Abstract
Background:
Azithromycin (AZM) is an antimicrobial agent and frequently used in the treatment of pediatric respiratory diseases due to its well-recognized clinical efficacy. Despite some favorable findings from many studies, there is a lack of research reports focusing on the safety profiles and adverse reactions.
Methods:
The randomized controlled trials of AZM in the treatment of pediatric respiratory diseases on internet databases were searched. The search databases included Chinese CNKI, Wanfang, VIP, PubMed, EMBASE, and Cochrane Library. Two researchers of this study independently assessed the eligibility, risk of bias, and extracted the data. The included literature was meta-analyzed and subgroup analyzed by revman 5.1 software.
Results:
A total of 14 eligible studies were included. The results of meta-analysis showed that the incidence of adverse reactions after AZM treatment was 24.20%, which was lower than 48.05% in the control group (OR = 0.42, 95% CI 0.12–0.72, P < .001). In the subgroup of sequential therapy, AZM had a lower incidence of adverse reactions in sequential therapy (OR = 0.29, 95% CI 0.09–0.60, P < .001). In the subgroup of intravenous administration, AZM had a lower the incidence of adverse reactions (OR = 0.57, 95% CI 0.12–0.84, P = .003). In the subgroup of oral administration, AZM had a lower the incidence of adverse reactions (OR = 0.45, 95% CI 0.13–0.69 P < .001). Overall, it was also found that the incidence of adverse reactions in the AZM subgroup was significantly lower than that in other treatment subgroup.
Conclusion:
AZM has fewer adverse reactions and better safety profiles, which make AZM a more attractive option in the treatment of pediatric respiratory diseases.
Keywords: adverse reactions, azithromycin, meta-analysis, pediatrics, respiratory diseases, systematic review
1. Introduction
Pediatric respiratory diseases are one of the most common diseases leading to pediatric hospitalization, and it accounts for about 25% of all pediatric consultations.[1] Also, pediatric respiratory diseases remain the leading cause of death worldwide in infants and young children with poor immunity and incomplete development of the respiratory system. Even though notable medical advances have been achieved in pediatric clinic in recent years, pediatric respiratory diseases still deserve heightened public awareness and pose a serious threat to children health.[2] Macrolides are antimicrobial agents with anti-inflammatory activities and are frequently used in the treatment of pediatric respiratory diseases. Among macrolides, azithromycin (AZM) has good tissue penetration and pharmacodynamic stability, and it deserves more popularity compared with erythromycin, clarithromycin, and other macrolides. Furthermore, its anti-infective, anti-inflammatory, and immunomodulatory properties also contribute to the preferable option and the wide use in clinical practice.[3]
In the past decades, researches on AZM and pediatric respiratory diseases are also on the rise. Most of the studies are aimed at analyzing the efficacy of AZM, but there is a lack of research reports on the comprehensive analysis of the safety profiles and adverse reactions of AZM. According to the existing reports, the adverse reactions of AZM mainly included gastrointestinal dysfunction, allergic reactions, nervous system abnormalities, and even cardiac function impairments, but inconsistent data about these adverse reactions frequently existed in related reports.[4,5] Therefore, more details should be further summarized to help the rational use of AZM and reduce the incidence of adverse reactions. In this study, our purpose was to systematically evaluate the adverse reactions of AZM in the treatment of pediatric respiratory diseases by conducting a systematic review and meta-analysis, so as to provide more reference data for clinicians in clinical practice.
2. Methodology
2.1. Literature search strategy
Literature search was conducted on databases such as Chinese CNKI, Wanfang, VIP, PubMed, EMBASE, Scopus, Web of science, and Cochrane Library. The full name keywords such as “azithromycin,” “pediatrics,” “adverse drug reaction (ADR),” “respiratory disease,” and “randomized controlled trial” were used for retrieval, and other word variations of aforementioned keywords such as “AZM,” “ADR or side effect,” “RCT” were used for supplementary search. The retrieval period was set from January 2010 to December 2020, and only randomized controlled studies were selected. For the purpose of this review, we defined “pediatrics” as individuals from birth up to 17 years old.
2.2. Inclusion and exclusion criteria of the literature
The included articles should meet the following criteria: Only clinical trials of pediatric respiratory diseases were included, and these articles should be published in English or Chinese; The treatment method used in the experimental group was AZM alone, not combined with other antibiotics; the control group was treated with other antibiotics or other treatment other than AZM. Exclusion criteria of this study were listed as below: non-randomized controlled trials, animal studies, reviews, and other meta-analysis studies; non peer-reviewed articles such as dissertation, conference proceeding, and others; in the literature research results, incomplete data, duplicate data, no relevant outcomes or fruitless presentation of adverse reactions.
2.3. Data extraction
According to the set inclusion and exclusion criteria, 2 researchers of this study independently searched the literature, and performed the assessment of eligibility, risk of bias, and data extraction. The extracted contents included the author, publication date, baseline data of participants, administration methods, dosage, and adverse reactions. Administration methods include oral administration, intravenous administration, sequential therapy, and other treatments. Sequential therapy of antibiotics refers to intravenous administration transiting to oral administration after obvious relieving of disease. When a disagreement appeared between 2 researchers, a discussion among all authors would be performed to solve it. If a full article or document data can not be obtained from internet databases, a request would be sent to the corresponding author. In deed, only one request was sent, and one reply was obtained.
2.4. Literature quality evaluation
The assessment of the quality of the literature was carried out with reference to the Cochrane risk of bias tool following Cochrane guidelines.[6] Evaluation indicators included the following items: Lack of the random allocation method or no allocation concealment; Absence of a double-blind method or blinding of outcome assessment; Evidence of selective reporting or inconsistencies in reported outcomes; High attrition rate without appropriate reason or without a clear explanation for lost visits and missing data; Detection of other sources of bias, such as significant baseline imbalances or conflict of interest not addressed.
2.5. Statistical method
Meta-analysis of the included literature was performed using revman 5.1 software. Mantel-Haenszel (M-H) method was selected for data calculation. The confidence interval (CI) was set as 95% CI, and I2 statistic was used to assess the heterogeneity, with lower values representing less heterogeneity. The literature included in the study has a mild or no heterogeneity, so it was analyzed by fixed effect model. If the literature heterogeneity was large and unacceptable, it would be analyzed by random effect model, and the results of meta-analysis would be reflected by forest plot. The publication bias of the study was tested by funnel plot and Galbraith plot. The stability of the results was analyzed by sensitivity test of subgroup analysis. The incidence of adverse effects between groups was tested by Z-test, P < .05 indicating that the difference was statistically significant, and all the P value was 2-sided.
3. Result
3.1. Document screening process and results
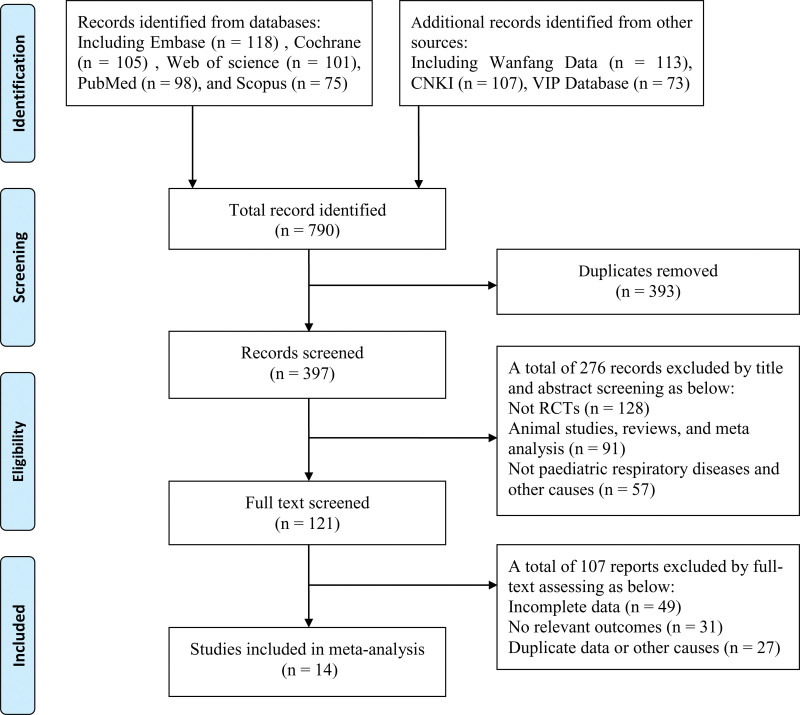
After preliminary literature search, a total of 614 articles related to the adverse reactions of AZM were retrieved, including 118 articles from Embase, 105 articles from Cochrane Library, 98 articles from PubMed, 113 articles from Wanfang database, 107 articles from Chinese CNKI, and 73 articles from VIP database. Then, after reading the title, abstract and full text of the literature, 14 eligible articles were finally included for research.[7–20] The process of document screening was shown in Figure 1.
Figure 1.
Document retrieval and screening process.
3.2. Basic information of included literature
The publication date of literature ranges from 2010 to 2020. The characteristic analysis of the literature in the study includes the author, publication year, age, indications, administration methods and treatment methods of the control group. A total of 14 studies were included, and the rest did not meet the inclusion criteria. The details of 14 eligible studies were shown in Table 1.
Table 1.
The basic information of 14 eligible studies in this meta-analysis.
| First author | Literature years | Indications | Age of AZM experimental group (yr) | Administration mode of AZM experimental group | Treatment methods of control group |
|---|---|---|---|---|---|
| Goyal V.[7] | 2018 | Acute exacerbation of bronchiectasis | 4~9 | Oral administration | Other treatment |
| Kneyber M.[8] | 2012 | Lower respiratory diseases | 0.3~6 | Intravenous administration | Other treatment |
| Vikas G.[9] | 2018 | Bronchiectasis | 1~17 | Sequential therapy | Other treatment |
| Valery PC[10] | 2013 | Bronchiectasis | 0.6~8 | Oral administration | Other treatment |
| Postma D.F.[11] | 2010 | Acquired pneumonia | 1~7 | Intravenous administration | Erythromycin |
| Hendricks[12] | 2016 | Mycoplasma pneumonia | 3~12 | Sequential therapy | Other treatment |
| Wilms E.B.[13] | 2012 | Cystic fibrosis | 0.6~12 | Intravenous administration | Other treatment |
| Bauer K.A.[14] | 2011 | Other symptoms | 1~10.5 | Intravenous administration | Erythromycin |
| Small S.M.[15] | 2018 | Acquired pneumonia | 1~10 | Sequential therapy | Erythromycin |
| To K.K.[16] | 2010 | Mycoplasma pneumonia | 2~10 | Sequential therapy | Other treatment |
| Yang D.[17] | 2018 | Mycoplasma pneumonia | 4~13 | Oral administration | Erythromycin |
| Lu M.P.[18] | 2013 | Mycoplasma pneumonia | 0.6~12 | Intravenous drip | Erythromycin |
| Han R.[19] | 2020 | Mycoplasma pneumonia | 4~9 | Sequential therapy | Erythromycin |
| Wang J.[20] | 2018 | Mycoplasma pneumonia | 0.3~12 | Oral administration | Other treatment |
Other treatment refers to amoxicillin, cefuroxime, and other antibiotics rather than macrolides.
3.3. Included in literature quality evaluation
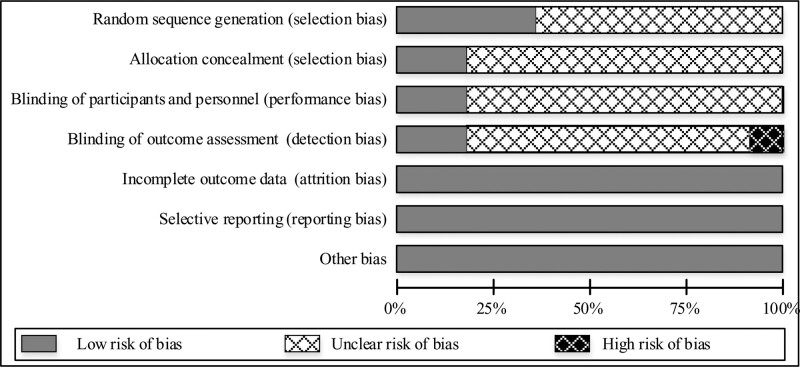
The risk of bias of the included literature was obtained through evaluating the quality of the literature. By analyzing the correctness of the random allocation method included in the literature, it can be seen that 6 of them accurately described the random allocation method, accounting for 40% of the total included literature, and showing a low risk of bias. As for whether the literature hides the allocation method, it can be seen that 3 of them mentioned the allocation method, accounting for 30% of the total literature, and the other 11 documents did not mention the hidden allocation method. When analyzing the use of the double-blind method, there were also 3 documents that clearly indicated the implementation of the double-blind method for experimenters and subjects. We also assessed the integrity of the data included in the literature in aspects of whether there was a midway withdrawal in the research, whether the literature clearly described the number of lost visits, and whether the analysis of the final processing results was made. Fortunately, there were no missing document data, and the included literature has complete data. The processing method of the results of the research object was fully expressed and there was no selective reporting of research results in the 14 included studies. As shown in Figure 2.
Figure 2.
Bias risk assessment of included literature.
3.4. Meta analysis results
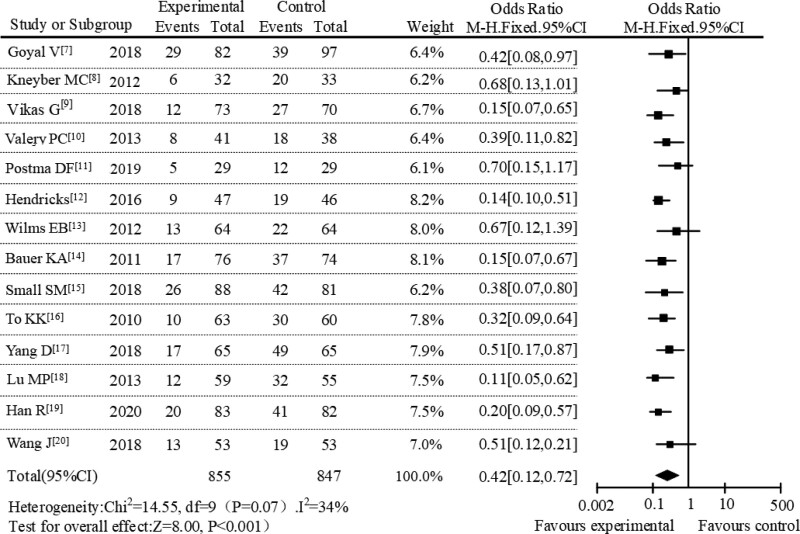
Among the 14 publications, 814 patients were involved in the AZM experimental group, of which 197 patients had adverse reactions. In addition, among 847 patients in the control group, 407 patients had adverse reactions. Overall, the incidence of adverse events treated with AZM was 24.20%, compared with 48.05% in the control group. The whole group meta-analysis was performed for all included literature, and the forest plot was drawn as shown in Figure 3. From the heterogeneity analysis, it can be concluded that the randomized controlled trials in the 14 included studies had slight heterogeneity (P = .07, I2 = 34%), with the fixed effect model used for analysis. Figure 3 showed that the diamond in the forest plot was on the left of the null vertical line with X = 1. This result meant that in the treatment of pediatric respiratory diseases, the incidence of adverse reactions after treatment with AZM was lower than that of the control group in the study. The magnitude of the combined effect was OR = 0.42, 95% CI (0.12–0.72), Z = 8.00, P < .001, and the difference was statistically significant (P < .05).
Figure 3.
Forest plot analysis of adverse reactions in the 2 groups.
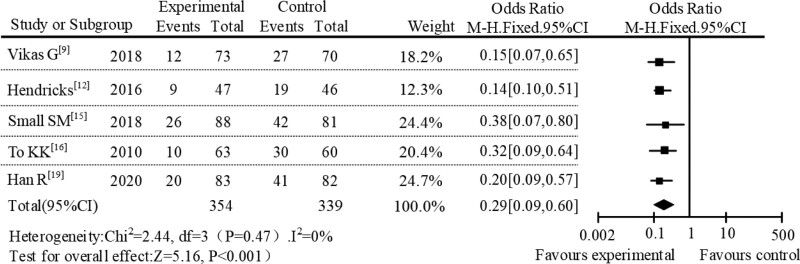
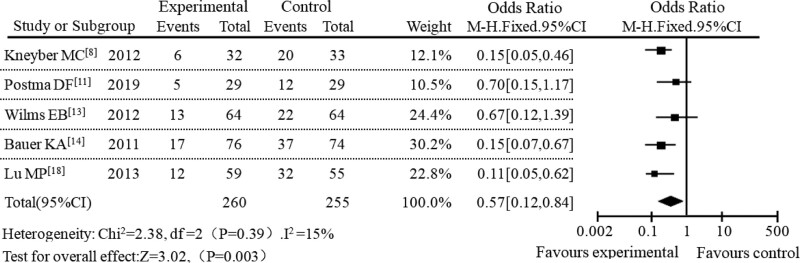
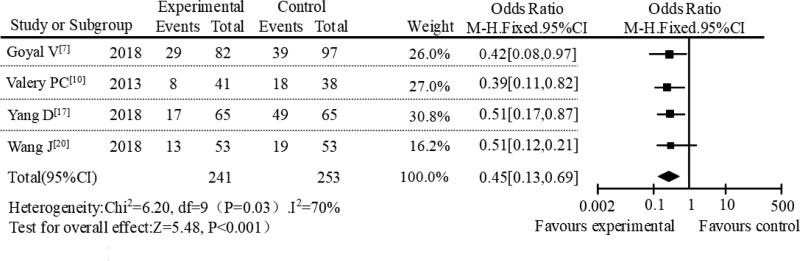
In the subgroup meta-analysis, a subgroup meta-analysis was performed based on different administration methods. A total of 14 studies were included, including 5 studies with sequential therapy, 5 studies with intravenous administration, and the remaining 4 studies with oral administration. In the subgroup of sequential therapy, the incidence of adverse reactions between the AZM experimental group and the control group was 11.37% and 37.92%, respectively. The meta-analysis results of this subgroup were shown in Figure 4. The result of heterogeneity analysis of sequential therapy was P = .47, I2 = 0%. The diamond in the forest plot was on the left of the null line. The results showed that AZM had a lower incidence of adverse reactions in sequential therapy with OR = 0.29, 95% CI [0.09–0.60], P < .001. In the study of intravenous administration, the incidence of adverse reactions was 17.83% in the AZM group and 52.09% in the control group. The subgroup meta-analysis results of the intravenous administration were shown in Figure 5. The heterogeneity analysis result of intravenous administration was P = .39, I2 = 15%. It can be seen from the forest plot that the diamond was on the left of the null line, which also showed that the incidence of adverse reactions in AZM intravenous administration was lower. In the study of oral administration, the incidence of adverse reactions between AZM experimental group and control group were 22.18% and 45.69%, respectively. The results of meta-analysis of subgroups of oral administration were shown in Figure 6. The results of heterogeneity analysis of oral administration were P = .03 and I2 = 70%. At the same time, it can be seen from the forest plot that the diamond was also on the left of the null line, which suggested that AZM had lower incidence of adverse reactions when it was administered orally.
Figure 4.
Forest plot analysis of adverse reactions under sequential therapy.
Figure 5.
Forest plot analysis of adverse reactions under intravenous administration.
Figure 6.
Forest plot analysis of adverse reactions under oral administration.
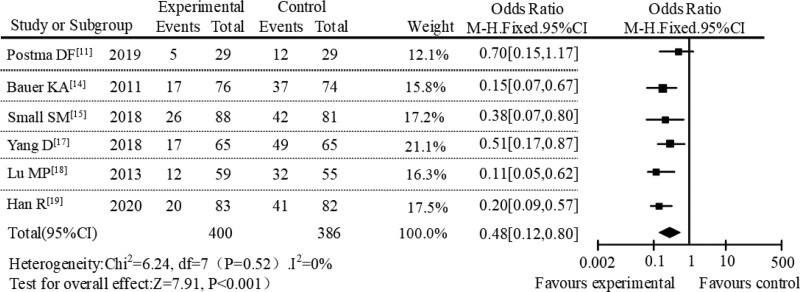
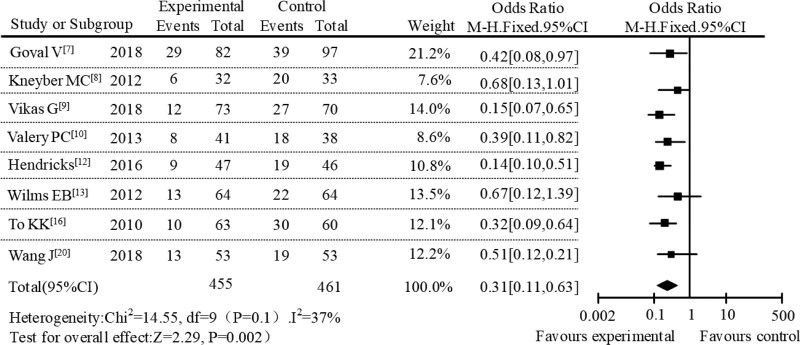
Then, a subgroup meta-analysis was conducted based on different treatments in the control group. From the literature included in the study, the extracted treatment measures were mainly erythromycins or other treatment. By analyzing the subgroups of erythromycin treatment, it can be found that the incidence of adverse reactions in AZM group and erythromycin group were 15.33% and 41.26%, respectively. As showed in Figure 7, the overall effect size of the incidence of adverse reactions after the treatment of pediatric respiratory diseases in the erythromycin subgroup analysis was OR = 0.48, 95% CI [0.12, 0.80], Z = 7.91, P < .001. At the same time, the result of heterogeneity analysis was P = .52, I2 = 0%, and the result from the forest plot showed that the diamond was located on the left of the null line, revealing that the incidence of adverse reactions of AZM treatment was lower than that in the erythromycin subgroup. Subgroup analysis was also performed in other treatments. From the data, the adverse reaction rate of the AZM treatment group and the other treatment group were 18.16% and 42.00%, respectively. We can see the results in the subgroup analysis of other treatments from Figure 8, showing that the combined effect of adverse reaction was OR = 0.31, 95% CI [0.11, 0.63], Z = 2.29, P = .002, and the result of heterogeneity analysis was P = .10, I2 = 37%. The position of the diamond in the forest plot was on the left of the null line. The results showed that the incidence of adverse reactions of AZM treatment was lower than that in the subgroup analysis of other treatments. According to the above results of subgroup analysis, it can be seen that AZM treatment have a lower incidence of adverse reactions in the subgroup analysis of different administration methods and different treatments in literature included in the study. This result was in great agreement with the aforementioned subgroup analysis. Therefore, the result of this systematic evaluation was that AZM in the treatment of pediatric respiratory diseases had lower adverse reactions and better safety profiles.
Figure 7.
Forest plot analysis of adverse reactions of erythromycin subgroup.
Figure 8.
Forest plot analysis of adverse reactions in other treatment subgroup.
3.5. Analysis of literature publication bias
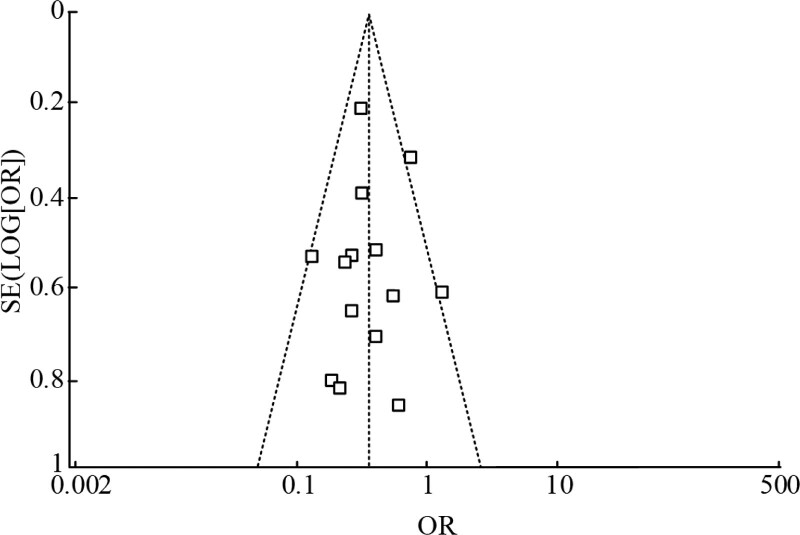
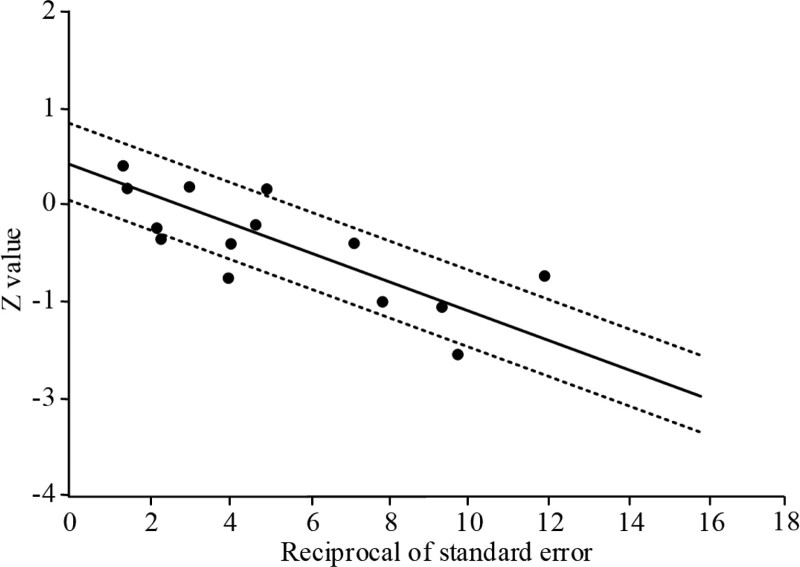
To examine the publication bias of the included literature, the data from the included literature were used to draw the funnel plot, and it was showed in Figure 9. The central axis of the funnel plot was OR = 0.42, the shape of the funnel plot did not reveal obvious evidence of asymmetry, indicating that the publication bias of the literature was low or nonexistent. At the same time, the Galbraith diagram was used to further analyze the publication bias of the literature included in our study. As shown in Figure 10, most articles included in this study were in the area between the dotted lines, which meant that the literature was within the 95% CI. These results further demonstrated that there was no evident publication bias in the literature included in this study.
Figure 9.
Funnel plot of publication bias in this meta-analysis.
Figure 10.
Galbraith plot of publication bias in this meta-analysis.
4. Discussion
In recent years, therapeutic effects of AZM have been widely recognized in pediatric clinic and many researchers have reported their favorable findings,[21–23] but there is a lack of systematic review and meta-analysis focusing on adverse reactions of AZM in the treatment of pediatric respiratory diseases. In this study, our results showed that the diamond was located on the left side of the null line, indicating that the use of AZM drugs had a lower incidence of adverse reactions compared with other drugs or other treatments in pediatric respiratory diseases. Although no homogeneous study was found in this field, some related findings were reported by other researchers. Pan X et al[24] demonstrated that AZM was beneficial in improving some clinical symptoms and lung functions in children over 6 years old with persistent asthma. Hiles SA et al[25] reported that maintenance use of AZM could reduce exacerbations in severe asthma patients with mild adverse reaction and well tolerance. In addition, a meta-analysis pointed out that no evidence of increased adverse events and mortality was found in the treatment of bronchiectasis patients with macrolides.[26] Overall, our results were supported by the findings from aforementioned studies to some extent.
In this study, it can be found from the whole review that in the treatment of pediatric respiratory diseases, the incidence of adverse events in children treated with AZM was 24.20%, compared with 48.05% in the control group. Besides, based on the subgroup analysis under different administration methods, the results showed that AZM had a lower incidence of adverse reactions in sequential therapy. In agreement with our study, Gao SY et al[27] observed the same results. They explored the clinical outcomes of sequential therapy with AZM and erythromycin for mycoplasma pneumonia in children and concluded that the sequential therapy with AZM is better than with erythromycin in clinical efficacy and adverse reactions. Furthermore, according to the subgroup data under different treatments, like erythromycin and other treatments, the incidence of adverse reactions in AZM group and erythromycin group were 15.33% and 41.26%, respectively, and the incidence of the AZM treatment group and other treatment group were 18.16% and 42.00%, respectively. All these data highlighted that the incidence of adverse reactions in AZM treatment was lower even under different administration methods and different treatment methods.
In the early studies, some researchers did several reports on the application of AZM and performed comparative analysis with other treatment methods in the treatment of children respiratory diseases. Referring to these literature, they proposed that the adverse reaction events of AZM in the treatment of pediatric respiratory diseases were fewer than those in the control group to a certain extent,[28–30] which was also consistent with the results of our study. Looking back at previous studies, a lot of studies conducted comparative analysis with erythromycin, azithromycin, and other treatments in the treatment of pediatric diseases, and these studies also clearly pointed out that the effectiveness of AZM was higher than that of erythromycin.[31–33] As others reported, AZM is an antibacterial drug with several advantages, including longer half-life time, better tolerance and therapeutic effect, and fewer contraindications and adverse reactions, and these advantages make AZM a preferred drug and extensively used in children with respiratory diseases.[34,35]
In our meta-analysis, we primarily underscored the overall incidence of adverse reactions after AZM treatment. While this provides a macroscopic understanding of its safety profiles, it is equally essential to delineate the specific side effects for a comprehensive interpretation. As a newer generation of macrolide antibiotics, AZM has demonstrated robust antimicrobial activity against a range of bacteria including staphylococcus, pneumococcus, enterococcus, mycoplasma, and chlamydia.[36] In clinical practice, the main adverse reactions of AZM is gastrointestinal complications such as nausea, vomiting, diarrhea, and abdominal pain, and followed by headache, sinusitis, and rash in some cases.[37] However, these adverse reactions are often mild and can be relieved by expectant treatment, which will not result treatment cessation in most patients. Moreover, these gastrointestinal complications can be prevented or alleviated by proton pump inhibitors, aluminum phosphate gel, and other mucosal protective drugs basen on other reports.[38,39] Although the therapeutic benefits of AZM are undeniable, clinicians need to be aware of its adverse reactions, and employing preventive measures would considerably reduce these adverse reactions, ensuring better therapeutic safety and clinical outcomes.
With rigorous systematic review methods, we did a comprehensive search of the literature, evaluated the quality of them with reference to the Cochrane risk of bias tool following Cochrane guidelines, and gave a deep insight into the safety of AZM in the treatment of pediatrics respiratory diseases. After assessment of publication bias via funnel plot and Galbraith plot, our results showed that most included literature were in the area between the dotted lines, which demonstrated that there was no evident publication bias in the literature of this study. However, some limitations and shortcomings of this study should be noted here. First, most of studies focused on the efficacy of AZM, while few about its adverse reactions were fully expressed, so there was a shortcoming regarding the presentation of all adverse drug reactions. Second, although our results showed that AZM had fewer adverse reactions and better safety profiles, some rare but severe adverse reactions such as arrhythmia,[40] cardiac arrest,[41] and even sudden cardiac death[42] were not fully analyzed due to data deficiency in included RCTs of our study. Therefore, we should not ignore these rare adverse reactions and potential risk events in clinical practice, even though these rare adverse reactions can only be found in a few of case reports or observational studies and have never been confirmed in high-quality literature.[43] Thus, further attention and well-designed RCTs with large sample size on this topic are needed to enrich the safety research of AZM and provide more reference for pediatricians.
5. Conclusion
In conclusion, the results from this systematic review and meta-analysis suggest that AZM has fewer adverse reactions and better safety profiles in the treatment of pediatric respiratory diseases. In consideration of other findings that AZM was as effective as or a preferable option with lower incidence of adverse reactions compared with other macrolides or antibiotics,[44,45] we can draw a conclusion that AZM is a more attractive option in the treatment of pediatric respiratory diseases. However, in view of existing limitations in this study, more high-quality studies are needed to verify our results, especially in the results of subgroup analysis.
Author contributions
Data curation: Yuan-hua Cen, Mu-heng Chen.
Writing – original draft: Ying-wen Sun.
Writing – review & editing: Xu-ke Yan, Xiao-fen Jin.
Abbreviations:
- AZM
- azithromycin
- CI
- confidence interval
- RCT
- randomized controlled trial
The authors have no funding and conflicts of interest to disclose.
Ethical approval is not required for the systematic review because all the data included had been published.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Sun Y-w, Cen Y-h, Chen M-h, Yan X-k, Jin X-f. Safety profiles and adverse reactions of azithromycin in the treatment of pediatric respiratory diseases: A systematic review and meta-analysis. Medicine 2023;102:48(e36306).
Contributor Information
Yuan-hua Cen, Email: keao8768710@yeah.ne.
Mu-heng Chen, Email: qiaoji0974498@yeah.net----han41386.
Xu-ke Yan, Email: keliao1900081@163.com.
Xiao-fen Jin, Email: qiaojing7451684@163.com.
References
- [1].Cutrera R, Baraldi E, Indinnimeo L, et al. Management of acute respiratory diseases in the pediatric population: the role of oral corticosteroids. Ital J Pediatr. 2017;43:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. 2020;8:585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chellew N, Chang AB, Grimwood K. Azithromycin prescribing by respiratory pediatricians in Australia and New Zealand for chronic wet cough: a questionnaire-based Survey. Front Pediatr. 2020;8:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Patel H, Calip GS, DiDomenico RJ, et al. Prevalence of Cardiac Risk Factors in Patients Prescribed Azithromycin before and after the 2012 FDA Warning on the Risk of Potentially Fatal Heart Rhythms. Pharmacotherapy. 2020;40:107–15. [DOI] [PubMed] [Google Scholar]
- [5].Zeng L, Xu P, Choonara I, et al. Safety of azithromycin in pediatrics: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:1709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Goyal V, Grimwood K, Byrnes CA, et al. Amoxicillin-clavulanate versus azithromycin for respiratory exacerbations in children with bronchiectasis (BEST-2): a multicentre, double-blind, non-inferiority, randomised controlled trial. Lancet. 2018;392:1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kneyber MC, Rimensberger PC. The need for and feasibility of a pediatric ventilation trial: reflections on a survey among pediatric intensivists. Pediatr Crit Care Med. 2012;13:632–8. [DOI] [PubMed] [Google Scholar]
- [9].Vikas G, Grimwood K, Ware RS, et al. Efficacy of oral amoxicillin-clavulanate or azithromycin for non-severe respiratory exacerbations in children with bronchiectasis (BEST-1): a multicentre, three-arm, double-blind, randomised placebo-controlled trial. Lancet Respir Med 2019;7:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Valery PC, Morris PS, Byrnes CA, et al. Long-term azithromycin for Indigenous children with non-cystic-fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med 2013;1:610–20. [DOI] [PubMed] [Google Scholar]
- [11].Postma DF, Spitoni C, van Werkhoven CH, et al. Cardiac events after macrolides or fluoroquinolones in patients hospitalized for community-acquired pneumonia: post-hoc analysis of a cluster-randomized trial. BMC Infect Dis. 2019;19:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hendricks EG, Hurst AL. Effect of real-time Mycoplasma pneumoniae polymerase chain reaction testing on azithromycin use in a pediatric intensive care unit. Pharmacotherapy. 2016;36:244. [Google Scholar]
- [13].Wilms EB, Touw DJ, Heijerman HG, et al. Azithromycin maintenance therapy in patients with cystic fibrosis: a dose advice based on a review of pharmacokinetics, efficacy, and side effects. Pediatr Pulmonol. 2012;47:658–65. [DOI] [PubMed] [Google Scholar]
- [14].Bauer KA, Brimhall AK, Chang TT. Drug reaction with eosinophilia and systemic symptoms (DRESS) associated with azithromycin in acute Epstein-Barr virus infection. Pediatr Dermatol. 2011;28:741–3. [DOI] [PubMed] [Google Scholar]
- [15].Small SM, Bacher RS, Jost SA. Disulfiram-like reaction involving ceftriaxone in a pediatric patient. J Pediatr Pharmacol Ther. 2018;23:168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].To KK, Chan KH, Fung YF, et al. Azithromycin treatment failure in macrolide-resistant Mycoplasma pneumoniae pneumonia. Eur Respir J. 2010;36:969–71. [DOI] [PubMed] [Google Scholar]
- [17].Yang D, Chen L, Chen Z. The timing of azithromycin treatment is not associated with the clinical prognosis of childhood Mycoplasma pneumoniae pneumonia in high macrolide-resistant prevalence settings. PLoS One. 2018;13:e0191951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu MP, Ma LY, Zheng Q, et al. Clinical characteristics of adenovirus associated lower respiratory tract infection in children. World J Pediatr. 2013;9:346–9. [DOI] [PubMed] [Google Scholar]
- [19].Han R, Yu Q, Zhang G, et al. Comparison of azithromycin and erythromycin in the treatment of mycoplasma pneumonia in children. Pak J Med Sci. 2020;36:156–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang J, Yang C. Clinical effect of sequential therapy with azithromycin in children mycoplasma pneumoniae pneumonia. Pak J Pharm Sci. 2018;31:1649–52. [PubMed] [Google Scholar]
- [21].Liu S, Zheng Y, Wu X, et al. Early target attainment of azithromycin therapy in children with lower respiratory tract infections. J Antimicrob Chemother. 2018;73:2846–50. [DOI] [PubMed] [Google Scholar]
- [22].Vicendese D, Yerkovich S, Grimwood K, et al. Long-term azithromycin in children with bronchiectasis unrelated to cystic fibrosis: treatment effects over time. Chest. 2023;163:52–63. [DOI] [PubMed] [Google Scholar]
- [23].Wang X, Luo J, Wang D, et al. The efficacy and safety of long-term add-on treatment of azithromycin in asthma: a systematic review and meta-analysis. Medicine (Baltim). 2019;98:e17190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pan X, Liu Y, Luo J, et al. The efficacy and safety of azithromycin in treatment for childhood asthma: a systematic review and meta-analysis. Pediatr Pulmonol. 2022;57:631–9. [DOI] [PubMed] [Google Scholar]
- [25].Hiles SA, McDonald VM, Guilhermino M, et al. Does maintenance azithromycin reduce asthma exacerbations? An individual participant data meta-analysis. Eur Respir J. 2019;54:1901381. [DOI] [PubMed] [Google Scholar]
- [26].Shi ZL, Peng H, Hu XW, et al. Effectiveness and safety of macrolides in bronchiectasis patients: a meta-analysis and systematic review. Pulm Pharmacol Ther. 2014;28:171–8. [DOI] [PubMed] [Google Scholar]
- [27].Gao SY, Zhu BW. Clinical efficacy and adverse reactions of erythromycin and azithromycin in sequential treatment of mycoplasma pneumoniae pneumonia in children. Guizhou Med J. 2022;46:1393–4. [Google Scholar]
- [28].Sie A, Bountogo M, Nebie E, et al. Neonatal azithromycin administration to prevent infant mortality: study protocol for a randomised controlled trial. BMJ Open. 2019;9:e031162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Naderi N, Assayag D, Mostafavi-Pour-Manshadi SM, et al. Long-term azithromycin therapy to reduce acute exacerbations in patients with severe chronic obstructive pulmonary disease. Respir Med. 2018;138:129–36. [DOI] [PubMed] [Google Scholar]
- [30].El Boustany P, Gachelin E, Colomban C, et al. A review of non-cystic fibrosis bronchiectasis in children with a focus on the role of long-term treatment with macrolides. Pediatr Pulmonol. 2019;54:487–96. [DOI] [PubMed] [Google Scholar]
- [31].Dawit G, Mequanent S, Makonnen E. Efficacy and safety of azithromycin and amoxicillin/clavulanate for otitis media in children: a systematic review and meta-analysis of randomized controlled trials. Ann Clin Microbiol Antimicrob. 2021;20:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li P, Jiang G, Shen X. Evaluation of 3-day azithromycin or 5-day cefaclor in comparison with 10-day amoxicillin for treatment of tonsillitis in children. Can J Physiol Pharmacol. 2019;97:939–44. [DOI] [PubMed] [Google Scholar]
- [33].Sié A, Dah C, Ourohiré M, et al. Azithromycin versus amoxicillin and malarial parasitemia among children with uncomplicated severe acute malnutrition: a randomized controlled trial. Am J Trop Med Hyg. 2021;106:351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhou P, Wang X, Zhang X, et al. Recommendations on off-label use of intravenous azithromycin in children. Int J Clin Pract. 2021;75:e14010. [DOI] [PubMed] [Google Scholar]
- [35].Bacharier LB. Azithromycin during wheezing illnesses among preschool children: does the airway microbiota provide insights into mechanism? Am J Respir Crit Care Med. 2021;204:115–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Heidary M, Ebrahimi Samangani A, Kargari A, et al. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J Clin Lab Anal. 2022; 36:e24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang D, Fu W, Dai J. Meta-analysis of macrolide maintenance therapy for prevention of disease exacerbations in patients with noncystic fibrosis bronchiectasis. Medicine (Baltim). 2019;98:e15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhou PX, Ran XL, Yan YY, et al. Safety analysis of azithromycin clinical application:Data based on 5-year spontaneous reports in Beijing. Chin Hosp Pharm J. 2020;40:809–14. [Google Scholar]
- [39].Chen JR, Guo Y. Aluminum phosphate gel in preventing gastrointestinal reactions caused by intravenous azithromycin in children. J Pediatr Pharm. 2018;24:20–1. [Google Scholar]
- [40].Espadas D, Castillo S, Moreno M, et al. Lack of effect of azithromycin on QT interval in children: a cohort study. Arch Dis Child. 2016;101:1079. [DOI] [PubMed] [Google Scholar]
- [41].Valdés SO, Kim JJ, Niu MC, et al. Cardiac arrest in pediatric patients receiving azithromycin. J Pediatr. 2017;182:311–314.e1. [DOI] [PubMed] [Google Scholar]
- [42].Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cook J, Pressler ML, Damle B, et al. The weight of evidence from electrophysiology, observational, and cardiovascular end point studies demonstrates the safety of azithromycin. Clin Transl Sci. 2021;14:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li D, Wang Y. Safety of azithromycin in pediatric infectious diseases: a clinical systematic review and meta-analysis. Transl Pediatr. 2021;10:2594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Duan X, Wang K, Wu J, et al. Comparative efficacy of Chinese herbal injections combined with azithromycin for mycoplasma pneumonia in children: a Bayesian network meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2019;44:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]