | 19 (13,5) | 0.57 |
| Female | 18 (12.8) | 19 (13.0) | 41 (29.2) | 54 (38.5) | 24 (17.1) | |||
| TOTAL n(%) | 26 (18,5) | 38 (27.1) | 76 (54.2) | 97 (69.2) | 43 (30.8) | |||
| Age group | Up to 75 | 21 (15.0) | 19 (13.5) | 17 (12.1) | 0.00 | 68 (48.5) | 21 (15.0) | 0.01 |
| 75 and over | 5 (3.5) | 19 (13.5) | 59 (42.1) | 29 (20.7) | 22 (15.7) | |||
| TOTAL n(%) | 26 (18.5) | 38 (27.1) | 76 (5402.0) | 97 (69.3) | 43 (30.7) | |||
| MNA † | Normal | 21 (15.0) | 27 (19.2) | 51 (3604.0) | 0.69 (*) |
78 (55.7) | 21 (15.0) | 0.00 (*) |
| Nutritional risk | 5 (3.5) | 10 (7.1) | 22 (1507.0) | 19 (13.5) | 18 (12.8) | |||
| Malnourished | 0(0.0) | 1(0.7) | 3(2.1) | 0(0.0) | 4(2.8) | |||
| TOTAL n(%) | 26(18.5) | 38(27.1) | 76(54.2) | 97(69.3) | 43(30.7) | |||
| Multimorbidities | Up to 5 | 24(17.1) | 30(21.4) | 44(31.4) | 0.00 | 65(46.4) | 32(22.8) | 0.32 |
| More than 5 | 2(1.4) | 8(5.7) | 32(21.4) | 33(23.5) | 10(7.1) | |||
| TOTAL n(%) | 26(18.5) | 38(27.2) | 76(52.8) | 98(70.0) | 42(30.0) | |||
*T2DM = Type 2 Diabetes Mellitus; †MNA = Mini Nutritional Assessment; *Chi-Square Test
Table 4 presents the multivariate logistic regression analysis of the frailty and sarcopenia assessment in older adults. The variables in the model were categorized as follows: BMI (underweight/normal weight and overweight); physical activity (physically active and sedentary); age group (up to 75 years and over 75 years); comorbidities (five or more morbidities); marital status (with a partner and without a partner); education (up to four years of education and more than four years of education); family income (up to 1 minimum wage and more than 1 minimum wage); nutritional assessment (normal nutritional status and nutritional risk/malnutrition).
Table 4 -. Multivariate logistic regression of frailty and sarcopenia according to sociodemographic and clinical variables in older adults with and without T2DM* (n = 140). Sinop, MT, Brazil, 2020.
| Variable | Odds Ratio | p † | 95% IC ‡ | ||
|---|---|---|---|---|---|
| Frailty | Body Mass Index | 0.18 | 0.00 | 0.06 | 0.54 |
| Physical inactivity | 13.64 | 0.00 | 3.29 | 56.55 | |
| Over 75 years age group | 9.18 | 0.00 | 3.30 | 27.82 | |
| Family income § | 9.58 | 0.00 | 1.80 | 50.98 | |
| More than 5 comorbidities | 3.77 | 0.01 | 1.36 | 10.48 | |
| Marital status | 1.29 | 0.59 | 0.49 | 0.54 | |
| Sarcopenia | Physical inactivity | 3.64 | 0.01 | 1.26 | 10.44 |
| Over 75 years age group | 1.45 | 0.71 | 0.51 | 4.10 | |
| Body Mass Index | 9.43 | 0.00 | 3.32 | 26.76 | |
| Education less than 4 years | 1.66 | 0.37 | 0.54 | 5.10 | |
| Family income less than 1 MW § | 1.59 | 0.71 | 0.44 | 5.72 | |
| Nutritional risk and/or malnourished | 3.16 | 0.01 | 1.30 | 7.70 | |
*T2DM = Type 2 Diabetes Mellitus; p† = Significance level; CI‡ = Confidence Interval; MW§ = minimum wage; Minimum wage (in Brazilian Reais): R$ 1,040.00, Brazil, 2020
The association between frailty in the older adults with and without T2DM and the BMI, physical inactivity, age group, family income, comorbidities, and marital status variables are presented in Table 4. Multivariate regression analysis shows that the older adults with and without T2DM who were physically inactive had a thirteen times (OR = 13.64) higher likelihood of developing frailty ( p = 0.00). The likelihood of an older adult with or without diabetes mellitus developing frailty in the age group over 75 years and with higher family income was nine times higher. The likelihood of an older adult with five or more comorbidities developing frailty was four times higher (OR = 3.77). In the Hosmer-Lemeshow test for Frailty ( N = 140), with five groups, Hosmer-Lemeshow Chi 2 = 0.69; Prob > Chi 2 = 0.8747 (not significant).
In the multivariate regression, sarcopenia in the older adults with and without T2DM was associated with the physical inactivity, age group, BMI, education, family income, and inadequate nutritional status variables. Physical inactivity increased the risk of an older adult developing sarcopenia by three times (OR = 3.64) ( p = 0.01). On the other hand, the over 75 years age group showed a low relationship with the development of sarcopenia ( p = 0.71). Regarding the BMI of the older adults, being underweight and/or normal weight increased the odds of developing sarcopenia by nine times (OR = 9.43) compared to overweight individuals ( p = 0.00). Older adults with less than four years of education and family income below one minimum wage did not show statistical significance for the development of sarcopenia. Older adults at nutritional risk and/or malnourished had a three times higher likelihood (OR=3.16) of developing sarcopenia than those with adequate nutritional status. In the Hosmer-Lemeshow test for sarcopenia (n = 140), with 5 groups, Hosmer-Lemeshow Chi 2 = 0.61; Prob > Chi 2 = 0.8941 (not significant).
Discussion
This study investigated frailty syndrome and sarcopenia in older adults with and without T2DM and their potentially related factors in a sample of older adults living in the Central-West region of Brazil. Although sex did not show an association with the presence of frailty and sarcopenia, the women presented a higher likelihood of developing them. Similarly, advancing age and the presence of multimorbidity were associated with a higher likelihood of developing frailty. Another important factor to highlight is the nutritional risk/malnutrition assessed through the nutritional evaluation, which showed a significant relationship with sarcopenia. Additionally, frailty in the older adults was associated with sedentary behavior, the over 75 years age group, low family income, and multimorbidity. Sarcopenia, on the other hand, was associated with sedentary behavior, low weight/eutrophic status, and malnutrition/nutritional risk.
The older adults with T2DM presented higher values for waist circumference, hip circumference, weight, BMI (Body Mass Index), glycated hemoglobin (HbA1c), and the number of comorbidities when compared to those without the disease, with only frailty syndrome showing a relationship with the presence of T2DM.
When analyzing the sociodemographic conditions of the older adults with and without T2DM, it was observed that the majority were in the 60 to 79 years age group, consisting of white individuals, married, and with up to four years of education. There was a predominance of older adults with and without the disease living with a spouse and with a family income of three minimum wages. Regarding clinical variables, the older adults with T2DM showed higher mean values of waist and hip circumference, body weight, BMI, glycated hemoglobin (HbA1c), and the number of comorbidities when compared to those without the disease. These results are in line with the literature on DM ( 21 ).
It is recognized that HbA1C is an important marker of glycemic control, requiring strict control, as altered values are associated with disease worsening and complications. In this study group, the mean value of HbA1C for the older adults with T2DM was 6.8%, and 5.6% for those without the disease. The study suggests that for healthy older adults, the desirable value is up to 7.5%, and for severely compromised older adults, it should be below 8.5%, which is higher than the limit for individuals without the disease, which is 5.6% ( 22 ). A value below 7% is considered adequate for individuals with T2DM, with complications tending to increase beyond this level. For older adults without the disease, the adequate value ranges from 4.0 to 5.6% ( 22 ).
In the analysis using the Chi-Square test, older adults classified as eutrophic/overweight, HbA1C above 6.5, and having more than five comorbidities showed statistical significance for the presence of T2DM. The results of the sociodemographic and clinical variables can contribute to identifying the risk of developing frailty and sarcopenia in older adults with T2DM.
When analyzing Frailty Syndrome, a higher percentage was found in the older adults with T2DM compared to those without the disease. Regarding the presence of sarcopenia, the older adults with and without the disease showed similar values, with no statistical significance according to the Chi-Square test.
The sex variable did not show statistical significance with regard to frailty or sarcopenia; however, the women had a higher percentage of frailty, classified as pre-frail and frail, as well as the presence of sarcopenia compared to the men. When analyzing the presence of sarcopenia in the older women, the results were in line with systematic review and meta-analysis studies. These studies assessed the global prevalence of sarcopenia using both versions of the European Working Group on Sarcopenia in Older People, the EWGSOP and the EWGSOP2, and showed that the prevalence was higher in women when using the EWGSOP (17% vs. 12%). On the other hand, the prevalence of sarcopenia in men was higher when using the EWGSOP2 (11% vs. 2%). These studies indicate that the results obtained from the SARC-F+CC and EWGSOP2 instruments are similar ( 23 ).
When analyzing the age group, it was found that older adults aged over 75 years showed statistical significance for both Frailty Syndrome and sarcopenia. It should be noted that the majority of older adults were classified as pre-frail and frail. Regarding the presence of sarcopenia, the results were similar in the two age groups investigated.
The relationship between frailty and age group is supported by the literature, and the results obtained corroborate those of other national and international studies. In one study conducted in Rio Grande do Sul, most older adults of higher age presented Frailty Syndrome, using the frailty phenotype criteria ( 17 ) ; however, in participants under 80 years of age, this condition was present in only half of the investigated sample ( 24 ). A longitudinal study conducted in Japan also showed that the incidence of frailty progressively increases with advancing age ( 25 ).
Regarding nutritional status, it was found that most of the frail older adults had a normal nutritional status. This result differs from a study conducted in southern India, which showed that inadequate nutrient intake was associated with frailty ( 26 ). Most sarcopenic older adults had an adequate nutritional status assessed through the MNA (Mini Nutritional Assessment). However, a study conducted in Asia with people on hemodialysis found that inadequate nutrition was associated with the risk of osteoporosis and sarcopenia, suggesting the importance of a proper nutritional assessment and management to prevent bone and muscle-related complications ( 27 ).
In the analysis of the comorbidities, it was found that the older adults with sarcopenia had a higher number of comorbidities compared to those without this condition. A longitudinal study conducted in the United Kingdom showed that comorbidities were associated with a higher risk of sarcopenia in 2,873 older adults over a 12-year follow-up period ( 28 ). The number of comorbidities in older adults can contribute to an increased degree of frailty. Accordingly, comorbidity and frailty appear to be similar and include a higher risk for healthcare utilization, disability, and mortality ( 29 ).
Multivariate regression analysis showed that the older adults with and without T2DM who were inactive, in the age group over 75 years, with a family income below 1 MW, and with more than five comorbidities, had a higher chance of developing frailty.
Regarding physical inactivity and the presence of Frailty Syndrome, in accordance with the literature, an association between these two variables was found. Increased time doing physical activity provides a protective factor against frailty; however, there is a scarcity of clinical trial studies to confirm these results ( 30 ). An integrative review study that investigated the evidence for an association between frailty, physical activity, and exercise in older adults highlighted a relationship between these variables in the literature, as well as the potential for reversing frailty and improving other covariates with physical activity interventions ( 31 ). Likewise, it indicated an association between low levels of physical activity and frailty, decreased cognitive function, functional disability, mortality, and the number of comorbidities ( 31 ).
Regarding family income, an association was found between low income and the occurrence of frailty. A study conducted in Pelotas, Rio Grande do Sul, Brazil, also found an association between frailty and low income ( 32 ). Similarly, another study also found frailty associated with sociodemographic conditions in older adults ( 33 ). It is evident that older individuals with low income face greater social vulnerability, difficulty accessing information, and challenges in accessing healthcare, nutrition, and leisure, factors that may explain the strong relationship between frailty and low income, as well as other factors associated with DM and other comorbidities.
Regarding the relationship between the number of comorbidities and frailty, a longitudinal study conducted in a geriatrics outpatient clinic highlighted the prevalence of frailty and comorbidities as 66.2%, with a mean of 3.22 ± 1.78 simultaneous chronic morbidities. This investigation also showed that older adults with frailty and comorbidities had a lower survival rate ( 34 ).
Another longitudinal study followed 6,425 older adults for 23 years and 6 months, concluding that 1,733 of them developed comorbidities, with 692 becoming frail and 611 developing some form of disability and passing away. The study found that comorbidities had a risk ratio of 2.38 for Frailty Syndrome ( 35 ). Comorbidities worsen the health of the person and generate difficulties in living their daily life independently ( 36 - 37 ). Therefore, it is recognized that managing older adults with type 2 diabetes mellitus is complicated due to comorbidities, reduced life expectancy, and exaggerated consequences of adverse effects of the treatment ( 38 ).
Multivariate regression analysis showed that the older adults with and without T2DM who were physically inactive, underweight and/or eutrophic, at nutritional risk and/or malnourished had a higher chance of developing sarcopenia.
Regarding the assessment of physical activity level, evaluated through the Minnesota Leisure Time Activity, it was found that the older adults had a higher risk of developing sarcopenia. In line with our results, another study showed that older adults with a higher level of physical activity had a lower risk of sarcopenia (24.2%). This data may indicate that higher levels of physical activity constitute a protective factor against sarcopenia in older adults ( 36 ). An integrative review study highlighted that sedentary behavior is an important risk factor for the development of sarcopenia in older adults and emphasized the importance of physical activity as a preventive measure ( 39 ). It is well-known that the literature provides evidence of the benefits of physical activity for health ( 40 - 41 ). However, a combination of factors, including organic and nutritional factors, is necessary to achieve good results in the maintenance and strengthening of muscle in older adults.
Another factor that may increase the occurrence of sarcopenia is advancing age. The results showed that the prevalence of sarcopenia according to age group was similar, with a slight increase in individuals above 75 years of age. A study conducted in Malaysia with 506 older adults with T2DM from primary healthcare showed that 28.5% of them presented sarcopenia. Among the associated factors were male gender and age ≥ 70 years, with ≥ 10 years of diabetes mellitus duration, low body mass index, involvement in light and moderate physical activities, and the use of fewer than five medications ( 42 ).
The body mass index (BMI) is a universal indicator to assess whether an individual is at the ideal weight. However, this variable showed a strong association with sarcopenia, indicating that low weight and/or eutrophic older adults had a higher chance of developing sarcopenia. A study showed that individuals with low weight (BMI < 22) had a six-fold increase in the development of sarcopenia ( 43 ). Another study that evaluated the association between sarcopenia and risk factors, with 396,283 participants in the UK Biobank Baseline Clinic, showed that individuals below the ideal weight had a higher chance of having this condition ( 44 ).
It is known that inadequate nutrition with insufficient nutritional and caloric quantities for muscle maintenance is a factor that contributes to the development of sarcopenia. Accordingly, the results of the present study show a strong relationship between older adults who presented malnutrition and/or nutritional risk and a higher chance of developing sarcopenia. Therefore, efforts should be made for older adults to consume an adequate portion of proteins for muscle maintenance as well as growth, which is beneficial for the metabolism ( 45 ). A review study highlighted the importance of physical activity in old age for the prevention and treatment of lean mass loss. This study showed that strength exercises in combination with protein supplementation for sarcopenic older adults provide various benefits and improve the quality of life, aiming for longevity and the prevention of malnutrition and other diseases ( 46 ).
However, poor nutrition, with inadequate caloric and/or nutritional quantities, harms both muscle health, leading to sarcopenia ( 47 ), and the development of other morbidities, leading to body frailty ( 48 ), compromising the older adult’s immune system ( 49 ). It is recognized that the practice of physical activity and adherence to a healthy diet are non-pharmacological measures for the good control of T2DM and also assist in the prevention and control of Frailty Syndrome and sarcopenia. However, it is necessary to include and reinforce specific recommendations in the clinical practice of Gerontology and Geriatrics care, aiming to improve the quality of life of older adults and prevent these syndromes, and others ( 50 ).
In summary, advanced age, low family income, and comorbidities are not changeable factors, however, are indicators that should be considered when planning care for preventing Frailty Syndrome and sarcopenia in older adults with and without T2DM.
The study’s limitations include: the cross-sectional study type, which did not allow causality to be established between variables; the sample size of older adults with and without DM, despite constituting the total registration in the PHU under study; the scarcity of literature on the relationship between Frailty Syndrome and sarcopenia in older adults with and without T2DM, and related factors that would allow for a more in-depth discussion of the results.
This study has the potential to contribute to developing innovative interdisciplinary approaches for ongoing care. It specifically emphasizes the importance of nurses in primary healthcare when consulting with older adults. Additionally, it highlights the significance of assessing Frailty Syndrome and sarcopenia in older adults with advanced age and T2DM as part of a comprehensive strategy for promoting health.
Conclusion
It can be concluded that the older adults with type 2 diabetes mellitus were more vulnerable to the development of Frailty Syndrome, necessitating the adoption of preventive measures in primary healthcare. The results of this study have significant potential in primary healthcare, as they indicate the need for the implementation of interdisciplinary educational actions regarding Frailty Syndrome and sarcopenia. The nurse plays a pivotal role in health promotion, ensuring proper glycemic control, providing nutritional guidance, and recommending physical activities for older adults with T2DM. The inclusion of a systematic assessment of frailty and sarcopenia is recommended for older adults with and without T2DM of advanced age, as a tool used for promoting the health of the older adult.
Acknowledgments
We would like to express our gratitude to the entire team at the Jardim Botânico Primary Health Unit and to the group of older adults who willingly participated in the study.
Footnotes
How to cite this article :Cunha AN, Zanetti ML, Santos JLF, Rodrigues RAP. Frailty Syndrome and sarcopenia in older adults with and without type 2 diabetes mellitus in the municipality of Sinop, Mato Grosso: an epidemiological study. Rev. Latino-Am. Enfermagem. 2023;31:e4077 [cited month day year]. Available from: URL . https://doi.org/10.1590/1518-8345.6677.4077
All authors approved the final version of the text.
Referencias
- 1.Sousa MC, Barroso ILD, Viana JA, Ribeiro KN, Lima LNF, Vanccin PDA, et al. The aging population: aspects of Brazil and the world, under the look of literature. Braz J Develop. 2020;29(8):61871–7. doi: 10.34117/bjdv6n8-564. [DOI] [Google Scholar]
- 2.Oliveira AS. Transition, epidemiological transition and population aging in Brazil. Rev Bras Geo Med Saude. 2019;15(32):69–7. doi: 10.14393/Hygeia153248614. [DOI] [Google Scholar]
- 3.Miyamura K, Fhon JRS, Bueno AA, Fuentes-Neira WL, Silva RCCP, Rodrigues RAP. Frailty syndrome and cognitive impairment in older adults: systematic review of the literature. Rev. Latino-Am. Enfermagem. 2019;27:e3202. doi: 10.1590/1518-8345.3189.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes Federation . Diabetes Atlas [Internet] 9. International Diabetes Federation; Brussels: 2019. [cited 2023 Jan 10]. Available from: https://diabetesatlas.org/atlas/ninth-edition/ [Google Scholar]
- 5.Dent E, Morley JE, Cruz-Jentoft AJ, Woodhouse L, Rodríguez-Mañas L, Fried LP, et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J Nutr Health Aging. 2019;23:771–87. doi: 10.1007/s12603-019-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenardt MH, Falcão AS, Hammerschmidt KSA, Barbiero MMA, Leta PRG, Sousa RL. Depressive symptoms and physical frailty in the older adults: an integrative review. Rev Bras Geriatr Gerontol. 2021;3:e210013. doi: 10.1590/1981-22562021024.210013. [DOI] [Google Scholar]
- 7.Bezerra PCL, Rocha BL, Monteiro GTR. Factors associated with frailty in older users of Primary Health Care services from a city in the Brazilian Amazon. Rev Bras Geriatr Gerontol. 2023;26:e230018. doi: 10.1590/1981-22562023026.230018.pt. [DOI] [Google Scholar]
- 8.Cruz-Jentoft AJ, Bahat G, Bauer F, Boirie Y, Bruyere O, Cederhom T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishikawa H, Fukunishi S, Asai A, Yokohama K, Ohama H, Nishiguchi S, et al. Sarcopenia, frailty and type 2 diabetes mellitus. Mol Med Rep. 2021;24(6):854. doi: 10.3892/mmr.2021.12494. [DOI] [PubMed] [Google Scholar]
- 10.Yanase Y, Yanagita I, Muta K, Nawata H. Frailty in elderly diabetes patients. Endocrine J. 2018;65:1–11. doi: 10.1507/endocrj.EJ17-0390. [DOI] [PubMed] [Google Scholar]
- 11.Izzo A, Massimino E, Riccardi G, Pepa GD. A Narrative Review on Sarcopenia in Type 2 Diabetes Mellitus: Prevalence and Associated Factors. Nutrients. 2021;1(13):1–18. doi: 10.3390/nu13010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong L, Zhao H, Fan J, Wang Q, Li J, Bai J, et al. Predictors of frailty among Chinese community-dwelling older adults with type 2 diabetes: a cross-sectional survey. BMJ Open. 2021 Mar 04;11(3):1–9. doi: 10.1136/bmjopen-2020-041578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampaio RX, Barros RS, Cera ML, Mendes FAS, Garcia PA. Association of clinical parameters of sarcopenia and cognitive impairment in older people: cross-sectional study. Rev Bras Geriatr Gerontol. 2023;26:1–13. doi: 10.1590/1981-22562023026.220181.pt. [DOI] [Google Scholar]
- 14.Ribeiro IA, Lima LR, Volpe CRG, Funghetto SS, Rhem TCMSB, Stival MM. Frailty syndrome in the elderly in elderly with chronic diseases in Primary Care. Rev Esc Enferm USP. 2019;53:1–9. doi: 10.1590/S1980-220X2018002603449. [DOI] [PubMed] [Google Scholar]
- 15.STROBE Initiative STROBE Statement - checklist of items that should be included in reports of observational studies [Internet] 2008. [cited 2023 Jan 10]. Available from: https://www.equator-network.org/wp-content/uploads/2015/10/STROBE_checklist_v4_combined.pdf . [DOI] [PubMed]
- 16.Ministério da Saúde (BR) Estatuto do Idoso. 2. Ministério da Saúde; Brasília: 2007. 70 [Google Scholar]
- 17.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol. 2001;56(3):146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 18.Barbosa-Silva TG, Menezes AMB, Bielemann RM, Malmstrom TK, Gonzalez MC. Enhancing SARC-F: Improving Sarcopenia Screening in the Clinical Practice. J Am Med Dir Assoc. 2016;17(12):1136–41. doi: 10.1016/j.jamda.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001;56(6):366–72. doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 20.Hosmer DW, Lemeshow JSA. Goodness of fit tests for the multiple logistic regression model. Commun Stat Theory Methods. 1980;(9):1043–69. doi: 10.1080/03610928008827941. [DOI] [Google Scholar]
- 21.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. American Diabetes Association. Introduction and Methodology: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S1–S4. doi: 10.2337/dc23-srev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sociedade Brasileira de Diabetes (BR) Diretrizes da Sociedade Brasileira de Diabetes 2023 [Homepage] Sociedade Brasileira de Diabetes; São Paulo: 2023. [cited 2023 Jul 17]. Available from: https://diretriz.diabetes.org.br/ [Google Scholar]
- 23.Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcop Muscle. 2021;13(1):86–99. doi: 10.1002/jcsm.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross CB, Kolankiewicz AC, Schmidt CR, Berlezi EM. Frailty levels of elderly people and their association with sociodemographic characteristics. Acta Paul Enferm. 2018;31(2):209–16. doi: 10.1590/1982-0194201800030. [DOI] [Google Scholar]
- 25.Wang C, Pu R, Li Z, Ji L, Li X, Ghose B, et al. Subjective health and quality of life among elderly people living with chronic multimorbidity and difficulty in activities of daily living in rural South Africa. Clin Interv Aging. 2019;14(19):1285–96. doi: 10.2147/CIA.S205734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shalini T, Chitra PS, Kumar BN, Madhavi G, Reddy GB. Frailty and Nutritional Status among Urban Older Adults in South India. J Aging Res. 2020:8763413. doi: 10.1155/2020/8763413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H, Kim K, Ahn J, Lee DR, Lee JH, Hwang SD. Association of nutritional status with osteoporosis, sarcopenia, and cognitive impairment in patients on hemodialysis. Asia Pacific J Clin Nutr. 2020;29(4):712–23. doi: 10.6133/apjcn.202012_29(4).0006. [DOI] [PubMed] [Google Scholar]
- 28.Veronese N, Smith L, Cereda E, Maggi S, Barbagallo M, Dominguez LJ, et al. Multimorbidity increases the risk for sarcopenia onset: Longitudinal analyses from the English Longitudinal Study of Ageing. Exp Gerontol. 2021;156(9):1116–24. doi: 10.1016/j.exger.2021.111624. [DOI] [PubMed] [Google Scholar]
- 29.Sinclair AJ, Abdelhafiz AH. Multimorbidity, Frailty and Diabetes in Older People-Identifying Interrelationships and Outcomes. J Personal Med. 2022;12(11):1911. doi: 10.3390/jpm12111911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kehler DS, Theou O. The impact of physical activity and sedentary behaviors on frailty levels. Mechan Ageing Develop. 2019;180:29–41. doi: 10.1016/j.mad.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Leta PRG, Lenardt MH, Betiolli SE, Melo J, Filho, Barbiero MMA, Binotto MA. Physical frailty in the elderly and its relationship with physical activity and exercise: an integrative review. [cited 2023 Jan 10];Cien Mov Biocien Saúde [Internet] 2021 23(47):111–26. Available from: https://www.metodista.br/revistas/revistas-ipa/index.php/CMBS/article/view/1208/972 . [Google Scholar]
- 32.Llano PMP, Carlos CL, Sequeira AC, Jardim VMR, Castro DSP, Santos F. Factors associated with frailty syndrome in the rural elderly. Rev Bras Enferm. 2019;72(2):14–21. doi: 10.1590/0034-7167-2017-0079. [DOI] [PubMed] [Google Scholar]
- 33.Grden CRB, Lenard MH, Sousa JAV, Kusomota L, Dellaroza MSG, Betiolli SE. Associations between frailty syndrome and sociodemographic characteristics in long-lived individuals of a community. Rev. Latino-Am. Enferm. 2017;25:e2886. doi: 10.1590/1518-8345.1770.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leme DEC, Thomaz RP, Borim FSA, Brenelli SL, Oliveira DV, Fattori A. Survival of elderly outpatients: effects of frailty, multimorbidity and disability. Cien Saude Colet. 2019;24(1):137–46. doi: 10.1590/1413-81232018241.04952017. [DOI] [PubMed] [Google Scholar]
- 35.Pillatt AP, Patias RS, Berlezi EM, Schneider RH. Which factors are associated with sarcopenia and frailty in elderly persons residing in the community? Rev Bras Geriatr Gerontol. 2018;21(6):781–92. doi: 10.1590/1981-22562018021.180165. [DOI] [Google Scholar]
- 36.Klompstra L, Ekdahs AW, Krevers B, Milberg A, Eckerblad J. Factors related to health-related quality of life in older people with multimorbidity and high health care consumption over a two-year period. BMC Geriatrics. 2019;19(1):1–8. doi: 10.1186/s12877-019-1194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira DV, Nascimento MA, Oliveira RF, Pivetta NRS, Kerber VL, Nascimento JRA., Júnior Physical activity and sedentary behavior in elderly in social groups: is there a difference because of the risk of sarcopenia? Saude Pesqui. 2022;15(2):104–16. doi: 10.17765/2176-9206.2022v15n2.e10416. [DOI] [Google Scholar]
- 38.Strain WD, Down S, Brown P, Puttanna A, Sinclair A. Diabetes and Frailty: An Expert Consensus Statement on the Management of Older Adults with Type 2 Diabetes. Diabetes Ther. 2021;12(5):1227–47. doi: 10.1007/s13300-021-01035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvalho DNR, Oliveira MS, Lima FC. Calf circumference assessment as a predictor for sarcopenia in the elderly and its relationship with sedentary lifestyle. [cited 2023 Jan 10];Rev Casos Consultoria [Internet] 2022 13(1):131–27847. Available from: https://periodicos.ufrn.br/casoseconsultoria/article/view/27847/15619 . [Google Scholar]
- 40.Faustino AM, Neves R. Benefits of physical activity in the elderly: a literature review. REAS. 2020;12(5):e3012. doi: 10.25248/reas.e3012.2020. [DOI] [Google Scholar]
- 41.Coelho-Ravagnani CF, Sandreschi PF, Piola TS, Santos L, Santos DL, Mazo GZ, et al. Physical activity for older adults: Physical Activity Guidelines for the Brazilian Population. Rev Bras Ativ Fís Saúde. 2021;26:1–8. doi: 10.12820/rbafs.26e0216. [DOI] [Google Scholar]
- 42.Dai X, Zhai L, Chen Q, Miller JD, Lu L, Hsue C, et al. Two-year-supervised resistance training prevented diabetes incidence in people with prediabetes: a randomised control trial. Diabetes Metab Res Rev. 2019;35(5):e31–43. doi: 10.1002/dmrr.3143. [DOI] [PubMed] [Google Scholar]
- 43.Nunes JD, Zacarin JF, Pavarini SCL, Zazzetta MS, Orlandi AAS, Orlandi FS. Factors associated with Sarcopenia in the older adults in the Community. Fisioter Pesqui. 2021;28(2):159–65. doi: 10.1590/1809-2950/20002828022021. [DOI] [Google Scholar]
- 44.Petermann-Rocha F, Chen M, Gray SR, Ho FK, Pell JP, Celis-Moralesa C. Factors associated with sarcopenia: A cross-sectional analysis using UK Biobank. Maturitas. 2020;133(67):60–7. doi: 10.1016/j.maturitas.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes, metabolic syndrome and obesity. Targets Ther. 2019;12:1057–72. doi: 10.2147/DMSO.S186600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nunes EAAC, Teixeira KG, Cruz MMN, Melo GSJ, Mallet ACT. Sarcopenia: os benefícios da suplementação proteica e a importância da atividade física na terceira idade. Rev Científica UBM. 2022;24(47):110–22. doi: 10.52397/rcubm.v24i47.1332. [DOI] [Google Scholar]
- 47.Alvernaz SC, Peixoto SR, Salerno PV, Viana GD, Bicalho SE. A importância da alimentação e da suplementação nutricional na prevenção e no tratamento da sarcopénia. J Investig Méd. 2022;3(1):73–86. doi: 10.29073/jim.v3i1.519. [DOI] [Google Scholar]
- 48.Oliveira AD, Reiners AAO, Azevedo RCS, Silva KM, Silva AMC. Pre-frailty in older adults: prevalence and associated factors. Texto Contexto Enferm. 2022;31 doi: 10.1590/1980-265X-TCE-2021-0157en. [DOI] [Google Scholar]
- 49.Lima LC., Júnior Alimentação saudável e exercícios físicos em meio à pandemia da COVID-19. Bol Conjuntura. 2020;3(9):33–41. doi: 10.5281/zenodo.3988664. [DOI] [Google Scholar]
- 50.Cechinel C, Lenardt MH, Rodrigues JAM, Binotto MA, Aristides MM, Kraus R. Frailty and delirium in hospitalized older adults: A systematic review with meta-analysis. Rev. Latino-Am. Enfermagem. 2022;30:1–15. doi: 10.1590/1518-8345.6120.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]


