Abstract
OBJECTIVES:
Although opioids are frequently used to treat pain, and are an important risk for ICU delirium, the association between ICU pain itself and delirium remains unclear. We sought to evaluate the relationship between ICU pain and delirium.
DESIGN:
Prospective cohort study.
SETTING:
A 32-bed academic medical-surgical ICU.
PATIENTS:
Critically ill adults (n = 4,064) admitted greater than or equal to 24 hours without a condition hampering delirium assessment.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Daily mental status was classified as arousable without delirium, delirium, or unarousable. Pain was assessed six times daily in arousable patients using a 0–10 Numeric Rating Scale (NRS) or the Critical Care Pain Observation Tool (CPOT); daily peak pain score was categorized as no (NRS = 0/CPOT = 0), mild (NRS = 1–3/CPOT = 1–2), moderate (NRS = 4–6/CPOT = 3–4), or severe (NRS = 7–10/CPOT = 5–8) pain. To address missingness, a Multiple Imputation by Chained Equations approach that used available daily pain severity and 19 pain predictors was used to generate 25 complete datasets. Using a first-order Markov model with a multinomial logistic regression analysis, that controlled for 11 baseline/daily delirium risk factors and considered the competing risks of unarousability and ICU discharge/death, the association between peak daily pain and next-day delirium in each complete dataset was evaluated.
RESULTS:
Among 14,013 ICU days (contributed by 4,064 adults), delirium occurred on 2,749 (19.6%). After pain severity imputation on 1,818 ICU days, mild, moderate, and severe pain were detected on 2,712 (34.1%), 1,682 (21.1%), and 894 (11.2%) of the no-delirium days, respectively, and 992 (36.1%), 513 (18.6%), and 27 (10.1%) of delirium days (p = 0.01). The presence of any pain (mild, moderate, or severe) was not associated with a transition from awake without delirium to delirium (aOR 0.96; 95% CI, 0.76–1.21). This association was similar when days with only mild, moderate, or severe pain were considered. All results were stable after controlling for daily opioid dose.
CONCLUSIONS:
After controlling for multiple delirium risk factors, including daily opioid use, pain may not be a risk factor for delirium in the ICU. Future prospective research is required.
Keywords: critical care, delirium, intensive care, Multiple Imputation by Chained Equations, opioid, pain
KEY POINTS
Question: Although opioids are frequently used to treat pain and are an important risk for ICU delirium, the association between ICU pain and delirium remains unclear. We sought to evaluate the relationship between ICU pain and delirium.
Findings: The presence of any pain (mild, moderate, or severe) was not associated with a daily transition from being awake without delirium to delirium. This result was stable after controlling for daily opioid exposure.
Meaning: After carefully accounting for data missingness, and rigorously accounting for multiple baseline and daily variables known to increase delirium, pain does not appear to be a risk factor for delirium in this cohort of critically ill adults.
Delirium occurs in up to 50% of critically ill adults and may result in serious ICU and post-ICU complications (1–3). A number of predisposing (e.g., age) and precipitating (e.g., illness severity) factors affect ICU delirium risk (4, 5). In a prior analysis of 4,075 adults, we reported daily opioid exposure to be a strong risk factor for developing delirium on the next ICU day (6). Although this analysis adjusted for maximum daily pain severity, it did not evaluate the independent relationship between level of daily ICU pain and next-day delirium. Furthermore, pain scores were found to be missing on 13.4% of the ICU days where the patient was arousable (i.e., without coma) and could be evaluated for pain.
Pain is highly individualized, can arise from different sources, and lead to variable patient responses (7). Disorientation, hyperarousability, and reduced cognition are manifestations that may be observed with pain and also with delirium (5, 8). Although postsurgical pain has been reported to be associated with increased delirium in adults who are not critically ill (9, 10), the relationship between pain and delirium in the ICU has been poorly researched. Pain in the ICU is usually more complex to detect and manage than on the floor given patient communication is often impaired, mental status is frequently altered, and disease severity commonly fluctuates (5, 11, 12). With pain usually being modifiable, the relationship between pain and delirium in critically ill adults is important to evaluate.
After using a Multiple Imputation by Chained Equations (MICEs) approach to address all pain and other data missingness (13, 14), we evaluated the association between ICU pain and next-day delirium occurrence, controlling for multiple baseline and daily ICU variables, including opioid use, that could affect this relationship.
MATERIALS AND METHODS
Study Population
Data for our study were prospectively collected by research personnel for patients admitted greater than or equal to 24 hours to the 32-bed mixed medical-surgical ICU at the University Medical Center (UMC) Utrecht (Utrecht, NL) from August 2011 to June 2013 and from May 2015 to March 2019. Research personnel were not available to collect data from June 2013 to April 2015. This study cohort has previously been used to evaluate the risk of ICU delirium with opioids (6), benzodiazepines (15), and corticosteroids (16). Patients were excluded if they had an acute neurologic condition precluding delirium assessment, were transferred from another ICU, or were managed with comfort care measures only. This research was approved by the UMC Utrecht Institutional Review Board (IRB) (number 010/056/c) on March 21, 2022. The need for informed consent was waived due to the retrospective nature of the research. All research procedures were conducted in accordance with the ethical standards of the Mass General Brigham IRB and the Helsinki Declaration of 1975 as most recently amended. The reporting of this study adheres to the STrengthening and Reporting of OBservational studies in Epidemiology statement (Supplemental Table 1, http://links.lww.com/CCX/B278) (17).
Exposures, Outcomes, and Other Variables
Pain, the primary study exposure, was assessed and recorded six times daily by the bedside nurse with a 0–10 Numeric Rating Scale (NRS) (18) if the patient was able to self-report (i.e., was wakeful) or the 0–8 Critical Care Pain Observation Tool (CPOT) if the patient was unable to self-report (5, 19). The highest daily pain score was categorized into four pain severity categories: no clinically significant pain (NRS = 0/CPOT = 0), mild pain (NRS = 1–3/CPOT = 1–2), moderate pain (NRS = 4–6/CPOT = 3–4), and severe pain (NRS = 7–10/CPOT = 5–8) (5, 20, 21). If a patient had both NRS and CPOT score results available on the same day, pain severity was based on the highest daily NRS score given patient’s self-report is the primary reference standard for pain measurement (22).
Patient wakefulness was evaluated using the Richmond Agitation and Sedation Scale (RASS) every 3 hours by bedside nurses, with a RASS less than or equal to –4 denoting an unarousable state (23). Delirium was assessed by the bedside nurses at least twice daily using the Confusion Assessment Method for the ICU (CAM-ICU) (24). Because a positive CAM-ICU is highly predictive for delirium (25), patients were classified as delirious on the day when greater than or equal to 1 CAM-ICU was positive. Individuals without delirium were then classified based on whether their RASS assessment qualified them as unarousable. The trained researcher assessed the mental status daily by reviewing all documented CAM-ICU scores and any delirium treatment that was initiated based on a previously validated algorithm (26). Mental status on each ICU day t was classified as: 1) awake without delirium, 2) delirium, or 3) an unarousable state. Mental status on each ICU day t + 1 (i.e., the next ICU day) was categorized as: 1) awake without delirium, 2) delirium, 3) an unarousable state, or 4) ICU discharge/death. In an effort to focus on the acute phase of critical illness (noting < 1% of patients [n = 22] first transitioned from awake without delirium to delirium after day 7), only data from the first seven ICU days were included.
Demographics, the presence of comorbidities, ICU admission characteristics (type of admission and admission diagnosis), daily physiologic measurements, and vital signs were prospectively collected by trained physicians (6). Daily severity of illness was assessed using the modified Sequential Organ Failure Assessment (mSOFA); the neurologic component was excluded to avoid adjusting for a component of the primary outcome (27). Agitation was defined as a RASS greater than or equal to +2 (5, 23). Medication data on opioid, benzodiazepine, clonidine, and gabapentin use were retrieved (28). All administered opioids were converted into IV milligrams of morphine-equivalent (MEQ) doses (Supplemental Table 2, http://links.lww.com/CCX/B278) (29–31). High opioid use at the time of ICU admission was defined as day 1 ICU opioid exposure greater than or equal to 25 IVMEQ, the median ICU daily value.
Multiple Imputation by Chained Equation Approach
We assumed ICU pain data missingness was related to observed, not unobserved values, and based on Strengthening Analytical Thinking For Observational Studies recommendations, chose MICE, rather than complete-case analysis or simple imputation, as the most appropriate approach to address this missingness (32). Through literature review and investigator consensus (5, 6, 12), we identified 14 reported predictors for pain: 1) baseline (admission): admission type, trauma admission, diagnosis of sepsis, and high opioid use, 2) ICU day t (each ICU day): mSOFA score, invasive mechanical ventilation use, agitation, opioid, clonidine, or gabapentin use, and 3) ICU day t – 1 (1 day before each ICU day): maximum daily pain severity, NRS (patient self-report) use only, CPOT (behavioral assessment by nurses only) use only, and presence of an unarousable state. These 14 predictors were combined with the remaining five baseline delirium risk variables from the prior Markov opioid delirium-risk model article (i.e., age, gender, Acute Physiology and Chronic Health Evaluation [APACHE] IV (33), Charlson Comorbidity Index (CCI) (34), and body mass index) (6) and imputed when daily pain severity was missing on any of the first seven complete ICU days (Supplemental Table 3, http://links.lww.com/CCX/B278). We excluded days spent in an unarousable state on day t for the purpose of imputation (but not for the Markov modeling) because no valid pain assessment methods exist for the unarousable patient. On these days we discarded the imputed values and kept the original observed values. Besides pain, missingness in any of the 19 predictors was also replaced with an imputed value. For variables with missingness on ICU day 1, both baseline and day t predictors were used.
The APACHE IV score and CCI were missing for 8.8% and 13.0% of patients, respectively. Pain severity was missing on 13.4% of unarousable (RASS ≥ –3) days. No other variable was missing for greater than 1% of ICU days. We then generated 25 imputed (i.e., “completed”) datasets using open-source R packages for MICE (35). Within the MICE procedure, logistic regression was used to impute binary variables, proportional odds model to impute ordinal variables, and predictive mean matching to impute continuous variables. The MICE algorithm involved multiple iterative series of imputing missing values with random draws from those subjects with observed values (13). We visually checked for convergence by plotting the mean and sd of the imputed values and found convergence was achieved. We studied the distribution of imputed versus observed data for each prediction and observed the distributions to be similar, thus indicating our imputation models fit the dataset well.
Markov Model With Multinomial Logistic Regression Analysis
To assess the association between day t pain severity and (next-day) mental status on day t + 1 for ICU days 1–7, a first-order Markov model with multinomial logistic regression analysis that controlled for each of the four possible next-day outcomes (awake without delirium, delirium, unarousable, and ICU discharge/death) and the same 11 covariables that could influence delirium occurrence used in the 2021 analysis (6, 32) (baseline: ICU admission type, age, gender, APACHE IV score (34), CCI (34) and body mass index; ICU day t: ICU day, mSOFA score (27), mechanical ventilator use, benzodiazepine use, and opioid use). Although the daily transition from being awake without delirium on day t to next-day delirium on day t + 1 was our main outcome of interest we also incorporated other daily mental status transitions within the statistical models (Supplemental Fig. 1, http://links.lww.com/CCX/B278). The daily “transition” from awake without delirium to awake without delirium (i.e., remaining awake without delirium) served as the reference standard.
Our primary pain-delirium analysis used the presence of any pain (i.e., mild, moderate, or severe) versus no pain on day t. Secondary analyses included the presence of either moderate or severe pain versus no or mild pain, severe pain only versus no/mild/moderate pain, severe pain only versus no pain, moderate pain only versus no pain, and mild pain only versus no pain. The exposure of each combined or individual pain severity measure in the primary or secondary analyses was modeled using an interaction term of pain presence (yes/no) on day t and mental status on day t. In the Markov models on arousable days missing pain severity was replaced with the imputed value and on unarousable days missing pain severity was deemed to be a no pain day (given the patient on this ICU day was excluded from MICE).
The outcomes of discharge alive from the ICU and death were combined into one category in the Markov model, given these represented few of the total daily transitions and neither was the outcome of interest (15, 32). Each Markov model was run for each of the 25 datasets generated from the MICE approach (36). The final results were obtained by pooling the estimates across the 25 datasets to obtain a single-point estimate for each variable using Rubin’s rule (37). For each of the six pain severity analyses, we performed three models for each pain severity level (i.e., inclusion of opioid use [yes or no], inclusion of opioid dose [by 10 IVMEQ], and exclusion of opioid use or dose). These pain severity models were run for both the inclusion and exclusion of opioids given patients in the ICU may receive opioids for nonpain reasons (e.g., sedation or to reduce respiratory drive).
We conducted three sensitivity analyses. Given complete-case analysis is frequently used to eliminate data missingness, we compared our results using complete-case analysis (vs. imputation using MICE). Given pain severity cannot be directly compared between NRS and CPOT-derived assessments (38) and therefore pain severity may be different based on the assessment instrument used, using the complete-case analysis cohort, we compared our results between ICU days where NRS (vs. CPOT) were used. Given that ICU practices focused on delirium recognition, prevention, and treatment may have incrementally changed over the 9-year study period, the cohort was divided into 3-year epochs to evaluate the stability of lack of a pain-delirium association we report over time (5, 39). A p value of less than 0.05 was deemed significant for all analyses. All analyses were performed using R, Version 4.0.3 (R Foundation for Statistical Computing, 2020).
RESULTS
Study Cohort Characteristics
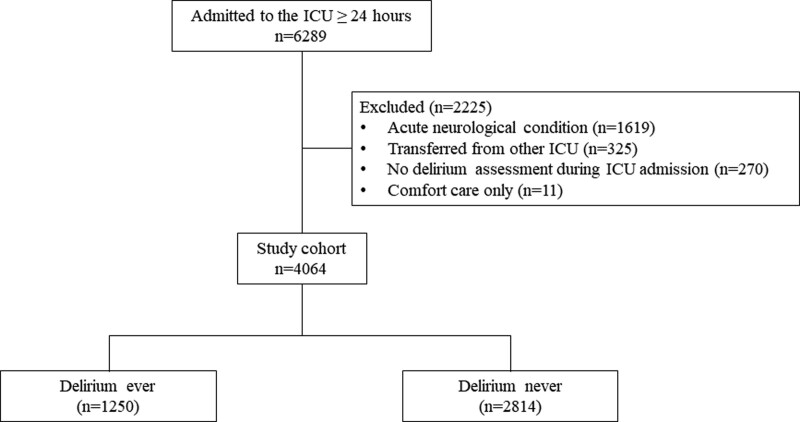
A total of 4,064 patients were included after patients with an acute neurologic condition (n = 1,619), who were transferred from an outside ICU (n = 325), had no delirium assessment during their entire ICU stay (n = 270), or were receiving comfort care measures only (n = 11) were excluded (Fig. 1). Data missingness before-and-after MICE use is described in Supplemental Table 4 (http://links.lww.com/CCX/B278). After MICE, most of the patients (n = 2,582; 63.5%) were male with a median (interquartile range [IQR]) age of 64 (53–72), a median APACHE IV of 55 (IQR 40–76), and a median maximum ICU mSOFA score of 6 (IQR 4–9) (Table 1). Most patients (91.2%) were invasively mechanically ventilated. Across all ICU days, 60.1% experienced pain (based on either NRS or CPOT assessments), and 83.3% received an opioid for a median of 2 days (IQR 1–4). The proportion of cohort patients with pain and each level of pain severity was not different after MICE use (Supplemental Table 5, http://links.lww.com/CCX/B278).
Figure 1.
Flowchart of the study cohort.
TABLE 1.
Characteristics of the Study Population During ICU Days 1–7 Based on Delirium Occurrence After Multiple Imputation by Chained Equations Use
| Total (n = 4,064) | Delirium Ever (n = 1,250) | Delirium Never (n = 2,814) | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, median (IQR), yr | 64 (53–72) | 65 (55–74) | 63 (51–71) |
| Sex, male, n (%) | 2,582 (63.5) | 832 (66.6) | 1,750 (62.2) |
| Charlson Comorbidity Scale Score, median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) |
| Body mass index, median (IQR) | 25.7 (22.9–29.1) | 25.5 (22.9–28.7) | 25.7 (22.8–29.3) |
| Admission type, n (%) | |||
| Medical | 1,531 (37.7) | 581 (46.5) | 950 (33.8) |
| Elective surgery | 1,744 (42.9) | 360 (28.8) | 1,384 (49.2) |
| Acute surgery | 789 (19.4) | 309 (24.7) | 480 (17.1) |
| Acute Physiology and Chronic Health Evaluation IV score, median (IQR) | 55 (40–76) | 68 (52–85) | 50 (36–69) |
| Daily ICU characteristics | |||
| Days of no pain, median (IQR) | 1 (1–3) | 2 (1–3) | 1 (1–2) |
| Presence of any pain, n (%) | 2,444 (60.1) | 744 (59.5) | 1,699 (60.4) |
| Days of mild pain, median (IQR) | 1.0 (1.0–2.0) | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) |
| Days of moderate pain, median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Days of severe pain, median (IQR) | 1.0 (1.0–1.0) | 1 (1–1.6) | 1.0 (1.0–1.0) |
| Maximum modified Sequential Organ Failure Assessment score, median (IQR) | 6 (4-9) | 8 (7–11) | 6 (4–8) |
| Use of invasive mechanical ventilation, n (%) | 3,734 (91.2) | 1,199 (95.9) | 2,535 (90.1) |
| Days of invasive mechanical ventilation, median (IQR) | 2 (1–5) | 5 (2–7) | 2 (1–3) |
| Benzodiazepine use, n (%) | 1982 (48.8) | 833 (66.6) | 1149 (40.8) |
| Days of benzodiazepine use, median (IQR) | 2(1–3) | 2 (1–4) | 1 (1–2) |
| Opioid use, n (%) | 3,384 (83.3) | 1,092 (87.4) | 2,292 (81.4) |
| Days of opioid use, median (IQR) | 2 (1–4) | 4 (2–6) | 1 (1–3) |
IQR = interquartile range.
Markov Modeling and Multinomial Regression Analyses
On the 14,013 ICU patient-days contributed by 4,064 patients (Fig. 1), delirium occurred in 1,250 (30.8%) of patients (Table 1) on 2,749 (19.6%) of their ICU days (Table 2). Patients with ICU delirium had higher APACHE IV (median [IQR], 68 [52–85] vs. 50 [36–69]) and mSOFA (8 [7–11] vs. 6 [4–8]) scores and spent more days mechanically ventilated (95.9% vs. 90.1%) or receiving benzodiazepine (66.6% vs. 40.8%) or opioid (87.4% vs. 81.4%) therapy. Although the overall presence of pain was similar between the delirium and no-delirium groups, the days spent at each pain severity category were different. On days with delirium (vs. no delirium) when pain was detected, the occurrence of mild pain was greater (992 [36.1%] vs. 2,712 [34.1%], p < 0.01), moderate pain less (513 [18.6%] vs. 1,682 [21.1%], p = 0.02) and severe pain was similar (277 [10.1%] vs. 894 [11.2%], p = 0.18) (Table 2).
TABLE 2.
Comparison of Individual ICU Days 1–7 Characteristics Between Each Mental State After Multiple Imputation by Chained Equations Use
| Mental Status Day t | ||||
|---|---|---|---|---|
| Characteristics on Day t | All ICU Patient-Days (n = 14,013) | Awake Without Delirium (n = 7,961) | Delirium (n = 2,749) | Unarousable Statea (n = 3,303) |
| No pain, n (%) | 4,747 (33.9) | 2,673 (33.6) | 967 (35.2) | 1,107 (33.5) |
| Mild pain, n (%) | 4,146 (29.6) | 2,712 (34.1) | 992 (36.1) | 442 (13.4) |
| Moderate pain, n (%) | 2,328 (16.6) | 1,682 (21.1) | 513 (18.6) | 133 (4.0) |
| Severe pain, n (%) | 1,211 (8.6) | 894 (11.2) | 277 (10.1) | 40 (1.2) |
| Modified Sequential Organ Failure Assessment, median (IQR) | 6 (4–8) | 5 (3–7) | 6 (4–9) | 8 (6–11) |
| Use of invasive mechanical ventilation, n (%) | 11,549 (82.4) | 6,075 (76.3) | 2,302 (83.7) | 3,172 (96.0) |
| Use of a benzodiazepine, n (%) | 4,666 (33.3) | 1,968 (24.7) | 908 (33.0) | 1,790 (54.2) |
| Use of any opioid, n (%) | 9,482 (67.7) | 4,892 (61.4) | 1,808 (65.8) | 2,782 (84.2) |
| Opioid dose (if any) in morphine-equivalent, median (IQR) | 24.0 (7.5–57.9) | 14.7 (5.6–42.0) | 24.7 (7.5–59.9) | 43.4 (22.5–191.5) |
IQR = interquartile range.
After Multiple Imputation by Chained Equations was performed, missing pain severity remained in 1,581 of 3,303 days (47.9%) where patient was unarousable.
Among the 7,961 ICU days patients were awake without delirium, 597 (7.5%) next-day transitions to delirium occurred. Any pain (either mild, moderate, or severe) versus no pain was found not to be associated with a transition from awake without delirium to delirium (adjusted odds ratio [aOR] 0.96; 95% CI, 0.76–1.21) (Table 3). Results were similar when days with only moderate or severe pain (aOR 0.91; 95% CI, 0.71–1.15), severe pain (aOR 0.94; 95% CI, 0.63–1.41), moderate pain (aOR 0.90; 95% CI, 0.66–1.24), or mild pain (aOR 1.00; 95% CI, 0.77–1.28) were separately evaluated. All results remained stable when either the daily opioid dose was controlled for or the covariate for opioid use was removed (Table 3). Our results remain unaffected by use of complete-case analysis (vs. MICE) (Supplemental Table 6, http://links.lww.com/CCX/B278), evaluation of pain using the NRS (vs. CPOT)(Supplemental Table 7, http://links.lww.com/CCX/B278) or by the 3-year study epoch patients were admitted to the ICU (Supplemental Table 8, http://links.lww.com/CCX/B278).
TABLE 3.
Pain Severity as a Risk Factor for the Transition to Delirium After Multiple Imputation by Chained Equations Use
| Mental Status | Pain | Controlling for Opioid Exposure and Dose | Adjusted ORa,b (95% CI), n = 14,013 | |
|---|---|---|---|---|
| Day t | Day t + 1 | |||
| Awake without delirium | Awake without delirium | No | Reference | Reference |
| Awake without delirium | Delirium | Mild/moderate/severe pain (vs. no pain) | No | 1.00 (0.80–1.26) |
| Yes | 0.96 (0.76–1.21) | |||
| 10 mg MEQ | 1.00 (0.80–1.26) | |||
| Awake without delirium | Delirium | Moderate/severe pain (vs. no/mild pain) | No | 0.95 (0.75–1.21) |
| Yes | 0.91 (0.71–1.15) | |||
| 10 mg MEQ | 0.95 (0.75–1.21) | |||
| Awake without delirium | Delirium | Severe pain (vs. no/mild/moderate pain) | No | 1.00 (0.69–1.44) |
| Yes | 0.95 (0.66–1.38) | |||
| 10 mg MEQ | 0.99 (0.69–1.44) | |||
| Awake without delirium | Delirium | Severe pain (vs. no pain) | No | 1.02 (0.69–1.52) |
| Yes | 0.94 (0.63–1.41) | |||
| 10 mg MEQ | 1.02 (0.68–1.52) | |||
| Awake without delirium | Delirium | Moderate pain (vs. no pain) | No | 0.95 (0.70–1.30) |
| Yes | 0.90 (0.66–1.24) | |||
| 10 mg MEQ | 0.94 (0.69–1.30) | |||
| Awake without delirium | Delirium | Mild pain (vs. no pain) | No | 1.02 (0.80–1.32) |
| Yes | 1.00 (0.77–1.28) | |||
| 10 mg MEQ | 1.02 (0.80–1.32) | |||
MEQ = morphine equivalent, OR = odds ratio.
Adjusted for time-fixed covariables, including admission category (medical, surgical, and trauma), age, sex, Acute Physiology and Chronic Health Evaluation IV score, body mass index, and Charlson Comorbidity Index.
Adjusted for time-varying covariables on day t, including day of ICU admission, modified Sequential Organ Failure Assessment score (without neurologic component), use of invasive mechanical ventilation, use of a benzodiazepine, and use of an opioid.
DISCUSSION
After employing a rigorous MICE approach to address data missingness, we found that exposure to any degree (severity) of pain may not be associated with a transition to delirium on the following day in awake nondelirious ICU patients in our cohort. Importantly, daily ICU opioid exposure did not change this association. Additionally, the pain-delirium association we report remained stable when complete-case analysis (vs. MICE) was used, pain was assessed with the NRS (vs. CPOT), and over the entire study period. Despite the methodological rigor of our time-dependent, multinomial analysis, our study should be considered exploratory in nature given it evaluated patients from only one center and the complex relationship between pain and delirium in critically ill adults. The results of our investigation need to be confirmed by additional multisite, prospective research.
Although we did not establish an epidemiologic association between pain and delirium in our analysis, future research is required to explore the potential mechanistic pathways between pain and delirium. When peripheral nociceptors are activated by noxious stimuli, pain signals are transmitted to the brain through neural circuits (40). Glutamate and norepinephrine, the primary neurotransmitters active during this process, have been shown to be associated with delirium (41). Furthermore, cortisol levels are elevated with both pain (given its role as a physiologic stressor) (42) and delirium (43). In cardiac surgery patients, the effect of cortisol on the hippocampus causes inattention and reduces cognition, two of the key symptoms associated with delirium (44). Acute pain is associated with reduced orientation and awareness; it remains unclear if this increases inattention (45). Pain-induced disrupted sleep increases delirium risk. Future research evaluating the relationship between pain and delirium in the ICU should consider these mechanistic pathways.
Importantly, even though our exploratory study suggests that a link between pain and delirium in critically ill adults may not exist, ICU clinicians should rigorously evaluate their patients for pain using valid assessment methods according to their capacity to communicate and treat it using guideline-driven, multimodal approaches when present (5, 46). In addition, all ICU patients should be regularly assessed for delirium; if delirium is present, modifiable delirium risk factors should be removed and evidence-based treatment practices should be administered (5).
Our results raise interesting questions as to why an association between pain and delirium has been found in investigations in perioperative and other non-ICU populations but not in critically ill adults (7, 8, 47–50). Unlike our analysis, many of these non-ICU studies failed to use time-dependent methods to confirm that pain occurred before delirium and did not account for the use of analgesics known to increase delirium (e.g., opioids). There may also be distinct factors in the ICU setting that affect the relationship between pain and delirium including a greater severity of illness, increased analgesic or sedative exposure, and a level of pain that frequently fluctuates (5, 6).
A secondary goal of our article is to encourage ICU researchers to carefully address and report data missingness, and if present, use rigorous methods like MICE to address it (50). Other approaches such as reducing datasets using a complete-case approach or simple imputation may result in biased estimates (13, 26, 51, 52). However, like all methods for missing data imputation, caution with MICE use must be exercised; missingness patterns must still meet missing at random (MAR) assumptions (i.e., missingness only deduced by observed values) (13, 14). In our cohort, we are confident a MAR assumption could be made given opioids are commonly used to treat or prevent pain in the ICU, and thus on days when patients were not receiving opioids they were less likely to have pain and pain would be less frequently recorded (i.e., greater missingness).
Our study has important strengths. We carefully addressed missing values and reasonably excluded imputed values for pain severity on days when patients were unarousable. In addition to its large sample size, patients were evaluated at least twice daily for delirium based on a validated assessment protocol, the model accounted for transitions to an unarousable state and to death/ICU discharge, and of the 11 model covariables considered, 5 were time-varying, which allowed us to consider daily changes in key confounding factors that could affect pain-delirium risk. We evaluated different daily pain severities and evaluated the effect of daily opioid exposure on our results.
Our study also has potential limitations. Although a validated method to assess pain in unarousable patients does not exist, the exclusion of patients on the ICU days they were unarousable from the imputation and the absence of pain in the Markov model on unarousable days may have affected our results. The CPOT score ranges we used for different levels of pain were proposed by one research team but never validated (30). Although we considered 14 predictors for pain in our MICE approach, other predictors for pain may be important. Although we evaluated the association between different maximum daily pain severity levels and delirium, we were not able to consider pain that may have rapidly changed over the course of a single ICU day or the degree of pain documented in the immediate period before delirium was positively assessed. Patient data were derived from one center; our results may be different at other hospitals. As with all observational studies, there might have been residual confounding. The majority of patients were surgical, the association between pain and delirium may be different in medical and trauma patients. We did not consider the potential causes for pain, the presence of symptoms associated with either pain or delirium, whether treatments for delirium were administered, or the presence of disrupted sleep. The very small number of patients who spent two consecutive days with severe pain while awake and without delirium and the limitations of averaging pain scores precluded us from evaluating the association between pain duration and delirium occurrence. Prospective evaluation of how long pain needs to be present before it potentially becomes a risk factor for delirium is an important area for future research.
CONCLUSIONS
After carefully accounting for data missingness, and rigorously accounting for multiple baseline and daily variables known to increase delirium, pain does not appear to be a risk factor for delirium in our cohort of critically ill adults. The results of our investigation should be confirmed by additional prospective multisite research.
Supplementary Material
Footnotes
Dr. Devlin disclosed he has received unrelated grant support from the National Institute of Aging, Agency for Healthcare Research and Quality, Sedana Medical, and BioXcel Therapeutics and has served as a consultant to BioExcel Therapeutics, Ceribell, and La Jolla Pharmaceuticals. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Drs. Wu, Vernooij, Duprey, Gélinas, Devlin, and A.F.C. conceived and designed the research. Drs. Zaal and Slooter were responsible for data acquisition. Drs. Wu, Vernooij, Duprey, and Devlin analyzed data. Drs. Wu and Devlin drafted the article. All authors approved the final article version.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Slooter AJC, Otte WM, Devlin JW, et al. : Updated nomenclature of delirium and acute encephalopathy: Statement of ten societies. Intensive Care Med 2020; 46:1020–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaal IJ, Slooter AJ: Delirium in critically ill patients: Epidemiology, pathophysiology, diagnosis and management. Drugs 2012; 72:1457–1471 [DOI] [PubMed] [Google Scholar]
- 3.Wolters AE, Slooter AJ, van der Kooi AW, et al. : Cognitive impairment after intensive care unit admission: A systematic review. Intensive Care Med 2013; 39:376–386 [DOI] [PubMed] [Google Scholar]
- 4.Zaal IJ, Devlin JW, Peelen LM, et al. : A systematic review of risk factors for delirium in the ICU. Crit Care Med 2015; 43:40–47 [DOI] [PubMed] [Google Scholar]
- 5.Devlin JW, Skrobik Y, Gelinas C, et al. : Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 6.Duprey MS, Dijkstra-Kersten SMA, Zaal IJ, et al. : Opioid use increases the risk of delirium in critically ill adults independently of pain. Am J Respir Crit Care Med 2021; 204:566–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeser JD, Treede RD: The Kyoto protocol of IASP basic pain terminology. Pain 2008; 137:473–477 [DOI] [PubMed] [Google Scholar]
- 8.Gelinas C, Boitor M, Puntillo K, et al. : Behaviors indicative of pain in brain-injured adult patient with different levels of consciousness in the intensive care unit. J Pain Symptom Manage 2019; 57:761–773 [DOI] [PubMed] [Google Scholar]
- 9.Vaurio LE, Sands LP, Wang Y, et al. : Postoperative delirium: The importance of pain and pain management. Anesth Analg 2006; 102:1267–1273 [DOI] [PubMed] [Google Scholar]
- 10.Lynch EP, Lazor MA, Gellis JE, et al. : The impact of postoperative pain on the development of postoperative delirium. Anesth Analg 1998; 86:781–785 [DOI] [PubMed] [Google Scholar]
- 11.Puntillo KA, Max A, Timsit JF, et al. : Determinants of procedural pain intensity in the intensive care unit: The Europain® study. Am J Respir Crit Care Med 2014; 189:39–47 [DOI] [PubMed] [Google Scholar]
- 12.Chanques G, Gelinas C: Monitoring pain in the intensive care unit. Intensive Care Med 2022; 48:1508–1511 [DOI] [PubMed] [Google Scholar]
- 13.Austin PC, White IR, Lee DS, et al. : Missing data in clinical research: A tutorial on multiple imputation. Can J Cardiol 2021; 37:1322–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JA, White IR, Carlin JB, et al. : Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009; 338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaal IJ, Devlin JW, Hazelbag M, et al. : Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med 2015; 41:2130–2137 [DOI] [PubMed] [Google Scholar]
- 16.Wolters AE, Veldhuijzen DS, Zaal IJ, et al. : Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med 2015; 43:e585–e588 [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative: Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007; 335:806–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chanques G, Viel E, Constantin JM, et al. : The measurement of pain in intensive care unit: Comparison of 5 self-report intensity scales. Pain 2010; 151:711–721 [DOI] [PubMed] [Google Scholar]
- 19.Gélinas C, Joffe AM, Szumita PM, et al. : A psychometric analysis update of behavioral pain assessment tools for noncommunicative, critically ill adults. AACN Adv Crit Care 2019; 30:365–387 [DOI] [PubMed] [Google Scholar]
- 20.Boonstra AM, Schiphorst Preuper HR, Balk GA, et al. : Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain 2014; 155:2545–2550 [DOI] [PubMed] [Google Scholar]
- 21.Severgnini P, Pelosi P, Contino E, et al. : Accuracy of critical care pain observation tool and behavioral pain scale to assess pain in critically ill conscious and unconscious patients: Prospective, observational study. J Intensive Care 2016; 4:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pain terminology: International Association for the Study of Pain (IASP). Available at: https://www.iasp-pain.org/resources/terminology/. Accessed May 12, 2023
- 23.Sessler CN, Gosnell MS, Grap MJ, et al. : The Richmond agitation-sedation scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166:1338–1344 [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Inouye SK, Bernard GR, et al. : Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286:2703–2710 [DOI] [PubMed] [Google Scholar]
- 25.van Eijk MM, van den Boogaard M, van Marum RJ, et al. : Routine use of the confusion assessment method for the intensive care unit: A multicenter study. Am J Respir Crit Care Med 2011; 184:340–344 [DOI] [PubMed] [Google Scholar]
- 26.Zaal IJ, Tekatli H, van der Kooi AW, et al. : Classification of daily mental status in critically ill patients for research purposes. J Crit Care 2015; 30:375–380 [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, de Mendonca A, Cantraine F, et al. : Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26:1793–1800 [DOI] [PubMed] [Google Scholar]
- 28.Varvel JR, Shafer SL, Hwang SS, et al. : Absorption characteristics of transdermally administered fentanyl. Anesthesiology 1989; 70:928–934 [DOI] [PubMed] [Google Scholar]
- 29.Patanwala AE, Duby J, Waters D, et al. : Opioid conversions in acute care. Ann Pharmacother 2007; 41:255–266 [DOI] [PubMed] [Google Scholar]
- 30.Kay B: A clinical investigation of piritramide in the treatment of postoperative pain. Br J Anaesth 1971; 43:1167–1171 [DOI] [PubMed] [Google Scholar]
- 31.Anderson R, Saiers JH, Abram S, et al. : Accuracy in equianalgesic dosing conversion dilemmas. J Pain Symptom Manage 2001; 21:397–406 [DOI] [PubMed] [Google Scholar]
- 32.Lee KJ, Tilling KM, Cornish RP, et al. ; STRATOS initiative: Framework for the treatment and reporting of missing data in observational studies: The treatment and reporting of missing data in observational studies framework. J Clin Epidemiol 2021; 134:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen PK, Geskus RB, de Witte T, et al. : Competing risks in epidemiology: Possibilities and pitfalls. Int J Epidemiol 2012; 41:861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerman JE, Kramer AA, McNair DS, et al. : Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today’s critically ill patients. Crit Care Med 2006; 34:1297–1310 [DOI] [PubMed] [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 36.van Buuren S, Groothuis-Oudshoorn K: Mice: Multivariate imputation by chained equations in R. J Stat Soft 2011; 45:1–67 [Google Scholar]
- 37.Rubin DB, Schenker N: Multiple imputation in health-care databases: An overview and some applications. Stat Med 1991; 10:585–598 [DOI] [PubMed] [Google Scholar]
- 38.Bouajram RH, Sebat CM, Love D, et al. : Comparison of self-reported and behavioral pain assessment tools in critically ill patients. J Intensive Care Med 2020; 35:453–460 [DOI] [PubMed] [Google Scholar]
- 39.Barr J, Fraser GL, Puntillo K, et al. ; American College of Critical Care Medicine: Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41:263–306 [DOI] [PubMed] [Google Scholar]
- 40.Peirs C, Seal RP: Neural circuits for pain: Recent advances and current views. Science 2016; 354:578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maldonado JR: Neuropathogenesis of delirium: Review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 2013; 21:1190–1222 [DOI] [PubMed] [Google Scholar]
- 42.Hannibal KE, Bishop MD: Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther 2014; 94:1816–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maclullich AM, Ferguson KJ, Miller T, et al. : Unravelling the pathophysiology of delirium: A focus on the role of aberrant stress responses. J Psychosom Res 2008; 65:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mu DL, Wang DX, Li LH, et al. : High serum cortisol level is associated with increased risk of delirium after coronary artery bypass graft surgery: A prospective cohort study. Crit Care 2010; 14:R238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Álvarez EA, Parada FJ: Association of pain during the evaluation of delirium in intensive care unit patients. Front Med (Lausanne) 2021; 8:722001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daoust R, Paquet J, Boucher V, et al. : Relationship between pain, opioid treatment, and delirium in older emergency department patients. Acad Emerg Med 2020; 27:708–716 [DOI] [PubMed] [Google Scholar]
- 47.Morrison RS, Magaziner J, Gilbert M, et al. : Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci 2003; 58:76–81 [DOI] [PubMed] [Google Scholar]
- 48.Kosar CM, Tabloski PA, Travison TG, et al. : Effect of perioperative pain and depressive symptoms on the development of postoperative delirium. Lancet Psychiatry 2014; 1:431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denny DL, Lindseth GN: Pain, opioid intake, and delirium symptoms in adults following joint replacement surgery. West J Nurs Res 2020; 42:165–176 [DOI] [PubMed] [Google Scholar]
- 50.Wu TT, Smith LH, Vernooij LM, et al. : Data missingness reporting and use of methods to address it in critical care cohort studies. Crit Care Explor 2023; 5:e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding X, Gao X, Chen Q, et al. : Preoperative acute pain is associated with postoperative delirium. Pain Med 2021; 22:15–21 [DOI] [PubMed] [Google Scholar]
- 52.Sharafoddini A, Dubin JA, Maslove DM, et al. : A new insight into missing data in intensive care unit patient profiles: Observational study. JMIR Med Inform 2019; 7:e11605. [DOI] [PMC free article] [PubMed] [Google Scholar]