ABSTRACT
Hematuria—either macroscopic hematuria or asymptomatic microscopic hematuria—is a clinical feature typical but not specific for immunoglobulin A nephropathy (IgAN). The only biomarker supported by the Kidney Disease: Improving Global Outcomes group as a predictor of progression, identifying patients needing treatment, is proteinuria >1 g/day persistent despite maximized supportive care. However, proteinuria can occur in the setting of active glomerulonephritis or secondary to sclerotic renal lesions. Microscopic hematuria is observed in experimental models of IgAN after IgA–IgG immunocomplex deposition, activation of inflammation and complement pathways. Oxidative damage, triggered by hemoglobin release, is thought to contribute to the development of proteinuria and progression. Despite being a clinical hallmark of IgAN and having a rational relationship with its pathophysiology, the value of microscopic hematuria in assessing activity and predicting outcomes in patients with IgAN is still debated. This was partly due to a lack of standardization and day-to-day variability of microhematuria, which discouraged the inclusion of microhematuria in large multicenter studies. More recently, several studies from Asia, Europe and the USA have highlighted the importance of microhematuria assessment over longitudinal follow-up, using a systematic approach with either experienced personnel or automated techniques.
We report lights and shadows of microhematuria evaluation in IgAN, looking for evidence for a more consistent consensus on its value as a marker of clinical and histological activity, risk assessment and prediction of treatment response. We propose that hematuria should be included as part of the clinical decision-making process when considering when to use immunosuppressive therapy and as part of criteria for enrollment into clinical trials to test drugs targeting the inflammatory reaction elicited by immune pathway activation in IgAN.
Keywords: biomarker, hematuria, IgA nephropathy, microhematuria, risk factor for progression
INTRODUCTION
Immunoglobulin A nephropathy (IgAN) has the unique histological pattern of IgA deposits in glomeruli, dominant or codominant with respect to the other immunoglobulins, detected by second-level analysis, including immunofluorescence or immunohistochemistry for immunoglobulins. The polymeric IgA1 subclass is prevalent in glomerular deposits [1]. However, the sophisticated diagnosis of IgAN is often anticipated in patients by a simple and inexpensive investigation, available all over the word, the urinalysis. A recent noninvasive artificial neural network model that included hematuria and serum IgA:C3 ratio was found to predict IgAN in the Chinese population with a sensitivity of 82.7% and a specificity of 84.8% [2]. Despite the role of hematuria as a key clinical feature in IgAN—either as bouts of macroscopic hematuria coincident with acute mucosal infections or clinically silent microscopic hematuria—the value of this biomarker is still debated and not fully supported by international recommendations, including the recent Kidney Disease: Improving Global Outcomes (KDIGO) 2021 edition [3].
Macroscopic hematuria was initially thought to be an acute and reversible event, characterizing children and young adults with nonprogressive IgAN [4]. This position was refuted considering the presence of gross hematuria in some rapidly progressive and crescentic cases [5]. More recently, acute kidney injury with possible irreversible changes was demonstrated in cases of prolonged massive macroscopic hematuria, particularly in older subjects. This event was found to produce interstitial damage consequent to blood casts obstruction or hemoglobin and heme pro-oxidant activity and chronic tissue damage with disease progression [6].
The most common clinical feature of IgAN at all ages is microscopic hematuria, and this review specifically addresses this debated issue, which is potentially of great value for clinicians. Although microscopic hematuria is not specific for IgAN, about half of glomerular diseases presenting with microscopic hematuria have a diagnosis of IgAN on renal biopsy [7]. Microscopic hematuria is detected in ≈70–80% of patients with IgAN enrolled in large cohort studies [8] and it is generally considered an undisputable biomarker of IgAN, although with insufficient specificity [9].
Recent studies suggest that microscopic hematuria in IgAN is the result of the glomerular damage of inflammation elicited by the pathogenetic events leading to full expression of the disease [10, 11]. It is hypothesized that abnormal production of galactose-deficient IgA1 (Gd-IgA1) induces the formation of unique antiglycan IgG antibodies, resulting in the circulation and renal accumulation of immune complexes [12]. Inflammatory mechanisms are locally activated with the release of cytokines and initiation of complement pathways. This proinflammatory milieu corresponds to the manifestation of microscopic hematuria [10]. Moreover, the release of hemoglobin induces oxidative damage, not only at a tubular level, but also on podocytes, triggering apoptosis [13]. Hence persistent heavy microscopic hematuria in IgAN is likely to be not only a biomarker of active glomerular damage, but also a factor contributing to the development of proteinuria and disease progression [14].
Despite being a clinical hallmark of IgAN and having a relationship with the pathophysiology of this disease, the value of microscopic hematuria in assessing activity of IgAN and predicting outcomes or in selecting patients to be treated with immunomodulating or suppressive drugs has not been universally established. A scientific approach to investigating the above unsolved issue has not been completed, partly due to a lack of standardization and day-to-day variability of microhematuria. The variability among different centers in performing urinalysis, and even in the same subject, was considered too high to include detection and quantification of microscopic hematuria in renal biopsy registries and large international collaborative databases, including the Oxford classification and Validation Study of the Oxford Classification for IgA Nephropathy cohorts [15]. Moreover, in the period 1980–2000, much of the interest was diverted towards proteinuria, which was found to be a major driver of IgAN progression [16], overshadowing the interest in microhematuria, which was thought to be just an unspecific manifestation of glomerular barrier damage. The value of proteinuria reduction as a surrogate marker for renal protection [17] has further consolidated proteinuria as the only biomarker to select patients at risk who need to be treated and to monitor disease progression. However, these studies did not consider differentiating proteinuria derived from disease not associated with glomerular inflammation, such as membranous nephropathy or primary focal segmental glomerulosclerosis, versus disease associated with inflammation, such as IgAN, where a clear glomerulonephritic component may be involved. And in that sense, they failed to evaluate the inflammatory component of the pathogenic process. The fact that hematuria is usually associated with proteinuria in IgAN (e.g. it was present in 75% of the patients in the STOP-IgAN study) may explain why previous studies found proteinuria as a driver of progression in IgAN, as the data were not adjusted for the impact of hematuria (its presence or its degree) when evaluating the effect of proteinuria on progression of the kidney disease. As such, the role of proteinuria alone (without hematuria) as a risk factor for progression of kidney disease was not evaluated. In other words, the studies failed in distinguishing proteinuria as a marker of chronic damage (i.e. scar) versus proteinuria associated with active glomerular inflammation when assessing the risk of kidney disease progression.
In the new millennium, several reports, initially from Asia and more recently from Europe and the USA, highlighted the relevant information provided by measurement of microhematuria using a more rational and systematic approach [11, 18]. This included longitudinal follow-up hematuria assessments and experienced personnel or reproducible automated techniques. The value of microscopic hematuria has been rescued by several studies without reaching a full and universal appreciation.
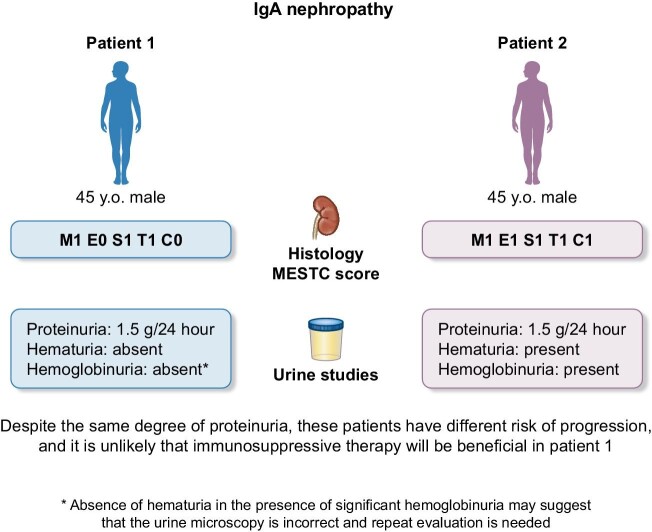
In this review, we will reason that the presence of microscopic hematuria is crucial in most patients with IgAN (Fig. 1) and will review lights and shadows of microhematuria evaluation in IgAN, trying to find the evidence for a more consistent consensus on its value as a marker of clinical and histological activity, risk assessment and prediction of treatment response.
Figure 1:
Proposed evaluation of patients with IgAN.
Microscopic hematuria origin in IgAN: insights from experimental models
In the first attempts to reproduce IgAN in experimental animals, the injection of aggregated polymeric IgA failed to show persistent IgA deposits and hematuria. Unphysiologically large amounts of antigen and specific antibody induced only transient and variable urinary abnormalities [19]. A mouse strain—ddY mice—producing high levels of circulating polymeric IgA were found to develop spontaneous IgA mesangial deposits with variable glomerular injury depending on the genetic background. However, these mice presented variable proteinuria but not hematuria [20].
A growing number of studies have shown in murine models of IgAN that for microhematuria to develop, complement activation is required (Fig. 2). In the active model of oral immunization in BALB/c mice, mesangial deposits containing IgA were noted, but no hematuria was detected [21]. Hematuria developed only after intravenous challenge with the same oral antigen when co-deposition of IgG/IgM and C3 were detected [22]. To further assess the role of complement activation in the pathogenesis of hematuria, a passive model of IgAN was induced by administration of immune complexes of monoclonal IgA/IgG antibodies with different complement-fixing activity. Despite comparable mesangial deposits of IgA, IgG and antigen, only mice given immune complexes containing the complement-fixing IgG had glomerular C3 and hematuria. Furthermore, in mice previously depleted of serum complement via cobra venom factor, no glomerular complement was observed and no hematuria ensued. The conclusion of this early seminal work was that complement activation by deposited IgA/IgG complexes is required for glomerular injury to occur that then manifests as microscopic hematuria. Also, the antigen composition was found to contribute to the severity of histopathologic lesions of hematuria manifestation in an experimental passive model of IgAN [23]. Mice developed severe diffuse mesangial proliferation, segmental necrosis and thrombotic microangiopathy when injected with pneumococcal C (PC) polysaccharide in addition to IgA/IgA immune complexes formed by dinitrophenyl-conjugated IgA antiphosphorylcholine, the latter functioning as PC-capturing antigen. Recent more sophisticated experimental models of IgAN also confirm that a reduction in C3 deposition is associated with a decrease in microhematuria. In the α1KI-CD89Tg mouse model of IgAN, which is a humanized mouse model that expresses human IgA1 and human IgA Fc receptor (CD89) and spontaneously develops IgAN, mesangial deposits of IgA1-CD89 were associated with C3 deposition and kidney inflammation that manifest with hematuria and proteinuria. Oral antigen challenge was found to modulate the intensity of IgA immune deposits as well as hematuria [24]. In this murine model, administration of recombinant IgA1 protease (a bacterial protein that selectively cleaves human IgA1) induced a marked decrease in IgA1 deposits as well as C3 deposition and inflammatory infiltrating cells. Notably, hematuria consistently decreased after this treatment [24, 25].
Figure 2:
Pathogenesis of IgAN and ensuing hematuria. Presence production of Gd-IgA1 results in generation of IgG anti-Gd-IgA1, which subsequently results in the formation of immune complexes that then get deposited in the kidney. The following inflammation due to the release of cytokines, chemokines, and activation of the complement cascade, results in damage to the glomerular capillary, passage of RBCs into the urinary space, and development of hematuria.
After the identification of the four-hit model, most of the interest was focused on Gd-IgA1, but the injection of Gd-gA1 alone did not induce microscopic hematuria, as antiglycan antibodies and complement activation are needed for the full IgAN pathology features to develop. However, in human IgAN, IgG co-deposition is not always detected and subclass analysis has mostly revealed IgG2, which in normal subjects has limited complement-activating properties. The role of IgG deposits and complement activation was recently addressed in an elegant experimental model where IgG and Gd-IgA1 were isolated from sera of IgAN patients and injected individually or in combination with immune complexes in immunodeficient mice [26]. While comparison animals injected with IgG or IgA from healthy subjects failed to develop IgAN, mice injected with Gd-IgA1 mixed with IgG autoantibodies from patients with IgAN displayed IgA, IgG and complement C3 glomerular deposits, with hematuria and proteinuria [26]. Confocal microscopy with IgG-specific nanobody confirmed the presence of IgG in all IgAN biopsies, including those without IgG by routine immunofluorescence microscopy [26]. These findings support the role of complement activation—selectively triggered by Gd-IgA1 and specific antiglycan IgG produced in patients with IgAN—in the development of microscopic hematuria in experimental IgAN.
Detection and quantification of microscopic hematuria: automated assays
Review of urine sediment by a trained nephrologist has been shown to yield higher accuracy compared with laboratory hospital staff [27]. However, review of urine sediment is increasingly done by laboratory staff, partly due to its time-consuming nature. Automated assays have been developed that help detect the presence of red blood cells (RBCs) via an automated urine particle analyzer (UF-5000, Sysmex, Lincolnshire, IL, USA) that has been shown to have sensitivity of 94.1% [28]. This may allow for more rapid detection of hematuria in the clinic. The UF-5000 uses fluorescent flow cytometry that fractionates urine particles and can express the quantity of hematuria [28]. The UF-5000 is able to recognize urine particles and further characterize RBCs based on their morphological appearance. Two subtypes, % small RBC (%sRBC) and lysed RBC have been shown to highly correlate with glomerular hematuria as seen in IgAN [28]. The %sRBC was highly correlated with the presence of dysmorphic RBCs and thus could be used as a clinical tool in place of manual microscopy [28].
Quantification of microscopic hematuria is assessed on centrifuged (2000 g) urine specimens resuspended after supernatant removal and examined microscopically at 400×. Degrees of microhematuria are mostly reported as 0, <3, 3–10, 11–20, 21–30, 31–40, 41–50, 51–100 or >100 RBC/HPF [11]. There is no consensus on what is considered ‘significant’ or ‘clinically meaningful’ hematuria and different studies have used different cutoffs. In general, mild microscopic hematuria is considered as 3–10 RBC/HPF, corresponding to 1+ on dipstick grading [29]; moderate hematuria is considered as 11–20 RBC/HPF, corresponding to 2–3+ on dipstick grading; and significant hematuria is considered as >21 RBC/HPF by some [11] or >28 RBC/HPF [30] or >31 RBC/HPF [31], while others have considered hematuria above the second tertile of the cohort as significant. We summarized in Table 1 our proposed classification of severity of hematuria based on literature review on standard microscopy and new automated methods.
Table 1:
Hematuria severity based on detection method
| Hematuria severity | Detection method Standard microscopy | Automated assay | Automated urine particle analyzer (for the detection method) |
|---|---|---|---|
| Minimal (1) | <5 | Negative | <20 RBC/microliter |
| Mild (2) | 5 ≤ 25 | 1+ | 201–00 RBC/microliter |
| Moderate (3) | 25 ≤ 50 | 2+ | >100 RBC/microliter |
| Severe (4) | >50 | 3+ | >100 RBC/microliter |
Standard microscopy: centrifuged (2000X) urine specimen resuspended after supernatant removal and examined at microscopy at x400 high power microscopic field (RBC/HPMF); Automated assay: dipstick + Automated urine particle analyzer (UF-5000) a third generation automated flow cytometry analyzer for urine sediment analysis [34].
As will be discussed below, when assessing hematuria in patients with IgAN, a one-time urinalysis and microscopy may have less value in predicting outcome than repeated evaluation of urine over time. As a result, the concept of time averaged (TA) hematuria has been developed to account for changes in hematuria over time in patients with IgAN. TA hematuria calculates the degree of hematuria over time. In some studies it is calculated as the area under the curve from microscopic measurements of RBCs (RBC/µl) during follow-up divided by the months of follow-up [30] and other studies have used the average of the mean RBC/HPF every 6 months [18, 32]. Either way, the presence of microhematuria over time is likely of more value than hematuria noted at one time point regardless of its severity.
Correlations between microhematuria and pathology features in IgAN, focus on crescents and endocapillary hypercellularity
Several studies have evaluated the association between microscopic hematuria and the histological findings of the kidney biopsy (Table 2). Most early studies considered the urinary evaluation available at renal biopsy while others considered the TA hematuria in association with active lesions. Not surprisingly, the results have repeatedly shown an association between hematuria and inflammatory lesions [according to the Oxford classification mesangial hypercellularity (M1), endocapillary hypercellularity (E1) and crescents (C1 and C)]. Crescentic lesions have most consistently been associated with hematuria. A Japanese study showed that patients with IgAN with severe hematuria (defined as ≥30 RBC/HPF) were more likely to have crescents {odds ratio [OR] 4.3 [95% confidence interval (CI) 1.7–10.9]} [31]. In the study by our group from the Mayo Clinic in which we evaluated 125 patients with IgAN who had an adequate biopsy sample, hematuria at the time of biopsy (defined as ≥21 RBC/HPF) was associated with the presence of M1, E1 and C1/2 lesions but had no association with the S or T score [11]. Notably, all patients who had an endocapillary hypercellularity had hematuria at baseline [11].
Table 2:
Microhematuria in predicting the risk of kidney disease progression
| Study | Hematuria assessment | Definition of significant hematuria | Renal outcome | Risk of progression, HR (95% CI) |
|---|---|---|---|---|
| Goto etal. [38] | At biopsy | 1–29 RBC/HPF | ESKD | 2.83 (1.89–4.25) |
| Iwasaki etal. [40] | At biopsy | Every 20 RBC/HPF increase | ESKD | 0.75 (0.55–1.00) |
| Tanaka etal. [41] | At biopsy | ≥20 RBC/HPF | ESKD | 1.06 (0.80–1.36) |
| Ebbestad etal. [29] | At biopsy | >10 RBC/HPF or 2–3+ on dipstick | 50% reduction in eGFR or ESKD | 26% versus 6.3% |
| Yu etal. [32] | TA hematuria | >5 RBC/HPF (manual) or >28 RBC/µl (automated) |
50% reduction in eGFR or ESKD | OR 1.14 (95% CI 1.13–1.87) |
| Bobart etal. [11] | TA hematuria | ≥21 RBC/HPF | Decline in eGFR | −3.99 ml/min/1.73 m2 (−6.94–1.04) |
| Sevillano etal. [18] | TA hematuria | >5 RBC/HPF | ESKD | 2.84 (1.06–7.3) |
| Huang etal. [34] | TA hematuria | Higher than the second tertile of the whole cohort | ESKD | 3.93 (1.33–11.6) |
| Weng etal. [30] | TA hematuria | >28 RBC/µl | ESKD | 0.004 (0.001–0.008) |
Another approach has been to consider the association of persistent TA microscopic hematuria and crescents. A Chinese study retrospectively evaluating 152 patients with IgAN showed that patients with persistent hematuria (defined as TA hematuria ≥28 RBC/HPF) were more likely to have crescents at the time of biopsy compared with those without persistent hematuria [30]. Another Chinese study [33] investigated a cohort of 169 patients with IgAN and crescents, matched by sex, age, estimated glomerular filtration rate (eGFR) and proteinuria, and compared them with IgAN patients without crescents. Crescents were found to be an independent predictor of outcome [50% decline in GFR or end-stage kidney disease (ESKD)]. Notably, persistent hematuria (as well as E1 and T1–2, TA proteinuria) was an independent risk factor for combined events in patients with crescents. In a Chinese cohort of 684 IgAN subjects, persistent hematuria (>24 RBC/μl) was assessed by an automated method [35]. Patients with high values of microscopic hematuria had a higher proportion of crescents (P = .003) and the degree of hematuria was a significant risk for progression when crescents were present but not when crescents were absent [35].
Taken together, the data suggest that relevant microscopic hematuria at baseline or persistently over time characterizes patients with IgAN with histological lesions that are typically associated with inflammation. Hematuria was more frequent in cases with severe destruction of the glomerular capillary basement membrane, which is thought to favor crescent formation [36, 37].
Value of microhematuria at biopsy and over time to predict progression
When it comes to assessing the value of microhematuria in predicting renal outcomes and progression to ESKD, there has been conflicting data over the years (Table 3). This has been partly due to a lack of standardized measurement when assessing hematuria with different threshold values for clinically meaningful microhematuria and to the concern regarding day-to-day variability. For example, in the recent international IgA prediction tool supported by the KDIGO 2021 guidelines, microscopic hematuria was not considered because hematuria data were not available, which reflects how little attention major centers pay in evaluating and documenting the presence of hematuria [3].
Table 3:
Association of microhematuria and renal histology
| Study | Hematuria assessment | Renal histology | Risk of association |
|---|---|---|---|
| Nagai etal. [31] | Pretreatment | C lesion | OR 4.3 (95% CI 1.7–10.9) |
| Bobart etal. [11] | At biopsy | C lesion E lesion M lesion |
86.6% versus 64.3% (P = .007) 30.9% versus 0% (P = .001) 32% versus 10.7% (P = .03) |
| Weng etal. [30] | TA hematuria | C lesion | 44.8% versus 19.6% (P = .002) |
| Huang etal. [34] | TA hematuria | C lesion | 65.4% versus 51.3% (P = .003) |
Some of the earlier studies focused on assessing the value of microhematuria at the time of biopsy, whereas more recent studies have looked at the value of persistent hematuria or TA hematuria in assessing the risk of progression to ESKD. A single-center study of 437 cases of IgAN followed for a mean of 107 months indicated the value of microscopic hematuria in the absence of recurrent macroscopic hematuria as a predictor of kidney failure [38].
One of the earliest studies that looked at the value of microhematuria was from Japan, which showed that patients with even mild microscopic hematuria (defined as 1–29 RBC/HPF) at the time of renal biopsy had an increased risk of kidney failure within 10 years of follow-up [39]. This was confirmed even when focusing on patients with isolated microhematuria and preserved eGFR (>60 ml/min/1.73 m2) [40]. Conversely, other studies failed to show an association between hematuria from the time of biopsy and long-term renal prognosis [41, 42]. These data were possibly affected by the use of a single urinary assessment at renal biopsy and the lack of quantification of the RBC count in urine to distinguish minimal from heavy microscopic hematuria. A Spanish national collaborative study showed excellent outcomes in patients with microhematuria who had minimal or no proteinuria, but again the degree of hematuria was not quantified [43].
Other studies that have quantified the degree of hematuria from the time of renal biopsy have shown that a higher degree of hematuria is indeed associated with worse renal outcome. Among the 95 Swedish patients followed for a median of 11 years, those with a high degree microscopic hematuria (>10 RBC or urine dipstick grade 2–3) had a higher predicted 5-year risk by the IgAN risk prediction tool (8.8% versus 5.6%) compared with patients with a lower degree of hematuria [29]. The composite outcome of a 50% reduction in eGFR or renal failure was detected in 26% of high-degree microhematuria versus 6.3% in low-microhematuria [29]. These differences did not reach statistical significance, possibly due to the limited number of patients [29].
Data has been more consistent when evaluating the role of persistent hematuria or TA hematuria on renal outcomes. In one of the largest cohorts from China in which 1333 patients with IgAN were evaluated and hematuria was assessed using both automated and manual methods, persistent hematuria was significantly associated with an increased risk of kidney failure [after adjusting for sex, blood pressure, eGFR, MEST-C score and the use of immunosuppressive therapy; OR using an automated method 1.46 (95% CI 1.13–1.87), P = .003]. Results were the same when urinalysis was completed manually [32]. Similar results were noted in the cohort from the Mayo Clinic, in which each increase in the degree of hematuria during follow-up was associated with a significant decrease in eGFR of −0.81 ml/min/1.73 m2 after adjusting for proteinuria and T score [11]. The results did not change after accounting for treatment and follow-up time [11]. The decrease in eGFR was most pronounced in those with hematuria ≥21 RBC/HPF [11]. Results were confirmed in a cohort of patients from Spain in which 112 patients with biopsy-proven IgAN were studied. After a mean follow-up of 14 years, the proportion of patients reaching renal failure was higher among those with persistent hematuria compared with those with no or minimal hematuria (30% versus 11%) [18]. TA hematuria was an independent predictor of progression to ESKD in the multivariable model [hazard ratio (HR) 2.84 (95% CI 1.06–7.3), P = .04] [18]. A recent meta-analysis of 5660 patients with IgAN concluded that persistent microscopic hematuria was associated with a significant 87% increase in the risk of kidney failure in the long term [44].
More recently, in another large study from China in which >600 patients with IgAN were studied, high-degree hematuria (defined as those with hematuria at baseline greater than the second tertile of the entire cohort) had a higher risk of progression to ESKD compared with those with low-degree hematuria after matching the groups based on age, sex, eGFR and follow-up time [HR 3.93 (95% CI 1.33–11.6)] [35]. RBC count was performed by automated urine analyzers at least three times in 6 months and TA hematuria was calculated for each 6-month period [35]. In another study, also from China, in which the automated method to measure urinary RBC was adopted, among 152 patients, 62% had persistent hematuria (>28 RBC/µl) lasting through the end of follow-up [30]. TA hematuria was independently associated with an increased risk of progression [30].
Despite the differences noted in the above studies, one theme does emerge when looking at all studies combined: the higher the degree of hematuria and the more persistent the hematuria, the higher the likelihood of kidney disease progression and the rate of ESKD over time, even after adjustment for baseline eGFR, proteinuria, age, sex and histology, suggesting that hematuria is a key biomarker in patients with IgAN and should be monitored over time.
Value of microhematuria remission
A few studies have looked at the value of hematuria remission in predicting renal outcome and have shown consistent results that improvement in hematuria is indeed associated with better renal outcome. The first was the study by Sevillano etal. [18] that looked at a Spanish cohort of 112 patients. As discussed above, the study showed that persistent hematuria and proteinuria were associated with poor renal outcome, but they also showed that disappearance of hematuria slows the rate of decline of renal function. Hematuria disappearance was defined as either an absence of RBCs or <5 RBC/HPF in all the urine sediment examinations performed during at least 3 years before the last outpatient visit. Of 112 patients, 52 had disappearance of hematuria. The rate of renal function decline in those who had hematuria disappearance significantly slowed from a rate of −6.45 ± 14.66 to −0.18 ±2.56 ml/min/1.73 m2. In patients who were treated with immunosuppression, hematuria disappeared faster than in those who were not treated. Note that is this study, the presence of proteinuria alone, without hematuria, was associated with a low risk for progression.
In a more recent study from a Japanese group that did a similar analysis, in 74 patients with IgAN, none of the patients who achieved hematuria remission (defined as a negative dipstick or <5 RBC/HPF) reached the primary outcome of a 50% decrease in renal function [45]. Patients with persistent hematuria and proteinuria had worse renal outcomes.
Value of microhematuria on treatment response
The Japanese nephrologists were among the first to consider hematuria remission as a benefit associated with therapy, as they reported that the most common therapy used in Japan for patients with IgAN—tonsillectomy in association with steroid pulses—was found to be effective for achieving hematuria remission in 67% of patients [46]. The repetition of steroid pulses induced remission of hematuria in 57% of the initial nonresponders. The presence of severe microscopic hematuria (often associated with crescents) was predictive for benefits of methylprednisolone pulses, as the eGFR trajectory after methylprednisolone pulses improved only in patients with severe pretreatment hematuria or C2 scores (P for interaction with time <.001) [31].
An international collaborative study enrolling patients from Spain and Argentina reported on the beneficial effects of corticosteroids and mycophenolic acid analogues in patients with progressive IgAN [47]. Patients were identified by a decrease in eGFR ≥10 ml/min/1.73 m2 in the last 12 months, proteinuria ≥0.75 g/24 h despite optimized renin–angiotensin system (RAS) inhibitor supportive care and persistent hematuria. The 25 patients included in the study had significant benefits from the combined immunosuppressive therapy on the rate of eGFR decline, with a decrease in proteinuria from 1.8 to 0.6 g/day at the end of a 2-year treatment (P = .01) and hematuria disappeared in 40% of patients.
One study enrolled 110 IgAN patients with persistent microhematuria and undetectable proteinuria to investigate the benefits of RAS blockade in the prevention of proteinuria [48]. This treatment decreased the risk of proteinuria development and increased the remission of hematuria.
CONCLUSIONS
The only biomarker validated and supported by the KDIGO as a predictor of progression, thus identifying patients needing treatment, is proteinuria >1 g/day persistent after maximized supportive care. However, this parameter cannot distinguish between active and sclerotic renal lesions, which can equally manifest with persistent proteinuria. Microscopic hematuria in repeated observations, detected by a well-trained observer or by an automated urine particle analyzer, has emerged as a biomarker of activity in patients with IgAN, independent of proteinuria, but even more so in the presence of proteinuria.
Microscopic hematuria at the time of biopsy and over time is associated with inflammatory glomerular lesions such as crescents and endocapillary hypercellularity. We showed that the higher the degree of hematuria and the more persistent over time (i.e. persistence of inflammation), the higher the rate of kidney function loss and progression to ESKD, even after adjusting for the degree of proteinuria, age, sex and baseline kidney function. Remission of hematuria portends a better outcome and correlates with treatment response.
In patients with membranous nephropathy, the discovery of anti-PLA2R antibodies, among others, has revolutionized how we diagnose and treat these patients. It is no longer possible to discuss membranous nephropathy without referring to these autoantibodies. Similarly, the discovery of antineutrophil cytoplasmic antibodies (ANCA) in the 1980s showed us that it is not enough to provide a diagnosis of a pauci-immune crescentic glomerulonephritis without stating if the patient is ANCA positive or negative. We do not have such a biomarker in IgAN, but we do have hematuria. As such, it is important to distinguish between a patient who has IgAN and proteinuria alone or IgAN with proteinuria AND hematuria.
Taken together, it is time to incorporate this cheap and easy test in our routine assessment of patients with IgAN and to consider it when deciding on initiation of immunosuppression in a specific patient. Hematuria should also be included for enrollment into clinical trials to test drugs targeting the inflammatory reaction elicited by immune pathway activation in IgAN.
Contributor Information
Ladan Zand, Division of Nephrology and Hypertension. Mayo Clinic. Rochester, MN, USA.
Fernando C Fervenza, Division of Nephrology and Hypertension. Mayo Clinic. Rochester, MN, USA.
Rosanna Coppo, Fondazione Ricerca Molinette, Regina Margherita Hospital, Turin, Italy.
FUNDING
This paper was published as part of a supplement funded by an educational grant from Otsuka America Pharmaceutical, Inc.
AUTHORS’ CONTRIBUTIONS
F.C.F.: received unrestricted grants from Genentech/Roche, Janssen Pharmaceuticals, and Travere, Morphosys Ag, consulting fees from Alexion Pharmaceuticals, BioCryst, Galapagos, GSK, Morphosys AG, Novartis, Ostuka Pharmaceuticals, Takeda, Travere, Amd Zyversa Therapeutics, Honoraria: UptoDate and advisory or leadership role, ASN, KI, NDT and UptoDate; L.Z.: received grants from Genentech and Janssen Pharmaceuticals; R.C.: Consultant/Advisory Boards/Steering Committees: Alnylam, Amgen, Argenx, Bayer, Calliditas, Chinook, Menarini, Novartis, Purespring, Otsuka-Visterra, Reata, STADApharm, Travere; Speakers' Bureau: STADApharm, Chinook Therapeutics and Travere; Data Safety and Monitoring Committees: Amgen and Bayer; Honoraria: UpToDate Session Editor.
DATA AVAILABILITY STATEMENT
No new data were generated or analyzed in support of this research.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Wyatt RJ, Julian BA.. IgA nephropathy. N Engl J Med 2013;368:2402–14. 10.1056/NEJMra1206793 [DOI] [PubMed] [Google Scholar]
- 2. Hou J, Fu S, Wang Xet al. A noninvasive artificial neural network model to predict IgA nephropathy risk in Chinese population. Sci Rep 2022;12:8296. 10.1038/s41598-022-11964-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021;100(4 Suppl):S1–276. 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 4. D'Amico G, Ferrario F, Colasanti Get al. IgA-mesangial nephropathy (Berger's disease) with rapid decline in renal function. Clin Nephrol 1981;16:251–7. [PubMed] [Google Scholar]
- 5. Bennett WM, Kincaid-Smith P. Macroscopic hematuria in mesangial IgA nephropathy: correlation with glomerular crescents and renal dysfunction. Kidney Int 1983;23:393–400. 10.1038/ki.1983.32 [DOI] [PubMed] [Google Scholar]
- 6. Sevillano AM, Diaz M, Caravaca-Fontan Fet al. IgA nephropathy in elderly patients. Clin J Am Soc Nephrol 2019;14:1183–92. 10.2215/CJN.13251118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park YH, Choi JY, Chung HSet al. Hematuria and proteinuria in a mass school urine screening test. Pediatr Nephrol 2005;20:1126–30. 10.1007/s00467-005-1915-8 [DOI] [PubMed] [Google Scholar]
- 8. Schena FP. Survey of the Italian Registry of Renal Biopsies. Frequency of the renal diseases for 7 consecutive years. The Italian Group of Renal Immunopathology. Nephrol Dial Transplant 1997;12:418–26. 10.1093/ndt/12.3.418 [DOI] [PubMed] [Google Scholar]
- 9. Coppo R, Fervenza FC. Persistent microscopic hematuria as a risk factor for progression of IgA nephropathy: new floodlight on a nearly forgotten biomarker. J Am Soc Nephrol 2017;28:2831–4. 10.1681/ASN.2017060639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moreno JL, Rodas LM, Draibe Jet al. Extracapillary proliferation scoring correlates with renal outcome and contributes to stratification in adult patients with immunoglobulin A nephropathy. Clin Kidney J 2021;14:284–90. 10.1093/ckj/sfz133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bobart SA, Alexander MP, Shawwa Ket al. The association of microhematuria with mesangial hypercellularity, endocapillary hypercellularity, crescent score and renal outcomes in immunoglobulin A nephropathy. Nephrol Dial Transplant 2021;36:840–7. 10.1093/ndt/gfz267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki H, Novak J. IgA glycosylation and immune complex formation in IgAN. Semin Immunopathol 2021;43:669–78. 10.1007/s00281-021-00883-8 [DOI] [PubMed] [Google Scholar]
- 13. Rubio-Navarro A, Sanchez-Nino MD, Guerrero-Hue Met al. Podocytes are new cellular targets of haemoglobin-mediated renal damage. J Pathol 2018;244:296–310. 10.1002/path.5011 [DOI] [PubMed] [Google Scholar]
- 14. Moreno JA, Sevillano A, Gutierrez Eet al. Glomerular hematuria: cause or consequence of renal inflammation? Int J Mol Sci 2019;20: 2205. 10.3390/ijms20092205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coppo R, Troyanov S, Bellur Set al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 2014;86:828–36. 10.1038/ki.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reich HN, Troyanov S, Scholey JWet al. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 2007;18:3177–83. 10.1681/ASN.2007050526 [DOI] [PubMed] [Google Scholar]
- 17. Inker LA, Heerspink HJL, Tighiouart Het al. Association of treatment effects on early change in urine protein and treatment effects on GFR slope in IgA nephropathy: an individual participant meta-analysis. Am J Kidney Dis 2021;78:340–9.e1. 10.1053/j.ajkd.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sevillano AM, Gutierrez E, Yuste Cet al. Remission of hematuria improves renal survival in IgA nephropathy. J Am Soc Nephrol 2017;28:3089–99. 10.1681/ASN.2017010108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rifai A, Chen A, Imai H. Complement activation in experimental IgA nephropathy: an antigen-mediated process. Kidney Int 1987;32:838–44. 10.1038/ki.1987.284 [DOI] [PubMed] [Google Scholar]
- 20. Imai H, Nakamoto Y, Asakura Ket al. Spontaneous glomerular IgA deposition in ddY mice: an animal model of IgA nephritis. Kidney Int 1985;27:756–61. 10.1038/ki.1985.76 [DOI] [PubMed] [Google Scholar]
- 21. Emancipator SN, Gallo GR, Lamm ME. Experimental IgA nephropathy induced by oral immunization. J Exp Med 1983;157:572–82. 10.1084/jem.157.2.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emancipator SN, Lamm ME. The role of IgG, IgM, and C3 in experimental murine IgA nephropathy. Semin Nephrol 1987;7:286–8. [PubMed] [Google Scholar]
- 23. Montinaro V, Esparza AR, Cavallo Tet al. Antigen as mediator of glomerular injury in experimental IgA nephropathy. Lab Invest 1991;64:508–19. [PubMed] [Google Scholar]
- 24. Papista C, Lechner S, Ben Mkaddem Set al. Gluten exacerbates IgA nephropathy in humanized mice through gliadin-CD89 interaction. Kidney Int 2015;88:276–85. 10.1038/ki.2015.94 [DOI] [PubMed] [Google Scholar]
- 25. Lechner SM, Abbad L, Boedec Eet al. IgA1 protease treatment reverses mesangial deposits and hematuria in a model of IgA nephropathy. J Am Soc Nephrol 2016;27:2622–9. 10.1681/ASN.2015080856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moldoveanu Z, Suzuki H, Reily Cet al. Experimental evidence of pathogenic role of IgG autoantibodies in IgA nephropathy. J Autoimmun 2021;118:102593. 10.1016/j.jaut.2021.102593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsai JJ, Yeun JY, Kumar VAet al. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis 2005;46:820–9. 10.1053/j.ajkd.2005.07.039 [DOI] [PubMed] [Google Scholar]
- 28. Mizuno G, Hoshi M, Nakamoto Ket al. Evaluation of red blood cell parameters provided by the UF-5000 urine auto-analyzer in patients with glomerulonephritis. Clin Chem Lab Med 2021;59:1547–53. 10.1515/cclm-2021-0287 [DOI] [PubMed] [Google Scholar]
- 29. Ebbestad R, Sanaei Nurmi M, Lundberg S. Long-term outcomes of patients with IgA nephropathy categorized by the International IgAN risk prediction tool and by the degree of hematuria at diagnosis. Nephron 2022;146:573–83. 10.1159/000525001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weng M, Lin J, Chen Yet al. Time-averaged hematuria as a prognostic indicator of renal outcome in patients with IgA nephropathy. J Clin Med 2022;11:6785. 10.3390/jcm11226785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagai M, Kobayashi N, Izumi Net al. Pre-treatment hematuria and crescents predict estimated glomerular filtration rate trajectory after methylprednisolone pulse therapy with tonsillectomy for IgA nephropathy. J Nephrol 2022;35:441–9. 10.1007/s40620-021-01064-4 [DOI] [PubMed] [Google Scholar]
- 32. Yu GZ, Guo L, Dong JFet al. Persistent hematuria and kidney disease progression in IgA nephropathy: a cohort study. Am J Kidney Dis 2020;76:90–9. 10.1053/j.ajkd.2019.11.008 [DOI] [PubMed] [Google Scholar]
- 33. Ma F, Liu L, Dong Ret al. Renal survival and risk factors in IgA nephropathy with crescents. Int Urol Nephrol 2020;52:1507–16. 10.1007/s11255-020-02457-3 [DOI] [PubMed] [Google Scholar]
- 34. Cho H, Yoo J, Kim Het al. Diagnostic characteristics of urinary red blood cell distribution incorporated in UF-5000 for differentiation of glomerular and non-glomerular hematuria. Ann Lab Med 2022;42:160–8. 10.3343/alm.2022.42.2.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang Z, Zhang J, Chen Bet al. Clinical significance of persistent hematuria degrees in primary IgA nephropathy: a propensity score-matched analysis of a 10-year follow-up cohort. Am J Nephrol 2023;54:62–73. 10.1159/000529650 [DOI] [PubMed] [Google Scholar]
- 36. Du Y, Chen S, Wang Fet al. The significance of crescents on the clinical features and outcomes of primary immunoglobin A nephropathy. Front Med 2022;9:864667. 10.3389/fmed.2022.864667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park S, Baek CH, Park SKet al. Clinical significance of crescent formation in IgA nephropathy—a multicenter validation study. Kidney Blood Press Res 2019;44:22–32. 10.1159/000497808 [DOI] [PubMed] [Google Scholar]
- 38. Manno C, Strippoli GF, D'Altri Cet al. A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. Am J Kidney Dis 2007;49:763–75. 10.1053/j.ajkd.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 39. Goto M, Wakai K, Kawamura Tet al. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrol Dial Transplant 2009;24:3068–74. 10.1093/ndt/gfp273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goto M, Kawamura T, Wakai Ket al. Risk stratification for progression of IgA nephropathy using a decision tree induction algorithm. Nephrol Dial Transplant 2009;24:1242–7. 10.1093/ndt/gfn610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iwasaki C, Moriyama T, Tanaka Ket al. Effect of hematuria on the outcome of immunoglobulin A nephropathy with proteinuria. J Nephropathol 2016;5:72–8. 10.15171/jnp.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanaka K, Moriyama T, Iwasaki Cet al. Effect of hematuria on the outcome of IgA nephropathy with mild proteinuria. Clin Exp Nephrol 2015;19:815–21. 10.1007/s10157-014-1068-9 [DOI] [PubMed] [Google Scholar]
- 43. Gutierrez E, Zamora I, Ballarin JAet al. Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J Am Soc Nephrol 2012;23:1753–60. 10.1681/ASN.2012010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He P, Wang H, Huang Cet al. Hematuria was a high risk for renal progression and ESRD in immunoglobulin a nephropathy: a systematic review and meta-analysis. Ren Fail 2021;43:488–99. 10.1080/0886022X.2021.1879852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsuzaki K, Suzuki H, Kawamura Tet al. Utility of remission criteria for the renal prognosis of IgA nephropathy. Clin Exp Nephrol 2021;25:988–95. 10.1007/s10157-021-02069-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toda M, Kume A, Hara Met al. Efficacy and limitations of additional steroid pulse therapy in IgA nephropathy patients whose hematuria did not remit on tonsillectomy and protocol steroid pulse therapy. Clin Exp Nephrol 2022;26:859–66. 10.1007/s10157-022-02226-9 [DOI] [PubMed] [Google Scholar]
- 47. Huerta A, Merida E, Medina Let al. Corticosteroids and mycophenolic acid analogues in immunoglobulin A nephropathy with progressive decline in kidney function. Clin Kidney J 2022;15:771–7. 10.1093/ckj/sfab244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen J, Liu S, Xu Het al. Retrospective analysis of clinical outcomes in patients with immunoglobulin A nephropathy and persistent hematuria following renin-angiotensin system blockade. Med Sci Monit 2020;26:e922839. 10.12659/MSM.922839 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.