Abstract
The selection of human monoclonal antibodies (MAbs) specific for human immunodeficiency virus (HIV) type 1 by binding assays may fail to identify Abs to quaternary epitopes on the intact virions. The HIV neutralization assay was used for the selection of human MAb 2909, which potently neutralizes SF162 and recognizes an epitope on the virus surface but not on soluble proteins. Three regions of gp120, the V2 and V3 loops and the CD4 binding domain, contribute to the epitope recognized by MAb 2909. The existence of such a unique MAb, which defines a complex epitope formed by a quaternary structure, suggests that there may be other new neutralizing HIV epitopes to target with vaccines.
Passive-immunization experiments with polyclonal and monoclonal reagents have shown that antibodies (Abs) can provide protection against infection with human immunodeficiency virus type 1 (HIV-1) (1, 10, 11, 34). Defining the nature of such protective Abs can best be done by studying the specificities and activities of monoclonal Abs (MAbs). Identifying those Abs which would optimally be induced with a vaccine is best analyzed by producing and characterizing human MAbs with potent neutralizing activities. Nonetheless, to date, the generation of human MAbs has been based entirely on the selection of MAbs that bind to infected cells or to peptides or proteins representing the viral envelope (4, 17, 33). Selection on the basis of binding, rather than functional activity, gives rise to many interesting but nonneutralizing MAbs (13) and fails to define all of the antigenic structures that exist on the surfaces of intact virus particles where quaternary interactions occur as the result of viral proteins forming multimeric structures in the lipid bilayer of the virus envelope.
In order to determine if human B cells can target these complex viral structures, and if Abs to these epitopes can neutralize primary HIV isolates, we replaced the various binding assays with a luciferase neutralization assay to screen for Ab-producing cells. Here we describe the first MAb selected by this technique, MAb 2909, which recognizes a quaternary structure present only on virions but does not react with proteins solubilized from the virion. This MAb may provide a new direction in the search for novel epitopes that can be targeted by vaccines for the production of protective anti-HIV Abs.
Generation of MAb.
MAb 2909 was produced by fusion of Epstein-Barr virus-transformed peripheral blood mononuclear cells (PBMCs) with heteromyeloma SHM-D33, as previously described (17). The PBMCs used were derived from an asymptomatic and antiretrovirus drug-naïve subject who had been infected with HIV for at least 15 years. At the time of study, his viral load was 4,627 copies/ml, and his CD4 T-cell count was 848/mm3. During the previous 5 years, the subject's viral load increased from undetectable to the reported level, and his CD4 T-cell counts were relatively constant (∼950/mm3).
To select the Epstein-Barr virus-transformed cells and then the heterohybridoma, culture supernatants were screened using a single-cycle infectivity assay for neutralization of pseudovirions SF162 (16). Pseudovirus SF162 was produced by cotransfection into 293T cells of plasmid pNL4-3.Luc.R−E− (NIH AIDS Research and Reference Reagent Program) and plasmid pLRB826 expressing the env fragment from SF162 (provided by A. Pinter) (6, 22). As previously described (16), the pseudovirus was preincubated with culture supernatants and this mixture was then added to HOS-CD4/CCR5 cells (NIH AIDS Research and Reference Reagent Program). The cultures were incubated for 3 days, and luciferase activity in the cell lysates was measured using a luminometer.
MAb 2909 belongs to immunoglobulin subclass G1 (IgG1) and bears lambda light chains. The variable domain of the heavy chain was sequenced by standard methods (20), and analysis of the gene sequence (http://imgt.cines.fr) showed that the heavy chain is a product of IgHV3-43, IgHJ6, and IgHD5-12.
Functional characteristics of MAb 2909.
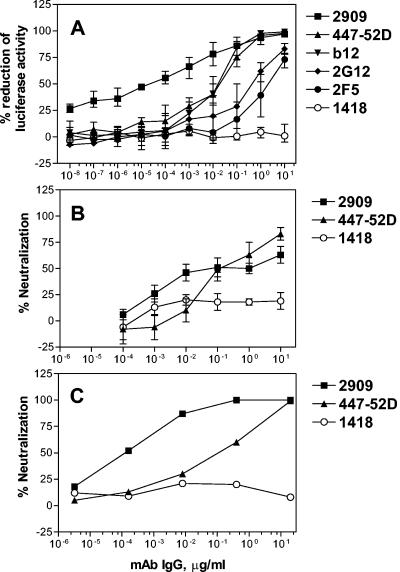
By the luciferase neutralization assay, 0.04 ng of MAb 2909 per ml was required to give 50% neutralization of pseudovirus SF162 (Fig. 1A). Simultaneous tests with a MAb to the CD4 binding domain (CD4bd), MAb IgG1b12 (4), required 30 ng/ml for 50% neutralization, while anti-V3 MAb 447-52D (18) required 35 ng/ml; a MAb to a complex carbohydrate epitope, MAb 2G12 (39), required 680 ng/ml, and an anti-gp41 MAb, 2F5 (28), required 3,900 ng/ml (Fig. 1A). Thus, the neutralizing activity of MAb 2909 against pseudovirus SF162 was 750- to 100,000-fold more potent than those of other well-characterized anti-HIV MAbs.
FIG. 1.
Neutralization of pseudotyped virus SF162 in the luciferase assay (A) and of parental SF162 virus (B and C) in the GHOST cell neutralization assay and the PHA-stimulated PBMC assay, respectively. MAbs 447-52D (anti-V3), b12 (anti-CD4bd), 2G12 (anti-complex carbohydrate), and 2F5 (anti-gp41) were used as positive controls, given their well-established neutralizing activity, while MAb 1418 (anti-parvovirus B19) served as a negative control. The error bars represent the standard deviations from the means of results from three (A) or five (B) experiments.
MAb 2909 was also tested for neutralization against the parental form of virus SF162 in the GHOST cell neutralization assay (5) and the phytohemagglutinin (PHA)-stimulated PBMC assay (14); 50% neutralizing dose levels for MAb 2909 were ∼100 ng/ml in the GHOST assay and <1.0 ng/ml in the PHA-stimulated PBMC assay and significantly lower than those for anti-V3 MAb 447-52D tested in parallel (Fig. 1B and C).
MAb 2909 was subsequently tested for neutralizing activity against several primary isolates, including three from clade A (VI191, CA1, and 92RW021), three from clade B (BX08, CA5, and BaL), one from clade C (95ZW2036), and two from clade F (93BR029 and CA20). None was neutralized when MAb 2909 was tested at 25 μg/ml. Virus neutralization was also tested using a virus isolated from the subject from whose cells MAb 2909 was isolated. This virus was isolated from PBMCs obtained 18 months after the specimen was drawn and from which heterohybridoma MAb 2909 was produced; this virus, which may represent an escape mutant, was not neutralized by MAb 2909 (data not shown). Thus, the neutralizing activity of MAb 2909 is quite narrow, although strictly speaking it is not isolate specific since it was induced in a patient whose virus is not closely related to SF162 on the basis of the sequences of the V1/V2 and C2 to V5 regions of env (data not shown).
Characterization of the epitope recognized by MAb 2909.
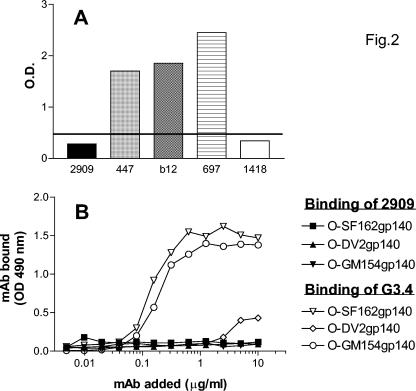
Surprisingly, this highly potent MAb 2909 was unreactive with gp120 derived from SF162. Thus, parental virus SF162 adjusted to a concentration of 100 ng of p24/ml was solubilized with Triton X-100 and the gp120 was captured on enzyme-linked immunosorbent assay (ELISA) plates with sheep antibody to the C terminus of gp120 (Cliniqa Corporation, Fallbrook, Calif.). MAb 2909 or other MAbs were then added at a concentration of 10 μg/ml, and binding of MAbs was detected using anti-human IgG conjugate, as previously described (15). No reactivity of MAb 2909 with gp120 was observed, while control MAbs 447-52D (anti-V3), IgG1b12 (anti-CD4bd), and anti-V2 MAb 697 (14) showed strong binding to the proteins (Fig. 2A). Additional ELISAs using oligomeric forms of SF162 gp140, which had not been exposed to detergent, also showed no reactivity with MAb 2909 (Fig. 2B).
FIG. 2.
Reactivities of MAbs in ELISA with detergent-solubilized gp120 (A) and with oligomeric SF162 proteins (B). Detergent-solubilized gp120 was reacted with human MAbs 2909, 447 (anti-V3), b12 (anti-CD4bd), 697 (anti-V2), and 1418 (anti-parvovirus B19). (A) The horizontal line represents the cutoff for the assay (mean optical density for MAb 1418 plus 3 standard deviations). Oligomeric SF162 gp140 (O-SF162gp140), V2 deletion oligomeric gp140 (O-DV2gp140), and a mutant of oligomeric SF162gp140 in which the glycosylation site at position 154 was eliminated (25) (O-GM154gp140) were reacted with human MAb 2909 or murine anti-V2 MAb G3.4 (19).
Subsequently, MAb 2909 was tested for its ability to bind to intact virions. We used a virus capture assay in which various MAbs were immobilized onto ELISA plates and free virus was added to each well and allowed to interact with the bound MAb. After extensive washing, the amount of bound virus was quantitated by measuring p24 released after virus lysis (29). MAb 2909 reacted with only 2 of 16 primary and T-cell line-adapted viruses from clade B: SF162 (Fig. 3 and 4) and a chimeric NL4-3 virus containing aBL-01 env that was not neutralized by MAb 2909 (data not shown) (15, 31). The ability of MAb to bind to SF162 virions and its inability to react with soluble or recombinant molecules derived from the SF162 envelope suggest that the MAb targets a quaternary structure present exclusively on the surfaces of intact virions.
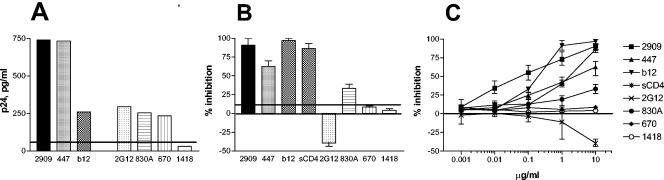
FIG. 3.
Mapping the 2909 epitope by ELISA. The ability of MAbs to bind intact SF162 virions is shown by the amount of p24 released from virions bound to the same human MAbs used for experiments shown in Fig. 2A. In addition, MAbs 2G12 (anti-carbohydrate complex), 830A (anti-V2), and 670 (anti-C5) were used. The horizontal line indicates the cutoff for the assay (mean level of binding to MAb 1418 plus 3 standard deviations) (A). The ability of various MAbs and sCD4, used at concentration of 20 μg/ml, to block the binding of SF162 virions to MAb 2909 is shown as “% inhibition” in panel B, where the horizontal line indicates the cutoff value (mean inhibition by MAb 1418 plus 3 standard deviations). MAb 2909 serves as its own positive control. MAb 2G12 enhanced the binding of SF162 virions to MAb 2909. (C) A dose-response curve in which various MAbs and sCD4 were used in the competition ELISA at concentrations from 0.001 to 10 μg/ml is shown.
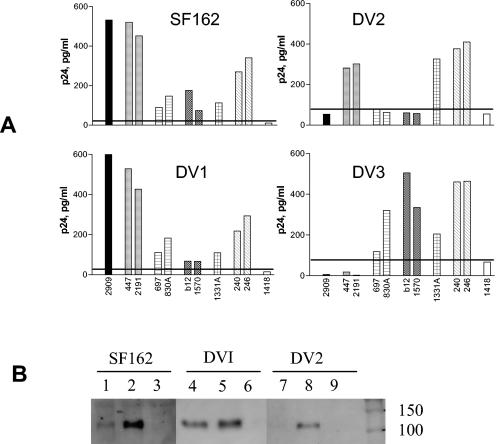
FIG. 4.
Binding of MAb 2909 to wild-type SF162 and its mutants with deletions of the V1 (DV1), V2 (DV2), and V3 (DV3) regions. (A) Binding of 2909 and control MAbs to intact virions was tested using the virus capture assay. The control MAbs were specific to V3 (447 and 2191), V2 (697 and 830A), CD4bd (b12 and 1570), C5 (1331A), gp41 (240 and 246), and parvovirus B19 (1418; negative control). The horizontal line indicates the cutoff (mean binding with MAb 1418 plus 3 standard deviations). (B) Immunoprecipitation analysis of MAb reactivities with wild-type SF162 and its mutants. MAb 2909 (lanes 1, 4, and 7) precipitated SF162 and DV1 but not DV2, while MAb 447-52D (lanes 2, 5, and 8) precipitated all three viruses. Control MAb 1418 (lanes 3, 6, and 9) showed no reactivity with any of the tested viruses.
To further characterize the epitope recognized by MAb 2909, we used a competition ELISA format in which 100 μl of a preparation of SF162 virions containing 100 ng of p24/ml was preincubated with 100 μl of various human MAbs at an IgG concentration of 20 μg/ml, namely, 2909, 447-52D, IgG1b12, 2G12 (anticarbohydrate complex) (3), 830A (anti-V2) (30), 670 (anti-C5) (43), soluble CD4 (Life Science Products, Boston, Mass.), or an irrelevant MAb 1418 (antiparvovirus B19) (12). The SF162-MAb complexes were then added to ELISA plates coated with 10 μg of MAb 2909 per ml. Subsequent to extensive washing, the amount of bound virus was measured as the amount of p24 released after addition of Triton X-100 to the wells (29). Percent inhibition is expressed as follows: p24 from SF162-MAb complexes divided by p24 from uncomplexed SF162 times 100. SF162 virions preincubated with MAb 2909 served as a positive control, and MAb 2909 was shown to cause 93% inhibition (Fig. 3B). Similar levels of competition were found with MAb IgG1b12 and soluble CD4 (sCD4), which inhibited SF162 binding to MAb 2909 by 97 and 89%, respectively. MAbs 447-52D (anti-V3) and 830A (anti-V2) also interfered with SF162 binding to MAb 2909 by 59 and 31%, respectively, whereas neither MAb 670, MAb 1418, nor MAb 2G12 had any blocking effect (Fig. 3B). MAb 2G12, in fact, had an effect opposite to those of all of the MAbs tested and enhanced SF162 binding to MAb 2909 by 39%. All MAbs tested, with the exception of MAb 1418, bound to the virus; as shown in Fig. 3A, the six anti-HIV MAbs could all bind significant levels of SF162, although MAbs 2909 and 447 were more efficient than the anti-CD4bd, anti-V2, and anti-C5 MAbs, as previously shown (30). The specificity of the competition assay is further demonstrated by the dose-dependent inhibitory or enhancing effects of the various MAbs when they were preincubated with SF162 virions at MAb concentrations ranging from 0.001 to 10 μg/ml (Fig. 3C).
The ELISA-based competition experiments suggested that the epitope recognized by MAb 2909 was composed of parts of three regions of gp120: the CD4bd and the V2 and V3 loops. Contribution of the CD4bd to the formation of the 2909 epitope might be dominant, as MAb b12 and sCD4 were most effective in competing with 2909 for binding to SF162. The enhancing effect of 2G12 on SF162 binding to 2909 is more difficult to explain but may be due to 2G12-induced conformational changes in gp120. This effect may be indirect, as 2G12 binds to an epitope dependent on glycosylation sites N295 in the C2 region, N332 in the C3 region, and N392 in the V4 loop (36).
To determine directly the contribution of the V2 and V3 loops to the 2909 epitope, the binding of MAb 2909 to three previously described mutants of SF162 was studied (37). As shown in Fig. 4A, MAb 2909 strongly bound to intact virions of wild-type SF162 and to mutants lacking the V1 region (DV1) but did not react at all with mutants lacking V2 (DV2) or V3 (DV3). Simultaneous control experiments using nine additional human anti-HIV MAbs directed against V2, V3, CD4bd, C5, and gp41 confirmed the presence of these regions on the wild-type and DV1 forms or SF162 and the absence of the V2 and V3 epitopes from DV2 and DV3 mutants, respectively (Fig. 4A).
The participation of V2 but not V1 in the 2909 epitope was confirmed using an immunoprecipitation technique. Intact wild-type SF162 virions and SF162 mutants (200 to 400 ng of p24 per ml) were incubated with human MAbs overnight at room temperature. The complexes were precipitated with protein G-Sepharose. After being washed with phosphate-buffered saline, the precipitate was resuspended in Laemmli buffer, boiled for 5 min, and then subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and electroblotted onto a nitrocellulose membrane. Membranes were immunoblotted with biotinylated anti-C5 MAb 1331A (2) and streptavidin conjugated with alkaline phosphatase. The proteins were detected using an enhanced-chemiluminescence detection system (Pierce, Rockford, Ill.) and then exposed to film. MAb 2909 reacted with SF162 and DV1 but not with DV2, while MAb 447 (anti-V3) bound to all three viruses. MAb1418, used as negative control, did not react with any of the viruses (Fig. 4B). The MAbs 2909 and 447 immunoprecipitated whole virions, not only the envelope glycoproteins which may have been shed from virions, since p24 was also coprecipitated and detected by biotinylated anti-p24 human MAb 241 (29; data not shown).
Conclusions.
An HIV neutralization assay was implemented as a screening tool with the specific purpose of selecting MAbs with neutralizing activities that preferentially target structures found on intact virus particles that may not exist on soluble viral proteins. The first MAb produced using this technique was MAb 2909, which has the characteristics that were sought: high neutralizing activity for primary isolate SF162 and specificity for a complex epitope consisting of V2, V3, and the CD4bd that is present exclusively on the surfaces of intact virions but not on soluble viral proteins.
From models of gp120 based on crystallographic data, all three envelope regions that contribute to the 2909 epitope are located close to each other on the neutralizing face of monomeric gp120 (23). Additional experimental data based on immunochemical analyses and the effect of envelope mutations on virus infectivity and tropism demonstrate interactions between V3 and C4 (a dominant part of the CD4bd) (27, 41), between V3 and V2 (21, 32), and between V2 and the CD4bd (26, 35).
The data presented here suggest that the relationship between V2, V3, and the CD4bd in the monomeric form of gp120 is further changed upon the oligomerization of the envelope proteins on the surface of the virus, resulting in the formation of an entirely new epitope. The existence of quaternary epitopes associated with trimeric envelope spikes was previously suggested by studies of polyclonal sera of chimpanzees infected with primary HIV isolate DH012; the epitope of the neutralizing Abs in that study was highly conformational, involving elements contributed by all of the variable domains of gp120. It was suggested that such an epitope might require the interaction of adjacent gp120 molecules since V1/V2 and V4, the most critical elements forming the epitope, are located on opposing sides of monomeric gp120 (7, 8). Similarly, the epitope recognized by MAb 2909 may be formed by the quaternary interactions of separate gp120 molecules in the trimeric protein spike on the surface of the virion.
The unusually high potency of MAb 2909 for SF162; the shallow shape of the neutralization curve (Fig. 1A); the involvement of V2, V3, and the CD4bd in the formation of the 2909 epitope; and the participation of these regions of the envelope in binding to the receptor and coreceptor on target cells suggest that MAb 2909 may block virus infectivity by preventing virus binding to both CD4 and the chemokine receptors. The data further suggest that the virus binding site either (i) is a small area, equal to that which can be recognized by an antibody paratope (∼700 to 900 Å2 [40]), a concept that complies with the constitutive association of CD4 and CCR5 in the cell membrane (38, 42), and/or (ii) is protected by overlapping flaps, i.e., variable loops, in the oligomeric form of the envelope glycoprotein spike. The latter hypothesis suggests that HIV utilizes modes employed by other viruses to protect their receptor binding sites and may explain the generally poor binding to intact virus particles of MAbs specific for the CD4bd and the CD4i epitope found in the gp120 bridging sheet (9, 24, 30).
Finally, the existence of a reagent that defines the native oligomeric form of gp120, and the promise of more such reagents selected with the new screening technique, may prove exceptionally useful in determining which candidate vaccines based on recombinant oligomers possess biologically and immunologically relevant structures, a potential criterion for accelerated movement through the vaccine pipeline.
Acknowledgments
This study was supported in part by NIH grants HL59725 and AI36085 (S.Z.-P), and AI47708 (L.S.); by the Immunology Core of the NYU Center for AIDS Research (NIH grant AI27742); and by research funds from the Department of Veterans Affairs.
REFERENCES
- 1.Andrus, L., A. M. Prince, I. Bernal, P. McCormack, D. H. Lee, M. K. Gorny, and S. Zolla-Pazner. 1998. Passive immunization with a human immunodeficiency virus type-1 neutralizing monoclonal antibody in Hu-PBL-SCID mice: isolation of a neutralization escape variant. J. Infect. Dis. 177:889-897. [DOI] [PubMed] [Google Scholar]
- 2.Bandres, J. C., Q. F. Wang, J. O'Leary, F. Baleaux, A. Amara, J. A. Hoxie, S. Zolla-Pazner, and M. K. Gorny. 1998. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J. Virol. 72:2500-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, A. Jungbauer, and H. Katinger. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10:359-369. [DOI] [PubMed] [Google Scholar]
- 4.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 5.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. N. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, B. K., K. Saksela, R. Andino, and D. Baltimore. 1994. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 68:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C. H., L. Jin, C. Zhu, S. Holz-Smith, and T. J. Matthews. 2001. Induction and characterization of neutralizing antibodies against a human immunodeficiency virus type 1 primary isolate. J. Virol. 75:6700-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho, M. W., M. K. Lee, C. H. Chen, T. Matthews, and M. A. Martin. 2000. Identification of gp120 regions targeted by a highly potent neutralizing antiserum elicited in a chimpanzee inoculated with a primary human immunodeficiency virus type 1 isolate. J. Virol. 74:9749-9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimmock, N. J. 1993. Neutralization of animal viruses. Curr. Top. Microbiol. Immunol. 183:1-149. [DOI] [PubMed] [Google Scholar]
- 10.Emini, E. A., W. A. Schleif, J. H. Nunberg, A. J. Conley, Y. Eda, S. Tokiyoshi, S. D. Putney, S. Matsushita, K. E. Cobb, C. M. Jett, J. W. Eichberg, and K. K. Murthy. 1992. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature 355:728-730. [DOI] [PubMed] [Google Scholar]
- 11.Gauduin, M. C., J. T. Safrit, R. Weir, M. S. Fung, and R. A. Koup. 1995. Pre- and postexposure protection against human immunodeficiency virus type 1 infection mediated by a monoclonal antibody. J. Infect. Dis. 171:1203-1209. [DOI] [PubMed] [Google Scholar]
- 12.Gigler, A., S. Dorsch, A. Hemauer, C. Williams, S. Kim, N. S. Young, S. Zolla-Pazner, H. Wolf, M. K. Gorny, and S. Modrow. 1999. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J. Virol. 73:1974-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorny, M. K., V. Gianakakos, S. Sharpe, and S. Zolla-Pazner. 1989. Generation of human monoclonal antibodies to HIV. Proc. Natl. Acad. Sci. USA 86:1624-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorny, M. K., J. P. Moore, A. J. Conley, S. Karwowska, J. Sodroski, C. Williams, S. Burda, L. J. Boots, and S. Zolla-Pazner. 1994. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J. Virol. 68:8312-8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorny, M. K., K. Revesz, C. Williams, B. Volsky, M. K. Louder, C. A. Anyangwe, C. P. Krachmarov, S. C. Kayman, A. Pinter, A. Nadas, P. N. Nyambi, J. R. Mascola, and S. Zolla-Pazner. 2004. The V3 loop is accessible on the surface of most human immunodeficiency virus type 1 primary isolates and serves as a neutralization epitope. J. Virol. 78:2394-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorny, M. K., C. Williams, B. Volsky, K. Revesz, S. Cohen, V. R. Polonis, W. J. Honnen, S. C. Kayman, C. P. Krachmarov, A. Pinter, and S. Zolla-Pazner. 2002. Human monoclonal antibodies specific for conformation-sensitive epitopes of V3 neutralize HIV-1 primary isolates from various clades. J. Virol. 76:9035-9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorny, M. K., J.-Y. Xu, V. Gianakakos, S. Karwowska, C. Williams, H. W. Sheppard, C. V. Hanson, and S. Zolla-Pazner. 1991. Production of site-selected neutralizing human monoclonal antibodies against the third variable domain of the HIV-1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA 88:3238-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorny, M. K., J.-Y. Xu, S. Karwowska, A. Buchbinder, and S. Zolla-Pazner. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J. Immunol. 150:635-643. [PubMed] [Google Scholar]
- 19.Ho, D. D., M. S. Fung, Y. Z. Cao, X. L. Li, C. Sun, T. W. Chang, and N. C. Sun. 1991. Another discontinuous epitope on glycoprotein gp120 that is important in human immunodeficiency virus type 1 neutralization is identified by a monoclonal antibody. Proc. Natl. Acad. Sci. USA 88:8949-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeffs, S. A., M. K. Gorny, C. Williams, K. Revesz, B. Volsky, S. Burda, X. H. Wang, J. Bandres, S. Zolla-Pazner, and H. Holmes. 2001. Characterization of human monoclonal antibodies selected with a hypervariable loop-deleted recombinant HIV-1(IIIB) gp120. Immunol. Lett. 79:209-213. [DOI] [PubMed] [Google Scholar]
- 21.Koito, A., L. Stamatatos, and C. Cheng-Mayer. 1995. Small amino acid sequence changes within the V2 domain can affect the function of a T-cell line-tropic human immunodeficiency virus type 1 envelope gp120. Virology 206:878-884. [DOI] [PubMed] [Google Scholar]
- 22.Krachmarov, C. P., S. C. Kayman, W. J. Honnen, O. Trochev, and A. Pinter. 2001. V3-specific polyclonal antibodies affinity purified from sera of infected humans effectively neutralize primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 17:1737-1748. [DOI] [PubMed] [Google Scholar]
- 23.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labrijn, A. F., P. Poignard, A. Raja, M. B. Zwick, K. Delgado, M. Franti, J. Binley, V. Vivona, C. Grundner, C. C. Huang, M. Venturi, C. J. Petropoulos, T. Wrin, D. S. Dimitrov, J. Robinson, P. D. Kwong, R. T. Wyatt, J. Sodroski, and D. R. Burton. 2003. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J. Virol. 77:10557-10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ly, A., and L. Stamatatos. 2000. V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J. Virol. 74:6769-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore, J. P., M. Thali, B. A. Jameson, F. Vignaux, G. K. Lewis, S. W. Poon, M. Charles, M. S. Fung, B. Sun, P. J. Durda, L. Akerblom, B. Wahren, D. D. Ho, Q. J. Sattentau, and J. Sodroski. 1993. Immunochemical analysis of the gp120 surface glycoprotein of human immunodeficiency virus type 1: probing the structure of the C4 and V4 domains and the interaction of the C4 domain with the V3 loop. J. Virol. 67:4785-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyambi, P. N., M. K. Gorny, L. Bastiani, G. van der Groen, C. Williams, and S. Zolla-Pazner. 1998. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J. Virol. 72:9384-9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyambi, P. N., H. A. Mbah, S. Burda, C. Williams, M. K. Gorny, A. Nadas, and S. Zolla-Pazner. 2000. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J. Virol. 74:7096-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohagen, A., A. Devitt, K. J. Kunstman, P. R. Gorry, P. P. Rose, B. Korber, J. Taylor, R. Levy, R. L. Murphy, S. M. Wolinsky, and D. Gabuzda. 2003. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J. Virol. 77:12336-12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinter, A., W. J. Honnen, Y. He, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2004. The V1/V2 domain of gp120 is a global regulator of sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 78:5205-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posner, M. R., T. Hideshima, T. Cannon, M. Mukherjee, K. H. Mayer, and R. Byrn. 1991. An IgG human monoclonal antibody that reacts with HIV-1/GP120, inhibits virus binding to cells, and neutralizes infection. J. Immunol. 146:4325-4332. [PubMed] [Google Scholar]
- 34.Prince, A. M., H. Reesink, D. Pascual, B. Horowitz, I. Hewlett, K. K. Murthy, K. E. Cobb, and J. W. Eichberg. 1991. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS Res. Hum. Retrovir. 7:971-973. [DOI] [PubMed] [Google Scholar]
- 35.Roben, P., J. P. Moore, M. Thali, J. Sodroski, C. F. Barbas III, and D. R. Burton. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68:4821-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of α1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamatatos, L., and C. Cheng-Mayer. 1998. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J. Virol. 72:7840-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staudinger, R., S. K. Phogat, X. Xiao, X. Wang, D. S. Dimitrov, and S. Zolla-Pazner. 2003. Evidence for CD4-enhanced signaling through the chemokine receptor CCR5. J. Biol. Chem. 278:10389-10392. [DOI] [PubMed] [Google Scholar]
- 39.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson, I. A., and R. L. Stanfield. 1993. Antibody-antigen interactions. Curr. Opin. Struct. Biol. 3:113-118. [DOI] [PubMed] [Google Scholar]
- 41.Wyatt, R., M. Thali, S. Tilley, A. Pinter, M. Posner, D. Ho, J. Robinson, and J. Sodroski. 1992. Relationship of the human immunodeficiency virus type 1 gp120 third variable loop to a component of the CD4 binding site in the fourth conserved region. J. Virol. 66:6997-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao, X., L. Wu, T. S. Stantchev, Y. R. Feng, S. Ugolini, H. Chen, Z. Shen, J. L. Riley, C. C. Broder, Q. J. Sattentau, and D. S. Dimitrov. 1999. Constitutive cell surface association between CD4 and CCR5. Proc. Natl. Acad. Sci. USA 96:7496-7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zolla-Pazner, S., J. O'Leary, S. Burda, M. K. Gorny, M. Kim, J. Mascola, and F. E. McCutchan. 1995. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J. Virol. 69:3807-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]