Abstract
Objective
Our study aimed to evaluate the efficacy and safety of Lenvatinib compared with Sorafenib for treating hepatocellular carcinoma (HCC) patients under real-world setting.
Methods
We retrieved relevant literature through the PubMed, Embase, Web of Science, and Cochrane Library databases from 1 January 2000 to 25 June 2022. The differences in overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR) as well as treatment adverse related events were evaluated between HCC patients treated with Lenvatinib and Sorafenib using fixed or random-effects models. The MINORS evaluation questionnaire was used to assess the quality of the included literature.
Results
This meta-analysis included a total of 9 single-arm studies and 6 comparative studies. In the meta-analysis, Lenvatinib showed significantly longer median OS than Sorafenib (P < 0.01, MD = 1.20, 95% CI [0.92–1.48]), as well as median PFS (P < 0.01, OR = 2.68, 95% CI [1.59–3.76]), and higher ORR(P < 0.01, OR = 5.36, 95% CI [3.42–8.40]), DCR(P < 0.01, OR = 2.17, 95% CI [1.64–2.86]). The occurrence of Hypertension was higher in Lenvatinib than in Sorafenib treatment (P < 0.01, MD = 5.27, 95% CI [2.38–11.66]), and there was no significant difference in Hand-foot syndrome between Lenvatinib and Sorafenib.
Conclusion
We found that treatment with Lenvatinib in HCC patients resulted in better OS, PFS, and higher ORR and DCR compared to Sorafenib. However, safety data indicated that Lenvatinib did not exhibit a significant advantage.
Keywords: HCC, Lenvatinib, real-world study, Sorafenib
Introduction
Hepatocellular carcinoma (HCC) is the primary malignancy of liver occurred from chronic liver disease and cirrhosis, the incidence of HCC has risen and is ranked 5th most common malignancy by WHO, it is also the leading cause of mortality in China [1]. Although some early-diagnosed HCC patients may be eligible for curative therapies, there is still a considerable proportion of patients who fail to meet the standards for curative therapy, such as portal vein thrombosis or distant metastasis [2]. These unresectable advanced HCC patients do not benefit from traditional surgery or loco-regional treatments. Previously, the main treatment option for these patients was oral multikinase inhibitor sorafenib (Nexavar, Bayer, Leverkusen, Germany) [3,4]. However, the narrow therapeutic window, high progression rates during treatment, and serious drug safety issues have limited the clinical application of sorafenib. Despite the more promising outcomes offered by Regorafenib in second-line therapy following sorafenib treatment progression, there is still a need for practical and effective alternative approaches to fill the treatment gap in first-line therapy [5].
Lenvatinib (Lenvima, Eisai Inc., Woodcliff Lake, NJ, USA) is a tyrosine kinase inhibitor (TKI). The mechanism of action of Lenvatinib involves the inhibition of vascular endothelial growth factor receptor (VEGFR)1-2 and 3, fibroblast growth factor receptor (FGFR) 1-2-3 and 4, platelet-derived growth factor receptors, c-KIT, and rearranged during transfection [6]. Based on the characteristics of Lenvatinib, it exhibits stronger inhibitory effects by simultaneously targeting both the VEGF and FGF pathways. This represents a unique advantage of Lenvatinib compared to sorafenib in its anti-FGFR functionality.
A previous Phase III multicenter randomized controlled trial (RCT) demonstrated that Lenvatinib is non-inferior to Sorafenib in terms of overall survival (OS) and safety in untreated advanced HCC patients [7]. Subsequent real-world studies (RWS) [8,9] comparing the two first-line treatment drugs have yielded consistent results with the findings of this study. In the Japanese subgroup [10], Lenvatinib demonstrated statistically significant improvements over Sorafenib in terms of progression-free survival (PFS) and objective response rate (ORR). In the covariate-adjusted analysis of the Phase III REFLECT trial, the original non-inferiority study might have underestimated the true impact of Lenvatinib on OS due to baseline covariate imbalances [11]. The comparative evaluation of the real efficacy and safety between Lenvatinib and Sorafenib is warranted for unresectable HCC patients.
While RCTs are considered the gold standard for assessing drug efficacy and safety, investigations conducted in ideal research settings often cannot be equated to real-world clinical practice. RWS refer to investigations conducted in real-world settings, targeting predefined clinical questions and collecting data related to the health status, diagnosis, treatment, and healthcare of study subjects (real-world data) or derived summary data based on such information. As a complement to RCTs, RWS have been widely applied in the assessment of efficacy and safety on larger sample sizes after product approval. Given the inconsistent conclusions from previous studies comparing the efficacy and safety of Lenvatinib and Sorafenib, we initiated this systematic review and meta-analysis to assess the practical differences between the two in a real-world setting.
Materials and methods
Literature search strategy
All English-language literature from Pubmed, Embase, Web of Science and Cochrane Library databases from 1 January 2000 to 25 June 2022 were searched. The search keywords or the medical subject headings (MeSH) terms were as follows: ‘Liver Neoplasms’, ‘Cancer of Liver’, ‘Hepatocellular Cancer’, ‘Hepatic Cancer’, ‘lenvatinib’, and ‘sorafenib’. The search strategy used in PubMed was as follows: (‘carcinoma, hepatocellular’[MeSH Terms] OR (‘carcinoma’[All Fields] AND ‘hepatocellular’[All Fields]) OR ‘hepatocellular carcinoma’[All Fields] OR (‘hepatocellular’[All Fields] AND ‘carcinoma’[All Fields])) AND (‘real’[All Fields] AND (‘world’[All Fields] OR ‘worlds’[All Fields] OR ‘worlds’[All Fields])) AND (‘lenvatinib’[Supplementary Concept] OR ‘lenvatinib’[All Fields] OR (‘lenvatinib’[Supplementary Concept] OR ‘lenvatinib’[All Fields] OR ‘e7080’[All Fields]) OR (‘lenvatinib’[Supplementary Concept] OR ‘lenvatinib’[All Fields] OR ‘lenvima’[All Fields])) AND (‘Sorafenib’[Supplementary Concept] OR ‘Sorafenib’[All Fields] OR (“Sorafenib “[Supplementary Concept] OR ‘Sorafenib’[All Fields] OR “ Nexavar “[All Fields]) OR (“Sorafenib “[Supplementary Concept] OR ‘Sorafenib’[All Fields] OR “ BAY5459085 “[All Fields])).
The retrieved literature was screened by title and abstract, and the full text of the article was read or relevant experts were consulted for those who were not sure of inclusion. The inclusion criteria for this study included: (1) all patients had advanced or unresectable HCC. (2) Lenvatinib was the only intervention for targeted patients. (3) Sorafenib was the comparison treatment for patients. (4) Outcome indicators included some or all of the following key indicators: OS, PFS, overall response rate (ORR), disease control rate (DCR), and treatment adverse related events (TAREs). (5) the type of included literature should be RWS. Exclusion criteria: (1) case reports, reviews, meta-analyses; (2) interventions were Lenvatinib in combination with other drugs or no Lenvatinib monotherapy group; (3) data for further analysis was not possible; (4) early-stage HCC or resectable HCC.
Data extraction and quality evaluation
Two researchers performed data extraction independently, and a third researcher compared the extracted results and discussed and unified the data where discrepancies existed. The underlying data information included: first author, publication year, country, study type, number of patients, the details of the intervention and control measures, and OS, PFS, ORR, DCR, and adverse events in both groups. The number of occurrences of safety indicators such as hand-foot skin reaction, decreased appetite, and hypertension were also extracted. The MINORS checklist was used to evaluate the quality of all included literature, and a score >17 was considered as high-quality [12].
Statistical analyses
Statistical analyses were performed using the Cochrane Review Manager software (RevMan, version 5.3). The primary endpoints were median OS and median PFS in this meta-analysis. the odds ratio (OR) and mean difference (MD) were used as effect measures for binary outcome variables and continuous outcome variables, respectively. Heterogeneity was regarded as significant when P < 0.1 or I2 > 50%, the fixed-effect model was selected when I2 < 50%, otherwise, random-effects model was selected for calculating pooled data. Sensitivity analysis was conducted by removing each study in turn. P < 0.05 was considered statistically different.
Results
Included literatures
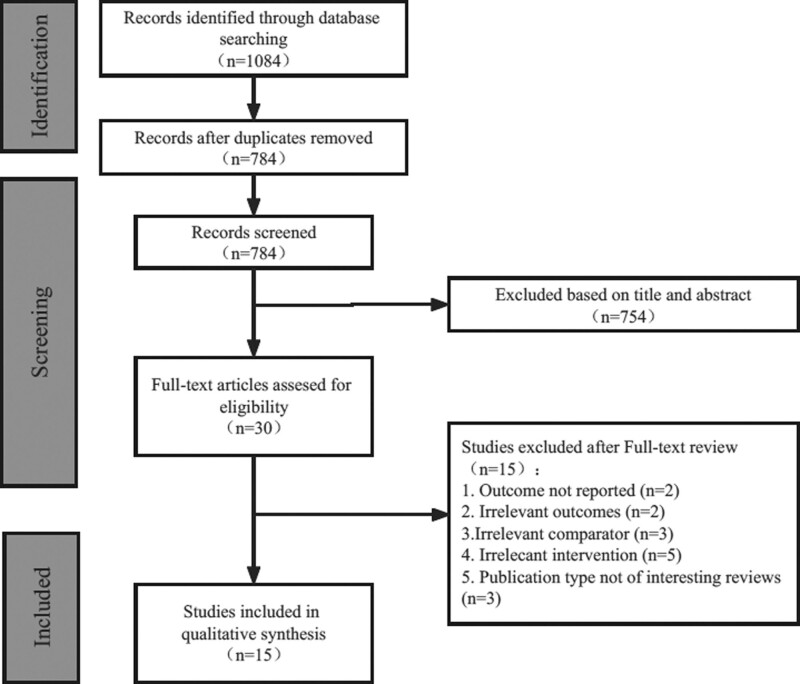
As shown in Fig. 1, a total of 784 articles were searched through PubMed, Web of Science, Embase, and Cochrane. Fifteen RWS [13–27], including 9 single-arm studies and 6 comparison studies were finally included.
Fig. 1.
Search and screening results of the literature included in the meta-analysis.
Basic characteristics of the included literature
Fifteen publications were eventually included (Supplement Table 1, Supplemental digital content 1, http://links.lww.com/EJGH/A935), all of which were RWS, with a final number of 2315 patients included and the quality evaluation was shown in Supplement Table 2, Supplemental digital content 2, http://links.lww.com/EJGH/A936. The intervention was Lenvatinib monotherapy in the experimental group and Sorafenib in the control group. Three comparison studies did not report the treatment dose of Lenvatinib, one single-arm study had a treatment dose of 8 mg daily or 4 mg daily gradually increasing to 12 mg daily, and the remaining 11 studies gave 12 mg daily for weight >60 kg and 8 mg daily for weight <60 kg.
Efficacy evaluation
Median OS
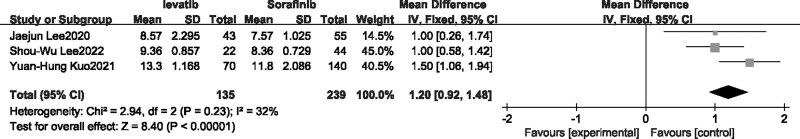
There were only two single-arm studies that reported median OS, which was not applicable for meta-analysis. Therefore, the meta-analysis of Median OS was only applied in three comparison studies [22,24,27]. Fixed-effects model was selected as there was no heterogeneity (I2 = 32%). The results revealed that the median OS of patients with advanced liver cancer was significantly longer with Lenvatinib treatment compared to Sorafenib. (P < 0.00001, MD = 1.20, 95% CI [0.92,1.48], Fig. 2).
Fig. 2.
Forest plot of overall survival.
Median PFS
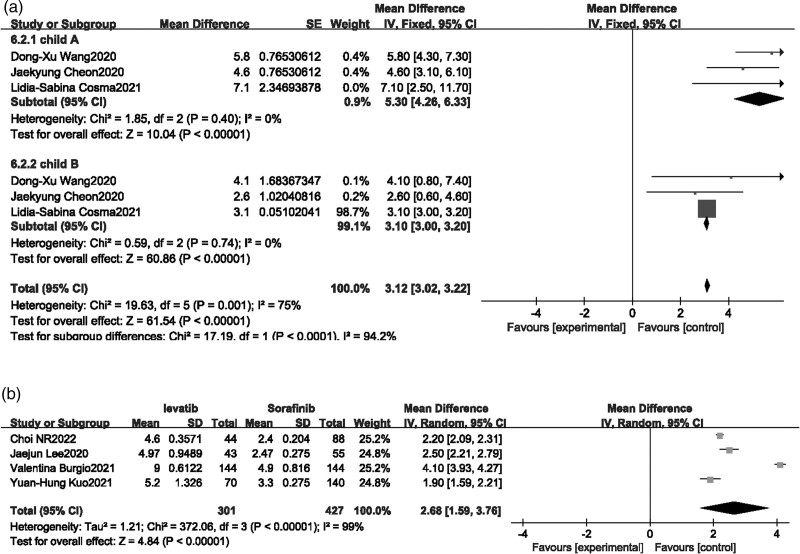
Three single-arm real studies [13,15,21] and four comparison studies [22–24,26] reported median PFS. In the meta-analysis including single-arm real studies (Fig. 3a), Lenvatinib treatment groups were divided into Child A and Child B subgroups based on liver function grading, there were no heterogeneity in both groups, and the fixed-effect model was selected, the results indicated that median PFS and corresponding 95% CI was 5.30 [4.26–6.33] and 3.10 [3.00–3.20] for child-A group and Child-B group, respectively, and combination median PFS with 95%CI was 3.12 [3.02–3.22] in included single-arm real studies. As for comparison studies, random-effect model was selected as there was high heterogeneity, results suggested that the median PFS of patients with advanced liver cancer was higher after receiving Lenvatinib treatment compared to Sorafenib (P < 0.00001, OR = 2.68, 95%CI [1.59–3.76], Fig. 3b).
Fig. 3.
Forest plot of median progression-free survival.
Objective response rate
Four single-arm studies [13,14,16,19] and six comparison experiments [22–27] reported ORR, respectively. Fixed-effects model was selected due to low heterogeneity in both meta-analysis of single arm (I2 = 0) and comparison studies (I2 = 45%). The result of single-arm studies suggested that the ORR after Lenvatinib treatment was 30% (Fig. 4a). The result of comparison studies suggested that the efficacy of Lenvatinib for advanced HCC was better than Sorafenib in terms of ORR, and the difference was statistically significant (P < 0.00001, OR = 5.36, 95% CI [3.42–8.40], Fig. 4b).
Fig. 4.
Forest plot of overall response rate (ORR).
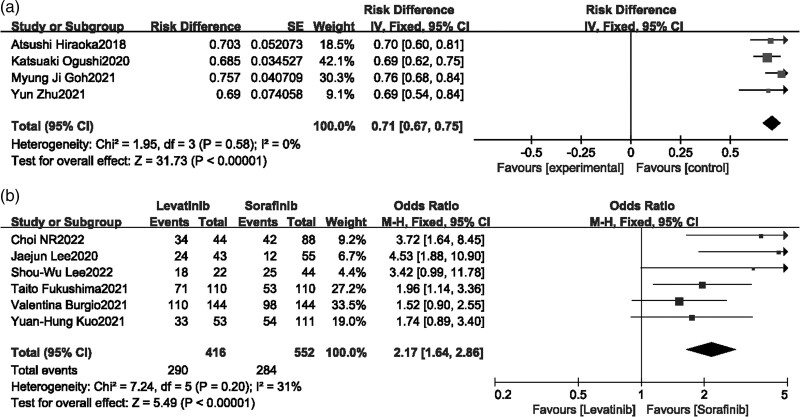
Disease control rate
Four single-arm studies [16,19,20,22] and six [22–27] comparison studies reported DCR. There was no heterogeneity in both meta-analysis of single arm and comparison studies, and fixed-effects model was applied. The results of single-arm studies indicated that the DCR of Lenvatinib for advanced HCC was 71% (Fig. 5a), and the results of comparison studies found that Lenvatinib had a statistically significant better DCR than Sorafenib for advanced HCC patients (P < 0.0001, OR = 2.17, 95% CI [1.64–2.86], Fig. 5b).
Fig. 5.
Forest plot of disease control rate.
Safety evaluation
Overall incidence of adverse reactions
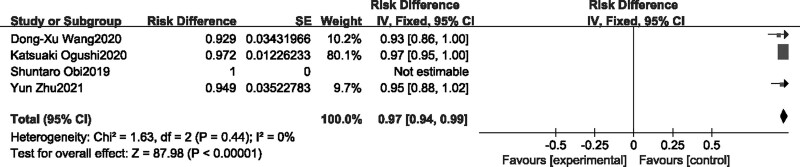
Four single-arm studies [13,14,16,19] reported overall adverse reaction rates (Fig. 6), fixed-effects model was selected as no heterogeneity was shown in the combined results. Meta-analysis showed that overall TAREs risk difference was 97% (95% CI [0.94–0.99]) after treating by Lenvatinib for advanced HCC patients.
Fig. 6.
Forest plot of overall incidence ta of TAREs.
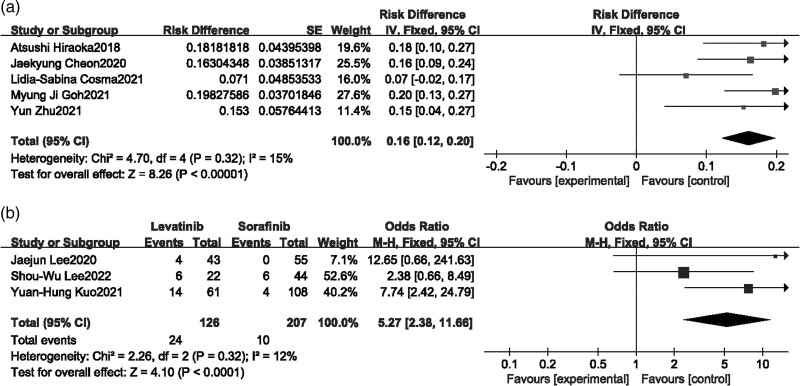
Hypertension
Four single-arm studies [15,16,20,21] and three comparison studies [22–24] reported hypertension, Fixed-effects model was selected as heterogeneity test showed mild heterogeneity in both combination, meta-analysis of single-arm studies showed that the incidence of hypertension after Lenvatinib treatment was 16% (95% CI [0.12–0.20]) (Fig. 7a). The meta-analysis results demonstrated that the incidence of hypertension during Lenvatinib treatment was higher than that of Sorafenib, and the difference was statistically significant (P < 0.00001, MD = 5.27, 95% CI [2.38–11.66]) (Fig. 7b).
Fig. 7.
Forest plot of hypertension.
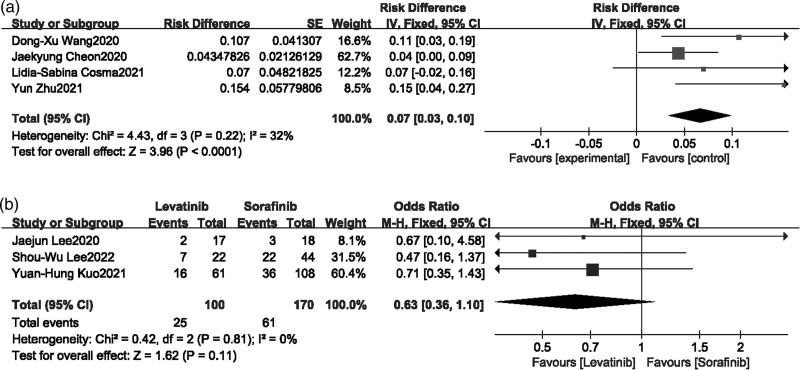
Hand-foot syndrome
Four single-arm studies [13,15,16,21] and three comparison studies [22,24,27] reported hand-foot syndrome, moderate heterogeneity was shown in single-arm studies and none heterogeneity performed in comparison studies after heterogeneity testing, Meta-analysis of single-arm studies showed that the incidence of hand-foot syndrome after Lenvatinib treatment was 7% (95% CI [0.03–0.10]) (Fig. 8a). Meta-analysis of comparison studies showed that there was no significant difference in hand-foot syndrome between Lenvatinib and Sorafenib treatment (P = 0.11, OR = 0.63, 95% CI [0.36–1.10]) (Fig. 8b).
Fig. 8.
Forest plot of hand-foot syndrome.
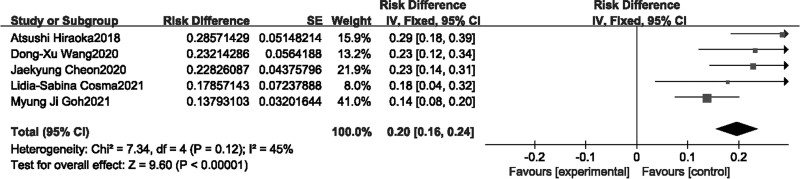
Decreased appetite
Five single-arm studies [13,15,16,20,21] reported Decreased appetite (Fig. 9), Fixed-effects model was selected due to moderate heterogeneity(I2 = 45%), and meta-analysis of single-arm studies showed that the incidence of Decreased appetite after Lenvatinib treatment was 20% (95% CI [0.16–0.24]).
Fig. 9.
Forest plot of decreased appetite.
Discussion
Over the past few decades, with the expansion of first-line treatment options for HCC, Sorafenib, Lenvatinib, and Bevacizumab + Atezolizumab have been recommended in international treatment guidelines [28,29]. Results from clinical trials demonstrated that Lenvatinib was non-inferior to sorafenib in efficacy and safety [6,10]. In addition, Andrew Briggs found that the effect of Lenvatinib may be underestimated due to imbalance of baseline covariates and the excessive use of post-treatment in the sorafenib group [11]. However, outside of RCTs, the effectiveness and safety of Lenvatinib in patients with advanced liver cancer in the real-world setting remain unclear. Meanwhile, Sorafenib has been consistently used as a comparator for evaluating clinical efficacy in the treatment of advanced liver cancer. Therefore, we conducted this systematic review and meta-analysis to assess the effectiveness and safety of Lenvatinib compared to Sorafenib in the real-world setting.
Our systemic review found that Lenvatinib demonstrated superior efficacy than Sorafenib in a real world settings, included longer OS, PFS and higher ORR, DCR. In contrast to previously published RCTs, we found that Lenvatinib, in a real-world setting, demonstrated a greater potential for prolonging the survival of patients with advanced HCC compared to Sorafenib. The results from the meta-analysis indicate that Lenvatinib may offer clinical survival benefits for patients with advanced liver cancer, although these findings still require further validation through large-scale clinical trials. In terms of PFS, the results were divided into Child A group and Child B group. The median PFS for Lenvatinib in Child A and Child B groups were 5.3 months and 3.1 months, respectively, both longer than the PFS time in the Sorafenib treatment group. These results are consistent with previous studies, but the PFS time observed in real-world settings is shorter than in clinical trials [10]. This indicated the need for further research to elucidate the true PFS of Lenvatinib in advanced HCC patients.
The ORR and DCR are key factors in assessing the anti-tumor efficacy of Lenvatinib. In meta-analysis including single-arm studies, the comprehensive ORR and DCR of Lenvatinib were 30 and 71%, respectively, which is consistent with the results of a real-world study conducted in Italy on Lenvatinib [23]. Compared to Sorafenib, Lenvatinib demonstrates superior performance in terms of ORR and DCR. This finding is consistently supported by meta-analyses conducted by Facciorusso [30] and Wenfeng Liu [31], further confirming the potent effect of Lenvatinib as evidenced by their research results. This may be attributed to the mechanism of action of Lenvatinib. Firstly, Lenvatinib targets multiple receptors including VEGFR1-3, FGFR1-4, and KIT, its broad-spectrum activity provides a high response rate during the treatment period [32]. Secondly, as a VEGF TKI, Lenvatinib exhibits a faster binding and relatively slower dissociation compared to other TKIs [33]. In addition, Lenvatinib also exerts regulatory effects on the immune microenvironment of liver cancer. Preclinical research also found that compared to Sorafenib, Lenvatinib exhibited more prominent anti-tumor effects in immune-deficient mice [34]. On the other hand, studies have found that Lenvatinib can promote the activation and infiltration of natural killer cells, thereby enhancing the efficacy of immune-based cancer therapies [35].
TAREs were considered to be an important factor that was associated in the interruption of drug usage. Based on our safety data, the overall incidence of treatment-related adverse events (TAREs) for Lenvatinib in real-world settings was 97% (95% CI [0.94–0.99]). The main drug-related adverse events were decreased appetite (20%), hypertension (16%), and hand-foot syndrome (7%). In the comparison between Lenvatinib and Sorafenib, Lenvatinib showed a higher proportion of hypertension compared to Sorafenib. However, there was no significant difference between Lenvatinib and Sorafenib in terms of hand-foot syndrome. Due to the limited number of studies available, no meta-analysis was conducted for decreased appetite. Lenvatinib showed higher drug-related TAREs than Sorafenib in real-world setting, which indicating that the realistic TAREs frequency of Lenvatinib may need to be reconsidered in clinical application and more precision treatment strategies were required [6,16]. On the other hand, in our data, the incidence of specific TAREs such as decreased appetite, hypertension, and hand-foot syndrome was not high. This may be attributed to regional and ethnic variations, and further research is needed to explore the possible reasons.
Our study has some limitations. First, the majority of studies were in real-world setting, which could lead to significant heterogeneity. Second, the number of RWS and the sample size were limited, which prevented the conduct of specific subgroup analyses and sensitivity analyses. Lastly, due to not reaching endpoints (such as OS in the study by Burgio et al. [23] with Lenvatinib treatment), some data were deemed ineligible. However, the primary outcomes received robust evidence and relatively low heterogeneity support.
Conclusion
Our meta-analysis compared the efficacy and safety of Lenvatinib and Sorafenib in a real-world setting. We found that Lenvatinib demonstrated superior OS, PFS, and higher ORR and DCR compared to Sorafenib. However, the safety results of Lenvatinib in the treatment of HCC patients were less satisfactory. Further large-scale, high-quality studies are still needed to validate our results, with a particular focus on safety issues.
Acknowledgements
This study is supported by the Guangzhou Science and Technology Program (Project No. 2023A03J0953).
XH, ZY and HG conceived the study, inside which, HX and HG identified included reviews and drafted the results. WC also helped to identify related reviews. XH and JL drafted the manuscript. All authors reviewed and approved the final manuscript.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Xuefeng Hua and Ziwei Yin contributed equally to the writing of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.eurojgh.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Tao ZW, Cheng BQ, Zhou T, Gao YJ. Management of hepatocellular carcinoma patients with portal vein tumor thrombosis: a narrative review. Hepatobiliary Pancreat Dis Int 2022; 21:134–144. [DOI] [PubMed] [Google Scholar]
- 3.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67:358–380. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al.; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390. [DOI] [PubMed] [Google Scholar]
- 5.Facciorusso A, Abd EAM, Sacco R. Efficacy of regorafenib in hepatocellular carcinoma patients: a systematic review and meta-analysis. Cancers (Basel) 2019; 12:36–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391:1163–1173. [DOI] [PubMed] [Google Scholar]
- 7.Vogel A, Qin S, Kudo M, Su Y, Hudgens S, Yamashita T, et al. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol 2021; 6:649–658. [DOI] [PubMed] [Google Scholar]
- 8.Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, et al. Sorafenib vs Lenvatinib as first-line therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Anticancer Res 2020; 40:2283–2290. [DOI] [PubMed] [Google Scholar]
- 9.Tomonari T, Sato Y, Tani J, Hirose A, Ogawa C, Morishita A, et al. Comparison of therapeutic outcomes of sorafenib and lenvatinib as primary treatments for hepatocellular carcinoma with a focus on molecular-targeted agent sequential therapy: a propensity score-matched analysis. Hepatol Res 2021; 51:472–481. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, et al. Reflect-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of japanese subset. J Gastroenterol 2020; 55:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briggs A, Daniele B, Dick K, Evans T, Galle PR, Hubner RA, et al. Covariate-adjusted analysis of the phase 3 reflect study of lenvatinib versus sorafenib in the treatment of unresectable hepatocellular carcinoma. Br J Cancer 2020; 122:1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. Anz J Surg 2003; 73:712–716. [DOI] [PubMed] [Google Scholar]
- 13.Wang DX, Yang X, Lin JZ, Bai Y, Long JY, Yang XB, et al. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: a retrospective, real-world study conducted in china. World J Gastroenterol 2020; 26:4465–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obi S, Sato T, Sato S, Kanda M, Tokudome Y, Kojima Y, et al. The efficacy and safety of lenvatinib for advanced hepatocellular carcinoma in a real-world setting. Hepatol Int 2019; 13:199–204. [DOI] [PubMed] [Google Scholar]
- 15.Cosma LS, Weigand K, Muller-Schilling M, Kandulski A. Lenvatinib as first-line treatment of hepatocellular carcinoma in patients with impaired liver function in advanced liver cirrhosis: real world data and experience of a tertiary hepatobiliary center. J Gastrointestin Liver Dis 2021; 30:247–253. [DOI] [PubMed] [Google Scholar]
- 16.Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Atsukawa M, Itobayashi E, et al.; Real-life Practice Experts for HCC (RELPEC) Study Group, HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: multicenter analysis. Cancer Med 2019; 8:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya K, Kurosaki M, Sakamoto A, Marusawa H, Kojima Y, Hasebe C, et al. The real-world data in japanese patients with unresectable hepatocellular carcinoma treated with lenvatinib from a nationwide multicenter study. Cancers (Basel) 2021; 13:2608–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singal AG, Nagar SP, Hitchens A, Davis KL, Iyer S. Real-world effectiveness of lenvatinib monotherapy among unresectable hepatocellular carcinoma patients in the usa. Future Oncol 2021; 17:2759–2768. [DOI] [PubMed] [Google Scholar]
- 19.Ogushi K, Chuma M, Uojima H, Hidaka H, Numata K, Kobayashi S, et al. Safety and ef fi cacy of lenvatinib treatment in child-pugh a and b patients with unresectable hepatocellular carcinoma in clinical practice: a multicenter analysis. Clin Exp Gastroenterol 2020; 13:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh MJ, Oh JH, Park Y, Kim J, Kang W, Sinn DH, et al. Efficacy and safety of lenvatinib therapy for unresectable hepatocellular carcinoma in a real-world practice in korea. Liver Cancer 2021; 10:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheon J, Chon HJ, Bang Y, Park NH, Shin JW, Kim KM, et al. Real-world efficacy and safety of lenvatinib in korean patients with advanced hepatocellular carcinoma: a multicenter retrospective analysis. Liver Cancer 2020; 9:613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo YH, Lu SN, Chen YY, Kee KM, Yen YH, Hung CH, et al. Real-world lenvatinib versus sorafenib in patients with advanced hepatocellular carcinoma: a propensity score matching analysis. Front Oncol 2021; 11:737767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgio V, Iavarone M, Di Costanzo GG, Marra F, Lonardi S, Tamburini E, et al. Real-life clinical data of lenvatinib versus sorafenib for unresectable hepatocellular carcinoma in italy. Cancer Manag Res 2021; 13:9379–9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Sung PS, Yang H, Lee SK, Nam HC, Yoo SH, et al. A real-world comparative analysis of lenvatinib and sorafenib as a salvage therapy for transarterial treatments in unresectable hcc. J Clin Med 2020; 9:4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukushima T, Morimoto M, Ueno M, Kubota K, Uojima H, Hidaka H, et al. Comparative study between sorafenib and lenvatinib as the first-line therapy in the sequential treatment of unresectable hepatocellular carcinoma in a real-world setting. JGH Open 2022; 6:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi NR, Kim JY, Hong JH, Hur MH, Cho H, Park MK, et al. Comparison of the outcomes between sorafenib and lenvatinib as the first-line systemic treatment for hbv-associated hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol 2022; 22:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SW, Yang SS, Lien HC, Peng YC, Ko CW, Lee TY. Efficacy of lenvatinib and sorafenib in the real-world first-line treatment of advanced-stage hepatocellular carcinoma in a taiwanese population. J Clin Med 2022; 11:1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimbach JK. Overview of the updated aasld guidelines for the management of hcc. Gastroenterol Hepatol (N Y) 2017; 13:751–753. [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29:iv238–iv255. [DOI] [PubMed] [Google Scholar]
- 30.Facciorusso A, Tartaglia N, Villani R, Serviddio G, Ramai D, Mohan BP, et al. Lenvatinib versus sorafenib as first-line therapy of advanced hepatocellular carcinoma: a systematic review and meta-analysis. Am J Transl Res 2021; 13:2379–2387. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Quan B, Lu S, Tang B, Li M, Chen R, et al. First-line systemic treatment strategies for unresectable hepatocellular carcinoma: a systematic review and network meta-analysis of randomized clinical trials. Front Oncol 2021; 11:771045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 2014; 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto K, Ikemori-Kawada M, Jestel A, von Konig K, Funahashi Y, Matsushima T, et al. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med Chem Lett 2015; 6:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-pd-1 antibody combination treatment activates cd8+ t cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One 2019; 14:e0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Liu H, Wang H, Lu M, Miao Y, Ding J, et al. Lenvatinib promotes antitumor immunity by enhancing the tumor infiltration and activation of nk cells. Am J Cancer Res 2019; 9:1382–1395. [PMC free article] [PubMed] [Google Scholar]