Abstract
Background
SWOG 0809 is the only prospective study of adjuvant chemotherapy followed by chemoradiation focusing on margin status in patients with extrahepatic cholangiocarcinoma (EHCC) and gallbladder cancer (GBCA). However, the effects of adjuvant therapy by nodal status have never been reported in this population.
Methods
Patients with resected EHCC and GBCA, stage pT2-4, N+ or margin-positive (R1) who completed four cycles of chemotherapy followed by radiotherapy were included. Cox regression were used to compare overall survival (OS), disease-free survival (DFS), local recurrence and distant metastasis by nodal status. DFS rates were compared to historical data via 1-sample t-test.
Results
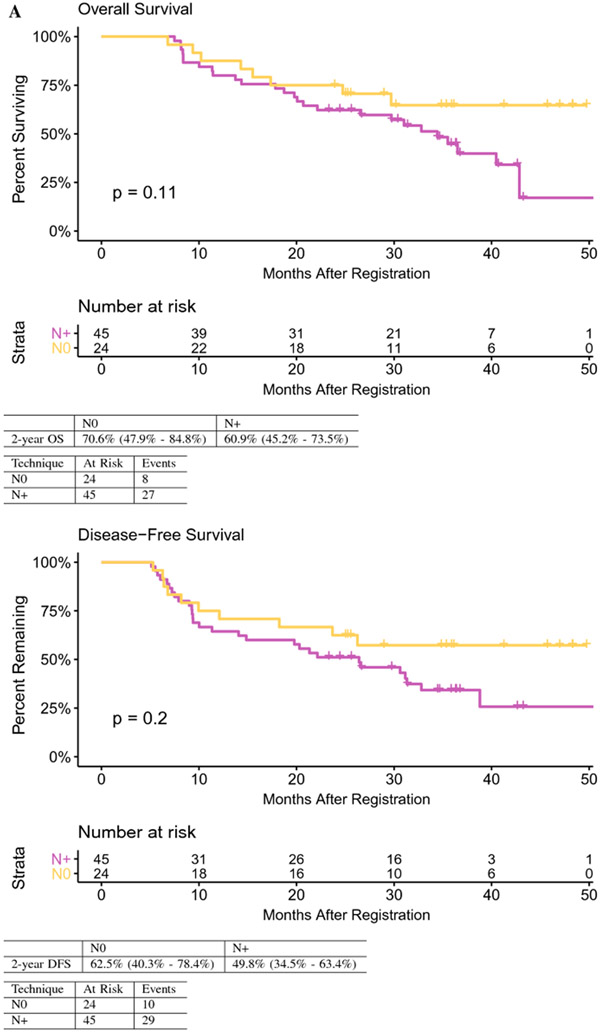
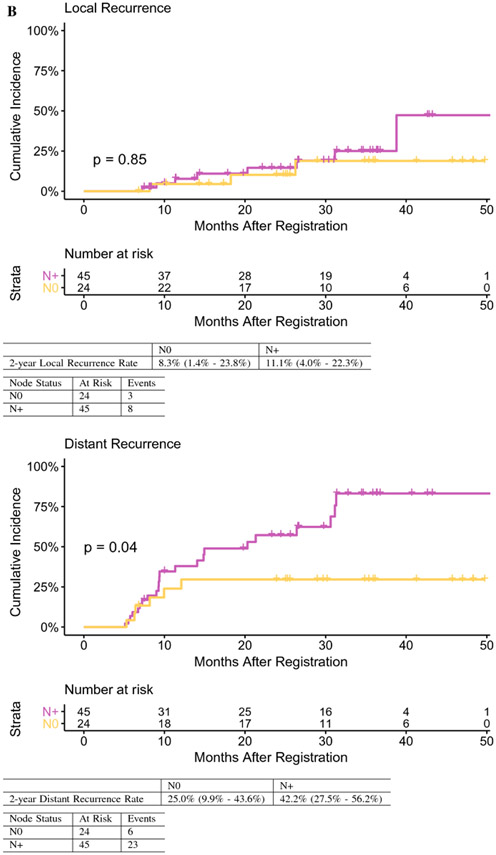
Sixty-nine patients [EHCC n=46 (66%); GBCA n= 23 (33%)] were evaluated with median age of 61.7 years and R0 (66.7%) and R1 (33.3%) rates. EHCC vs GBCA were more likely to be node positive (N+) (73.9% vs 47.8%, p=0.03). Nodal status did not significantly impact OS (HR 1.98, 95% CI 0.86-4.54, p=0.11) or DFS (HR 1.63, 95% CI 0.77-3.44, p=0.20). Two-year OS was 70.6% for node negative (N0) and 60.9% for N+ disease; 2-year DFS was 62.5% for N0 and 49.8% for N+ tumors. N+ vs N0 tumors showed higher rates of distant failure (42.2% vs 25.0%, p=0.04). The two-year DFS rate in N+ tumors was significantly higher than in historic controls (49.8% vs. 29.7%, p=0.004).
Conclusions
Adjuvant therapy is associated with favorable outcome independent of nodal status and may impact local control in N+ patients. These data could serve as benchmark for future adjuvant trials, including molecular targeted agents.
Introduction
Biliary tract cancers (BTC) including gallbladder cancer (GBCA), extrahepatic and intrahepatic cholangiocarcinoma (CCA), are a rare and heterogeneous group of tumors with poor prognosis1-3 The majority of patients present with locally advanced disease.4, 5 Only a minority of patients (less than 10% for GBCAs and 25% for CCAs) are candidates for radical resection, the only potential curative treatment.6, 7 Without further treatment, local recurrence rates remained high (60-75%) at a median 2-year follow up8, 9 following curative resection. This has led to the emerging role of adjuvant systemic therapy and chemoradiation for patients with resected BTC based on the assumption that improving locoregional and systemic disease control may improve survival.10-15
Given the rarity and heterogeneity of these tumors, the role of adjuvant systemic plus chemoradiation therapy for BTC has not been well established. Most data are derived from small, retrospective studies that have shown conflicting results. Nassour et al. suggested that adjuvant chemotherapy and chemoradiotherapy were associated with improved survival in resected perihilar cholangiocarcinoma in a retrospective analysis with propensity matching using the National Cancer Database.16 However, others have described minimal benefit from adjuvant radiotherapy on survival for patients with extrahepatic cholangiocarcinoma using Surveillance, Epidemiological, and End Results (SEER).17,18 Similar conflicting findings have been reported for patients with GBCA.8,19-25 Most of these studies show a short-term survival benefit of adjuvant radiotherapy in resected GBCA patients; however, this benefit did not persist at 5-year follow up.24, 25 These discordant results created the need for a prospective study to evaluate the effect of adjuvant chemotherapy and chemoradiotherapy in patients with resected BTC.
To date, SWOG 0809 remains the only prospective clinical trial to evaluate the efficacy of adjuvant radiation in conjunction with systemic chemotherapy in patients with resected BTC. This phase II, single arm trial included 79 patients with T2-4 EHCC or GBCA, irrespective of margin and nodal status, who received adjuvant chemotherapy with concurrent chemoradiation after curative resection and showed improved survival compared to historical controls.26 Median overall survival was 35 months (R0, 34 months; R1, 35 months).
Furthermore, it has been well established that lymph node status is a prognostic factor for recurrence in BTC. Recent meta-analyses have shown improved locoregional recurrence rates in this patient population receiving adjuvant radiation following resection.27, 28 A recent meta-analysis reported improved pooled locoregional control of 52.1% versus 34.9% (p = 0.014) for patients with and without adjuvant radiation. In the sensitivity analysis on 14 eligible studies, the authors showed lower margin negative rate (36.8% vs. 63.2%, p = 0.02) and a trend towards higher rate of node positive disease (47.4% vs. 34.9%, p = 0.08) in the group of patients receiving adjuvant radiotherapy. Similar favorable outcomes using adjuvant radiation have been described in other studies, with the greatest benefit in the node positive group.28-30 SWOG 0809 reported on the effect of adjuvant chemotherapy followed by radiation with concurrent chemotherapy with a focus on margin status. However, the effect of adjuvant chemoradiation by lymph node status for BTC has never been reported in a prospective clinical trial. Therefore, the objective for this study was to perform a secondary analysis of SWOG 0809 and delineate the effect of adjuvant chemoradiation on DFS and OS according to lymph node status following resection. We hypothesized that adjuvant chemotherapy and chemoradiation provided a clinical benefit to patients with node positive disease compared to historic controls.
Methods
Patients
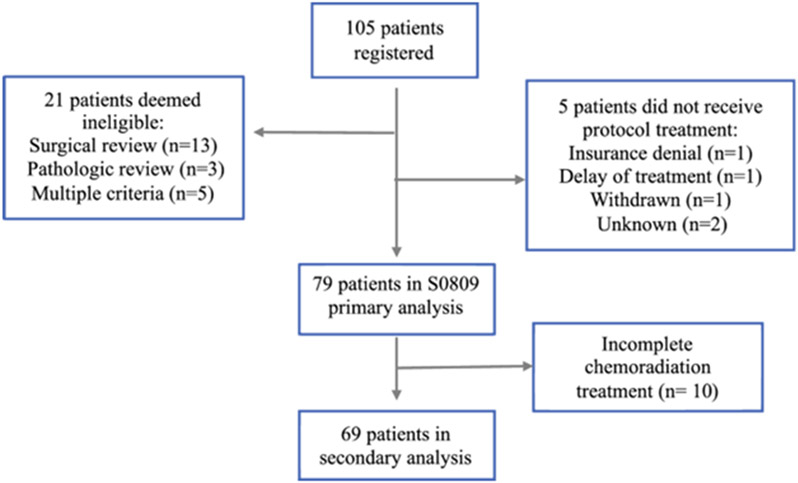
SWOG 0809 included patients with a diagnosis of EHCC or GBCA (pathologic stage T2-4 irrespective of nodal or margin status) who underwent complete resection.26 Eligibility criteria for the original trial included 1) no prior systemic therapy for EHCC or GBCA or prior radiation to the upper abdomen, 2) favorable performance status (KPS 0,1), and 3) an absolute neutrophil count ≥ 1,500/mcl, platelets ≥ 100,000/mcl, serum creatinine ≤ 1.5 mg/dL, total bilirubin ≤ 1.5 × institutional upper limit of normal, and either AST or ALT ≤ 2.5 × institutional upper limit of normal. Patients were followed every 3 months after completion of adjuvant therapy, with surveillance imaging of the chest, abdomen, and pelvis (CT or MRI) every 6 months for 2 years. The primary trial analysis of SWOG 0809 included 79 eligible and evaluable patients (Figure 1). Ten patients from the original trial were excluded from this secondary analysis (EHCC, n = 8; GBCA, n = 2) as they did not complete radiation therapy (early progression, n = 5; personal reasons, n = 3; toxicity, n = 1; unknown reason, n = 1).
Fig. 1.
Study Cohort
Treatment
Adjuvant treatment consisted of four cycles of chemotherapy with gemcitabine (1,000 mg/m2 intravenously on days 1 and 8) and capecitabine (1,500 mg/m2 per day on days 1 to 14, in divided doses twice daily) every 21 days. If no progression was seen on interval imaging, patients then proceeded with capecitabine (1,330 mg/m2 per day, in divided doses twice daily, 7 days per week) and concurrent radiotherapy (45 Gy to regional lymph nodes [retropancreaticoduodenal, celiac, and portal vein nodes] and 54 to 59.4 Gy to the preoperative tumor bed). Radiation therapy using three-dimensional planning was dosed at 54 Gy in 30 fractions. For patients who underwent image guided intensity-modulated radiotherapy (IMRT), a concurrent boost was added for a total dose of 52.5 Gy in 25 fractions. Details of the radiation therapy were summarized previously.26
Statistical considerations
Statistical differences between baseline demographic and clinical characteristics according to lymph node status and disease site were assessed via Mann-Whitney and chi-squared tests. Probabilities of OS and DFS were estimated using the Kaplan-Meier method. The probabilities of local recurrence (LR) and distant relapse were summarized using cumulative incidence estimates; death without recurrence was treated as a competing risk for recurrence events. Data from patients last known to be alive and/or free of disease were censored at date of last contact. Statistical differences in event rates between groups according to lymph node status were assessed via Cox regression models with stratification for disease site. The strength of associations between treatment characteristics and incidence of LR were tested via Fisher’s exact test. Observed DFS rates were compared to historical data via 1-sample t-test, with historical rates calculated from previous studies32-34 (median for N+, 13.7 months vs N0, 39.3 months); median DFS of 13.7 months is equivalent to a 2-year DFS of 29.7%.
Results
Patient characteristics
A total of 69 patients [EHCC n=46 (66%); GBCA n=23 (33%)] with a median age of 61.7 (range 26.1-80.6 years) received adjuvant therapy and were included in this secondary analysis (Table 1). The majority of node negative patients were female (17/24, 70.8%), whereas most node positive patients were male (25/45, 55.6%; p=0.04). Patients with EHCC were more likely than those with GBCA to have node positive disease (73.9% vs 47.8%, p=0.03). Patients with GBCA were more likely than those with EHCC to have KPS=1 (56.5% vs 26.1%, p=0.02) (Table 2). Eighty one percent of patients underwent IMRT, whereas only 19% received three-dimensional planning. Median dose to R0 and R1 patients was 52.5Gy and 54Gy, respectively. Treatment interruptions were seen in 21 patients (while receiving radiotherapy and concurrent chemotherapy, n = 7; radiotherapy only, n = 1; chemotherapy only, n = 13).
Table 1:
Baseline Characteristics by Lymph Node Status (n=69)
| N0 (n=24) | N+ (n=45) | p-value* | |
|---|---|---|---|
| Age: median (range) | 60.1 (26.1-80.6) | 61.7 (26.7-80.3) | 0.98 |
| Sex | 0.04 | ||
| Female | 17 (70.8%) | 20 (44.4%) | |
| Male | 7 (29.2%) | 25 (55.6%) | |
| Hispanic | 0.78 | ||
| Yes | 2 (8.3%) | 2 (4.4%) | |
| No | 19 (79.2%) | 38 (84.5%) | |
| Unknown | 3 (12.5%) | 5 (11.1%) | |
| Race | 0.92 | ||
| Black | 2 (8.3%) | 5 (11.1%) | |
| Asian | 1 (4.2%) | 3 (6.7%) | |
| White | 20 (83.3%) | 36 (80%) | |
| Unknown | 1 (4.2%) | 1 (2.2%) | |
| Disease Site | 0.03 | ||
| Gallbladder | 12 (50%) | 11 (24.4%) | |
| Bile duct | 12 (50%) | 34 (75.6%) | |
| Performance Status | 0.57 | ||
| 0 | 14 (58.3%) | 23 (51.1%) | |
| 1 | 10 (41.7%) | 22 (48.9%) | |
| Resection Margin | 0.99 | ||
| R0 | 16 (66.7%) | 30 (66.7%) | |
| R1 | 8 (33.3%) | 15 (33.3%) | |
| Radiation Modality | 0.94 | ||
| IMRT | 19 (79.2%) | 36 (80%) | |
| 3D | 5 (20.8%) | 9 (20%) |
Mann-Whitney U-test for age and Chi-squared test for categorical variables
Abbreviations: N0, node negative; N+, node positive; IMRT, intensity modulated radiation therapy; 3D, three-dimensional conformal radiation therapy
Table 2:
Baseline Characteristics by Disease Site
| Bile duct (n=46) | Gallbladder (n=23) |
p-value* | |
|---|---|---|---|
| Age: median (range) | 60.2 (26.1 – 80.3) | 67.7 (41 – 80.6) | 0.15 |
| Sex | 0.001 | ||
| Female | 18 (39.1%) | 19 (82.6%) | |
| Male | 28 (60.9%) | 4 (17.4%) | |
| Hispanic | 0.34 | ||
| Yes | 2 (4.4%) | 2 (8.7%) | |
| No | 37 (80.4%) | 20 (86.9%) | |
| Unknown | 7 (15.2%) | 1 (4.4%) | |
| Race | 0.12 | ||
| Black | 2 (4.4%) | 5 (21.7%) | |
| Asian | 3 (6.4%) | 1 (4.4%) | |
| White | 39 (84.8%) | 17 (73.9%) | |
| Unknown | 2 (4.4%) | 0 | |
| Lymph node | 0.03 | ||
| N0 | 12 (26.1%) | 12 (52.2%) | |
| N+ | 34 (73.9%) | 11 (47.8%) | |
| Performance Status | 0.02 | ||
| 0 | 20 (43.5%) | 17 (73.9%) | |
| 1 | 26 (56.5%) | 6 (26.1%) | |
| Resection Margin | 0.37 | ||
| R0 | 29 (63%) | 17 (73.9%) | |
| R1 | 17 (37%) | 6 (26.1%) | |
| Radiation Modality | 0.67 | ||
| IMRT | 36 (78.3%) | 19 (82.6%) | |
| 3D | 10 (21.7%) | 4 (17.4%) |
Mann-Whitney U-test for age and Chi-squared test for categorical variables
Abbreviations: IMRT, intensity modulated radiation therapy; 3D, three-dimensional conformal radiation therapy
Clinical outcomes
OS and DFS were greater in patients with node negative compared to node positive disease, although the differences did not reach statistical significance. Two-year OS was 70.6% for node negative and 60.9% for node positive disease (HR 1.98, 95% CI 0.86-4.54, p=0.11). Two-year DFS was 62.5% for node negative and 49.8% for node positive disease (HR 1.63, 95% CI 0.77-3.44, p=0.20) (Figure 2A). The observed 2-year DFS in patients with node positive tumors was significantly higher than the historical rate of 29.7% (p=0.004).
Fig. 2.
A Overall and disease-free survival by lymph node status.
B Local recurrence and distant recurrence by lymph node status. OS overall survival
A total of 11 patients developed LR, of whom eight experienced a concurrent distant relapse; 21 patients developed distant-only relapse (Table 3). Node positive vs. node negative tumors showed a higher rate of 2-year distant failure (42.2% vs 25.0%; HR 2.57, 95% CI: 1.04-6.38, p=0.04) (Figure 2B) but similar LR rates (11.1% vs 8.3%; HR 1.13, 95% CI 0.30-4.28, p=0.85), suggesting improved local control for node positive patients receiving adjuvant radiation therapy.
Table 3.
Pattern of First Relapse*
| Recurrence | EHCC Distal (n=31) |
EHCC Hilar (n=13) |
GBCA (n=23) |
|---|---|---|---|
| Local only | 2 (6) | 1 (8) | 0 |
| Local plus distant | 4 (13) | 2 (15) | 2 (9) |
| Distant only | 11 (35) | 1 (8) | 9 (39) |
| Total | 17 (54) | 4 (31) | 11 (48) |
2 patients for whom complete data was not available were excluded
Abbreviations: EHCC, extra hepatic cholangiocarcinoma; GBCA, gallbladder carcinoma
Discussion
Biliary tract cancers, which include GBCA and EHCC, are a heterogeneous group of rare, biologically aggressive tumors characterized by high frequency of regional lymph node and distant metastatic spread.35, 36 Due to the relative rarity of BTC and limited prospective clinical trials, evidence-based treatment regimens targeting these tumors are not well established. To date, SWOG 0809 has been the only Phase II clinical trial that assessed the efficacy of adjuvant chemotherapy and chemoradiation for BTC patients.26 Here, we present a secondary analysis of the SWOG 0809 clinical trial data. Specifically, we examine the relationships between lymph node status, recurrence patterns, and survival.
Nodal involvement in both EHCC and GBCA37, 38 has been well established as playing a significant role on survival. Several retrospective studies demonstrated that lymph node status predicts survival in BTC patients.39-44 Only a few prospective clinical trials focused on BTC report on lymph node status and survival.32, 45 However, a meta-analysis of 20 studies analyzing 6712 patients with BTC proposed lymph node positivity as an indication for adjuvant therapy.28 In that report, adjuvant therapy was associated with a higher survival rate compared with surgery alone in patients with either node positive or margin positive resections, but this association did not reach statistical significance (OR 0.74, 95% CI 0.55–1.01, p= 0.06). This improvement was statistically significant with adjuvant chemotherapy or chemoradiotherapy but not with radiotherapy alone. Looking at specific subgroups, a pooled analysis of nine studies confirmed a significant survival benefit of any adjuvant therapy in patients with node positive disease (OR 0.49, 95% CI 0.30-0.80, p = .004). Of note, less than a third of patients received combination chemoradiotherapy. Additionally, a nomogram developed for GBCA using the SEER-Medicare database suggests a survival advantage using adjuvant chemoradiation for T2 and node-positive patients, and that chemoradiotherapy provides greater benefit than chemotherapy alone in all patient subsets46. Finally, the recent BILCAP study showed a survival benefit of adjuvant capecitabine compared with observation alone in patients with margin and node positive BTC in a per-protocol analysis, although the intention-to treat analysis did not show a statistically significant difference. Although the BILCAP study did not meet its primary endpoint of improving survival in the intention-to-treat population, the prespecified per-protocol analysis suggest a potential survival benefit and has been considered standard of care. However, the benefit of adjuvant capecitabine was not seen in the hilar cholangiocarcinoma subtype on subgroup analysis. In our secondary analysis of SWOG 0809, lymph node status did not significantly impact OS (HR 2.03, p=0.08) or DFS (HR 1.75, p=0.13). However, the node positive patients had higher DFS rates compared to historical controls.
It has been well described in the literature that the predominant pattern of initial treatment failure is locoregional disease for extrahepatic bile duct tumors.47 Based on this rationale, adjuvant radiation has been offered to this patient population despite no clear evidence from randomized, phase III trials. In our clinical trial cohort, we observed a higher proportion of tumor with nodal involvement in patients with EHCA (75.6%) compared to GBCA (24.4%). With regards to patterns of recurrence, local-only recurrence was a rare event. In fact, the 2-year local recurrence was not statistically different between node negative patients (8%) and node positive patients (11%). However, distant recurrence rates differed by disease subtype and appeared to be more dependent on nodal status with node positive patients experiencing a 42% distant recurrence rate compared to 25% of node negative patients. For example, the distant only failure rates were significantly higher in the patients with GBCA (39%) compared to only 8% for patients with hilar cholangiocarcinoma despite the latter having a higher incidence of node positivity. Although limited by sample size and single-arm design, one can speculate that these low local-only recurrence rates could be associated with treatment effects of adjuvant chemoradiation. This hypothesis remains to be formally studied in larger controlled trials. In an earlier trial of adjuvant chemotherapy for resected BTC, mitomycin-C appeared to confer a benefit to GBCA patients but not bile duct cancer patients. However, this effect was not significant in intention-to-treat analysis. Interestingly, nodal positivity was nearly universal in both cohorts (>80%), and a significant portion of patients underwent non-curative intent surgery.48 Given these potential biologic differences between gallbladder cancer and bile duct cancer, we would strongly advocate for disease and site-specific trials.
Identifying relevant historical survival data from patients undergoing resection for BTC presents a challenge as results vary based on tumor type, stage, residual disease at time of surgical exploration, and additional adjuvant therapy. For example, Butte et al. reported a median DFS of 15 vs. 41 months for patients with and without nodal disease at time of exploration for GBCA.34 A slightly higher median DFS was reported by the same group for patients with resected GBCA (N0: 34 mo, N+: 19 mo). Of note, about 13-18% of patients in both studies received adjuvant chemotherapy. Similarly, worse survival data have been reported for patients with hilar cholangiocarcinoma undergoing resection with positive lymph nodes with median DFS of only 7 months. The historical 2-year DFS for patients with node positive disease was 29.7%.32,33,34 We show that SWOG 0809 node positive patients treated with adjuvant chemotherapy and chemoradiation experienced significantly longer DFS (49.8%, p=0.004). Together, our findings suggest that a patient’s lymph node status could inform treatment recommendations.
Our study is limited by several factors, including a small sample size, tumor heterogeneity (as with most BTC studies), a single-arm design, and two radiation modalities. However, this is the first analysis to our knowledge that evaluates the impact of nodal disease on survival in patients with EHCC and GBCA who received adjuvant radiation in a prospective clinical trial setting. With the advancement of more effective systemic regimens (gemcitabine, cisplatin and nab-paclitaxel), immune-based regimens with systemic chemotherapy, and multiple novel targeted drugs for actionable mutations for BTC, it becomes even more critical to improve local control rates in this disease. Given the explosion of molecular profiling and FDA-approval of multiple targeted agents, our findings could serve as a baseline comparison for future clinical trials.
Synopsis:
Lymph node status is a strong predictor for recurrence in biliary cancers. A secondary analysis of S0809 was performed to estimate survival benefits of adjuvant chemoradiation based on nodal status in this population compared to historic controls.
Acknowledgments:
We thank all the patients, their families, and the investigators who participated in the study.
Funding:
This study was supported by grants from the National Institutes of Health for design and conduct of the study, data collection, management, analysis, and interpretation of the data (all authors); grant U10CA180888 from NCI to (Dr Blanke); grant U10CA180819 from NCI to (Dr LeBlanc).
Role of the Funder/Sponsor:
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosure: The authors have declared no conflicts of interest.
References:
- 1.Goetze TO, Gallbladder carcinoma: Prognostic factors and therapeutic options. World journal of gastroenterology 2015, 21 (43), 12211–12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarz RE; Smith DD, Lymph node dissection impact on staging and survival of extrahepatic cholangiocarcinomas, based on U.S. population data. J Gastrointest Surg 2007, 11 (2), 158–65. [DOI] [PubMed] [Google Scholar]
- 3.Jong M. C.d. ; Nathan H; Sotiropoulos GC; Paul A; Alexandrescu S; Marques H; Pulitano C; Barroso E; Clary BM; Aldrighetti L; Ferrone CR; Zhu AX; Bauer TW; Walters DM; Gamblin TC; Nguyen KT; Turley R; Popescu I; Hubert C; Meyer S; Schulick RD; Choti MA; Gigot J-F; Mentha G; Pawlik TM, Intrahepatic Cholangiocarcinoma: An International Multi-Institutional Analysis of Prognostic Factors and Lymph Node Assessment. Journal of Clinical Oncology 2011, 29 (23), 3140–3145. [DOI] [PubMed] [Google Scholar]
- 4.Azizi AA; Lamarca A; McNamara MG; Valle JW, Chemotherapy for advanced gallbladder cancer (GBC): A systematic review and meta-analysis. Crit Rev Oncol Hematol 2021, 163, 103328. [DOI] [PubMed] [Google Scholar]
- 5.Marcano-Bonilla L; Mohamed EA; Mounajjed T; Roberts LR, Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol 2016, 5 (5), 61. [DOI] [PubMed] [Google Scholar]
- 6.Fong Y; Wagman L; Gonen M; Crawford J; Reed W; Swanson R; Pan C; Ritchey J; Stewart A; Choti M, Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg 2006, 243 (6), 767–71; discussion 771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Vugt JLA; Gaspersz MP; Coelen RJS; Vugts J; Labeur TA; de Jonge J; Polak WG; Busch ORC; Besselink MG; IJzermans JNM; Nio CY; van Gulik TM; Willemssen FEJA; Groot Koerkamp B, The prognostic value of portal vein and hepatic artery involvement in patients with perihilar cholangiocarcinoma. HPB (Oxford) 2018, 20 (1), 83–92. [DOI] [PubMed] [Google Scholar]
- 8.Jarnagin WR; Ruo L; Little SA; Klimstra D; D'Angelica M; DeMatteo RP; Wagman R; Blumgart LH; Fong Y, Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003, 98 (8), 1689–700. [DOI] [PubMed] [Google Scholar]
- 9.Park SW; Park YS; Chung JB; Kang JK; Kim KS; Choi JS; Lee WJ; Kim BR; Song SY, Patterns and relevant factors of tumor recurrence for extrahepatic bile duct carcinoma after radical resection. Hepatogastroenterology 2004, 51 (60), 1612–8. [PubMed] [Google Scholar]
- 10.González González D; Gerard JP; Maners AW; De la Lande-Guyaux B; Van Dijk-Milatz A; Meerwaldt JH; Bosset JF; Van Dijk JD, Results of radiation therapy in carcinoma of the proximal bile duct (Klatskin tumor). Semin Liver Dis 1990, 10 (2), 131–41. [DOI] [PubMed] [Google Scholar]
- 11.Kresl JJ; Schild SE; Henning GT; Gunderson LL; Donohue J; Pitot H; Haddock MG; Nagorney D, Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys 2002, 52 (1), 167–75. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald OK; Crane CH, Palliative and postoperative radiotherapy in biliary tract cancer. Surg Oncol Clin N Am 2002, 11 (4), 941–54. [DOI] [PubMed] [Google Scholar]
- 13.Mahe M; Romestaing P; Talon B; Ardiet JM; Salerno N; Sentenac I; Gerard JP, Radiation therapy in extrahepatic bile duct carcinoma. Radiother Oncol 1991, 21 (2), 121–7. [DOI] [PubMed] [Google Scholar]
- 14.Mahe M; Stampfli C; Romestaing P; Salerno N; Gerard JP, Primary carcinoma of the gall-bladder: potential for external radiation therapy. Radiother Oncol 1994, 33 (3), 204–8. [DOI] [PubMed] [Google Scholar]
- 15.Todoroki T; Ohara K; Kawamoto T; Koike N; Yoshida S; Kashiwagi H; Otsuka M; Fukao K, Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys 2000, 46 (3), 581–7. [DOI] [PubMed] [Google Scholar]
- 16.Nassour I; Mokdad AA; Porembka MR; Choti MA; Polanco PM; Mansour JC; Minter RM; Wang SC; Yopp AC, Adjuvant Therapy Is Associated With Improved Survival in Resected Perihilar Cholangiocarcinoma: A Propensity Matched Study. Ann Surg Oncol 2018, 25 (5), 1193–1201. [DOI] [PubMed] [Google Scholar]
- 17.Shinohara ET; Mitra N; Guo M; Metz JM, Radiotherapy is associated with improved survival in adjuvant and palliative treatment of extrahepatic cholangiocarcinomas. Int J Radiat Oncol Biol Phys 2009, 74 (4), 1191–8. [DOI] [PubMed] [Google Scholar]
- 18.Vern-Gross TZ; Shivnani AT; Chen K; Lee CM; Tward JD; MacDonald OK; Crane CH; Talamonti MS; Munoz LL; Small W, Survival outcomes in resected extrahepatic cholangiocarcinoma: effect of adjuvant radiotherapy in a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys 2011, 81 (1), 189–98. [DOI] [PubMed] [Google Scholar]
- 19.Czito BG; Hurwitz HI; Clough RW; Tyler DS; Morse MA; Clary BM; Pappas TN; Fernando NH; Willett CG, Adjuvant external-beam radiotherapy with concurrent chemotherapy after resection of primary gallbladder carcinoma: a 23-year experience. Int J Radiat Oncol Biol Phys 2005, 62 (4), 1030–4. [DOI] [PubMed] [Google Scholar]
- 20.Baeza M; Reyes J; del Castillo C, Post-operative adjuvant radiochemotherapy in the treatment of gallbladder cancer. Presented at the 47th Annual Meeting of the American Society of Therapeutic Radiation Oncology, Denver, CO. 2005. [Google Scholar]
- 21.Gold DG; Miller RC; Haddock MG; Gunderson LL; Quevedo F; Donohue JH; Bhatia S; Nagorney DM, Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys 2009, 75 (1), 150–5. [DOI] [PubMed] [Google Scholar]
- 22.Cho SY; Kim SH; Park SJ; Han SS; Kim YK; Lee KW; Lee WJ; Woo SM; Kim TH, Adjuvant chemoradiation therapy in gallbladder cancer. J Surg Oncol 2010, 102 (1), 87–93. [DOI] [PubMed] [Google Scholar]
- 23.Mojica P; Smith D; Ellenhorn J, Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. J Surg Oncol 2007, 96 (1), 8–13. [DOI] [PubMed] [Google Scholar]
- 24.Mantripragada KC; Hamid F; Shafqat H; Olszewski AJ, Adjuvant Therapy for Resected Gallbladder Cancer: Analysis of the National Cancer Data Base. J Natl Cancer Inst 2017, 109 (2). [DOI] [PubMed] [Google Scholar]
- 25.Hyder O; Dodson RM; Sachs T; Weiss M; Mayo SC; Choti MA; Wolfgang CL; Herman JM; Pawlik TM, Impact of adjuvant external beam radiotherapy on survival in surgically resected gallbladder adenocarcinoma: a propensity score-matched Surveillance, Epidemiology, and End Results analysis. Surgery 2014, 155 (1), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Josef E; Guthrie KA; El-Khoueiry AB; Corless CL; Zalupski MM; Lowy AM; Thomas CR; Alberts SR; Dawson LA; Micetich KC; Thomas MB; Siegel AB; Blanke CD, SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol 2015, 33 (24), 2617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi SH; Rim CH; Shin IS; Yoon WS; Koom WS; Seong J, Adjuvant Radiotherapy for Extrahepatic Cholangiocarcinoma: A Quality Assessment-Based Meta-Analysis. Liver Cancer 2021, 10 (5), 419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horgan AM; Amir E; Walter T; Knox JJ, Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012, 30 (16), 1934–40. [DOI] [PubMed] [Google Scholar]
- 29.Gerhards MF; van Gulik TM; González González D; Rauws EA; Gouma DJ, Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg 2003, 27 (2), 173–9. [DOI] [PubMed] [Google Scholar]
- 30.Todoroki T; Kawamoto T; Otsuka M; Koike N; Yoshida S; Takada Y; Adachi S; Kashiwagi H; Fukao K; Ohara K, Benefits of combining radiotherapy with aggressive resection for stage IV gallbladder cancer. Hepatogastroenterology 1999, 46 (27), 1585–91. [PubMed] [Google Scholar]
- 31.Rosati L; Charu V; Hacker-Prietz A; Zheng L; Cosgrove D; Pawlik T; Herman J, Adjuvant Chemoradiation Therapy in Cholangiocarcinoma: A Single-Institution Experience. International Journal of Radiation Oncology, Biology, Physics 2015, 93 (3), E177. [Google Scholar]
- 32.Ito F; Agni R; Rettammel RJ; Been MJ; Cho CS; Mahvi DM; Rikkers LF; Weber SM, Resection of Hilar Cholangiocarcinoma: Concomitant Liver Resection Decreases Hepatic Recurrence. Annals of Surgery 2008, 248 (2), 273–279. [DOI] [PubMed] [Google Scholar]
- 33.Ito H; Ito K; D'Angelica M; Gonen M; Klimstra D; Allen P; DeMatteo RP; Fong Y; Blumgart LH; Jarnagin WR, Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg 2011, 254 (2), 320–5. [DOI] [PubMed] [Google Scholar]
- 34.Butte JM; Kingham TP; Gönen M; D’Angelica MI; Allen PJ; Fong Y; DeMatteo RP; Jarnagin WR, Residual Disease Predicts Outcomes after Definitive Resection for Incidental Gallbladder Cancer. Journal of the American College of Surgeons 2014, 219 (3), 416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartlett DL, Gallbladder cancer. Seminars in Surgical Oncology 2000, 19 (2), 145–155. [DOI] [PubMed] [Google Scholar]
- 36.Burke EC; Jarnagin WR; Hochwald SN; Pisters PW; Fong Y; Blumgart LH, Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg 1998, 228 (3), 385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aoba T; Ebata T; Yokoyama Y; Igami T; Sugawara G; Takahashi Y; Nimura Y; Nagino M, Assessment of Nodal Status for Perihilar Cholangiocarcinoma: Location, Number, or Ratio of Involved Nodes. Annals of Surgery 2013, 257 (4), 718–725. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto Y; Fujii H; Aoyama H; Yamamoto M; Sugahara K; Suda K, Surgical treatment of primary carcinoma of the gallbladder based on the histologic analysis of 48 surgical specimens. The American Journal of Surgery 1992, 163 (2), 239–245. [DOI] [PubMed] [Google Scholar]
- 39.Ma W-J; Wu Z-R; Hu H-J; Wang J-K; Yin C-H; Shi Y-J; Li F-Y; Cheng N. s., Extended Lymphadenectomy Versus Regional Lymphadenectomy in Resectable Hilar Cholangiocarcinoma. Journal of Gastrointestinal Surgery 2020, 24 (7), 1619–1629. [DOI] [PubMed] [Google Scholar]
- 40.Giuliante F; Ardito F; Guglielmi A; Aldrighetti L; Ferrero A; Calise F; Giulini SM; Jovine E; Breccia C; De Rose AM; Pinna AD; Nuzzo G, Association of Lymph Node Status With Survival in Patients After Liver Resection for Hilar Cholangiocarcinoma in an Italian Multicenter Analysis. JAMA Surgery 2016, 151 (10), 916–922. [DOI] [PubMed] [Google Scholar]
- 41.Kitagawa Y; Nagino M; Kamiya J; Uesaka K; Sano T; Yamamoto H; Hayakawa N; Nimura Y, Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg 2001, 233 (3), 385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X; Lin H; Sun Y; Gong J; Feng H; Tu J, Prognostic Significance of the Lymph Node Ratio in Surgical Patients With Distal Cholangiocarcinoma. Journal of Surgical Research 2019, 236, 2–11. [DOI] [PubMed] [Google Scholar]
- 43.Kiriyama M; Ebata T; Aoba T; Kaneoka Y; Arai T; Shimizu Y; Nagino M; Group NSO, Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br J Surg 2015, 102 (4), 399–406. [DOI] [PubMed] [Google Scholar]
- 44.Shirai Y; Sakata J; Wakai T; Ohashi T; Ajioka Y; Hatakeyama K, Assessment of lymph node status in gallbladder cancer: location, number, or ratio of positive nodes. World J Surg Oncol 2012, 10, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Primrose JN; Fox RP; Palmer DH; Malik HZ; Prasad R; Mirza D; Anthony A; Corrie P; Falk S; Finch-Jones M; Wasan H; Ross P; Wall L; Wadsley J; Evans JTR; Stocken D; Praseedom R; Ma YT; Davidson B; Neoptolemos JP; Iveson T; Raftery J; Zhu S; Cunningham D; Garden OJ; Stubbs C; Valle JW; Bridgewater J; group B. s., Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019, 20 (5), 663–673. [DOI] [PubMed] [Google Scholar]
- 46.Wang SJ; Lemieux A; Kalpathy-Cramer J; Ord CB; Walker GV; Fuller CD; Kim JS; Thomas CR, Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 2011, 29 (35), 4627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koo T; Park HJ; Kim K, Radiation therapy for extrahepatic bile duct cancer: Current evidences and future perspectives. World J Clin Cases 2019, 7 (11), 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takada T; Amano H; Yasuda H; Nimura Y; Matsushiro T; Kato H; Nagakawa T; Nakayama T, Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 2002, 95 (8), 1685–95. [DOI] [PubMed] [Google Scholar]