Abstract
Anti-rabies virus immunoglobulin combined with rabies vaccine protects humans from lethal rabies infections. For cost and safety reasons, replacement of the human or equine polyclonal immunoglobulin is advocated, and the use of rabies virus-specific monoclonal antibodies (MAbs) is recommended. We produced two previously described potent rabies virus-neutralizing human MAbs, CR57 and CRJB, in human PER.C6 cells. The two MAbs competed for binding to rabies virus glycoprotein. Using CR57 and a set of 15-mer overlapping peptides covering the glycoprotein ectodomain, a neutralization domain was identified between amino acids (aa) 218 and 240. The minimal binding region was identified as KLCGVL (aa 226 to 231), with key residues K-CGV- identified by alanine replacement scanning. The critical binding region of this novel nonconformational rabies virus epitope is highly conserved within rabies viruses of genotype 1. Subsequently, we generated six rabies virus variants escaping neutralization by CR57 and six variants escaping CRJB. The CR57 escape mutants were only partially covered by CRJB, and all CRJB-resistant variants completely escaped neutralization by CR57. Without exception, the CR57-resistant variants showed a mutation at key residues within the defined minimal binding region, while the CRJB escape viruses showed a single mutation distant from the CR57 epitope (N182D) combined with mutations in the CR57 epitope. The competition between CR57 and CRJB, the in vitro escape profile, and the apparent overlap between the recognized epitopes argues against including both CR57 and CRJB in a MAb cocktail aimed at replacing classical immunoglobulin preparations.
Lethal rabies is prevented by postexposure prophylaxis (PEP) through the combined administration of a rabies virus vaccine and rabies virus immunoglobulin (RIG). Two types of RIG are used: human RIG (HRIG) and equine RIG (ERIG), both derived from pooled sera of human donors or horses vaccinated against rabies virus, respectively. The need to replace these hyperimmune serum preparations is widely recognized (1), and MAbs that neutralize rabies virus offer the opportunity to do so.
Mouse monoclonal antibodies (MAbs) as well as human MAbs have been shown to protect rodents from a lethal rabies virus challenge (8, 11, 14, 15, 20, 25, 26). One of the most potent of the human antibodies neutralizing a variety of rabies virus strains was described by Dietzschold et al. (8). This human antibody (MAb57) was subsequently included in a cocktail of three human antibodies, SOJA, SOJB, and SO57, that was shown to be as effective as HRIG in protection of mice from a lethal dose of rabies virus (25).
We considered two criteria to be of crucial importance for the inclusion of human MAbs into a cocktail aimed at effectively blocking rabies virus infections acquired from wildlife animals. Firstly, the MAbs should target distinct, nonoverlapping epitopes and preferably should not compete for binding to rabies virus glycoprotein. Secondly, in vitro-generated antibody-resistant rabies virus variants selected using one antibody should be neutralized by the nonselecting other antibody in the cocktail (and vice versa), thus addressing the issue of natural variation among rabies virus field isolates.
In the present study, the variable heavy- and light-chain coding regions of the SOJA, SOJB, and SO57 antibody genes were synthesized, introduced into a single human immunoglobulin G1 (IgG1) expression vector, and expressed in human PER.C6 cells (17). This yielded the antibodies CR57, CRJB, and CRJA. The potency of CR57 was significantly greater than that of CRJB, while the potency of CRJA was poor and therefore was not included in further studies. Binding analyses revealed that CR57 and CRJB compete for binding to rabies virus glycoprotein. Using CR57, we identified a novel linear epitope on the rabies virus glycoprotein by scanning the complete extracellular domain for peptide recognition using Pepscan technology (13, 28). The key residues of the epitope were identified next. Subsequently, rabies virus variants were generated that escaped neutralization by either CR57 or CRJB. The glycoprotein gene of these antibody-resistant variants was sequenced to identify critical amino acid residues involved in the binding region of each of these antibodies. Variant residues were introduced in peptides mimicking the epitope and were tested for loss of MAb binding. An updated antigenic map of the rabies virus glycoprotein is included that incorporates the novel CR57 epitope.
MATERIALS AND METHODS
Cells.
Mouse neuroblastoma (NA) cells were grown at 37°C and 5% CO2 in RPMI 1640 (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS). BSR cells (a subclone of baby hamster kidney cells) were grown at 37°C and 5% CO2 in Dulbecco's modified Eagle medium (DMEM; Gibco) supplemented with 10% FBS. PER.C6 cells (12) were grown at 37°C and 10% CO2 in DMEM (Gibco) supplemented with 10% FBS and 10 mM MgCl2.
Antibodies.
The heavy and light chains of the antibodies CR57, CRJB, and CRJA, as described previously (25), were cloned indirectly into the pcDNA3002 vector (17) via shuttle vectors containing the constant domains of the IgG1 heavy chain, the kappa light chain, and the lambda light chain, respectively. Antibodies CR57, CRJB, and CRJA were expressed in PER.C6 cells and purified by protein A chromatography. Antibodies were buffered with phosphate-buffered saline (PBS) (Gibco), filter sterilized, and stored at −20°C. Biotinylation of antibodies was performed using EZ-link Sulfo NHS-SS-biotin (Pierce) according to standard laboratory procedures.
Virus.
Monolayers of BSR cells were infected with CVS-11 (challenge virus standard) at a multiplicity of infection (MOI) of 0.1 for 1 h at 37°C and 5% CO2. The virus inoculum was then removed, fresh medium was added to the cells, and the mixture was incubated for 72 h at 34°C and 5% CO2. The culture supernatants were collected and stored at −80°C until further use.
RFFIT.
Modified rapid fluorescent focus inhibition neutralization test (RFFIT) neutralization assays were performed as described previously (8).
Competition experiments.
Enzyme-linked immunosorbent assay (ELISA) plates coated with purified rabies virus glycoprotein (ERA strain; 1 μg/ml) were incubated with 50 μg of unlabeled anti-rabies virus glycoprotein IgG/ml for 1 h at room temperature. Fifty microliters of biotinylated anti-rabies virus glycoprotein IgG (2.5 μg/ml) was then added to each well, incubated for 5 min at room temperature, and immediately washed five times with 100 μl of PBS-0.05% Tween-20. Subsequently, wells were incubated for 1 h at room temperature with 50 μl of a 1:2,000 dilution of streptavidin-horseradish peroxidase (HRP) (Becton Dickinson), washed, and developed by addition of 100 μl of OPD reagent (Sigma). The reaction was stopped by adding 50 μl of 1 M H2SO4 before measuring the optical density at 492 nm (OD492). Alternatively, competition experiments were performed using PER.C6 cells expressing rabies virus glycoprotein. Cells were transiently transfected using Fugene (Gibco) with a plasmid encoding the full-length rabies virus glycoprotein of the ERA strain. Two days after transfection, the cells were harvested and used in the competition assay. Cells were then incubated with saturating amounts of unlabeled antibody at 4°C before washing and addition of 2.5 μg of biotinylated antibodies/ml. Bound biotinylated antibodies were visualized after 5 min with streptavidin-phycoerythrin (PE) conjugate (Becton Dickinson), and samples were analyzed by flow cytometry.
Generation of escape viruses.
Serial dilutions (0.5 ml) of CVS-11 ranging from 10−1 to 10−8 focus-forming units (FFU)/ml were incubated with a constant amount (∼4 IU/ml) of antibody CR57 or CRJB (0.5 ml) for 1 h at 37°C and 5% CO2. The mixtures were added to 2-day-old NA cells or BSR cells in 12-well plates. After 3 days of incubation in the presence of a 4-IU/ml concentration of either human monoclonal antibody CR57 or CRJB, medium (1 ml) containing potential escape viruses was harvested and stored at 4°C until further use. Subsequently the cells were acetone fixed for 20 min at 4°C and stained overnight at 37°C and 5% CO2 with an anti-rabies virus N-fluorescein isothiocyanate (FITC) antibody conjugate (Centocor). The number of foci per well was scored by immunofluorescence, and supernatants from wells infected with the lowest dilution of virus which produced one to six fluorescent foci were chosen for escape virus amplification. The number of foci for each escape virus differed, ranging from one to six foci/well. All E57 escape viruses were generated from a single focus, except E57B1 (three foci). EJB viruses were isolated from one focus (EJB3F), three foci (EJB2B), four foci (EJB2C), five foci (EJB2E, EJB2F), or six foci (EJB2D), respectively.
Determination of the NI.
Escape virus (2 ml) was incubated at 106 FFU/ml with either CR57 or CRJB (∼4 IU/ml) for 1 h at 37°C and 5% CO2. Monolayers of 2-day-old NA cells in T25 flasks were washed with PBS (Gibco); the virus-MAb mixture was added to the cells and incubated for 1 h at 37°C and 5% CO2. After virus attachment, the medium containing virus-antibody mix was removed, and cells were washed twice with medium and incubated for 48 h at 34°C and 5% CO2 in medium supplemented with 4 IU of their respective antibody/ml. Harvested medium was used to titrate the virus on NA cells to determine the focus-forming units per milliliter. The neutralization index (NI) was calculated with the formula NI = log[FFU/ml − IgG] − log[FFU/ml + IgG]. An index lower than 2.5 was considered evidence of escape from neutralization by the antibody.
cDNA sequencing.
Amplified virus stocks were used to inoculate a monolayer of NA cells (MOI = 1.0). Two days postinfection the cells were harvested, and total RNA was isolated using a QIAGEN RNeasy mini kit according to the manufacturer's instructions. Subsequently, reverse transcription-PCR (RT-PCR) was performed using rabies virus-specific primers and the One-Step SuperScript RT-PCR with Platinum Taq DNA polymerase (Invitrogen) according to the manufacturer's recommendations. cDNA was then sequenced by standard procedures.
Epitope mapping.
The 15-mer linear overlapping peptides (overlap by 14 amino acids) were synthesized spanning the extracellular domain of the G protein of the rabies virus strain ERA (amino acids [aa] 20 to 458; GenBank accession no. J02293) and screened using credit card format mini-PEPSCAN cards as described previously (28). Additionally, 8-mer peptides were synthesized that overlapped by 7 amino acids spanning only the region reactive with CR57. Binding of the antibodies to each linear peptide was tested in a PEPSCAN-based ELISA. The 455-well credit card format polypropylene cards, containing the covalently linked peptides, were incubated with the human anti-rabies virus antibody (10 μg/ml; diluted in blocking solution, which contains 5% [vol/vol] horse serum and 5% [wt/vol] ovalbumin) (4°C, overnight). After washing, the peptides were incubated with anti-human antibody peroxidase at a dilution of 1/1,000 for 1 h at 25°C. The peptides were then washed, and the peroxidase substrate 2,2′-azino-di-3-ethylbenzthiazoline sulfonate (ABTS) and 2 μl of 3% H2O2/ml were added. Controls were incubated with anti-human antibody peroxidase only. After 1 h, the color development of the ELISA was quantified with a CCD camera and an image-processing system using the image-processing software package Optimas, version 6.5 (Media Cybernetics, Silver Spring, Md.).
Epitope alignment.
The minimal binding region of the CR57 epitope was aligned using glycoprotein amino acid sequences of the 229 rabies virus isolates (for GenBank accession numbers, see supplementary material).
RESULTS
Rabies virus-neutralizing activity of human monoclonal antibodies.
Heavy- and light-chain sequences of the antibodies SOJA, SOJB, and SO57 were cloned into our human IgG1 expression vector pcDNA3002. The antibodies, designated CRJA, CRJB, and CR57, were produced in PER.C6 cells and purified by affinity chromatography. The CRJB antibody was originally of IgG3 format but was converted into an IgG1 molecule, because IgG1 molecules have a longer half-life than IgG3 molecules. To establish the neutralizing activity of these MAbs, the antibodies were tested in a modified RFFIT using CVS-11 rabies virus (Table 1). The neutralizing activities of CR57 and CRJB were higher than previously reported for the antibodies produced using a rhabdoviral vector (25). In contrast, CRJA had such a low potency (0.5 IU/mg) that it was excluded from further experiments.
TABLE 1.
Neutralizing potencies of anti-rabies virus antibodies
| Rhabdoviral systema
|
PER.C6
|
||
|---|---|---|---|
| IgG | Potency (IU/mg) | IgG | Potency (IU/mg) |
| SO57 | 2,200 | CR57 | 3,800 |
| SOJB | 240 | CRJB | 620 |
| SOJA | 30 | CRJA | 0.5 |
See reference 25.
Competition between rabies virus-neutralizing MAbs.
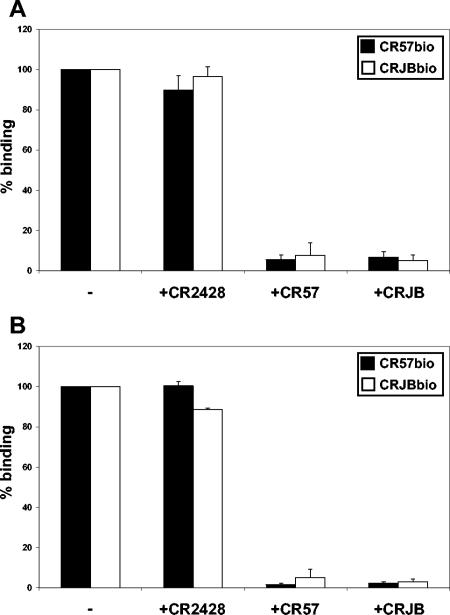
To investigate whether the antibodies CR57 and CRJB compete with each other for binding to rabies virus glycoprotein, we performed a set of competition experiments using either an ELISA (fixed antigen conformation) or flow cytometry (native antigen conformation) format. Analysis of competition in an ELISA format on immobilized glycoprotein showed that the negative control antibody, CR2428, did not block the binding of either biotinylated antibody CR57bio or CRJBbio (Fig. 1A). In contrast, when CR57bio and CRJBbio were coincubated with their unlabeled counterpart IgGs, the binding of the former was completely eliminated, thereby serving as an internal positive control. Both CR57 and CRJB also blocked binding of CRJBbio and CR57bio, respectively, indicating that the two antibodies compete for binding to rabies virus glycoprotein. These experiments were also performed on immobilized inactivated rabies virus (Pitman-Moore strain), and identical results were obtained (data not shown).
FIG. 1.
CR57 and CRJB compete for binding to glycoprotein. Saturating amounts of unlabeled IgG (indicated on x axes) were allowed to bind to (A) immobilized glycoprotein or (B) glycoprotein expressing cells before addition of biotinylated competitor IgG CR57bio (filled bars) or CRJBbio (open bars). Binding is expressed as the percentage of the binding (ELISA signal or mean fluorescence intensity) of the biotinylated antibody alone.
Antibody competition was next studied by a flow cytometry-based assay using rabies virus glycoprotein-expressing cells. In this format, the glycoprotein is expressed on the cell surface and can still undergo conformational changes, thereby mimicking more closely the natural situation on the virion. Figure 1B confirms that the binding of either CR57bio or CRJBbio is blocked by the unlabeled CRJB or CR57, respectively, whereas the negative control antibody CR2428 did not interfere with binding.
Identification of a novel rabies virus-neutralizing epitope.
In contrast to the conformational epitope that is recognized by CRJB, the SO57 MAb has been reported to react with denatured rabies glycoprotein on Western blotting, suggesting interaction with a linear epitope (8). To identify the region of the glycoprotein that is recognized by CR57, a PEPSCAN-ELISA was performed. Binding of the antibody to 15-mer linear peptides (overlapping by 14 amino acids) spanning the extracellular domain of the mature protein was determined (data not shown). CR57 recognized linear peptides in the region SLKGACKLKLCGVLGLRLMDGTW (aa 218 to 240). To further identify the core amino acids within the binding region of CR57, an 8-mer peptide PEPSCAN-ELISA was performed (Fig. 2A). CR57 showed reactivity with three peptides: KLKLCGVL, LKLCGVLG, and KLCGVLGL. This demonstrates a minimal binding region, KLCGVL (aa 226 to 231), within the rabies virus glycoprotein. Subsequently, we performed an alanine replacement scan through the LKLCGVLG peptide to identify the residues within the core peptide that are critical for binding of the CR57 antibody. Figure 2B shows that the lysine (K) as well as the central CGV triplet are key residues within the minimal binding region for CR57 recognition (K-CGV-).
FIG. 2.
CR57 recognizes a linear epitope on rabies virus glycoprotein. (A) CR57 was tested using an 8-mer peptide PEPSCAN-ELISA in a region of the rabies virus glycoprotein ectodomain identified using overlapping 15-mer peptides. (B) Alanine replacement scan through the core 8-mer peptide containing the minimal binding region as identified in panel A. Relative ELISA signals are shown for each peptide.
Generation and characterization of escape viruses.
To further define and/or confirm the critical residues on the rabies virus glycoprotein that are, through conformational changes, directly or indirectly involved in binding of CR57 and CRJB, we generated a number of antibody-resistant escape viruses using both CR57 and CRJB, designated E57 and EJB escape viruses hereafter. A total of six distinct escape viruses were isolated for each antibody. Cross-neutralization of the E57 and EJB escape viruses by CRJB and CR57, respectively, was examined by determination of the neutralization index (NI) for the escape viruses. Each virus was grown in the absence and presence of either selecting or nonselecting antibody. Subsequently, the virus titers were determined and used to calculate the NI as described in Materials and Methods (Table 2). None of the E57 viruses were neutralized by CR57, and two viruses (E57A2 and E57B2) were also not neutralized by CRJB. In contrast, EJB viruses were not covered by either CRJB or CR57. This suggested that the epitopes recognized by CR57 and CRJB overlap. The glycoprotein-encoding genes from the escape viruses were analyzed by direct sequencing of cDNA that was obtained from infected NA cells. DNA sequence analysis revealed several mutations in the glycoprotein gene (Fig. 3, Table 3). Overall, four unique escape viruses for CR57 and two unique escape viruses for CRJB were isolated based on glycoprotein amino acid sequence. The mutations K226E, K226N, and G229E observed in the E57 viruses were located in the CR57 minimal binding region (aa 226 to 231), as was identified in the PEPSCAN-ELISA (Fig. 2). Moreover, the mutations exactly coincided with the key amino acids within the core peptide identified through the alanine replacement scan. As expected, the introduction of the mutations observed in the E57 viruses into the core wild-type 8-mer peptide eliminated binding of the CR57 MAb (data not shown). EJB viruses also harbored mutations (G229E and L231P) in the CR57 minimal binding region, thereby confirming the competition results described above. In addition, all EJB viruses also had an extra mutation at position 182 (N182D). This further suggests that the mutation in position 182 and in the region 229 to 231 destabilizes the conformational epitope recognized by CRJB.
TABLE 2.
Neutralization index of wild-type CVS-11 and escape virusesa
| Virus | NI (CR57) | NI (CRJB) | Escape of virus |
|---|---|---|---|
| CVS-11 | 6.8 | 6.8 | |
| E57A2 | 0.8 | 0 | CR57 + CRJB |
| E57A3 | 0.1 | 3.1 | CR57 |
| E57B1 | 0 | 2.8 | CR57 |
| E57B2 | 0 | 2.1 | CR57 + CRJB |
| E57B3 | 0 | 4.1 | CR57 |
| E57C3 | 0.8 | 3.9 | CR57 |
| EJB2B | 0.2 | 0.1 | CRJB + CR57 |
| EJB2C | 0 | 0.4 | CRJB + CR57 |
| EJB2D | 0 | 0.1 | CRJB + CR57 |
| EJB2E | 0 | 0.1 | CRJB + CR57 |
| EJB2F | 0 | 0 | CRJB + CR57 |
| EJB3F | 0.5 | 0.2 | CRJB + CR57 |
Neutralization index (NI) is shown as the log10 value for each antibody. NI < 2.5 was considered escape.
FIG. 3.
Amino acid sequences of the E57 and EJB escape viruses. Escape viruses were generated, amplified, and characterized as described in Materials and Methods. (A) E57 escape virus glycoprotein sequences. (B) EJB escape virus glycoprotein sequences. The relevant amino acids harboring the mutations of each escape virus are depicted. Boxed amino acids indicate the identified minimal binding region required for CR57 recognition.
TABLE 3.
Characterization of E57 and EJB escape virusesa
| Virus | Amino acid no. | Amino acid change | Codon change |
|---|---|---|---|
| E57A2 | 229 | G to E | GGA to GAA |
| E57A3 | 20 | H to Q | CAC to CAA |
| 226 | K to E | AAG to GAG | |
| E57B1, E57B3 | 226 | K to E | AAG to GAG |
| E57B2, E57C3 | 226 | K to N | AAG to AAT |
| EJB2B, 2C, 2F | 182 | N to D | AAT to GAT |
| 229 | G to E | GGA to GAA | |
| 231 | L to P | CTT to CCT | |
| EJB2D, 2E, 3F | 182 | N to D | AAT to GAT |
| 231 | L to P | CTT to CCT |
Amino acid numbering is from the mature protein minus the signal peptide. Wild-type CVS-11 sequence was determined in a similar fashion from the original virus stock used to generate the escape viruses.
Conservation of the novel epitope within rabies viruses.
We subsequently aligned the CR57 minimal binding region of glycoprotein sequences of 229 genotype 1 rabies virus isolates to assess the conservation of the epitope (Table 4). The sample set contained human isolates, bat isolates, and isolates from canines or from domestic animals most likely bitten by rabid canines. Frequency analysis of the amino acids at each position within the minimal binding region revealed that the critical residues constituting the epitope were highly conserved. The lysine at position one was conserved in 99.6% of the isolates, while in only 1 out of 229 isolates a conservative K-to-R mutation was observed. Positions two and three (L and C, respectively) were completely conserved. The glycine at position four was conserved in 98.7% of the isolates, while in 3 out of 229 isolates mutations towards charged amino acids (R, 1 out of 229; E, 2 out of 229) were observed. The fifth position was also conserved, with the exception of one isolate where a conservative V-to-I mutation was observed. At the sixth position, which is not a critical residue as determined by the alanine replacement scan, significant heterogeneity is observed in the street isolates: L in 70.7%, P in 26.7%, and S in 2.6% of the strains, respectively. Alignment of the minimal binding region with representative lyssaviruses belonging to genotypes 2 to 7 showed that within these genotypes the epitope is less well conserved (Table 5). However, at the critical residues, genotypes 4 to 7 have conserved amino acid changes, suggesting potential recognition by CR57.
TABLE 4.
Conservation of the CR57 minimal binding region within rabies virusesa
| K | L | C | G | V | L |
|---|---|---|---|---|---|
| K (99.6%) | L (100%) | C (100%) | G (98.7%) | V (99.6%) | L (70.7%) |
| R (0.4%) | E (0.9%) | I (0.4%) | P (26.7%) | ||
| R (0.4%) | S (2.6%) |
Percentage of occurrence of each amino acid is shown in parentheses for 229 rabies virus isolates.
TABLE 5.
Conservation of the CR57 minimal binding region within Lyssaviruses
DISCUSSION
Present PEP treatment of rabies virus-infected people involves the use of HRIG. Blood-derived products, like HRIG, have potential health risks normally associated with those products. Also, HRIG can display batch-to-batch variation and may be limited in availability in case of sudden mass exposure (5, 6). An alternative to HRIG would be to use human monoclonal antibodies produced according to industrial standards. The concern regarding blood-derived products could thereby be circumvented, and consistent batches of antibodies could be produced in large quantities. Therefore, we initiated a study to identify and characterize human MAbs to be included in such a cocktail. We focused on the human MAbs previously described by Prosniak et al. (25), which displayed potent neutralization of several rabies virus strains.
For expression of the antibodies, we used the human cell line PER.C6, which produces consistently high levels of antibody and can easily be adapted for industrial use. This method contrasts with that of the rhabdoviral antibody system (22), which involves the error-prone rhabdoviral RNA polymerase and is therefore less suitable as an industrial standard for the production of a consistent, high-quality antibody. Reformatting of the antibodies SOJA, SOJB, and SO57 into the PER.C6 expression system resulted in improved neutralizing potency of the antibodies, with the exception of CRJA. We decided to exclude CRJA from further experiments due to its low potency. The low potency would result in exorbitant protein content if CRJA were to be used in a future cocktail of MAbs. Therefore, CR57 and CRJB were further investigated.
Earlier data suggested that CR57 and CRJB show a distinct pattern of coverage of different lyssavirus genotypes, which may be based on qualitative or quantitative characteristics of the respective MAbs (25). Competition experiments using both immobilized rabies virus glycoprotein and glycoprotein expressed on cells showed that CR57 and CRJB recognize competing, overlapping epitopes and cannot bind simultaneously to the rabies virus glycoprotein (Fig. 1). CR57 was tested in a PEPSCAN ELISA to determine the epitope and was shown to recognize a linear peptide of the rabies virus glycoprotein (Fig. 2), which agreed with the finding that CR57 is able to bind to reduced denatured rabies virus glycoprotein on Western blotting. Detailed analysis of the binding region of CR57 revealed a minimal binding region of 6 amino acids (KLCGVL, aa 226 to 231) critical for CR57 binding, with K-CGV as key residues (Fig. 2). This finding was confirmed by analysis of the amino acid sequences of the escape viruses generated with CR57. Specific nonsilent point mutations (K226E, K226N, and G229E) were detected in this minimal binding region (Fig. 3, Table 3). This underlines the importance of these residues for binding of CR57 to the rabies virus glycoprotein. Analysis of the glycoprotein open reading frame of EJB viruses showed point mutations in two different spots of the glycoprotein, including mutations (G229E and L231P) within the minimal binding region of CR57, indicating that amino acids within this epitope are important for binding of CRJB to the rabies virus glycoprotein. Furthermore, these data suggest that CRJB recognizes a nonlinear, conformational epitope that partially overlaps with the minimal binding region of CR57. This is also in agreement with the competition data described above.
Another goal of the escape virus studies was to mimic the theoretical event of escape in vivo. Antibody escape viruses could occur from naturally existing quasispecies present in the virus inoculum as well as from antibody-dependent selection of a neutralization-resistant variant as a consequence of a humoral immune response against the virus. However, the latter is not likely to occur, as was demonstrated by the observed conservation of the antigenic sites on rabies virus glycoprotein (2).
The antigenic structure of the rabies virus glycoprotein was initially defined by Lafon et al. (18). The antigenic sites were identified using a panel of mouse MAbs and their respective MAb-resistant virus variants. Since then, the antigenic sites have been mapped by identification of the amino acid mutations in the glycoprotein of MAb-resistant variants (3, 24, 27). The majority of rabies virus-neutralizing MAbs are directed against antigenic site II (3), which is a discontinuous conformational epitope comprising aa 34 to 42 and aa 198 to 200 (24). Antigenic site III is a continuous conformational epitope at aa 330 to 338 and harbors two charged residues, K330 and R333, that affect viral pathogenicity (7, 10, 27). The conformational antigenic site I was defined by only one MAb, 509-6, located at aa 231 (3, 18). Antigenic site IV is known to harbor overlapping linear epitopes (4, 19, 23). Benmansour et al. (3) also described the presence of a minor site located at position 342 to 343, which is distinct from antigenic site III despite its close proximity.
Alignment of the CR57 epitope with the presently known linear and conformational neutralizing epitopes on rabies virus glycoprotein (Fig. 4) revealed that the CR57 epitope is located in the same region as the conformational antigenic site I, defined by the single MAb 509-6 (3, 10, 18). Competition experiments described within this study revealed that CR57 and 509-6 competed for binding to ERA G in both ELISA and fluorescence-activated cell sorting (data not shown). Interestingly, neutralization of E57 escape viruses by 509-6 could be demonstrated (data not shown), which agreed with earlier data by Dietzschold et al. (8) showing neutralization of an SO57 escape variant by the 509-6 antibody. Coverage of 509-6 escape viruses by CR57 could not be performed, as this escape virus was not available. However, based on literature, it is expected that CR57 does neutralize 509-6 escape viruses. CR57 neutralizes silver-haired bat rabies viruses (SHBRV) (25), which all harbor a proline at position 231 (9, 21). This proline was identical to the mutation L231P observed in the 509-6 escape virus (3). Thus, SHBRV resemble the 509-6 escape virus with respect to antigenic site I, suggesting that CR57 is capable of neutralizing 509-6 escape viruses. Overall, antigenic site I might be redefined as a region harboring both conformational (MAbs CRJB and 509-6) and linear epitopes (CR57), as indicated in Fig. 4.
FIG. 4.
Neutralizing epitopes on rabies virus glycoprotein. A schematic drawing of the rabies virus glycoprotein is shown depicting the antigenic sites including the novel CR57 epitope. The signal peptide (19 aa) and transmembrane domain are indicated by black boxes. Disulfide bridges are indicated. Amino acid numbering is from the mature protein minus the signal peptide.
We analyzed the glycoprotein sequence of a large set of rabies virus isolates to assess the occurrence of quasispecies that might have a mutation in the CR57 epitope. We observed a high level of conservation within the CR57 epitope, which was underlined by experiments showing that a panel of rabies street viruses were all neutralized by CR57 in a standard RFFIT (data not shown). This may be explained in part by the presence of the central cysteine residue, which is structurally involved in glycoprotein folding and is conserved among all lyssaviruses (2). From the 229 analyzed naturally occurring rabies virus isolates, only 3 isolates (AF346857, AF346861, and U72050) contained nonconserved amino acid changes at key residues within the epitope that would eliminate antibody binding. Hence, approximately 99% of the rabies viruses that can be encountered are predicted to be recognized by the CR57 antibody.
In two bat virus isolates (AF346857 and AF346861), the amino acid changes within the CR57 epitope were identical to those observed in some of the EJB viruses (i.e., KLCEVP). However, none of the 229 rabies virus isolates contained an aspartic acid at position 182 of the mature glycoprotein, as was observed in the EJB viruses. Apparently, such genotype 1 rabies viruses do not exist in nature. In contrast, Mokola and Lagos bat viruses do contain an aspartic acid at position 182 in combination with a proline at position 231. In vitro analysis of neutralizing activity of CR57 towards representative viruses of genotypes 2 to 7 revealed that CR57 neutralizes genotypes 4 to 7 but not genotypes 2 and 3 (data not shown). The results contradict earlier reports showing that CR57 only covers genotype 1 viruses (8, 25). This might be explained by the mutations we observed in the rhabdoviral pSPBN SO57 vectors compared to the originally described MAb 57 sequence (16), which potentially could result in altered antigen binding properties. Because the PER.C6-produced CR57 completely matches the MAb 57-amino-acid sequence, the studies formally cannot be compared because different MAbs were used. In addition, it could be explained by the use of different virus isolates as representatives of lyssavirus genotypes 2 to 7. Apparently, mutation of the key residue K to R in genotypes 4 and 5 (Table 5) at position 1 of the minimal binding region of CR57 is tolerated as well as an isoleucine at position 5 (genotypes 4, 6, and 7). In contrast, changing key residues K to T at position one and V to K at position five eliminates neutralization of Mokola and Lagos bat viruses by CR57.
Our study showed that one of the most potent rabies virus-neutralizing human MAbs recognizes a novel highly conserved epitope on the glycoprotein of rabies viruses. This epitope was shown to be linear and to contain four key residues for binding of the antibody. All rabies virus variants escaping neutralization by this MAb had nonsilent mutations in the key residues of the epitope. The replacement of the wild-type amino acids within a peptide that encompasses the minimal binding region with those observed in the glycoprotein of the escape eliminated antibody binding. The second antibody that we characterized, CRJB, recognized a conformational epitope that partially overlapped with the CR57 epitope, identifying a neutralization epitope complex. Rabies virus mutants escaping from CRJB completely escaped CR57 neutralization. These results argue for the inclusion of only one of these two antibodies in a cocktail product aimed at replacing HRIG.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Anonymous. 2002. W.H.O. consultation on a monoclonal antibody cocktail for rabies post exposure treatment. World Health Organization, Geneva, Switzerland.
- 2.Badrane, H., and N. Tordo. 2001. Host switching in Lyssavirus history from the Chiroptera to the Carnivora orders. J. Virol. 75:8096-8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benmansour, A., H. Leblois, P. Coulon, C. Tuffereau, Y. Gaudin, A. Flamand, and F. Lafay. 1991. Antigenicity of rabies virus glycoprotein. J. Virol. 65:4198-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunschoten, H., M. Gore, I. J. Claassen, F. G. Uytdehaag, B. Dietzschold, W. H. Wunner, and A. D. Osterhaus. 1989. Characterization of a new virus-neutralizing epitope that denotes a sequential determinant on the rabies virus glycoprotein. J. Gen. Virol. 70:291-298. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1999. Mass treatment of humans who drank unpasteurized milk from rabid cows-Massachusetts, 1996-1998. Morb. Mortal. Wkly. Rep. 48:228-229. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1999. Multiple human exposures to a rabid bear cub at a petting zoo and barnwarming-Iowa, August 1999. Morb. Mortal. Wkly. Rep. 48:761. [PubMed] [Google Scholar]
- 7.Coulon, P., J. P. Ternaux, A. Flamand, and C. Tuffereau. 1998. An avirulent mutant of rabies virus is unable to infect motoneurons in vivo and in vitro. J. Virol. 72:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietzschold, B., M. Gore, P. Casali, Y. Ueki, C. E. Rupprecht, A. L. Notkins, and H. Koprowski. 1990. Biological characterization of human monoclonal antibodies to rabies virus. J. Virol. 64:3087-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietzschold, B., K. Morimoto, D. C. Hooper, J. S. Smith, C. E. Rupprecht, and H. Koprowski. 2000. Genotypic and phenotypic diversity of rabies virus variants involved in human rabies: implications for postexposure prophylaxis. J. Hum. Virol. 3:50-57. [PubMed] [Google Scholar]
- 10.Dietzschold, B., W. H. Wunner, T. J. Wiktor, A. D. Lopes, M. Lafon, C. L. Smith, and H. Koprowski. 1983. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc. Natl. Acad. Sci. USA 80:70-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enssle, K., R. Kurrle, R. Kohler, H. Muller, E. J. Kanzy, J. Hilfenhaus, and F. R. Seiler. 1991. A rabies-specific human monoclonal antibody that protects mice against lethal rabies. Hybridoma 10:547-556. [DOI] [PubMed] [Google Scholar]
- 12.Fallaux, F. J., A. Bout, I. van der Velde, D. J. van den Wollenberg, K. M. Hehir, J. Keegan, C. Auger, S. J. Cramer, H. van Ormondt, A. J. van der Eb, D. Valerio, and R. C. Hoeben. 1998. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum. Gene Ther. 9:1909-1917. [DOI] [PubMed] [Google Scholar]
- 13.Geysen, H. M., R. H. Meloen, and S. J. Barteling. 1984. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc. Natl. Acad. Sci. USA 81:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanlon, C. A., C. A. DeMattos, C. C. DeMattos, M. Niezgoda, D. C. Hooper, H. Koprowski, A. Notkins, and C. E. Rupprecht. 2001. Experimental utility of rabies virus-neutralizing human monoclonal antibodies in post-exposure prophylaxis. Vaccine 19:3834-3842. [DOI] [PubMed] [Google Scholar]
- 15.Hanlon, C. A., M. Niezgoda, and C. E. Rupprecht. 2002. Postexposure prophylaxis for prevention of rabies in dogs. Am. J. Vet. Res. 63:1096-1100. [DOI] [PubMed] [Google Scholar]
- 16.Ikematsu, H., N. Harindranath, Y. Ueki, A. L. Notkins, and P. Casali. 1993. Clonal analysis of a human antibody response. II. Sequences of the VH genes of human IgM, IgG, and IgA to rabies virus reveal preferential utilization of VHIII segments and somatic hypermutation. J. Immunol. 150:1325-1337. [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, D., N. Kroos, R. Anema, B. van Montfort, A. Vooys, S. van der Kraats, E. van der Helm, S. Smits, J. Schouten, K. Brouwer, F. Lagerwerf, P. van Berkel, D. J. Opstelten, T. Logtenberg, and A. Bout. 2003. High-level expression of recombinant IgG in the human cell line PER.C6. Biotechnol. Prog. 19:163-168. [DOI] [PubMed] [Google Scholar]
- 18.Lafon, M., T. J. Wiktor, and R. I. Macfarlan. 1983. Antigenic sites on the CVS rabies virus glycoprotein: analysis with monoclonal antibodies. J. Gen. Virol. 64:843-8451. [DOI] [PubMed] [Google Scholar]
- 19.Luo, T. R., N. Minamoto, H. Ito, H. Goto, S. Hiraga, N. Ito, M. Sugiyama, and T. Kinjo. 1997. A virus-neutralizing epitope on the glycoprotein of rabies virus that contains Trp251 is a linear epitope. Virus Res. 51:35-41. [DOI] [PubMed] [Google Scholar]
- 20.Montano-Hirose, J. A., M. Lafage, P. Weber, H. Badrane, N. Tordo, and M. Lafon. 1993. Protective activity of a murine monoclonal antibody against European bat lyssavirus 1 (EBL1) infection in mice. Vaccine 11:1259-1266. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto, K., M. Patel, S. Corisdeo, D. C. Hooper, Z. F. Fu, C. E. Rupprecht, H. Koprowski, and B. Dietzschold. 1996. Characterization of a unique variant of bat rabies virus responsible for newly emerging human cases in North America. Proc. Natl. Acad. Sci. USA 93:5653-5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morimoto, K., M. J. Schnell, R. Pulmanausahakul, J. P. McGettigan, H. D. Foley, M. Faber, D. C. Hooper, and B. Dietzschold. 2001. High level expression of a human rabies virus-neutralizing monoclonal antibody by a rhabdovirus-based vector. J. Immunol. Methods 252:199-206. [DOI] [PubMed] [Google Scholar]
- 23.Ni, Y., Y. Tominaga, Y. Honda, K. Morimoto, S. Sakamoto, and A. Kawai. 1995. Mapping and characterization of a sequential epitope on the rabies virus glycoprotein which is recognized by a neutralizing monoclonal antibody, RG719. Microbiol. Immunol. 39:693-702. [DOI] [PubMed] [Google Scholar]
- 24.Prehaud, C., P. Coulon, F. LaFay, C. Thiers, and A. Flamand. 1988. Antigenic site II of the rabies virus glycoprotein: structure and role in viral virulence. J. Virol. 62:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prosniak, M., M. Faber, C. A. Hanlon, C. E. Rupprecht, D. C. Hooper, and B. Dietzschold. 2003. Development of a cocktail of recombinant-expressed human rabies virus-neutralizing monoclonal antibodies for postexposure prophylaxis of rabies. J. Infect. Dis. 188:53-56. [DOI] [PubMed] [Google Scholar]
- 26.Schumacher, C. L., B. Dietzschold, H. C. Ertl, H. S. Niu, C. E. Rupprecht, and H. Koprowski. 1989. Use of mouse anti-rabies monoclonal antibodies in postexposure treatment of rabies. J. Clin. Investig. 84:971-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seif, I., P. Coulon, P. E. Rollin, and A. Flamand. 1985. Rabies virulence: effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J. Virol. 53:926-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slootstra, J. W., W. C. Puijk, G. J. Ligtvoet, J. P. Langeveld, and R. H. Meloen. 1996. Structural aspects of antibody-antigen interaction revealed through small random peptide libraries. Mol. Divers. 1:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.