Abstract
Background:
Intravenous ketorolac is commonly used for treating migraine headaches in children. However, the prerequisite placement of an intravenous line can be technically challenging, time-consuming, and associated with pain and distress. Intranasal ketorolac may be an effective alternative that is needle-free and easier to administer. We aimed to determine whether intranasal ketorolac is non-inferior to intravenous ketorolac for reducing pain in children with migraine headaches.
Methods:
We conducted a randomized double-blind non-inferiority clinical trial. Children aged 8– 17 years with migraine headaches, moderate to severe pain, and requiring parenteral analgesics received intranasal ketorolac (1 mg/kg) or intravenous ketorolac (0.5 mg/kg). Primary outcome was reduction in pain at 60 min after administration measured using the Faces Pain Scale-Revised (scored 0– 10). Non-inferiority margin was 2/10. Secondary outcomes included time to onset of clinically meaningful decrease in pain; ancillary emergency department outcomes (e.g. receipt of rescue medications, headache relief, headache freedom, percentage improvement); 24-h follow-up outcomes; functional disability; and adverse events.
Results:
Fifty-nine children were enrolled. We analyzed 27 children who received intranasal ketorolac and 29 who received intravenous ketorolac. The difference in mean pain reduction at 60 min between groups was 0.2 (95% CI −0.9, 1.3), with the upper limit of the 95% CI being less than the non-inferiority margin. There were no statistical differences between groups for secondary outcomes.
Conclusions:
Intranasal ketorolac was non-inferior to intravenous ketorolac for reducing migraine headache pain in the emergency department.
INTRODUCTION
Ketorolac is an analgesic commonly used to treat migraine headaches in children in the emergency department (ED).1 Ketorolac is a nonsteroidal anti-inflammatory drug (NSAID) frequently given by the intravenous (IV) route, which requires a needle-stick for administration. Needle-related procedures are one of the most feared medical experiences reported by children and are associated with pain and distress that, when inadequately managed, can result in both short-and long-term consequences.2–12 In addition, the placement of an IV line can be technically challenging and time consuming; analgesics may take longer to administer when using the IV route compared to other routes that do not require IV access.13
Ketorolac can be given by the intranasal (IN) route, which does not require a needle-stick or IV access for administration.14–18 Analgesics and sedatives administered by the IN route have been shown to have comparable efficacy and time to onset of action compared to IV administration.19–23 The IN route takes advantage of the highly-vascularized respiratory epithelium in the nasal cavity for systemic absorption and transports medications directly to the brain through the olfactory and trigeminal nerves, also known as the “nose-brain pathway.”24 This allows some medications administered by the IN route to produce both faster central nervous system effects and higher drug concentrations in the central nervous system than after IV administration alone.17,19,25–27
Intranasal ketorolac may be an effective alternative to IV ketorolac that is both needle-sparing and easier to administer. However, these benefits are immaterial if the analgesic effectiveness of IN ketorolac is not comparable to IV ketorolac. Therefore, the primary aim of our study was to determine if IN ketorolac is non-inferior to IV ketorolac for reducing pain intensity in children with migraine headaches. Our secondary aims were to identify differences in time to onset of a clinically meaningful reduction in pain intensity; ancillary ED outcomes (i.e. receipt of rescue medication, headache relief, headache freedom, percentage improvement); 24-h follow-up outcomes; functional disability; and adverse events.
METHODS
Study design and setting
We conducted a prospective, double-blind, randomized, parallel, 1:1, non-inferiority clinical trial comparing IN ketorolac with IV ketorolac. We enrolled patients presenting to a single tertiary-care children’s hospital ED during one of three recruitment periods between June 2015 and March 2021. Enrollment was paused from May 2016 to March 2018 due to funding limitations, and from March 2020 to September 2020 due to hospital-wide COVID-19 restrictions on research activities. The study was closed March 2021 due to a persisting decline in eligible patients associated with an overall reduction in pediatric ED visits.28,29 The decision to close the study was made prior to unblinding and data analysis. Our institutional review board approved this study with written informed consent and assent. This trial was registered at clinicaltrials.gov (NCT02358681).
Selection of participants
We enrolled children who were aged 8– 17 years; presented with a migraine headache as defined by the modified Irma’s ED Criteria (Table S1); had a self-reported pain score of ≥4/10 (representing moderate to severe pain); and required any IV analgesic for the headache pain as per the treating physician.30 Exclusion criteria included any contraindication to receiving ketorolac; receipt of any NSAID within previous 6 h; presence of IN obstruction that could not be readily cleared; inability to complete self-report measures of pain or questionnaires (e.g. developmental delay, autism spectrum disorder, neurological impairment); history of intracranial surgery, structural abnormalities, or risk factors for intracranial abnormality (e.g. coagulopathy; pseudotumor cerebri; pregnancy); chronic disease associated with pain other than migraine headaches (e.g. sickle cell disease, fibromyalgia); underlying medical condition necessitating multiple painful procedures (e.g. malignancy, complex congenital heart disease); known liver or kidney problems; critical illness; use of any medication for headaches on more than 10 days per month; or did not speak English or Spanish.
Interventions
Patients were randomized to receive either IN ketorolac (1 mg/kg) and IV normal saline (placebo) or IV ketorolac (0.5 mg/kg) and IN normal saline (placebo), with a maximum ketorolac dose of 30 mg. The dose of IN ketorolac was chosen based on its bioavailability and in consultation with a clinical pharmacologist.15 A 30 mg/ml concentration of ketorolac was used for both IN and IV administration. Intranasal medications were administered first using a mucosal atomization device (Wolfe-Tory Medical, Inc.). Total volumes were divided into two equal aliquots, with each aliquot administered into a different nostril. With the maximum dose and concentration used, the largest possible volume of administration for each nostril using this technique was 0.5 ml. The IV medication was administered over 30– 60 s immediately after completing IN administration, followed by a 20 ml/kg normal saline bolus (maximum 1 L) over 60 min. All IVs were placed before any study medications were administered. No other analgesics (e.g. dopamine antagonists) were administered in the ED before or concurrently with the study medications. Treating clinicians administered rescue medications (i.e. additional parenteral analgesics administered in response to inadequate improvement in pain) when deemed clinically indicated.
We randomized patients using computer-generated blocks of eight. Allocation was concealed using sequentially numbered, sealed, opaque envelopes. The random allocation sequence was created and maintained by a research administrator not involved with study procedures, and was not available to the investigator or study team until completion of the study. We ensured blinding of treatment assignment by using syringes with identical volume, color and odor. The treating clinicians, study team members who assessed outcomes, patient, and family members were all blinded to the treatment assignment.
Measurements and outcomes
Outcomes were measured by a study team member at 10, 30, 60, and 120 min after completion of the IV study medication administration. Pain associated with IN administration was assessed immediately after completing IN administration of study medication (i.e. ketorolac or placebo). Pain intensity was measured at 10, 30, 60, and 120 min after study medication administration. Qualitative descriptors of pain intensity (i.e. none, mild, moderate, or severe), functional disability, and adverse events were assessed at 60 and 120 min.31 Twenty four-hour follow-up outcomes were assessed via telephone by a study team member within 24–48 h after study medication administration.
The primary outcome was the difference in pain intensity reduction 60 min after study medication administration, measured using the Faces Pain Scale – Revised (FPS-R).32,33 The FPS-R is a self-reported pain scale scored from 0 to 10 and comprised of 6 faces, each representing an increasing degree of pain intensity. The FPS-R has strong validity and reliability for assessing pain intensity in children aged 4– 17 years and is recommended for research in children.33,34 The 60-min time point was chosen based on International Headache Society (IHS) recommendations for the study of parenteral medications for treating migraine headaches.31 Secondary outcomes included: (a) difference in pain intensity reduction 10, 30, and 120 min after administration; (b) time to onset of clinically meaningful reduction in pain; (c) ancillary ED outcomes (i.e. receipt of rescue medication, headache relief, headache freedom, percentage improvement); (d) 24-h follow-up outcomes; (e) functional disability; and (f) adverse events.35,36
Time to onset of clinically meaningful reduction in pain intensity was determined by identifying the time that pain was first observed to have decreased by a minimum clinically significant difference (i.e. 2 on the FPS-R) and by performing a Kaplan-Meier distribution analysis.35,36 As per IHS recommendations, ancillary ED outcomes included: (a) receipt of rescue medications in the ED after study medication administration; (b) headache relief, defined as change within 120 min of the patient’s headache from severe to moderate to either mild or none, without receipt of rescue medications; (c) headache freedom, defined as achieving a headache level of none within 120 min, without receipt of rescue medications; and (d) percentage improvement in pain intensity between baseline and 60 min, defined as: (baseline pain intensity – 60 min pain intensity) / baseline pain intensity. We also evaluated treatment success as a reduction of 50% or greater in pain intensity at 30 or 60 min after study medication administration, or complete resolution of pain.37 Degree of pain intensity associated with IN administration was assessed immediately after administration of the IN medication using the FPS-R.
Twenty four-hour follow-up outcomes included: (a) patient’s overall assessment of efficacy and tolerability, expressed as a dichotomous response to the question, “The next time you come to the emergency department with a headache or migraine, do you want to be given the same medication?”; (b) sustained headache relief, defined as achieving headache relief and maintaining this level for 24 h without the use of rescue medications after ED discharge; (c) sustained headache freedom, defined as achieving headache freedom, and maintaining this level for 24 h without the use of rescue medications after ED discharge; and (d) use of outpatient rescue medications during the 24-h period after ED discharge.31
Functional disability was assessed using a question standard in headache research but modified for the pediatric population (Table S2).31 Responses were categorized as none, mild, moderate, and severe functional disability. Functional disability was assessed at baseline, 60 and 120 min after study medication administration, and during the 24-h follow-up assessment. Adverse events were assessed at the same three time points.
Missed eligible patient review
We identified missed eligible patients (i.e. eligible but not enrolled) by reviewing the electronic medical record and identifying patients with a chief complaint of headache or migraine who received a parenteral analgesic (e.g. ketorolac, metoclopramide, prochlorperazine). Data collected for comparison to enrolled patients included the patient’s age, sex, initial pain score, receipt of rescue medications, and disposition.
Data analysis
For our primary outcome, we compared the difference in FPS-R score reduction between IN and IV ketorolac 60 min after study medication administration using the independent samples t-test. The predetermined margin of non-inferiority was 2, which represents a minimum clinically significant difference in pain intensity in children when using the FPS-R.35,36 A margin of 1.8 was used for the sample size determination, which was based on reducing our predetermined margin by 10% in order to be conservative. Using a standard deviation of 2.725, a planned sample size of 40 patients per group was chosen to provide 90% power to detect non-inferiority using a one-sided independent sample t-test with an alpha of 0.05.38,39 All enrolled randomized patients with outcomes measured were analyzed. To evaluate our secondary outcomes, we used the independent samples t-test to compare continuous variables and the chi-square test to compare categorical variables. Kaplan-Meier curves were compared using a log rank test. P values <0.05 were considered statistically significant. Analyses were conducted using SPSS (version 26; IBM Corporation).
RESULTS
Characteristics of study participants
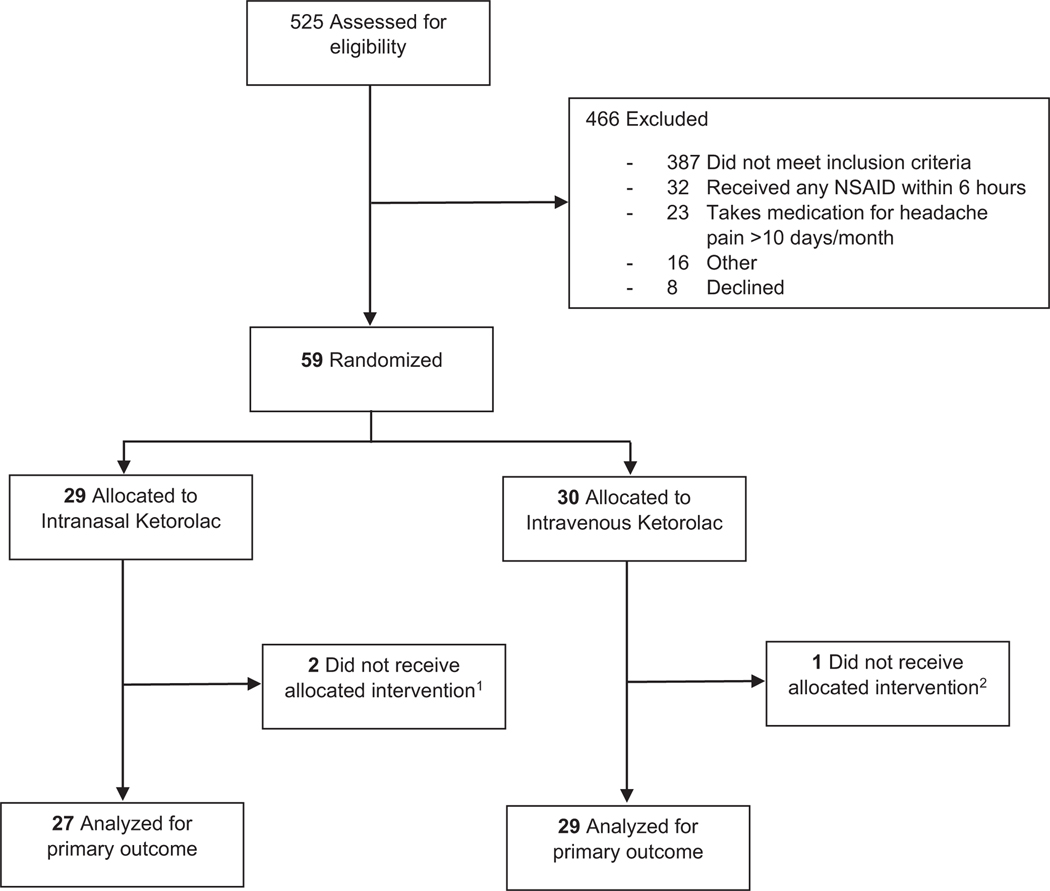
We assessed 525 children for eligibility and excluded 466 (Figure 1). Fifty-nine patients were enrolled and randomized. Three patients were withdrawn before study medication administration due to either resolution of headache prior to study medication administration or identification of an exclusion criterion after randomization; no outcome measures were assessed for these patients. The patient characteristics of the 56 children analyzed are shown in Table 1. Missed eligible patients were similar to those enrolled in age, sex, headache pain intensity at ED presentation, proportion who received rescue medications, and ED disposition (Table S3).
FIGURE 1.
Enrollment flow diagram. 1One patient did not receive allocated intervention because headache pain resolved prior to study drug administration; the other patient had an exclusion criterion identified after enrollment and intervention was not administered. 2Patient did not receive allocated intervention because headache pain resolved prior to study drug administration
TABLE 1.
Patient characteristics
| Intranasal Ketorolac n = 27 | Intravenous Ketorolac n = 29 | |
|---|---|---|
| Age, median (IQR), years | 14 (11, 16) | 15 (11, 16) |
| Female, No. (%) | 17 (63) | 21 (72.4) |
| Weight, mean (SD), kg | 57.7 (16.9) | 63.7 (26.8) |
| Ethnicity/race, No. (%) | ||
| Hispanic | 24 (88.9) | 26 (89.7) |
| Black | 2 (7.4) | 0 |
| White | 0 | 3 (10.3) |
| Don’t know | 1 (3.7) | 0 |
| Primary language, No. (%) | ||
| English | 24 (88.9) | 28 (96.6) |
| Spanish | 3 (11.1) | 1 (3.4) |
| Headache history, No. (%) | ||
| First headache of life | 5 (18.5) | 5 (17.2) |
| Headaches for <1 year, not first headache of life | 8 (29.6) | 9 (31.1) |
| Headaches for ≥1 year | 14 (51.9) | 15 (51.7) |
| Number of days per month with a headache, median (IQR)a | 3 (1, 6) | 3 (2, 6) |
| Number of days per month requiring medication for headache pain, median (IQR)a | 1 (1, 4) | 2 (1, 4) |
| Type of medication taken at home for headache prior to ED presentation No. (%) | ||
| Over-the-counter analgesic only | 26 (96.3) | 28 (96.6) |
| Prescription analgesic (+/− over-the-counter analgesic)b | 1 (3.7) | 1 (3.4) |
| Headache pain intensity at ED presentation, mean (SD)c | 6.3 (1.6) | 6.3 (1.8) |
| Functional disability at ED presentation, No. (%) | ||
| None | 3 (11.1) | 0 |
| Mild | 9 (33.3) | 9 (31) |
| Moderate | 9 (33.3) | 12 (41.4) |
| Severe | 6 (22.3) | 8 (27.6) |
| Family history of migraine headaches, No. (%)d | 20 (74.1) | 21 (72.4) |
Abbreviations: ED, emergency department; IQR, interquartile range; SD, standard deviation.
Does not include patients with first headache of life; intranasal n = 22, intravenous = 22.
Over-the-counter analgesics include acetaminophen, ibuprofen, naproxen, and combination analgesics (e.g. aspirin/acetaminophen/caffeine). Prescription analgesics include sumitriptan, metoclopramide, and topiramate.
Measured using the Faces Pain Scale – Revised.
First-or second-degree relatives (parents, siblings; grandparents, uncles, aunts) with migraine headaches.
Main results
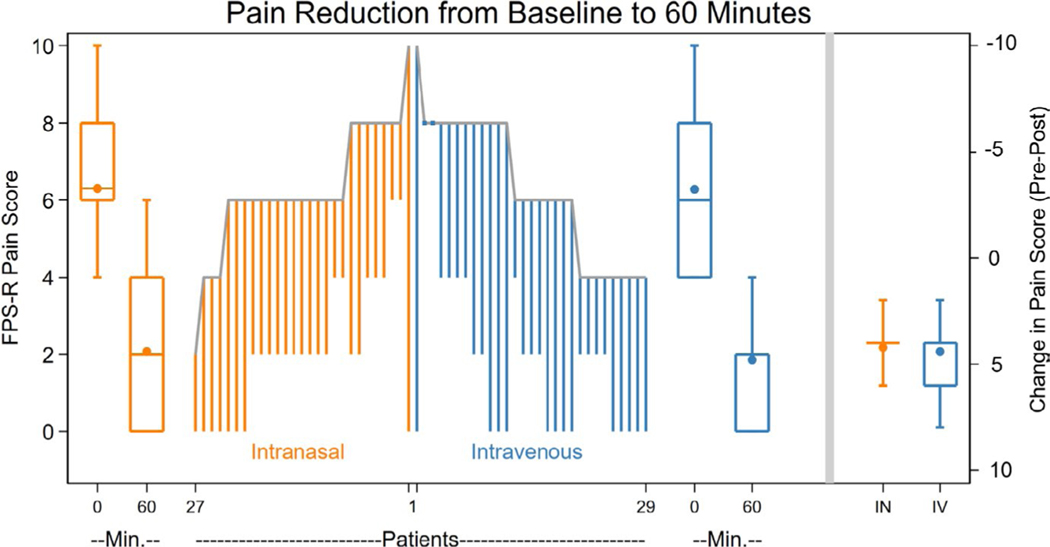
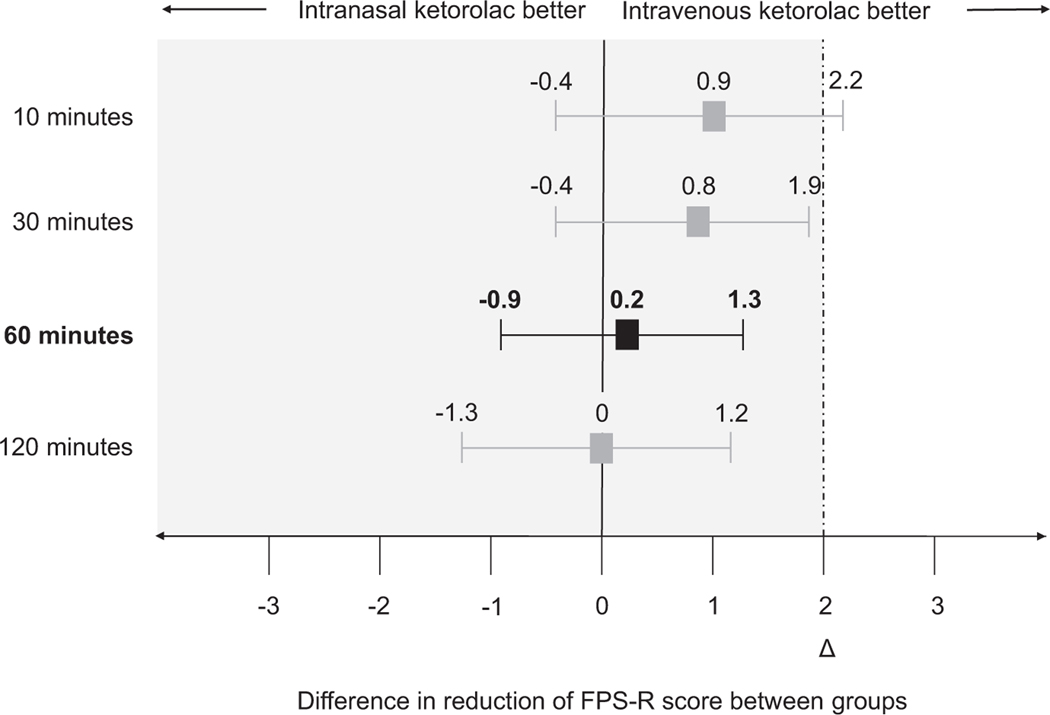
The decrease in pain intensity associated with IN and IV ketorolac at 60 min is shown in Figure 2, with IN ketorolac being non-inferior to IV ketorolac for reducing pain intensity (p < 0.001) (Figure 3).40 Table 2 shows the decrease in pain intensity associated with IN and IV ketorolac at 10, 30, and 120 min. Intranasal ketorolac was also non-inferior to IV ketorolac at 30 and 120 min, but the non-inferiority determination was inconclusive at 10 min (Figure 3). There was no statistical difference between groups in time to onset of a minimum clinically significant difference in pain, and ancillary ED outcomes (Table 2, Figure S1). No patients received rescue medications prior to the 60-min assessment. All patients achieved at least a minimum clinically significant difference in pain by 60 min. Treatment success, defined as a reduction of 50% or greater in pain intensity at 30 or 60 min after study medication administration, or complete resolution of pain, was achieved by 24 (88.9%) and 27 (93.1%) patients who received IN and IV ketorolac, respectively; the mean difference between groups was −4.2% (95% CI −19.2, 10.8).
FIGURE 2.
Pain reduction associated with intranasal ketorolac and intravenous ketorolac at 60 min after study medication administration. The length of lines in the parallel line plot represents the magnitude of change in pain intensity for each patient. The boxplots to the left and right of the parallel line plot represent the pain scores at 0 and 60 min, with the middle line of each box representing the median, the box representing the interquartile range, the whiskers representing the range, and the dot representing the mean. The box plots on the far right represent the change in pain score from baseline to 60 min; a single line is portrayed for the IN group because of overlapping median and quartiles. FPS-R, Faces Pain Scale – Revised; IN, intranasal; IV, intravenous; Min., Minutes
FIGURE 3.
Differences in pain reduction between intranasal and intravenous ketorolac. Pain intensity was measured using the FPS-R (scored 0–10). The upper limit of the 95% confidence interval for differences in mean pain reduction at 30, 60, and 120 min were less than the non-inferiority margin of 2, demonstrating non-inferiority(primary outcome = difference in pain reduction at 60 min). Non-inferiority determination at 10 min was inconclusive. Δ, Non-inferiority margin; FPS-R, Faces Pain Scale-Revised
TABLE 2.
Secondary headache-related outcomes
| Intranasal Ketorolac | Intravenous Ketorolac | Difference in Means or Proportions |
|
|---|---|---|---|
| Decrease in pain intensity, mean (95% CI), unitsa | |||
| 10 min | 1.7 (0.9, 2.5) | 2.6 (1.6, 3.6) | 0.9 (−0.4, 2.2) |
| 30 min | 2.9 (2.2, 3.6) | 3.7 (2.9, 4.5) | 0.8 (−0.4, 1.9) |
| 60 min (Primary outcome) | 4.2 (3.6, 4.8) | 4.4 (3.5, 5.3) | 0.2 (−0.9, 1.3) |
| 120 minutesb | 4.9 (4, 5.8) | 4.9 (4.1, 5.7) | 0 (−1.3, 1.2) |
| Time to minimum clinically significant decrease in pain, mean (95% CI), mina | 21.9 (15, 28.8) | 18.6 (12.7, 25) | −3.3 (−12.5, 6) |
| Ancillary ED outcomesa | |||
| Receipt of ED rescue medications, No. (%) | 6 (22.2) | 5 (17.2) | −5 (−25.8, 15.8) |
| Headache relief, No. (%)c | 25 (92.6) | 26 (89.7) | −2.9 (−17.7, 11.9) |
| Headache freedom, No. (%)d | 11 (40.7) | 17 (58.6) | 17.9 (−7.9, 43.7) |
| Percentage improvement at 60 min, mean (95% CI)e |
69.8 (60.6, 78.9) | 71.8 (60.7, 82.9) | 2 (−12.7, 16.9) |
| 24-h follow-up outcomesf | |||
| Want same medication again, No. (%)g | 19 (79.2) | 21 (95.4) | 16.2 (−2.3, 34.7) |
| Sustained headache relief, No. (%)h | 7 (29.2) | 12 (52.2) | 23 (−4.3, 50.3) |
| Sustained headache freedom, No. (%)i | 5 (20.8) | 8 (34.8) | 14 (−11.4, 39.4) |
| Use of rescue medications after ED discharge, No. (%)j |
12 (66.7) | 8 (42.1) | −24.6 (−52.2, 3) |
| Functional disabilityk | |||
| 60 min, No. (%)a | |||
| None or mild | 25 (92.5) | 28 (96.5) | 4 (−8, 16) |
| None | 15 (55.6) | 13 (44.8) | −10.8 (−36.9, 15.3) |
| Mild | 10 (37) | 15 (51.7) | 14.7 (−11, 40.4) |
| Moderate | 2 (7.4) | 1 (3.5) | −3.9 (−15.8, 8) |
| Severe | 0 | 0 | NA |
| 120 min No. (%)l | |||
| None or mild | 18 (94.7) | 22 (100) | 5.3 (−4.8, 15.4) |
| None | 11 (57.9) | 16 (72.7) | 14.8 (−14.2, 43.8) |
| Mild | 7 (36.8) | 6 (27.3) | −9.5 (−38.1, 19.1) |
| Moderate | 0 | 0 | NA |
| Severe | 1 (5.3) | 0 | −5.3 (−15.4, 4.8) |
| 24-h follow up, No. (%)m | |||
| None or mild | 7 (100) | 12 (100) | NA |
| None | 7 (100) | 11 (91.7) | −8.3 (−23.9, 7.3) |
| Mild | 0 | 1 (8.3) | 8.3 (−7.3, 23.9) |
| Moderate | 0 | 1 (8.3) | 8.3 (−7.3, 23.9) |
| Severe | 0 | 0 | NA |
Abbreviations: CI, confidence interval; ED, emergency department; NA, not applicable.
The use of italicized values for functional disability were to distinguish the composite values from the non-composite values.
Times listed are number of minutes after study drug administration at which pain intensity was assessed. Intranasal ketorolac = 27, intravenous ketorolac = 29.
Analyzed patients who did not receive rescue medication or were not discharged prior to 120-minute assessment. Intranasal ketorolac = 20, intravenous ketorolac = 23.
Change within 2 h of the patient’s description of headache from severe or moderate to either mild or none, without the use of ED rescue medications.
Achieving a headache description of none within 2 h, without the use of ED rescue medications.
(Baseline pain intensity – 60 min pain intensity)/baseline pain intensity.
Patients who completed 24-h follow-up. Intranasal ketorolac = 24, intravenous ketorolac = 23.
One patient in intravenous ketorolac group who completed follow-up did not give an answer to this question, so only 22 patients in intravenous ketorolac group analyzed.
Patients whose headaches changed to either mild or none in the ED without the use of ED rescue medications, and maintained this level of relief (or better) without use of ED rescue medication or rescue medication after ED discharge.
Patients who achieved a level of “none” within 2 h of study medication administration, and maintained this level for 24 h without use of ED rescue medication or rescue medication after ED discharge.
Analyzed patients who completed 24-h follow-up and did not receive a rescue medication in the ED. Intranasal ketorolac = 18, intravenous ketorolac = 19.
Assessed using standardized question detailed in Table S2.
Patients removed from analysis if data missing, discharged home, or received ED rescue medications prior to 120-minute assessment. Intranasal ketorolac = 19, intravenous ketorolac = 22.
Analyzed patients who completed 24-hour follow-up and did not receive ED rescue medication or rescue medication after ED discharge. Intranasal ketorolac = 7, intravenous ketorolac = 12.
There was no statistical difference between groups for the 24-h follow-up outcomes, although the group that received IV ketorolac had a larger proportion of children with sustained headache relief and headache freedom and a smaller proportion who used rescue medications after ED discharge (Table 2). There was no difference between groups in proportion of children who experienced none or mild functional disability when assessed at 60 and 120 min after study medication administration and when assessed at 24-h follow-up. There were very few children who reported moderate or severe functional disability in either group at these same time points (Table 2).
There were no serious adverse events, including no upper or lower gastrointestinal bleeding. Four children who received IN ketorolac reported 5 adverse events; 6 who received IV ketorolac reported 6 adverse events (Table 3). The most common adverse events were nausea and dizziness. The mean pain intensity associated with IN administration of ketorolac and placebo was 6.7 (95% CI 6.5, 6.9) and 0.6 (95% CI 0.5, 0.7), respectively; the mean difference between groups was 6.1 (95% CI 4.8, 7.3).
TABLE 3.
Adverse events
| Adverse Events, No. | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Totala | Emergency Departmentb | 24-hour Follow-Up | ||||
|
|
|
|
||||
| Symptoms | Intranasal Ketorolac | Intravenous Ketorolac | Intranasal Ketorolac | Intravenous Ketorolac | Intranasal Ketorolac | Intravenous Ketorolac |
| Nausea | 2 | 1 | 0 | 1 | 2 | 0 |
| Dizziness | 1 | 1 | 0 | 0 | 1 | 1 |
| Sleepiness | 0 | 1 | 0 | 1 | 0 | 0 |
| Otherc | 2 | 3 | 2 | 3 | 0 | 0 |
| Total | 5 | 6 | 2 | 5 | 3 | 1 |
For the intranasal ketorolac group, 4 patients reported 5 adverse events. For the intravenous ketorolac group, 6 patients reported 6 events. Adverse events documented were not present prior to initial assessment at 60 min after study medication administration.
Includes adverse events identified at 60 and 120 min after study medication administration.
Other includes “feeling cold”, “less focus, feel off”, transient extremity sensory complaints.
DISCUSSION
In this randomized clinical trial, we found that IN ketorolac was non-inferior to IV ketorolac for treating pain in children with migraine headaches at 60 min after medication administration. Ketorolac administered by either route was effective in treating migraine headaches in children when assessed using a number of clinically important headache-related outcome measures.
This is the first randomized clinical trial of IN ketorolac in children, and the first trial comparing IN ketorolac to a parenteral analgesic for treating migraine headaches. This is also the first study of which we are aware utilizing the parenteral formulation of ketorolac for IN administration outside of the dental and post-operative setting.41,42 The vast majority of prior studies of IN ketorolac have utilized an IN formulation that combines ketorolac with lidocaine, the latter of which may also have analgesic effects.16,43–52 This lidocaine-containing formulation has been shown to be superior to placebo and non-inferior to IN sumitriptan for reducing migraine headache pain in adults.16,48 Our findings further support the effectiveness of IN ketorolac for treating migraine headaches, specifically in the pediatric population and when using the parenteral formulation of ketorolac that does not include lidocaine and is readily available in the ED setting.
Ketorolac, when given by either route, appeared to be effective in our study for treating migraine headaches in children based on a number of clinically important headache-related outcomes. A percent reduction of pain intensity at 60 min of ~70% was greater than percent reductions associated with an ideal clinically significant difference (i.e. 60% reduction) and children declining additional analgesia because of adequate pain relief (i.e. 40% reduction).36 Ketorolac treatment was also associated with headache relief in ~90% and mild or no functional disability in greater than 90% of patients. Approximately 20% of patients who received IN or IV ketorolac received rescue medications and between 40 and 60% achieved headache freedom within 2 h, which are proportions comparable to those described in four prior studies evaluating other parenteral analgesics in children. These studies of ketorolac, prochlorperazine, propofol, and a combination of ketorolac/dopamine antagonist/diphenhydramine/IV fluids reported that 5– 37% of patients received rescue medications, and 7– 60% experienced headache freedom at similar time points.37,53–55 Our results are comparable to those reported in the randomized clinical trial comparing ketorolac and prochlorperazine for treating migraine headaches in children: the proportion of patients in our study who achieved treatment success with both IN and IV ketorolac was no less than that reported for IV ketorolac (55.2%) and IV prochlorperazine (84.8%).37 In addition, we observed that both IN and IV ketorolac were comparable to a combination of ketorolac/dopamine antagonist/diphenhydramine/IV fluids with regard to percent pain reduction 60 min after administration (59%) and proportion of children who received ED rescue medications (22.2%).53
The implementation of IN ketorolac may be limited by the moderate degree of nasal pain associated with IN administration. Pain associated with IN administration has also been described with IN midazolam, which is commonly used for anxiolysis for children in the ED setting.56–59 This associated pain, however, has not precluded the use of IN midazolam. Rather, it has prompted the study of different strategies for treating this pain so that children can still benefit from its favorable properties (e.g. rapid onset, needle-free administration, effective anxiolysis), such as with the pre-treatment or co-administration with lidocaine.57,60–63 The administration of the IN formulation of ketorolac containing lidocaine has been associated with nasal pain in 5– 20% of patients, with the degree of pain intensity rated as “mild” in one study.14,48,49,51 However, further research is necessary to better describe the effect of lidocaine or other strategies for decreasing the pain associated with IN administration of ketorolac in children. Until then, there should be shared decision-making with patients and families weighing the benefits and drawbacks of IN administration with those associated with IV administration of ketorolac.
This study demonstrated non-inferiority of IN ketorolac compared to IV ketorolac, but it does not address whether IN ketorolac alone is non-inferior to a regimen consisting of IV ketorolac and a normal saline bolus. Intravenous fluids are commonly given to children as part of their migraine headache treatment in the ED.64 Since one of the advantages of using IN ketorolac would be to avoid placing an IV line, patients receiving IN ketorolac would be unlikely to receive a normal saline bolus. Although hydration could be achieved orally, nausea and vomiting associated with migraine headaches could potentially be prohibitive. Therefore, clinical practice could be informed by future studies comparing IN ketorolac alone to a regimen of IV ketorolac and a normal saline bolus. However, the benefit of a normal saline bolus for decreasing pain in patients with migraine headaches is unclear. To date, there are only two prospective trials of a normal saline bolus for treating migraine headaches in patients presenting to the ED. One study of children aged 5– 17 years demonstrated that the overall decrease in pain associated with a 10 ml/kg normal saline bolus was small and not clinically significant.65 Similarly, one study of adults showed no difference in pain intensity improvement between patients who received a one-liter normal saline bolus and those who did not.66
LIMITATIONS
First, we were unable to achieve our planned sample size. However, we were able to enroll a sufficient number of patients to provide adequate power to achieve our primary aim and demonstrate non-inferiority due to the conservative estimates used when calculating the sample size. Specifically, we determined the sample size using a standard deviation (2.725) that was larger than those actually observed in the IN ketorolac and IV ketorolac groups (1.695 and 2.353, respectively). Second, we did not include patients who used medications for more than 10 days a month, which may limit generalizability by excluding patients who may have a more established or refractory history of migraine headaches. This decision was based on IHS recommendations to avoid enrolling patients who may be taking excessive medications for headaches and, therefore, have altered pathophysiology and response to treatment.31 Finally, our sample size was not powered to identify differences between groups for secondary outcomes. Although a number of these outcomes were clinically similar between groups (e.g. percentage improvement at 60 min, proportion who experienced headache relief and received rescue mediations, time to achieve a minimum clinically significant decrease in pain), there were potentially meaningful differences between groups that did not achieve statistical significance but may favor IV ketorolac (e.g. proportion who experienced headache freedom, sustained headache relief or headache freedom, use of rescue medications after ED discharge, and wanting same medication again). Further study is required to definitively determine whether there are differences in these headache-related outcomes.
CONCLUSION
Intranasal ketorolac was non-inferior to IV ketorolac for reducing pain intensity in children with migraine headaches at 60 min after medication administration. Ketorolac administered by either route was effective in treating migraine headaches in children when assessed using a number of clinically important headache-related outcome measures.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Marc Vindas, Julie Ochs, Mona Bugaighis, Vartan Pahalyants and Leonor Suarez for their assistance with patient enrollment; Josh Kriger for his assistance with study design; Dr. Serge Cremers for his pharmacologic consultation; and Dr. David Schriger for his assistance preparing a graphical representation of our data.
Funding information
This study was funded by Columbia University’s CTSA grant No. UL1TR000040 from NCATS/NIH and the Migraine Research Foundation (New York, NY).
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
CONFLICTS OF INTEREST
None of the authors have any conflicts of interest to disclose.
REFERENCES
- 1.Sheridan DC, Meckler GD, Spiro DM, Koch TK, Hansen ML. Diagnostic testing and treatment of pediatric headache in the emergency department. J Pediatr 2013;163(6):1634–1637. [DOI] [PubMed] [Google Scholar]
- 2.Fradet C, McGrath PJ, Kay J, Adams S, Luke B. A prospective survey of reactions to blood tests by children and adolescents. Pain 1990;40(1):53–60. [DOI] [PubMed] [Google Scholar]
- 3.Humphrey GB, Boon CM, van Linden van den Heuvell GF, van de Wiel HB. The occurrence of high levels of acute behavioral distress in children and adolescents undergoing routine venipunctures. Pediatrics 1992;90(1 Pt 1):87–91. [PubMed] [Google Scholar]
- 4.Cummings EA, Reid GJ, Finley GA, McGrath PJ, Ritchie JA. Prevalence and source of pain in pediatric inpatients. Pain 1996;68(1):25–31. [DOI] [PubMed] [Google Scholar]
- 5.Van Cleve L, Johnson L, Pothier P. Pain responses of hospitalized infants and children to venipuncture and intravenous cannulation. J Pediatr Nurs 1996;11(3):161–168. [DOI] [PubMed] [Google Scholar]
- 6.Goodenough B, Thomas W, Champion DG, et al. Unravelling age effects and sex differences in needle pain: ratings of sensory intensity and unpleasantness of venipuncture pain by children and their parents. Pain 1999;80(1– 2):179–190. [DOI] [PubMed] [Google Scholar]
- 7.Carlson KL, Broome M, Vessey JA. Using distraction to reduce reported pain, fear, and behavioral distress in children and adolescents: a multisite study. J Soc Pediatr Nurs 2000;5(2):75–85. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995;41(2):169–175. [PubMed] [Google Scholar]
- 9.Pate JT, Blount RL, Cohen LL, Smith AJ. Childhood medical experience and temperament as predictors of adult functioning in medical situations. Child Health Care 1996;25(4):281–298. [Google Scholar]
- 10.Bijttebier P, Vertommen H. The impact of previous experience on children’s reactions to venipunctures. J Health Psychol 1998;3(1):39–46. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy AM, Kleiber C. A conceptual model of factors influencing children’s responses to a painful procedure when parents are distraction coaches. J Pediatr Nurs 2006;21(2):88–98. [DOI] [PubMed] [Google Scholar]
- 12.Broome ME, Bates TA, Lillis PP, McGahee TW. Children’s medical fears, coping behaviors, and pain perceptions during a lumbar puncture. Oncol Nurs Forum 1990;17(3):361–3 67. [PubMed] [Google Scholar]
- 13.Borland ML, Clark L-J, Esson A. Comparative review of the clinical use of intranasal fentanyl versus morphine in a paediatric emergency department. Emerg Med Australas 2008;20(6):515–520. [DOI] [PubMed] [Google Scholar]
- 14.Drover DR, Hammer GB, Anderson BJ. The pharmacokinetics of ketorolac after single postoperative intranasal administration in adolescent patients. Anesth Analg 2012;114(6):1270–1276. [DOI] [PubMed] [Google Scholar]
- 15.McAleer SD, Majid O, Venables E, Polack T, Sheikh MS. Pharmacokinetics and safety of ketorolac following single intranasal and intramuscular administration in healthy volunteers. J Clin Pharmacol 2007;47(1):13–18. [DOI] [PubMed] [Google Scholar]
- 16.Rao AS, Gelaye B, Kurth T, Dash PD, Nitchie H, Peterlin BL. A randomized trial of ketorolac vs. sumatripan vs. placebo nasal spray (KSPN) for acute migraine. Headache 2016;56(2):331–3 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vadivelu N, Gowda AM, Urman RD, et al. Ketorolac tromethamine -routes and clinical implications. Pain Pract 2015;15(2):175–193. [DOI] [PubMed] [Google Scholar]
- 18.Garnock-Jones KP. Intranasal ketorolac: for short-term pain management. Clin Drug Investig 2012;32(6):361–3 71. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe TR, Braude DA. Intranasal medication delivery for children: a brief review and update. Pediatrics 2010;126(3):532–537. [DOI] [PubMed] [Google Scholar]
- 20.Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ 2000;321(7253):83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borland M, Jacobs I, King B, O’Brien D. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med 2007;49(3):335–340. [DOI] [PubMed] [Google Scholar]
- 22.Tabibi SE, Needham TE, Zia H. Nasal systemic drug delivery. By Yie W. Chien, Kenneth S.E. SU, and Shyi-Feu Chang. Marcel Dekker: New York. 1989. J Pharm Sci 1991;80(1):97–98. [Google Scholar]
- 23.Tsze DS, Pan SS, DePeter KC, Wagh AM, Gordon SL, Dayan PS. Intranasal hydromorphone for treatment of acute pain in children: a pilot study. Am J Emerg Med 2019;37(6):1128–1132. [DOI] [PubMed] [Google Scholar]
- 24.Pires A, Fortuna A, Alves G, Falcão A. Intranasal drug delivery: how, why and what for? J Pharm Pharm Sci 2009;12(3):288–311. [DOI] [PubMed] [Google Scholar]
- 25.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002;5(6):514–516. [DOI] [PubMed] [Google Scholar]
- 26.Illum L. Is nose-to-b rain transport of drugs in man a reality? J Pharm Pharmacol 2004;56(1):3–17. [DOI] [PubMed] [Google Scholar]
- 27.Lötsch J, Walter C, Parnham MJ, Oertel BG, Geisslinger G. Pharmacokinetics of non-intravenous formulations of fentanyl. Clin Pharmacokinet 2013;52(1):23–36. [DOI] [PubMed] [Google Scholar]
- 28.DeLaroche AM, Rodean J, Aronson PL, et al. Pediatric emergency department visits at US children’s hospitals during the COVID-19 Pandemic. Pediatrics [Internet] 2021 [cited 2021 Mar 28]; Available from: https://pediatrics.aappublications.org/content/early/2021/03/17/peds.2020-039628 [DOI] [PubMed] [Google Scholar]
- 29.Pines JM, Zocchi MS, Black BS, et al. Characterizing pediatric emergency department visits during the COVID-19 pandemic. Am J Emerg Med 2021;41:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trottier ED, Bailey B, Lucas N, Lortie A. Diagnosis of migraine in the pediatric emergency department. Pediatr Neurol 2013;49(1):40–45. [DOI] [PubMed] [Google Scholar]
- 31.Tfelt-Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012;32(1):6–38. [DOI] [PubMed] [Google Scholar]
- 32.Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain 2001;93(2):173–183. [DOI] [PubMed] [Google Scholar]
- 33.Tsze DS, von Baeyer CL, Bulloch B, Dayan PS. Validation of self-report pain scales in children. Pediatrics 2013;132(4):e971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain 2006;125(1– 2):143–157. [DOI] [PubMed] [Google Scholar]
- 35.Tsze DS, Hirschfeld G, von Baeyer CL, Bulloch B, Dayan PS. Clinically significant differences in acute pain measured on self-report pain scales in children. Acad Emerg Med 2015;22(4):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsze DS, Hirschfeld G, von Baeyer CL, Suarez LE, Dayan PS. Changes in Pain Score associated with clinically meaningful outcomes in children with acute pain. Acad Emerg Med 2019;26(9):1002–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brousseau DC, Duffy SJ, Anderson AC, Linakis JG. Treatment of pediatric migraine headaches: a randomized, double-blind trial of prochlorperazine versus ketorolac. Ann Emerg Med 2004;43(2):256–262. [DOI] [PubMed] [Google Scholar]
- 38.Julious SA. Sample sizes for clinical trials with normal data. Stat Med 2004;23(12):1921–1986. [DOI] [PubMed] [Google Scholar]
- 39.Chow S-C, Wang H, Shao J. Sample Size Calculations in Clinical Research, Second Edition, 2nd ed. Chapman and Hall/CRC, 2007. [Google Scholar]
- 40.Schriger DL. Graphic portrayal of studies with paired data: a tutorial. Ann Emerg Med 2018;71(2):239–246. [DOI] [PubMed] [Google Scholar]
- 41.Turner CL, Eggleston GW, Lunos S, Johnson N, Wiedmann TS, Bowles WR. Sniffing out endodontic pain: use of an intranasal analgesic in a randomized clinical trial. J Endod 2011;37(4):439–444. [DOI] [PubMed] [Google Scholar]
- 42.Moodie JE, Brown CR, Bisley EJ, Weber HU, Bynum L. The safety and analgesic efficacy of intranasal ketorolac in patients with postoperative pain. Anesth Analg 2008;107(6):2025–2031. [DOI] [PubMed] [Google Scholar]
- 43.Yazdani J, Khorshidi-Khiavi R, Nezafati S, et al. Comparison of analgesic effects of intravenous and intranasal ketorolac in patients with mandibular fracture-A Randomized Clinical Trial. J Clin Exp Dent 2019;11(9):e768–e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watts K, Balzer S, Drum M, et al. Ibuprofen and acetaminophen versus intranasal ketorolac (Sprix) in an untreated endodontic pain model: a randomized, Double-blind Investigation. J Endod 2019;45(2):94–98. [DOI] [PubMed] [Google Scholar]
- 45.Stentz D, Drum M, Reader A, Nusstein J, Fowler S, Beck M. Effect of a combination of intranasal ketorolac and nitrous oxide on the success of the inferior alveolar nerve block in patients with symptomatic irreversible pulpitis: a prospective, Randomized, Double-blind Study. J Endod 2018;44(1):9–13. [DOI] [PubMed] [Google Scholar]
- 46.Pollack CV, Diercks DB, Thomas SH, et al. Patient-reported outcomes from a national, prospective, observational study of emergency department acute pain management with an intranasal nonsteroidal anti-inflammatory drug, opioids, or both. Acad Emerg Med 2016;23(3):331–341. [DOI] [PubMed] [Google Scholar]
- 47.Bullingham R, Juan A. Comparison of intranasal ketorolac tromethamine pharmacokinetics in younger and older adults. Drugs Aging 2012;29(11):899–904. [DOI] [PubMed] [Google Scholar]
- 48.Pfaffenrath V, Fenzl E, Bregman D, Färkkila M. Intranasal ketorolac tromethamine (SPRIX(R)) containing 6% of lidocaine (ROX-828) for acute treatment of migraine: safety and efficacy data from a phase II clinical trial. Cephalalgia Int J Headache 2012;32(10):766–777. [DOI] [PubMed] [Google Scholar]
- 49.Singla N, Singla S, Minkowitz HS, Moodie J, Brown C. Intranasal ketorolac for acute postoperative pain. Curr Med Res Opin 2010;26(8):1915–1923. [DOI] [PubMed] [Google Scholar]
- 50.Grant GM, Mehlisch DR. Intranasal ketorolac for pain secondary to third molar impaction surgery: a randomized, double-blind, placebo-controlled trial. J Oral Maxillofac Surg 2010;68(5):1025–1031. [DOI] [PubMed] [Google Scholar]
- 51.Brown C, Moodie J, Bisley E, Bynum L. Intranasal ketorolac for postoperative pain: a phase 3, double-blind, randomized study. Pain Med 2009;10(6):1106–1114. [DOI] [PubMed] [Google Scholar]
- 52.Dagenais R, Zed PJ. Intranasal lidocaine for acute management of primary headaches: a systematic review. Pharmacotherapy 2018;38(10):1038–1050. [DOI] [PubMed] [Google Scholar]
- 53.Sheridan DC, Hansen ML, Lin AL, Fu R, Meckler GD. Low-dose propofol for pediatric migraine: a prospective. Randomized Controlled Trial. J Emerg Med 2018;54(5):600–606. [DOI] [PubMed] [Google Scholar]
- 54.Trottier ED, Bailey B, Lucas N, Lortie A. Prochlorperazine in children with migraine: a look at its effectiveness and rate of akathisia. Am J Emerg Med 2012;30(3):456–463. [DOI] [PubMed] [Google Scholar]
- 55.Kabbouche MA, Vockell AL, LeCates SL, Powers SW, Hershey AD. Tolerability and effectiveness of prochlorperazine for intractable migraine in children. Pediatrics 2001;107(4):E62. [DOI] [PubMed] [Google Scholar]
- 56.Tsze DS, Ieni M, Flores-Sanchez PL, et al. Quantification of pain and distress associated with intranasal midazolam administration in children and evaluation of validity of four observational measures. Pediatr Emerg Care 2021;37:e17–e20. [DOI] [PubMed] [Google Scholar]
- 57.Chiaretti A, Barone G, Rigante D, et al. Intranasal lidocaine and midazolam for procedural sedation in children. Arch Dis Child 2011;96(2):160–163. [DOI] [PubMed] [Google Scholar]
- 58.Yealy DM, Ellis JH, Hobbs GD, Moscati RM. Intranasal midazolam as a sedative for children during laceration repair. Am J Emerg Med 1992;10(6):584–587. [DOI] [PubMed] [Google Scholar]
- 59.Tsze DS, Ieni M, Fenster DB, et al. Optimal volume of administration of intranasal midazolam in children: a randomized clinical trial. Ann Emerg Med 2017;69(5):600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Connell NC, Woodward HA, Flores-Sanchez PL, et al. Comparison of preadministered and coadministered lidocaine for treating pain and distress associated with intranasal midazolam administration in children: a randomized clinical trial. J Am Coll Emerg Physicians Open 2020;1(6):1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith D, Cheek H, Denson B, Pruitt CM. Lidocaine pretreatment reduces the discomfort of intranasal midazolam administration: a randomized, double-blind, Placebo-controlled Trial. Acad Emerg Med 2017;24(2):161–167. [DOI] [PubMed] [Google Scholar]
- 62.Antonio C, Zurek J, Creighton P, Johnson K, Heard C. Reducing the pain of intranasal drug administration. Pediatr Dent 2011;33(5):415–419. [PubMed] [Google Scholar]
- 63.Khalil W, Raslan N. The effectiveness of topical lidocaine in relieving pain related to intranasal midazolam sedation: a randomized, placebo-controlled clinical trial. Quintessence Int 2020;51(2):162–167. [DOI] [PubMed] [Google Scholar]
- 64.Bachur RG, Monuteaux MC, Neuman MI. A comparison of acute treatment regimens for migraine in the emergency department. Pediatrics 2015;135(2):232–238. [DOI] [PubMed] [Google Scholar]
- 65.Richer L, Craig W, Rowe B. Randomized controlled trial of treatment expectation and intravenous fluid in pediatric migraine. Headache 2014;54(9):1496–1505. [DOI] [PubMed] [Google Scholar]
- 66.Jones CW, Remboski LB, Freeze B, Braz VA, Gaughan JP, McLean SA. Intravenous fluid for the treatment of emergency department patients with migraine headache: a randomized controlled trial. Ann Emerg Med 2019;73(2):150–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.