Abstract
Neurotropic coronavirus-induced encephalitis was used to evaluate recruitment, functional activation, and retention of peripheral bystander memory CD8+ T cells. Mice were first infected with recombinant vaccinia virus expressing a non-cross-reactive human immunodeficiency virus (HIV) epitope, designated p18. Following establishment of an endogenous p18-specific memory CD8+ T-cell population, mice were challenged with coronavirus to directly compare recruitment, longevity, and activation characteristics of both primary coronavirus-specific and bystander memory populations trafficking into the central nervous system (CNS). HIV-specific memory CD8+ T cells were recruited early into the CNS as components of the innate immune response, preceding CD8+ T cells specific for the dominant coronavirus epitope, designated pN. Although pN-specific T-cell numbers gradually exceeded bystander p18-specific CD8+ T-cell numbers, both populations peaked concurrently within the CNS. Nevertheless, coronavirus-specific CD8+ T cells were preferentially retained. By contrast, bystander CD8+ T-cell numbers declined to background numbers following control of CNS virus replication. Furthermore, in contrast to highly activated pN-specific CD8+ T cells, bystander p18-specific CD8+ T cells recruited to the site of inflammation maintained a nonactivated memory phenotype and did not express ex vivo cytolytic activity. Therefore, analysis of host CD8+ T-cell responses to unrelated infections demonstrates that bystander memory CD8+ T cells can comprise a significant proportion of CNS inflammatory cells during virus-induced encephalitis. However, transient CNS retention and the absence of activation suggest that memory bystander CD8+ T cells may not overtly contribute to pathology in the absence of antigen recognition.
Sequential unrelated infections of multiple peripheral tissues have demonstrated that a virus not only activates antigen-specific CD8+ T-cell responses but may also mobilize memory CD8+ T cells of irrelevant specificities (9, 12, 26, 38, 42). The biological consequences depend on the strength and type of stimulus received. Irrelevant memory T cells may merely be recruited to inflammatory sites without expressing effector function (38). However, cytokines, particularly type I interferons (IFNs), or T-cell receptor (TCR) engagement as a result of cross-reactivity may provide additional activation signals (7, 44). Promiscuous memory cell recruitment to inflammatory sites to sample foreign antigens provides a distinct biological advantage to the host when a microbial antigen is recognized. Pathogen cross-reactivity and activation of effector function may indeed provide heterologous protection (42); however, cross-reactivity with self may enhance chronic inflammation and autoimmune disease. Although it is evident that the history of previous heterologous infections may significantly impact the outcome of a new infection (42), the role of antigen versus cytokine- or chemokine-driven effects remains elusive and low-level cross-reactivity between epitopes derived from pathogens or self-antigens is difficult to exclude in vivo. Furthermore, the in vitro requirement for class I peptide-dependent TCR triggering for expression of many T-cell effector functions (43) may be overcome in vivo by specific cytokine combinations or cross-talk via adhesion and costimulatory molecules (24).
Control of T-cell entry, expression of T-cell effector function, and antigen specificity are of particular importance within the central nervous system (CNS), where limiting virus- and immune system-mediated damage is critical to host survival. The CNS is protected by a unique vasculature comprising the blood brain barrier that minimizes the passage of cells and macromolecules into the CNS parenchyma (19, 20). Although it is widely accepted that T cells survey the resting CNS (19), T-cell migration and/or extravasation in the absence of inflammatory signals is minimal compared to that of other nonlymphoid tissues such as liver or lung tissues (27, 35). Furthermore, although activated T cells have an enhanced capacity for migration into noninflamed tissues compared to memory T cells (35), they rapidly disappear from the CNS in the absence of cognate antigen (8, 20, 22). Such transient accumulation, in the absence of inflammatory signals, by the use of adoptive transfers into hosts harboring or devoid of antigen within the CNS has been described for both activated CD4+ and CD8+ T cells (8, 20, 22). By contrast, activated CD8+ T cells specific for an antigen widely expressed in the recipient produced liver inflammation but no inflammation in other tissues, including CNS tissues (1), suggesting restricted access. However, direct intracerebral (i.c.) injection resulted in necroinflammatory disease of the choroid plexus in antigen-transgenic, but not control, littermates (1). Thus, despite promiscuous trafficking, the potential for immunopathology within the CNS appears negligible in the absence of cognate antigen recognition, especially considering the paucity of major histocompatibility complex (MHC) expression by CNS resident cells (40).
In contrast to results showing restricted T-cell entry into the naïve CNS, viral infection induces vigorous T-cell responses (14, 18, 32). However, the extent of virus-specific versus “irrelevant” bystander CD8+ T-cell recruitment and activation during virus-induced inflammation is largely unknown. Adoptive transfer studies during Sindbis virus-induced encephalitis suggest that lymphocyte entry is independent of antigen specificity but that virus-specific T cells are selectively retained (22). Inflammation in this model is largely CD4+ T cell mediated. During neurotropic coronavirus-induced inflammation, primary antigen-specific CD8+ T cells access the CNS, reach a peak that is delayed relative to that of viral replication, and are retained within the CNS for prolonged periods following viral clearance (4, 32). Approximately 50% of CD8+ T cells are virus specific, and the relative ratio of virus-specific to total CD8+ T cells remains relatively stable over time (4, 32, 39). Other models, involving viral CNS challenge of immune mice to avoid lethality, also demonstrate efficient recruitment and survival of virus-specific CD8+ T cells within the CNS for many months (18, 46). However, the relative frequencies of irrelevant antigen specificities, as well as the dynamics within CD8+ T-cell populations persisting in the CNS, has not been studied extensively (4, 18, 33, 36, 46). Similarly, the specificities of the relatively restricted TCR repertoire of CD8+ T cells found in CNS lesions of multiple sclerosis patients are unresolved (2). Activation of non-specifically recruited memory cells prior to or within the CNS environment following microbial infection may influence subsequent CNS inflammation and/or enhance pathogenesis. Indeed, the ability of highly activated bystander CD8+ T cells to mediate enhanced CNS pathology in a TCR-transgenic mouse has recently been demonstrated during neurotropic coronavirus infection (15). By contrast, recruitment of CD8+ T cells monospecific for an irrelevant antigen did not significantly contribute to CNS damage in a transgenic model of persistent lymphocytic choriomeningitis virus (LCMV) infection (36).
To gain insights into how bystander CD8+ T cells may influence pathological events in the CNS of mice with a natural TCR repertoire, the recruitment, activation state, and retention of a defined, irrelevant peripheral memory CD8+ T-cell population were characterized in the context of acute neurotropic coronavirus infection. CNS infection with the neurotropic JHM strain of mouse hepatitis virus (JHMV) results in nonfatal encephalomyelitis (13). JHMV replicates in the CNS parenchyma in astrocytes, microglia-macrophages, and oligodendroglia (48) and induces a vigorous inflammatory response (3, 31). CD8+ T-cell infiltration peaks within the CNS between days 8 and 10 postinfection (p.i.) and plays a significant role in eliminating infectious virus (3, 4, 31). Although the majority of CD8+ T cells recruited into the CNS during acute infection are virus specific, a consistent proportion is not accounted for by specificities for T-cell epitopes identified to date (4, 32, 33). To determine whether irrelevant T cells are derived from a preexisting memory pool, a non-cross-reactive indicator memory CD8+ T-cell population was established prior to JHMV challenge by peripheral infection with a recombinant vaccinia virus (rVV) expressing a Dd-restricted epitope derived from human immunodeficiency virus type 1 (HIV-1) IIIB gp160, designated p18 (6). CD8+ T-cell infiltration within the CNS of mice undergoing acute rVV infection was transient, suggesting that activated CD8+ T cells access the naïve CNS but lack survival signals for prolonged retention. Subsequent JHMV CNS infection, induced after p18-specific CD8+ T-cell levels declined below detection within the CNS, mobilized peripheral memory T cells into the infected CNS independent of antigen specificity. However, in contrast to highly activated CD8+ T cells specific for the dominant JHMV pN epitope (5), p18-specific CD8+ T cells recruited to the site of inflammation maintained a nonactivated memory phenotype and did not express ex vivo cytolytic activity. Taking advantage of a CNS-restricted infection in a host harboring a naturally established memory T-cell population, the data indicate that CD8+ bystander T cells are indiscriminately recruited into the CNS as a component of the innate cellular response. The data also indicate that in the absence of cognate or cross-reactive antigen, accumulation is transient and therefore unlikely to contribute to immune pathology.
MATERIALS AND METHODS
Mice and viruses.
BALB/c (H-2d) mice were purchased from the National Cancer Institute (Frederick, Md.) at 6 weeks of age and housed in microisolator cages in an accredited animal facility at the University of Southern California. Mice were immunized by intraperitoneal (i.p.) injection with 5 × 107 PFU of an rVV expressing the HIV-1 IIIB gp160-derived 10-mer epitope, designated p18 (vac-p18), or a control rVV expressing β-galactosidase (vSC8) (6). Acute sublethal encephalitis was induced at 30 to 40 days postimmunization by i.c. injection with 500 PFU of the 2.2v-1 monoclonal antibody (MAb)-derived variant of JHMV in a 30-μl volume of endotoxin-free phosphate-buffered saline (PBS) as previously described (4, 32, 33).
Isolation of lymphocytes.
Mononuclear cells were obtained from pooled brains or spleens of four to eight mice/group perfused with PBS as described previously (32, 33). For isolation of inflammatory cells from the CNS, brains were disrupted in RPMI medium containing 25 mM HEPES (pH 7.2) by the use of ice-cold Tenbroeck tissue homogenizers. Homogenates were adjusted to 30% Percoll (Pharmacia, Uppsala, Sweden), and the mononuclear cells were isolated by centrifugation at 800 × g at 4°C for 25 min onto a 70% Percoll cushion. CNS-derived cells were collected from the 30% Percoll/70% Percoll interface, washed three times, and resuspended in RPMI 1640 containing 25 mM HEPES and 1% fetal calf serum prior to analysis. Yields varied from 1 × 106 to 3 × 106 cells per brain, with maximum yields obtained between days 8 and 12 p.i.
Flow cytometry and intracellular cytokine staining.
Cells were suspended in PBS containing 0.1% bovine serum albumin for analysis by flow cytometry. Nonspecific binding was blocked by preincubation with FCγIII/IIR MAb (2.4G2; BD PharMingen, San Diego, Calif.) and 1% mouse serum for 20 min at 4°C. Inflammatory cells were separated from CNS resident cells on the basis of a CD45hi phenotype (3). PerCP anti-CD45 (BD PharMingen) and PE anti-F4/80 (Serotec, Raleigh, N.C.) were used to distinguish microglia from infiltrating macrophages. MHC class II expression on CD45low microglia was determined with fluorescein isothiocyanate anti-I-A/I-E (2G9; BD PharMingen). Neutrophils were identified with allophycocyanin anti-Ly-6G/6C (1A8; BD PharMingen) and the absence of I-A/I-E expression. JHMV-specific CD8+ T cells within the CD45hi population were detected using the Ld-pN tetramer (4) and Cy-chrome anti-CD8 MAb (53-6.7; BD PharMingen). The p18-specific CD8+ T cells were detected using the Dd-p18 tetramer (47). CD44 (IM7; BD PharMingen), CD43 (1B11; BD PharMingen), and CD127 (SB/199; BD PharMingen) expression on CD8+ T cells was used to differentiate memory and effector CD8+ T-cell populations. Samples were analyzed on a FacsCalibur flow cytometer (Becton Dickinson, Mountain View, Calif.). Forward- and side-scatter signals were used to establish a gate containing intact lymphocytes, macrophages, and neutrophils while excluding dead cells and tissue debris. A minimum of 106 viable cells were stained and ∼105 events were analyzed per sample.
IFN-γ production in response to antigen was analyzed by incubating 1 × 106 splenocytes or CNS-derived cells with J774.1 cells (2 × 105) in 100 μl of RPMI 1640 supplemented with 10% fetal calf serum, 1 μM of the Ld-restricted pN peptide derived from the JHMV nucleocapsid protein (APTAGAFFF) or the p18 gp160 derived Dd-restricted peptide (RGRGRAFVTI), and 1 μl of Golgi Stop (BD PharMingen)/ml for 5 h at 37°C (3). Peptides were synthesized by the University of Southern California Norris Cancer Center Microchemistry Laboratory, and purity was assessed by high-pressure liquid chromatography and mass spectrometry. Cells were stained with Cy-chrome anti-CD8 MAb, fixed, permeabilized, and analyzed for intracellular gamma interferon (IFN-γ) by the use of XMG1.2 MAb (BD PharMingen).
Cytotoxic T-lymphocyte assays.
Cytotoxic T-lymphocyte assays were performed directly ex vivo using cells isolated from the CNS as described previously (4). Briefly, J774.1 (H-2d) target cells were labeled with 100 μCi of Na51CrO4 (New England Nuclear, Boston, Mass.) and coated with 1 μM of the p18 or pN peptide. CNS-derived cells were added at the indicated effector-to-target cell (E:T) ratios (4). Supernatants (100 μl) were removed after 4 h of incubation at 37°C. Specific lysis was defined as 100 × (experimental release − spontaneous release)/(detergent release − spontaneous release). Maximum spontaneous release values were <10% of the total release values. Specific cytolysis per tetramer-positive CD8+ T cell was calculated by adjusting E:T ratios to tetramer-positive CD8+ T-cell levels.
RESULTS
Transient accumulation of activated p18-specific CD8+ T cells within the CNS during peripheral inflammation.
Activated CD8+ T cells not only localize to the site(s) of infection but also traffic to uninfected nonlymphoid organs, where they establish an effector memory pool (34, 35). However, the recruitment and longevity characteristics of activated CD8+ T cells in the CNS during an infection restricted to the periphery are unclear. To examine CNS entry and retention of activated CD8+ T cells during an acute peripheral infection, mice were infected with vac-p18 expressing the Dd-restricted HIV-1-derived p18 epitope (6). Following i.p. injection, rVV replicates prominently in ovaries but does not infect the CNS (25). During acute infection p18-specific CD8+ T cells comprise 8 to 10% of total splenic CD8+ T cells and subsequently decline to 3 to 5% following viral clearance (47). Examination of the CNS during acute peripheral infection indeed revealed recruitment of CD8+ T cells (Fig. 1). CD8+ T cells were detected by 4 days p.i. and increased to day 7 p.i., coincident with peak peripheral expansion (47; data not shown). CD8+ T cells remained elevated within the CNS to day 14 p.i. but decreased to barely detectable numbers by day 30 p.i. To monitor the p18-specific population, CNS-derived CD8+ T cells were stimulated with peptide and analyzed by intracellular IFN-γ staining. Although p18-specific CD8+ T-cell levels were below detection at day 4 p.i., small numbers were recovered at both 7 and 14 days p.i., coinciding with maximal total CD8+ T-cell levels (Fig. 1). Nevertheless, maximal total p18-specific CD8+ T-cell levels barely reached 4,000 cells per brain. By day 30 p.i., no p18-specific CD8+ T cells were detected within the low number of CD8+ T cells retained in the CNS (Fig. 1). These data demonstrate that activated CD8+ T cells are transiently recruited into the CNS during peripheral infection and that very few are retained within the CNS in the absence of inflammatory signals. Importantly, p18-specific CD8+ T cells decreased to undetectable levels within the CNS by 30 days following peripheral infection.
FIG. 1.
Transient CD8+ T-cell recruitment into the CNS during peripheral infection. Mice were infected i.p. with vac-p18. At days 4, 7, 14, and 30 p.i., CNS cells were analyzed for levels of p18-specific CD8+ T cells following peptide stimulation and intracellular IFN-γ staining. Bars indicate total p18-specific CD8+ T cells within the CNS, and symbols denote total CD8+ T cells per brain. The data are representative of two separate experiments using three mice per group.
Recruitment of bystander CD8+ T cells during CNS inflammation.
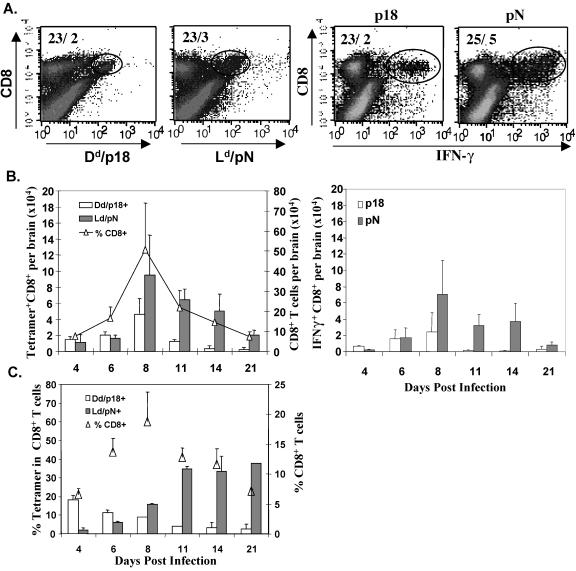
The peripheral p18-specific memory pool established following vac-p18 clearance provided a marker population to monitor recruitment and activation of resting, irrelevant bystander cells during an infection confined to the CNS. No cross-reactivity between the dominant JHMV pN epitope and p18 epitope determined by either IFN-γ production or the ability of in vitro-stimulated memory cells to lyse targets presenting the heterologous epitope was detectable (data not shown). Furthermore, the inability to detect p18-specific CD8+ T cells within the CNS at the time of CNS challenge suggested that any accumulation of p18-specific cells following JHMV CNS infection results from recruitment. To examine the relative recruitment of primary JHMV and irrelevant memory cells into the CNS, mice immunized by peripheral infection with vac-p18 or the vSC8 control virus were infected with JHMV at 30 to 45 days post-primary infection. Both vac-p18 and vSC8 immune mice mounted vigorous CD8+ T-cell inflammatory responses to JHMV infection (Fig. 2). The relative proportions of CD8+ T cells specific for the JHMV pN epitope and bystander p18 CD8+ T cells were assessed using class I tetramers and intracellular IFN-γ staining following stimulation with either peptide (Fig. 2A). CD8+ T cells were initially detected at day 4 p.i., peaked at day 8 p.i., and gradually declined after day 8 p.i. but remained detectable to 21 days p.i. (Fig. 2B). Analysis of JHMV-pN- and HIV-p18-specific CD8+ T-cell subsets by tetramer staining revealed that total numbers of p18-specific cells dominated pN-specific cells at day 4 p.i. (Fig. 2B, left panel). Although the two subsets were comparable at day 6 p.i., pN-specific CD8+ T cells prevailed by day 8 p.i. and at subsequent time points. Although only ∼4 × 103 pN-specific CD8+ T cells were detected at day 4 p.i., they exceeded the level of p18-specific CD8+ T cells by twofold at day 8 p.i. Maximal total numbers of both populations coincided with the peak of JHMV-induced inflammation at day 8 p.i. At day 11 p.i., p18-specific CD8+ T-cell levels decreased dramatically.
FIG. 2.
Recruitment of primary homologous (pN) and bystander memory (p18) CD8+ T cells into the CNS. Vac-p18 immune mice were infected i.c. with JHMV. At 4, 6, 8, 11, 14, and 21 days p.i., CNS-derived cells were analyzed for levels of p18- or pN-specific CD8+ T cells by the use of class I tetramers and peptide-induced IFN-γ staining. (A) Representative flow cytometric density plots, gated on CD8+ T cells, showing either Dd/p18- and Ld/pN tetramer-positive CD8+ cells or intracellular p18- or pN-specific IFN-γ+ cells in the CD8+ population at day 8 p.i. Numbers indicate percentages of CD8+ T cells and percentages of epitope-specific CD8+ T cells within total CNS-derived cells. (B) Bars represent total numbers of Dd/p18 or Ld/pN tetramer+ CD8+ T cells (left panel) and total numbers of peptide-specific IFN-γ+ CD8+ T cells (right panel) per brain. Results represent the averages for two separate experiments containing three mice per group ± standard deviations. (C) Bars indicate percentages of either Dd/p18 or Ld/pN tetramer+ cells within the CD8+ T-cell population (left y axis); symbols indicate the CD8+ T-cell percentages within total brain-derived cells (right y axis).
In similarity to the results seen with infection of naïve mice (3), pN-specific CD8+ T cells were selectively retained, although JHMV titers are substantially reduced by day 11 p.i. As tetramer staining may lead to underestimation of antigen-specific cells due to TCR downregulation, intracellular IFN-γ production following peptide stimulation was also examined. Slightly reduced, albeit similar, numbers of both pN- and p18-specific IFN-γ-producing T cells were obtained (Fig. 2B, right panel). The inverse dynamics of the p18- and pN-specific CD8+ T-cell populations are most evident when the relative percentages within the CD8+ T-cell population are calculated (Fig. 2C). Bystander p18-specific CD8+ T cells comprised ∼20% of the CD8+ T cells infiltrating the CNS, whereas the pN-specific proportion was barely detectable at day 4 p.i. However, the percentage of p18-specific memory cells steadily declined to the limits of detection after day 8 p.i. By contrast, the proportion of pN-specific cells increased gradually to day 11 p.i. and remained elevated at days 14 and 21 p.i. Although kinetics of total CD8+ T-cell- and pN-specific inflammation were similar within the CNS of JHMV-infected vSC8 immune mice, no p18-specific CD8+ T cells were detected, confirming tetramer specificity (data not shown). On the basis of the observation that similar numbers of CD8+ T cells entered the CNS of vSC8 immune mice, the specificity of the CD8+ T cells not accounted for by tetramer staining is unknown but may involve additional VV epitopes (17). These data suggest that bystander memory CD8+ T cells enter the CNS rapidly during an acute inflammatory response but are gradually displaced by pathogen-specific primary T cells.
The extent of recruitment of bystander cells and their role during inflammatory responses are controversial. Although bystander cells may acquire an effector phenotype during heterologous infection, activation may be attributed to antigen cross-reactivity (7). The vast majority of cells recruited to the inflamed CNS, including those recruited during JHMV infection, exhibit an activated-memory phenotype, characterized by a CD44hi CD62Llo CD11ahi CD69+ expression profile (4, 18, 46). Although naïve subsets are thus excluded, differentiation of effector and memory cells has been difficult. Nevertheless, expression of a glycoform of CD43, recognized by MAb 1B11, and of CD127, the interleukin-7Rα chain, has recently been shown to correlate with highly differentiated effector functions (16, 21, 23). CD43 is expressed at low levels on naïve CD8+ T cells, highly expressed on activated T cells, and downregulated on memory cells (16). CD127 is constitutively expressed by naïve cells, downregulated on the majority of antigen-specific CD8+ T cells during T-cell activation, and expressed on memory CD8+ T cells (21, 23). Thus, whereas highly activated, lytic CD8+ T cells are characterized by a CD44hi CD43hi CD127lo phenotype, memory cells express a CD44hi CD43lo/int CD127hi phenotype. These markers were thus used in combination with intracellular IFN-γ to discern distinct activation phenotypes in the memory p18-specific CD8+ T cells and primary activated JHMV-specific CD8+ T cells (Fig. 3).
FIG. 3.
Phenotypic differences between bystander memory and JHMV-specific CD8+ T cells. Flow cytometric density plots showing intracellular IFN-γ+ p18- and pN-specific cells in the CD8+ T-cell population prior to JHMV challenge (A), at day 6 p.i. in spleen (B), and at days 6 and 8 p.i. in brain (C). Numbers indicate the percentages of IFN-γ-producing CD8+ T cells expressing high and low levels of CD44, CD127, and CD43, as indicated. Boxes separate high- and low-level populations of CD44, CD127, and CD43 within the IFN-γ-specific CD8+ T cells. Results are representative of two separate experiments using three mice per group.
Prior to JHMV challenge, p18-specific splenic CD8+ T cells were CD44hi and the vast majority were CD127hi and CD43lo/int, confirming a memory phenotype (Fig. 3A). Furthermore, both the splenic pN- and p18-specific CD8+ T cells exhibited the CD44hi activated-memory phenotype at day 6 p.i. (Fig. 3B). However, whereas CD127 was expressed by >70% of p18-specific CD8+ T cells, the majority of pN-specific CD8+ T cells were CD127−. In similarity to their prechallenge phenotype, the majority of p18-specific CD8+ T cells remained CD43lo/int. By contrast, pN-specific CD8+ T cells were CD43hi, consistent with an activated effector phenotype. Thus, splenic p18 memory cells showed little evidence of activation during expansion of primary JHMV-specific CD8+ T cells. To confirm that mobilization and recruitment into the CNS did not alter the p18-specific CD8+ T-cell phenotype, CNS-derived CD8+ T cells were analyzed in a similar fashion. The phenotypes of the p18- versus pN-specific populations remained distinct in the CNS throughout days 6 and 8 p.i. (Fig. 3C). CD127 expression remained detectable on ∼70% of p18-specific CD8+ T cells, whereas only 35 to 42% of pN-specific CD8+ T cells retained the “long-lived” memory marker. Whether the noticeably higher percentage of CD127+ CNS cells compared to that of the splenic pN population reflects preferential recruitment or survival remains to be investigated. Nevertheless, the predominant CD43lo/int versus CD43hi phenotype in the p18 versus pN populations, respectively, further confirmed selective activation of the primary CD8+ T-cell responders within the CNS. These results demonstrate that despite acute encephalitis, bystander CD8+ T cells express a predominantly memory phenotype in both the periphery and CNS.
Functional analysis of CD8+ T cells during CNS inflammation.
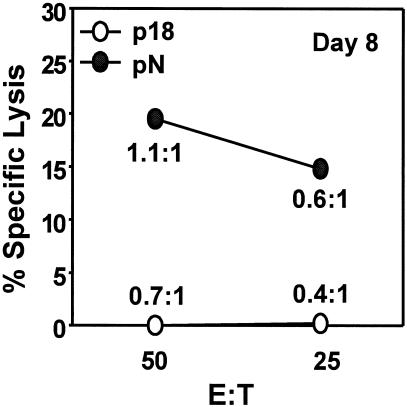
Activated bystander CD8+ T cells increase myelin loss in JHMV-infected mice (15). A potential mechanism may reside in their expression of cytolytic activity; however, phenotypic analysis of p18-specific memory T cells recruited during JHMV CNS infection suggested limited effector function (Fig. 3). To confirm that p18-specific memory CD8+ T cells recruited into the CNS are not activated in the absence of homologous antigen, CNS-derived cells were compared for pN- and p18-specific ex vivo cytolytic activity. JHMV-specific cytolysis by mononuclear cells isolated from the CNS of p18 immune mice was apparent at day 8 p.i., the peak of T-cell inflammation (Fig. 4), in similarity to the results seen with cells derived from the CNS of infected naïve mice (39). By contrast, no detectable p18-specific cytolysis was detected (Fig. 4). To accommodate differences in relative frequencies, E:T ratios were recalculated on the basis of the frequency of epitope-specific CD8+ T cells as determined by both tetramer staining and IFN-γ production. These data demonstrate that pN-specific CD8+ T cells induce cytolysis at E:T ratios < 1 (Fig. 4). In stark contrast, p18-specific cytolytic activity was undetectable at the single-cell level. Analysis at day 6 p.i., when both populations were present at equal frequencies (Fig. 2), confirmed the absence of p18-specific cytolysis (data not shown). These results suggest that memory CD8+ T cells randomly recruited to the CNS are not sufficiently activated in the presence of an inflammatory environment to exert detectable ex vivo cytolytic function. Although the potential expression of effector function in vivo cannot be excluded, the absence of ex vivo cytolytic activity supports the concept that TCR triggering is essential for effector function (43) and/or pathology (36).
FIG. 4.
Differential expression of ex vivo cytolytic activity. CNS-derived cells from vac-p18 immune mice infected with JHMV were analyzed for ex vivo cytolytic activity at day 8 p.i. J774.1 (H-2d) target cells were coated with either the p18 or pN peptides at a final concentration of 1 μM before addition of effectors. E:T ratios determined on the basis of total cell numbers are shown on the x axis. Ratios in the graph represent epitope-specific CD8+ T cells enumerated on the basis of intracellular IFN-γ staining results. Data are representative of three separate experiments using six to eight mice per group.
Preimmune status does not alter inflammation.
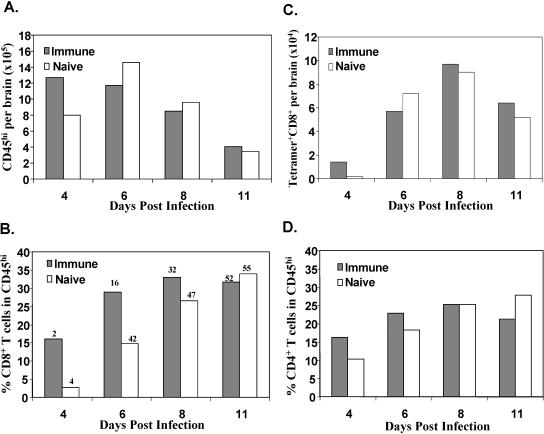
During acute JHMV infection of naïve mice, pN tetramer-positive cells consistently comprise 30 to 40% of CNS-derived CD8+ T cells by day 8 p.i. (3, 4, 32, 39). The relative delay in the peak pN-specific cell recruitment into the CNS of vac-p18 immune mice (Fig. 2) suggested that recruitment of memory cells may delay extravasation of primary activated T cells due to competition or space restrictions at entry sites. To determine whether bystander T-cell recruitment alters inflammatory cell composition or delays access of newly primed effector T cells, CNS infiltration in rVV immune mice was compared to that of naïve age-matched mice infected with JHMV. Total brain cell yields and infiltrating cells were similar in both groups except at day 4 p.i., when CD45hi inflammatory cell levels were increased in immunized mice (Fig. 5A). Analysis of the infiltrating cell composition revealed significantly higher levels of CD8+ T cells at days 4 and 6 p.i. in immune mice (Fig. 5B). Coincident with the prominent difference in CD8+ T-cell percentages, the relative proportion of pN-specific cells remained significantly reduced compared to the results seen with naïve mice infected with JHMV throughout day 8 p.i., indicating an apparent delay in recruitment. However, adjustment to total numbers of Ld/pN tetramer-positive CD8+ cells per brain revealed that the increased CD8+ T-cell influx compensated for the apparent lower frequency of pN-specific T cells (Fig. 5C). In contrast to CD8+ T-cell results, CD4+ T-cell recruitment was only slightly increased in the CNS of immune mice (Fig. 5D), consistent with prominent CD8+ T-cell activation-memory following rVV infection (47). At day 4 p.i., the reduced CD8+ T-cell percentages in infected naïve mice were compensated for by a relative increase in neutrophils and macrophages (3, 51). The kinetics and peak infiltration of other cell types were not significantly different in comparisons of the two groups (data not shown). CD8+ T cells are the prominent antiviral effectors controlling acute JHMV infection (3, 31). Comparison of antiviral effector functions in vac-p18 immune and naïve mice confirmed that the kinetics of viral clearance were identical in both groups (data not shown). These data suggest that recruitment of irrelevant bystander memory CD8+ T cells into the CNS during acute encephalitis does not delay the recruitment of newly primed antigen-specific effector T cells. Therefore, bystander recruitment provides a mechanism for the host to probe and respond rapidly to previously encountered microbial antigen without jeopardizing primary responses to a novel CNS antigen.
FIG. 5.
Immune status affects CD8+ T-cell inflammation. CNS cells derived from vac-p18 immune and age-matched naïve mice infected with JHMV were analyzed for CD45, CD8, CD4, and Ld/pN tetramer expression by flow cytometry at 4, 6, 8, and 11 days p.i. (A) Total infiltrating cells per brain identified by the CD45hi phenotype. (B) Relative percentages of CD8+ T cells within CD45hi infiltrates. Numbers above columns indicate relative percentages of Ld/pN tetramer+ cells within the CD8 population. (C) Total Ld/pN+CD8+ T cells per brain. (D) Relative percentages of CD4+ T cells within the CD45hi infiltrates. Data are representative of three separate experiments using three mice per group.
DISCUSSION
The presence of memory CD8+ T cells in many peripheral organs (34, 27) and their recruitment to sites of infection provides an opportunity for the host to mount a rapid protective immune response. However, bystander memory cells may also contribute to immune pathology or delay entry of newly primed T cells to sites of infection. Their protective, pathogenic, or passive role is difficult to predict, as several parameters, including cross-reactivity to heterologous pathogen or self-antigen, the site of infection, and competition for space, influence biological function. Although memory cells do not circulate from blood into the CNS (27), our results suggest that infection induces recruitment of random memory CD8+ T cells into the CNS and that this recruitment precedes the activation and extravasation of activated CD8+ T cells specific for the pathogen. However, as the numbers of JHMV-specific CD8+ T cells increased in response to the presence of antigen, the numbers of the non-cross-reactive bystander CD8+ T cells declined. The delay in pathogen-specific primary CD8+ T-cell recruitment, relative to the rapid recruitment of bystander CD8+ T cells, is surprisingly similar to that seen during pulmonary infection with respiratory syncytial virus (RSV) in LCMV immune mice (38). Although the relative percentage of RSV-specific CD8+ T cells was reduced following challenge of LCMV immune mice, increased total CD8+ T-cell inflammation resulted in similar total RSV-specific T-cell numbers in the lungs during peak inflammation. However, while RSV clearance was delayed in LCMV immune mice (38), JHMV clearance from the CNS was not affected by bystander recruitment. Furthermore, the observation that JHMV-specific CD8+ T cells were retained within the CNS whereas bystander CD8+ T-cell levels ultimately decreased to below detection limits is reminiscent of a heterologous respiratory infection model in which influenza virus infection of Sendai virus immune mice induces recruitment of nondividing Sendai virus-specific memory CD8+ T cells (12). In similarity to results seen with the CNS, as influenza virus infection of the lung progressed, Sendai virus-specific memory CD8+ T cells subsequently disappeared.
The mechanisms by which memory CD8+ T cells are recruited into the CNS remain undetermined. Restrictions imposed by the blood brain barrier clearly limit access of memory CD8+ T cells from the blood into the naïve CNS (19, 27). However, activated CD4+ T cells traffic into the naïve CNS without apparent signals provided from the parenchymal side (20). Similarly, activated TCR-transgenic CD8+ T cells accumulate in the CNS parenchyma only in the presence of cognate antigen (8). The transient accumulation of p18-specific CD8+ T cells in the CNS during acute peripheral rVV infection supports the concept that activated CD8+ T cells traffic into the CNS. However, in contrast to the apparent maintenance of CD8+ T cells in other peripheral organs (34), CD8+ T cells in the CNS decreased to undetectable levels following rVV clearance. Although this observation is consistent with the paucity of memory CD8+ T cells to access the naïve CNS (19), proinflammatory signals induced by JHMV CNS infection rapidly mobilize recruitment of heterologous memory CD8+ T cells. JHMV viral infection upregulates the expression of CCL5/RANTES, CXCL9/monokine induced by IFN-γ, and CXCL10/IP-10 (28-30). These cytokines-chemokines are necessary for the induction of inflammatory responses and attract T cells and macrophages to the site of infection. In contrast to the results seen with naïve T cells, activated and memory T cells express the chemokines receptors CXCR3 and CCR5 (10, 49). Rapid induction of cytokines-chemokines following CNS infection thus provides a likely mechanism for the early recruitment of bystander memory CD8+ T cells independent of antigen (10, 45).
The signals driving selective retention of pathogen-specific T cells after infectious virus is cleared are as yet unclear. One simplistic explanation is that activated antigen-specific T cells undergo prominent peripheral antigen-driven expansion and outcompete the bystander population. However, the consequences of local antigen recognition or differential activation states are unclear. Despite significant cross-reactivity between CD8+ T-cell epitopes among some heterologous viruses (7, 41, 50), there is no evidence for cross-reactivity between the p18 epitope and the dominant JHMV pN epitope. Furthermore, in similarity to the results seen with the splenic population, the majority of p18-specific CD8+ T cells isolated from the JHMV-infected CNS maintained a CD44hi CD127+ CD43lo/int memory phenotype. By contrast, pN-specific CD8+ T cells in both the spleen and CNS were highly activated, as demonstrated by the presence of a CD44hi CD127− CD43hi phenotype, which may confer enhanced adhesion facilitating extravasation into the CNS (37). Cognate antigen recognition within the CNS appears to further enhance accumulation of antigen-specific T cells. The idea of a role for antigen in T-cell retention is supported by the disappearance of JHMV-specific T cells in the CNS of mice that clear virus (32) as well as by adoptive transfer studies using other models of CNS inflammation (1, 8, 22).
In both herpes virus-induced stromal keratitis and the peripheral LCMV models, bystander CD8+ T cells traffic to the site of infection and contribute to disease pathology (9, 11, 42). Our results demonstrate that although virus-induced encephalitis recruits heterologous memory T cells, they are not activated in the process and do not acquire expression of ex vivo cytolytic activity. These data suggest that bystander CD8+ T-cell recruitment alone is unlikely to directly result in immune pathology in the absence of cognate or cross-reactive antigen. Indeed, no evidence for enhanced clinical disease was noted in immunized mice compared to infected naïve mice (data not shown). These results are consistent with highly selective destruction of antigen-expressing choroid epithelial cells (1) and astrocytes (8) by reactive cognate CD8+ T cells. In both models, no evidence for bystander damage was detected and cell loss was strictly dependent on antigen expression (8). Similarly, bystander CD8+ T cells recruited during LCMV CNS infection did not appear to contribute to CNS damage, even after activation (36). Although these results contrast with the enhanced pathology mediated by activated LCMV antigen-specific TCR-transgenic bystander CD8+ T cells in JHMV-infected TCR/Rag 2−/− mice (15), a consistent theme in all models is that only highly activated CD8+ T cells mediated a pathogenic affect with the CNS.
In conclusion, the data demonstrate that preimmune status can alter the composition of infiltrates into the CNS, especially very early following viral infection. While heterologous memory CD8+ T cells are indiscriminately recruited into the CNS as a component of the innate cellular response, they are rapidly displaced by pathogen-specific effector T cells. Furthermore, in the absence of cognate or cross-reactive antigen within the CNS, bystander T cells retain a memory phenotype, accumulate transiently, do not exert ex vivo cytolytic function, and are unlikely to contribute to immune pathology.
Acknowledgments
This work was supported by National Institutes of Health grants NS40667 and NS18146.
REFERENCES
- 1.Ando, K., L. G. Guidotti, A. Cerny, T. Ishikawa, and F. V. Chisari. 1994. CTL access to tissue antigen is restricted in vivo. J. Immunol. 153:482-488. [PubMed] [Google Scholar]
- 2.Babbe, H., A. Roers, A. Waisman, H. Lassmann, N. Goebels, R. Hohlfeld, M. Friese, R. Schroder, M. Deckert, S. Schmitdt, R. Ravid, and K. Rajewsky. 2000. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 192:393-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann, C. C., B. Parra, D. R. Hinton, R.Chandran, M. Morrison, S. A. Stohlman. 2003. Perforin mediated effector function within the CNS requires IFN-γ mediated MHC upregulation. J. Immunol. 170:3204-3213. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the CNS. J. Immunol. 163:3379-3387. [PubMed] [Google Scholar]
- 5.Bergmann, C. C., M. McMillan, and S. A. Stohlman. 1993. Characterization of the Ld-restricted cytotoxic T lymphocyte epitope in the mouse hepatitis virus nucleocapsid protein. J. Virol. 67:7041-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann, C. C., S. A. Stohlman, and M. Mcmillan. 1993. An endogenously synthesized decamer peptide efficiently primes cytotoxic T cells specific for the HIV-1 envelope glycoprotein. Eur. J. Immunol. 23:2777-2781. [DOI] [PubMed] [Google Scholar]
- 7.Brehm, M. A., A. K. Pinto, K. A. Daniels, J. P. Schneck, R. M. Welsh, and L. K. Selin. 2002. T cell immunodominance and maintenance of memory regulated by unexpectedly cross reactive pathogens. Nat. Immunol. 3:627-634. [DOI] [PubMed] [Google Scholar]
- 8.Cabarrocas, J., J. Bauer, E. Piaggio, R. Liblau, and H. Lassman. 2003. Effective and selective immune surveillance of the brain by MHC class I-restricted cytotoxic T lymphocytes. Eur. J. Immunol. 33:1174-1182. [DOI] [PubMed] [Google Scholar]
- 9.Chen, H. D., A. E. Fraire, I. Joris, M. A. Brehm, R. M. Welsh, and L. K. Selin. 2001. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat. Immunol. 2:1067-1076. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, J. E., A. Nansen, T. Moos, B. Lu, C. Gerard, J. P. Christensen, and A. R. Thomsen. 2004. Efficient T-cell surveillance of the CNS requires expression of the CXC chemokine receptor 3. J. Neurosci. 24:4849-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshpande, S., M. Zheng, S. Lee, K. Banerjee, S. Gangappa, U. Kumaraguru, and B. Rouse. 2001. Bystander activation involving T lymphocytes in herpetic stromal keratitis. J. Immunol. 167:2902-2910. [DOI] [PubMed] [Google Scholar]
- 12.Ely, K. H., L. S. Cauley, A. D. Roberts, J. W. Brennan, T. Cookenham, and D. L. Woodland. 2003. Non-specific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J. Immunol. 170:1423-1429. [DOI] [PubMed] [Google Scholar]
- 13.Fleming, J. O., M. Trousdale, F. E. Zactarim, S. A. Stohlman, and L. P. Weiner. 1986. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J. Virol. 58:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin, D. E. 2003. Immune responses to RNA-virus infections of the CNS. Nat. Rev. Immunol. 3:493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haring, J. S., L. L. Pewe, and S. Perlman. 2002. Bystander CD8+ T cell-mediated demyelination after viral infection of the central nervous system. J. Immunol. 169:1550-1555. [DOI] [PubMed] [Google Scholar]
- 16.Harrington, L. E., M. Galvan, L. G. Baum, J. D. Altman, and R. Ahmed. 2000. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J. Exp. Med. 191:1241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington, L. E., R. van der Most, J. L. Whitton, and R. Ahmed. 2002. Recombinant vaccinia virus-induced T-cell immunity: quantification of the response to the virus vector and the foreign epitope. J. Virol. 76:3329-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawke, S., P. G. Stevenson, S. Freeman, and C. R. Bangham. 1998. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J. Exp. Med. 187:1575-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickey, W. F. 2001. Basic principles of immunological surveillance of the normal central nervous system. Glia 36:118-124. [DOI] [PubMed] [Google Scholar]
- 20.Hickey, W. F., B. F. Hsu, and H. Kimura. 1991. T lymphocyte entry into the central nervous system. J. Neurosci. Res. 28:254-260. [DOI] [PubMed] [Google Scholar]
- 21.Huster, K. M., V. Busch, M. Schiemann, K. Linkemann, K. M. Kerksiek, H. Wagner, and D. H. Busch. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 101:5610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irani, D. N., and D. E. Griffin. 1996. Regulation of lymphocyte homing into the brain during viral encephalitis at various stages of infection. J. Immunol. 156:3850-3857. [PubMed] [Google Scholar]
- 23.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 24.Kambayashi, T., E. Assarsson, A. E. Lukacher, H. G. Ljunggren, and P. E. Jensen. 2003. Memory CD8+ T cells provide an early source of IFN-γ. J. Immunol. 170:2399-2408. [DOI] [PubMed] [Google Scholar]
- 25.Karupiah, G., B. Coupar, B., I. Ramshaw, D. Boyle, R. Blanden, and M. Andrew. 1990. Vaccinia virus-mediated damage of murine ovaries and protection by virus-expressed interleukin-2. Immunol. Cell Biol. 68:325-333. [DOI] [PubMed] [Google Scholar]
- 26.Kim, S. K., M. A. Brehm, R. M. Welsh, and L. K. Selin. 2002. Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J. Immunol. 169:90-98. [DOI] [PubMed] [Google Scholar]
- 27.Klonowski, K. D., K. J. Williams, A. L. Marzo, D. A. Blair, E. G. Lingenheld, and L. Lefrancois. 2004. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20:551-562. [DOI] [PubMed] [Google Scholar]
- 28.Lane, T. E., M. Liu, B. P. Chen, V. C. Asensio, R. M. Samawi, A. D. Paoletti, I. L. Campbell, S. L. Kunkel, H. S. Fox, and M. J. Buchmeier. 2000. A central role for CD4+ T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J. Virol. 74:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. Hamilton, and T. E. Lane. 2000. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165:2327-2330. [DOI] [PubMed] [Google Scholar]
- 30.Liu, M. T., D. Armstrong, T. A. Hamilton, and T. E. Lane. 2001. Expression of Mig (monokine induced by interferon-γ) is important in T lymphocyte recruitment and host defense following viral infection of the central nervous system. J. Immunol. 166:1790-1795. [DOI] [PubMed] [Google Scholar]
- 31.Marten, N. W., S. A. Stohlman, and C. C. Bergmann. 2001. MHV infection of the CNS: mechanisms of immune-mediated control. Viral. Immunol. 14:1-18. [DOI] [PubMed] [Google Scholar]
- 32.Marten, N. W., S. A. Stohlman, and C. C. Bergmann. 2000. Role of viral persistence in retaining CD8+ T cells within the CNS. J. Virol. 74:7903-7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marten, N. W., S. A. Stohlman, W. Smith-Begolka, S. D. Miller, E. Dimicali, Q. Yao, S. Stohl, J. Goverman, and C. C. Bergmann. 1999. Selection of CD8+ cells with highly focused specificity during viral persistence in the central nervous system. J. Immunol. 162:3905-3914. [PubMed] [Google Scholar]
- 34.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 35.Masopust, D., V. Vezys, E. J. Usherwood, L. S. Cauley, S. Olson, A. L. Marzo, R. L. Ward, D. Woodland, and L. Lefrancois. 2004. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 172:4875-4882. [DOI] [PubMed] [Google Scholar]
- 36.McGavern, D. B., and P. Truong. 2004. Rebuilding an immune-mediated central nervous system disease: weighing the pathogenicity of antigen-specific versus bystander T cells. J. Immunol. 173:4779-4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onami, T. M., L. E. Harrington, M. A. Williams, M. Galvan, C. P. Larsen, T. C. Pearson, N. Manjunath, L. G. Baum, B. D. Pearce, and R. Ahmed. 2002. Dynamic regulation of T cell immunity by CD43. J. Immunol. 168:6022-6031. [DOI] [PubMed] [Google Scholar]
- 38.Ostler, T., H. Pircher, and S. Ehl. 2003. “Bystander” recruitment of systemic memory T cells delays the immune response to respiratory virus infection. Eur. J. Immunol. 33:1839-1848. [DOI] [PubMed] [Google Scholar]
- 39.Ramakrishna, C., S. A. Stohlman, R. D. Atkinson, M. J. Shlomchik, and C. C. Bergmann. 2002. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J. Immunol. 168:1204-1211. [DOI] [PubMed] [Google Scholar]
- 40.Sedgwick, J. D., and W. F. Hickey. 1997. Antigen presentation in the central nervous system, p. 364. Immunology of the central nervous system. Oxford University Press, New York, N.Y.
- 41.Selin, L. K., K. Vergilis, R. M. Welsh, and S. R. Nahill. 1996. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J. Exp. Med. 183:2489-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selin, L. K., S. M. Varga, L. C. Wong, and R. M. Welsh. 1998. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J. Exp. Med. 188:1705-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slifka, M. K., F. Rodriguez, and J. L. Whitton. 1999. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature 401:76-79. [DOI] [PubMed] [Google Scholar]
- 44.Tough, D. F., P. Borrow, and J. Sprent. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272:1947-1950. [DOI] [PubMed] [Google Scholar]
- 45.Trifilo, M. J., C. C. Bergmann, W. A. Kuziel, and T. Lane. 2003. CC chemokine ligand 3 (CCL3) regulates CD8+-T-cell effector function and migration following viral infection. J. Virol. 77:4004-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van der Most, R. G., K. Murali-Krishna, and R. Ahmed. 2003. Prolonged presence of effector-memory CD8+ T cells in the central nervous system after dengue virus encephalitis. Int. Immunol. 15:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villacres, M. C., J. Zuo, and C. C. Bergmann. 2000. Maintenance of CD8+ T cell memory following infection with recombinant sindbis and vaccinia viruses. Virology 270:54-64. [DOI] [PubMed] [Google Scholar]
- 48.Wang, R. I., D. R. Hinton, W. Gilmore, M. D. Trousdale, and J. O. Fleming. 1992. Sequential infection of glial cells by the murine hepatitis virus JHM strain (MHV-4) leads to a characteristic distribution of demyelimnation. Lab. Investig. 66:744-754. [PubMed] [Google Scholar]
- 49.Ward, S. G., K. Bacon, and J. Westwick. 1998. Chemokines and T lymphocytes: more than an attraction. Cell 9:1-11. [DOI] [PubMed] [Google Scholar]
- 50.Wedemeyer, H., E. Mitukoshi, A. R. Davis, J. R. Bennink, and B. Rehermann. 2001. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J. Virol. 75:11392-11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou, J., S. A. Stohlman, D. R. Hinton, and N. W. Marten. 2003. Neutrophils promote mononuclear cell infiltration during viral-induced encephalitis. J. Immunol. 170:3331-3336. [DOI] [PubMed] [Google Scholar]