ABSTRACT
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis worldwide. Recent years have witnessed significant improvements in the understanding of the pathogenesis of IgAN and particularly, the pathogenic role of complement activation. The alternative complement pathway is the major complement cascade activator in IgAN, and glomerular C3 deposition has been shown to correlate with disease progression. In addition, several studies have provided insight into the pathogenic role of factor H–related proteins -1 and -5 in IgAN, as independent players in complement dysregulation. The lectin pathway has also been shown to be associated with the severity of IgAN. Glomerular deposition of C4d has been associated with increased histologic disease activity, faster decline in estimated glomerular filtration rate and higher risk of kidney failure. On the other hand, although overlooked in the Oxford classification, numerous studies have shown that the coexistence of thrombotic microangiopathy in IgAN is a significant indicator of a poorer prognosis. All the breakthroughs in the understanding of the contributing role of complement in IgAN have paved the way for the development of new complement-targeted therapies in this disease. Several ongoing trials are evaluating the efficacy of new agents against factor B (iptacopan, Ionis-FB-LRX), C3 (pegcetacoplan), factor D (vemircopan, pelecopan), C5 (ravulizumab, cemdisiran) and C5a receptor 1 (avacopan). In this study, we provide a comprehensive review of the role of complement in IgAN, including the emerging mechanisms of complement activation and the promising potential of complement inhibitors as a viable treatment option for IgAN.
Keywords: alternative pathway, IgA nephropathy, lectin pathway, thrombotic microangiopathy
INTRODUCTION
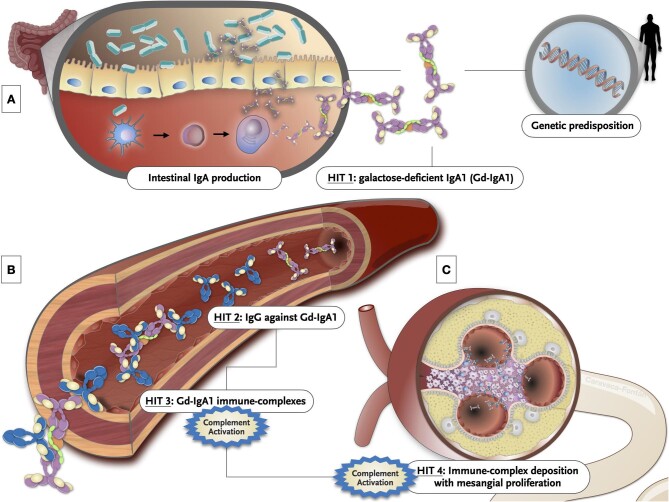
Immunoglobulin A nephropathy (IgAN), first described in 1968 [1], is the most common primary glomerulonephritis worldwide in patients undergoing kidney biopsy [2]. IgAN is an autoimmune disease characterized by abnormal synthesis and glycosylation of IgA1, resulting in increased circulating levels of galactose-deficient IgA1 (gd-IgA1) [3, 4]. Multiple genetic, ethnic and environmental factors contribute to and modulate kidney damage. The currently accepted model of IgAN pathogenesis is the “four-hit” model [4] (Fig. 1). This “four-hit hypothesis” starts with the production of gd-IgA1 (first hit), followed by the overproduction of autoantibodies that specifically recognize gd-IgA1 (second hit), and the formation of circulating immune complexes composed of gd-IgA1 and anti-gd-IgA1 autoantibodies (gd-IgA-IC). The deposition of these immune-complexes in the mesangium (third hit) induces mesangial proliferation and glomerular inflammation (fourth hit) [5, 6].
Figure 1:
Graphical illustration of the multi-hit hypothesis. (A) The production of mucosal IgA may be induced by T-cell-dependent or T-cell-independent mechanisms. The secretion of several cytokines such as interleukin-6, interleukin-10, B-cell activation factor of the TNF family (BAFF) and a proliferation-inducing ligand (APRIL) by dendritic cells induce B cells to undergo class switching recombination from IgM to IgA1. IgA-secreting plasma cells migrate to the mucosal lamina propria and release dimeric IgA1 into the lumen. Some misdirected IgA-secreting cells are released to the systemic compartment where they take up residence in systemic sites and secrete poorly O-galactosylated IgA1 to systemic circulation. Several genetic and environmental factors may predispose IgA patients to mount abnormal immune responses to certain common pathogens. This genetic background can also modulate the IgAN phenotype. (B) Specific reactive IgG and IgA antibodies against gd-IgA1 are formed and ultimately result in immune complex formation. This can induce complement activation. (C) Immune complexes get trapped in the mesangium through an increased affinity of gd-IgA1 for extracellular matrix components and trigger inflammatory pathways, which result in glomerular injury and tubulointerstitial scarring.
In the following sections we review the role of complement in IgAN, thrombotic microangiopathy (TMA) in IgAN patients, and the future landscape for the use of complement inhibitors in the management of IgAN.
ROLE OF COMPLEMENT IN IgAN PATHOGENESIS
Tissue deposition of gd-IgA1-IC can trigger local complement activation, but how this occurs is not well understood [7]. Complement activation (local and systemic) in IgAN patients was described five decades ago [8], although its pathogenic significance was not immediately recognized.
The complement system is an important component of innate immunity and can be activated through three different pathways [9]: the classical pathway (CP), lectin pathway (LP) and alternative pathway (AP), all of them converging in the terminal pathway (TP). Several studies have provided evidence for activation of AP, LP and TP as effector mechanisms of kidney injury in IgAN [10, 11]. It should be noted that AP plays an important role in complement amplification even when other pathways are primarily activated. Conversely, the absence of C1q in most IgAN kidney biopsies suggests that CP is not significantly involved in its pathogenesis [11] (Fig. 2).
Figure 2:
The complement system. Diagram of the two main activation pathways in IgAN, which eventually converge in the terminal pathway with the formation of the membrane attack complex. The main regulatory factors at each stage are represented. C4BP: C4-binding protein; CR1: complement receptor type 1; DAF: decay accelerating factor; MCP: membrane cofactor protein.
Alternative pathway
The AP is the main activator of the complement cascade in IgAN, and is primarily responsible for C3 deposition. Glomerular C3 deposition may be observed in 71%–100% (∼90%) of IgAN patients [7]. Glomerular C3 deposits correlate with progression of the disease, and may help differentiate IgAN from those isolated and asymptomatic deposits of IgA (“lanthanic IgA”) found in up to 4%–16% of the population [12]. Other AP components and regulators such as complement factor H (FH, 30%–90%), properdin (75%–100%) and FH-related proteins (FHR) are also found in kidney biopsies of IgAN patients [10, 13]. Serum C3 levels are characteristically normal in IgAN, although some studies have shown that increased plasma concentrations of C3 activation markers, such as C3b, C3dg and iC3b, are associated with worse kidney prognosis [14, 15]. Likewise, a high serum IgA/C3 ratio is correlated with poorer kidney survival [16, 17].
Factor B is a crucial cofactor for C3 activation and for AP activity. A positive correlation has been observed between plasma levels of factor Ba and levels of gd-IgA1, whereas a negative correlation was found between factor Ba and kidney function [18]. In this study, immunosuppressive treatment induced a rapid decrease of factor Ba, C5a and Gd-IgA1, accompanied by a decrease in proteinuria and stabilization of kidney function [18]. However, when evaluating variations in the levels of different AP factors, it should be considered that this pathway can be secondarily activated after a primary activation of the LP.
Membrane cofactor protein (MCP; CD46) is one of the most important membrane-bound complement regulators [19]. A significant correlation between lower expression of CD46 messenger RNA in peripheral white blood cells and estimated glomerular filtration rate (eGFR) decline has been reported in patients with IgAN enrolled by the European collaborative study group Validation of the Oxford Classification of IgAN (VALIGA) [20].
Important recent contributions have provided insight into the pathogenic involvement of FHR1 and FHR5 in IgAN [21, 22]. The interplay between FH and the different FHRs provides a sophisticated network that regulates AP activation in a context-dependent manner [23]. FHRs modulate the regulatory effect of FH by competing with ligands, resulting in complement activation on cell surfaces.
Genome-wide association studies have identified protective associations for IgAN within the CFH locus on chromosome 1q32. Deletion of CFHR1 and CFHR3 genes (delCFHR1-3) is associated with protection against IgAN and may partly explain the geographical distribution of this disease [24–26]. An association between delCFHR1-3 and reduced glomerular immune deposits has been reported in a large cohort of white IgAN patients, although no association with CKD progression was found [27]. Two independent studies showed that FHR1 plasma levels were significantly higher in IgAN patients than in controls, and the authors also observed a negative correlation between FHR1 levels and eGFR [21, 22]. Plasma levels of FHR5 were higher in IgAN patients than in controls in two large cohorts from Europe and Asia. In addition, serum FHR5 levels correlated with histological markers of kidney damage in both cohorts [15, 17]. FHR1 and FHR5 have been observed in kidney biopsies of IgAN with complement-activating products, suggesting that they are important and independent players in AP dysregulation [3]. Notably, it has been reported that IgAN patients receiving a kidney graft and carrying two copies of CFHR1-3 had a worse graft outcome, with increased glomerular and tubulo-interstitial FHR1 deposits [28].
The mechanism underlying IgA-induced AP activation is poorly understood, but IgA polymerization appears to be critical (polymeric but not monomeric IgA) [29]. Hence, it remains a challenge to unravel the precise mechanisms linking glomerular IgA1 deposition to complement activation, inflammation and the spectrum of clinical features encompassed by IgAN.
Lectin pathway
The LP is activated by the binding of pattern recognition molecules, including mannan-binding lectin (MBL), ficolins and collectins, to pathogen-associated molecular patterns [9] (Fig. 2). IgA and the LP are important mediators of innate immunity in the respiratory and gastrointestinal tracts. Glomerular deposition of the LP is associated with IgAN severity. In a European cohort of 60 patients with IgAN, glomerular deposition of MBL and L-ficolin was associated with more severe histologic damage as evidenced by increased mesangial proliferation, extracapillary proliferation, sclerosed glomeruli and interstitial infiltration, together with significantly increased proteinuria [30].
Mesangial deposition of C4, and particularly C4d activation fragment, has been identified in kidney biopsies from patients with progressive IgAN, reflecting LP activation. Two well-characterized studies showed that positive glomerular C4d staining was a significant predictor of kidney failure [31, 32]. Specifically, the authors found that glomerular deposition of C4d was associated with increased histologic disease activity, a more rapid decline in eGFR and, consequently, a higher risk of kidney failure [31, 32]. A systematic review and meta-analysis of studies about C4d deposition in IgAN concluded that glomerular C4d deposits are associated with worse clinical and histologic characteristics, and are an independent risk factor for end-stage kidney disease (ESKD) in IgAN [33].
On the other hand, circulating MBL and mannan-binding lectin–associated serine protease-3 (MASP-3) levels have been associated with disease outcomes in IgAN, although the information is still limited and controversial [34–36]. An association between higher urinary C4d/creatinine levels and higher proteinuria, more severe MEST scores and higher risk of developing ESKD has been reported [37].
Terminal pathway
The TP also appears to be important in the pathogenesis of IgAN. C5b-9 deposition is associated with kidney inflammation and progression of glomerulosclerosis [38] and mesangial C5b-9 staining correlates significantly with mesangial C3 fragments [36]. In addition, glomerular deposition of C5b-9 may contribute to podocyte damage and the subsequent development of proteinuria [39].
In summary, a larger and more intense deposition of the different complement components correlates with worse kidney prognosis (lower eGFR, more proteinuria and more histologic damage) in IgAN. However, it is difficult to obtain all these histologic markers in routine clinical practice. C3 deposition is virtually universal, but deposition of the remaining complement proteins is less homogeneous. An open question remains as to whether new complement-targeted therapies should be selected based on the presence or absence of these histologic markers. In addition to the prognostic implications of complement deposits, plasma levels of complement proteins, regulators and complement activation products could become valuable biomarkers of clinical application in IgAN. The urinary excretion of complement factors could also be a useful biomarker to monitor complement activation in glomerular diseases [40, 41], although conclusive studies are needed in this regard.
TMA IN IgAN AND ITS RELATIONSHIP WITH COMPLEMENT HYPERACTIVITY
Thrombi occluding the glomerular capillaries and/or kidney arterioles, together with mesangiolysis, endothelial swelling, and intimal thickening and concentric lamination (onion skin lesions) of the arterioles, are the most frequently observed TMA lesions [42–44]. Different studies have reported microangiopathy (without glomerular or arteriolar thrombi), or TMA in kidney biopsies of IgAN patients. The proportion of biopsies showing these lesions oscillates between 2% and 50% [45–55] and the reasons underlying such differences are not clear.
Since TMA and vascular lesions are not included in the Oxford pathologic classification, there is a lack of systematic report and analysis of these lesions in IgAN, but they seem to be similar to those found in other causes of TMA [47]. Despite the relatively high prevalence of TMA in IgAN, the percentage of patients presenting laboratory evidence of TMA (microangiopathic hemolytic anemia, thrombocytopenia, elevated lactate dehydrogenase, decreased or undetectable serum haptoglobin, schistocytes in peripheral blood smear) seems to be low compared with other entities causing TMA [56], and the reason for this discrepancy is not clear.
From a clinical standpoint, patients with TMA have a more aggressive presentation [45–55]: greater proteinuria, worse kidney function and, particularly, much more pronounced hypertension, which in many cases meets the defining criteria of malignant hypertension (extremely high blood pressure accompanied by bilateral retinal flame-shaped hemorrhages and/or exudates with or without papilledema). In fact, it has been shown that IgAN is a relatively frequent cause of malignant hypertension [56].
The finding of TMA lesions in IgAN patients has a significant impact on kidney outcomes [45, 46, 48–50] and the prognosis is particularly dismal in patients presenting with malignant hypertension [51, 56]. A severe kidney function impairment at presentation is common and, in a substantial proportion of cases, blood pressure control is not accompanied by a recovery of kidney function. Some studies have reported ESKD rates of more than 50% at 5 years [45, 46, 48, 49], with some patients reaching ESKD even faster, in a few months [50]. Immunosuppressive drugs do not seem to modify the unfavorable prognosis of IgA patients with TMA [50], although no controlled studies have been performed. Blockade of complement terminal complex with eculizumab and plasma exchange have been tried in some patients with disparate results [57–59]. These are, however, anecdotal clinical case reports. Table 1 summarizes the most important studies on TMA in IgAN.
Table 1:
Summary of studies addressing TMA lesions in IgAN patients, and the reported outcomes.
| Reference | N | TMA (%) | Hypertension in patients with TMA (%) | Malignant hypertension in patients with TMA (%) | ESKD in patients with TMA (%) |
|---|---|---|---|---|---|
| Neves et al. [46] | 118 | 18 | 100 | 71a | |
| Zhang et al. [48] | 1683 | 26 | 28b | ||
| El Karoui et al. [45] | 128 | 53 | 71 | 26 | 48c |
| Chua et al. [53] | 128 | 18 | 77 | 8 | 49d |
| Faria et al. [52] | 126 | 29 | |||
| Chang et al. [50] | 435 | 2.3 | 100 | 60 | 60e |
| Cai et al. [49] | 944 | 20 | 67 | 10 | 39f |
ESKD: kidney replacement therapy requirement. Median follow-up 65 months.
ESKD or 50% reduction in renal function. Mean follow-up 40 months.
Mean follow-up 44 months.
Kidney replacement therapy required. Median follow-up 48 months.
Kidney replacement therapy required.
ESKD: a >50% reduction in eGFR, ESKD or death. Median follow-up 50 months.
Severe hypertension has been traditionally considered as a cause of TMA [60]. However, recent studies have shown that many patients with kidney TMA-like primary and secondary atypical hemolytic uremic syndromes (entities in which complement dysregulation plays a crucial pathogenic role) or scleroderma renal crisis present with severe and malignant hypertension [61–63]. In this view, severe hypertension would be a manifestation of TMA, and not its cause. A similar mechanism could be invoked to explain the frequent finding of severe and malignant hypertension in IgAN patients with TMA lesions in the kidney biopsy, considering that recent studies have suggested a role of complement dysregulation in IgAN-associated TMA.
A more intense deposition of C4d, C3d and C5b-9 has been described in IgAN patients with TMA as compared with patients with other types of vascular lesions or patients without histological vascular damage lesions [53, 54]. Complement deposits, particularly C4d, were preferentially arteriolar in IgAN patients with TMA lesions, and a significant association between C4d deposition in kidney arterioles and the presence of vascular lesions has been reported [52, 54]. On the other hand, potentially pathogenic rare variants in complement genes have been reported in IgAN patients with microangiopathic lesions and in IgAN patients presenting with malignant hypertension [54, 56].
However, it should be noted that poorly galactosylated IgA can itself cause direct endothelial damage [47], and that patients with IgAN with microangiopathic lesions had higher levels of galactose-deficient IgA than patients without this type of vascular lesion [54]. A genetically determined predisposition to complement activation could amplify the endothelial damage initiated by an aberrant circulating IgA [10, 47, 54]. In this complex interplay between abnormal circulating IgA and activated complement, the coagulation cascade also plays a pathogenic role. Endothelial damage caused by C5b-9 increases the local production of von Willebrand factor which contributes to platelet activation and thrombus formation [47].
Overall, although vascular lesions are neglected in the Oxford classification, cumulative data suggest that the presence of TMA constitutes a poor prognostic marker in IgAN and that complement hyperactivity plays an important role in the pathogenesis of these vascular lesions. IgAN patients with TMA, and particularly those presenting with severe and malignant hypertension accompanying these vascular lesions, may be priority candidates for the complement blockers currently being evaluated in this disease. Studies specifically focused on this subset of IgAN patients are needed.
COMPLEMENT-TARGETED THERAPIES IN IgAN
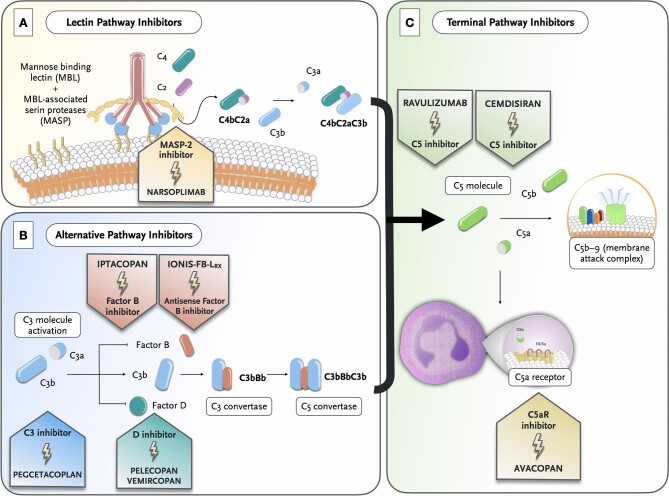
The dramatic improvements in the understanding of the role of complement in IgAN in recent years—as summarized above—have paved the way for the development and use of new complement-targeted therapies in this disease. To date, several ongoing trials are evaluating the safety and efficacy of several anti-complement agents in IgAN [64], and the main targets include MASP-2, factor B, factor D, C3, C5 and C5a receptor 1 (C5aR1) [7] (Table 2, Fig. 3). While definitive results from ongoing trials are still awaited, preliminary information have been made available.
Table 2:
Summary of clinical trials with complement inhibitors in IgAN.
| Agent | Target/mechanism of action | Compound/route | Company | Trial registration no./trial name | Phase | Design | Primary outcome | Results/status |
|---|---|---|---|---|---|---|---|---|
| Narsoplimab (OMS721) |
MASP-2/LP inhibition | Monoclonal antibody against MASP-2/intravenous injection | Omeros | NCT02682407 | 2 | Substudy 1: single-arm open-label study | Safety and tolerability | - Safe and well tolerated |
| Substudy 2: RCT followed by open-label | - Proteinuria reduction with preserved eGFR | |||||||
| NCT03608033/ARTEMIS-IGAN | 3 | Randomized, double-blind, placebo-controlled | Change of proteinuria from baseline at 36 weeks | Ongoing | ||||
| Iptacopan (LNP023) | Factor B/AP inhibition | Small molecule/orally administered | Novartis | NCT03373461 | 2 | Randomized, double-blind, dose-ranging, parallel-group adaptive design | Safety and tolerability | - Well tolerated |
| - Reduction in proteinuria | ||||||||
| - Strong inhibition of alternative pathway | ||||||||
| NCT04578834/APPLAUSE-IgAN | 3 | Multi-center, randomized, double-blind, placebo-controlled | Ratio to baseline in UPCR (9 months) and annualized total eGFR slope (24 months) |
Ongoing | ||||
| IONIS-FB-LRX (RG6299) |
Factor B/antisense inhibitor of complement factor B | Oligonucleotide/subcutaneous injection | Ionis | NCT04014335 | 2 | Single-arm, open-label study | Change of proteinuria from baseline at 29 weeks | Ongoing |
| Pegectacoplan (APL-2) | C3/AP inhibition | Pegylated peptide/subcutaneous injection | Apellis | NCT03453619 | 2 | Single-arm, open-label study | Safety and efficacy in reduction of proteinuria at Week 48 | Ongoing |
| Pelecopan (BCX9930) |
Factor D/AP inhibition | Small molecule/orally administered | BioCryst Pharmaceuticals | NCT05162066 | 2 | Open-label, proof-of-concept study | Safety and tolerability | Terminated, no results available |
| Percent change from baseline in UPCR | ||||||||
| Vemircopan (ALXN2050) |
Factor D/AP inhibition | Small molecule/orally administered | Alexion | NCT05097989 | 2 | Randomized, double-blind, placebo-controlled study | Percentage proteinuria change at Week 26 | Ongoing |
| Ravulizumab (ALXN1210) |
C5/TP inhibition | Monoclonal antibody/intravenous injection | Alexion/AstraZeneca | NCT04564339 | 2 | Randomized, double-blind, placebo-controlled study | Percentage proteinuria change at Week 26 | Ongoing |
| Cemdisiran (ALN-CC5) | C5/TP inhibition | Small interfering RNA/subcutaneous injection | Alnylam | NCT03841448 | 2 | Randomized, double-blind, placebo-controlled study | Percentage proteinuria change at Week 32 | Ongoing |
| Avacopan (CCX168) |
C5aR1/inhibition of anaphylatoxin | Small molecule/orally administered | Chemocentryx | NCT02384317 | 2 | Single-arm open-label study | Change in slope of the UPCR from the 8-week run-in period through the 12 weeks | Improvement in UPCR slope, with ∼50% improvement in 3/7 patients |
eGFR: estimated glomerular filtration rate; MASP-2: Mannan-associated lectin-binding serine protease-2; RCT: randomized controlled trial; UPCR: urinary protein-to-creatinine ratio.
Figure 3:
Landscape of complement inhibitors currently evaluated by ongoing clinical trials in IgAN and their site of action.
The increasingly recognized role of the LP in the pathogenesis of IgAN [30, 65, 66] has provided rationale for evaluating an agent against MASP-2, the effector enzyme of this pathway. Narsoplimab (OMS721) is a fully humanized IgG4 monoclonal antibody to MASP-2 that inhibits the LP, allowing C3 activation through the CP or AP.
Narsoplimab was evaluated in a phase 2 study (NCT02682407) that assessed the safety and effectiveness of this agent in the disease [67]. This trial included two substudies: Substudy 1 was a single-arm open-label study that enrolled 4 patients with corticosteroid-dependent IgAN who received corticosteroids (at a dose >10 mg/day) for at least 12 weeks, and then received narsoplimab infusions once weekly for 12 weeks (during which corticosteroid doses were tapered down); and Substudy 2 enrolled 12 patients who were not receiving corticosteroids, and patients were randomized 1:1 to receive once-weekly narsoplimab or vehicle infusions for 12 weeks. After 6 weeks of follow-up, both Substudy 2 groups could continue in an open-label extension, receiving one or more narsoplimab courses at the investigator's discretion. Patients enrolled in Substudy 1 achieved a proteinuria reduction ranging from 54% to 95% compared with baseline, at Week 18. Conversely, in Substudy 2, proteinuria reduction was similar between narsoplimab and placebo at Week 18, although eight patients who continued the extension study showed a median proteinuria reduction of 61%, suggesting a potential benefit in IgAN.
Narsoplimab was being evaluated in a phase 3 randomized, double-blind, placebo-controlled study (ARTEMIS-IgA Nephropathy trial, NCT03608033) [68], which initially planned to recruit 450 patients (225 per arm) with biopsy-proven IgAN, a proteinuria >1 g/day and eGFR ≥30 mL/min/1.73 m2. Patients were randomized 1:1 to weekly intravenous narsoplimab or placebo during an initial treatment period (Weeks 1–12) and, based on proteinuria response, patients were monitored or received additional 6-week blinded treatment during the response evaluation period (Weeks 13–36). The primary endpoint was the change in proteinuria from baseline at 36 weeks. However, the trial was discontinued in 2023, as treatment with narsoplimab did not result in a statistically significant reduction in proteinuria compared with placebo.
Selective inhibition of the AP is also being evaluated in IgAN patients, as a key driver of glomerular inflammation [64]. Among the agents, two drugs that specifically target factor B are currently being tested. Iptacopan (LNP023) is a selective and highly potent oral drug that binds to the active site of factor B, preventing the activation of this pathway and its amplification loop [69].
Iptacopan was evaluated in a phase 2 study (NCT03373461) that enrolled IgAN patients with proteinuria ≥0.75 g/day [70]. The study comprised two parts: Part 1 which enrolled 46 patients for a 3-month period; and Part 2 which enrolled 66 patients for a 6-month period. Patients were randomized to one of the four iptacopan arms [10, 50, 100 (in Part 2 only) and 200 mg twice daily] or placebo. Sustained AP inhibition was observed with all doses of iptacopan and no serious infections occurred. A reduction of proteinuria was observed from baseline up to 6 months in the twice-daily iptacopan 200 mg arm by up to 40% and at least 28% versus placebo [70]. An ongoing phase 3 trial (APPLAUSE-IgA Nephropathy trial, NCT04578834) will analyze the efficacy and safety of iptacopan compared with placebo on proteinuria reduction (primary outcome at 9 months), and slowing disease progression in IgAN patients (annualized eGFR slope over 24 months).
The other anti–factor B agent also under investigation is IONIS-FB-LRX (NCT04014335), an oligonucleotide targeting the complement factor B gene (CFB), thereby reducing circulating levels of complement factor B. This is a phase 2, single arm open-label clinical study in up to 25 participants that consists of a 24-week treatment period, an optional treatment extension period of up to an additional 48 weeks, and a 12-week post-treatment follow-up evaluation period. The main outcome is proteinuria reduction. Preliminary results revealed in abstract form showed selective reduction of plasma factor B levels and serum AP activity, together with a proteinuria reduction of –1.09 g/day corresponding to a 44% reduction.
Selective inhibition of C3 is also being tested in IgAN. Pegcetacoplan (APL-2) is a subcutaneous compstatin derivative which blocks C3 preventing the generation of C3b [71]. Pegcetacoplan (Empaveli™) has already been approved for paroxysmal nocturnal hemoglobinuria and, in fact, recent reports suggest that this agent may provide clinical benefits as first-line treatment compared with ravulizumab or eculizumab [72, 73]. Pegcetacoplan is currently being evaluated in IgAN, in a phase 2 study (NCT03453619) which aims to assess the safety and efficacy in terms of proteinuria reduction from baseline to Week 48.
Selective inhibition of factor D is another target that is being investigated in IgAN, as well as in other glomerular/systemic diseases. Factor D is a serine protease largely produced in adipocytes and, due to its low serum concentrations, represents the limiting enzyme in the activation sequence of the AP [74]. Two compounds are currently being tested. Pelecopan (BCX9930), a selective oral factor D inhibitor, has been evaluated in a proof-of-concept basket study (NCT05162066) in patients with IgAN, membranous nephropathy or C3 glomerulopathy [75]. The trial was stopped at the sponsor's decision and no results are available at the time of writing. On the other hand, vemircopan (ALXN2050) is currently being evaluated in an ongoing phase 2 trial including patients with IgAN and proliferative lupus nephritis (NCT05097989). This study is designed to assess the safety and efficacy, with the change in proteinuria from baseline to Week 26, as the primary endpoint.
While results with eculizumab, a humanized monoclonal antibody that selectively inhibits C5, in IgAN have been inconsistent [76–78], a newer long-acting C5-inhibitor named ravulizumab is currently being evaluated in IgAN a phase 2 study (NCT04564339). The primary outcome of this study is percentage proteinuria change from baseline to Week 26.
Moreover, a small-interfering RNA that suppresses liver production of C5, cemdisiran (ALN-CC5), is also being tested in IgAN in a phase 2, randomized, double-blind, placebo-controlled trial (NCT03841448).
Finally, the potential efficacy of C5aR1 blockade is also being evaluated in IgAN. Evidence on the deleterious effects of kidney expression of C3aR and C5aR1 in IgAN patients—in terms of both disease activity and severity of kidney injury—provide rationale for pharmacological blockade of these receptors [79, 80].
An open-label phase 2 pilot trial evaluated the effects of avacopan in adult patients with biopsy-proven IgAN [81], urinary protein:creatinine ratio (UPCR) >1 g/g and an eGFR >60 mL/min/1.73 m2. If the UPCR remained >1 g/g after an 8-week run-in period, patients started avacopan 30 mg twice daily. Seven patients received avacopan: six of the seven patients had numerical improvement in the UPCR during the avacopan treatment period, three of whom had a numerical improvement of about 50% at Week 12 [81]. These results in IgAN, although significant, were more modest compared with those found in ANCA vasculitis [82], although it is likely that the long-term effects of avacopan, avoiding or reducing corticosteroid exposure should be explored.
In summary, all the breakthroughs in the understanding of the contributing role of complement in IgAN have led to the development of novel promising anti-complement therapies that, combined with other newer agents, will likely move the management of IgAN towards a more personalized approach. On the other hand, ongoing trials with the new complement blockers will provide relevant information to compare the potential risks of each complement inhibition approach: individual blockade of the LP and AP, blockade of C3 or C5aR, or blockade of TP.
CONCLUSIONS AND FUTURE PERSPECTIVE
The last decade has seen dramatic improvements in our understanding of the pathogenesis underlying IgAN. Landmark studies have underpinned the pathogenic importance of complement activation in IgAN—namely the AP and LP—which has fostered the development of newer complement-targeted therapies. However, despite the accumulation of data, several uncertainties remain in both the management and monitoring of these patients. The great heterogeneity in clinical presentation and evolution of IgAN patients makes it difficult to cluster patients into specific risk profiles of who would benefit the most from these new therapeutic approaches. Hence, all these unmet clinical challenges will require further research.
In conclusion, all the breakthroughs in the understanding of IgAN and the development of novel complement-targeted therapies are likely to usher in a new era of personalized treatment. With the advent of precision medicine, the possibilities for tailored approaches based on a patient's individual characteristics offer hope for improved outcomes in IgAN patients.
Contributor Information
Fernando Caravaca-Fontán, Department of Nephrology, Instituto de Investigación Hospital 12 de Octubre (imas12), Madrid, Spain.
Eduardo Gutiérrez, Department of Nephrology, Hospital Universitario 12 de Octubre (imas12), Madrid, Spain.
Ángel M Sevillano, Department of Nephrology, Hospital Universitario 12 de Octubre (imas12), Madrid, Spain.
Manuel Praga, Department of Nephrology, Instituto de Investigación Hospital 12 de Octubre (imas12), Madrid, Spain; Department of Medicine, Complutense University, Madrid, Spain.
FUNDING
This paper was published as part of a supplement funded by an educational grant from Otsuka America Pharmaceutical, Inc.
DATA AVAILABILITY STATEMENT
No new data were generated or analyzed in support of this research.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris) 1968;74:694–5. [PubMed] [Google Scholar]
- 2. McGrogan A, Franssen CFM, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011;26:414–30. 10.1093/ndt/gfq665 [DOI] [PubMed] [Google Scholar]
- 3. Gutiérrez E, Carvaca-Fontán F, Luzardo Let al. A personalized update on IgA nephropathy: a new vision and new future challenges. Nephron 2020;144:555–71. 10.1159/000509997 [DOI] [PubMed] [Google Scholar]
- 4. Suzuki H, Kiryluk K, Novak Jet al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol 2011;22:1795–803. 10.1681/ASN.2011050464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Novak J, Julian BA, Mestecky Jet al. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin Immunopathol 2012;34:365–82. 10.1007/s00281-012-0306-z [DOI] [PubMed] [Google Scholar]
- 6. Ohyama Y, Renfrow MB, Novak Jet al. Aberrantly glycosylated IgA1 in IgA nephropathy: what we know and what we don't know. J Clin Med 2021;10:3467. 10.3390/jcm10163467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poppelaars F, Faria B, Schwaeble Wet al. The contribution of complement to the pathogenesis of IgA nephropathy: are complement-targeted therapies moving from rare disorders to more common diseases? J Clin Med 2021;10:4715. 10.3390/jcm10204715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans DJ, Williams DG, Peters DKet al. Glomerular deposition of properdin in Henoch-Schönlein syndrome and idiopathic focal nephritis. BMJ 1973;3:326–8. 10.1136/bmj.3.5875.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merle NS, Church SE, Fremeaux-Bacchi Vet al. Complement system part I—molecular mechanisms of activation and regulation. Front Immunol 2015;6:1–30. 10.3389/fimmu.2015.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tortajada A, Gutierrez E, Pickering MCet al. The role of complement in IgA nephropathy. Mol Immunol 2019;114:123–32. 10.1016/j.molimm.2019.07.017 [DOI] [PubMed] [Google Scholar]
- 11. Maillard N, Wyatt RJ, Julian BAet al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol 2015;26:1503–12. 10.1681/ASN.2014101000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki K, Honda K, Tanabe Ket al. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 2003;63:2286–94. 10.1046/j.1523-1755.63.6s.2.x [DOI] [PubMed] [Google Scholar]
- 13. Medjeral-Thomas NR, Cook HT, Pickering MC. Complement activation in IgA nephropathy. Semin Immunopathol 2021;43:679–90. 10.1007/s00281-021-00882-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zwirner J, Burg M, Schulze Met al. Activated complement C3: a potentially novel predictor of progressive IgA nephropathy. Kidney Int 1997;51:1257–64. 10.1038/ki.1997.171 [DOI] [PubMed] [Google Scholar]
- 15. Janssen U, Bahlmann F, Köhl Jet al. Activation of the acute phase response and complement C3 in patients with IgA nephropathy. Am J Kidney Dis 2000;35:21–8. 10.1016/S0272-6386(00)70296-4 [DOI] [PubMed] [Google Scholar]
- 16. Mizerska-Wasiak M, Małdyk J, Rybi-Szumińska Aet al. Relationship between serum IgA/C3 ratio and severity of histological lesions using the Oxford classification in children with IgA nephropathy. Pediatr Nephrol 2015;30:1113–20. 10.1007/s00467-014-3024-z [DOI] [PubMed] [Google Scholar]
- 17. Kawasaki Y, Maeda R, Ohara Set al. Serum IgA/C3 and glomerular C3 staining predict severity of IgA nephropathy. Pediatr Int 2018;60:162–7. 10.1111/ped.13461 [DOI] [PubMed] [Google Scholar]
- 18. Chiu Y-L, Lin W-C, Shu K-Het al. Alternative complement pathway is activated and associated with galactose-deficient IgA1 antibody in IgA nephropathy patients. Front Immunol 2021;12:638309. 10.3389/fimmu.2021.638309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol 2013;33:479–92. 10.1016/j.semnephrol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coppo R, Peruzzi L, Loiacono Eet al. Defective gene expression of the membrane complement inhibitor CD46 in patients with progressive immunoglobulin A nephropathy. Nephrol Dial Transplant 2019;34:587–96. 10.1093/ndt/gfy064 [DOI] [PubMed] [Google Scholar]
- 21. Medjeral-Thomas NR, Lomax-Browne HJ, Beckwith Het al. Circulating complement factor H–related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int 2017;92:942–52. 10.1016/j.kint.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tortajada A, Gutiérrez E, Goicoechea de Jorge Eet al. Elevated factor H–related protein 1 and factor H pathogenic variants decrease complement regulation in IgA nephropathy. Kidney Int 2017;92:953–63. 10.1016/j.kint.2017.03.041 [DOI] [PubMed] [Google Scholar]
- 23. Lucientes-Continente L, Márquez-Tirado B, Goicoechea de Jorge E. The Factor H protein family: the switchers of the complement alternative pathway. Immunol Rev 2023;313:25–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gharavi AG, Kiryluk K, Choi Met al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet 2011;43:321–7. 10.1038/ng.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holmes LV, Strain L, Staniforth SJet al. Determining the population frequency of the CFHR3/CFHR1 deletion at 1q32. PLoS One 2013;8:e60352. 10.1371/journal.pone.0060352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yeo SC, Goh SM, Barratt J. Is immunoglobulin A nephropathy different in different ethnic populations? Nephrology (Carlton) 2019;24:885–95. 10.1111/nep.13592 [DOI] [PubMed] [Google Scholar]
- 27. Jullien P, Laurent B, Claisse Get al. Deletion variants of CFHR1 and CFHR3 associate with mesangial immune deposits but not with progression of IgA nephropathy. J Am Soc Nephrol 2018;29:661–9. 10.1681/ASN.2017010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pesce F, Stea ED, Divella Cet al. DelCFHR3-1 influences graft survival in transplant patients with IgA nephropathy via complement-mediated cellular senescence. Am J Transplant 2021;21:838–45. 10.1111/ajt.16350 [DOI] [PubMed] [Google Scholar]
- 29. Stad RK, Bruijn JA, Van Gijlswijk-Janssen DJet al. An acute model for IgA-mediated glomerular inflammation in rats induced by monoclonal polymeric rat IgA antibodies. Clin Exp Immunol 2008;92:514–21. 10.1111/j.1365-2249.1993.tb03430.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roos A, Rastaldi MP, Calvaresi Net al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol 2006;17:1724–34. 10.1681/ASN.2005090923 [DOI] [PubMed] [Google Scholar]
- 31. Espinosa M, Ortega R, Sánchez Met al. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin J Am Soc Nephrol 2014;9:897–904. 10.2215/CJN.09710913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Segarra A, Romero K, Agraz Iet al. Mesangial C4d deposits in early IgA nephropathy. Clin J Am Soc Nephrol 2018;13:258–64. 10.2215/CJN.02530317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang Y, Zan J, Shi Set al. Glomerular C4d deposition and kidney disease progression in IgA nephropathy: a systematic review and meta-analysis. Kidney Med 2021;3:1014–21. 10.1016/j.xkme.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo W, Zhu L, Meng Set al. Mannose-binding lectin levels could predict prognosis in IgA nephropathy. J Am Soc Nephrol 2017;28:3175–81. 10.1681/ASN.2017010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ouyang Y, Zhu L, Shi Met al. A rare genetic defect of MBL2 increased the risk for progression of IgA nephropathy. Front Immunol 2019;10:537. 10.3389/fimmu.2019.00537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Medjeral-Thomas NR, Troldborg A, Constantinou Net al. Progressive IgA nephropathy is associated with low circulating mannan-binding lectin–associated serine protease-3 (MASP-3) and increased glomerular factor H–related protein-5 (FHR5) deposition. Kidney Int Rep 2018;3:426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Z, Jiang Y, Chen Pet al. The level of urinary C4d is associated with disease progression in IgA nephropathy with glomerular crescentic lesions: a cohort study. Nephrol Dial Transplant 2022;37:2119–27. 10.1093/ndt/gfac024 [DOI] [PubMed] [Google Scholar]
- 38. Stangou M, Alexopoulos E, Pantzaki Aet al. C5b-9 glomerular deposition and tubular α3β1-integrin expression are implicated in the development of chronic lesions and predict renal function outcome in immunoglobulin A nephropathy. Scand J Urol Nephrol 2008;42:373–80. 10.1080/00365590801943241 [DOI] [PubMed] [Google Scholar]
- 39. Xu L, Yang H-C, Hao C-Met al. Podocyte number predicts progression of proteinuria in IgA nephropathy. Mod Pathol 2010;23:1241–50. 10.1038/modpathol.2010.110 [DOI] [PubMed] [Google Scholar]
- 40. Medjeral-Thomas NR. Can urinary complement proteins stratify patients to therapeutic complement inhibitors? Kidney Int Rep 2022;7:939–41. 10.1016/j.ekir.2022.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Genest DS, Bonnefoy A, Khalili Met al. Comparison of complement pathway activation in autoimmune glomerulonephritis. Kidney Int Rep 2022;7:1027–36. 10.1016/j.ekir.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med 2014;371:654–66. 10.1056/NEJMra1312353 [DOI] [PubMed] [Google Scholar]
- 43. Goodship THJ, Cook HT, Fakhouri Fet al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2017;91:539–51. 10.1016/j.kint.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 44. Manenti L, Gnappi E, Vaglio Aet al. Atypical haemolytic uraemic syndrome with underlying glomerulopathies. A case series and a review of the literature. Nephrol Dial Transplant 2013;28:2246–59. 10.1093/ndt/gft220 [DOI] [PubMed] [Google Scholar]
- 45. El Karoui K, Hill GS, Karras Aet al. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol 2012;23:137–48. 10.1681/ASN.2010111130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neves PDM de M, Souza RA, Torres FMet al. Evidences of histologic thrombotic microangiopathy and the impact in renal outcomes of patients with IgA nephropathy. PLoS One 2020;15:e0233199. 10.1371/journal.pone.0233199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trimarchi H, Coppo R. Glomerular endothelial activation, C4d deposits and microangiopathy in immunoglobulin A nephropathy. Nephrol Dial Transplant 2021;36:581–6. 10.1093/ndt/gfz241 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Y, Sun L, Zhou Set al. Intrarenal arterial lesions are associated with higher blood pressure, reduced renal function and poorer renal outcomes in patients with IgA nephropathy. Kidney Blood Press Res 2018;43:639–50. 10.1159/000489290 [DOI] [PubMed] [Google Scholar]
- 49. Cai Q, Shi S, Wang Set al. Microangiopathic lesions in IgA nephropathy: a cohort study. Am J Kidney Dis 2019;74:629–39. 10.1053/j.ajkd.2019.03.416 [DOI] [PubMed] [Google Scholar]
- 50. Chang A, Kowalewska J, Smith KDet al. A clinicopathologic study of thrombotic microangiopathy in the setting of IgA nephropathy. Clin Nephrol 2006;66:397–404. 10.5414/CNP66397 [DOI] [PubMed] [Google Scholar]
- 51. Sevillano ÁM, Cabrera J, Gutiérrez Eet al. Malignant hypertension: a type of IgA nephropathy manifestation with poor prognosis. Nefrologia 2015;35:42–9. [DOI] [PubMed] [Google Scholar]
- 52. Faria B, Canão P, Cai Qet al. Arteriolar C4d in IgA nephropathy: a cohort study. Am J Kidney Dis 2020;76:669–78. 10.1053/j.ajkd.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 53. Chua JS, Zandbergen M, Wolterbeek Ret al. Complement-mediated microangiopathy in IgA nephropathy and IgA vasculitis with nephritis. Mod Pathol 2019;32:1147–57. 10.1038/s41379-019-0259-z [DOI] [PubMed] [Google Scholar]
- 54. Li J, Guo L, Shi Set al. The role of complement in microangiopathic lesions of IgA nephropathy. Kidney Int Rep 2022;7:1219–28. 10.1016/j.ekir.2022.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haas M, Mirocha J. Thrombotic microangiopathy in IgA nephropathy. Kidney Dis 2018;4:165–6. [Google Scholar]
- 56. Cavero T, Auñón P, Caravaca-Fontán Fet al. Thrombotic microangiopathy in patients with malignant hypertension. Nephrol Dial Transplant 2023;38:1217–26. [DOI] [PubMed] [Google Scholar]
- 57. Nakamura H, Anayama M, Makino Met al. Atypical hemolytic uremic syndrome associated with complement factor H mutation and IgA nephropathy: a case report successfully treated with eculizumab. Nephron 2018;138:324–7. 10.1159/000485194 [DOI] [PubMed] [Google Scholar]
- 58. Wang Z, Zhang JJ, Zuo Let al. Efficacy of plasma exchange in severe crescentic IgA nephropathy: a multicentered, cohort study. Beijing Da Xue Xue Bao 2022;54:1038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matsumura D, Tanaka A, Nakamura Tet al. Coexistence of atypical hemolytic uremic syndrome and crescentic IgA nephropathy treated with eculizumab: a case report. Clin Nephrol Case Stud 2016;4:24–8. 10.5414/CNCS108889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van den Born BJH, Honnebier UPF, Koopmans RPet al. Microangiopathic hemolysis and renal failure in malignant hypertension. Hypertension 2005;45:246–51. 10.1161/01.HYP.0000151620.17905.ee [DOI] [PubMed] [Google Scholar]
- 61. Cavero T, Arjona E, Soto Ket al. Severe and malignant hypertension are common in primary atypical hemolytic uremic syndrome. Kidney Int 2019;96:995–1004. 10.1016/j.kint.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 62. Timmermans SAMEG, Abdul-Hamid MA, Vanderlocht Jet al. Patients with hypertension-associated thrombotic microangiopathy may present with complement abnormalities. Kidney Int 2017;91:1420–5. 10.1016/j.kint.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 63. El Karoui K, Boudhabhay I, Petitprez Fet al. Impact of hypertensive emergency and rare complement variants on the presentation and outcome of atypical hemolytic uremic syndrome. Haematologica 2019;104:2501–11. 10.3324/haematol.2019.216903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cheung CK, Dormer JP, Barratt J. The role of complement in glomerulonephritis—are novel therapies ready for prime time? Nephrol Dial Transplant 2023;38:1789–97. [DOI] [PubMed] [Google Scholar]
- 65. Rizk DV, Maillard N, Julian BAet al. The emerging role of complement proteins as a target for therapy of IgA nephropathy. Front Immunol 2019;10:504. 10.3389/fimmu.2019.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Espinosa M, Ortega R, Gomez-Carrasco JMet al. Mesangial C4d deposition: a new prognostic factor in IgA nephropathy. Nephrol Dial Transplant 2008;24:886–91. 10.1093/ndt/gfn563 [DOI] [PubMed] [Google Scholar]
- 67. Lafayette RA, Rovin BH, Reich HNet al. Safety, tolerability and efficacy of narsoplimab, a novel MASP-2 inhibitor for the treatment of IgA nephropathy. Kidney Int Rep 2020;5:2032–41. 10.1016/j.ekir.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lafayette R, Rovin B, Floege Jet al. POS-132 trial design: phase 3 randomized, double-blind, placebo-controlled study of narsoplimab safety and efficacy in IGA nephropathy (artemis-IGAN). Kidney Int Rep 2022;7:S57. 10.1016/j.ekir.2022.01.144 [DOI] [Google Scholar]
- 69. Schubart A, Anderson K, Mainolfi Net al. Small-molecule factor B inhibitor for the treatment of complement-mediated diseases. Proc Natl Acad Sci USA 2019;116:7926–31. 10.1073/pnas.1820892116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Barratt J, Rovin B, Zhang Het al. POS-546 efficacy and safety of iptacopan in IgA nephropathy: results of a randomized double-blind placebo-controlled phase 2 study at 6 months. Kidney Int Rep 2022;7:S236. 10.1016/j.ekir.2022.01.577 [DOI] [Google Scholar]
- 71. Hoy SM. Pegcetacoplan: first approval. Drugs 2021;81:1423–30. 10.1007/s40265-021-01560-8 [DOI] [PubMed] [Google Scholar]
- 72. Hillmen P, Szer J, Weitz Iet al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 2021;384:1028–37. 10.1056/NEJMoa2029073 [DOI] [PubMed] [Google Scholar]
- 73. Wong R, Fishman J, Wilson Ket al. Comparative effectiveness of pegcetacoplan versus ravulizumab and eculizumab in complement inhibitor-naïve patients with paroxysmal nocturnal hemoglobinuria: a matching-adjusted indirect comparison. Adv Ther 2023;40:1571–89. 10.1007/s12325-023-02438-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gavriilaki E, Papakonstantinou A, Agrios KA. Novel insights into factor D inhibition. Int J Mol Sci 2022;23:7216. 10.3390/ijms23137216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nester C, Nast C, Appel Get al. POS-045 Evaluating BCX9930, an oral factor D inhibitor for treatment of complement-mediated kidney disease: a proof-of-concept study (RENEW). Kidney Int Rep 2022;7:S457–8. 10.1016/j.ekir.2022.04.067 [DOI] [Google Scholar]
- 76. Rosenblad T, Rebetz J, Johansson Met al. Eculizumab treatment for rescue of renal function in IgA nephropathy. Pediatr Nephrol 2014;29:2225–8. 10.1007/s00467-014-2863-y [DOI] [PubMed] [Google Scholar]
- 77. Ring T, Pedersen BB, Salkus Get al. Use of eculizumab in crescentic IgA nephropathy: proof of principle and conundrum? Clin Kidney J 2015;8:489–91. 10.1093/ckj/sfv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Herzog AL, Wanner C, Amann Ket al. First treatment of relapsing rapidly progressive IgA nephropathy with eculizumab after living kidney donation: a case report. Transplant Proc 2017;49:1574–7. 10.1016/j.transproceed.2017.02.044 [DOI] [PubMed] [Google Scholar]
- 79. Liu L, Zhang Y, Duan Xet al. C3a, C5a renal expression and their receptors are correlated to severity of IgA nephropathy. J Clin Immunol 2014;34:224–32. 10.1007/s10875-013-9970-6 [DOI] [PubMed] [Google Scholar]
- 80. Zhang Y, Yan X, Zhao Tet al. Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin A nephropathy in mice. Clin Exp Immunol 2017;189:60–70. 10.1111/cei.12961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bruchfeld A, Magin H, Nachman Pet al. C5a receptor inhibitor avacopan in immunoglobulin A nephropathy—an open-label pilot study. Clin Kidney J 2022;15:922–8. 10.1093/ckj/sfab294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jayne DRW, Merkel PA, Schall TJet al. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med 2021;384:599–609. 10.1056/NEJMoa2023386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.