Abstract
During inhibition of cell growth by weak acids, there is substantial accumulation of the weak acid anions in the cytoplasm. This study was undertaken to determine the impact of anion accumulation on cellular pools. At pH 6, growth in the presence of 8 mM acetate led to an internal pool of greater than 240 mM acetate anion and resulted in reduced levels of glutamate in the cell, but there were no significant changes in K+ and Na+ levels. At low osmolarity, the change in the glutamate pool compensated for only a small fraction of the accumulated acetate anion. However, at high osmolarity, glutamate compensated for over half of the accumulated acetate. Recovery of the normal cytoplasmic pH after the removal of acetate was dependent on the synthesis of glutamate.
Growth of the bacterial cell is best achieved when the cell is able to maintain an outwardly directed turgor pressure and a cytoplasmic pH (pHi) in the neutral to slightly alkaline range (1, 5, 9). Turgor pressure is generated through the accumulation of K+ salts, of which K+ glutamate is the dominant species in most gram-negative organisms (9, 15). Primary turgor regulation is through K+ accumulation, with accompanying anions being either synthesized or transported from the environment (9, 15). At high osmolarity, the bacterial cell maintains positive turgor pressure mainly by the accumulation of potassium and glutamate ions (9, 15). However, at low osmolarity, the free K+ is balanced by other anions, with glutamate as a relatively minor component; the identity of the other anions has not been established (15). Regulation of pHi is tied to the maintenance of low membrane permeability to protons and other cations, such as K+ and Na+ (5). Perturbation of cation and anion pools is expected to cause a reduction in growth potential and eventually to impair viability (5, 6, 9, 19).
Organic acids, such as acetate, benzoate, and sorbate, are frequently used in the preservation of foods because of their capacity to perturb cellular homeostasis (3, 6–8, 10, 14). Lipid-permeable, weak acids, such as acetic acid and benzoic acid, dissociate in the cytoplasm, leading to the lowering of pHi and the accumulation of anions (18). The accumulation of anions can cause an osmotic problem for the cell if it leads to an increase in cell turgor pressure. For example, if the transmembrane pH gradient is 1.8 (pHi 7.8, external pH [pHo] 6), then the addition of 8 mM acetate can cause the accumulation of acetate anions at concentrations of over 500 mM in the cytoplasm, assuming there is no change in pHi. The actual accumulation is lower, but still substantial, due to a decrease in pHi caused by the protons liberated by the dissociation of the weak acid. Such an accumulation of anions causes an increase in the internal osmotic pressure of the cell that must be compensated for if turgor is to be maintained at a constant level (5, 6, 9). What has been less clear is whether, and how, bacterial cells accommodate the anions that accumulate as a consequence of weak acid entry into the cell. In this study, we have sought to analyze this problem and the mechanisms by which Escherichia coli cells adjust to the accumulation of anions in the cytoplasm. We demonstrate that the addition of weak acids to E. coli cells results in displacement of glutamate from the cells. Correspondingly, when cells are transferred from the weak acid to media devoid of this acid, restoration of the normal pHi is dependent on glutamate synthesis. At both high and low osmolarity, the reduction of the glutamate pool is insufficient to account for the acetate anion accumulated. No changes in Na+ or K+ content were detected, suggesting that acetate accumulation is achieved by replacement of physiological anions.
MATERIALS AND METHODS
Materials.
All chemicals were supplied by either BDH or Sigma and were Analar grade or better. Peptone, yeast extract, and agar were supplied by Oxoid. Radiochemicals were supplied by ICN ([1-14C]acetate) and NEN ([7-14C]benzoate and [3H]inulin).
Bacterial strains.
The two strains used in this study were E. coli Frag1 (F− thi rha lac gal) and E. coli Frag83 (F− kdpABC5 thi rha lacZ gltB31 gdh-1 zdi276::Tn10) (15).
Growth media.
The growth experiments in this study were all carried out in defined media based upon citrate-phosphate buffer at pHo 6 containing 17.2 g of Na2HPO4 liter−1, 7.1 g of citric acid liter−1, 0.87 g of K2HPO4 liter−1, 0.1 g of MgSO4 · 7H2O liter−1, 1 g of (NH4)2SO4 liter−1, 0.02 mg of (NH4)2SO4 · FeSO4 liter−1, and 1 μg of thiamine ml−1. The potassium concentration of the medium was 5 mM for all experiments. For growth of the glutamate auxotroph, sodium aspartate was added to the medium at 0.03% (wt/vol). Measurements of growth were made by monitoring the optical density of 1-ml samples at 650 nm (OD650). A single colony of E. coli was used as an inoculum for overnight growth under limiting glucose conditions (0.04% [wt/vol]). Cells were subsequently supplemented with glucose (0.2% [wt/vol]) and allowed one cell-doubling step prior to dilution in fresh medium to an OD650 of 0.05.
pHi measurements.
The pHi was determined by using the distribution of a radiolabelled weak acid according to a previously described centrifugation method (13) which uses bromodecane to separate the cell pellet from the supernatant (15). The pHi was determined by using [7-14C]benzoic acid (4.5 μM; 0.1 μCi · ml−1) and [3H]inulin (1.0 μM; 1 μCi · ml−1) as extracellular markers. The pHi value was calculated as described previously (13). The intracellular concentration of acetate and the pHi in the presence of 8 mM acetate were determined by using either [1-14C]acetate (0.5 μCi · ml−1) or [7-14C]benzoate (4.5 μM; 0.1 μCi · ml−1) and [3H]inulin (1.0 μM; 1 μCi · ml−1) as extracellular markers.
Measurement of acetate pools.
Acetate pools were determined by the method used for pHi determination. Cells were grown to mid-exponential phase with 8 mM acetate, a 10-ml sample was removed and transferred to a stirred glass pot incubated at 37°C, and [1-14C]acetate (0.5 μCi · ml−1) plus [3H]inulin (1.0 μM; 1 μCi · ml−1) was added. After incubation for 5 min to allow equilibration of the acid with the preestablished pools, three 1-ml samples were taken and the cells were separated from the supernatant by centrifugation through bromodecane as described above. Samples were then treated as described previously (13), and the internal acetate concentration was calculated from knowledge of the pK of acetate (pK 4.7), the pHo, and the accumulation ratio. A cytoplasmic volume of 1.6 μl · mg−1 (dry weight) (13) was assumed for calculation of pHi, acetate pool levels, and amino acid pool levels.
Amino acid pool measurements.
Cells were harvested for amino acid pool analysis at mid-exponential phase (OD650 = 0.6) by filtration of 1 ml through a Whatman membrane filter (cellulose nitrate; 0.45-μm pore size) under vacuum. Filters were washed immediately, with slightly hypertonic glucose solution added in individual drops to the filter. The filter papers containing the cells were then placed into Eppendorf tubes containing ice-cold 0.1% trifluoroacetic acid (TFA). Norleucine (250 pmol · ml−1) was added to the TFA as an internal standard. The Eppendorf tubes were left on ice for 30 min to allow extraction of amino acids, after which the filters were removed and the supernatant was stored at −20°C. The analysis was performed as described previously (2).
Measurements of intracellular potassium and sodium levels.
Cultures were grown as described above. A 1-ml aliquot of a mid-exponential-phase culture (OD650 = 0.5) was harvested by filtration with Whatman filters (cellulose nitrate; 0.45-μm pore size; diameter, 2.5 cm). Cells were then treated in one of two ways. For measurements of total intracellular potassium levels, cells were washed with warm (37°C), slightly hypertonic glucose solution added drop-wise from a pipette. For determination of bound potassium, cells were first washed with warm, slightly hypertonic glucose solution followed by ice-cold distilled water, also added drop-wise, to subject the cells to osmotic shock (15). The resulting filter papers were then placed in individual beakers and dried in an oven at 120°C for 12 h. One milliliter of ultrapure water was then added to each of the beakers to dissolve the potassium. The samples were left for 20 min and then analyzed by flame photometry (Corning 400). Sodium ions were measured by preparing the samples as described for pHi measurements by spinning the cells through bromodecane oil and suspending the pellets in milli-Q water. This technique was preferred to the filtration technique, as it minimized contamination by Na+ in the medium. The Na+ content was determined by flame photometry after the samples had been boiled and allowed to cool.
RESULTS
Growth inhibition by weak acids.
Growth of E. coli can be inhibited by weak acids in a pH- and concentration-dependent manner (18). To establish standard conditions that would allow the cytoplasmic pools to be investigated, E. coli Frag1 was grown in McIlvaine’s medium at pH 6. Under these conditions, low concentrations of acetate and benzoate are inhibitory and the acids are more than 95% dissociated, which minimizes the contribution to growth inhibition by the undissociated acid (6). Addition of 2 mM sodium benzoate or 8 mM sodium acetate decreased the specific growth rate (μ) of Frag1 from 0.7 ± 0.03 h−1 (mean ± standard deviation) (n = 26) to 0.35 ± 0.01 h−1 (n = 25) and 0.35 ± 0.02 h−1 (n = 25), respectively. Thus, on a molar basis, sodium benzoate had a much greater effect on the growth of Frag1 than did sodium acetate, in accordance with previous studies (18). The cytoplasmic pH was reduced by growth in the presence of these acids; after steady-state exponential growth had been established, the control cultures exhibited a pHi of 7.85 ± 0.05 (n = 40), compared with pHi 7.26 ± 0.06 (n = 20) and pHi 7.48 ± 0.05 (n = 20) for cultures with 2 mM benzoate and 8 mM acetate, respectively. Thus, the cultures achieve the same growth rate despite different values of pHi, and acetate-treated cells exhibit greater inhibition than would be expected simply from changes in the pHi. These data suggest that other factors contribute to growth inhibition by acetate.
Anion accumulation in weak-acid-treated cells.
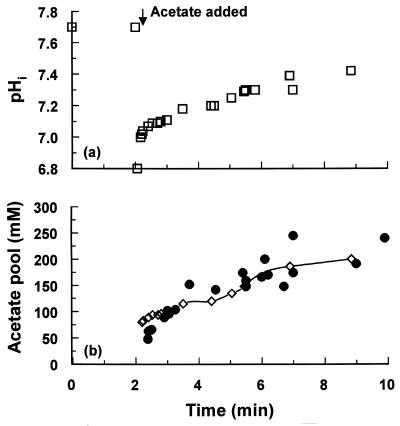
As described above, anion accumulation in the cytoplasm may be a potential cause of growth inhibition; thus, the consequences of anion accumulation were investigated. As described above, addition of 2 mM benzoate lowered the pHi to 7.26 ± 0.06, which, at the steady state, would correspond to an accumulation of 36 ± 5 mM benzoate anions. In contrast, 8 mM acetate reduced the pHi to 7.48 ± 0.05, which would give rise to the accumulation of 232 ± 25 mM acetate anions in the cytoplasm. Benzoate accumulation, therefore, represents only a small osmotic problem for the cell, but the accumulation of acetate creates a potential increase in cell turgor. Adjustments to cell physiology for acetate anion accumulation may be a factor in reducing the growth rate of cells. To determine the effects of acetate on cell pools, the kinetics of the change in pHi were determined. Upon addition of acetate, the pHi rapidly declined to 7.0 and then recovered to the steady-state value of 7.4 (Fig. 1a). The recovery of pHi was biphasic, with an initial rapid recovery over the first 50 s and then a slower phase over the next 5 to 10 min. To monitor changes in the acetate pool, radiolabelled acetate was added and the change in acetate pools was determined (Fig. 1b). The measured acetate accumulation rose to approximately 200 mM over the 10-min incubation period and reached 230 mM after 20 min. These figures correspond with the calculated value based upon the pK of acetate, the pHo, and pHi measured by [14C]benzoate distribution (Fig. 1b).
FIG. 1.
Changes in pHi and acetate pool levels upon addition of 8 mM acetate to E. coli Frag1 at pH 6. Acetate (8 mM) was added to E. coli cells growing at pH 6. (a) pHi was determined by the distribution of radioactive benzoate (□), as described in Materials and Methods. (b) Acetate pool levels (•) were determined as described in Materials and Methods. The predicted acetate pool level (◊) based upon a pK of 4.7 for acetate, the free acetate anion concentration outside the cells (7.6 mM), and the pHi determined by benzoate distribution is indicated. The data shown are composites from several independent experiments in which the time courses were overlapped to enable rapid sampling.
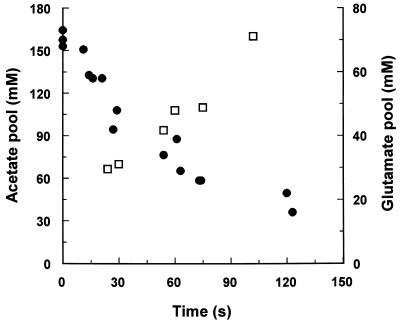
The high levels of acetate accumulation observed suggested that other anion pools in the cell might be significantly decreased to compensate. It has been suggested that E. coli cells have approximately equivalent levels of fixed anions, such as nucleic acids and free carboxyl groups of proteins; free, osmotically-active anions, such as glutamate; and intermediates in glycolysis and the tricarboxylic acid cycle following growth in media similar to those utilized in this study (15). At low osmolarity, glutamate is the largest single pool of osmotically active anions, constituting 25% of the total anion pool (15). Treatment of E. coli Frag1 with acetate or benzoate significantly reduced the glutamate and aspartate pool levels (Table 1). Cells in steady-state growth at pH 6 in the presence of 8 mM acetate exhibited pool reductions of approximately 50 mM and 3 mM for the glutamate and aspartate pools, respectively. Much smaller decreases in the pools were observed with benzoate. Rapid sampling was utilized to analyze the kinetics of glutamate pool reduction during acetate accumulation (Fig. 2). At the first time point (approximately 20 s after addition of acetate), the intracellular concentration of acetate was approximately 65 mM, but it rapidly rose to 100 mM within 1 min (Fig. 2). The rapid phase of acetate uptake coincides with the decrease in the glutamate pool; however, the increase in acetate levels was approximately double the rate of reduction in the glutamate pool. These data suggest that during the recovery of pHi, the lowering of the glutamate pool is significant but is not the sole compensating mechanism for acetate accumulation.
TABLE 1.
Major changes in amino acid pool levels consequent upon growth with acetate or benzoate
| Amino acid | Pool size (mM)a
|
||
|---|---|---|---|
| Control | Benzoate | Acetate | |
| Glutamate | 60.0 ± 6.0 | 44.4 ± 3.0 | 10.0 ± 2.0 |
| Aspartate | 3.1 ± 0.6 | 1.2 ± 0.2 | 0.3 ± 0.2 |
| Lysine | 1.8 ± 0.7 | 0.9 ± 0.1 | 1.1 ± 0.4 |
| Arginine | 4.8 ± 1.1 | 2.9 ± 0.1 | 0.7 ± 0.4 |
| Glutamine | 1.2 ± 0.5 | 0.6 ± 0.4 | 0.2 ± 0.3 |
| Total amino acid pool | 90 ± 7.4 | 65 ± 6.5 | 25 ± 4.4 |
Calculated on the basis of an intracellular volume of 1.6 μl · mg of cells−1 (13). Values are means ± standard deviations.
FIG. 2.
Glutamate and acetate pool levels in E. coli cells upon treatment with acetate. Pools of acetate and glutamate were measured in parallel incubations at pHo 6, as described in Materials and Methods. Acetate (8 mM) was added at zero time. Symbols: •, glutamate; □, acetate. The data are drawn from several experiments with overlapping sample times to enable rapid kinetics determinations to be performed.
Small decreases in the pool levels of glutamine, lysine, and arginine (Table 1), which are directly linked to glutamate and aspartate via metabolism, were also observed. However, these pools were initially markedly smaller than the glutamate pool. Other pools, such as serine, glycine, and proline, which were relatively small, remained unchanged (data not shown), and the total change in the amino acid pool corresponded closely with the change in the four amino acids mentioned above (Table 1). The pools appeared to be well regulated, since growth of Frag1 cells with aspartate did not change the pool sizes of any of the amino acids derived from this amino acid and did not change the pattern of amino acid pool reduction observed with acetate (data not shown). These data suggest that the changes in the physiological anion pools were specific and did not arise from nonspecific balancing of turgor via stretch-activated channels (4). These results suggest that reductions in glutamate and aspartate pools may compensate for acetate and benzoate entering the cell; however, the changes in amino acid pool levels did not correspond with the measured accumulation of benzoate or acetate.
Changes in cation content.
Previous work (15) has shown a close interdependence of potassium and glutamate pools during osmotic upshock. We sought to determine whether the changes in acetate pool levels in the cell affected the cytoplasmic pools of K+ and Na+. Total and bound K+ were determined to allow the calculation of free K+ within the cell, which is the fraction that balances the charge of osmotically active anions (15). We saw no significant difference in the concentration of free or bound K+ in cells treated with weak acids compared to untreated control cells (Table 2). The distributions of the K+ pool between the fixed and mobile anion pools in the presence and absence of acetate were similar (Table 2). Similarly, there was no detectable change in the free Na+ pool or in the pools of polyamines (data not shown). Together, these data are consistent with no overall change in the total free inorganic cation pools in the cell. Further, it is notable that the accumulation of acetate observed at the steady state is similar in magnitude to the free K+ pool, with approximately 230 to 240 mM acetate and 240 to 250 mM K+ (Table 2).
TABLE 2.
Potassium pool levels in cells treated with acetate and benzoatea
| Growth condition | Total K+ (mM) | Bound K+ (mM) | Free K+ (mM) |
|---|---|---|---|
| Control | 483 ± 23 | 229 ± 19 | 254 ± 42 |
| 8 mM acetate | 492 ± 33 | 248 ± 17 | 244 ± 50 |
| 2 mM benzoate | 492 ± 25 | 252 ± 7 | 240 ± 32 |
Cells were grown to exponential phase, and samples were taken for K+ analysis as described in Materials and Methods. The data are means ± standard deviations from four replicate experiments.
Compensation for acetate accumulation at high osmolarity.
In the above experiments, which were conducted at low osmolarity, the small size of the glutamate pool prevented it from fully compensating for acetate accumulation. Cells accumulate larger pools of glutamate and K+ ions when grown at high osmolarity in the absence of compatible solutes (15). To determine the maximum compensation by glutamate pool reduction, the pHi and glutamate pool levels were measured during steady-state growth in cells grown at pH 6 over a range of osmolarities, in the presence and absence of 8 mM acetate (Table 3). The growth rate declined as the osmolarity of the medium was increased, and incubation with 8 mM acetate further diminished the growth rate; overall, the percent inhibition by acetate declined as the osmolarity was raised (Table 3). At higher osmolarities, the steady-state pHi was slightly lower than that of cells in the basic medium and was further diminished by the addition of acetate (Table 3). As expected, the glutamate pool levels were a function of the osmolarity of the medium. Incubation with acetate lowered the glutamate pool level. The greatest compensation for acetate accumulation by lower glutamate pool levels occurred in the presence of 400 mM glucose, where the reduction of the glutamate pool accounted for approximately 72% of the acetate accumulated (Table 3), suggesting that the depletion of other anions must also contribute to the compensation for acetate accumulation. However, even under these conditions it was notable that acetate-treated cells retained higher glutamate pool levels than at low osmolarity, which suggests that there is no specific, coupled exchange of acetate for glutamate.
TABLE 3.
Effects of increased osmolarity on pHi and glutamate pool levels in the presence of acetatea
| Glucose (mM) | Acetate | Acetate anion (mM)b | μ (h−1) | Inhibition (%) | pHi | Glutamate (mM) |
|---|---|---|---|---|---|---|
| 0 | − | 0.69 ± 0.03 | 7.85 ± 0.05 | 60 ± 6 | ||
| + | 243 ± 28 | 0.34 ± 0.01 | 51 | 7.48 ± 0.05 | 10 ± 2 | |
| 200 | − | 0.53 ± 0.02 | 7.59 ± 0.01 | 117 ± 2 | ||
| + | 161 ± 26 | 0.30 ± 0.04 | 44 | 7.30 ± 0.07 | 48 ± 1 | |
| 400 | − | 0.44 ± 0.03 | 7.61 ± 0.03 | 160 ± 3 | ||
| + | 130 ± 9 | 0.28 ± 0.05 | 36 | 7.21 ± 0.03 | 62 ± 2 |
Cells were grown to steady state in the presence or absence of 8 mM acetate; pHi and glutamate pool levels were determined as described in Materials and Methods. The experiments were carried out three times, and the pHi and glutamate pool levels for each experiment were means of three determinations.
Acetate pool levels were calculated from a knowledge of the pHi, the external concentrations of acetate anion (7.6 mM), and the pHo of 6.
Cellular glutamate pool levels recover when the weak acid is removed.
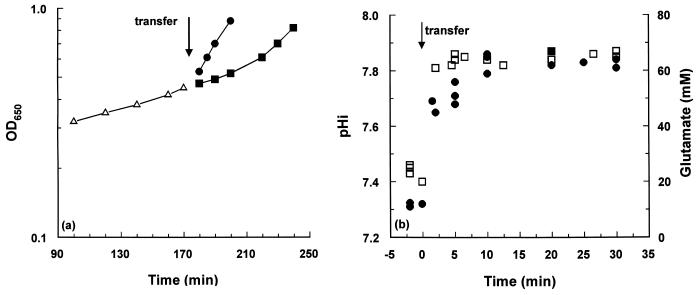
Benzoate-treated cells reestablished the same growth rate as untreated cultures almost immediately on transfer to fresh, prewarmed, benzoate-free medium (Fig. 3a). In contrast, acetate-treated cells retained the lower growth rate for approximately 20 min after removal of acetate and achieved the same rate as the control incubation only after 60 min (Fig. 3a). In view of the substantial drop in the glutamate pool in the presence of acetate, we sought to determine whether the sustained growth inhibition was due to an inability to restore the pHi or the glutamate pool. Cells were treated with acetate, and the pHi and glutamate pools were determined prior to and following filtration, washing, and transfer to fresh medium lacking acetate. After the transfer, the pHi recovered to the normal level within 5 min and the glutamate pool level was 90% of normal at the same time, achieving its maximum value 10 min after transfer to fresh growth medium (Fig. 3b). Therefore, the failure of acetate-treated cells to restore normal growth immediately after transfer to fresh growth medium cannot be attributed to failure to reestablish the normal values for the pHi and glutamate pool levels.
FIG. 3.
Changes in growth rate, pHi, and glutamate pool levels upon suspension of acetate-treated cells in acetate-free medium. (a) Cultures were grown to exponential phase in pH 6 medium containing either acetate or benzoate. The growth data for both acetate-treated and benzoate-treated cultures obtained prior to transfer to acid-free medium are represented by a single set of data points (▵). After approximately 3 h of incubation, the cells were rapidly filtered and transferred into pH 6 medium without the weak acid, and the incubation was continued. Symbols: ▪, acetate-treated cells; •, benzoate-treated cells. (b) Cells were pregrown at pH 6 in the presence of 8 mM acetate, and the cytoplasmic pH (□) and glutamate pool levels (•) were determined as described in Materials and Methods. After a 5-min preincubation period, cells were transferred into acetate-free medium, pHo 6, and the changes in pHi and glutamate pool levels were recorded.
Interdependence of glutamate pool levels and pHi.
To determine the interdependence of the recovery of the pHi and of glutamate pool levels, we utilized a gdh gltB double mutant of E. coli, Frag83 (15), which is unable to synthesize glutamate in the absence of a suitable precursor. The growth of Frag83 was always slower than the parent, Frag1, with specific growth rates (μ) of approximately 0.35 h−1 and <0.18 h−1 when cells were supplemented with aspartate and glutamate, respectively (data not shown). The internal pH of Frag83 was lower than the values observed with Frag1, with steady-state pHi values of 7.5 ± 0.03 (n = 5) for cells grown with aspartate compared with 7.88 ± 0.06 for Frag1 (Table 4). Acetate caused a reduction of the steady-state pHi in both Frag1 and Frag83 (Table 4). For Frag83, recovery of pHi after acetate treatment was dependent on aspartate. When cells of Frag83 were resuspended in aspartate-free medium, the pHi did not recover and the glutamate pool level remained very low (Table 4). Addition of aspartate led to the reestablishment of a high pHi value and complete restoration of the glutamate pool (Table 4). The kinetics of pHi and glutamate pool recovery for Frag83 were similar to those for Frag1 (data not shown). Thus, recovery of the normal pHi is dependent upon the synthesis of glutamate.
TABLE 4.
Interdependence of glutamate synthesis and pHi recoverya
| Strain | Acetate (8 mM) | Aspartate (0.03% [wt/vol]) | pHi | Glutamate pool concn (mM) |
|---|---|---|---|---|
| Frag1 | − | − | 7.85 ± 0.05 | 60 ± 6 |
| − | + | 7.88 ± 0.06 | 61 ± 5 | |
| + | − | 7.48 ± 0.05 | 11 ± 2 | |
| −b | − | 7.84 ± 0.04 | 63 ± 6 | |
| Frag83 | − | + | 7.5 ± 0.03 | 50 ± 1.7 |
| + | + | 7.2 ± 0.04 | 12 ± 0.5 | |
| −b | − | 7.2 ± 0.08 | 1.4 ± 0.2 | |
| −b | + | 7.58 ± 0.04 | 59 ± 0.9 |
Experiments were performed three times and the data were averaged; five pHi measurements were taken per experiment, and three glutamate pool measurements were taken per experiment.
Glutamate pool and pHi after washing of cells to remove acetate and incubation in acetate-free medium for 15 min.
DISCUSSION
In this study, we have demonstrated that E. coli cells grow both at low and at high osmolarity, with the majority of the glutamate pool replaced by acetate. These cells do not achieve their maximum growth rates, but this may reflect the changes in other anion pools and/or the lowering of the pHi. The pHi is not the sole determinant of growth inhibition, since benzoate-treated cells and acetate-treated cells establish different steady-state values of pHi but grow at the same rate. Clearly, also, in the case of growth with acetate, there are changes in either gene expression or cell physiology that lead to a transient period of continued inhibition even after removal of the acid (Fig. 3a). In a previous study, we showed that acetate can have profound effects on gene expression at pH 6, with effects on both two-component-controlled systems and those regulated by supercoiling and the ion balance of the cell (20). Others have reported that weak acids cause increased expression of RpoS (19), and it is possible that other general stress-regulated systems are operational in acetate-treated cells. Clearly, however, there are major differences between acetate-treated and benzoate-treated cells (Fig. 3a), despite the same exponential growth rate; thus, one must seek some explanation for these specific effects in the influence of the acids on the pHi and anion pool levels.
During steady-state growth, E. coli cells had a glutamate pool concentration of approximately 60 mM (Table 1), which represents only 25% of the measured pool of free K+ (Table 2). These data imply the existence of a substantial pool of other unidentified anions that are most probably generated by metabolism, in accordance with previous studies (15). The measured steady-state acetate pool concentration was approximately 230 ± 25 mM, which is similar to that of the free K+ pool (Table 2); this suggests that acetate has almost completely replaced the pools of other anions that normally balance free K+. The lowering of the pool levels of these other anionic metabolites may be one of the reasons that acetate causes the same growth inhibition as benzoate, when the pHi is higher in the presence of acetate. The slow recovery of growth after acetate has been washed out of E. coli cells may be related to the altered pools and possible subsequent changes in enzyme activity that unbalance metabolism once the acetate is removed.
For cells grown at low osmolarity, such as those used for most of this study, there are three major classes of anion pools: fixed anions (DNA and protein), acidic amino acids, and metabolic intermediates in the main catabolic and anabolic pathways. Analysis of the distribution of the K+ pool in E. coli between bound and free pools led to the conclusion that there are approximately equal contributions from the fixed (nucleic acids and protein) and the mobile (amino acids and metabolic intermediates) anions (15). For the fixed anions, nucleic acids predominate since, firstly, the phosphate backbone of the DNA and RNA corresponds to approximately 300 to 400 mM phosphate (with a pK of 6.4), which represents considerable buffering capacity. Secondly, the pK values of the acidic amino acids (nominally pK 3.9 and 4.07 for aspartate and glutamate, respectively) on proteins are outside the range of pH changes observed and thus should not contribute substantially to the pH changes observed. The RNA and DNA of the cell are balanced by approximately 250 mM K+ and an excess of arginine and lysine in proteins equivalent to approximately 80 mM (calculated from data reported in reference 16). The initial fall in pHi of approximately 0.8 to 0.9 units within 10 to 15 s of acetate addition may lead to a change in the ionization of the backbone equivalent to 60 to 80 mM phosphate. The measured pool of acetate at this time is 60 to 65 mM; therefore, this increase in the acetate anion might be accommodated by protonation of the phosphate groups of DNA and RNA consequent upon lowering of the pHi. However, as the pHi recovers, the phosphate is deprotonated and, at the steady state, the net gain from changes in phosphate ionization is only 20 to 30 mM. At the steady state, the acetate pool is approximately 240 mM; therefore, we have concluded that an exchange of physiological anions for acetate ions must occur to maintain electrical neutrality. These metabolites are derived from acids with pK values that are similar to, or below, that for acetate; their exchange for acetate anions has the potential to contribute to the recovery of an alkaline pHi (see below). Glutamate loss is a major compensatory mechanism.
It is not known whether the glutamate pool level is lowered due to rapid metabolism by reentry into the tricarboxylic acid cycle, by efflux, or by glutamate decarboxylase, which has been implicated in survival at acid pHs (11, 14). A gadC mutant, which lacks the permease for the decarboxylated product derived from glutamate (11), exhibited the same glutamate pool reduction upon addition of acetate (data not shown). This suggests that the glutamate decarboxylase pathway is not responsible for the lowering of the glutamate pool level upon the addition of acetate.
The regulation of pHi is a complex phenomenon that is poorly understood despite much effort to identify individual components of the regulatory system (5, 13, 17). The transfer of acetic acid across the membrane and its subsequent dissociation into a proton and an acetate ion causes the pHi to decline from 7.8 to 7. The recovery to between pHi 7.4 and 7.5 must involve a number of processes. Previously, we have found that recovery from transient acidification is dependent upon K+ transport, though no net K+ accumulation was detected (5). Thus, pH changes do not equate directly with alterations in the K+ pool. Other cation transport systems, notably Na+/H+ antiports, have been implicated in pH homeostasis in alkaliphiles, but the evidence for their role in pH regulation in E. coli is limited (17). Exchange of glutamate and other anions for acetate may affect the pHi due to the difference in pKa values. Using the standard pKa values and the Henderson-Hasselbach equation, one may calculate that a 1:1 exchange of acetate for glutamate would raise the pH of a solution by 0.4 to 0.5 units. However, in the cell, these gains have to be set against the buffering capacity of the phosphate backbone of the nucleic acids referred to above. Cumulatively, the large scale of the exchange of physiological anions for acetate must contribute to the steady-state pHi achieved.
Our data suggest that glutamate synthesis and pHi homeostasis are closely linked. After suspension of acetate-treated cells in acetate-free medium, the pHi of Frag83, a strain unable to synthesize glutamate without a suitable precursor, did not recover unless aspartate was provided to facilitate glutamate synthesis. Similarly, suspension of acetate-treated Frag1 cells in acetate-free medium led to a rapid rise in pHi and in the magnitude of the glutamate pool (Fig. 3b). The mechanisms operating to effect recovery of pHi are unclear. As was noted above, reaccumulation of glutamate at the expense of acetate will itself cause a change in the pHi, but in this instance this would lead to further slight acidification. Clearly, net proton extrusion is required to effect the recovery of the pHi to a higher value.
In conclusion, growth inhibition by acetate and benzoate cannot be accounted for simply by their effects on the release of protons in the cytoplasm, despite the fact that both acids do provoke significant changes in the pHi. It is clear that while changes in the pHi are important consequences of incubation with the acids, accumulation of the acid anion has significant effects on the anion balance of the cell, and growth inhibition is a complex result of these effects.
ACKNOWLEDGMENTS
A.J.R. is supported by a BBSRC CASE studentship in collaboration with Unilever Research, Colworth House; D.M. is supported by a Wellcome Trust Programme grant; and I.R.B. is a Wellcome Trust Research Leave Fellow.
We thank J. Slonczewski for supplying the gadC mutant of E. coli and Heather Wallace for analysis of polyamine pools.
REFERENCES
- 1.Ahmed S, Booth I R. The effect of β-galactosides on the protonmotive force and growth of Escherichia coli. J Gen Microbiol. 1983;129:2521–2529. doi: 10.1099/00221287-129-8-2521. [DOI] [PubMed] [Google Scholar]
- 2.Amezaga M R, Davidson I, McLaggan D, Verheul A, Abee T, Booth I R. The role of peptide metabolism in the growth of Listeria monocytogenes ATCC 23074 at high osmolarity. Microbiology. 1995;141:41–49. doi: 10.1099/00221287-141-1-41. [DOI] [PubMed] [Google Scholar]
- 3.Baik H S, Bearson S, Dunbar S, Foster J W. The acid tolerance response of Salmonella typhimurium provides protection against organic acids. Microbiology. 1996;142:3195–3200. doi: 10.1099/13500872-142-11-3195. [DOI] [PubMed] [Google Scholar]
- 4.Blount P, Sukharev S I, Moe P C, Nagle S K, Kung C. Towards an understanding of the structural and functional properties of MscL, a mechanosensitive channel in bacteria. Biol Cell. 1996;87:1–8. [PubMed] [Google Scholar]
- 5.Booth I R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M H, Booth I R. Acidulants and pH. In: Russell N J, Gould G W, editors. Food preservatives. Glasgow, United Kingdom: Blackie; 1991. pp. 22–43. [Google Scholar]
- 7.Cherrington C A, Hinton M, Pearson G R, Chopra I. Short chain organic acids at pH 5.0 kill Escherichia coli and Salmonella spp. without causing membrane perturbation. J Appl Bacteriol. 1991;70:161–165. doi: 10.1111/j.1365-2672.1991.tb04442.x. [DOI] [PubMed] [Google Scholar]
- 8.Cherrington C A, Hinton M, Mead G C, Chopra I. Organic acids: chemistry, antibacterial activity and practical applications. Adv Microb Physiol. 1991;32:87–108. doi: 10.1016/s0065-2911(08)60006-5. [DOI] [PubMed] [Google Scholar]
- 9.Epstein W. Osmoregulation by potassium-transport in Escherichia coli. FEMS Microbiol Rev. 1986;39:73–78. [Google Scholar]
- 10.Ferguson G P, McLaggan D, Booth I R. Potassium channel activation by glutathione-S-conjugates in Escherichia coli: protection against methylglyoxal is mediated by cytoplasmic acidification. Mol Microbiol. 1995;17:1025–1033. doi: 10.1111/j.1365-2958.1995.mmi_17061025.x. [DOI] [PubMed] [Google Scholar]
- 11.Hersch B M, Farooq F T, Barstad D N, Blankenshorn D L, Slonczewski J L. A glutamate-dependent acid resistance gene in Escherichia coli. J Bacteriol. 1996;178:3978–3981. doi: 10.1128/jb.178.13.3978-3981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabara J J, Eklund T. Organic acids and esters. In: Russell N J, Gould G W, editors. Food preservatives. Glasgow, United Kingdom: Blackie; 1991. pp. 44–71. [Google Scholar]
- 13.Kroll R G, Booth I R. The role of potassium transport in the generation of a pH gradient in Escherichia coli. Biochem J. 1981;198:691–698. doi: 10.1042/bj1980691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin J, Smith M P, Chapin K C, Baik H S, Bennett G N, Foster J W. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1996;62:3094–3100. doi: 10.1128/aem.62.9.3094-3100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaggan D, Naprstek J, Buurman E T, Epstein W. Interdependence of K+ and glutamate accumulation during osmotic adaptation of Escherichia coli. J Biol Chem. 1994;269:1911–1917. [PubMed] [Google Scholar]
- 16.Neidhardt F C, Ingraham J L, Schaechter M, editors. The physiology of the bacterial cell: a molecular approach. Sunderland, Mass: Sinauer Associates; 1990. [Google Scholar]
- 17.Padan E, Schuldiner S. N+/H+ antiporters, molecular devices that couple the Na+ and H+ circulation in cells. J Bioenerg Biomembr. 1993;25:647–669. doi: 10.1007/BF00770252. [DOI] [PubMed] [Google Scholar]
- 18.Salmond C V, Kroll R G, Booth I R. The effect of food preservatives on pH homeostasis in Escherichia coli. J Gen Microbiol. 1984;130:2845–2850. doi: 10.1099/00221287-130-11-2845. [DOI] [PubMed] [Google Scholar]
- 19.Schellhorn H E, Stones V L. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J Bacteriol. 1992;174:4769–4776. doi: 10.1128/jb.174.14.4769-4776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas A D, Booth I R. The regulation of porin gene expression by acid pH. J Gen Microbiol. 1992;138:1829–1835. doi: 10.1099/00221287-138-9-1829. [DOI] [PubMed] [Google Scholar]