Keywords: AKI, clinical epidemiology, pediatric intensive care medicine, pediatric nephrology
Abstract
Key Points
The incidence of AKI while undergoing ECMO in pediatric patients is high and independently increases mortality.
Laboratory markers consistent with intravascular hemolysis increase the hazard of a composite outcome of AKI or RRT while undergoing ECMO.
Further research into appropriate monitoring or treatment of ECMO-associated hemolysis may lead to important interventions to prevent AKI.
Background
AKI is common in patients requiring extracorporeal membrane oxygenation (ECMO), with a variety of proposed mechanisms. We sought to describe the effect of laboratory evidence of ECMO-associated intravascular hemolysis on AKI and RRT.
Methods
This retrospective cohort study included patients treated with ECMO at a single center over 10 years. The primary outcome was a composite of time to RRT or AKI (by creatinine-based Kidney Disease Improving Global Outcomes criteria) after ECMO start. Serum creatinine closest to ECMO start time was considered the pre-ECMO baseline and used to determine abnormal kidney function at ECMO start. The patient's subsequent creatinine values were used to identify AKI on ECMO. Multivariable cause-specific Cox proportional hazards models were used to assess the effect of separate markers of intravascular hemolysis on the time to the composite outcome after controlling for confounders.
Results
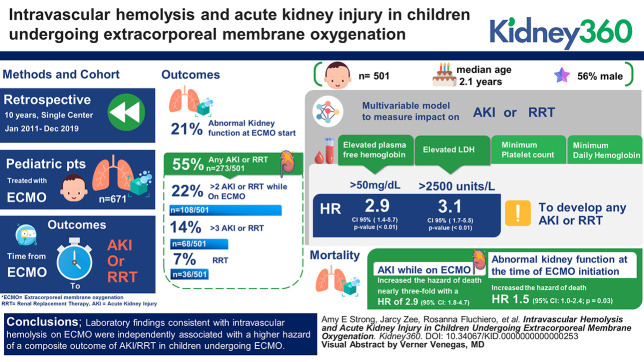
Five hundred and one children were evaluated with a median age 1.2 years, 56% male. Four separate multivariable models, each with a different marker of hemolysis (plasma-free hemoglobin, lactate dehydrogenase (LDH), minimum platelet count, and minimum daily hemoglobin), were used to examine the effect on the composite outcome of AKI/RRT. An elevated plasma-free hemoglobin, the most specific of these hemolysis markers, demonstrated an almost three-fold higher adjusted hazard for the composite outcome (hazard ratio [HR], 2.9; P value < 0.01; 95% confidence interval [CI], 1.4 to 5.6). Elevated LDH was associated with an adjusted HR of 3.1 (P value < 0.01; 95% CI, 1.7 to 5.5). Effect estimates were also pronounced in a composite outcome of only more severe AKI, stage 2+ AKI/RRT: HR 6.6 (P value < 0.01; 95% CI, 3.3 to 13.2) for plasma-free hemoglobin and 2.8 (P value < 0.01; 95% CI, 1.5 to 5.6) for LDH.
Conclusions
Laboratory findings consistent with intravascular hemolysis on ECMO were independently associated with a higher hazard of a composite outcome of AKI/RRT in children undergoing ECMO.
Introduction
The incidence of AKI is high in pediatric patients who receive extracorporeal membrane oxygenation (ECMO), with reported rates of 60%–74%,1 and evidence shows that AKI is independently associated with morbidity and mortality.2 Typical conditions for neonatal patients in the intensive care unit who require ECMO include congenital diaphragmatic hernia, meconium aspiration, primary pulmonary hypertension, and congenital heart disease.1 Older pediatric patients in the pediatric intensive care unit or cardiac intensive care unit (CICU) have common indications, including acute respiratory failure, cardiac failure, and sepsis.3
Multiple etiologies have been considered for the high incidence of AKI associated with ECMO. Potential mechanisms include hemodynamic instability secondary to cardiac or respiratory failure and circuit-specific factors, such as the nonpulsatile flow of venoarterial (VA) ECMO or mechanically induced intravascular hemolysis.4–7 Although free hemoglobin deposition is a well-established risk factor for acute tubular injury in a range of clinical contexts,6,8–10 less is known about the effect of hemolysis on AKI in children receiving ECMO.
The objectives of this study were to assess the incidence of AKI and RRT while undergoing ECMO and examine the association between laboratory markers of intravascular hemolysis on these outcomes. We hypothesized that there would be a high incidence of patients whose kidney function worsened after ECMO initiation and that there would be a correlation with laboratory markers of intravascular hemolysis and AKI/RRT.
Methods
Study Population
This was a retrospective cohort study of patients treated with ECMO from January 2011 through December 2019 at a single pediatric center with a high-volume ECMO program. The initial cohort was identified using data collected for the Extracorporeal Life Support Organization (ELSO) registry. The ELSO registry is a leading source of data on patients treated with ECMO because it compiles data from hundreds of international centers that provide extracorporeal therapy.11 Once eligible patients were identified from the ELSO cohort, study data were extracted from the electronic medical record (EMR). Only patients who underwent ECMO after their respective hospital units were fully transitioned to the EMR and were included in the study. Patients were excluded if they were transferred to our center already on ECMO (n=2). If patients underwent multiple ECMO runs (n=14), only the first ECMO run was included in the primary analysis. This study was reviewed by the Institutional Review Board at the Children's Hospital of Philadelphia and determined to meet the criteria for exemption per 45 Code Federal Regulations 46.104(d) 4(iii) because of the use of existing clinical data.
Outcomes
The primary outcome of interest was a composite of time from ECMO start to AKI or RRT. A time-to-event analysis was used to consider the association with laboratory markers of hemolysis preceding the AKI/RRT only. Composite outcomes were used given the relatively low number of patients requiring RRT. Models were fit using two composite outcomes: (1) any stage AKI or RRT and (2) stage ≥2 AKI or RRT. Time from ECMO start to death was considered a secondary outcome and was not included in the primary composite outcome, given the high mortality in this population, which may be unrelated to kidney dysfunction. For the primary outcome of AKI or RRT, patients were excluded if they were receiving RRT at ECMO start (n=5); however, these patients were included in the analysis of mortality.
For all AKI definitions, the serum creatinine–based Kidney Disease Improving Global Outcomes criteria was used with the neonatal supplement.12 All serum creatinine values were extracted from the EMR. Urine output criteria were not considered, given the lack of consistently available data in the EMR. A binary indicator (yes/no) of abnormal kidney function at the start of ECMO was defined by first identifying the creatinine value drawn within 72 hours before and closest to the ECMO start time, which was considered the pre-ECMO baseline creatinine. If no creatinine value was obtained within this time window before ECMO start (n=65), the earliest value within their ECMO run was used as this baseline creatinine. To define abnormal creatinine at ECMO start, the value was compared with pediatric normative values that were validated in an intensive care population.13 For patients older than 18 years, a creatinine value of 0.8 mg/dl was used as an expected reference. A patient was considered to have abnormal kidney function at ECMO start if their baseline creatinine value was 0.3 mg/dl higher or 1.5-fold greater than the published reference value.13 Published reference values were used to standardize this variable across the cohort because 76% of patients did not have a creatinine value available before their admission.
Given that the aim of the study was to determine whether kidney function worsened while undergoing ECMO therapy, this pre-ECMO baseline creatinine value was used, and to meet Kidney Disease Improving Global Outcomes criteria for AKI, the creatinine had to rise 0.3 mg/dl higher or 1.5-fold greater than this baseline level before censoring. Patients were censored 24 hours after the ECMO run ended or at time of death for all outcomes. RRT was identified using billing data, and start times were obtained through chart review. All patients who met criteria for RRT underwent continuous RRT after ECMO start and before censoring. The earliest of creatinine-based AKI or RRT initiation was used to define the time to the composite outcome.
Primary Exposures
Intravascular hemolysis was the exposure of interest for the primary outcome, and laboratory values that clinically reflect hemolysis were extracted from the EMR. The highest daily plasma-free hemoglobin (mg/dl), highest daily lactate dehydrogenase (LDH) (units/L), lowest daily hemoglobin (g/dl), lowest daily platelet count (103/ul), and lowest daily haptoglobin (mg/dl) were extracted for all days during which the patient was on ECMO. Haptoglobin was not included in the final analyses given the sparsity of available values.
Plasma-free hemoglobin and LDH were categorized given the frequency in which they were unmeasured daily. Unmeasured plasma-free hemoglobin or LDH was used as the reference group because an unmeasured value was believed to reflect low clinical suspicion of hemolysis. The cutoffs for high and low were chosen on the basis of clinical relevance and the distribution of the data. A cutoff of 50 mg/dl for plasma-free hemoglobin was used because this is well established in the ECMO literature.6,11,14,15 For LDH, 2500 units/L was chosen because there were no values <600 units/L when LDH was measured, and 2500 units/L was the upper limit of the lowest tertile of results. Hemoglobin and platelet counts were treated as continuous variables. For the secondary analyses of mortality, the primary exposure of interest was AKI while on ECMO, as defined above.
Covariates
Time-varying covariates were similarly collected for each day of the ECMO run. These included average daily lactate (mmol/L), average daily pH, median mean arterial pressure (mm Hg through an arterial line or cuff), and daily administered pressor medication count. Lactate and pH values were carried forward from the previous day if missing for a given day (n=20) to conserve as much data as possible. Other potential risk factors for AKI included daily administered nephrotoxic medication count and daily ECMO mode ([VA] versus venovenous). Nephrotoxic medications were defined using the amended nephrotoxic medication list from the “Nephrotoxic Injury Negated by Just-in-Time Action” program.16 Fixed covariates included age at start of ECMO, sex, and hospital unit (neonatal patients in the intensive care unit, pediatric intensive care unit, and CICU). If a patient was transferred between hospital units, the ECMO run was assigned to a single unit on the basis of where most time was spent on ECMO. For descriptive purposes, all diagnoses associated with the index hospital admission were extracted from the EMR and categorized on the basis of common indications for ECMO therapy and other relevant underlying diseases or comorbidities. The presence of cardiac arrest was included as a descriptive diagnosis in a similar fashion and not included in the models because EMR data were not consistently accurate for this variable (Supplemental Table 1). ECMO pump type (centrifugal versus roller) was collected as well and used for descriptive purposes given little effect on model outcomes.
Statistical Analyses
Descriptive statistics included median and interquartile range for continuous variables and counts and percentages for categorical variables. Cause-specific Cox proportional hazards regression was used to model the association between hemolysis markers and the composite AKI/RRT primary outcome, with censoring at the competing event of death. The relationship between the primary composite outcome and each index of hemolysis was initially analyzed in unadjusted models. Separate multivariable models for each hemolysis marker were subsequently adjusted for the covariates listed above. Time-varying covariates were considered to be time-dependent confounders alone and not affected by previous exposures. For these models, unit and ECMO mode were combined into a single variable given that most patients undergoing ECMO in the CICU received VA-ECMO. For patients with an outcome within 24 hours after the end of their ECMO run, time-varying variables were carried until the end of follow-up. The proportional hazards assumption was tested using Schoenfeld residuals for each hemolysis marker. Because hemoglobin and platelets were used as continuous variables, linearity of their associations with the composite outcome was assessed by martingale residual plots.
We conducted several sensitivity analyses: (1) evaluating the effect of using the creatinine measure closest in absolute value time to ECMO start as the baseline value without priority for pre-ECMO values, (2) excluding patients without creatinine value obtained before their documented ECMO start time, (3) excluding patients with diagnosis codes consistent with CKD before ECMO start (Supplemental Table 1), and (4) including both ECMO runs for the 14 patients who underwent two runs of ECMO, allowing them to contribute data for both runs. For this last sensitivity analysis, sandwich-type clustered SEMs were estimated to account for multiple ECMO runs within the same patient.
A multivariable Cox proportional hazards model was used to assess the association between AKI while on ECMO and death. The model was adjusted for age, sex, unit/ECMO mode, and abnormal kidney function at ECMO initiation. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 16.
Results
Sample Characteristics
Six hundred and seventy-one patients were identified as having undergone ECMO during the study period. After applying exclusion criteria (Figure 1), 506 patients were eligible to meet the outcome of mortality and 501 were eligible to meet the composite outcome of time to AKI/RRT. There was great variability in demographic features across subgroups (Table 1).
Figure 1.
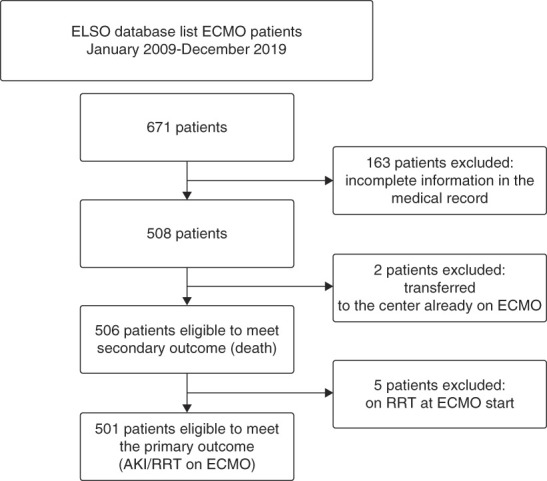
Sample selection. ECMO, extracorporeal membrane oxygenation; ELSO, Extracorporeal Life Support Organization.
Table 1.
Cohort characteristics by age (<1 month versus ≥1 month) and intensive care unit (cardiac versus pediatric or neonatal)
| N=506 | NICU/PICU Neonatal |
CICU Neonatal |
NICU/PICU Pediatric |
CICU Pediatric |
|---|---|---|---|---|
| n=200 | n=105 | n=71 | n=130 | |
| Male sex | 114 (57.0%) | 65 (61.9%) | 34 (47.9%) | 69 (53.1%) |
| Age (yr) | — | — | 4.4 (0.8 to 7.6) | 0.6 (0.2 to 4.7) |
| Age (d) | 1 (0.4 to 2) | 4.2 (2.9 to 12) | — | — |
| Abnormal creatinine at ECMO start | 18 (9.0%) | 8 (7.6%) | 27 (38.0%) | 48 (36.9%) |
| ECMO mode a | ||||
| VV | 41 (20.5%) | 1 (1.0%) | 39 (54.9%) | 1 (0.8%) |
| VA/VVA | 157 (78.5%) | 104 (99.0%) | 32 (45.1%) | 128 (99.2%) |
| ECMO pump type b | ||||
| Cardiohelp | 0 (0%) | 2 (2.0%) | 30 (42.9%) | 5 (3.9%) |
| Roller (Jostra/Stockert) | 187 (97.9%) | 71 (69.6%) | 27 (38.6%) | 79 (62.2%) |
| Sorin centrifugal | 4 (2.1%) | 29 (28.4%) | 13 (18.6%) | 43 (33.9%) |
| ECMO duration (d) | 10.4 (7 to 18.2) | 4.2 (2.5 to 6.7) | 6.5 (2.5 to 12.0) | 5.7 (2.8 to 10.0) |
| Diagnosis categories | ||||
| Cardiac arrest | 6 (3.1%) | 40 (38%) | 21 (30%) | 35 (27%) |
| Congenital heart disease | 78 (40%) | 100 (95%) | 9 (13%) | 95 (73%) |
| Other cardiac dysfunction | 35 (18%) | 53 (50%) | 21 (30%) | 109 (84%) |
| CDH | 43 (22%) | 0 (0%) | 0 (0%) | 0 (0%) |
| PPHN | 51 (26%) | 3 (2.9%) | 0 (0%) | 5 (3.8%) |
| Meconium aspiration | 45 (23%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Acute resp failure | 153 (79%) | 58 (55%) | 47 (67%) | 53 (41%) |
| Pneumonia/Pneumonitis | 15 (7.8%) | 3 (2.9%) | 39 (56%) | 18 (14%) |
| Sepsis | 43 (22%) | 15 (14%) | 23 (33%) | 17 (13%) |
| Oncologic/BMT | 0 (0%) | 0 (0%) | 13 (19%) | 1 (0.8%) |
| Solid organ transplant | 0 (0%) | 1 (1.0%) | 2 (2.9%) | 9 (6.9%) |
| CKD/CAKUT | 7 (3.6%) | 0 (0%) | 6 (8.6%) | 1 (0.8%) |
BMT, bone marrow transplantation; CDH, congenital diaphragmatic hernia; CAKUT, congenital anomalies of the kidney and urinary tract; CICU, cardiac intensive care unit; ECMO, extracorporeal membrane oxygenation; NICU, neonatal patients in the intensive care unit; PPHN, persistent pulmonary hypertension; PICU, pediatric intensive care unit; VVA, veno-veno-arterial; VV, venovenous; VA, venoarterial.
N=2 ECMO mode unknown.
N=9 ECMO pump type unknown.
AKI/RRT
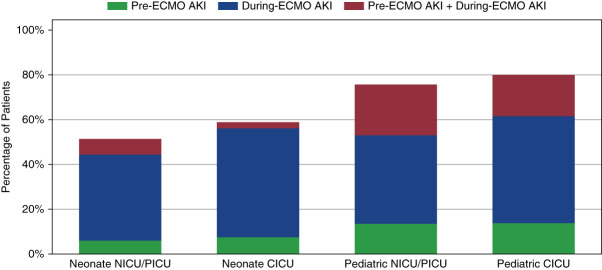
Comparisons between the presence of abnormal kidney function at ECMO start versus AKI during ECMO are shown in Figure 2. Abnormal kidney function at the time of ECMO start was present in 103 of 501 patients (21%) and varied between subgroups. 56 of 501 patients (11%) had abnormal kidney function at the start of ECMO with creatinine that increased while undergoing ECMO therapy and met criteria for AKI. Of 501 patients, 273 (55%) met criteria for the composite outcome of any stage AKI or RRT, 108 (22%) developed stage ≥2 AKI or RRT while on ECMO, and 68 patients (14%) developed stage ≥3 AKI or RRT. RRT was initiated during the ECMO run in 36 patients (7%). The median time to the event in those that met criteria for the composite outcome was 53.2 hours (95% confidence interval [CI], 43.3 to 72.4 hours).
Figure 2.
Incidence and timing of AKI by subgroups. CICU, cardiac intensive care unit; NICU, neonatal patients in the intensive care unit; PICU, pediatric intensive care unit.
Table 2 shows the results from multivariable cause-specific Cox proportional hazards models for the primary composite outcome of time to any stage AKI or RRT, with each laboratory marker of intravascular hemolysis as the primary exposure of interest. Both an elevated plasma-free hemoglobin (>50 mg/dl) and elevated LDH (>2500 units/L) were significantly associated with an increased hazard ratio (HR) for the primary outcome of later developing any stage AKI/RRT: HR 2.9 (P value < 0.01; 95% CI, 1.4 to 5.7) and HR 3.1 (P value < 0.01; 95% CI, 1.7 to 5.5), respectively, compared with not having these markers measured.
Table 2.
Cause-specific Cox proportional hazards models for time to composite outcome of any stage AKI or RRT and each marker of hemolysis
| Covariate | Model 1: Plasma-Free Hemoglobin (mg/dl) (Ref: Unmeasured Value) |
Model 2: LDH (U/L) (Ref: Unmeasured Value) |
Model 3: Minimum Platelets (per 100/ul) | Model 4: Minimum Hemoglobin (per 1 g/dl) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| Hemolysis marker | <50 | 1.12 (0.26 to 4.81) | 0.88 | <2500 | 0.78 (0.19 to 3.24) | 0.73 | 0.96 (0.93 to 1.00) | 0.06 | 0.96 (0.89 to 1.04) | 0.30 |
| >50 | 2.86 (1.44 to 5.66) | <0.01 | >2500 | 3.07 (1.72 to 5.48) | <0.01 | |||||
| Agea (ref: <1 mo) | 0.02 | <0.01 | <0.01 | 0.03 | ||||||
| 1 mo to 1 yr | 1.63 (1.01 to 2.64) | 1.60 (0.99 to 2.59) | 1.67 (1.03 to 2.71) | 1.62 (1.00 to 2.63) | ||||||
| >1–5 yr | 1.42 (0.82 to 2.44) | 1.40 (0.81 to 2.39) | 1.57 (0.91 to 2.72) | 1.42 (0.82 to 2.46) | ||||||
| >5–12 yr | 1.96 (1.01 to 3.80) | 1.90 (0.98 to 3.67) | 2.37 (1.21 to 4.64) | 1.92 (0.98 to 3.75) | ||||||
| >12–18 yr | 3.02 (1.60 to 5.73) | 3.26 (1.75 to 6.09) | 3.41 (1.77 to 6.56) | 2.99 (1.56 to 5.72) | ||||||
| >18 yr | 2.49 (1.04 to 5.99) | 2.67 (1.11 to 6.40) | 2.66 (1.10 to 6.41) | 2.41 (0.98 to 5.92) | ||||||
| Male sex (versus female) | 1.15 (0.88 to 1.49) | 0.31 | 1.15 (0.88 to 1.50) | 0.29 | 1.14 (0.87 to 1.49) | 0.34 | 1.17 (0.90 to 1.52) | 0.25 | ||
| Hospital unit/ECMO mode (ref: CICU) | <0.01 | <0.01 | <0.01 | <0.01 | ||||||
| NICU/VV | 0.29 (0.14 to 0.59) | 0.29 (0.14 to 0.61) | 0.28 (0.13 to 0.60) | 0.30 (0.14 to 0.62) | ||||||
| NICU/VA | 0.41 (0.27 to 0.61) | 0.41 (0.27 to 0.62) | 0.42 (0.28 to 0.64) | 0.41 (0.27 to 0.62) | ||||||
| PICU/VV | 1.15 (0.66 to 2.02) | 1.16 (0.66 to 2.04) | 1.25 (0.71 to 2.22) | 1.18 (0.67 to 2.09) | ||||||
| PICU/VA | 1.83 (1.03 to 3.26) | 1.78 (1.00 to 3.16) | 1.77 (0.99 to 3.17) | 1.75 (0.98 to 3.12) | ||||||
| Abnormal Cr ECMO start | 0.77 (0.55 to 1.09) | 0.14 | 0.75 (0.53 to 1.06) | 0.10 | 0.75 (0.53 to 1.06) | 0.10 | 0.78 (0.55 to 1.11) | 0.17 | ||
| Average pH | 0.37 (0.04 to 3.28) | 0.37 | 0.40 (0.04 to 3.59) | 0.41 | 0.38 (0.04 to 3.99) | 0.42 | 0.40 (0.05 to 3.57) | 0.41 | ||
| Average lactate | 1.12 (1.07 to 1.16) | <0.01 | 1.11 (1.07 to 1.16) | <0.01 | 1.11 (1.07 to 1.16) | <0.01 | 1.11 (1.07 to 1.16) | <0.01 | ||
| Median MAPb | 0.99 (0.97 to 1.00) | 0.10 | 0.99 (0.97 to 1.00) | 0.10 | 0.99 (0.97 to 1.00) | 0.12 | 0.99 (0.97 to 1.00) | 0.09 | ||
| Nephrotoxic medication countc | 1.03 (0.85 to 1.23) | 0.79 | 1.04 (0.87 to 1.25) | 0.69 | 1.02 (0.85 to 1.23) | 0.84 | 1.03 (0.86 to 1.24) | 0.77 | ||
CI, confidence interval; CICU, cardiac intensive care unit; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; LDH, lactate dehydrogenase; MAP, mean arterial pressure; NICU, neonatal patients in the intensive care unit; NINJA, Nephrotoxic Injury Negated by Just-in-Time Action; PICU, pediatric intensive care unit; VA, venoarterial; VV, venovenous.
Age at time of ECMO start.
MAP (mean arterial pressure).
On the basis of the NINJA list of nephrotoxins.
Similarly, elevated plasma-free hemoglobin and LDH were significantly associated with the composite outcome of time to stage ≥2 AKI or RRT, with an elevated plasma-free hemoglobin associated with a more than six-fold higher hazard for this outcome (Table 3).
Table 3.
Cause-specific Cox proportional hazards models for time to composite outcome of stage ≥2 AKI or RRT and each marker of hemolysis
| Covarite | Model 1: Plasma-Free Hemoglobin (mg/dl) (Ref: Unmeasured Value) |
Model 2: LDH (U/L) (Ref: Unmeasured Value) |
Model 3: Minimum Platelets (per 100/ul) | Model 4: Minimum Hemoglobin (per 1 g/dl) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| Hemolysis marker | <50 | 0.83 (0.11 to 6.31) | 0.85 | <2500 | 0.27 (0.04 to 2.13) | 0.21 | 0.97 (0.92 to 1.02) | 0.22 | 0.90 (0.80 to 1.00) | 0.05 |
| >50 | 6.57 (3.27 to 13.2) | <0.01 | >2500 | 2.84 (1.45 to 5.57) | <0.01 | |||||
| Agea (ref: <1 mo) | 0.28 | 0.07 | 0.09 | 0.40 | ||||||
| 1 mo to 1 yr | 1.19 (0.57 to 2.48) | 1.13 (0.54 to 2.35) | 1.16 (0.55 to 2.47) | 1.02 (0.49 to 2.13) | ||||||
| >1–5 yr | 1.62 (0.76 to 3.45) | 1.70 (0.81 to 3.55) | 1.97 (0.90 to 4.31) | 1.45 (0.68 to 3.12) | ||||||
| >5–12 yr | 1.94 (0.78 to 4.83) | 1.90 (0.76 to 4.74) | 2.10 (0.81 to 5.44) | 1.59 (0.64 to 3.93) | ||||||
| >12–18 yr | 2.61 (1.11 to 6.13) | 3.26 (1.41 to 7.50) | 3.47 (1.42 to 8.50) | 2.37 (1.00 to 5.63) | ||||||
| >18 yr | 2.61 (0.88 to 7.74) | 2.96 (1.00 to 8.78) | 3.04 (1.01 to 9.13) | 2.06 (0.67 to 6.30) | ||||||
| Male sex (versus female) | 0.93 (0.63 to 1.37) | 0.71 | 0.92 (0.62 to 1.36) | 0.67 | 0.82 (0.55 to 1.22) | 0.33 | 0.91 (0.62 to 1.34) | 0.63 | ||
| Hospital unit/ECMO mode (ref: CICU) | <0.01 | <0.01 | <0.01 | <0.01 | ||||||
| NICU/VV | 0.09 (0.01 to 0.65) | 0.09 (0.01 to 0.68) | 0.10 (0.01 to 0.75) | 0.09 (0.01 to 0.68) | ||||||
| NICU/VA | 0.28 (0.14 to 0.54) | 0.28 (0.15 to 0.54) | 0.29 (0.14 to 0.56) | 0.27 (0.14 to 0.53) | ||||||
| PICU/VV | 1.56 (0.79 to 3.11) | 1.53 (0.76 to 3.09) | 1.62 (0.79 to 3.36) | 1.68 (0.82 to 3.42) | ||||||
| PICU/VA | 1.53 (0.68 to 3.40) | 1.32 (0.60 to 2.92) | 1.48 (0.66 to 3.33) | 1.29 (0.58 to 2.89) | ||||||
| Abnormal Cr ECMO start | 1.30 (0.83 to 2.05) | 0.25 | 1.18 (0.75 to 1.86) | 0.48 | 1.16 (0.72 to 1.87) | 0.53 | 1.28 (0.81 to 2.02) | 0.29 | ||
| Average pH | 0.01 (0.01 to 1.18) | <0.01 | 0.01 (0.01 to 0.26) | <0.01 | 0.02 (0.01 to 0.41) | 0.01 | 0.01 (0.01 to 0.23) | <0.01 | ||
| Average lactate | 1.17 (1.11 to 1.23) | <0.01 | 1.16 (1.10 to 1.22) | <0.01 | 1.16 (1.10 to 1.23) | <0.01 | 1.16 (1.10 to 1.22) | <0.01 | ||
| Median MAPb | 0.99 (0.97 to 1.01) | 0.26 | 0.99 (0.97 to 1.01) | 0.28 | 0.98 (0.96 to 1.01) | 0.20 | 0.99 (0.97 to 1.01) | 0.46 | ||
| Nephrotoxic medication countc | 0.82 (0.62 to 1.08) | 0.16 | 0.83 (0.63 to 1.10) | 0.19 | 0.81 (0.61 to 1.08) | 0.15 | 1.81 (0.61 to 1.08) | 0.15 | ||
CI, confidence interval; CICU, cardiac intensive care unit; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; LDH, lactate dehydrogenase; MAP, mean arterial pressure; NICU, neonatal patients in the intensive care unit; NINJA, Nephrotoxic Injury Negated by Just-in-Time Action; PICU, pediatric intensive care unit; VA, venoarterial; VV, venovenous.
Age at time of ECMO start.
MAP (mean arterial pressure).
On the basis of the NINJA list of nephrotoxins.
Mortality
In a multivariable model controlling for age, sex, and unit/ECMO mode, AKI while on ECMO increased the hazard of death nearly three-fold with an HR of 2.9 (95% CI, 1.8 to 4.7; P value < 0.01). Abnormal kidney function at the time of ECMO initiation increased the hazard of death by 1.5 (95% CI, 1.0 to 2.4; P = 0.03), although this is a direct effect only, as part of the total effect is likely mediated by AKI while on ECMO.
Sensitivity Analyses
Two sensitivity analyses related to the choice of the pre-ECMO baseline creatinine value were performed. In the first, we used the creatinine value measured closest in absolute value of time to the ECMO start time, without preference for pre-ECMO values. These results yielded a slightly higher frequency of patients with abnormal kidney function at ECMO start in the pediatric patients (36%) but slightly less for the neonatal patients (9%). This did not demonstrate a substantial effect on the model findings, and all four hemolysis marker effect estimates were similar (Supplemental Table 2a/2b). In the second sensitivity analysis, we excluded all patients for whom there was no creatinine value obtained within 72 hours before their ECMO start time. Again, there was no significant effect on the model findings, and effect estimates were similar (Supplemental Table 3a/3b). Given the aim of the study was to assess the change in creatinine values after ECMO had started, pre-ECMO creatinine was chosen preferentially as the baseline for the primary analysis.
A sensitivity analysis was also performed including both runs of ECMO for those who underwent multiple runs, increasing the total number of ECMO runs to 514. This analysis revealed similar effect estimates as the primary analysis (Supplemental Table 4a/4b). Finally, all patients with diagnosis codes related to CKD during their ECMO admission were manually chart reviewed. Those with evidence of CKD stage II or greater before ECMO start were excluded. This analysis also revealed similar effect estimates as the primary analysis (Supplemental Table 5a/5b).
Discussion
Our findings support the existing literature regarding the high frequency of AKI experienced by pediatric patients treated with ECMO, as well as the increased risk of mortality associated with AKI in this population. Hypothesized circuit-specific factors that may affect the risk of ECMO-associated AKI included the nonpulsatile blood flow of VA-ECMO and circuit-induced intravascular hemolysis. Our results did not show a significant effect on the risk for either composite outcome on the basis of the type of ECMO (venovenous versus VA) used. However, multiple laboratory indices of intravascular hemolysis demonstrated an association with both composite AKI/RRT outcomes. We consistently found that high plasma-free hemoglobin–the most specific marker of intravascular hemolysis—significantly increased the hazard of AKI/RRT. High LDH levels also consistently showed a strong positive effect in multivariable models for both composite AKI/RRT outcomes.
Multiple mechanisms by which hemolysis induces kidney injury have been studied. With red cell hemolysis, both free hemoglobin and heme are released into the circulation and cause direct renal tubular damage. Free heme also binds to renal tubular cells and accumulates, leading to the generation of reactive oxygen species, intracellular oxidative stress, and subsequent tubular toxicity.17,18 Heme oxygenase 1, an enzyme responsible for heme degradation, has been shown to be upregulated in many types of AKI.19 As heme toxicity is believed to contribute to the mechanism of several nephropathies, many protective approaches for these deleterious effects are currently being developed. Although heme nephrotoxicity is well described in the literature, the direct clinical effect of ECMO-induced intravascular hemolysis on AKI has been unclear. There are no standardized approaches for monitoring for hemolysis on ECMO. There is also variability in addressing the presence of intravascular hemolysis on ECMO because some centers have standardized ECMO circuit changes or provide plasmapheresis to clear free hemoglobin at certain levels of hemolysis.13 Our study supports the plausibility of this mechanism's contribution to AKI/RRT during ECMO, and as such, considerations for the optimal monitoring regimen and efficacy of treatment are an important area of further research.
This study is distinct from previous work in this population, as the commonly used ELSO database defines AKI by the binary outcome of creatinine >1.5 mg/dl. Using this cutoff, AKI is likely to be underrecognized in pediatric patients, particularly in neonatal patients for whom a normal creatinine value should be as low as 0.2 mg/dl. For this study, we used data collected from our center for the ELSO database to define the cohort, followed by collecting all creatinine values from the EMR, allowing us to identify AKI more accurately and comprehensively. This study is also novel in its evaluation of the association of multiple laboratory markers of hemolysis in a time-dependent fashion, enhancing the ability to detect a mechanistic relationship between intravascular hemolysis and AKI.
For this study, serum creatinine was used for all AKI definitions because this is the most widely accepted and readily available measure of kidney function. We should consider, however, the known limitations of serum creatinine as it pertains to identifying the timing of AKI. Patients were censored 24 hours after ECMO cessation; however, this may fail to detect creatinine elevations that may be delayed but reflect an injury sustained while on ECMO therapy. Further prospective work should consider the use of AKI biomarkers or alternate AKI definitions for more specificity, specifically to consider the likely time of injury. Perhaps the most significant limitation of this study is that given the retrospective nature of the data, there is substantial missingness of laboratory markers used for monitoring for intravascular hemolysis. More specific laboratory parameters such as plasma-free hemoglobin, LDH, and haptoglobin were likely to be obtained only with clinical suspicion for intravascular hemolysis because these are not obtained routinely for patients on ECMO at our center. As such, we considered a day in which these values were unmeasured as a reference, in which there was a low clinical suspicion for intravascular hemolysis and thus the clinician did not send these tests. In addition, in this study, a laboratory marker of hemolysis would have been excluded if the outcome of AKI/RRT had already been met before its measurement. Both of these sources of missing hemolysis marker data would potentially bias our study results toward the null. A prospective study in this population with protocolized laboratory assessment would help further elucidate this mechanisms effect on AKI on ECMO.
This study raises important considerations regarding our current understanding of the risk factors for AKI while undergoing ECMO therapy. Although hemodynamic instability is a well-understood risk factor for kidney injury, our findings suggest that the addition of intravascular hemolysis may contribute to this risk. Further research into the contribution of this mechanism may yield opportunities for prevention and mitigation of the high burden of kidney injury in these critically ill children.
Supplementary Material
Disclosures
M.R. Denburg reports consultancy agreement with TriSalus Life Sciences (spouse); ownership interest in In-Bore LLC (spouse) and Precision Guided Interventions LLC (spouse); research funding from Instylla (spouse) and Mallinckrodt; patents or royalties with In-Bore LLC; advisory or leadership role on the editorial board of Kidney International Reports, KDIGO Executive Committee, and TriSalus Life Sciences Scientific Advisory Board (spouse); and other interests or relationships include American Society of Pediatric Nephrology Research and Program Committees. B.L. Laskin reports ownership interest in Acorda therapeutics, Charter Communications, Comcast, DHT Holdings, Duke Energy, Electra Battery Materials, Ford Motor, Happiness Development, Johnson Controls, Medtronic, Nu Holdings, AT&T, TE Connectivity, Verizon, and Warner Brothers Discovery; research funding from Viracor Eurofins, research sample testing free of charge; and patent on Compositions and Methods for Treatment of HSCT-Associated Thrombotic Microangiopathy. United States Patent Number PCT/US2014/055922, 2014. J. Zee reports honoraria from Booz Allen Hamilton. All remaining authors have nothing to disclose.
Funding
This work is supported by NIH from 2T32DK00700647 (A.E. Strong) and Carole Marcus Mid-Career Award to Promote Career Development and Mentoring in Pediatric Research from (T32) University of Pennsylvania School of Medicine (M.R. Denburg).
Author Contributions
Conceptualization: Michelle R. Denburg, Todd J. Kilbaugh, Benjamin L. Laskin, Amy E. Strong, Jarcy Zee.
Data curation: Diego Campos, James Connelly, Michelle R. Denburg, Rosanna Fulchiero, Amy E. Strong.
Formal analysis: Michelle R. Denburg, Spandana Makeneni, Amy E. Strong, Jarcy Zee.
Funding acquisition: Michelle R. Denburg, Amy E. Strong.
Investigation: Amy E. Strong.
Methodology: Spandana Makeneni, Amy E. Strong, Jarcy Zee.
Software: Amy E. Strong.
Supervision: Michelle R. Denburg, Benjamin L. Laskin, Jarcy Zee.
Visualization: Spandana Makeneni.
Writing – original draft: Amy E. Strong.
Writing – review & editing: Michelle R. Denburg, Benjamin L. Laskin, Amy E. Strong, Jarcy Zee.
Data Sharing Statement
Anonymized data created for the study are or will be available in a persistent repository on publication. Generated data; observational data. Iowa Research Online Data Repository.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A401.
Supplemental Table 1. Diagnosis code categories.
Supplemental Table 2a. Cause-specific Cox proportional hazards models for time to composite outcome of any stage AKI or RRT using ECMO start creatinine closest in absolute value without priority for the pre-ECMO creatinine value.
Supplemental Table 2b. Cause-specific Cox proportional hazards models for time to composite outcome of stage ≥2 AKI or RRT using ECMO start creatinine closest in absolute value without priority for the pre-ECMO creatinine value.
Supplemental Table 3a. Cause-specific Cox proportional hazards models for time to composite outcome of any stage AKI or RRT excluding patients without a serum creatinine value obtained in the 72 hours pre-ECMO.
Supplemental Table 3b. Cause-specific Cox proportional hazards models for time to composite outcome of any ≥stage 2 AKI or RRT excluding patients without a serum creatinine value obtained in the 72 hours pre-ECMO.
Supplemental Table 4a. Cause-specific Cox proportional hazards models for time to composite outcome of all stage AKI or RRT using all eligible ECMO runs, with a single patient contributing multiple runs.
Supplemental Table 4b. Cause-specific Cox proportional hazards models for time to composite outcome of stage ≥2 AKI or RRT using all eligible ECMO runs, with a single patient contributing multiple runs.
Supplemental Table 5a. Cause-specific Cox proportional hazards models for time to composite outcome of all stage AKI or RRT after excluding patients with diagnosis code(s) consistent with CKD and known baseline eGFR <90 ml/min per 1.73 m2.
Supplemental Table 5b. Cause-specific Cox proportional hazards models for time to composite outcome of stage ≥2 AKI or RRT after excluding patients with diagnosis code(s) consistent with CKD and known baseline eGFR <90 ml/min per 1.73 m2.
References
- 1.Maratta C, Potera RM, van Leeuwen G, Castillo Moya A, Raman L, Annich GM. Extracorporeal life support organization (ELSO): 2020 pediatric respiratory ELSO guideline. ASAIO J. 2020;66(9):975–979. doi: 10.1097/mat.0000000000001223 [DOI] [PubMed] [Google Scholar]
- 2.Askenazi DJ Ambalavanan N Hamilton K, et al. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12(1):e1–e6. doi: 10.1097/PCC.0b013e3181d8e348 [DOI] [PubMed] [Google Scholar]
- 3.Etchill EW, Dante SA, Garcia AV. Extracorporeal membrane oxygenation in the pediatric population—who should go on, and who should not. Curr Opin Pediatr. 2020;32(3):416–423. doi: 10.1097/mop.0000000000000904 [DOI] [PubMed] [Google Scholar]
- 4.Jenks C, Raman L, Dhar A. Review of acute kidney injury and continuous renal replacement therapy in pediatric extracorporeal membrane oxygenation. Indian J Thorac Cardiovasc Surg. 2021;37(suppl 2):254–260. doi: 10.1007/s12055-020-01071-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenks CL, Zia A, Venkataraman R, Raman L. High hemoglobin is an independent risk factor for the development of hemolysis during pediatric extracorporeal life support. J Intensive Care Med. 2019;34(3):259–264. doi: 10.1177/0885066617708992 [DOI] [PubMed] [Google Scholar]
- 6.Borasino S Kalra Y Elam AR, et al. Impact of hemolysis on acute kidney injury and mortality in children supported with cardiac extracorporeal membrane oxygenation. J Extra Corpor Technol. 2018;50(4):217–224. doi: 10.1051/ject/201850217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gbadegesin R, Zhao S, Charpie J, Brophy PD, Smoyer WE, Lin JJ. Significance of hemolysis on extracorporeal life support after cardiac surgery in children. Pediatr Nephrol. 2009;24(3):589–595. doi: 10.1007/s00467-008-1047-z [DOI] [PubMed] [Google Scholar]
- 8.Qian Q, Nath KA, Wu Y, Daoud TM, Sethi S. Hemolysis and acute kidney failure. Am J Kidney Dis. 2010;56(4):780–784. doi: 10.1053/j.ajkd.2010.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvanajscak Z Walker PD Cossey LN, et al. Hemolysis-associated hemoglobin cast nephropathy results from a range of clinicopathologic disorders. Kidney Int. 2019;96(6):1400–1407. doi: 10.1016/j.kint.2019.08.026 [DOI] [PubMed] [Google Scholar]
- 10.Houston S, Patel S, Badheka A, Lee-Son K. Clearance of severely elevated plasma free hemoglobin with total plasma exchange in a pediatric ECMO patient. Perfusion. 2022;37(5):515–518. doi: 10.1177/02676591211021946 [DOI] [PubMed] [Google Scholar]
- 11.ECLS International Summary of Statistics. https://www.elso.org/Registry/InternationalSummaryandReports/InternationalSummary.aspx [Google Scholar]
- 12.Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012;24(2):191–196. doi: 10.1097/MOP.0b013e32834f62d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald JC Basu RK Fuhrman DY, et al. Renal dysfunction criteria in critically ill children: the PODIUM consensus conference. Pediatrics. 2022;149(suppl 1):S66–S73. doi: 10.1542/peds.2021-052888J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalton HJ Cashen K Reeder RW, et al. Hemolysis during pediatric extracorporeal membrane oxygenation: associations with circuitry, complications, and mortality. Pediatr Crit Care Med. 2018;19(11):1067–1076. doi: 10.1097/pcc.0000000000001709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu JH, Sarathy S, Ramesh S, Rudolph K, Raghavan ML, Badheka A. Risk factors for hemolysis with centrifugal pumps in pediatric extracorporeal membrane oxygenation: is pump replacement an answer? Perfusion. 2023;38(4):771–780. doi: 10.1177/02676591221082499 [DOI] [PubMed] [Google Scholar]
- 16.Goldstein SL Dahale D Kirkendall ES, et al. A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int. 2020;97(3):580–588. doi: 10.1016/j.kint.2019.10.015 [DOI] [PubMed] [Google Scholar]
- 17.Higdon AN Benavides GA Chacko BK, et al. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. Am J Physiol Heart Circ Physiol. 2012;302(7):H1394–H1409. doi: 10.1152/ajpheart.00584.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabibzadeh N Estournet C Placier S, et al. Plasma heme-induced renal toxicity is related to a capillary rarefaction. Sci Rep. 2017;7:40156. doi: 10.1038/srep40156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zager RA, Johnson AC, Becker K. Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol. 2012;23(6):1048–1057. doi: 10.1681/ASN.2011121147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data created for the study are or will be available in a persistent repository on publication. Generated data; observational data. Iowa Research Online Data Repository.