Abstract
Perianal/perineal rhabdomyosarcomas (PRMS) is rare, and the outcome is poor. A 29-year-old female presented with perineal rhabdomyosarcomas revealed metastases to inguinal lymph nodes on the bilateral side. Disease progression was discovered when the patient got adjuvant epirubicin, ifosfamide, and bevacizumab for 2 cycles. After 3 cycles of nivolumab, dacarbazine, cisplatin, and vinblastine therapy, a partial response was identified in the patient. The surgical resection was performed. The patient received neoadjuvant chemotherapy before surgery and was weak after surgery, so he did not receive chemoradiotherapy. The patient succumbed after 11 months postoperatively due to widespread intraabdominal metastasis.
Keywords: operation, perianal/perineal rhabdomyosarcomas, surgery, treatment
1. Introduction
Rhabdomyosarcoma (RMS) is a malignancy that typically affects the head and neck, followed by the urogenital tract, the extremities, and, very infrequently, the perianal/perineal area. It develops from mesenchymal cells. RMS is classified mainly into 2 subtypes: alveolar rhabdomyosarcoma (ARMS) and embryonal rhabdomyosarcoma (ERMS). Perianal rhabdomyosarcoma is usually ARMS. Rhabdomyosarcoma is a rare tumor, occurring in 4 cases per 1 million population per year, with an incidence of 4.5 per 1 million children. Approximately 400 cases are diagnosed each year in young adults aged 0 to 19 years in Europe. Rhabdomyosarcoma has an overall 5-year survival rate of approximately 70% and requires treatment with a combination of surgical resection, radiotherapy, and chemotherapy.[1–3] A patient’s prognosis is poor if they have ARMS and are susceptible to regional lymph node metastases. The PAX3–FOXO1 fusion protein consists of 2 N-terminal DNA-binding domains of PAX3 (the paired box domain and the paired homeodomain) and the C-terminal trans-activation domain of FOXO1. Through the PAX3 domain, the protein binds to more than 1000 sites in the genome, most of which are promoter distal enhancer regions enriched for the PAX3 motif, resulting in the transcriptional activation of hundreds of target genes.[4] An adult patient with perianal rhabdomyosarcoma was admitted to the Second Department of Surgery, Fourth Hospital of Hebei Medical University. The present report describes a case of PRMS in an adult patient and reviews the diagnosis, treatment, and prognosis of rhabdomyosarcoma. The patient received standard clinical treatment. Mortality was expected in this type of cases.[5,6]
2. Case report
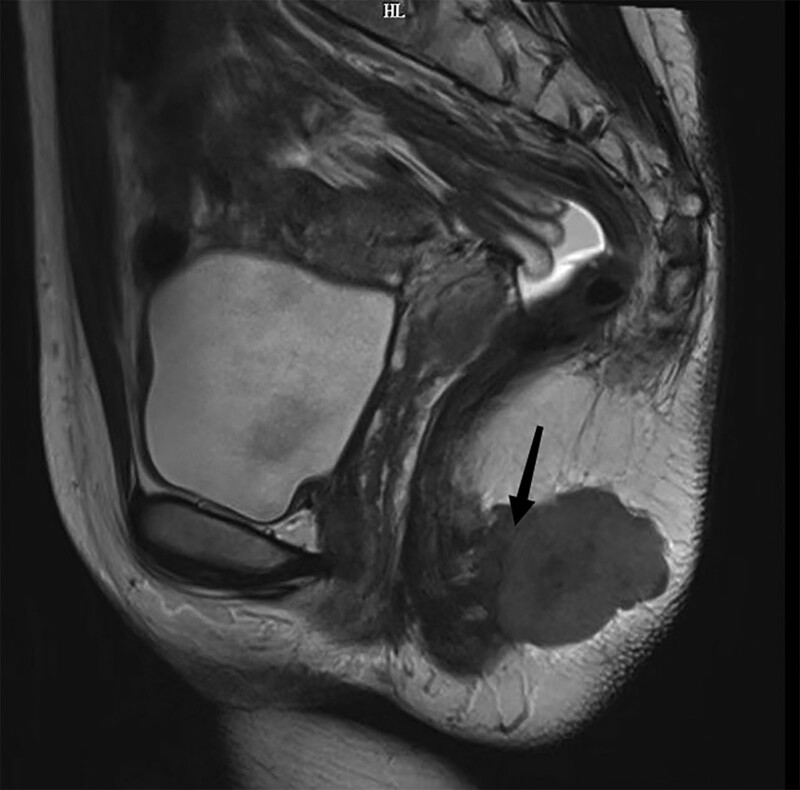
A perianal mass affected a 29-year-old woman for around 11 months. During the rectal examination, the rectovaginal septum felt normal, the activity was okay, and the anus seemed expected (knee-chest position). The finger inserted into the anus at about 3 cm could palpate a firm, 3 cm in diameter mass at the 5 o’clock position. She had a pelvic magnetic resonance imaging, which revealed several lymph node metastases in the anterior sacrum, bilateral iliac blood vessels, and bilateral groin region. Magnetic resonance imaging revealed a soft tissue mass posterior to anal canal (Fig. 1). Blood tests were within normal range, including biochemistry, routine, and serum tumor markers. Hemoglobin: 120 g/L, platelet: 212 × 109/L, white blood cell: 5.53 × 109/L, red blood cell: 4.08 × 1012/L (Data S1, Supplemental Digital Content, http://links.lww.com/MD/K848). There was no family history of cancer, alcoholism, or smoking. A network of transcription factors for cancer must include the chimeric transcription factor PAX3–FOXO1. In numerous cell cultures and xenograft tumor models, PAX3–FOXO1 increases the development and proliferation of human cells. Although PAX3–FOXO1 expression alone does not induce transformation in normal human cells, it can promote tumor development in conjunction with other events. In addition to its tumorigenic activity, PAX3–FOXO1 enhances cell invasion and migration.[7] Research has shown that PAX3/7–FOXO1 fusions can be detected and have prognostic significance in adult RMS patients.[8]
Figure 1.
The pelvic contrast-enhanced CT findings showed a malignant soft-tissue mass 3 cm in diameter (arrow) at 5 o’clock. CT = computed tomography.
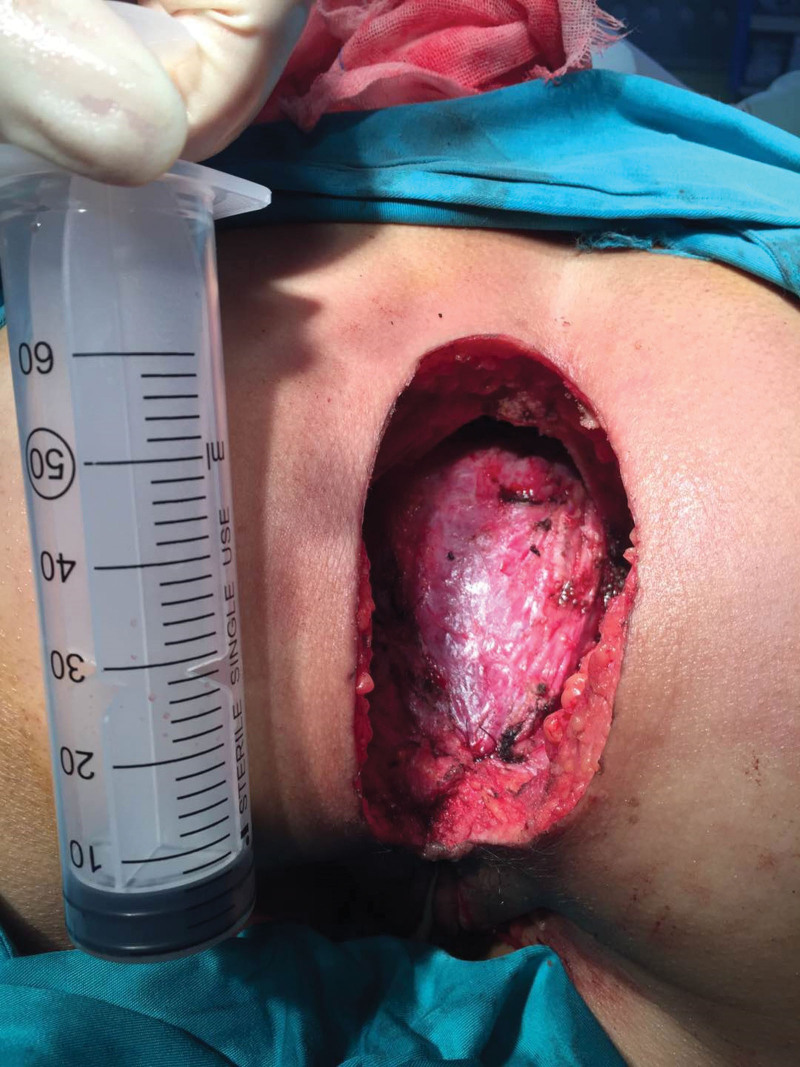
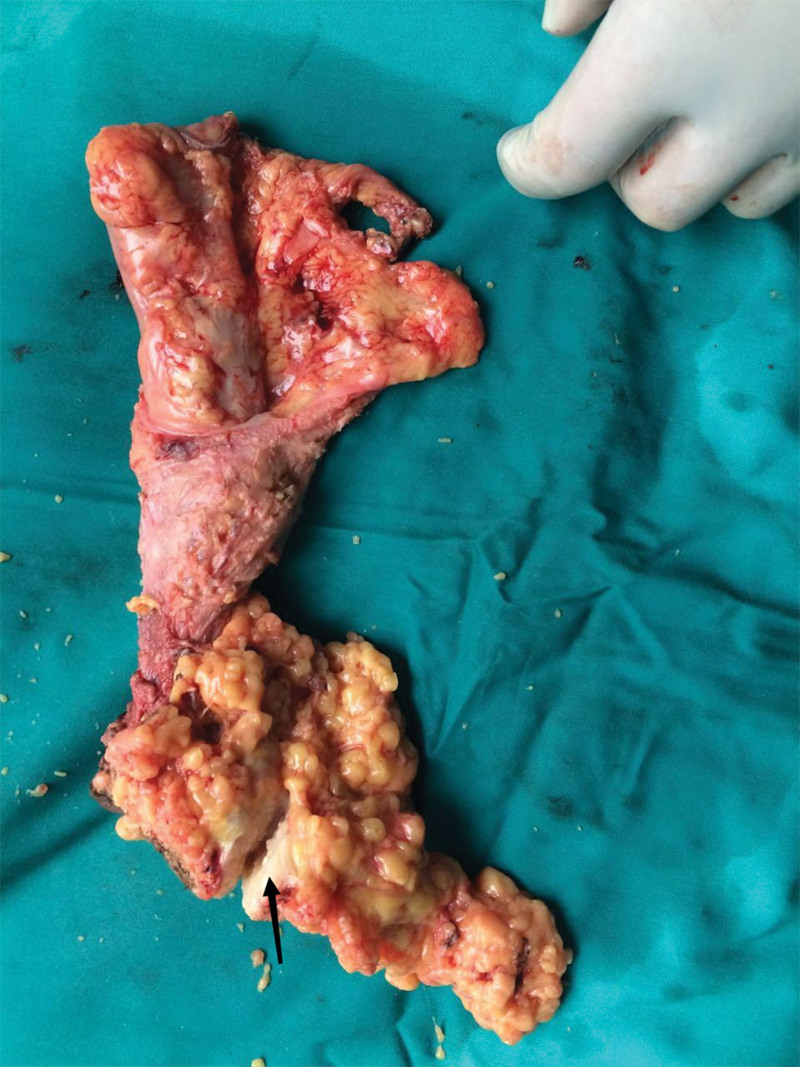
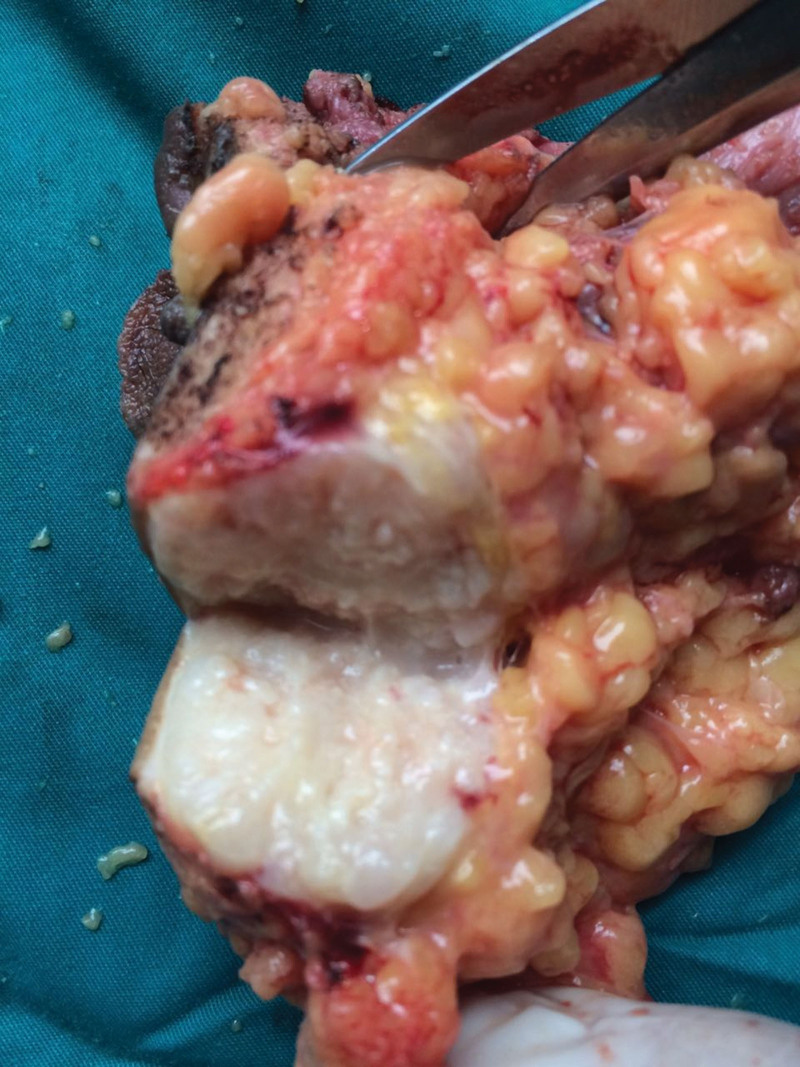
She underwent surgery and mass lesion was removed along with the inguinal lymph nodes. PRMS, the alveolar subtype, was found in the postoperative pathology. As previously indicated, the inspection recommended that she undergo 4 cycles of neoadjuvant chemotherapy. Bevacizumab (7.5 mg/m2), epirubicin hydrochloride (100 mg/m2 d1), and ifosfamide (7.5 g/m2) were the specific medications. The treatment process went smoothly; the patient found slightly smaller perianal nodules. The efficacy was evaluated as partial remission after 2 treatment cycles and stable disease after 4 cycles. But in less than a month, the patient felt the anus mass compared with the previous slightly larger recently. Anal examination found nodules enlarged slightly. It was precise because of the changes in conditions that previous programs have been replaced by second-line therapy. 3 cycles of chemotherapy with nivolumab (200 mg d1), dacarbazine (200 mg/m2 d1–3), cisplatin (50 mg/m2 d4.5), and vincristine (1.4 mg/m2 d1.8).[9] She subsequently underwent bilateral pelvic and bilateral inguinal lymphadenectomy surgery. Considering functional preservation in the surgery. Combined radiotherapy is an option. There was an about 12 cm incision (Fig. 2) and a grossly massive mass at the mesentery outside the bowel wall, measuring 5.5 cm × 3.5 cm × 3.0 cm (Fig. 3). On section, it was gray-white, tenacious, and slightly exquisite (Fig. 4). Perianal mass biopsy has been performed. Pathologic examination was consistent with ARMS, which has confirmed with pathologic review (Fig. 5). No metastases were seen in the rectal lymph nodes, mesenteric lymph nodes, and bilateral pelvic lymph nodes—surgical margin-negative. The pathology scale is described below.
Figure 2.
There is a 12 cm incision around the anus.
Figure 3.
A huge mass (arrow) at the mesentery outside the bowel wall, measuring 5.5 cm × 3.5 cm × 3.0 cm.
Figure 4.
On section, the mass was gray-white, tenacious, and slightly exquisite.
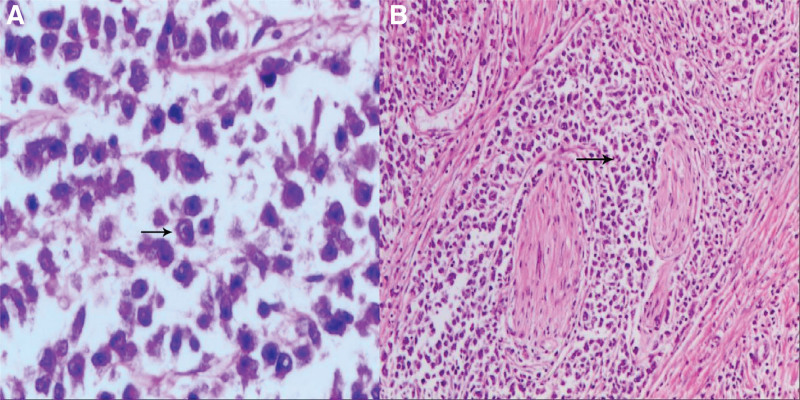
Figure 5.
Naive rhabdomyoblasts with abundant cytoplasm, eosinophilic. (A) The nucleus is oval or irregular and oversized nucleoli (arrow, high magnification: eyepiece 10× objective 40). (B) The tumor cells appeared with a nest-like distribution. Nest sizes were variable, and some presented as an acinar pattern (arrow, low magnification eyepiece 10× objective 10).
The Fourth Hospital of Hebei Medical University
Pathology consultation report
Consultation number: H16-00724
Name: Jing Shao
Gender: Female
Age: 29
Samples were sent to the testing hospital: The 260th Hospital of the Chinese People’s Liberation Army
Original pathology number: 57480
Materials to be examined: HE × 1, wax block × 1
Site of examination: inguinal lymph node
Consultation advice: Immunohistochemical results: CK (part+), CD3 (–), CD20 (–), Ki67 (70% positive), CgA (–), S100 (–), CD56 (+), EMA (–), Vimentin (+), CK7 (–), CK20 (–), P40 (–), Villin (–), Bcl-2 (–), Syn (–), HMB45 (–), MART-1 (–), CD99 (–), Desmin (+), myoglobic (NS).
Consistent with rhabdomyosarcoma
Reporting Doctor: Yanning Chen/Yan Ding
On the 10th day after surgery, the patient had intermittent distending pain in the lower abdomen without radiation pain. Nausea and vomiting of stomach content and bile, stop flatus, and defecation. The abdomen was soft and moderately distended, with mild tenderness in the lower quadrant. Bowel sounds are active. High-pitched bowel sounds can be heard. Computed tomography of the abdomen showed intestinal obstruction. Despite gastrointestinal decompression, fasting, parenteral feeding, dexamethasone medication, and somatostatin injection, the symptoms do not disappear. Transnasal obstructive catheter placement on postoperative day 26 and symptoms are not relieved too. The patient was treated with an exploratory laparotomy on postoperative day 31. There were dense adhesions between the pelvic side wall peritoneum and small bowel and difficult to detach. We performed small bowel decompression, resection, and anastomosis. The patient made an uneventful recovery. The patient was given semiliquid diet after operation, and the symptoms of abdominal distension occurred after taking normal diet. So the semiliquid diet is mainly, eating poorly. The patient cannot tolerate chemotherapy and radiotherapy after surgery because of frailty. The patient died postoperatively after 11 months, and widespread intraperitoneal metastasis is the cause of mortality. The lungs, kidneys, and liver had no metastatic lesions (Fig. S1, Supplemental Digital Content, http://links.lww.com/MD/K849). For patients with intraperitoneal metastasis after surgery for malignant tumors, the diagnosis is easier. Patients with unexplained abdominal mass or ascites as the initial symptom, especially those with multiple masses with or without ascites, should make full use of routine and imaging examinations, and at the same time draw ascites for repeated exfoliative cell examination to further confirm the diagnosis. Laparoscopy or early laparotomy can also be performed for early diagnosis and treatment. The main symptoms of intraperitoneal metastasis were massive peritoneal effusion, peritoneal thickening, abdominal mass and weight loss. Intraperitoneal metastasis may also often cause symptoms such as anemia (Fig. S2, Supplemental Digital Content, http://links.lww.com/MD/K850). The symptoms of intraperitoneal metastasis vary from patient to patient, but are usually accompanied by loss of appetite, nausea, and diarrhea.
3. Discussion
RMS includes 2 major histological subtypes: ARMS and ERMS, accounting for 20% and 70% of RMS, respectively.[10] ERMS is correlated with a good Prognosis of RMS.ARMS is more invasive due to its propensity to metastasize and recur.[11] PRMS is mostly ARMS. RMS has 2 evident genotypes characterized by the PAX3/7-FOXO1 fusion, and those without these fusions are closely related to tumor progression, prognosis, and clinical features.[12] Most fusion RMS is characterized by a PAX3–FOXO1 fusion, but a few have a PAX7–FOXO1 fusion.[13]
PRMS is rare and accounts for only 2% of RMS.[14] Some PRMS are misdiagnosed as perianal abscesses that delay treatment leading to local or distant metastasis.[15] Because magnetic resonance imaging indicates that PRMS is more closely associated with the external anal sphincter.[16] Miles operation of the rectum is often the most reliable choice. These patients underwent laparoscopic miles operation of the rectum and pelvic lymph node dissection. Previous studies documented that for patients with limited lesions and little or no involvement of external anal sphincter, local radical resection with anal preservation can be performed.[17]
The studies advocate the combination treatment of PRMS, involving surgical excision, radiotherapy, chemotherapy, tumor-targeted therapy, and immune therapy.[18] The patient received molecular targeted therapy, chemotherapy, radiotherapy, immunotherapy, and surgery. The patient presented with postoperative bowel obstruction, and on the 31st postoperative day, the patient underwent surgery. This case is a rare disease, and the patient developed adhesive intestinal obstruction after surgery. This patient was treated with an immune checkpoint inhibitor, which is considered to be related to immunosuppressive therapy. Immune checkpoint inhibitors have been reported to simultaneously compromise the autoimmune system and cause intestinal obstruction. Immune checkpoint inhibitors have been associated with colitis complicated with inflammatory intestinal obstruction.[19] Immune checkpoint inhibitor therapy results in aberrant immune activation, inflammatory response to injury smooth muscle cells, and intestinal canal motility, leading to intestinal obstruction.[20,21] Nivolumab is a monoclonal antibody for the treatment of cancer. The nivolumab increases the activation of T cells while decreasing the responsiveness of self-antigens, leading to autoimmune intestinal injury and clinical manifestations, including erosions to ulcers and obstruction.[22] Nivolumab has been reported to have efficacy in metastatic sarcoma.[23] Corticosteroids may provide some protection against inflammatory intestinal obstruction.[24] By accelerating the return of blood flow to the intestinal wall and promoting the healing of inflammation, somatostatin can considerably reduce intestinal dilatation and ischemia brought on by the buildup of digestive juice in the intestinal canal above the blocked segment.[25] In this patient, conservative treatment of intestinal obstruction failed, and surgical treatment was performed; intestinal stenosis, severe and widespread belly adhesions, and difficulties separating. As ICIs become more common in cancer treatment, irAEs will inevitably increase.
Lymph node metastases were frequently observed in patients with PRMS (46%), and alveolar PRMS had an exceptionally high risk of lymph node spread (78%).[26] In the past 30 years, the prognosis for individuals with advanced and metastatic RMS has not been much improved. The 5-year OS of patients with PRMS is 47.8%, and the prognosis of patients > 15 years old is inferior.[26] The patient was 29 years old, and the postoperative survival time was only 11 months. Currently, the research of targeted therapy and immunotherapy applied to RMS is ascendant.PAX-FOXO1 is the most immediate and promising target and immune antigen. It has been reported that liposome-protamine-siRNA particles targeting PAX3–FOXO1 are efficiently delivered to ARMS cell lines in vitro and downregulate PAX3–FOXO1 and its target genes, resulting in tumor growth delay and inhibition of ARMS xenografts.[27]
4. Conclusion
Immunotherapy research, molecularly targeted drugs, and other therapeutic modalities have raised hopes for improved PRMS diagnosis and treatment and more prolonged survival. PRMS requires comprehensive treatment, such as surgery, chemotherapy, and radiotherapy.
5. Limitations of the study
Although a case of adult perianal rhabdomyosarcoma was reported in detail in this study, only normal treatment of the patient was described, and the patient’s condition did not recover. PAX3–FOXO1 fusion gene testing and treatment were not performed in this patient. Therefore, future studies should increase the number of patients and summarize to provide new ideas for the treatment of perianal rhabdomyosarcoma.
Author contributions
Conceptualization: Ning Yang.
Data curation: Yabin Liu.
Formal analysis: Yabin Liu.
Funding acquisition: Ning Yang.
Investigation: Dexian Kong, Xv Wang.
Methodology: Dexian Kong, Xv Wang.
Software: Dexian Kong, Xv Wang.
Writing – original draft: Yabin Liu.
Writing – review & editing: Ning Yang, Yabin Liu.
Supplementary Material
Abbreviations:
- ARMS
- alveolar rhabdomyosarcoma
- EAS
- external anal sphincter
- ERMS
- embryonal rhabdomyosarcoma
- MRI
- magnetic resonance imaging
- PRMS
- perineal rhabdomyosarcomas
- RMS
- rhabdomyosarcoma
The study was supported financially by a grant from the Science and Research planning of Health Committee of Hebei Province, China (No. 20221233), and the Natural Scientific Foundation of Hebei Province (Grant number: H2021206177).
Patients and their families provided informed consent to participate in the study. The patient and families were informed in writing and consented to publication.
The Ethics Committee approved the study of the Fourth Hospital of Hebei Medical University (2019038).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Yang N, Kong D, Wang X, Liu Y. Perianal rhabdomyosarcoma in an adult: A case report and review of the literature. Medicine 2023;102:48(e36276).
Contributor Information
Ning Yang, Email: yangning780829@163.com.
Dexian Kong, Email: sun1101192021@163.com.
Xv Wang, Email: 1975503372@qq.com.
References
- [1].Kaseb H, Kuhn J, Babiker HM. Rhabdomyosarcoma. Treasure Island, FL: StatPearls; 2022. [Google Scholar]
- [2].Rajagopalan A, Christenberry SC, Ramachandran V. Rhabdomyosarcoma. Pediatr Rev. 2022;43:599–600. [DOI] [PubMed] [Google Scholar]
- [3].Agaram NP. Evolving classification of rhabdomyosarcoma. Histopathology. 2022;80:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cao L, Yu Y, Bilke S, et al. Genome-wide identification of PAX3-FKHR binding sites in rhabdomyosarcoma reveals candidate target genes important for development and cancer. Cancer Res. 2010;70:6497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Casey DL, Wexler LH, LaQuaglia MP, et al. Patterns of failure for rhabdomyosarcoma of the perineal and perianal region. Int J Radiat Oncol Biol Phys. 2014;89:82–7. [DOI] [PubMed] [Google Scholar]
- [6].Lautz TB, Chi YY, Li M, et al. Benefit of delayed primary excision in rhabdomyosarcoma: a report from the Children’s Oncology Group. Cancer. 2021;127:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nguyen TH, Barr FG. Therapeutic approaches targeting PAX3–FOXO1 and its regulatory and transcriptional pathways in rhabdomyosarcoma. Molecules. 2018;23:2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dumont SN, Lazar AJ, Bridge JA, et al. PAX3/7–FOXO1 fusion status in older rhabdomyosarcoma patient population by fluorescent in situ hybridization. J Cancer Res Clin Oncol. 2012;138:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Davis KL, Fox E, Merchant MS, et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020;21:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pal A, Chiu HY, Taneja R. Genetics, epigenetics and redox homeostasis in rhabdomyosarcoma: emerging targets and therapeutics. Redox Biol. 2019;25:101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hoang NT, Acevedo LA, Mann MJ, et al. A review of soft-tissue sarcomas: translation of biological advances into treatment measures. Cancer Manag Res. 2018;10:1089–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gallego S, Zanetti I, Orbach D, et al. Fusion status in patients with lymph node-positive (N1) alveolar rhabdomyosarcoma is a powerful predictor of prognosis: experience of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG). Cancer. 2018;124:3201–9. [DOI] [PubMed] [Google Scholar]
- [13].Azorsa DO, Bode PK, Wachtel M, et al. Immunohistochemical detection of PAX–FOXO1 fusion proteins in alveolar rhabdomyosarcoma using breakpoint specific monoclonal antibodies. Mod Pathol. 2021;34:748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Behera S, Mahajan JK, Bansal D. Pediatric perianal rhabdomyosarcoma: multimodal therapy for tumor control. Pediatr Blood Cancer. 2022;69:e29677. [DOI] [PubMed] [Google Scholar]
- [15].Maas M, Tielbeek J, Stoker J. Staging of anal cancer: role of MR imaging. Magn Reson Imaging Clin N Am. 2020;28:127–40. [DOI] [PubMed] [Google Scholar]
- [16].Guo Y, Hu B, Huang D, et al. Perianal and perineal rhabdomyosarcomas: a retrospective multicenter study of 35 cases. BMC Surg. 2021;21:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guo YY, Hu B, Wang XH, et al. [Clinical characteristics of perianal/perineal rhabdomyosarcoma-a report of 15 cases]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:1100–3. [DOI] [PubMed] [Google Scholar]
- [18].Fuchs J, Dantonello TM, Blumenstock G, et al. Treatment and outcome of patients suffering from perineal/perianal rhabdomyosarcoma: results from the CWS trials--retrospective clinical study. Ann Surg. 2014;259:1166–72. [DOI] [PubMed] [Google Scholar]
- [19].Tan S, Zhu G, Fan J, et al. Immune checkpoint inhibitor-induced colitis complicated with inflammatory intestinal obstruction: a case report and literature review. Transl Cancer Res. 2022;11:2443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Farro G, Gomez-Pinilla PJ, Di Giovangiulio M, et al. Smooth muscle and neural dysfunction contribute to different phases of murine postoperative ileus. Neurogastroenterol Motil. 2016;28:934–47. [DOI] [PubMed] [Google Scholar]
- [21].Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15:458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Iranzo I, Huguet JM, Suárez P, et al. Endoscopic evaluation of immunotherapy-induced gastrointestinal toxicity. World J Gastrointest Endosc. 2018;10:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Paoluzzi L, Cacavio A, Ghesani M, et al. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res. 2016;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Collins M, Michot JM, Danlos FX, et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol. 2017;28:2860–5. [DOI] [PubMed] [Google Scholar]
- [25].Wu Z, Wang S, Yuan S, et al. Clinical efficacy and safety of somatostatin in the treatment of early postoperative inflammatory small bowel obstruction: a protocol for systematic review and meta analysis. Medicine (Baltim). 2020;99:e20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rogers T, Zanetti I, Coppadoro B, et al. Perianal/perineal rhabdomyosarcoma: results of the SIOP MMT 95, Italian RMS 96, and EpSSG RMS 2005 studies. Pediatr Blood Cancer. 2022;69:e29739. [DOI] [PubMed] [Google Scholar]
- [27].Rengaswamy V, Zimmer D, Süss R, et al. RGD liposome-protamine-siRNA (LPR) nanoparticles targeting PAX3-FOXO1 for alveolar rhabdomyosarcoma therapy. J Control Release. 2016;235:319–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.