Abstract
Most patients receiving dialysis rely on dietary restriction and phosphate binders to minimize the risk of hyperphosphatemia, which is associated with increased mortality. However, dietary restriction is difficult because of hidden phosphate additives in processed foods and medications. Restriction of dietary phosphate sources such as protein may increase the risk of malnutrition. Phosphate binders, the only pharmacologic option for phosphate management since aluminum salts were introduced several decades ago, are often insufficient for binding the 1400–2500 mg of phosphate potentially consumed daily. Over the last decade, serum phosphate levels in the United States have risen, and >69% of patients receiving dialysis exhibited a most recent phosphate level >4.5 mg/dl (above the normal range), indicating an urgent need for new, more effective therapies to manage phosphate burden. Novel, nonbinder therapies such as transcellular and paracellular phosphate absorption inhibitors may be used for phosphate management, and future studies should examine whether they allow fewer dietary restrictions for patients receiving dialysis, potentially improving patient quality of life and nutritional status. It is imperative that we collaborate to move beyond the restrictive approaches available today and provide patients and clinicians with an array of strategies so that they may choose the most appropriate patient-centered therapy.
Keywords: phosphate burden, dietary phosphate restrictions, suboptimal efficacy of phosphate binders, phosphate absorption inhibitors, phosphate binders, phosphate uptake
There Is an Urgent Need for New Classes of Phosphate-Lowering Therapies
CKD is estimated to affect 15% of the adult population in the United States.1 In the later stages of CKD, phosphate retention occurs because compensatory biologic mechanisms that both limit phosphate absorption and increase phosphate excretion become insufficient to maintain phosphate homeostasis, which may lead to hyperphosphatemia.2 Elevated serum phosphate levels are associated with several adverse outcomes including vascular calcification, cardiovascular disease, secondary hyperthyroidism, and an increased risk of all-cause mortality.3–6 Patients with CKD G3a-G5d are advised to lower phosphate levels toward the normal range.3 However, there is still a lack of data that show an improvement in clinical outcomes when interventions aimed at lowering serum phosphate are used in patients with CKD.3 Today, approximately 600,000 patients with ESKD are undergoing dialysis in the United States,1 most of whom likely rely on dietary restrictions and phosphate binders to avoid the negative consequences of phosphate retention and hyperphosphatemia.
Although dietary restrictions are a foundational part of phosphate management, sustained adherence can take a toll on patients. Patients receiving dialysis are typically advised by dietitians and nephrologists to adhere to a diet low in phosphate, potassium, sodium, and liquids to avoid the negative consequences associated with electrolyte overload.7 While updated Kidney Disease Outcomes Quality Initiative guidelines acknowledge the need to create individualized dietary plans based on patient needs and clinical judgment, with consideration given to the bioavailability of dietary phosphate sources, these nutritional guidelines no longer specify a target phosphate intake range because of a lack of demonstrated clinical efficacy for target-based strategies.8 To decrease the risk of hyperphosphatemia and electrolyte overload, patients receiving dialysis cannot consume a long list of otherwise healthy foods, and there is concern that many patients receiving dialysis do not consume a heart-healthy diet.9 Phosphorus and protein intake correlate significantly; thus, a major risk of certain restrictive diets is protein malnutrition.10 Along with the stress of daily self-management, dietary restrictions can negatively affect the quality of life of patients by limiting family and social interactions.11
The burden of constant dietary self-management is exacerbated by the ubiquity of hidden phosphate additives in modern processed foods and in medications. Dietary phosphates may be considered hidden because manufacturers are not required to include the quantity of phosphate from food additives on labels.7,12 The reality of industrialized food systems with insufficient food labeling practices and the resultant challenge that presents to managing dietary phosphate sources is reflected in language added in the 2020 updates to the nutritional guidelines provided by Kidney Disease Outcomes Quality Initiative. New guidance states that individualized dietary management plans should also take into consideration the source of dietary phosphate because the bioavailability of phosphate may vary between organic (animal or vegetal) and inorganic (food additives) sources.8 While absorption of phosphate from organically derived foods do vary depending on the source, the inorganic phosphate from additives used in processed foods is more readily absorbed than organic sources overall.12,13 Quantities of inorganic phosphate that may also be present as inactive ingredients in medications which are not required to be disclosed provide yet another hidden source of phosphate. However, medicinal sources have the potential to represent a significant contribution to the sum phosphate intake for dialysis patients because medicines commonly prescribed to patients at dialysis clinics in the United States may contain up to 200 mg phosphate/tablet.14,15
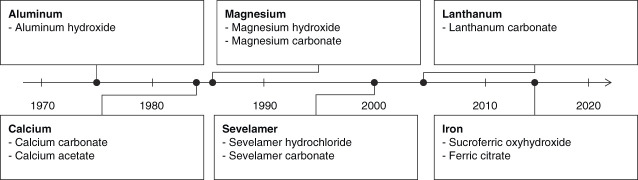
Unlike treatments for some disease states that regularly see innovations in therapeutic classes, phosphate binders have been the only pharmacologic option to manage phosphate burden since aluminum salts were introduced in the 1970s.16,17 (Figure 1) Although phosphate binders are shown to effectively reduce serum phosphate concentrations in patients receiving dialysis,18–20 the binding capacities of each pill could be maxed out in the face of high dietary phosphate intake. Binding capacities for the recommended starting daily dose of each binder ranges from 63 to 234 mg of phosphates (Table 1).18–22 Even high doses of phosphate binders can typically only remove up to 300 mg of phosphate per day.12 This is insufficient to keep up with an estimated daily dietary phosphate load of 1400–2500 mg23–25 (Figure 2).
Figure 1.
Timeline of phosphate management strategies. Over the last 50 years, minor changes in the phosphate binder drug class have been introduced through use of different active ingredients.
Table 1.
Recommended dose and binding capacities for select phosphate binders
| Phosphate Binder | Recommended Starting Dose (per day) | Binding Capacity (mg Phosphate/g Binder) | Daily Binding Capacity of Starting Dose |
|---|---|---|---|
| Sevelamer carbonate20–22 | 3–6× 0.8 g pills or 0.8 g packets | 26.3 mg/g | 63–126 mg |
| Lanthanum carbonate18,22 | 3× 0.5 g pills or 2× 750 mg packets | 78–156 mg/g | 117–234 mg |
| Calcium acetate19,21 | 6 gelcaps (667 mg calcium acetate/gelcap) | 45 mg/g | 180 mg |
Figure 2.
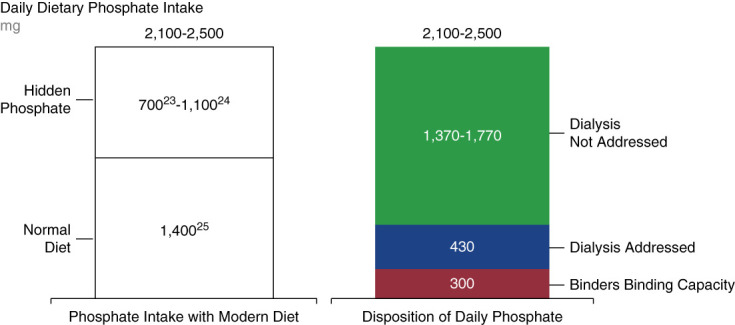
Phosphate binders are insufficient to match daily dietary phosphate intake.18–25,48 The binding capacity of phosphate binders is too low to account for daily phosphate intake, particularly for diets high in hidden phosphates.
Phosphate binders can negatively affect patient quality of life in a variety of ways. The addition of phosphate binders to an already extensive medication regimen can substantially increase pill burden. Chiu et al. found that phosphate binders account for approximately 50% of the total daily pill burden for patients receiving dialysis, with a median daily count of nine phosphate binder pills, and many may take up to 15–20 binders per day.26 This study of the effect of pill burden on quality-of-life outcomes in dialysis patients reported that only approximately 40% of patients were adherent to the phosphate binder therapy,26 potentially because regularly taking high quantities of large, hard-to-swallow pills may be both unpleasant and logistically difficult for patients. Patients must take many binders with each meal and snack, and timing pill ingestion is critical for binders to work as well, and patients may easily forget to take binders on time, particularly if they are at work, at a restaurant, or away from home. In addition to the pill burden phosphate binders may add with each meal and snack, they are also associated with gastrointestinal adverse events, most commonly nausea, vomiting, diarrhea, and constipation18–20,27 (Figure 3). An analysis of reasons for phosphate binder discontinuation found that 11% of patients stopped treatment because of nontolerance, and within this subgroup, 48% discontinued because of gastrointestinal upset.28
Figure 3.
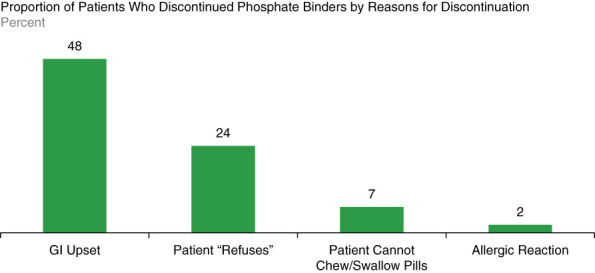
Reasons for phosphate binder discontinuation.28 Approximately half of patients who discontinued phosphate binders cited gastrointestinal (GI) upset as the reason for discontinuation.
Despite extensive dietary restriction and prescription of phosphate binders, phosphate control has worsened for patients with CKD in the United States over recent years. Of patients receiving dialysis in January 2021, 69% were found to have serum phosphate levels above normal range (>4.5 mg/dl), and the mean phosphate levels in patients receiving dialysis was 5.6 mg/dl after having seen a decrease in phosphate levels over the decade spanning 2002–2012.29,30 While the most recent Kidney Disease Improving Global Outcomes guidelines (2017) did not provide specific serum phosphate targets, it is recommended that patients with CKD G3a-G5D with persistently elevated phosphate levels should lower levels toward a normal range of 2.8–4.5 mg/dl.3,30 This lack of improvement in overall patient phosphate levels suggests that current therapies for phosphate management are not effective for all patients receiving dialysis. Thus, there is a clear and urgent need for new classes of therapies for phosphate management to improve outcomes.
New Classes of Phosphate Management Therapies Would Give Patients and Clinicians Freedom of Choice
New phosphate-lowering therapies that reduced both pill burden and adverse events could potentially improve patient quality of life and may help patients reach therapeutic goals. The effect of treatment innovation has been demonstrated in potassium management, where within the past decade, two different potassium binders have been developed for daily use that are safe and well-tolerated.31,32 These new agents have become enablers of life-saving/kidney-preserving agents like renin–angiotensin aldosterone inhibitors that normally could not be given to people with stages 4 and 5 CKD.33,34
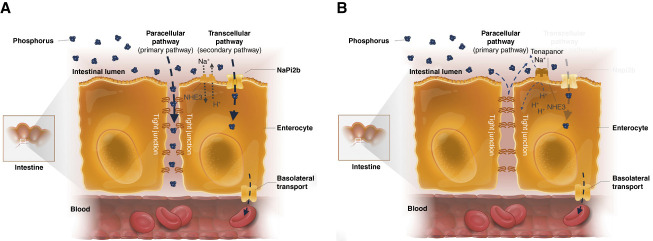
New classes of therapies for phosphate management are being developed that instead of binding ingested phosphates, target one of two intestinal phosphate absorption pathways: the paracellular or transcellular pathway (Figure 4).2,35–39 In brief, the transcellular absorption pathway uses specific phosphate transporters such as the sodium-dependent phosphate cotransporter 2b (NaPi2b) and type-III inorganic phosphate (Pi) transporters PiT-1 and PiT-2, whereas the paracellular pathway relies on the passive transport of phosphate through tight junction complexes between cells according to concentration gradients.2,40 Because these therapies have different mechanisms of action from that of phosphate binders, there is hope that they will provide necessary therapeutic innovation.
Figure 4.
Intestinal phosphate absorption pathways.2 (A) Intestinal phosphate absorption occurs through the transcellular and paracellular pathways. Absorption through the secondary transcellular pathway is facilitated by the sodium-dependent phosphate transporter NaPi2b. In the primary paracellular pathway, phosphate is absorbed passively along the concentration gradient through tight junctions. (B) Tenapanor reduces permeability of tight junctions to phosphate, reducing paracellular phosphate absorption. NaPi2b, sodium-dependent phosphate transporter 2b; NHE3, sodium/hydrogen exchanger isoform 3.
Several transcellular phosphate absorption inhibitors are in development, and clinical trial data so far are mixed. A phase 1 trial on standardized phosphate diet of the novel drug EOS789, an inhibitor of NaPi-2b, PiT-1, and PiT-2, demonstrated encouraging results in patients receiving hemodialysis.35 Nicotinamide seems to inhibit gastrointestinal NaPi2b cotransporters, thereby reducing phosphate-specific transcellular permeability.36 However, there was a lack of significant reductions in phosphate or FGF23 in nondialysis patients with CKD treated by lanthanum carbonate and/or nicotinamide during a 12-month trial.37 A phase 1 study of the NaPi2b inhibitor ASP3325 showed that this therapy was not effective in reducing serum phosphate levels in patients with ESKD.38
Studies have shown that the paracellular pathway is the predominant pathway of intestinal phosphate absorption in humans.41–43 Tenapanor (Ardelyx Inc.) is a paracellular phosphate absorption inhibitor that has been shown to reduce serum phosphate concentrations in clinical trials. Its mechanism of action inhibits the sodium/hydrogen exchanger isoform 3 in the gastrointestinal tract, which blocks paracellular phosphate permeability by reducing sodium absorption and increasing intracellular proton retention, causing conformational changes in tight junction proteins.41 In a phase 3 study of ESKD patients with hyperphosphatemia receiving maintenance dialysis, tenapanor administration (after a phosphate binder washout period) lowered serum phosphate by ≥1.0 mg/dl at 8 weeks in each of the three dose groups studied.39 The results from a similarly designed, long-term phase 3 study showed that at 26 weeks, tenapanor administered as one tablet twice a day as a replacement therapy to phosphate binders lowered serum phosphate in participants (n=248) from baseline concentrations by a mean (SD) of 1.4 (1.8) mg/dl.44 These studies indicate that twice-daily tenapanor could potentially replace phosphate binders, thereby substantially reducing pill burden. However, no prespecified head-to-head analyses comparing tenapanor as monotherapy compared with other phosphate control therapies have been conducted.
Tenapanor administered in conjunction with phosphate binders can have a more significant effect than binders alone. Data from a trial that compared the effectiveness of a combination of tenapanor and binder versus placebo and binder showed that tenapanor plus binder resulted in a 0.65 mg/dl larger mean serum phosphate reduction from baseline compared with placebo plus binder.45 The study included 236 patients undergoing maintenance dialysis with hyperphosphatemia (defined in this trial as serum phosphate 5.5–10 mg/dl inclusive), despite receiving phosphate binder therapy (sevelamer, nonsevelamer, sevelamer plus nonsevelamer, or multiple nonsevelamer binders).45 In addition, almost twice as many patients treated with tenapanor and binder achieved serum phosphate <5.5 mg/dl compared with patients treated with placebo and binder (37%–50% versus 18%–24%, P < 0.05).45 This dual-mechanism approach may be particularly relevant for patients with persistent hyperphosphatemia.45
It should be noted, that while tenapanor has been shown to effectively reduce serum phosphate in most patients,39,44,45 a clinical benefit of tenapanor for patients with CKD has not been established, as is the case with other serum phosphate-reducing medications, including phosphate binders.3 While tenapanor was safe and generally well-tolerated (as a monotherapy or in combination with other binders), in the published studies to date, 5%–7% of patients who either switched to tenapanor or added tenapanor to a preexisting phosphate binder regimen discontinued because of hyperphosphatemia, and 4%–20% discontinued because of adverse events or tolerability issues.39,44,45 Diarrhea has been the most common adverse event in these studies and was mostly reported as mild to moderate in severity.
Despite the potential for adverse events and the relatively small population of nonresponders, tenapanor has positive effects on patient-reported outcomes. Preliminary data from congress presentations of the OPTIMIZE trial show that 85.4% of patients with hyperphosphatemia previously treated with binders who switched to a new treatment plan that reduced their binder dose by ≥50% and added tenapanor (30 mg twice daily) reported an improvement in their overall phosphate management experience.46 The primary reason attributed to a change in perception was a change in medication burden, related to a reduction in the size, number, frequency, or diversity of pills patients used to manage phosphate.46 (Figure 5) Preliminary efficacy results from the OPTIMIZE trial show that serum phosphate was lowered to <5.5 mg/dl in approximately half of all participants that either added tenapanor to a 50% reduced binder regimen or switched completely to only tenapanor.47
Figure 5.
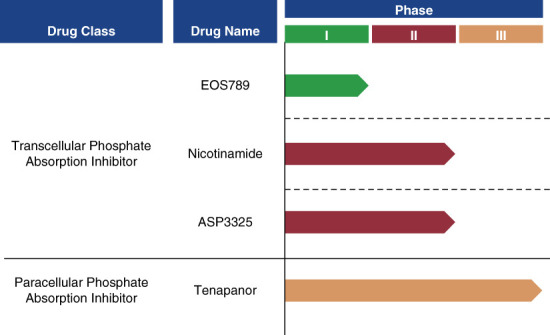
Phosphate absorption inhibitor trials. Two new classes of phosphate management drugs, transcellular and paracellular phosphate absorption inhibitors, are in development.
New Treatment Options Could Give Patients the Freedom to Eat More Healthy Foods and Improve Their Quality of Life
Given the need for improved phosphate control in patients receiving dialysis and the lack of treatment choice regarding classes of agents, new therapies with alternative mechanisms of action are needed to broaden the armamentarium for hyperphosphatemia therapy. New classes of pharmacologic therapies would also allow for combination therapies, with the dual benefits of possibly improving efficacy while lowering potential side effects. Furthermore, the benefit to patient health outcomes vis-à-vis lower phosphate concentrations and potentially lower cardiovascular event rates only describes the clinical/medical side of patient care. The average hemodialysis patient may take 20 pills/d with phosphate binders comprising approximately half the pill burden.26 The addition of phosphate transport blockers to replace all or a portion of the phosphate binder regimen could significantly reduce pill burden.
Often overlooked from the patient's point of view is significantly improved quality of life that would result from additional freedom of food choices and meals if more effective pharmacologic interventions were available. Importantly, more flexible protein intake would also reduce the risk of malnutrition and protein-energy wasting. In addition, patients would experience the social and emotional benefits of being able to participate in meals with family and friends again, instead of preparing and eating separately. We should strive to allow patients and their clinicians the opportunity to tailor appropriate combinations of therapeutic approaches from as wide a field of choices as possible, provided these therapies are deemed safe and effective in reducing serum phosphate in patients with CKD who are receiving dialysis.
Acknowledgments
In memory of Derek Forfang. Medical writing support provided by Xelay Acumen Group, Inc and Ashfield MedComms, an Inizio company, and was funded by Ardelyx, Inc.
Footnotes
Author is deceased.
Disclosures
G. Bakris reports the following—consultancy: Alnylam, AstraZeneca, Bayer, Ionis, Janssen, KBP Biosciences, Medscape, Novo Nordisk; Honoraria: Alnylam, AstraZeneca, Bayer, Ionis, KBP Biosciences, Merck, and Novo Nordisk; advisory or leadership role: KBP Biosciences, American J Nephrology, Editor, Diabetes Care, Assoc. Ed.; American Heart Assoc.; UpToDate-Nephrology; and other interests or relationships: American Diabetes Association, American Heart Association, Blood Pressure Council. D. Forfang reports the following—employer: ASN Kidney Health Initiative (KHI); consultancy: Ardelyx Inc Scientific Advisory Board, ASN, CareDX, HSAG, Responsum, and University of North Carolina Kidney Center; honoraria: Health Service Advisory Group; advisory or leadership role: HSAG ESRD Network #17 Board Member; National Forum of ESRD Networks, Board Member; National Forum of ESRD Networks, Kidney Patient Advisory Council, Chair; Kidney Health Initiative, Patient Advisory Committee; National Kidney Foundation, SONG Group, European Association for Dialysis, Arbor Research and Unity Health Toronto OPPUS, UCSF Kidney Project Patient Advisor; and other interests or relationships: volunteer for the Forum of ESRD Networks as Kidney Patient Advisory Council Chair and Board Member; volunteer for ESRD Network #17 as Patient Advisory Committee Chair and Network Board Member; volunteer for the NKF as a member of their Public Policy Committee; volunteer for the NKF as a Regional Leader of their Kidney Advocacy Committee, KHI PFPC Member. K. Kalantar-Zadeh reports the following—consultancy: Akebia; Ardelyx, Inc.; Fresenius Medical Care Renal Therapies; OPKO. K.J. Martin reports the following—consultancy: Amgen, Applied Therapeutics, Ardelyx; Honoraria: Amgen, Applied Therapeutics, Ardelyx and advisory or leadership role: Amgen, Ardelyx, Clinical Nephrology; DMC for Tricida, Applied Therapeutics. S.M. Moe reports the following—consultancy: Amgen, Ardelyx, Sanifit/Vifor, Inozyme; ownership interest: Eli Lilly (stock); research funding: NIH—research grant; Keryx—research grant; honoraria: Ardelyx, Inozyme, Sanifit; and advisory or leadership role: Editorial Board: AJNephrology, AJ Nutrition. S.M. Sprague reports the following—consultancy: Amgen, Ardelyx, Bayer, Fresenius, Horizon, Litholink Corp, OPKO, Shire, Vifor; ownership interest: individually owned stocks; Apple, Baxter, Bristol Myers, Coca Cola, First Australia Fund, lBM, Walgreens; research funding: Amgen, Ardelyx, OPKO, Reata, Takeda; honoraria: Amgen, Ardelyx, Bayer, Fresenius, Horizon, OPKO, Vifor; advisory or leadership role: National Kidney Foundation of Illinois, American Journal of Nephrology, International Federation of Clinical Chemistry and Laboratory Medicine-Work Group for Parathyroid Hormone, American Association of Endocrine Surgeons; and speakers bureau: Amgen, Bayer, Fresenius, Horizon, OPKO.
Funding
This work was supported by Ardelyx, Inc.
Author Contributions
Conceptualization: George Bakris, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Data curation: George Bakris, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Formal analysis: George Bakris, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Investigation: George Bakris, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Methodology: George Bakris, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Project administration: George Bakris, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Resources: George Bakris, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Software: George Bakris, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Supervision: George Bakris, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Validation: George Bakris, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Writing – original draft: George Bakris, Derek Forfang, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Writing – review & editing: George Bakris, Derek Forfang, Kamyar Kalantar-Zadeh, Kevin J. Martin, Sharon M. Moe, Stuart M. Sprague.
Data Sharing Statement
All data is included in the manuscript and/or supporting information.
References
- 1.USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- 2.Yee J, Rosenbaum D, Jacobs JW, Sprague SM. Small intestinal phosphate absorption: novel therapeutic implications. Am J Nephrol. 2021;52(7):522–530. doi: 10.1159/000518110 [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease Improving Global Outcomes KDIGO CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1–59. doi: 10.1016/j.kisu.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper K, Quarles D, Kubo Y, Tomlin H, Goodman W. Relationship between reductions in parathyroid hormone and serum phosphorus during the management of secondary hyperparathyroidism with calcimimetics in hemodialysis patients. Nephron Clin Pract. 2012;121(3-4):c124–c130. doi: 10.1159/000345164 [DOI] [PubMed] [Google Scholar]
- 5.Goodman WG Goldin J Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–1483. doi: 10.1056/NEJM200005183422003 [DOI] [PubMed] [Google Scholar]
- 6.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16(6):1788–1793. doi: 10.1681/ASN.2004040275 [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K Tortorici AR Chen JL, et al. Dietary restrictions in dialysis patients: is there anything left to eat? Semin Dial. 2015;28(2):159–168. doi: 10.1111/sdi.12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikizler TA Burrowes JD Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(3 suppl 1):S1–S107. doi: 10.1053/j.ajkd.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 9.Khoueiry G, Waked A, Goldman M, El-Charabaty E, Dunne E, Smith M. Dietary intake in hemodialysis patients does not reflect a heart healthy diet. J Ren Nutr. 2011;21(6):438–447. doi: 10.1053/j.jrn.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 10.Rufino M de Bonis E Martín M, et al. Is it possible to control hyperphosphataemia with diet, without inducing protein malnutrition? Nephrol Dial Transplant. 1998;13(suppl 3):65–67. doi: 10.1093/ndt/13.suppl_3.65 [DOI] [PubMed] [Google Scholar]
- 11.Woolley K, Fishbach A, Wang RM. Food restriction and the experience of social isolation. J Pers Soc Psychol. 2020;119(3):657–671. doi: 10.1037/pspi0000223 [DOI] [PubMed] [Google Scholar]
- 12.Cupisti A, Kalantar-Zadeh K. Management of natural and added dietary phosphorus burden in kidney disease. Semin Nephrol. 2013;33(2):180–190. doi: 10.1016/j.semnephrol.2012.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe MT, Araujo RM, Vogt BP, Barretti P, Caramori JCT. Most consumed processed foods by patients on hemodialysis: alert for phosphate-containing additives and the phosphate-to-protein ratio. Clin Nutr ESPEN. 2016;14:37–41. doi: 10.1016/j.clnesp.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 14.Sawin DA Ma L Stennett A, et al. Phosphates in medications: impact on dialysis patients. Clin Nephrol. 2020;93(4):163–171. doi: 10.5414/CN109853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int. 2015;87(6):1097–1099. doi: 10.1038/ki.2015.67 [DOI] [PubMed] [Google Scholar]
- 16.Malindretos P, Cozzolino M. Phosphate binders, past – present – future. A critical appraisal. Expert Opin Pharmacother. 2016;17(3):297–300. doi: 10.1517/14656566.2016.1133593 [DOI] [PubMed] [Google Scholar]
- 17.Friedman EA. An introduction to phosphate binders for the treatment of hyperphosphatemia in patients with chronic kidney disease. Kidney Int Suppl. 2005;68(96):S2–S6. doi: 10.1016/s0085-2538(15)51226-x [DOI] [PubMed] [Google Scholar]
- 18.FOSRENOL® (Lanthanum Carbonate) [Prescribing Information]. Shire US Inc.; 2004. [Google Scholar]
- 19.PhosLo ® Gelcaps (Calcium Acetate): 667 mg [Prescribing Information]. Fresenius Medical Care North America; 2011. [Google Scholar]
- 20.RENVELA ® (Sevelamer Carbonate) [Prescribing Information]. Genzyme Corp.; 2000. [Google Scholar]
- 21.Daugirdas JT, Finn WF, Emmett M, Chertow GM, Frequent Hemodialysis Network Trial Group. The phosphate binder equivalent dose. Semin Dial. 2011;24(1):41–49. doi: 10.1111/j.1525-139X.2011.00849.x [DOI] [PubMed] [Google Scholar]
- 22.Martin P Wang P Robinson A Poole L, et al. Comparison of dietary phosphate absorption after single doses of lanthanum carbonate and sevelamer carbonate in healthy volunteers: a balance study. Am J Kidney Dis. 2011;57(5):700–706. doi: 10.1053/j.ajkd.2010.11.028 [DOI] [PubMed] [Google Scholar]
- 23.León JB, Sullivan CM, Sehgal AR. The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J Ren Nutr. 2013;23(4):265–270.e2. doi: 10.1053/j.jrn.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell RR, Draper HH, Tzeng DY, Shin HK, Schmidt GR. Physiological responses of human adults to foods containing phosphate additives. J Nutr. 1977;107(1):42–50. doi: 10.1093/jn/107.1.42 [DOI] [PubMed] [Google Scholar]
- 25.McClure ST, Chang AR, Selvin E, Rebholz CM, Appel LJ. Dietary sources of phosphorus among adults in the United States: results from NHANES 2001-2014. Nutrients. 2017;9(2):95. doi: 10.3390/nu9020095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–1096. doi: 10.2215/CJN.00290109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.AURYXIA® (Ferric Citrate) Tablets [Prescribing Information]. Keryx Biopharmaceuticals Inc.; 2017. [Google Scholar]
- 28.Wang S, Anum EA, Ramakrishnan K, Alfieri T, Braunhofer P, Newsome B. Reasons for phosphate binder discontinuation vary by binder type. J Ren Nutr. 2014;24(2):105–109. doi: 10.1053/j.jrn.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 29.Serum Phosphorus (Most Recent), Categories. Accessed August 18. https://www.dopps.org/DPM-HD/Files/phosphmgdl_c_overallTAB.htm [Google Scholar]
- 30.Guedes M, Bieber B, Dasgupta I, Vega A, Nitta K, Brunelli S. Serum phosphorus level rises in US hemodialysis patients over the past decade: a DOPPS special report. Kidney Med. 2023;5(2):100584. doi: 10.1016/j.xkme.2022.100584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VELTASSA® (Patiromer) for Oral Suspension [Prescribing Information]. Relypsa; 2015. [Google Scholar]
- 32.LOKELMA™ (Sodium Zirconium Cyclosilicate) for Oral Suspension [Prescribing Information]. AstraZeneca; 2018. [Google Scholar]
- 33.Epstein M. Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl (2011). 2016;6(1):20–28. doi: 10.1016/j.kisu.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weir MR Bakris GL Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–221. doi: 10.1056/NEJMoa1410853 [DOI] [PubMed] [Google Scholar]
- 35.Hill Gallant KM Stremke ER, et al. EOS789, a broad-spectrum inhibitor of phosphate transport, is safe with an indication of efficacy in a phase 1b randomized crossover trial in hemodialysis patients. Kidney Int. 2021;99(5):1225–1233. doi: 10.1016/j.kint.2020.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eto N, Miyata Y, Ohno H, Yamashita T. Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant. 2005;20(7):1378–1384. doi: 10.1093/ndt/gfh781 [DOI] [PubMed] [Google Scholar]
- 37.Ix JH Isakova T Larive B, et al. Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: the COMBINE trial. J Am Soc Nephrol. 2019;30(6):1096–1108. doi: 10.1681/ASN.2018101058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsson TE Kameoka C Nakajo I, et al. NPT-IIb inhibition does not improve hyperphosphatemia in CKD. Kidney Int Rep. 2018;3(1):73–80. doi: 10.1016/j.ekir.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Block GA, Rosenbaum DP, Yan A, Chertow GM. Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: a randomized phase 3 trial. J Am Soc Nephrol. 2019;30(4):641–652. doi: 10.1681/ASN.2018080832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fishbane SN, Nigwekar S. Phosphate absorption and hyperphosphatemia management in kidney disease: a physiology-based review. Kidney Med. 2021;3(6):1057–1064. doi: 10.1016/j.xkme.2021.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King AJ Siegel M He Y, et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med. 2018;10(456):eaam6474. doi: 10.1126/scitranslmed.aam6474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marks J, Lee GJ, Nadaraja SP, Debnam ES, Unwin RJ. Experimental and regional variations in Na+-dependent and Na+-independent phosphate transport along the rat small intestine and colon. Physiol Rep. 2015;3(1):e12281. doi: 10.14814/phy2.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knöpfel T, Himmerkus N, Günzel D, Bleich M, Hernando N, Wagner CA. Paracellular transport of phosphate along the intestine. Am J Physiol Gastrointest Liver Physiol. 2019;317(2):G233–G241. doi: 10.1152/ajpgi.00032.2019 [DOI] [PubMed] [Google Scholar]
- 44.Block GA Bleyer AJ Silva AL, et al. Safety and efficacy of tenapanor for long-term serum phosphate control in maintenance dialysis: a 52-week randomized phase 3 trial (PHREEDOM). Kidney360. 2021;2(10):1600–1610. doi: 10.34067/KID.0002002021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pergola PE, Rosenbaum DP, Yang Y, Chertow GM. A randomized trial of tenapanor and phosphate binders as a dual-mechanism treatment for hyperphosphatemia in patients on maintenance dialysis (AMPLIFY). J Am Soc Nephrol. 2021;32(6):1465–1473. doi: 10.1681/ASN.2020101398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patient-Reported Experience With Tenapanor in the OPTIMIZE Trial. Kidney Week; 2021. [Google Scholar]
- 47.Fishbane S Rosenbaum D Yang Y, et al. A randomized, open-label study to evaluate potential real-world use of tenapanor as the core therapy in the treatment of hyperphosphatemia in patients with chronic kidney disease on dialysis (OPTIMIZE). 58th Annual ERA-EDTA Congress June 5-6, 2021; Virtual; 2021. [Google Scholar]
- 48.Hou SH Zhao J Ellman CF, et al. Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis. 1991;18(2):217–224. doi: 10.1016/s0272-6386(12)80882-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is included in the manuscript and/or supporting information.