Abstract
Baseline trachoma surveys in Côte d'Ivoire (2019) identified seven evaluation units (EUs) with a trachomatous inflammation–follicular (TF) prevalence ≥10%, but a trachomatous trichiasis (TT) prevalence in individuals ≥15 y of age below the elimination threshold (0.2%). Two of these EUs, Bondoukou 1 and Bangolo 2, were selected for a follow-up survey to understand the epidemiology of trachoma using additional indicators of Chlamydia trachomatis infection (DNA from conjunctival swabs) and exposure (anti-Pgp3 and Ct694 antibodies from dried blood spots [DBSs]). A two-stage cluster sampling methodology was used to select villages and households. All individuals 1–9 y of age from each selected household were recruited, graded for trachoma and had a conjunctival swab and DBS collected. Conjunctival swabs and DBSs were tested using Cepheid GeneXpert and a multiplex bead assay, respectively. The age-adjusted TF and infection prevalence in 1- to 9-year-olds was <1% and <0.3% in both EUs. Age-adjusted seroprevalence was 5.3% (95% confidence interval [CI] 1.5 to 15.6) in Bondoukou 1 and 8.2% (95% CI 4.3 to 13.7) in Bangolo 2. The seroconversion rate for Pgp3 was low, at 1.23 seroconversions/100 children/year (95% CI 0.78 to 1.75) in Bondoukou 1 and 1.91 (95% CI 1.58 to 2.24) in Bangolo 2. Similar results were seen for CT694. These infection, antibody and clinical data provide strong evidence that trachoma is not a public health problem in either EU.
Keywords: alternative indicators, Chlamydia trachomatis, elimination, seroconversion, trichiasis, trachoma infection
Introduction
Trachoma is the leading infectious cause of blindness worldwide and is caused by particular strains of the bacterium Chlamydia trachomatis (Ct). Ct can be transmitted directly from eye or nasal discharges during close contact or indirectly on fomites and by eye-seeking flies.1–3 Infection can cause inflammation of the conjunctiva, known as trachomatous inflammation–follicular (TF). Repeated infections may lead to scarring of the inner eyelids. Scarring causes a loss of elasticity of the upper eyelid and potentially in-turning of the eyelashes, which may rub against the eyeball. When one or more eyelashes touch the eyeball because of trachoma, it is known as trachomatous trichiasis (TT). TT can lead to blindness if not treated.4,5 According to the World Health Organization (WHO), in June 2022, an estimated 125 million people lived in trachoma-endemic areas and were at risk of trachoma-related blindness.6 Children <10 y of age are the principal reservoirs of Ct infection.7,8 Environmental factors such as hygiene, sanitation, access to water and latrine use are associated with transmission intensity of the trachoma bacterium.9–13
The WHO has set a target for elimination of trachoma as a public health problem by 203014 through implementation of the SAFE (surgery for trichiasis, antibiotics for infection, facial cleanliness and environmental improvement) strategy.15 Elimination is said to be achieved when three targets are met: a TT prevalence of <0.2% in adults ≥15 y of age; a system able to identify and manage incident TT cases, using defined strategies, with evidence of appropriate financial resources to implement those strategies; and a TF prevalence <5% in children ages 1–9 y achieved and sustained for at least 2 y in the absence of ongoing antibiotic mass drug administration (MDA) in each formerly endemic district.16 However, TF is used as a proxy indicator of Ct infection and there are several issues with relying solely on this indicator, including the fact that follicles can be caused by other pathogens17 and follicles can last for years after infection has been cleared.18 Also, intergrader agreement can be an issue.19,20
The presence of intermittent chlamydial antigens elicits a chronic immune response following recurrent TF, leading to TT. Nonetheless, in areas with significant TF and no prior interventions, it is expected that there would be some TT cases. In Côte d'Ivoire, baseline surveys indicated no or very low levels of TT (<0.2%) even though TF prevalence estimates were high (>28% in the two evaluation units [EUs] selected for this study). Similar discrepancies between TT and TF have been reported in Australia, Fiji, Papua New Guinea, Solomon Islands and Vanuatu.21–27 In cases where the TF and TT prevalence do not correlate, other indicators may be used to guide decision making.28 These indicators include nucleic acid amplification testing of conjunctival swabs, used as a marker of current infection with Ct, and testing for the presence of antibodies against Ct antigens in blood to measure exposure to Ct. This study therefore aimed to use alternative indicators to ascertain the epidemiological situation of trachoma in this area.
Methods
Study area
This study was conducted in two EUs: Bangolo 2, in the western part of Côte d'Ivoire, and Bondoukou 1, in the east (Figure 1). The Bondoukou 1 EU was composed of three districts—Nassian, Sandégué and part of Bondoukou—while the Bangolo 2 EU was made up of part of the Bangolo district. Both EUs had a TF prevalence of 28.3% and a TT prevalence <0.2% at the baseline survey (2019) prior to any trachoma interventions. Bondoukou 1 and Bangolo 2 had total populations of 222 636 and 192 516, respectively, with the proportion of children ages 1–9 y estimated at 28% of the total population (Côte d'Ivoire population Statistics, 2014). Both EUs had received a round of MDA prior the present survey.
Figure 1.
Study area. The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the authors, or the institutions with which they are affiliated, concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Study design
This was a population-based cross-sectional study conducted between July and October 2021, with Tropical Data support (https://www.tropicaldata.org/) for routine survey methodology elements and village and household selection, following WHO recommendations.29,30 A two-stage cluster sampling method was used to select the villages (primary sampling unit) and households (secondary sampling unit) in each EU.
Sample size
A total of 1701 children ages 1–9 y were estimated to be required for each EU, based on an expected TF prevalence of 10%, 95% confidence, an absolute precision of 3%, a design effect of 3.6931 and an inflation factor of 1.2 to account for non-response.30 In addition to the children selected, 10% of adults were also sampled (approximately 11 adults per village) as a control to ensure anti-Ct antibodies were stable in dried blood spots (DBSs).
Cluster and household selection
In each EU, a full list of villages was prepared using the district census lists. Thirty villages were sampled with probability proportional to size, and within each village, 30 households were selected using compact segment sampling.29 When the number of households did not reach the required number in a segment, the team continued the selection in the neighbourhood adjacent to the selected one. All children ages 1–9 y from each selected household were invited to be included in the study. To select the 10% of adults, the numbers 1–30, corresponding to the 30 households, were written on pieces of paper, folded and placed in a bowl. Eleven households from the list were randomly selected from the bowl. When the field team visited the selected house, an adult from the house was sampled. In households with more than one adult present at the time of the visit, one of them was randomly selected by writing the initials of their names on pieces of paper that were folded and then one of the team members picked one. This gave a total number of 315 adults per EU.
Specimen collection and storage
The survey team was composed of Tropical Data–certified trachoma graders and recorders and laboratory technicians. The graders performed the clinical examination and the laboratory technicians collected the conjunctival swabs and DBSs. The recorders registered the data into an Android smartphone.
Eye examination and swab collection
After obtaining consent and participant registration, both eyes of children ages 1–9 y were examined for the presence or absence of TF and trachomatous inflammation–intense (TI) according to the WHO simplified grading system, using 2.5× binocular loupes and follicle size guides.29,32–34 After grading, a polyester-coated cotton swab was passed three times over the everted left conjunctiva with a 120-degree rotation made between each pass. The swab was placed directly in an empty sterile polyethylene tube with a barcoded label specific to the individual's sample. The swabs were placed in a cooler containing icepacks and at the end of each day they were transported to the District Service laboratories in each EU where they were refrigerated at 4°C. By the third day, the samples were transferred to a freezer at −20°C for long-term storage at the Institute Pasteur (IP) laboratory in Côte d'Ivoire. At the end of the sample collection, ocular swabs were shipped on dry ice to the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, USA, where they were stored at −20°C prior to testing.
Blood collection
The tip of the middle finger of the left hand was then cleaned with cotton and 70% alcohol and pricked using a lancet. The first blood drop was wiped off and subsequent flow was collected on a TropBio filter paper disc containing six circular extensions calibrated to hold 10 µl of blood (TropBio, Townsville, QLD, Australia). The filter paper was affixed with a barcoded label for each participant to link data. The samples were air dried and stored in sealable plastic bags with desiccant at room temperature in the district laboratories and on the third day they were transported to the IP laboratory, where they were stored at −20°C. The DBSs were shipped to the CDC at ambient temperature and stored at −20°C prior to testing.
Sample analysis
Infection testing
Testing of ocular swabs was done according to a previously published methodology35 using the Cepheid GeneXpert CT/NG kit (Cepheid, Sunnyvale, CA, USA). Swabs were eluted in 1 ml of diethyl pyrocarbonate (DEPC) water for >1 h at 4°C. A total of 200 µl of each specimen elution was added to a tube containing Cepheid transport media, then 260 µL of this solution was added to a 2 ml tube to create pools of five specimens each. A total of 1200 µl of each pool was added immediately to the sample chamber of the Cepheid CT/NG cartridge and run according to the manufacturer's instructions. Samples that were part of each positive pool were subsequently tested individually to identify positive specimens. Each run included a sample processing control, sample adequacy control and probe check control included with each kit. Pools and specimens from invalid runs were retested.
Multiplex bead assay
The DBSs were tested for antibodies against the Ct antigens Pgp3 and CT694 by multiplex bead assay as previously described.36 Briefly, each DBS extension was eluted overnight at 4°C in 1X phosphate-buffered saline (PBS) containing 0.5% casein, 0.3% Tween 20, 0.5% polyvinyl alcohol, 0.8% polyvinylpyrrolidone, 0.02% sodium azide and 3 µg/ml Escherichia coli extract (Buffer B). DBS eluates were diluted in Buffer B to a final serum dilution of 1:400. Pgp3- and CT694-coupled beads (1000 per antigen per well) were incubated for 1.5 h with 50 µl of diluted DBS eluate. Wells were washed three times with PBST (1X PBS+0.3% Tween 20) and incubated with 50 ng biotinylated mouse anti-human immunoglobulin G (IgG) (Southern BioTech, Birmingham, AL, USA) and 40 ng biotinylated mouse anti-human IgG4 (Southern BioTech) for 45 min to detect any Pgp3- or CT694-specific IgG bound to the beads. After three washes with PBST, wells were incubated with 250 ng phycoerythrin-labelled streptavidin (Invitrogen, South San Francisco, CA, USA) for 30 min. Wells were washed three times with PBST and incubated with 50 µl PBS containing 0.5% BSA, 0.05% Tween 20 and 0.02% sodium azide to remove any loosely bound antibodies. After one more wash with PBST, wells were suspended in 100 µl PBS and plates were stored overnight at 4°C. The next day, plates were read on a MAPGIX instrument equipped with xPONENT software (Luminex, Austin, TX, USA). The median fluorescence intensity (MFI) with the background from the blank well (Buffer B alone) subtracted out (MFI-bg) was recorded for each antigen for each sample. The cut-off of positivity was established as an MFI-bg of 168 for Pgp3 and 200 for CT694 by using receiver operating characteristics curve analysis on a panel of 122 samples from ocular Ct PCR-positive individuals from the United Republic of Tanzania and 74 paediatric samples from New York, NY, USA, that tested negative by a chlamydial micro-immunofluorescence assay.
Data collection and analysis
All routine prevalence survey field data were collected and managed electronically using the Tropical Data system and EU-level age-adjusted TF and TI prevalences were calculated as the mean of the adjusted village-level proportions.29 Non-routine survey data management and analysis were conducted by the CDC. Overall seroprevalence estimates for 1- to 9-year-olds and individuals ≥15 y were calculated for each antigen using age-adjusted EU-level seroprevalence proportions. Seroconversion rates were calculated for each antigen using Bayesian serocatalytic models in R (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria) as previously described.37 The prevalence of infection for 1- to 9-year-olds was calculated using age-adjusted EU-level infection prevalence proportions.
Results
Descriptive statistic
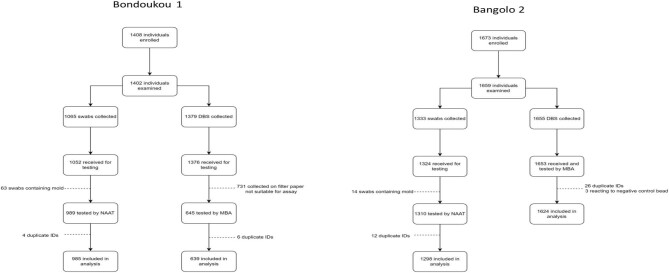
A total of 1639 households were examined from the 60 selected villages in the two EUs combined. In Bondoukou 1, a total of 1408 individuals were enrolled in the study (Figure 2). Of these individuals, 1402 (99.6%) were examined, with 1065 (76%) children providing a swab sample and 1379 (98.4%) children and adults providing a DBS sample (Table 1, Figure 2). In Bangolo 2, 1673 individuals were enrolled in the study. Of these, 1659 (99.2%) were examined, with 1333 (80.3%) children providing swabs and 1655 (99.8%) children and adults providing DBSs (Table 1, Figure 2). Almost 25% (n=731) of the samples collected could not be analysed because the filter papers used were not suitable for the assay. The age-adjusted prevalence of TF in 1- to 9-year-olds was 0.6% (95% confidence interval [CI] 0.0 to 1.4) in Bondoukou 1 and 0.6% (95% CI 0.2 to 1.2) in Bangolo 2. The age-adjusted prevalence of conjunctival Ct infection was <0.5% in each EU (0.2% and 0.3% for Bondoukou 1 and Bangolo 2, respectively).
Figure 2.
Flow chart for sample collection and analysis in each EU. Abbreviations: 1) NAAT = Nucleic Acid Amplification Testing; 2) MBA = Multiplex Bead Assay; 3) DBS = Dried Blood Spots.
Table 1.
Households and individuals enrolled and examined, TF and TI prevalence in children ages 1–9 y in Bondoukou 1 and Bangolo 2, Côte d'Ivoire, 2021
| EU | Households surveyed/enrolled, n | Age category (years) | Swabs, n | DBSs, n | Age-adjusted prevalence of TF in 1- to 9-year-olds (95% CI) | Age-adjusted prevalence of TI in 1- to 9-year-olds (95% CI) |
|---|---|---|---|---|---|---|
| Bondoukou 1 | 803/ 1408 |
1–9 | 1065 | 1065 | 0.59 (0.05 to 1.40) | 0 (–) |
| ≥15 | – | 314 | – | – | ||
| Bangolo 2 | 836/ 1673 |
1–9 | 1333 | 1333 | 0.61 (0.19 to 1.22) | 0.12 (0.00 to 0.35) |
| ≥15 | – | 322 | – | – |
Serology
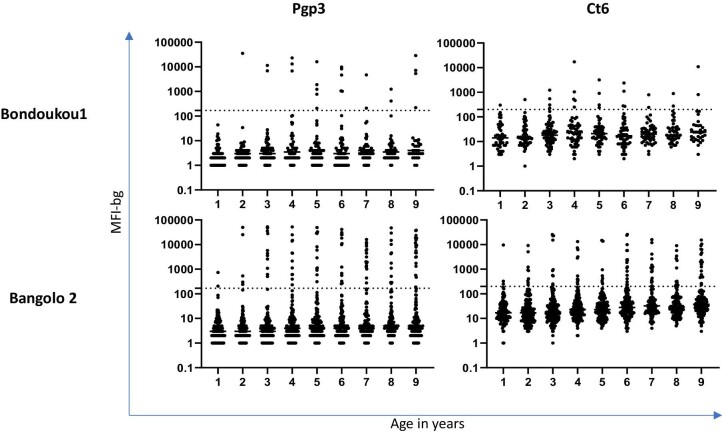
Table 2 shows the age-adjusted seroprevalence for Pgp3 and Ct694 in 1- to 9-year-olds and individuals ≥15 y for each EU. Seroprevalence was <10% for 1- to 9-year-olds in both districts by both antigens. Seroprevalence in individuals ≥15 y of age was >50% in both EUs for Pgp3 and >30% in both EUs for Ct694. The intensity of antibody response, represented by MFI-bg, by year for age is shown in Figure 3.
Table 2.
Age-adjusted prevalence of antibodies to Pgp3 and Ct694 antigens, Bondoukou 1 and Bangolo 2, Côte d'Ivoire, 2021
| Age-adjusted prevalence of Pgp3, % (95% CI) | Age-adjusted prevalence of Ct694, % (95% CI) | |||
|---|---|---|---|---|
| EU | 1–9 y | ≥15 y | 1–9 y | ≥15 y |
| Boudoukou 1 | 5.3 (1.5 to 15.6) |
57.0 (39.1 to 74.0) |
5.2 (1.2 to 15.6) |
33.8 (19.4 to 53.4) |
| Bangolo 2 | 8.2 (4.7 to 13.7) |
73.6 (61.0 to 83.8) |
8.7 (5.1 to 14.3) |
56.8 (44.4 to 69.0) |
Figure 3.
Intensity of antibody responses by age in 1- to 9-year-olds in each EU. Each point represents a single study participant. Abbreviations: 1) MFI-bg = Median Fluorescence Intensity with background: 2) Pgp = Plasmid Gene Protein; 3) Ct = Chlamydia trachomatis.
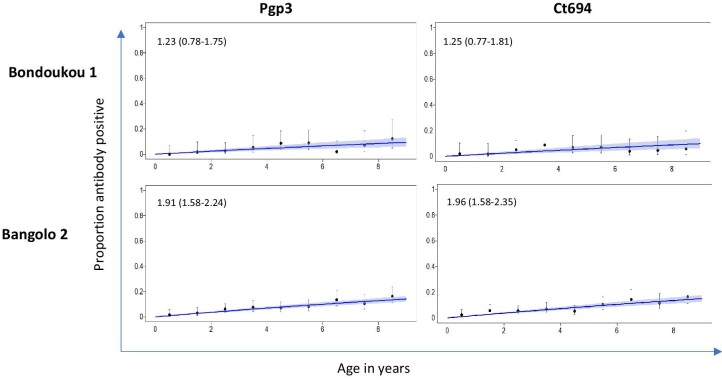
Figure 4 shows the proportion of antibody positive individuals by year of age in 1- to 9-year-olds and the seroconversion rate (SCR) estimates per 100 children for each antigen in each EU. The SCR was <2 seroconversion events per 100 children per year for each antigen in each EU.
Figure 4.
Proportion of antibody positive individuals by age in 1- to 9-year-olds in each EU. Seroconversion rates per 100 individuals with 95% CIs are shown on each graph. Seroconversion rates per 100 individuals for each graph with 95% CI in parenthesis.
Discussion
In this study we showed that despite high baseline TF prevalence in children, updated prevalence of TF and prevalence of Ct infection from conjunctival swabs and exposure from DBSs indicate that trachoma is not a public health problem in the two EUs surveyed. There was higher seroprevalence in older age groups, indicating trachoma may have been a public health problem in previous years. However, as the tests cannot differentiate between ocular and urogenital Ct infection and exposure, it is unclear how much past infection was due to trachoma, especially in the context of low TT prevalence. These data indicate the value of utilizing multiple indicators to better understand the epidemiology of trachoma.
The TF prevalence estimates generated here contrast with the baseline TF prevalence. While azithromycin MDA was given before the present survey, this would likely not have been sufficient to reduce the TF prevalence below the 5% elimination threshold or to have impacted the serological profile.38,39 Given the cross-validation of all markers used in the repeat survey, this discrepancy is possibly due to an overestimation of the baseline TF prevalence. The overestimation may be due to issues regarding grading, data collection or other field processes. Although Tropical Data has quality assurance measures throughout the process,40 including the use of follicle size guides to help standardize the diagnosis,34 TF is subjective in nature. In addition, training for TF grading is becoming increasingly difficult as TF becomes rarer and more districts achieve the elimination thresholds.29 This challenge necessitates the need to explore alternatives to grading children during training, such as the use of photography. Photography could also help with quality assurance and supervision during fieldwork.41 Alternatively, the discrepancy could be due to follicles at the baseline survey being caused by something other than ocular Ct infection or resolution of TF lagging behind the clearance of Ct infection.18
Results of this study demonstrate the utility of collecting data on complementary indicators to estimate trachoma prevalence in settings with contradictory survey findings. The need for alternative indicators has also been demonstrated by other studies in areas of unusual trachoma epidemiology.21–26 In these settings, data from these alternative indicators provided information suggesting that the elevated TF levels found were not necessarily due to Ct, thus MDA treatments were not recommended. Similarly, our study prompted Côte d'Ivoire's National NTD Program and its partners to evaluate the need to continue MDA in these EUs as well as the utility of including further infection/serology testing in other EUs as part of decision-making processes. Although MDA may no longer be required, as indicated by our study, MDA of azithromycin has been demonstrated to have off-target benefits, such as reduced infant mortality, perhaps as a result of treating respiratory infections42–44 and malaria.45,46 Conversely, unnecessary MDA of antibiotics to whole districts necessitates appropriate monitoring of antimicrobial resistance (AMR), although no Ct AMR has been detected.47
Our study has some limitations. First, the coronavirus disease 2019 pandemic delayed the date at which the present survey could be done; the increased intervening period with the baseline survey introduces possible confounders in interpreting the results. Second, some of the filter papers used for the DBSs were not suitable for the assay (N=731). As a result, the blood collected with these filter papers could not be processed, resulting in a reduced sample size for the serology analysis. Third, although collecting data on these additional indicators can help give a fuller epidemiological profile to facilitate programmatic decision making, there are as yet no recommended thresholds for programmatic decision making with these indicators. Ongoing research will help inform these much-needed recommendations.
Conclusions
In Côte d'Ivoire, as in many countries, trachoma remains a public health problem.6 In this study, prompted by an unusual epidemiological profile of high baseline TF prevalence in children not accompanied by a high TT prevalence in adults, we have demonstrated the utility of measuring multiple indicators to better understand the epidemiology of trachoma and inform programmatic decision making. The updated TF prevalence plus infection and antibody data all suggest that trachoma is not a public health problem in the two EUs surveyed and that no further interventions for trachoma are required.
Contributor Information
Kareen Atekem, Department of Entomology, Center for Infectious Disease Dynamics, Pennsylvania State University, University Park, PA, USA; Sightsavers.
Emma M Harding-Esch, London School of Hygiene and Tropical Medicine, London, UK.
Diana L Martin, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Philip Downs, Sightsavers.
Stephanie L Palmer, FHI 360, Washington, DC, USA.
Achille Kaboré, FHI 360, Washington, DC, USA.
Michaela Kelly, Sightsavers.
Anoma Bovary, Ministry of Health, Abidjan, Côte d'Ivoire.
Astou Sarr, Sightsavers.
Konan Nguessan, Sightsavers.
Fiona James, Sightsavers.
Sarah Gwyn, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Karana Wickens, Oak Ridge Institute for Science and Education, Atlanta, GA, USA.
Ana Bakhtiari, International Trachoma Initiative, Task Force for Global Health, Decatur, GA, USA.
Sarah Boyd, International Trachoma Initiative, Task Force for Global Health, Decatur, GA, USA.
Ange Aba, FHI 360, Washington, DC, USA.
Laura Senyonjo, Sightsavers.
Paul Courtright, Sightsavers; Kilimanajaro Centre for Community Ophthalmology, Division of Ophthalmology, University of Cape Town, Cape Town, South Africa.
Aboulaye Meite, Ministry of Health, Abidjan, Côte d'Ivoire.
Authors’ contributions:
AM, DLM, PC, PD, AK, SLP and EMHE were responsible for conception and design. KA, MK, ABovary, AS, KN, FJ and LS were responsible for implementation and coordination. KA, KN, ABovary, DLM, SG, KW, ABakhtiari, SB and AA were responsible for data collection, analysis and interpretation. KA, EMHE, SLP, DLM, PD, MK, ABakhtiari, LS and PC were responsible for drafting and revising of article. All authors approved the final version of the manuscript.
Acknowledgements:
We thank the Côte d'Ivoire's National NTD Program for facilitating the implementation of this research. We are also thankful to the members of the various EUs for agreeing to take part in this work. Our gratitude goes to Dr Sarr Boubacar and Dr Kadjo Gamael Koizan for training the survey team and the survey team for their active contribution in the field. We equally thank Tropical Data for providing the data collection platform, IP for sample storage and transportation and the CDC for performing laboratory analysis. Finally, we thank Sightsavers for the overall support and coordination of the research implementation.
Funding:
Core Tropical Data funding was provided by the International Trachoma Initiative, Sightsavers and RTI International through the USAID Act to End NTDs East program. In addition to funding, Sightsavers supported additional sample collection (training and consumables) and the CDC provided shipping and analysis. AB and SB are employed by the International Trachoma Initiative at the Task Force for Global Health, which receives an operating budget and research funds from Pfizer, the manufacturer of Zithromax (azithromycin). EMH-E is chief scientist for Tropical Data and receives salary support from the International Trachoma Initiative.
Competing interests:
KA, MK and PC are Guest Editors of this supplement but had no role in the review of this manuscript.
Ethical approval:
Approval for conducting this study was obtained from the Comité National d'Ethique des Sciences de la Vie et de la Sante, Côte d’Ivoire (approval 155-21/MSHP/CNESVS-km). Ethical approval for Tropical Data survey support was provided by the London School of Hygiene and Tropical Medicine (16105). Written consent was obtained from parents or guardians of children ages 1–9 y to be enrolled in the survey. Furthermore, both consent and assent were obtained from individuals ages 15–17 y and consent from all adults ≥18 y. Verbal consent for examination was also recorded in the Android smartphone app. CDC staff did not interact with study participants or have access to identifying information.
Data availability
All relevant data are presented within the manuscript.
References
- 1. Miller K, Pakpour N, Yi Eet al. . Pesky trachoma suspect finally caught. Br J Ophthalmol. 2004;88(6):750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Last A, Versteeg B, Shafi Abdurahman Oet al. . Detecting extra-ocular Chlamydia trachomatis in a trachoma-endemic community in Ethiopia: identifying potential routes of transmission. PLoS Negl Trop Dis. 2020;14(3):e0008120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Versteeg B, Vasileva H, Houghton Jet al. . Viability PCR shows that non-ocular surfaces could contribute to transmission of Chlamydia trachomatis infection in trachoma. PLoS Negl Trop Dis. 2020;14(7):e0008449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu VH, Harding-Esch EM, Burton MJet al. . Epidemiology and control of trachoma: systematic review. Trop Med Int Health. 2010;15(6):673–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowman RJ, Jatta B, Cham Bet al. . Natural history of trachomatous scarring in The Gambia: results of a 12-year longitudinal follow-up. Ophthalmology. 2001;108(12):2219–24. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization . WHO Alliance for the Global Elimination of Trachoma by the year 2021: progress report on elimination of trachoma. Wkly Epidemiol Rec. 2022;97(31):353–64. [Google Scholar]
- 7. Solomon AW, Holland MJ, Burton MJet al. . Strategies for control of trachoma: observational study with quantitative PCR. Lancet. 2003;362(9379):198–204. [DOI] [PubMed] [Google Scholar]
- 8. Nash SD, Chernet A, Moncada Jet al. . Ocular Chlamydia trachomatis infection and infectious load among pre-school aged children within trachoma hyperendemic districts receiving the SAFE strategy, Amhara region, Ethiopia. PLoS NeglTrop Dis. 2020;14(5):e0008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emerson PM, Cairncross S, Bailey RLet al. . Review of the evidence base for the ‘F’ and ‘E’ components of the SAFE strategy for trachoma control. Trop Med Int Health. 2000;5(8):515–27. [DOI] [PubMed] [Google Scholar]
- 10. Burton MJ, Holland MJ, Faal Net al. . Which members of a community need antibiotics to control trachoma? Conjunctival Chlamydia trachomatis infection load in Gambian villages. Invest Ophthalmol Vis Sci. 2003;44(10):4215–22. [DOI] [PubMed] [Google Scholar]
- 11. Last AR, Burr SE, Weiss HAet al. . Risk factors for active trachoma and ocular Chlamydia trachomatis infection in treatment-naïve trachoma-hyperendemic communities of the Bijagós Archipelago, Guinea Bissau. PLoS Negl Trop Dis. 2014;8(6):e2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garn JV, Boisson S, Willis Ret al. . Sanitation and water supply coverage thresholds associated with active trachoma: modeling cross-sectional data from 13 countries. PLoS Negl Trop Dis. 2018;12(1):e0006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sullivan KM, Harding-Esch EM, Keil APet al. . Exploring water, sanitation, and hygiene coverage targets for reaching and sustaining trachoma elimination: G-computation analysis. PLoS Negl Trop Dis. 2023;17(2):e0011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization; 2020. [Google Scholar]
- 15. Planning for the Global Elimination of Trachoma. Consultation (1996: Geneva, Switzerland) & World Health Organization. Programme for the Prevention of Blindness and Deafness . Planning for the Global Elimination of Trachoma (GET): report of a WHO Consultation, Geneva, Switzerland, 25 and 26 November 1996. World Health Organization. 1997.https://apps.who.int/iris/handle/10665/66169.
- 16. World Health Organization . Validation of elimination of trachoma as a public health problem. Geneva: World Health Organization; 2016. [Google Scholar]
- 17. Burr SE, Hart JD, Edwards Tet al. . Association between ocular bacterial carriage and follicular trachoma following mass azithromycin distribution in The Gambia. PLoS Negl Trop Dis. 2013;7(7):e2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grassly NC, Ward ME, Ferris Set al. . The natural history of trachoma infection and disease in a Gambian cohort with frequent follow-up. PLoS Negl Trop Dis. 2008;2(12):e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rahman SA, Yu SN, Amza Aet al. . Reliability of trachoma clinical grading—assessing grading of marginal cases. PLoS Negl Trop Dis. 2014;8(5):e2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gebresillasie S, Tadesse Z, Shiferaw Aet al. . Inter-rater agreement between trachoma graders: comparison of grades given in field conditions versus grades from photographic review. Ophthalmic Epidemiol. 2015;22(3):162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butcher RMR, Sokana O, Jack Ket al. . Low prevalence of conjunctival infection with Chlamydia trachomatis in a treatment-naïve trachoma-endemic region of the Solomon Islands. PLoS Negl Trop Dis. 2016;10(9):e0004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macleod CK, Butcher R, Mudaliar Uet al. . Low prevalence of ocular Chlamydia trachomatis infection and active trachoma in the western division of Fiji. PLoS Negl Trop Dis. 2016;10(7):e0004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mathew AA, Keeffe JE, Le Mesurier RTet al. . Trachoma in the Pacific Islands: evidence from trachoma rapid assessment. Br J Ophthalmol. 2009;93(7):866–70. [DOI] [PubMed] [Google Scholar]
- 24. Lynch KD, Brian G, Ahwang Tet al. . Assessing the prevalence of trachoma: lessons from community screening with laboratory testing in Australia's Torres Strait Islands. Ophthalmic Epidemiol. 2022;doi: 10.1080/09286586.2022.2136389. [DOI] [PubMed] [Google Scholar]
- 25. Lynch KD, Morotti W, Brian Get al. . Clinical signs of trachoma and laboratory evidence of ocular Chlamydia trachomatis infection in a remote Queensland community: a serial cross-sectional study. Med J Aust. 2022;217(10):538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lynch KD, Brian G, Ahwang Tet al. . Discord between presence of follicular conjunctivitis and Chlamydia trachomatis infection in a single Torres Strait Island community: a cross-sectional survey. Aust N Z J Public Health. 2022;46(2):155–60. [DOI] [PubMed] [Google Scholar]
- 27. Butcher R, Handley B, Garae Met al. . Ocular Chlamydia trachomatis infection, anti-Pgp3 antibodies and conjunctival scarring in Vanuatu and Tarawa, Kiribati before antibiotic treatment for trachoma. J Infect. 2020;80(4):454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization . Informal consultation on end-game challenges for trachoma elimination, Task Force for Global Health, Decatur, United States of America, 7–9 December 2021. Geneva: World Health Organization; 2022. [Google Scholar]
- 29. Solomon AW, Pavluck AL, Courtright Pet al. . The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization . Design parameters for population-based trachoma prevalence surveys. Geneva: World Health Organization; 2018. [Google Scholar]
- 31. Macleod CK, Bailey RL, Dejene Met al. . Estimating the intracluster correlation coefficient for the clinical sign “Trachomatous inflammation-follicular” in population-based trachoma prevalence surveys: results from a meta-regression analysis of 261 standardized preintervention surveys carried out in Ethiopia, Mozambique, and Nigeria. Am J Epidemiol. 2020;189(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thylefors B, Dawson CR, Jones BRet al. . A simple system for the assessment of trachoma and its complications. Bull World Health Org. 1987;65(4):477–83. [PMC free article] [PubMed] [Google Scholar]
- 33. Solomon AW, Kello AB, Bangert Met al. . The simplified trachoma grading system, amended. Bull World Health Org. 2020;98(10):698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solomon AW, Le Mesurier RT, Williams WJ. A diagnostic instrument to help field graders evaluate active trachoma. Ophthalmic Epidemiol. 2018;25(5–6):399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dize L, West S, Williams JAet al. . Comparison of the Abbott m2000 RealTime CT assay and the Cepheid GeneXpert CT/NG assay to the Roche Amplicor CT assay for detection of Chlamydia trachomatis in ocular samples from Tanzania. J Clin Microbiol. 2013;51(5):1611–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goodhew EB, Priest JW, Moss DMet al. . CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis. 2012;6(11):e1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinsent A, Solomon AW, Bailey RLet al. . The utility of serology for elimination surveillance of trachoma. Nat Commun. 2018;9(1):5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodhew EB, Morgan SM, Switzer AJet al. . Longitudinal analysis of antibody responses to trachoma antigens before and after mass drug administration. BMC Infect Dis. 2014;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. West SK, Munoz B, Kaur Het al. . Longitudinal change in the serology of antibodies to Chlamydia trachomatis pgp3 in children residing in a trachoma area. Sci Rep. 2018;8(1):3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solomon AW, Willis R, Pavluck ALet al. . Quality assurance and quality control in the Global Trachoma Mapping Project. Am J Trop Med Hyg. 2018;99(4):858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ICTC . Use of photography for support of trachoma grading: progress report. Available from: https://www.trachomacoalition.org/resources/use-photography-support-trachoma-grading-progress-report.
- 42. Bacharier LB, Guilbert TW, Mauger DTet al. . Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314(19):2034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Donde S, Mishra A, Kochhar P. Azithromycin in acute bacterial upper respiratory tract infections: an Indian non-interventional study. Indian J Otolaryngol Head Neck Surg. 2014;66(Suppl 1):225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Keenan JD, Bailey RL, West SKet al. . Azithromycin to reduce childhood mortality in sub-Saharan Africa. N Engl J Med. 2018;378(17):1583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bruce D, Gaynor AA, Kadri Bet al. . Impact of mass azithromycin distribution on malaria parasitemia during the low-transmission season in Niger: a cluster-randomized trial. Am J Trop Med Hyg. 2014;90(5):846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oldenburg CE, Amza A, Cooley Get al. . Biannual versus annual mass azithromycin distribution and malaria seroepidemiology among preschool children in Niger: a sub-study of a cluster randomized trial. Malar J. 2019;18(1):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang SA, Papp JR, Stamm WEet al. . Evaluation of antimicrobial resistance and treatment failures for Chlamydia trachomatis: a meeting report. J Infect Dis. 2005;191(6):917–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are presented within the manuscript.