Abstract
Patients with reflux-like symptoms (heartburn and regurgitation) are often not well advised on implementing individualised strategies to help control their symptoms using dietary changes, lifestyle modifications, behavioural changes or fast-acting rescue therapies. One reason for this may be the lack of emphasis in management guidelines owing to ‘low-quality’ evidence and a paucity of interventional studies. Thus, a panel of 11 gastroenterologists and primary care doctors used the Delphi method to develop consolidated advice for patients based on expert consensus. A steering committee selected topics for literature searches using the PubMed database, and a modified Delphi process including two online meetings and two rounds of voting was conducted to generate consensus statements based on prespecified criteria (67% voting ‘strongly agree’ or ‘agree with minor reservation’). After expert discussion and two rounds of voting, 21 consensus statements were generated, and assigned strength of evidence and Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) rating. Eleven statements achieved the strongest (100%) agreement: five are related to diet and include identification and avoidance of dietary triggers, limiting alcohol, coffee and carbonated beverages, and advising patients troubled by postprandial symptoms not to overeat; the remaining six statements concern advice around smoking cessation, weight loss, raising the head-of-the-bed, avoiding recumbency after meals, stress reduction and alginate use. The aim of developing the consensus statements is that they may serve as a foundation for tools and advice that can routinely help patients with reflux-like symptoms better understand the causes of their symptoms and manage their individual risk factors and triggers.
Keywords: advice, brain-gut, diet, gastro-oesophageal reflux disease, lifestyle, oesophagus, symptoms
Introduction
Heartburn and regurgitation are common symptoms, experienced regularly by up to 20–50% of individuals [1,2]. Individuals with mild heartburn frequently use over-the-counter antacids, while those with more severe symptoms often resort to long-term use of acid-suppressant medications. This acid-centred approach, which is highly effective for healing reflux oesophagitis, ignores the more complex aetiology of oesophageal symptoms, wherein dietary habits, reflux hypersensitivity, psychosocial factors and obesity, may also play a role [3–5]. However, the impracticality of exploring this complex symptom aetiology compared with the pragmatic approach of using proton pump inhibitors (PPIs) has led to widespread, open-ended PPI use. Unfortunately, this also means that reflux-like symptoms, the severity of which exhibits only a weak relationship with pathological oesophageal acid exposure, continue to impact the day-to-day lives of a large proportion of individuals [1,6].
The concept that dietary and lifestyle factors can influence the likelihood of reflux-like symptoms is well accepted. However, patients are generally given minimal (and inconsistent) advice about implementing diet, lifestyle and behavioural changes [7–10], despite these being low-harm, pragmatic approaches to self-management. One reason for this is a historical lack of emphasis in evidence-based clinical guidelines, which stems from a paucity of high-quality interventional studies. Ideally, guidelines and clinical advice should be based on rigorous evidence, for which randomised controlled trials (RCTs) are the gold standard. However, a lack of RCT evidence does not negate the value of ‘lifestyle’ recommendations. Hence, the Delphi method, with greater emphasis on clinical experience and expert consensus, was used to develop a set of consensus statements of advice to help providers and patients target the physical and perceptual factors contributing to reflux-like symptoms. These statements can serve as a basis for facilitating doctor-patient communication as well as aiding the development of self-management tools to better support the wellbeing of patients with reflux-like symptoms.
Methods
Study design
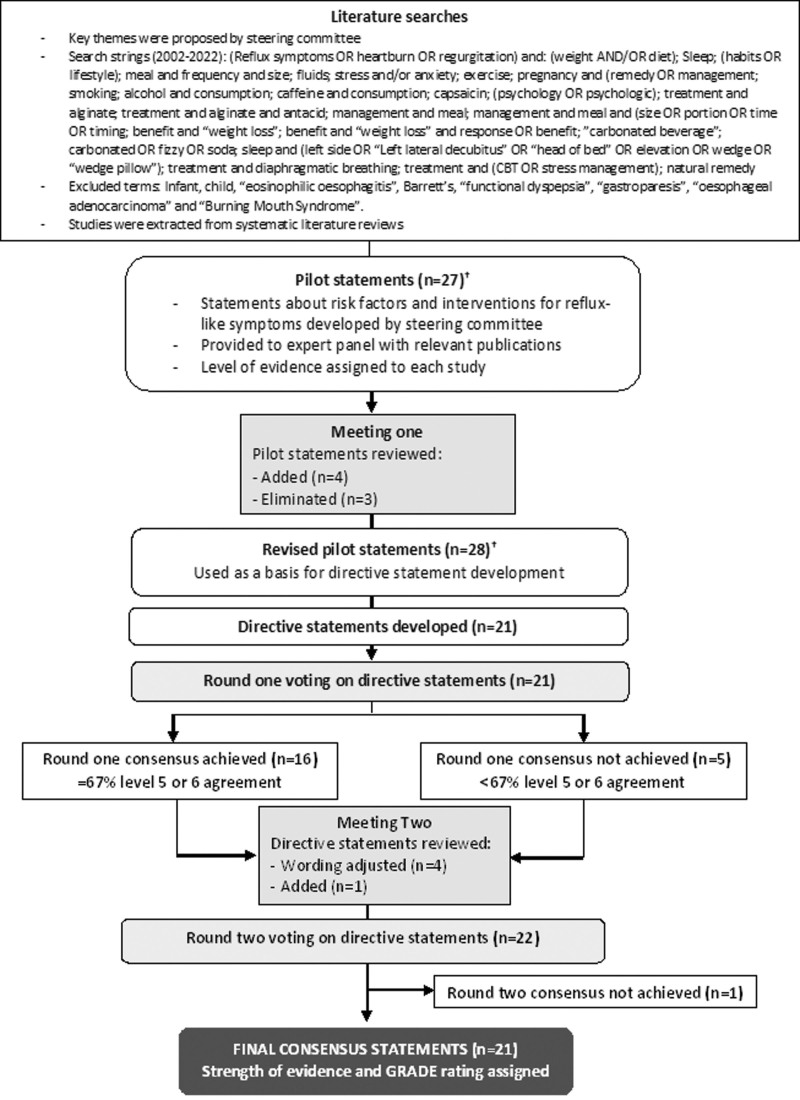
The consensus procedure was based on a literature review and rating of statements by an expert panel (summarised in Fig. 1). Consensus statements were developed over the course of two online meetings and two rounds of voting. A meeting facilitator and medical writer attended the meetings and collated the results (Lumanity, UK).
Fig. 1.
Consensus procedure flow chart. †Pilot statements and revisions are listed in Supplemental digital content 1, http://links.lww.com/EJGH/A950.
The steering committee, comprising two experienced gastroenterologists and a general practitioner with a specialist interest in gastroenterology, defined key themes for literature searches and selected eight further panel members (five gastroenterologists and three primary care doctors) who were experienced in the management of reflux-like symptoms (average 28 years in practice, range 13–45 years), and involved in gastroenterology research and education (held positions in national/regional gastroenterological societies and/or have previously been involved in guideline development). The panel represented seven countries, including the USA, Italy, Spain, Greece, the Netherlands, Romania and the UK.
Targeted literature searches
Literature searches using the PubMed database were conducted in June 2022 by an information specialist using keywords identified by the steering committee to identify relevant publications (excluding editorials, commentary, consensus statements, case reports, letters and response papers) in English from 2002 to 2022. The search strings for terms in the title and abstract are shown in Fig. 1. The terms 'infant', 'child', ‘eosinophilic oesophagitis’, 'Barrett’s', ‘functional dyspepsia’, ‘gastroparesis’, ‘oesophageal adenocarcinoma’ and ‘Burning Mouth Syndrome’ were excluded. The reference lists of retrieved articles were used to identify any further studies of interest. Throughout the consensus process, panel members were also encouraged to contribute supporting evidence.
Consensus procedure
The literature searches identified 406 articles. After the removal of 50 duplicates and the exclusion of nonrelevant studies, a total of 135 references were reviewed and used by the steering committee to develop 27 pilot statements around risk factors and interventions for reflux-like symptoms (Supplemental digital content 1, http://links.lww.com/EJGH/A950). The pilot statements and supporting articles were shared with the expert panel together with the level of supporting evidence for each study which had been assigned by the steering committee using the scale (1–6), as described previously [11] (Table 1). In the first consensus meeting, statements and evidence were reviewed and, based on panel discussion and agreement, three statements were added and four were eliminated (Supplemental digital content 1, http://links.lww.com/EJGH/A950). The revised pilot statements were then used as a basis for the steering committee to develop a set of 21 directive statements, which were shared (with supporting evidence) for anonymous voting using the Qualtrics online platform. For each statement, the experts, including the steering committee, indicated their level of agreement on a scale from 1 to 6 (1 = strongly disagree; 2 = disagree with major reservation; 3 = disagree with minor reservation; 4 = agree with major reservation; 5 = agree with minor reservation; and 6 = strongly agree). Results were analysed by the meeting facilitator. The consensus threshold was set a priori as agreement (level 5 or 6) by at least 67% of respondents.
Table 1.
Strength of evidence
| Level or GRADE | Criteria |
|---|---|
| Evidence | |
| 1+ | Systematic review or meta-analysis of randomised controlled trials |
| 1 | Randomised controlled trial with adequate power |
| 2+ | Randomised controlled trial that does not meet level 1 criteria |
| 3 | Nonrandomised clinical trial or cohort study |
| 4 | Systematic review including observational studies, before-after studies, cross-sectional studies, cohort studies with non-contemporaneous controls, case-control study |
| 5 | Case series with controls |
| 6 | Case series without controls |
| Recommendation | |
| A | Supported by level 1 or 1+ evidence plus consensus |
| B | Supported by level 2 evidence plus consensus |
| C | Supported by level 3 evidence plus consensus |
| D | Any lower level of evidence plus consensus |
| Grade | |
| High | Further research is unlikely to change our confidence in the estimate of effect |
| Moderate | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
| Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| Very low | Any estimate of effect is very uncertain |
During the second meeting, round one voting results were reviewed. Statements that did not reach consensus were discussed and revised and an additional statement was added ahead of a second round of voting. After the second round of voting, each of the consensus statements was assigned strength of evidence (A–D) by the steering committee and were rated using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) system [12] (high, moderate, low, very low, see Table 1).
Results
Consensus was achieved for 21 of the final 22 statements (Table 2). For each consensus statement, the result of the final vote and the grade of supporting evidence are given, followed by a discussion of key evidence. The statements have been separated into three categories of advice, (1) diet and exercise, (2) lifestyle and behaviour and (3) other interventions to target potential reflux-like symptom pathophysiology. The level of voting agreement achieved in each round is summarised in Figs. 2–4.
Table 2.
Consensus recommendations for the management of reflux-like symptoms
| Diet and exercise | |
|---|---|
| 1. | Identification and avoidance of specific dietary triggers (e.g. citrus, tomatoes, highly spiced foods, fatty foods, fried foods and chocolate) should be recommended to individuals with postprandial reflux-like symptoms |
| 2. | Individuals who experience reflux-like symptoms after consuming alcoholic beverages should be advised to limit intake of alcoholic beverages |
| 3. | Individuals who experience reflux-like symptoms after consuming coffee should be advised to limit intake of coffee |
| 4. | Individuals who experience reflux-like symptoms after consuming carbonated beverages should be advised to limit intake of carbonated beverages |
| 5. | A low-fat diet should be recommended to individuals with reflux-like symptoms |
| 6. | Individuals with postprandial reflux-like symptoms should be advised not to overeat |
| 7. | Moderate, regular exercise should be recommended for overweight and obese individuals with reflux-like symptoms |
| Lifestyle and behaviour advice | |
| 8. | Individuals who experience reflux-like symptoms should be advised to avoid wearing items that are tight around their waist |
| 9. | Smoking cessation should be recommended to individuals with reflux-like symptoms who smoke tobacco products |
| 10. | Weight loss should be recommended to individuals with reflux-like symptoms who are overweight, obese or have recently gained weight |
| 11. | Head of the bed elevation should be recommended to individuals with night-time reflux-like symptoms |
| 12. | Sleeping in the left lateral decubitus position should be recommended to individuals with night-time reflux-like symptoms |
| 13. | Avoiding recumbence after meals and leaving a 3-hour interval between an evening meal and going to bed should be recommended to individuals with postprandial and/or night-time reflux-like symptoms |
| Other interventions to target reflux-like symptom pathophysiology | |
| 14. | Diaphragmatic breathing training is a treatment option for individuals with reflux-like symptoms, especially regurgitation |
| 15. | Stress management strategies should be recommended to individuals with reflux-like symptoms and stress |
| 16. | Brain-gut behavioural therapies (e.g. CBT or gut-directed hypnotherapy) should be recommended to individuals with reflux-like symptoms and signs of oesophageal hypervigilance and/or psychological distress |
| 17. | Disordered sleep is associated with an increased likelihood of having reflux-like symptoms |
| 18. | Alginate-antacid combinations are an effective treatment for reflux-like symptoms |
| 19. | Antacids are an effective treatment for reflux-like symptoms |
| 20. | Antacids and alginate-antacid combinations are an effective treatment option for reflux-like symptoms during pregnancy |
| 21. | Products containing hyaluronic acid and chondroitin sulphate are an effective treatment option for reflux-like symptoms |
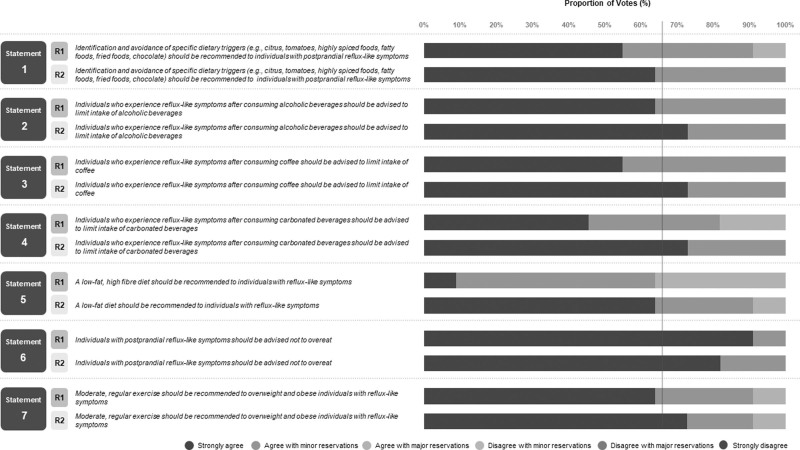
Fig. 2.
Breakdown of voting agreement for statements regarding diet and exercise advice for reflux-like symptoms.
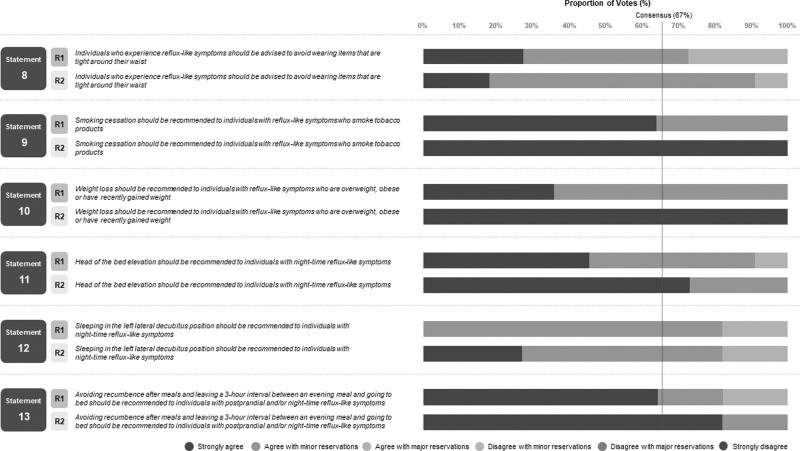
Fig. 4.
Breakdown of voting agreement for statements concerning other interventions to target reflux-like symptom pathophysiology.
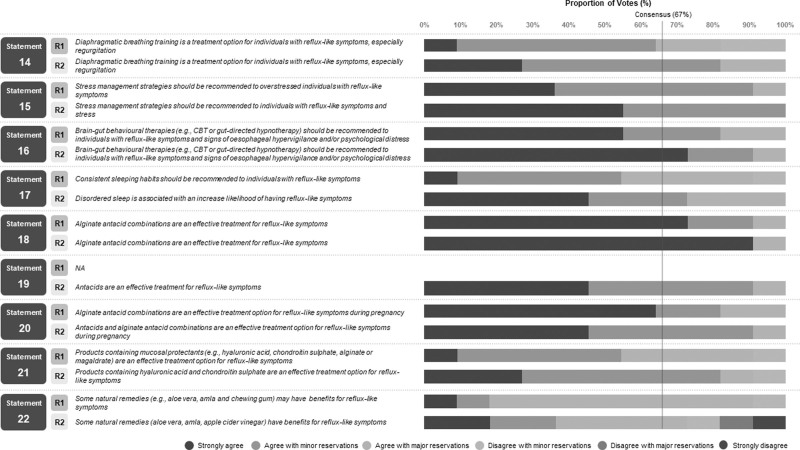
Fig. 3.
Breakdown of voting agreement for statements regarding lifestyle and behaviour advice for reflux-like symptoms.
Diet and exercise advice
Statement 1: Identification and avoidance of specific dietary triggers (e.g. citrus, tomatoes, highly spiced foods, fatty foods, fried foods and chocolate) should be recommended to individuals with postprandial reflux-like symptoms. Agreement: 100% (6, 63.6%; 5, 36.4%; grade of evidence: D; strength of recommendation: high).
Sixteen studies (one randomised crossover trial [13], one prospective cohort study [14], one before-after study [15], two case-control studies [16,17], eleven cross-sectional studies [18–28]) and two systematic reviews [29,30] supported the association of one or more dietary factors with reflux-like symptoms. The only study assessing a dietary intervention was a before-after study in patients presenting with reflux-like symptoms for the first time in primary care [15]. Reflux-like symptoms decreased significantly from baseline when patients were helped to identify and fully/partially eliminate dietary triggers, and 45% of patients agreed to continue symptom management with dietary intervention alone [15]. One cross-sectional study showing increased heartburn perception after ingestion of chili in patients with sleep disturbance (Pittsburgh Sleep Quality Index Score score >5) versus normal sleep [27], emphasizes how the impact of dietary triggers may be influenced by other lifestyle factors.
Statement 2: Individuals who experience reflux-like symptoms after consuming alcoholic beverages should be advised to limit their intake of alcoholic beverages. Agreement: 100% (6, 72.7%; 5, 27.3%; grade of evidence: D; strength of recommendation: high).
Seven studies (one RCT [31], one prospective cohort [14] and five cross-sectional [32–36]) and a meta-analysis [37] support a positive association between alcohol consumption and reflux-like symptoms. No interventional studies evaluating alcohol abstinence or reduction were identified. The meta-analysis by Pan et al. [37] found a linear association between alcohol consumption and gastro-oesophageal reflux disease (GORD; diagnosed by the presence of oesophagitis or symptoms), with erosive reflux disease having a stronger association compared with nonerosive reflux disease (NERD).
Statement 3: Individuals who experience reflux-like symptoms after consuming coffee should be advised to limit their intake of coffee. Agreement: 100% (6, 72.7%; 5, 27.3%; grade of evidence: C; strength of recommendation: moderate).
Five studies (two prospective cohort [14,38], two randomised crossover studies in coffee-sensitive individuals [39,40] and one cross-sectional study [41]) support a positive association between coffee consumption and reflux-like symptoms. However, a recent systematic review shows that the data are particularly conflicting, with multiple studies finding no increased risk of reflux-like symptoms in coffee drinkers [30]. We did not identify any interventional studies assessing the effect of reduced coffee intake on symptoms. However, substitution analysis in the cohort study conducted by Mehta et al. [38] showed that replacing two servings of coffee with water would reduce the risk of reflux-like symptoms [hazard ratio (HR), 0.96; 95% confidence interval (CI), 0.92–1.00] and a small, randomised study demonstrated significant symptom reduction and antacid use when coffee-sensitive individuals switched to dewaxed coffee (containing less caffeine and chlorogenic acids) [39].
Statement 4: Individuals who experience reflux-like symptoms after consuming carbonated beverages should be advised to limit their intake of carbonated beverages. Agreement: 100% (6, 72.7%; 5, 27.2%; grade of evidence: D; strength of recommendation: moderate).
Eight studies (two prospective cohort [38,42], one case-control [16], and five cross-sectional [22,24,33,41,43]) suggested that carbonated drinks are associated with an increase in reflux-like symptoms. The prospective cohort study conducted by Mehta et al. [38] reported an increased risk of reflux-like symptoms (HR, 1.29; 95% CI, 1.05–1.58; P < 0.0001) for those with the highest intake of soda (more than 6 servings/day) compared with the lowest intake (0 servings/day). Substitution analysis showed a reduced risk (HR, 0.92; 95% CI, 0.89–0.96) when 2 servings of soda were replaced with water. Two of the studies (one prospective cohort [42] and one cross-sectional [43]) found an association between carbonated beverage consumption and nocturnal reflux-like symptoms [42,43].
Statement 5: A low-fat diet should be recommended to individuals with reflux-like symptoms. Agreement: 90.9% (6, 63.6%; 5, 27.3%; grade of evidence: D; strength of recommendation: moderate).
Eight studies (one prospective cohort [14], one case-control [16] and six cross-sectional [22,24,25,43–45]) suggested that high dietary fat intake is associated with reflux-like symptoms and increased nocturnal reflux-like symptom severity. Three cross-sectional studies indicated that high saturated fat is an important risk factor [22,25,44], while a further cross-sectional study in an Albanian population [45], found that a predominantly non-Mediterranean diet (processed foods high in sugar, salt and saturated fats) was positively related to reflux-like symptoms versus a Mediterranean diet (frequent consumption of fresh fruit and vegetables, olive oil and fish) (adjusted OR, 2.3; 95% CI, 1.2–4.5). No interventional study assessing reflux-like symptoms after switching from a high-fat to a low-fat diet was identified.
Statement 6: Individuals with postprandial reflux-like symptoms should be advised not to overeat. Agreement: 100% (6, 81.8%; 5, 18.2%; grade of evidence: C; strength of recommendation: high).
One prospective cohort study supported overeating as a risk factor for reflux-like symptoms [14]. Multivariate regression analysis revealed that the habit of eating beyond fullness (continuing to eat beyond a sensation of fullness until unable to eat anymore) was a risk factor for reflux-like symptoms (OR, 2.85; 95% CI, 2.18–3.73). Symptomatic patients were defined as those with a reflux disease questionnaire score ≥12 and either a positive oesophageal endoscopy or a positive PPI test (>80% response to 2 weeks of PPI) suggesting acid-related aetiology was likely.
Statement 7: Moderate, regular exercise should be recommended to overweight and obese individuals with reflux-like symptoms. Agreement: 90.9% (6, 72.7%; 5, 18.2%; grade of evidence: D; strength of recommendation: high).
One case-control study [17] and five cross-sectional studies [19,21,46–48] provided evidence that lack of physical activity is a risk factor for reflux-like symptoms. Nilsson et al. [17] showed a linear association between increasing frequency of physical exercise sessions (e.g. running or swimming for at least 30 min) and reduced reflux-like symptoms (P value for linear trend 0.0001). Djärv et al. [48] suggested that physical exercise once a week for at least 30 min can significantly reduce the risk of reflux-like symptoms, while Eslami et al. [21] suggested that regular physical exercise for more than 2 h per week will reduce the risk of symptoms.
Lifestyle and behaviour advice
Statement 8: Individuals who experience reflux-like symptoms should be advised to avoid wearing items that are tight around their waist. Agreement: 90.9% (6, 18.2%; 5, 72.7%; grade of evidence: C; strength of recommendation: low).
One prospective cohort study found ‘wearing tight girdles or corsets’ to be among the most robust risk factors for reflux-like symptoms on multivariate analysis (OR 2.19; 95% CI: 1.42–3.38) [14].
Statement 9: Smoking cessation should be recommended to individuals with reflux-like symptoms who smoke tobacco products. Agreement: 100% (6, 100%; grade of evidence: D; strength of recommendation: high).
Seventeen studies (one prospective cohort [14], one case-control [17] and 15 cross-sectional studies [19,23,26,32,33,35,43,46,47,49–54]) and a meta-analysis [55] provided evidence that smoking tobacco products is a risk factor for reflux-like symptoms. A further population-based cohort study [56] found that cessation of daily tobacco smoking was associated with improvement in reflux-like symptoms among individuals within the normal range of BMI (OR, 5.67; 95% CI, 1.36–23.64), but not among overweight individuals.
Statement 10: Weight loss should be recommended to individuals with reflux-like symptoms who are overweight, obese or have recently gained weight. Agreement: 100% (6, 100%; grade of evidence: C; strength of recommendation: high).
Seven studies (one nonrandomised controlled trial [57], five cohort studies [58–62] and a case-control study [63]) demonstrated that weight loss has benefits for controlling reflux-like symptoms and discontinuation of PPI. The population-based nord-trøndelag health study study demonstrated a dose-dependent association between weight loss and reduced reflux-like symptoms (P value for trend P < 0.001), with the odds of symptom loss increasing four-fold (OR, 3.95; 95% CI, 2.03–7.65) in participants on medication who achieved a decrease in BMI of >3.5 units versus <0.5 units [59].
Statement 11: Head-of-the-bed elevation should be recommended to individuals with night-time reflux-like symptoms. Agreement: 100% (6, 72.7%; 5, 27.2%; grade of evidence: C; strength of recommendation: high).
Five systematic reviews [64–68] and five studies (two randomised crossover [69,70] two before-after [71,72] and one cross-sectional [46]) concluded that head-of-the-bed elevation may reduce oesophageal acid exposure and reflux-like symptoms. Methods used to raise the head-of-the-bed included wooden blocks (20 cm) [69,71], a 20 cm height/20-degree foam wedge pillow [70] and a positional device [72] (elevating the head/torso to 15 to 20 degrees together with maintaining left-side positioning).
Statement 12: Sleeping in the left lateral decubitus position should be recommended to individuals with night-time reflux-like symptoms. Agreement: 81.8% (6, 27.2%; 5, 54.6%; grade of evidence: B; strength of recommendation: moderate).
A systematic review [65] and two studies (one randomised sham-controlled [73] and one before-after study [72]) concluded that left lateral decubitus positioning has benefits for reducing nocturnal gastro-oesophageal reflux symptoms. Further studies have demonstrated significantly shorter acid exposure and clearance times in the left lateral versus right lateral and supine positions [74,75].
Statement 13: Avoiding recumbence after meals and leaving a 3-hour interval between an evening meal and going to bed should be recommended to individuals with postprandial and/or night-time reflux-like symptoms. Agreement: 100% (6, 81.8%; 5, 18.2%; grade of evidence: B; strength of recommendation: Moderate)
Three studies (one randomised crossover [76], one case-control [77] and one cross-sectional [46]) supported this statement. The randomised crossover study [76] demonstrated significantly greater supine acid reflux following a standardised meal consumed late in the evening (2 h before bed) compared with earlier in the evening (6 h before bed), especially in overweight patients, although no significant difference in symptom score was detected. The matched case-control study found that the odds of reflux-like symptoms increased seven-fold in patients whose dinner-to-bed time was less than 3 h versus an interval of 4 h or more (OR, 7.45; 95% CI, 3.38–16.4) [77], and the cross-sectional study showed that dinner within 2 h of going to bed was significantly associated with reflux symptoms (adjusted OR, 6.98; 95% CI, 5.36–9.08) [46].
Other interventions to target reflux-like symptom pathophysiology
The initial focus of the consensus procedure was to evaluate interventions for reflux-like symptoms beyond acid reduction/neutralisation strategies. However, an antacid statement was included at the second round of voting to acknowledge its role in the self-management of symptoms.
Statement 14: Diaphragmatic breathing training may reduce reflux-like symptoms. Agreement: 81.8% (6, 27.2%; 5, 54.6%; grade of evidence: B; strength of recommendation: moderate).
A systematic review [78] and five studies (three randomised controlled pilot studies [79–81], one nonrandomised controlled study [82] and one case-control study [83]) provided evidence that diaphragmatic breathing has benefits for patients with reflux-like symptoms, including symptom reduction [79,81,82], increased lower oesophageal sphincter pressure/crural diaphragm tension [81,83], reduced reflux/acid reflux [79,80], improved quality of life [79,82,83] and reduced use of acid suppression medication [79,81].
Statement 15: Stress management strategies should be recommended to individuals with reflux-like symptoms and stress. Agreement: 100% (6, 54.6%; 5, 45.4%; grade of evidence: D; strength of recommendation: low).
Only one nonrandomised controlled study [84] was identified assessing the effectiveness of mindfulness-based stress reduction in PPI-treated patients with reflux-like symptoms reporting symptoms of depression or distress. Self-help mindfulness training significantly improved symptoms, overall health-related quality of life, and reduced levels of depression after 3 months compared with PPI alone (all P < 0.001).
Statement 16: Brain-gut behavioural therapies (e.g. cognitive behavioural therapy or gut-directed hypnotherapy) should be recommended to individuals with reflux-like symptoms and signs of oesophageal hypervigilance and/or psychological distress. Agreement: 90.9% (6, 72.7%; 5, 18.2%; grade of evidence: D; strength of recommendation: low).
Two before-after studies [85,86] have assessed brain-gut behavioural therapies in patients with upper gastrointestinal GI symptoms (functional heartburn [86] and supragastric belching [85]). The study in patients with functional heartburn found that weekly oesophageal-directed hypnotherapy sessions for 7 weeks were associated with a significant decrease in visceral anxiety and symptom severity, a significant increase in emotional quality of life, and a trend for reduced heartburn catastrophizing [86]. The study in patients with supragastric belching found that cognitive behavioural therapy (CBT; 4 sessions, as described previously [87]) showed significant reductions in acid exposure time and symptoms [85], which was maintained for 6–12 months after the completion of CBT [88].
Statement 17: Disordered sleep is associated with an increased likelihood of having reflux-like symptoms. Agreement: 72.7% (6, 45.5%; 5, 27.3%; grade of evidence: C; strength of recommendation: low).
Two cross-sectional studies indicated an association between poor sleep quality and reflux-like symptoms [52,89]. Three interventional studies (two small randomised controlled crossover studies [90,91] and one before-after study [92]) indicated that sleep deprivation may be hyperalgesic by demonstrating greater acid exposure and increased acid perfusion sensitivity score on the day following a night of disturbed sleep [90–92].
Statement 18: Alginate-antacid combinations are an effective treatment for reflux-like symptoms. Agreement: 100% (6, 90.9%; 5, 9.1%; grade of evidence: A; strength of recommendation: high).
Alginates form a buoyant precipitate on top of gastric contents creating a physical barrier to reflux. Seven RCTs have demonstrated symptomatic relief with alginate formulations versus placebo [93–100] and antacid alone [93,95,101], and one RCT suggested that alginate-antacids reduce night-time symptoms in PPI-treated patients [98]. Three meta-analyses of RCTs confirmed alginates are more effective than placebo (relative benefit increase up to 60% versus placebo [102]) or antacid alone for treating reflux-like symptoms [102–104] and three studies suggested noninferiority to omeprazole [94,105,106].
Statement 19: Antacids are an effective treatment for reflux-like symptoms. Agreement: 90.9% (6, 45.5%; 5, 45.5%; grade of evidence: A; strength of recommendation: moderate).
Most antacid research was conducted before the 2002 cut-off point for the current literature search. A meta-analysis of these RCTs reported an 11% relative benefit increase compared with placebo [102]. Two randomised, controlled, crossover studies conducted more recently demonstrated that a calcium carbonate gum [107] and hydrotalcite (aluminium hydroxide, magnesium hydroxide and carbonate) chewable tablets [108] effectively reduce heartburn.
Statement 20: Antacids and alginate-antacid combinations are an effective treatment option for reflux-like symptoms during pregnancy. Agreement 90.9% (6, 45.5%; 5, 45.5%; grade of evidence: B; strength of recommendation: high).
Antacids and alginate-antacids are considered safe in pregnant and breastfeeding women as they are not systemically absorbed and have a long history of clinical use [109], although formulations with sodium bicarbonate as the main active ingredient are generally avoided given the potential for metabolic alkalosis [110]. Three studies (one RCT [111] and two-phase IV studies [112,113]) reported reduced frequency and intensity of heartburn in pregnant women, without any treatment-related adverse events or changes in serum sodium.
Statement 21: Products containing hyaluronic acid and chondroitin sulphate are an effective treatment option for reflux-like symptoms. Agreement: 81.8% (6, 82%; 5, 18%; grade of evidence: B; strength of recommendation: low).
Bioadhesive formulations target reflux-like symptoms by directly protecting the oesophageal mucosa. One RCT [114] two pilot RCTs [115,116] and an exploratory postmarketing study [117] have demonstrated symptom reduction with several different formulations in patients with reflux-like symptoms. Two formulations were assessed in RCTs, one consisted of hyaluronic acid, amino acids and rice extract [115] and the other, hyaluronic-chondroitin sulphate [114,116]. Both demonstrated reflux-like symptom reduction versus placebo in NERD patients.
A statement regarding natural remedies [some natural remedies (aloe vera, amla or apple cider vinegar) may have benefits for reflux-like symptoms] failed to reach consensus after the second round of voting, with only 36.4% voting agreement at level 5 or 6 (Fig. 4). The supporting evidence from three RCTs [118–120] was considered weak, and the expert group was not able to share any clinical experience of efficacy for such products.
Discussion
GORD and dyspepsia management guidelines, such as the recently updated American College of Gastroenterology guidelines [121], generally include lifestyle recommendations as a first approach for symptomatic patients, but they are not always implemented in practice. This Delphi-style process builds on the published recommendations by providing greater analysis of the published evidence combined with clinical experience, with the goal of developing education for providers and patients. Consensus was achieved for 21 statements according to the prespecified criteria (67% voting either ‘strongly agree’ or ‘agree with minor reservation’). Eleven statements achieved the strongest (100%) agreement; five are related to diet and include identification and avoidance of dietary triggers, limiting alcohol, coffee and carbonated beverages, and advising patients troubled by postprandial symptoms not to overeat. The remaining six statements with 100% consensus concern advice around smoking cessation, weight loss, raising the head-of-the-bed, avoiding recumbency after meals, stress reduction and alginate use.
The wording of the consensus statements regarding food and beverage advice reflects the fact that dietary risk factors are highly individualised, which is often not emphasised in guidelines and patient brochures. Even for a given individual, the ability of dietary factors to trigger symptoms may depend on other factors, such as sleep disturbance or stress, which have the potential to increase symptom perception. A statement on tea drinking was not included based on the findings of a meta-analysis suggesting no overall relationship with GORD [122]. However, the amount and type of tea drunk worldwide is highly variable and stratification by geographical region suggests that it may be a more important risk factor in Eastern Asia [29,122].
Advising patients not to overeat was given 100% consensus, even though the evidence is sparse [14] and no interventional study to assess smaller meal sizes was identified. Rather, the confidence in this statement is based on clinical experience and the known pathophysiology of gastro-oesophageal reflux, whereby the gastric distention associated with a large meal stimulates acid secretion, triggers transient lower oesophageal sphincter relaxations, and makes reflux from the proximal postprandial acid pocket more likely. The efficacy of alginate-antacid combinations for reflux-like symptoms also gained 100% consensus. Alginate formulations are especially suited as a treatment for postprandial symptoms owing to their mode of action which physically displaces the acid pocket further from the oesophagus while the meal empties from the stomach [123].
Despite recommendations in guidelines, evidence suggests that even simple dietary and lifestyle advice is not communicated in real-world practice. In physician surveys, 17.5% [9] to 51% [10] claim to recommend lifestyle modifications, while in a survey of patients in the UK, only 28% reported being advised in primary care to elevate the head of bed and/or avoid food and drink before going to bed [124]. A retrospective chart review in the USA indicated lifestyle advice counselling, including smoking and alcohol cessation, dietary changes, head-of-the-bed elevation and postprandial avoidance of recumbency, was provided to just 12% of patients [7]. This lack of doctor-patient dialogue regarding self-management of the potential causes of reflux-like symptoms may contribute to the well-recognised problem of PPI overuse [125]. Thus, while some of the consensus statements may seem intuitive, the hope is that consolidation of this advice will help facilitate a shift in management to one that is more patient-centred, with routine counselling to help patients make associations between their symptoms and lifestyle/behaviours. One reason for the lack of advice may be the perceived difficulty in effecting a long-term change in patients’ habits and behaviours. In a survey of general practitioners, half believed that only 10% or less of patients would be prepared to make lifestyle modifications [9]. During focus group sessions of patients with obesity and GORD enrolled in a personalised health education and goal-setting programme(The Reflux Improvement and Monitoring Programme; TRIM [62]), it was revealed that many patients did not independently recognise the link between weight and reflux-like symptoms before starting the programme. TRIM was associated with sustained loss of excess body weight, and improvements in patient-reported symptom severity and quality of life. Thus, better-informed patients may be more willing to accept referrals and engage with services for interventions such as weight loss, smoking cessation or increasing exercise. This will help improve overall health and wellbeing, while also supporting appropriate PPI prescribing and de-escalation practices [125].
Statements assigned a low strength of recommendation emphasize areas where further study is warranted. These include the link between sleep disturbance and reflux-like symptom perception, wearing tight clothing or items around the waist, mucosal protectants and behavioural interventions (e.g. stress reduction, CBT). The use of bioadhesive agents aimed at potentiating the defensive properties of the oesophageal mucosa has gained traction in recent years owing to evidence of altered mucosal integrity in symptomatic NERD patients. Recent research with alginates suggests that they may owe their efficacy, in part, to their bioadhesive properties and a direct protective effect on the oesophageal mucosa. Newer compounds containing hyaluronic acid with chondroitin sulphate have shown promising results in RCTs, and further clinical experience and large-scale clinical trials with these agents will increase the strength of recommendation. While the evidence base for gut-directed hypnotherapy and CBT for disorders of gut-brain interaction is growing, further assessment is required for these interventions when directed toward the oesophagus. However, a rate-limiting step for these therapies is patient access to psychologists/trained providers or through virtual platforms. Acupuncture also has reported efficacy for the treatment of reflux-like symptoms and disorders of gut-brain interactions, but the current clinical experience and quality of evidence for this intervention were not considered sufficient for its inclusion in the statements of advice [126,127].
Unlike other chronic conditions with a lifestyle or behavioural element, there are generally no standardised programs in place to help patients with reflux-like symptoms, and it can be challenging to counsel patients within the context of brief appointments in primary care. However, pilot programs of dietitian- and nurse-led initiatives for patients with reflux-like symptoms have reported high levels of patient satisfaction, with patients appreciating the opportunity to learn about their condition and strategies for self-management, as well as reducing referrals and facilitating PPI deprescribing [128–131].
The current work has limitations. The number of experts in the consensus panel was kept relatively small to allow every participant to be involved in productive discussions during the two meetings, and, although seven different countries were represented, all were from North America and Europe. A systematic review/meta-analysis was not conducted owing to the heterogeneity of the limited evidence. The targeted literature searches were conducted using a single database (PubMed) and a detailed screen of abstracts for study inclusion was conducted by a single medical writer.
The aim of developing the consensus statements is that they may serve as a foundation for standardised patient tools and advice that can routinely help patients with reflux-like symptoms better understand and manage their condition. While there has been no patient involvement to validate the advice to date, further work on the acceptability and pragmatic utility of these measures, particularly beyond the short term, is planned.
Acknowledgements
Editorial assistance was provided by Lisa O’Rourke PhD of Lumanity, UK, and funded by Reckitt Benckiser Healthcare Ltd.
Peter J. Kahrilas is the guarantor of the article.
All authors meet the ICMJE authorship criteria. All authors approved the final version of the article, including the authorship list. P.J.K., P.H. and R.Y. drafted the article. All authors critically reviewed and revised the article.
Data sharing is not applicable – no new data is generated.
Conflicts of interest
All authors attended the online meetings funded by Reckitt Benckiser Healthcare Ltd. A.P.H. has served as a consultant for Sandoz and Tillotts, and Chair of Rome IV Primary Care Committee. R.Y. has served as a consultant for Medtronic, Phathom Pharmaceuticals, StatLinkMD, and Medscape, has received research support from Ironwood Pharmaceuticals and is on the advisory board with stock options for RJS Mediagnostix. F.A. is a member of the Programme for NASH in Primary Health Care at the University of Crete, funded by Gilead. A.B. has received research funding from Nutricia, Norgine, Dr Falk Pharma, Thelial, and SST and received speaker and/or consulting fees from Laborie, Medtronic, Dr Falk Pharma, Reckitt, Alimentiv, Sanofi/Regeneron and AstraZeneca. H.E. has received research funding from Phathom. P.F. has received conference fees from Schwabe Pharma. J.M.M. has participated in educational activities funded by Reckitt Benckiser Healthcare Ltd. E.V.S. has served as speaker for Abbvie, Agave, AGPharma, Alfasigma, Aurora Pharma, CaDiGroup, Celltrion, Dr Falk Pharma, for example, Stada Group, Fenix Pharma, Fresenius Kabi, Galapagos, Janssen, JB Pharmaceuticals, Innovamedica/Adacyte, Malesci, Mayoly Biohealth, Omega Pharma, Pfizer, Reckitt Benckiser, Sandoz, SILA, Sofar, Takeda, Tillots, Unifarco; has served as a consultant for Abbvie, Agave, Alfasigma, Biogen, Bristol-Myers Squibb, Celltrion, Diadema Farmaceutici, Dr Falk Pharma, Fenix Pharma, Fresenius Kabi, Janssen, JB Pharmaceuticals, Merck & Co, Reckitt Benckiser, Regeneron, Sanofi, SILA, Sofar, Synformulas GmbH, Takeda, Unifarco; he received research support from Pfizer, Reckitt Benckiser, SILA, Sofar, Unifarco, Zeta Farmaceutici. D.S. has received research grants from Reckitt Benckiser Healthcare Ltd, Jinshan Technology (China), and Alfa Sigma (Italy). M.U. has served as speaker for Sanofi, Terapia, Servier, Berlin-Chemie, Ipsen, Bayer, Abbvie. P.J.K. has served as a consultant for Ironwood and Reckitt.
Supplementary Material
Footnotes
A. Pali Hungin and Rena Yadlapati shared co-first authorship.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.eurojgh.com.
References
- 1.Delshad SD, Almario CV, Chey WD, Spiegel BMR. Prevalence of gastroesophageal reflux disease and proton pump inhibitor-refractory symptoms. Gastroenterology 2020; 158:1250–1261.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014; 63:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Yadlapati R. Pathophysiology and treatment options for gastroesophageal reflux disease: looking beyond acid. Ann N Y Acad Sci 2021; 1486:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hungin APS, Scarpignato C, Keefer L, Corsetti M, Anastasiou F, Muris JWM, et al. Review article: rethinking the ‘ladder’ approach to reflux-like symptom management in the era of PPI ‘resistance’ - a multidisciplinary perspective. Aliment Pharmacol Ther 2022; 55:1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Savarino E, Anastasiou F, Bredenoord AJ, Corsetti M, Lagergren J, et al. The tapestry of reflux syndromes: translating new insight into clinical practice. Br J Gen Pract 2021; 71:470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther 2010; 32:720–737. [DOI] [PubMed] [Google Scholar]
- 7.Salyers WJ, Jr, Mansour A, El-Haddad B, Golbeck AL, Kallail KJ. Lifestyle modification counseling in patients with gastroesophageal reflux disease. Gastroenterol Nurs 2007; 30:302–304. [DOI] [PubMed] [Google Scholar]
- 8.Kang JH, Kang JY. Lifestyle measures in the management of gastro-oesophageal reflux disease: clinical and pathophysiological considerations. Ther Adv Chronic Dis 2015; 6:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowak M, Büttner P, Raasch B, Daniell K, McCutchan C, Harrison S. Lifestyle changes as a treatment of gastroesophageal reflux disease: a survey of general practitioners in North Queensland, Australia. Ther Clin Risk Manag 2005; 1:219–224. [PMC free article] [PubMed] [Google Scholar]
- 10.Reimer C, Bytzer P. Perceptions and beliefs concerning gastroesophageal reflux disease: physicians and patients disagree. Digestion 2007; 76:229–234. [DOI] [PubMed] [Google Scholar]
- 11.Hanley DA, Cranney A, Jones G, Whiting SJ, Leslie WD; Guidelines Committee of the Scientific Advisory Council of Osteoporosis Canada. Vitamin D in adult health and disease: a review and guideline statement from Osteoporosis Canada (summary). CMAJ 2010; 182:1315–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al.; GRADE Working Group. Grading quality of evidence and strength of recommendations. Bmj 2004; 328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patcharatrakul T, Kriengkirakul C, Chaiwatanarat T, Gonlachanvit S. Acute effects of red chili, a natural capsaicin receptor agonist, on gastric accommodation and upper gastrointestinal symptoms in healthy volunteers and gastroesophageal reflux disease patients. Nutrients 2020; 12:3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan LZ, Yi P, Wang GS, Tan SY, Huang GM, Qi LZ, et al. Lifestyle intervention for gastroesophageal reflux disease: a national multicenter survey of lifestyle factor effects on gastroesophageal reflux disease in China. Therap Adv Gastroenterol 2019; 12:1756284819877788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tosetti C, Savarino E, Benedetto E, De Bastiani R; Study Group for the Evaluation of GERD Triggering Foods. Elimination of dietary triggers is successful in treating symptoms of gastroesophageal reflux disease. Dig Dis Sci 2020; 66:1565–1571. [DOI] [PubMed] [Google Scholar]
- 16.Asl SF, Mansour-Ghanaei F, Samadi H, Joukar F. Evaluations of life style factors and the severity of Gastroesophageal reflux disease; a case-control study. Int J Mol Epidemiol Genet 2015; 6:27–32. [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Lifestyle related risk factors in the aetiology of gastro-oesophageal reflux. Gut 2004; 53:1730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atta MM, Sayed MH, Zayed MA, Alsulami SA, Al-Maghrabi AT, Kelantan AY. Gastro-oesophageal reflux disease symptoms and associated risk factors among medical students, Saudi Arabia. Int J Gen Med 2019; 12:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cela L, Kraja B, Hoti K, Toçi E, Muja H, Roshi E, et al. Lifestyle characteristics and gastroesophageal reflux disease: a population-based study in Albania. Gastroenterol Res Pract 2013; 2013:936792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du J, Liu J, Zhang H, Yu CH, Li YM. Risk factors for gastroesophageal reflux disease, reflux esophagitis and non-erosive reflux disease among Chinese patients undergoing upper gastrointestinal endoscopic examination. World J Gastroenterol 2007; 13:6009–6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eslami O, Shahraki M, Bahari A, Shahraki T. Dietary habits and obesity indices in patients with gastro-esophageal reflux disease: a comparative cross-sectional study. BMC Gastroenterol 2017; 17:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu C, Olszewski T, Vaezi MF, Niswender KD, Silver HJ. Objective ambulatory pH monitoring and subjective symptom assessment of gastroesophageal reflux disease show type of carbohydrate and type of fat matter. Therap Adv Gastroenterol 2022; 15:17562848221101289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kariri AM, Darraj MA, Wassly A, Arishi HA, Lughbi M, Kariri A, et al. Prevalence and risk factors of gastroesophageal reflux disease in Southwestern Saudi Arabia. Cureus 2020; 12:e6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubo A, Block G, Quesenberry CP, Jr, Buffler P, Corley DA. Dietary guideline adherence for gastroesophageal reflux disease. BMC Gastroenterol 2014; 14:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro M, Green C, Bautista JM, Dekel R, Risner-Adler S, Whitacre R, et al. Assessment of dietary nutrients that influence perception of intra-oesophageal acid reflux events in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2007; 25:93–101. [DOI] [PubMed] [Google Scholar]
- 26.Alkhathami AM, Alzahrani AA, Alzhrani MA, Alsuwat OB, Mahfouz MEM. Risk factors for gastroesophageal reflux disease in Saudi Arabia. Gastroenterology Res 2017; 10:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi CH, Lei WY, Hung JS, Liu TT, Orr WC, Chen CL. Sleep disturbance and enhanced esophageal capsaicin sensitivity in patients with gastroesophageal reflux disease. J Gastroenterol Hepatol 2016; 31:1940–1945. [DOI] [PubMed] [Google Scholar]
- 28.Yi CH, Lei WY, Hung JS, Liu TT, Chen CL, Pace F. Influence of capsaicin infusion on secondary peristalsis in patients with gastroesophageal reflux disease. World J Gastroenterol 2016; 22:10045–10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Hou Z-K, Huang Z-B, Chen X-L, Liu F-B. Dietary and lifestyle factors related to gastroesophageal reflux disease: a systematic review. Ther Clin Risk Manag 2021; 17:305–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidarzadeh-Esfahani N, Soleimani D, Hajiahmadi S, Moradi S, Heidarzadeh N, Nachvak SM. Dietary intake in relation to the risk of reflux disease: a systematic review. Prev Nutr Food Sci 2021; 26:367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Leary C, McCarthy J, Humphries M, Shanahan F, Quigley E. The prophylactic use of a proton pump inhibitor before food and alcohol. Aliment Pharmacol Ther 2003; 17:683–686. [DOI] [PubMed] [Google Scholar]
- 32.Gong Y, Zeng Q, Yan Y, Han C, Zheng Y. Association between lifestyle and gastroesophageal reflux disease questionnaire scores: a cross-sectional study of 37 442 Chinese adults. Gastroenterol Res Pract 2019; 2019:5753813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghoshal UC, Singh R, Rai S. Prevalence and risk factors of gastroesophageal reflux disease in a rural Indian population. Indian J Gastroenterol 2021; 40:56–64. [DOI] [PubMed] [Google Scholar]
- 34.Rosaida MS, Goh KL. Gastro-oesophageal reflux disease, reflux oesophagitis and non-erosive reflux disease in a multiracial Asian population: a prospective, endoscopy based study. Eur J Gastroenterol Hepatol 2004; 16:495–501. [DOI] [PubMed] [Google Scholar]
- 35.Yamamichi N, Mochizuki S, Asada-Hirayama I, Mikami-Matsuda R, Shimamoto T, Konno-Shimizu M, et al. Lifestyle factors affecting gastroesophageal reflux disease symptoms: a cross-sectional study of healthy 19864 adults using FSSG scores. BMC Med 2012; 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma A, Sharma PK, Puri P. Prevalence and the risk factors of gastro-esophageal reflux disease in medical students. Med J Armed Forces India 2018; 74:250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan J, Cen L, Chen W, Yu C, Li Y, Shen Z. Alcohol consumption and the risk of gastroesophageal reflux disease: a systematic review and meta-analysis. Alcohol Alcohol 2019; 54:62–69. [DOI] [PubMed] [Google Scholar]
- 38.Mehta RS, Song M, Staller K, Chan AT. Association between beverage intake and incidence of gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol 2020; 18:2226–2233.e4. [DOI] [PubMed] [Google Scholar]
- 39.Polese B, Izzo L, Mancino N, Pesce M, Rurgo S, Tricarico MC, et al. Effect of dewaxed coffee on gastroesophageal symptoms in patients with GERD: a randomized pilot study. Nutrients 2022; 14:2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiBaise JK. A randomized, double-blind comparison of two different coffee-roasting processes on development of heartburn and dyspepsia in coffee-sensitive individuals. Dig Dis Sci 2003; 48:652–656. [DOI] [PubMed] [Google Scholar]
- 41.Arivan R, Deepanjali S. Prevalence and risk factors of gastro-esophageal reflux disease among undergraduate medical students from a southern Indian medical school: a cross-sectional study. BMC Res Notes 2018; 11:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fass R, Quan SF, O’Connor GT, Ervin A, Iber C. Predictors of heartburn during sleep in a large prospective cohort study. Chest 2005; 127:1658–1666. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Hou XH, Zou XP, Li RZ, Wang CD, Sun J, et al. Survey of nocturnal reflux in patients with gastroesophageal reflux disease in China. J Dig Dis 2019; 20:589–595. [DOI] [PubMed] [Google Scholar]
- 44.El-Serag HB, Satia JA, Rabeneck L. Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut 2005; 54:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mone I, Kraja B, Bregu A, Duraj V, Sadiku E, Hyska J, et al. Adherence to a predominantly Mediterranean diet decreases the risk of gastroesophageal reflux disease: a cross-sectional study in a South Eastern European population. Dis Esophagus 2016; 29:794–800. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed S, Jamil S, Shaikh H, Abbasi M. Effects of Life style factors on the symptoms of gastro esophageal reflux disease: a cross sectional study in a Pakistani population. Pak J Med Sci 2020; 36:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murao T, Sakurai K, Mihara S, Marubayashi T, Murakami Y, Sasaki Y. Lifestyle change influences on GERD in Japan: a study of participants in a health examination program. Dig Dis Sci 2011; 56:2857–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Djärv T, Wikman A, Nordenstedt H, Johar A, Lagergren J, Lagergren P. Physical activity, obesity and gastroesophageal reflux disease in the general population. World J Gastroenterol 2012; 18:3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi JM, Yang JI, Kang SJ, Han YM, Lee J, Lee C, et al. Association between anxiety and depression and gastroesophageal reflux disease: results from a large cross-sectional study. J Neurogastroenterol Motil 2018; 24:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedenberg FK, Rai J, Vanar V, Bongiorno C, Nelson DB, Parepally M, et al. Prevalence and risk factors for gastroesophageal reflux disease in an impoverished minority population. Obes Res Clin Pract 2010; 4:e247–e342. [DOI] [PubMed] [Google Scholar]
- 51.Kulig M, Nocon M, Vieth M, Leodolter A, Jaspersen D, Labenz J, et al. Risk factors of gastroesophageal reflux disease: methodology and first epidemiological results of the ProGERD study. J Clin Epidemiol 2004; 57:580–589. [DOI] [PubMed] [Google Scholar]
- 52.Matsuki N, Fujita T, Watanabe N, Sugahara A, Watanabe A, Ishida T, et al. Lifestyle factors associated with gastroesophageal reflux disease in the Japanese population. J Gastroenterol 2013; 48:340–349. [DOI] [PubMed] [Google Scholar]
- 53.Sharma PK, Ahuja V, Madan K, Gupta S, Raizada A, Sharma MP. Prevalence, severity, and risk factors of symptomatic gastroesophageal reflux disease among employees of a large hospital in northern India. Indian J Gastroenterol 2011; 30:128–134. [DOI] [PubMed] [Google Scholar]
- 54.Shimamoto T, Yamamichi N, Kodashima S, Takahashi Y, Fujishiro M, Oka M, et al. No association of coffee consumption with gastric ulcer, duodenal ulcer, reflux esophagitis, and non-erosive reflux disease: a cross-sectional study of 8,013 healthy subjects in Japan. PLoS One 2013; 8:e65996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut 2018; 67:430–440. [DOI] [PubMed] [Google Scholar]
- 56.Ness-Jensen E, Lindam A, Lagergren J, Hveem K. Tobacco smoking cessation and improved gastroesophageal reflux: a prospective population-based cohort study: the HUNT study. Am J Gastroenterol 2014; 109:171–177. [DOI] [PubMed] [Google Scholar]
- 57.de Bortoli N, Guidi G, Martinucci I, Savarino E, Imam H, Bertani L, et al. Voluntary and controlled weight loss can reduce symptoms and proton pump inhibitor use and dosage in patients with gastroesophageal reflux disease: a comparative study. Dis Esophagus 2016; 29:197–204. [DOI] [PubMed] [Google Scholar]
- 58.Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA, Jr. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006; 354:2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ness-Jensen E, Lindam A, Lagergren J, Hveem K. Weight loss and reduction in gastroesophageal reflux A prospective population-based cohort study: the HUNT study. Am J Gastroenterol 2013; 108:376–382. [DOI] [PubMed] [Google Scholar]
- 60.Park SK, Lee T, Yang HJ, Park JH, Sohn CI, Ryu S, et al. Weight loss and waist reduction is associated with improvement in gastroesophageal disease reflux symptoms: a longitudinal study of 15 295 subjects undergoing health checkups. Neurogastroenterol Motil 2017; 29:e13009. [DOI] [PubMed] [Google Scholar]
- 61.Singh M, Lee J, Gupta N, Gaddam S, Smith BK, Wani SB, et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: a prospective intervention trial. Obesity (Silver Spring) 2013; 21:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yadlapati R, Pandolfino JE, Alexeeva O, Gregory DL, Craven MR, Liebovitz D, et al. The reflux improvement and monitoring (TRIM) program is associated with symptom improvement and weight reduction for patients with obesity and gastroesophageal reflux disease. Am J Gastroenterol 2018; 113:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA 2003; 290:66–72. [DOI] [PubMed] [Google Scholar]
- 64.Albarqouni L, Moynihan R, Clark J, Scott AM, Duggan A, Del Mar C. Head of bed elevation to relieve gastroesophageal reflux symptoms: a systematic review. BMC Fam Pract 2021; 22:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schuitenmaker JM, Kuipers T, Smout A, Fockens P, Bredenoord AJ. Systematic review: clinical effectiveness of interventions for the treatment of nocturnal gastroesophageal reflux. Neurogastroenterol Motil 2022; 34:e14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerson LB, Fass R. A systematic review of the definitions, prevalence, and response to treatment of nocturnal gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2009; 7:372–8; quiz 367. [DOI] [PubMed] [Google Scholar]
- 67.Ness-Jensen E, Hveem K, El-Serag H, Lagergren J. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2016; 14:175–82.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971. [DOI] [PubMed] [Google Scholar]
- 69.Villamil Morales IM, Gallego Ospina DM, Otero Regino WA. Impact of head of bed elevation in symptoms of patients with gastroesophageal reflux disease: a randomized single-blind study (IBELGA). Gastroenterol Hepatol 2020; 43:310–321. [DOI] [PubMed] [Google Scholar]
- 70.Huang HC, Chang YJ, Tseng YL, Fang SY. Effect of head-of-bed elevation on nocturnal reflux symptoms of esophageal cancer patients with esophagectomy and reconstruction. Cancer Nurs 2021; 44:244–250. [DOI] [PubMed] [Google Scholar]
- 71.Khan BA, Sodhi JS, Zargar SA, Javid G, Yattoo GN, Shah A, et al. Effect of bed head elevation during sleep in symptomatic patients of nocturnal gastroesophageal reflux. J Gastroenterol Hepatol 2012; 27:1078–1082. [DOI] [PubMed] [Google Scholar]
- 72.Allampati S, Lopez R, Thota PN, Ray M, Birgisson S, Gabbard SL. Use of a positional therapy device significantly improves nocturnal gastroesophageal reflux symptoms. Dis Esophagus 2017; 30:1–7. [DOI] [PubMed] [Google Scholar]
- 73.Schuitenmaker JM, Kuipers T, Oude Nijhuis RAB, Schijven MP, Smout A, Fockens P, et al. Sleep positional therapy for nocturnal gastroesophageal reflux: a double-blind, randomized, sham-controlled trial. Clin Gastroenterol Hepatol 2022; 20:2753–62.e2. [DOI] [PubMed] [Google Scholar]
- 74.Schuitenmaker JM, van Dijk M, Oude Nijhuis RAB, Smout A, Bredenoord AJ. Associations between sleep position and nocturnal gastroesophageal reflux: a study using concurrent monitoring of sleep position and esophageal ph and impedance. Am J Gastroenterol 2022; 117:346–351. [DOI] [PubMed] [Google Scholar]
- 75.Person E, Rife C, Freeman J, Clark A, Castell DO. A novel sleep positioning device reduces gastroesophageal reflux: a randomized controlled trial. J Clin Gastroenterol 2015; 49:655–659. [DOI] [PubMed] [Google Scholar]
- 76.Piesman M, Hwang I, Maydonovitch C, Wong RK. Nocturnal reflux episodes following the administration of a standardized meal. Does timing matter? Am J Gastroenterol 2007; 102:2128–2134. [DOI] [PubMed] [Google Scholar]
- 77.Fujiwara Y, Machida A, Watanabe Y, Shiba M, Tominaga K, Watanabe T, et al. Association between dinner-to-bed time and gastro-esophageal reflux disease. Am J Gastroenterol 2005; 100:2633–2636. [DOI] [PubMed] [Google Scholar]
- 78.Casale M, Sabatino L, Moffa A, Capuano F, Luccarelli V, Vitali M, et al. Breathing training on lower esophageal sphincter as a complementary treatment of gastroesophageal reflux disease (GERD): a systematic review. Eur Rev Med Pharmacol Sci 2016; 20:4547–4552. [PubMed] [Google Scholar]
- 79.Eherer AJ, Netolitzky F, Högenauer C, Puschnig G, Hinterleitner TA, Scheidl S, et al. Positive effect of abdominal breathing exercise on gastroesophageal reflux disease: a randomized, controlled study. Am J Gastroenterol 2012; 107:372–378. [DOI] [PubMed] [Google Scholar]
- 80.Halland M, Bharucha AE, Crowell MD, Ravi K, Katzka DA. Effects of diaphragmatic breathing on the pathophysiology and treatment of upright gastroesophageal reflux: a randomized controlled trial. Am J Gastroenterol 2021; 116:86–94. [DOI] [PubMed] [Google Scholar]
- 81.Sun X, Shang W, Wang Z, Liu X, Fang X, Ke M. Short-term and long-term effect of diaphragm biofeedback training in gastroesophageal reflux disease: an open-label, pilot, randomized trial. Dis Esophagus 2016; 29:829–836. [DOI] [PubMed] [Google Scholar]
- 82.Ong AM, Chua LT, Khor CJ, Asokkumar R, V SON, Wang YT. Diaphragmatic breathing reduces belching and proton pump inhibitor refractory gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol 2018; 16:407–16.e2. [DOI] [PubMed] [Google Scholar]
- 83.Ahmadi M, Amiri M, Rezaeian T, Abdollahi I, Rezadoost AM, Sohrabi M, et al. Different effects of aerobic exercise and diaphragmatic breathing on lower esophageal sphincter pressure and quality of life in patients with reflux: a comparative study. Middle East J Dig Dis 2021; 13:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chandran S, Raman R, Kishor M, Nandeesh HP. The effectiveness of mindfulness meditation in relief of symptoms of depression and quality of life in patients with gastroesophageal reflux disease. Indian J Gastroenterol 2019; 38:29–38. [DOI] [PubMed] [Google Scholar]
- 85.Glasinovic E, Wynter E, Arguero J, Ooi J, Nakagawa K, Yazaki E, et al. Treatment of supragastric belching with cognitive behavioral therapy improves quality of life and reduces acid gastroesophageal reflux. Am J Gastroenterol 2018; 113:539–547. [DOI] [PubMed] [Google Scholar]
- 86.Riehl ME, Pandolfino JE, Palsson OS, Keefer L. Feasibility and acceptability of esophageal-directed hypnotherapy for functional heartburn. Dis Esophagus 2016; 29:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Katzka DA. Simple office-based behavioral approach to patients with chronic belching. Dis Esophagus 2013; 26:570–573. [DOI] [PubMed] [Google Scholar]
- 88.Sawada A, Anastasi N, Green A, Glasinovic E, Wynter E, Albusoda A, et al. Management of supragastric belching with cognitive behavioural therapy: factors determining success and follow-up outcomes at 6-12 months post-therapy. Aliment Pharmacol Ther 2019; 50:530–537. [DOI] [PubMed] [Google Scholar]
- 89.Ju G, Yoon IY, Lee SD, Kim N. Relationships between sleep disturbances and gastroesophageal reflux disease in Asian sleep clinic referrals. J Psychosom Res 2013; 75:551–555. [DOI] [PubMed] [Google Scholar]
- 90.Schey R, Dickman R, Parthasarathy S, Quan SF, Wendel C, Merchant J, et al. Sleep deprivation is hyperalgesic in patients with gastroesophageal reflux disease. Gastroenterology 2007; 133:1787–1795. [DOI] [PubMed] [Google Scholar]
- 91.Yamasaki T, Quan SF, Fass R. The effect of sleep deficiency on esophageal acid exposure of healthy controls and patients with gastroesophageal reflux disease. Neurogastroenterol Motil 2019; 31:e13705. [DOI] [PubMed] [Google Scholar]
- 92.Dickman R, Green C, Fass SS, Quan SF, Dekel R, Risner-Adler S, et al. Relationships between sleep quality and pH monitoring findings in persons with gastroesophageal reflux disease. J Clin Sleep Med 2007; 3:505–513. [PMC free article] [PubMed] [Google Scholar]
- 93.Deraman MA, Abdul Hafidz MI, Lawenko RM, Ma ZF, Wong MS, Coyle C, et al. Randomised clinical trial: the effectiveness of Gaviscon Advance vs non-alginate antacid in suppression of acid pocket and post-prandial reflux in obese individuals after late-night supper. Aliment Pharmacol Ther 2020; 51:1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim JH, Lee YC, Kim EH, Park JC, Shin SK, Lee SK, et al. The clinical efficacy of a pure alginate formulation (Lamina G) for controlling symptoms in individuals with reflux symptoms: a randomized clinical study. Gut Liver 2019; 13:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giannini EG, Zentilin P, Dulbecco P, Iiritano E, Bilardi C, Savarino E, et al. A comparison between sodium alginate and magaldrate anhydrous in the treatment of patients with gastroesophageal reflux symptoms. Dig Dis Sci 2006; 51:1904–1909. [DOI] [PubMed] [Google Scholar]
- 96.Thomas E, Wade A, Crawford G, Jenner B, Levinson N, Wilkinson J. Randomised clinical trial: relief of upper gastrointestinal symptoms by an acid pocket-targeting alginate-antacid (Gaviscon Double Action) - a double-blind, placebo-controlled, pilot study in gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2014; 39:595–602. [DOI] [PubMed] [Google Scholar]
- 97.Wilkinson J, Wade A, Thomas SJ, Jenner B, Hodgkinson V, Coyle C. Randomized clinical trial: a double-blind, placebo-controlled study to assess the clinical efficacy and safety of alginate-antacid (Gaviscon Double Action) chewable tablets in patients with gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol 2019; 31:86–93. [DOI] [PubMed] [Google Scholar]
- 98.Reimer C, Lødrup AB, Smith G, Wilkinson J, Bytzer P. Randomised clinical trial: alginate (Gaviscon Advance) vs placebo as add-on therapy in reflux patients with inadequate response to a once daily proton pump inhibitor. Aliment Pharmacol Ther 2016; 43:899–909. [DOI] [PubMed] [Google Scholar]
- 99.Manabe N, Haruma K, Ito M, Takahashi N, Takasugi H, Wada Y, et al. Efficacy of adding sodium alginate to omeprazole in patients with nonerosive reflux disease: a randomized clinical trial. Dis Esophagus 2012; 25:373–380. [DOI] [PubMed] [Google Scholar]
- 100.Yuan YZ, Fang JY, Zou DW, Levinson N, Jenner B, Wilkinson J. Alginate antacid (Gaviscon DA) chewable tablets reduce esophageal acid exposure in Chinese patients with gastroesophageal reflux disease and heartburn symptoms. J Dig Dis 2016; 17:725–734. [DOI] [PubMed] [Google Scholar]
- 101.Lai IR, Wu MS, Lin JT. Prospective, randomized, and active controlled study of the efficacy of alginic acid and antacid in the treatment of patients with endoscopy-negative reflux disease. World J Gastroenterol 2006; 12:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tran T, Lowry AM, El-Serag HB. Meta-analysis: the efficacy of over-the-counter gastro-oesophageal reflux disease therapies. Aliment Pharmacol Ther 2007; 25:143–153. [DOI] [PubMed] [Google Scholar]
- 103.Leiman DA, Riff BP, Morgan S, Metz DC, Falk GW, French B, et al. Alginate therapy is effective treatment for GERD symptoms: a systematic review and meta-analysis. Dis Esophagus 2017; 30:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao CX, Wang JW, Gong M. Efficacy and safety of alginate formulations in patients with gastroesophageal reflux disease: a systematic review and meta-analysis of randomized controlled trials. Eur Rev Med Pharmacol Sci 2020; 24:11845–11857. [DOI] [PubMed] [Google Scholar]
- 105.Pouchain D, Bigard MA, Liard F, Childs M, Decaudin A, McVey D. Gaviscon® vs omeprazole in symptomatic treatment of moderate gastroesophageal reflux a direct comparative randomised trial. BMC Gastroenterol 2012; 12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chiu CT, Hsu CM, Wang CC, Chang JJ, Sung CM, Lin CJ, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther 2013; 38:1054–1064. [DOI] [PubMed] [Google Scholar]
- 107.Collings KL, Rodriguez-Stanley S, Proskin HM, Robinson M, Miner PB, Jr. Clinical effectiveness of a new antacid chewing gum on heartburn and oesophageal pH control. Aliment Pharmacol Ther 2002; 16:2029–2035. [DOI] [PubMed] [Google Scholar]
- 108.Holtmeier W, Holtmann G, Caspary WF, Weingärtner U. On-demand treatment of acute heartburn with the antacid hydrotalcite compared with famotidine and placebo: randomized double-blind cross-over study. J Clin Gastroenterol 2007; 41:564–570. [DOI] [PubMed] [Google Scholar]
- 109.Ali RAR, Hassan J, Egan LJ. Review of recent evidence on the management of heartburn in pregnant and breastfeeding women. BMC Gastroenterol 2022; 22:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.National Institute for Health and Care Excellence. Clinical Knowledge Summaries. Dyspepsia - pregnancy associated. 2022. [Google Scholar]
- 111.Meteerattanapipat P, Phupong V. Efficacy of alginate-based reflux suppressant and magnesium-aluminium antacid gel for treatment of heartburn in pregnancy: a randomized double-blind controlled trial. Sci Rep 2017; 7:44830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Strugala V, Bassin J, Swales VS, Lindow SW, Dettmar PW, Thomas EC. Assessment of the safety and efficacy of a raft-forming alginate reflux suppressant (liquid gaviscon) for the treatment of heartburn during pregnancy. ISRN Obstet Gynecol 2012; 2012:481870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lindow SW, Regnéll P, Sykes J, Little S. An open-label, multicentre study to assess the safety and efficacy of a novel reflux suppressant (Gaviscon Advance) in the treatment of heartburn during pregnancy. Int J Clin Pract 2003; 57:175–179. [PubMed] [Google Scholar]
- 114.Savarino V, Pace F, Scarpignato C; Esoxx Study Group. Randomised clinical trial: mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease - efficacy of Esoxx, a hyaluronic acid-chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther 2017; 45:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ribaldone DG, Rajesh P, Chandradhara D, Astegiano M, Pellicano R. A randomized, double-blind, placebo-controlled pilot study to evaluate the efficacy and tolerability of a novel oral bioadhesive formulation for the treatment of nonerosive reflux disease-related symptoms. Eur J Gastroenterol Hepatol 2021; 32:163–170. [DOI] [PubMed] [Google Scholar]
- 116.Palmieri B, Merighi A, Corbascio D, Rottigni V, Fistetto G, Esposito A. Fixed combination of hyaluronic acid and chondroitin-sulphate oral formulation in a randomized double blind, placebo controlled study for the treatment of symptoms in patients with non-erosive gastroesophageal reflux. Eur Rev Med Pharmacol Sci 2013; 17:3272–3278. [PubMed] [Google Scholar]
- 117.Boarino V, Raguzzi I, Marocchi M, Merighi A. Symptomatic response to GERDOFF® in patients with gastro-esophageal reflux disease and poor response to alginates: an exploratory, post-market, open-label study. Turk J Gastroenterol 2020; 31:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Panahi Y, Khedmat H, Valizadegan G, Mohtashami R, Sahebkar A. Efficacy and safety of Aloe vera syrup for the treatment of gastroesophageal reflux disease: a pilot randomized positive-controlled trial. J Tradit Chin Med 2015; 35:632–636. [DOI] [PubMed] [Google Scholar]
- 119.Karkon Varnosfaderani S, Hashem-Dabaghian F, Amin G, Bozorgi M, Heydarirad G, Nazem E, et al. Efficacy and safety of Amla (Phyllanthus emblica L) in non-erosive reflux disease: a double-blind, randomized, placebo-controlled clinical trial. J Integr Med 2018; 16:126–131. [DOI] [PubMed] [Google Scholar]
- 120.Dabos KJ, Sfika E, Vlatta LJ, Frantzi D, Amygdalos GI, Giannikopoulos G. Is chios mastic gum effective in the treatment of functional dyspepsia? A prospective randomised double-blind placebo controlled trial. J Ethnopharmacol 2010; 127:205–209. [DOI] [PubMed] [Google Scholar]
- 121.Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2022; 117:27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao H, Huang X, Zhi X, Han C, Li L, Li Y. Association between tea consumption and gastroesophageal reflux disease: a meta-analysis. Medicine (Baltim) 2019; 98:e14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kwiatek MA, Roman S, Fareeduddin A, Pandolfino JE, Kahrilas PJ. An alginate-antacid formulation (Gaviscon Double Action Liquid) can eliminate or displace the postprandial ‘acid pocket’ in symptomatic GERD patients. Aliment Pharmacol Ther 2011; 34:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kang JY. Lifestyle measures and reflux. Aliment Pharmacol Ther 2000; 14:1103–1103. [DOI] [PubMed] [Google Scholar]
- 125.Savarino E, Anastasiou F, Labenz J, Hungin APS, Mendive J. Holistic management of symptomatic reflux: rising to the challenge of proton pump inhibitor overuse. Br J Gen Pract 2022; 72:541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu J, Guo Y, Liu S, Su X, Li Y, Yang Y, et al. Acupuncture for the treatment of gastro-oesophageal reflux disease: a systematic review and meta-analysis. Acupunct Med 2017; 35:316–323. [DOI] [PubMed] [Google Scholar]
- 127.Liu J, Song G, Huang Y, Lv C, Wang Y, Wu D, et al. Placebo response rates in acupuncture therapy trials for functional dyspepsia: a systematic review and meta-analysis. J Clin Gastroenterol 2022; 56:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mutsekwa RN, Larkins V, Canavan R, Ball L, Angus RL. A dietitian-first gastroenterology clinic results in improved symptoms and quality of life in patients referred to a tertiary gastroenterology service. Clin Nutr ESPEN 2019; 33:188–194. [DOI] [PubMed] [Google Scholar]
- 129.Ryan D, Pelly F, Purcell E. The activities of a dietitian-led gastroenterology clinic using extended scope of practice. BMC Health Serv Res 2016; 16:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murie J, Allen J, Simmonds R, de Wet C. Glad you brought it up: a patient-centred programme to reduce proton-pump inhibitor prescribing in general medical practice. Qual Prim Care 2012; 20:141–148. [PubMed] [Google Scholar]
- 131.Coyle C, Symonds R, Allan J, Dawson S, Russell S, Smith A, et al. Sustained proton pump inhibitor deprescribing among dyspeptic patients in general practice: a return to self-management through a programme of education and alginate rescue therapy. A prospective interventional study. BJGP Open 2019; 3:bjgpopen19X101651. [DOI] [PMC free article] [PubMed] [Google Scholar]