Abstract
Adenovirus vectors based on human serotype 5 (Ad5) have successfully been used as gene transfer vectors in many gene therapy-based approaches to treat disease. Despite their widespread application, many potential therapeutic applications are limited by the widespread prevalence of vector-neutralizing antibodies within the human population and the inability of Ad5-based vectors to transduce important therapeutic target cell types. In an attempt to circumvent these problems, we have developed Ad vectors based on human Ad serotype 11 (Ad11), since the prevalence of neutralizing antibodies to Ad11 in humans is low. E1-deleted Ad11 vector genomes were generated by homologous recombination in 293 cells expressing the Ad11-E1B55K protein or by recombination in Escherichia coli. E1-deleted Ad11 genomes did not display transforming activity in rodent cells. Transduction of primary human CD34+ hematopoietic progenitor cells and immature dendritic cells was more efficient with Ad11 vectors than with Ad5 vectors. Thirty minutes after intravenous injection into mice that express one of the Ad11 receptors (CD46), we found, in a pattern and at a level comparable to what is found in humans, Ad11 vector genomes in all analyzed organs, with the highest amounts in liver, lung, kidney, and spleen. Neither Ad11 genomes nor Ad11 vector-mediated transgene expression were, however, detected at 72 h postinfusion. A large number of Ad11 particles were also found to be associated with circulating blood cells. We also discovered differences in in vitro transduction efficiencies and in vivo biodistributions between Ad11 vectors and chimeric Ad5 vectors possessing Ad11 fibers, indicating that Ad11 capsid proteins other than fibers influence viral infectivity and tropism. Overall, our study provides a basis for the application of Ad11 vectors for in vitro and in vivo gene transfer and for gaining an understanding of the factors that determine Ad tropism.
Since the concept of using adenoviruses (Ads) as vehicles for gene transfer was first proposed by Berkner and Sharp in 1983 (3), many groups have investigated the therapeutic potential of using recombinant Ads for gene delivery. Ad vectors, predominantly derived from human serotype Ad5, have seen widespread use in gene therapy applications for the treatment of many diseases; however, many inherent problems still remain within this gene delivery system. These problems include an inability to transduce certain target cell populations, an intrinsic acute Ad-mediated toxicity in vivo, and a high prevalence of vector-neutralizing antibodies (VNAbs) within the human population.
In an attempt to address the problems associated with vectors based on Ad5, several studies have investigated the development of vectors derived from different Ads. These vector systems may be based on Ad5 but incorporate components of another Ad (45) or may be entirely based on another Ad serotype that may be of human (33) (38) or animal (20, 28, 39) origin. In one such strategy we (57, 61) along with others (13, 17, 27, 62) have developed chimeric Ad5 vectors with the fiber from a human group B Ad. Unlike human subgroups A, C, D, E, and F Ads, which utilize the coxsackie and Ad receptor (CAR) as a primary attachment receptor (47), group B Ads employ CD46 as a cellular receptor (12, 50, 58). Ads containing group B fibers are able to efficiently infect cell types expressing no or low levels of CAR, including important gene therapy target cells (13, 17, 27, 52, 57, 61, 62). More recently, vectors based entirely on the group B serotypes Ad7 and Ad35 have also been developed (14, 41, 49, 51, 69).
In order to facilitate development of a group B Ad11 vector system, we recently cloned and sequenced the genome of strain Ad11p (63). Since previous studies have shown that Ad11p can bind and infect therapeutic target cells like dendritic cells (DCs), tumor cells, or hematopoietic progenitor cells with high efficiency (46, 59, 61, 73) and that the prevalence of Ad11-neutralizing antibodies is low (10), we hypothesized that Ad11 would make a promising gene transfer vector for treatment of hematopoietic and metastatic disease. In this article we introduce a simple method for generating Ad11 vectors and compare their in vitro and in vivo characteristics with those of standard Ad5 vectors and Ad5 vectors containing the Ad11 fiber (Ad5/11).
MATERIALS AND METHODS
Plasmids.
Construction of the plasmid pBGwtAd11 has been described previously (63). The plasmid pAd11-shuttle was constructed as follows. A PCR fragment containing the left 388 bp of Ad11 (inverted terminal repeat [ITR] and packaging signal) was amplified by using the primers Ad11LITRpsiF (5′-GATCCTCGAGGGCCGGCCGTTTAAACGAATTCCATCATCAATAATATACCTTATAG-3′) and Ad11LITRpsiR (5′-GATCTTCGAACTCCACGTAATGGGTCAAAGTCTAC-3′) and cloned into XhoI/BstBI-linearized pGEM7Zf (Promega, Madison, Wis.) to produce p7Zf-Ad11-left. Primers Ad11LF (5′-GATCGAGCTCGCTGTCATGAGTGGAAACGCTTC-3′) and Ad11LR (5′-GATCATGCATGAACATTCATACCCCAATCTGG-3′) were used to amplify a PCR fragment corresponding to Ad11 nucleotides 3477 to 4624 that was subsequently cloned into SacI/NsiI-linearized p7Zf-Ad11-left to generate p7Zf-Ad11-left-pIX. An NsiI/PstI fragment from the plasmid pPBGshAd11-2 (63) containing Ad11 nucleotides 32801 to 34794 was cloned into the NsiI site of p7Zf-Ad11-left-pIX with the right Ad11 terminus in reverse orientation to the left terminus, generating p7Zf-Ad11-left-pIX-right. The simian virus 40 (SV40) promoter was amplified by PCR from the plasmid pLXSN (provided by A. Dusty Miller, Fred Hutchinson Cancer Research Center, Seattle, Wash.) by using the primers SV40F (5′-GATCGAGCTCCAGCTGTGGAATGTGTGTC-3′) and SV40R (5′-GATCGAGCTCGTGCTTCAGCTGGAAGCTTTTTGC-3′) and cloned into the SacI site of p7Zf-Ad11-left-pIX-right to generate pAd11-shuttle. To generate pAd11-left end-shuttle, a PCR product corresponding to Ad11 nucleotides 4251 to 7928 was amplified by using the primers Ad11polF (5′-GATCACCGGTTGAGCTGGGATGGGTGCATTC-3′) and Ad11polR (5′-GATCTTAATTAAGTTTAAACGGCCGGCCCGCAGAAACTTCTACTTCCATC-3′) and cloned into the AgeI/PacI sites of pAd11-shuttle replacing the Ad11 right-end fragment. In order to generate pAd11-left-CMV-GFP (where CMV is cytomegalovirus and GFP is green fluorescent protein) the enhanced GFP (EGFP) cDNA was extracted from the plasmid pEGFP (Clontech, Palo Alto, Calif.) as a BamHI/NotI fragment and cloned into the BamHI/NotI sites of pcDNA3.1 (Invitrogen, Carlsbad, Calif.) to generate pcDNA-EGFP. The CMV-EGFP-BGHpA (where BGH is bovine growth hormone) expression cassette was then extracted as a BglII/DraIII fragment, blunted, and then cloned in forward orientation into the SwaI site of pAd11-left end-shuttle to generate pAd11-left-CMV-GFP. To generate pAd11-shuttle-CMV-GFP, the BglII/DraIII CMV-GFP-BGHpA-containing fragment from pcDNA-EGFP was blunt cloned into the SwaI site of pAd11-shuttle. To generate pAd11-CMV-GFP the plasmid pAd11-shuttle-CMV-GFP was linearized with BssHII and then recombined with wild-type Ad11 (wtAd11) DNA in Escherichia coli BJ5183 cells. The plasmid pPGK-Ad11E1B55K-SV40pA (where PGK is phosphoglycerate kinase) was constructed as follows. The Ad11 E1B55K open reading frame (ORF) corresponding to Ad11 nucleotides 1915 to 3399 was amplified by PCR from the plasmid pBGwtAd11 by using the primers Ad1155KF-XhoI (5′-AACTCGAGAATGGATCCCGCAGACT-3′) and Ad1155KR-KpnI (5′-AATGGTACCTTAGTCAGTTTCTTCTCCAC-3′) and cloned into the vector phPGKLgfp as an XhoI/KpnI fragment to generate pPGK-Ad11E1B55K-SV40pA. Within pPGK-Ad11E1B55K-SV40pA, the Ad11 E1B55K gene is preceded by the human PGK promoter and followed by the SV40 polyadenylation signal. The plasmid pAd11-FRT-helper is an E1-deleted Ad11 genome containing a truncated packaging signal flanked by minimal FLP recombinase target (FRT) sites (D. Stone and A. Lieber, unpublished data).
Cell culture.
HEK-293 (293) cells were obtained from Microbix Biosystems Inc, Toronto, Canada. HuH7 cells have been described previously (40). HeLa (ATCC CCL-2), HEp-2 (ATCC CCL-23), A549 (ATCC CCL-185), HT29 (ATCC HTB-38), K562 (ATCC CCL-243), PC3 (ATCC CRL-1435), and SK-OV-3 (ATCC HTB-77) cells were obtained from the American Type Tissue Collection (ATCC). CHO-CAR cells, which express the human coxsackie and Ad receptor, were provided by J. Bergelson (Children's Hospital of Philadelphia, Philadelphia, Pa.). CHO-C2 cells, which express the C2 isoform of human CD46, were provided by J.P. Atkinson (Washington University, St. Louis, Mo.). CHO-pcDNA cells, stably transfected with the plasmid pcDNA, were used as controls and were provided by J. Bergelson. Cell lines 293, HEp-2, and A549 were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Cell lines HuH7, HT29, K562, PC3, CHO-pcDNA, CHO-CAR, and CHO-C2 were grown in the same medium supplemented with 1 mM sodium pyruvate and 1× minimal essential medium (MEM) nonessential amino acids. SK-OV-3 cells were grown in McCoy's 5A medium supplemented with 10% FCS and 1.5 mM l-glutamine.
Complementing cell line.
A 293-based complementing cell line was constructed that stably expresses the Ad11 E1B55K protein. Briefly, low passage HEK-293 cells were transfected with the plasmid pPGK-Ad11E1B55K-SV40pA and left for 3 weeks under G418 selection at a concentration of 400 μg/ml. Individual clones were seeded and amplified in a 96-well plate, and the presence of Ad11 E1B55K mRNA transcripts in individual clones was confirmed by semiquantitative reverse transcription PCR by using the primers Ad1155KF-XhoI and Ad1155KR-KpnI (data not shown). Clone 293D7 was chosen for further studies and designated 293-Ad11-E1B55K.
Viruses.
The virus used to generate all Ad11-based plasmids was of genome type Ad11p (Slobitski strain) and was obtained from the ATTC (VR-12). Wild-type Ad5 was obtained from the ATCC (VR-5). Wild-type Ad5 and Ad11 were propagated in 293 and HEp-2 cells, respectively, and purified in CsCl gradients by using previously described methods (31). Viral DNA was extracted by using a previously described method (31). The sequence of human Ad11 has been reported previously (63) and assigned GenBank accession number AY163756.
Ad vectors Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad5/35-CMV-GFP have been described previously (57, 61) and contain a CMV-EGFP-BGHpA expression cassette in the E3 region. E1-deleted Ad11 vectors were propagated in the cell line 293-Ad11-E1B55K. Viruses were rescued following calcium phosphate transfection of respective plasmids into subconfluent 10-cm dishes of 293-Ad11-E1B55K cells. Since 293-Ad11-E1B55K cells do not tolerate long periods under agar, viruses were rescued in a single 10-cm dish without overlay and subsequently plaque purified. Single virus plaques were amplified, screened for correct genomes by restriction analysis, and purified on cesium chloride gradients as previously described (31), before dialysis against 10% glycerol, 150 mM NaCl, 10 mM Tris-HCl (pH 8), and 10 mM MgCl2. Ad11-CMV-GFP was generated by cotransfection of pAd11-left-CMV-GFP with NheI/PmeI-linearized pAd11-right-shuttle, and recombinant plaques appeared by approximately 3 weeks. Ad11-CMV-GFP was also generated by transfection of FseI-linearized pAd11-CMV-GFP, and plaques appeared by approximately 3 weeks. Ad11-CMV-GFP is an E1-deleted Ad11 recombinant virus with a genome of 34,321 bp and has a CMV-EGFP expression cassette inserted in the E1 region.
Electron microscopy analysis.
Cesium chloride-purified Ad stocks were thawed and diluted with 0.5% glutaraldehyde. Grids were prepared as described earlier (36). After staining with 2% methylamine tungstate (Nanoprobes, Stony Brook, N.Y.), the carbon coated grids were evaluated and photomicrographed with a Philips 410 electron microscope, operated at 80 kV (original magnification, ×31,000).
VNAb assay.
Cervical cancer-positive, breast cancer-positive, or cancer-negative serum samples from Senegalese patients were provided by Nancy Kiviat (Department of Pathology, University of Washington, Seattle, Wash.). Serum samples from cancer patients were taken before patients underwent chemotherapy. Briefly, 293 cells were plated in 96-well plates at 4 × 104 cells per well and placed at 37°C. The following day, serum samples were heat inactivated at 56°C for 30 min and then serially diluted from 1:2 to 1:1,204 in MEM containing 2% FCS. A total of 20 PFU per cell of wild-type Ad5 or Ad11 in 10 μl of MEM was incubated with 100 μl of each serum dilution for 1 h at 37°C. Medium was removed from cells plated the previous day, and 55 μl of virus-containing serum was added to cells along with 45 μl of 293 growth medium in duplicate. A further 100 μl of 293 growth medium was added to cells 3 and 6 days postinfection. At 8 days postinfection, cells were analyzed for the presence of cytopathic effect (CPE), and serum samples were scored positive for the presence of VNAbs if no CPE was seen at a dilution of 1:2 or higher.
Ad thermostability assay.
The day before infection 293-Ad11-E1B55K cells were plated in 24-well plates at 5 × 104 cells per well. On the day of the experiment Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP were incubated at 37 or 48°C for 0, 5, 15, or 30 min in 0.5 ml of growth medium. Virus was then used to infect 293-Ad11-E1B55K cells at a multiplicity of infection (MOI) of 400 virus particles (VP)/cell, and 48 h later the number of GFP-positive cells was analyzed by flow cytometry.
Transforming activity assay.
Cultures of baby rat kidney (BRK) cells were isolated from kidneys of 7-day-old rats by either trypsin or collagenase digestion according to previously published protocols (43, 65) and in collaboration with Daniel Moralejo and Ake Lernberg (Department of Medicine, University of Washington). Cells were plated at two densities (106 and 3 × 106 cells per dish) in 10-cm dishes, and 48 h later cultures were calcium phosphate transfected with 2 μg of Ad genome plasmids. Calcium phosphate precipitate was left on cells overnight, and medium was changed the following day and every third day thereafter. After 5 weeks cells were stained with crystal violet, and transformed foci of cells were visually assessed by light microscopy. For each Ad genome plasmid, both trypsin-and collagenase-isolated cells were used at two different densities. The total number of transformed foci in four dishes was counted. Cultures were transfected with recombinant Ad genome plasmids pHVAd1 (Ad5 E1+E3-E4+ genome; DeveloGen AG, Göttingen, Germany), pAdHM4(Ad5 E1-E3-E4+ genome [37]), pBGwtAd11 (Ad11 E1+E3+E4+ genome [63]), and pAd11-FRT-helper (Ad11 E1-E3+E4+ genome; see “Plasmids” above).
Infection blocking studies.
The method for infection blocking studies has been described previously (12). Briefly, 5 × 104 HeLa, A549, CHO-pcDNA, CHO-CAR, or CHO-C2 cells were plated in 24-well plates and incubated the following day with phosphate-buffered saline (PBS) or recombinant Ad5, Ad11p, or Ad35 fiber knobs in 100 μl of PBS at a concentration of 10 μg/ml for 15 min at room temperature. A total of 25 PFU/cell (Fig. 2) or 500 VP/cell (Fig. 5) of Ad5-CMV-GFP, Ad5/11-CMV-GFP, Ad11-CMV-GFP, or Ad5/35-CMV-GFP in MEM containing 2% FCS was then added to cells for 30 min at room temperature before medium was replaced with 500 μl of MEM containing 10% FCS. Cell transduction, as assessed by GFP fluorescence, was analyzed by flow cytometry by using a FACscan instrument (Becton Dickinson) 24 h after infection. Ad5, Ad11p, and Ad35 fiber knob domains were prepared as described earlier (12).
FIG. 2.
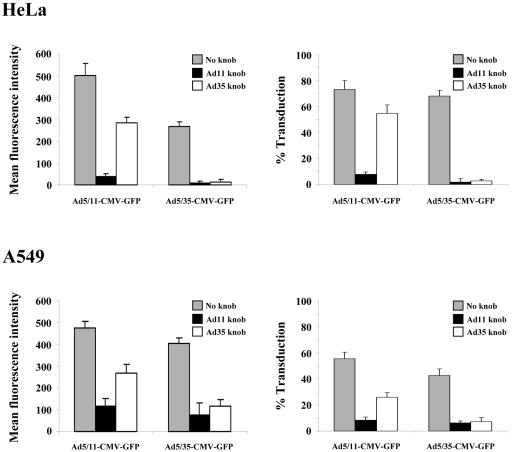
Fiber chimeric Ad infection blocking studies. A549 and HeLa cells were preincubated with Ad11p or Ad35 fiber knobs (10 μg/ml) and then infected with Ad5/11-CMV-GFP or Ad5/35-CMV-GFP at an MOI of 25 PFU/cell (roughly equivalent to 500 VP/cell). Mean fluorescent intensity (left panels) and percentage of transduced cells (right panels) were assessed by flow cytometry of GFP expression 24 h after infection.
FIG. 5.
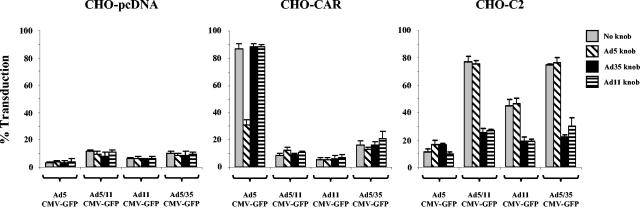
CD46-dependent infection of group B fiber targeted Ad vectors. CHO-pcDNA, CHO-CAR, or CHO-C2 cells were preincubated with Ad5, Ad11p, or Ad35 fiber knobs (10 μg/ml) and then infected with Ad5-CMV-GFP, Ad5/11-CMV-GFP, Ad11-CMV-GFP, or Ad5/35-CMV-GFP at an MOI of 500 VP/cell. The following day cell transduction was assessed by flow cytometry of GFP expression.
Tumor cell infection studies.
Tumor cell lines A549, HuH7, PC3, HT29, SK-OV-3, and K562 were plated in 24-well plates at a density of 5 × 104 cells per well. The following day, cells were incubated with Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP at 500, 2,500, 10,000, and 25,000 VP/cell in growth medium for 3 h before medium was changed. At 24 h postinfection GFP expression was assessed by flow cytometry.
Primary cell isolation and infection.
Human CD34-positive cells were provided by Shelly Heimfeld (Fred Hutchinson Cancer Research Center). Cells were isolated from peripheral blood of granulocyte colony-stimulating factor-mobilized donors on a CliniMACSs machine (Miltenyi Biotec, Auburn, Calif.), and aliquots were stored in liquid nitrogen. The day before the experiment, cells were recovered from frozen stock and incubated in Iscove modified Dulbecco medium supplemented with 20% FCS, 10−4 M β-mercaptoethanol, 100 μg of DNase I per ml, 2 mM l-glutamine, 10 U of interleukin-3 per ml, 50 ng of stem cell factor per ml, and 2 ng of thrombopoietin per ml. On the day of infection 2.5 × 105 cells were incubated with 250, 500, 1,000 and 4,000 VP/cell of Ad5-CMV-GFP, Ad5/11-CMV-GFP, or Ad11-CMV-GFP in 500 μl of growth medium and then left at 37°C overnight. At 24 h postinfection GFP expression was assessed by flow cytometry.
Human DCs were derived from peripheral blood mononuclear cells obtained after patient leukapheresis. Peripheral blood mononuclear cells were provided by Nora Dissis (Tumor Vaccine Group, Department of Oncology, University of Washington). Mononuclear cells were resuspended in serum-free AIMV medium containing L-glutamine, streptomycin sulfate, and gentamycin sulfate (Invitrogen, Carlsbad, Calif.) and plated in six-well plates at 107 cells/well for 1 h. After 1 h, nonadherent cells were washed off, and AIMV medium containing 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 800 U of granulocyte-macrophage colony-stimulating factor (Avigen, Seattle, Wash.) per ml, and 500 IU of interleukin-4 (RandD Systems, Minneapolis, Minn.) per ml was added. Cells were incubated for 4 days and medium was replaced on day 3. On the day of the experiment, 105 DCs were plated in 24-well plates and incubated with Ad5-CMV-GFP, Ad5/11-CMV-GFP, or Ad11-CMV-GFP at 500, 2,500, 5,000, 10,000, and 50,000 VP/cell overnight. At 24 h postinfection, GFP expression was assessed by flow cytometry.
Biodistribution and blood clearance studies.
All experiments involving animals were conducted in accordance with the institutional guidelines set forth by the University of Washington. All in vivo biodistribution and blood clearance studies were done in hCD46Ge mice, which are transgenic for human CD46 (25), housed in specific-pathogen-free facilities. For analysis of vector biodistribution, mice were injected in the tail vein with 1011 VP of Ad5-CMV-GFP, Ad5/11-CMV-GFP, or Ad11-CMV-GFP in 200 μl of PBS. At 30 min and 72 h after virus infusion, animals were sacrificed, blood was flushed from the circulation by cardiac saline perfusion, and organs were collected for analysis. For vector blood clearance studies, mice were injected in the tail vein with 1011 VP of Ad5-CMV-GFP, Ad5/11-CMV-GFP, or Ad11-CMV-GFP in 200 μl of PBS. At 3, 15, and 120 min after virus injection, blood was collected retro-orbitally for serum or blood cell extraction.
Southern blot analysis.
Isolation of DNA for vector genome titration and analysis by Southern blotting was carried out as described elsewhere (6). Isolation of cellular DNA from mouse livers and Southern analysis were performed as described elsewhere (32). A 32P-labeled GFP fragment, corresponding to the entire GFP gene, was used for hybridization to specifically detect genomic DNA from all Ad vectors in organs. A 32P-labeled mouse β-glucoronidase fragment was used as a specific probe for a housekeeping gene.
Immunohistochemistry.
GFP expression after infusion of Ad5-CMV-GFP, Ad5/11-CMV-GFP, or Ad11-CMV-GFP virus was visualized on paraffin sections by immunohistochemistry with an anti-GFP antibody (1:200; Clontech, BD Biosciences, San Diego, Calif.). Binding was visualized by using a goat anti-mouse immunoglobulin G conjugated with peroxidase or with Alexa Fluor 488 (green) (1:200; Molecular Probes, Inc., Eugene, Oreg.).
Quantitative vector genome PCR.
Heparinized blood, collected from animals 3, 15, and 120 min after Ad vector injection, was centrifuged, and the virus load in plasma was analyzed by quantitative PCR. Samples were diluted 1:1,000, and quantitative PCR was performed with primers specific for GFP (5′-AACGAGAAGCGCGATCACATGGTCCTGCTG-3′;sense) and the BGH poly(A) signal (5′-CCCAATCCTCCCCCTTGCTGTCCTGCCCCA-3′; antisense) by using a SYBR green kit (QIAGEN, Valencia, Calif.) for the Light Cycler (Roche, Indianapolis, Ind.) and external standards for GFP (15s at 95°C, 5s at 57°C, and 17s at 72°C). Plasma levels of viral genomes are the mean of values from four mice.
RESULTS
Anti-Ad neutralizing antibodies.
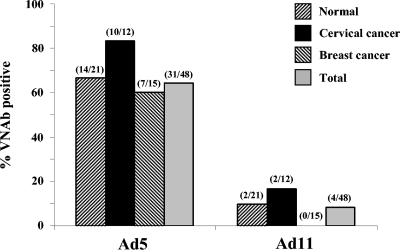
One of the existing problems for Ad vectors is the presence of anti-VNAbs within the human population due to widespread exposure to common strains of Ad such as Ad5. We decided to compare the prevalence of Ad5 and Ad11 VNAbs in serum from healthy, breast cancer, or cervical cancer patients, since tumor transduction in vivo is one of the goals of our laboratory. The prevalence of Ad5 VNAbs in healthy, breast cancer, and cervical cancer patients was higher than Ad11 VNAbs, with combined prevalences of 66 and 10% for Ad5 and Ad11, respectively (Fig. 1). Since it is known that Ad11 is often isolated from patients with suppressed immunity, it was thought that Ad11 VNAb prevalence could potentially be higher in cancer patients, but no significant difference was seen in comparison to prevalence in healthy patients. These findings are in agreement with previous studies demonstrating a lower prevalence of some B group VNAbs within humans and support our hypothesis that Ad11 vectors may be useful for intravenous injection and ex vivo transduction of stem cells or DCs in the presence of human serum.
FIG. 1.
Prevalence of anti-Ad neutralizing antibodies. Serum from nonimmunocompromised, cervical cancer-positive, or breast cancer-positive Senegalese patients was evaluated for its ability to inhibit wild-type Ad5 or Ad11 infection. Serially diluted serum was incubated with wild-type virus and used to infect 293 cells at an MOI of 20 PFU/cell, and the CPE seen was used to determine the presence of VNAbs. Samples were scored positive if no CPE was seen 8 days after infection. Total numbers of positive samples per total number tested are shown above each bar.
Fiber chimeric Ad infection.
To further assess serotype Ad11 as a basis for new gene transfer vectors, we compared attachment receptor usage of Ad5 vectors possessing Ad11p or Ad35 fibers, i.e., Ad5/11-CMV-GFP and Ad5/35-CMV-GFP, respectively. HeLa and A549 cells were incubated with recombinant Ad11p or Ad35 fiber knobs before infection with Ad5/11-CMV-GFP or Ad5/35-CMV-GFP. In the absence of recombinant fiber knobs, efficient infection of HeLa and A549 cells was seen with both Ad5/11-CMV-GFP and Ad5/35-CMV-GFP, although higher levels of gene transfer were seen in both cell lines with Ad5/11-CMV-GFP (Fig. 2). When cells were preincubated with recombinant Ad11p fiber knob, infection by both Ad5/11-CMV-GFP and Ad5/35-CMV-GFP was almost completely inhibited. In contrast, preincubation of cells with recombinant Ad35 fiber knob almost completely inhibited infection of Ad5/35-CMV-GFP but only partially blocked infection of Ad5/11-CMV-GFP. The higher levels of infection by Ad5/11-CMV-GFP, along with the inability of Ad35 fiber to completely block Ad11p-mediated infection, suggests that Ad11p may utilize an additional receptor for in vitro cell infection.
E1-deleted Ad11 vector production and rescue.
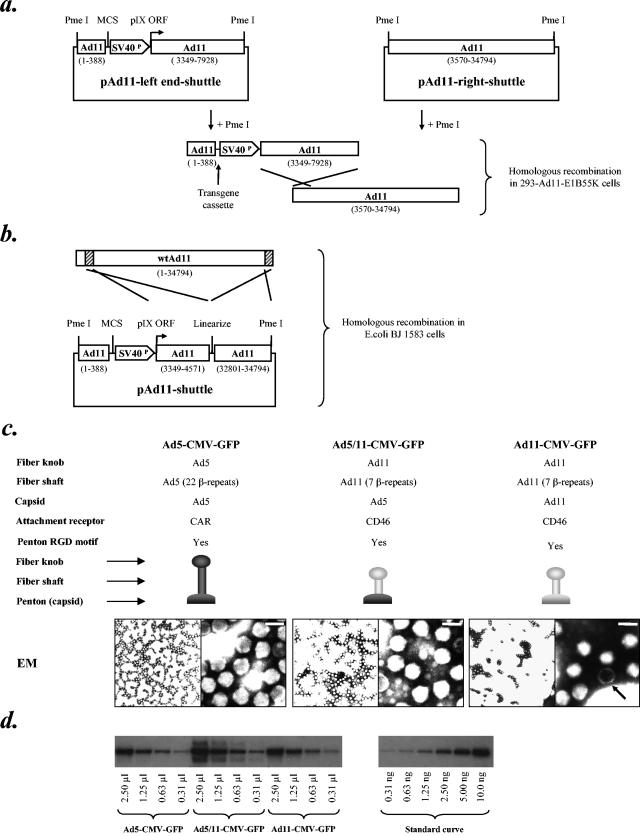
A series of vector plasmids and a complementing cell line were constructed for production and amplification of E1-deleted first-generation Ad11 vectors. Ad11 shuttle plasmids were constructed with the Ad11 left ITR and packaging signal, a multiple cloning site, a complete deletion of E1A and E1B genes, and an SV40 promoter upstream of the Ad11 pIX ORF (Fig. 3A, B). (Since upstream regulatory elements in the viral genomes that regulate pIX expression might have been removed together with the E1 genes, the SV40 promoter was inserted to ensure high-level pIX expression). Complementary plasmids were designed so that E1-deleted Ad11 genomes could be rescued by homologous recombination in a complementing cell line (Fig. 3A) or by E. coli recombination (Fig. 3B) in BJ5183 cells according to the method of Chartier et al. (8). In order to propagate E1-deleted Ad11 vectors, a 293-based complementing cell line expressing the Ad11-E1B55K protein was generated (293-Ad11-E155K), since E1-deleted vectors based on the highly homologous serotype Ad35 are known to replicate in cells expressing Ad5 E1 proteins and Ad35-E1B55K (69). The Ad11-E1B55K expression construct was designed so that no regions of homology were present between the matched vector backbones and the cellular Ad11-E1B55K ORF, which was flanked by a human PGK promoter and an SV40 polyadenylation signal.
FIG.3.
Generation of recombinant E1-deleted Ad11 vectors. (a) Schematic representation of plasmids used to generate recombinant Ad11 genomes by homologous recombination in 293-Ad11-E1B55K cells. Unique PmeI restriction sites used for linearization of Ad genome plasmids, Ad11p sequences (based on GenBank sequence AY163756), multiple cloning site, and SV40 promoter driving expression of the pIX ORF are indicated. (b) Schematic representation of the plasmid used to generate recombinant Ad11 genomes by E. coli recombination in BJ5183 cells. Unique PmeI restriction sites used for linearization of Ad genome plasmids, Ad11p sequences (based on GenBank sequence AY163756), multiple cloning site, and SV40 promoter driving expression of the pIX ORF are indicated. (c) Capsid compositions of Ad vectors Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP along with transmission electron microscopic images of negatively stained vector particles. Scale bar (top right of right-hand images) represents 100 nm. An arrow indicates a defective particle. (d) Determination of virus titer by Southern blotting. A 10-μl aliquot of each viral stock was mixed with 2 × 105 293 cells as a carrier, and total DNA was extracted and resuspended in 50 μl. A standard curve from 0 to 10 ng of a linearized, same size plasmid containing GFP (∼34 kb) was run on a 0.8% agarose gel alongside 2.5, 1.25, 0.63, and 0.31 μl of the extracted DNA. The whole GFP gene was used as a probe and the blot was subsequently analyzed with a phosphorimager. The determined titer was corrected for the volume lost during the extraction process. The genome titers of the Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP preparations shown were 1.51 × 1012, 3.24 × 1012, and 2.16 × 1012 genomes/ml, respectively. SV40P, SV40 promoter, MCS, multiple cloning site; EM, electron micrograph; RGD, arginine, glycine, and aspartic acid.
In order to demonstrate rescue of recombinant virus, a CMV-EGFP-BGHpA expression cassette was cloned into the multiple cloning sites of pAd11-left end-shuttle and pAd11-shuttle in forward orientation. These plasmids were then used to rescue E1-deleted recombinant Ad11-CMV-GFP by either homologous recombination in 293-Ad11-E1B55K cells or E. coli recombination (Fig. 3A and B). By using either technique for virus rescue, GFP-positive viral plaques were seen in 293-Ad11-E1B55K cells at around 3 weeks posttransfection. Virus was amplified from single plaques, and the predicted genome structure was confirmed by restriction analysis of viral genomes (data not shown). Efficient growth and amplification of Ad11-CMV-GFP were seen in 293-Ad11-E1B55K cells, with preparations of greater than 1012 VP/ml generated. On cesium chloride gradients the ratio of Ad11 particles containing full-length genomes to defective or empty particles was approximately 1:1.5 from cells harvested 48 h postinfection, but after 72 h this ratio increased to approximately 1:1 (this was also seen with wild-type Ad11). The concentration of virus genomes or particles in individual preparations was measured by either optical density spectrophotometry or quantitative Southern blotting (Fig. 3D). The plaque-forming activity of virus was titered by end point dilution in 293-Ad11-E1B55K cells. Individual virus preparations were found to have particle-to-PFU ratios in the range of 150:1 to 250:1. Analysis of negatively stained particles by transmission electron microscopy revealed no structural abnormalities in Ad5, chimeric Ad5/11, or Ad11 vector particles (Fig. 3C). Efficient replication of Ad11-CMV-GFP was also seen in VK109 cells, which express Ad5 E1 and E4 proteins (29), while 293 cells were not able to efficiently support virus replication.
Stability of Ad11 vectors.
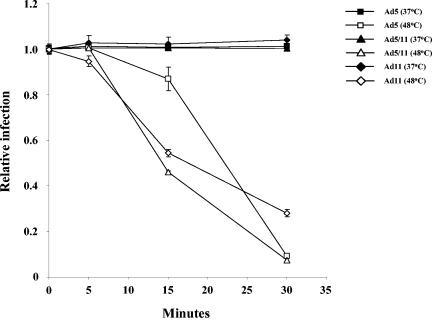
A potential reason for the limited distribution of Ad11 in the human population could be related to a lower stability of Ad11 particles. To assess the thermostability of Ad11 vectors, we employed a protocol that is routinely used in characterization of Ads (5). We incubated Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP at 48°C for 5, 15, and 30 min and measured the number of infectious, GFP-expressing particles (Fig. 4). While the loss in Ad5 infectivity during the first 15 min of incubation at 48°C was less than for the other vectors, more infectious Ad11 particles than Ad5 and Ad5/11 particles remained after 30 min of incubation at 48°C. Overall, all vectors showed similar stability profiles, indicating that Ad11 vectors are as thermostable as standard Ad5 vectors.
FIG. 4.
Ad thermostability. A total of 400 VP/cell of Ad5-CMV-GFP, Ad5/11-CMV-GFP, or Ad11-CMV-GFP was incubated at 37 or 48°C for 0, 5, 15, or 30 min in 0.5 ml of medium. After incubation virus was used to infect 293-Ad11-E1B55K cells in 24-well plates, and the number of GFP-positive cells was analyzed by flow cytometry 48 h after infection (n = 3). The relative infection level was normalized to the level of transduction seen with each virus at 0 min. A Student's t test gave the following P values: Ad11 (37°C, 5 min) versus Ad11 (48°C, 5 min), P > 0.3; Ad5 (37°C, 15 min) versus Ad5 (48°C, 15 min), P = 0.0422; Ad5 (48°C, 15 min) versus Ad11 (48°C, 15 min), P > 0.0221; Ad5/11 (48°C, 15 min) versus Ad11 (48°C, 15 min), P > 0.0136; Ad5 (48°C, 30 min) versus Ad11 (48°C, 30 min), P > 0.0012; Ad5/11 (48°C, 30 min) versus Ad11 (48°C, 30 min), P > 0.0045.
Ad transforming activity.
It has previously been shown that wild-type group B Ad serotypes 3, 7, 14, 16, and 21 are mildly oncogenic in rodents (15, 16, 22, 30), while Ad serotype 5 is not oncogenic in rodents (7). In order to assess the transforming potential of Ad11 in the context of an Ad vector backbone, cellular transformation assays were carried out in BRK cells with recombinant Ad5 and Ad11 vector genome plasmids with or without E1 sequences. Plasmids were transfected into collagenase-or trypsin-isolated BRK cells, and the ability to induce cellular transformation was assessed after 5 weeks. The highest number of transformed colonies was seen in cells transfected with the E1 containing Ad5 genome plasmid pHV.Ad1 (Table 1). Plates that were mock-transfected or transfected with the E1-deleted Ad5 genome plasmid pAd.HM4, the E1-containing Ad11 genome plasmid pBGwtAd11, or the E1-deleted Ad11 genome plasmid pAd11-FRT-helper had significantly fewer or no colonies (Table 1). We hypothesize that the low level of transforming activity from the plasmid containing Ad11 E1, along with the absence of transforming activity from the E1-deleted Ad11 plasmid, implies that E1-deleted Ad11 vectors will be nononcogenic in human cells.
TABLE 1.
Ad transforming activitya
| Characteristics | Plasmid
|
Untransfectedb | |||
|---|---|---|---|---|---|
| pAd.HM4 | pHV.Ad1 | pAd11-FRT-helper | pBGwtAd11 | ||
| Serotype | Ad5 | Ad5 | Ad11 | Ad11 | NA |
| E1 | E1(−), 342-3523 | Wild type | E1(−), 389-3348 | Wild type | NA |
| E3 | E3(−), 28133-30818 | E3(−), 28133-30818 | Wild type | Wild type | NA |
| E4 | Wild type | Wild type | Wild type | Wild type | NA |
| No. of collagenase colonies | 0 | 10 | 0 | 0 | 0 |
| No. of trypsin colonies | 0 | 4 | 0 | 1 | 1 |
| Total no. of colonies | 0 | 14 | 0 | 1 | 1 |
The cell transforming activity of Ad genome plasmids was assayed in BRK cells isolated from 7 day-old rats by either collagenase or trypsin digestion. BRK cells were transfected with 2 μg of Ad5 or Ad11 vector genome plasmids (with or without E1 sequences); after 5 weeks cells were stained with crystal violet and transformed foci were counted. E1- or, E3-deleted sequences (based on wild-type Ad5 or, Ad11 genome sequences) are indicated by nucleotide positions. The transformation efficiency of BRK cells was assessed by transfection with the plasmid pAd11-CMV-GFP and found to be ∼20%.
NA, not applicable.
CD46-dependent infection.
Although it has been shown that wild-type Ad11 is able to use CD46 as a primary attachment receptor (12, 50), we decided to confirm that Ad11 vectors can also infect cells in a CD46-dependent, CAR-independent manner. Infection competition studies were carried out in CHO-based cell lines expressing either CD46 or CAR. Cell lines were incubated with recombinant Ad5, Ad11p, or Ad35 fiber knobs before infection with CAR-dependent (Ad5-CMV-GFP) or CD46-dependent (Ad5/11-CMV-GFP, Ad5/35-CMV-GFP, or Ad11-CMV-GFP) viruses. In CHO-pcDNA cells Ad5-CMV-GFP, Ad5/11-CMV-GFP, Ad5/35-CMV-GFP, and Ad11-CMV-GFP showed inefficient infection (Fig. 5). In CHO-CAR cells only Ad5-CMV-GFP showed efficient infection, and this infection could be blocked with recombinant Ad5 fiber knob but not Ad11p or Ad35 fiber knobs. In CHO-C2 cells Ad5/11-CMV-GFP, Ad11-CMV-GFP, and Ad5/35-CMV-GFP showed efficient infection, and this infection could be blocked with recombinant Ad11p and Ad35 fiber knobs but not Ad5 fiber knob. These data demonstrate that, like wild-type Ad11, Ad5/11 vectors, and Ad5/35 vectors, Ad11 vectors infect cells in a CAR-independent, CD46-dependent manner.
Transduction of established cell lines of different tissue origin.
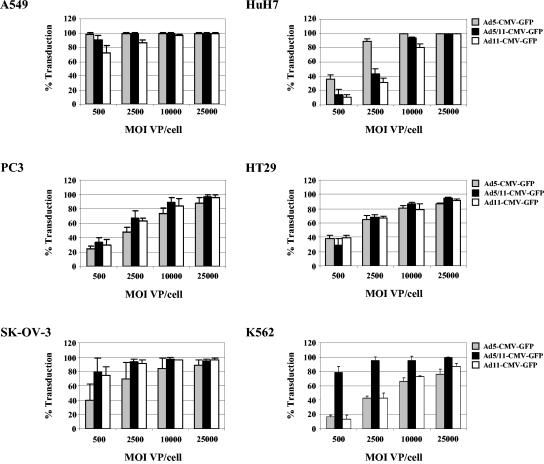
While CD46 is a major receptor for Ad11 fiber-containing vectors, other cellular receptor(s) might be involved in transduction with these vectors, as our competition studies indicate (Fig. 2). We have also previously shown that intracellular trafficking of Ad vectors is affected by cell type-specific factors (55). We therefore tested the ability of Ad11 vectors to transduce a series of human cell lines of different tissue origin that theoretically have different levels of surface receptors. Cell lines derived from lung (A549), liver (HuH7), prostate (PC3), colon (HT29), ovarian (SK-OV-3), or blood cells (K562) were infected with Ad vectors at MOIs of 500, 2,500, 10,000, and 25,000 VP/cell for 2 h, and cell transduction was assessed by flow cytometry of GFP expression 24 h later. In A549 cells, greater than 70% of cells were infected by all vectors at the lowest MOI, with the highest number of infected cells seen with Ad5-CMV-GFP. At higher MOIs, comparable levels of infection were seen for all vectors (Fig. 6). In HuH7 cells, Ad5-CMV-GFP infected significantly more cells than Ad5/11-CMV-GFP and Ad11-CMV-GFP at MOIs of 500 and 2,500, while greater than 80% of cells were infected by all vectors at MOIs of 10,000 and 25,000. In PC3 and HT29 cell lines, the number of cells transduced was comparable for all vectors and increased in direct correlation to vector MOI. In SK-OV-3 cells, Ad5/11-CMV-GFP and Ad11-CMV-GFP infections were more efficient than Ad5-CMV-GFP infection, although more than 80% of cells were infected by all vectors at a MOIs of 10,000 and 25,000. In K562 cells, infection by Ad5/11-CMV-GFP was more efficient than Ad5-CMV-GFP and Ad11-CMV-GFP at all MOIs, although the number of cells infected by Ad5-CMV-GFP and Ad11-CMV-GFP increased in direct correlation to vector MOI. In summary, while the transduction efficiency of the Ad11 vector was comparable to that of the Ad5/11 vector in most cell lines tested, interesting differences in the transduction of leukemia K562 cells were observed between these two vectors.
FIG. 6.
Transduction analysis of tumor cell lines. Tumor cell lines A549, HuH7, PC3, HT29, SK-OV-3, and K562 were infected with Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP at MOIs of 500, 2,500, 10,000 and 25,000 VP/cell. Cell transduction was assessed by flow cytometry of GFP expression 24 h after infection.
Primary cell transduction.
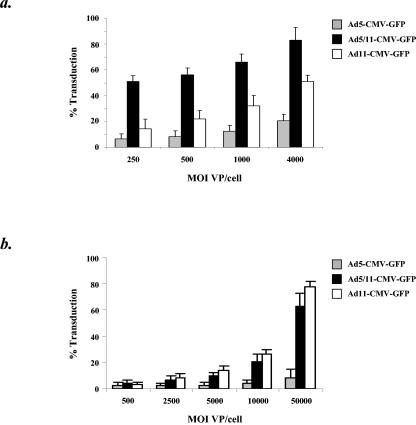
Transduction of primary human hematopoietic stem cells (HSCs) and immature DCs by Ad5-based vectors is known to be inefficient (2, 42, 70). In contrast, it has been shown that fiber chimeric Ad5/11, Ad5/35, and Ad5/50 vectors as well as E1-deleted Ad35 vectors can readily infect both HSCs and DCs (26, 46, 48, 57, 61, 69). To test the efficiency of E1-deleted Ad11 vector transduction in these cell types, human peripheral blood-derived CD34-positive cells and human peripheral blood-derived immature DCs were infected with Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP. CD34+ cells and immature DCs were incubated with vectors at different MOIs, and cell transduction was assessed by flow cytometry of GFP expression 24 h later. In human CD34+ cells inefficient infection by Ad5-CMV-GFP was seen, with maximal infection levels of ∼20% seen at an MOI of 4,000 VP/cell (Fig. 7A). Infection by Ad11-CMV-GFP was more efficient, with maximal infection levels of ∼51% seen at an MOI of 4,000 VP/cell, while Ad5/11-CMV-GFP showed the most efficient infection, with maximal infection levels of ∼83% seen at an MOI of 4,000 VP/cell. In human immature DCs, infection by both Ad5/11-CMV-GFP and Ad11-CMV-GFP was more efficient than infection by Ad5-CMV-GFP (Fig. 7B). The highest levels of expression were seen at an MOI of 50,000 VP/cell, with Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP infecting ∼10, ∼63, and ∼78% of immature DCs, respectively. The levels of CD34+ cell and DC transduction by Ad11 vectors suggest that they will be more effective than Ad5 vectors for treatment of genetic blood disorders or in immunotherapy strategies involving ex vivo transduction of DCs. Notably, differences in transduction of CD34+ cells were seen between Ad11 and Ad5/11 vectors.
FIG. 7.
Transduction analysis of human primary cells. (a) Human hematopoietic progenitor cell transduction. Peripheral blood-derived CD34-positive cells were infected with Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP at MOIs of 250, 500, 1,000 and 4,000 VP/cell. Cell transduction was assessed by flow cytometry of GFP expression 24 h after infection. (b) Human immature DC transduction. Peripheral blood-derived immature DCs were infected with Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP at MOIs of 500, 2,500, 5,000, 10,000 and 50,000 VP/cell. Cell transduction was assessed by flow cytometry of GFP expression 24 h after infection.
In vivo biodistribution.
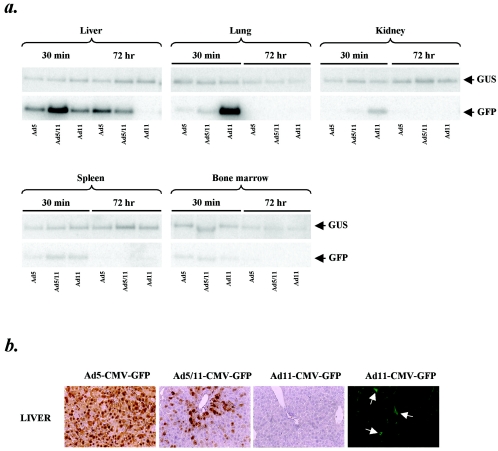
We (4, 54) along with others (51) have previously shown that group B Ads can efficiently infect human but not mouse cells. This is likely because human CD46 is found on all nucleated cells, while mouse CD46 expression is limited to the testes (64). This lack of CD46 expression in mice implies that in vivo biodistribution studies in wild-type mice will inadequately reflect the in vivo transduction properties of Ad11 in humans. In order to address this problem, transgenic mice expressing human CD46 were used to study the biodistribution of Ad11 vectors following systemic delivery. Mice were injected in the tail vein with 1011 VP of Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP and then sacrificed 30 min or 72 h later. Tissue DNA was analyzed by Southern blotting with a GFP-specific probe for presence of vector genomes. GFP expression was analyzed by immunohistochemistry on tissue sections. Southern analysis of liver DNA revealed that genomes from all vectors were present at 30 min postinjection, but by 72 h, in contrast to Ad5-CMV-GFP and Ad5/11-CMV-GFP, Ad11-CMV-GFP genomes could not be detected (Fig. 8A). At 30 min postinjection a significant number of Ad11-CMV-GFP genomes were detected in the lung, in contrast to the low levels of Ad5-CMV-GFP and Ad5/11-CMV-GFP genomes, while at 72 h postinjection no vector genomes were detected from any group. In kidney, no Ad5-CMV-GFP genomes and low levels of Ad5/11-CMV-GFP and Ad11-CMV-GFP vector genomes were detected at 30 min postinjection, while no vector genomes were detected from any group at 72 h after virus infusion. In both spleen and bone marrow, low levels of all vector genomes were detected at 30 min postinjection, while no vector genomes were detected from any group at 72 h after virus injection. Analysis of GFP expression in organs harvested 72 h after virus injection revealed very few GFP-positive cells in organs of mice injected with Ad11-CMV-GFP (Fig. 8B). As seen before for Ad5/35 vectors (60), liver transduction with the Ad5/11 vector was less efficient than with the Ad5 vector and mostly localized to periportal hepatocytes. In contrast to results with Ad5-CMV-GFP and Ad5/11-CMV-GFP, no GFP-positive parenchymal cells were seen in the livers of mice injected with Ad11-CMV-GFP, with only sparse nonparenchymal cells transduced. In the spleen, a few GFP-positive cells were seen in the marginal zone surrounding germinal centers of all virus-injected groups (data not shown). GFP-positive cells were only sparsely detected in the red pulp of the spleen from all virus-injected animals (data not shown). For Ad11-CMV-GFP very few GFP-positive cells were seen in kidney and lung (data not shown). Taken together, the Southern blot and GFP immunohistochemical analyses demonstrate that Ad11 vectors show an in vivo biodistribution that is markedly different from that of Ad5-CMV-GFP and Ad5/11-CMV-GFP in CD46 transgenic mice.
FIG. 8.
In vivo biodistribution. hCD46Ge mice were injected in the tail vein with 1011 viral particles of Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP. Organs were obtained from individual mice at 30 min and 72 h postinjection. (a) Tissue biodistribution of vector genomes. Genomic DNA was isolated from organs harvested at 30 min and 72 h postinjection, and the presence of Ad vector genomes in liver, lung, kidney, spleen, and bone marrow was analyzed by Southern blotting with a GFP-specific probe. Membranes were stripped of GFP probe and rehybridized with a housekeeping mouse β-glucoronidase (GUS)-specific probe. (b) In vivo gene transfer. Vector mediated expression of GFP in liver harvested 72 h postinjection was detected with a monoclonal anti-GFP antibody. Binding of primary antibody was visualized with fluorescein isothiocyanate-conjugated secondary antibody and a fluorescence microscope (green) or with horseradish peroxidase-conjugated secondary antibody and diaminobenzidine tetrahydrochloride (brown). Arrows indicate GFP-positive nonparenchymal liver cells. Magnification, ×20 (left three images) and ×40 (right image).
In vivo blood clearance.
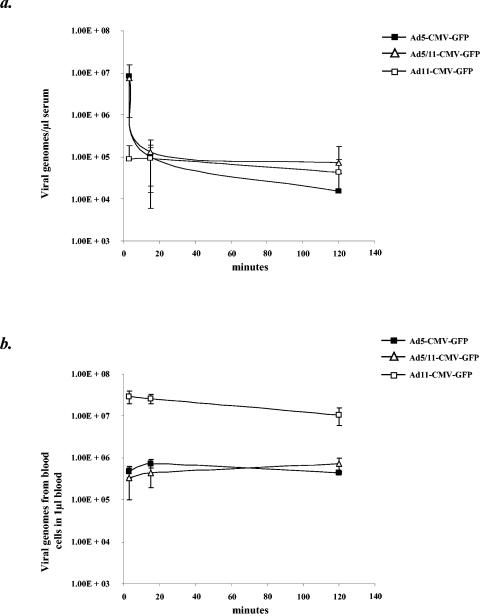
To investigate the kinetics of Ad11 vector blood clearance, studies were performed in transgenic mice expressing human CD46. Mice were injected in the tail vein with 1011 VP of Ad5-CMV-GFP, Ad5/11-CMV-GFP, or Ad11-CMV-GFP, and serum from blood drawn at 3, 15, and 120 min was analyzed for the presence of vector genomes by quantitative PCR. Serum extracted from mice at 3 min after virus injection revealed that similar levels of Ad5-CMV-GFP and Ad5/11-CMV-GFP genomes were present, while levels of Ad11-CMV-GFP genomes were more than 1.5 orders of magnitude lower (Fig. 9A). At 15 min postinjection, the concentration of Ad11-CMV-GFP genomes was similar to that seen at 3 min, and the levels of Ad5-CMV-GFP and Ad5/11-CMV-GFP genomes were comparable to the level of Ad11-CMV-GFP. At 120 min after virus injection the concentrations of Ad5/11-CMV-GFP and Ad11-CMV-GFP genomes were similar to the concentration seen at 15 min, and the levels of Ad5-CMV-GFP were approximately half an order of magnitude lower. Since the number of Ad11-CMV-GFP genomes present in serum at 3 min postinjection was significantly lower than for Ad5-CMV-GFP and Ad5/11-CMV-GFP, we decided to investigate whether vector genomes were associated with blood cells 3 min after injection. CD46 transgenic mice were injected with 1011 VP of each virus, and blood was taken at 3, 15, and 120 min postinjection. After collection 50 μl of blood was spun at 16,000 × g in a microcentrifuge for 2 min, and serum was then extracted. Total DNA was then isolated from the remaining cell fraction and analyzed for the presence of vector genomes by quantitative PCR. At 3 min after virus injection, the number of Ad11-CMV-GFP genomes associated with blood cells was more than 1.5 orders of magnitude higher than for Ad5-CMV-GFP and Ad5/11-CMV-GFP, which showed comparable levels (Fig. 9B). The number of genomes associated with blood cells for Ad5-CMV-GFP and Ad5/11-CMV-GFP remained constant at 15 and 120 min postinjection, while the number of Ad11-CMV-GFP genomes decreased marginally at 15 and 120 min postinjection. These data demonstrate that, following intravenous delivery, Ad11-CMV-GFP associates with blood cells to a much higher degree than Ad5-CMV-GFP and Ad5/11-CMV-GFP.
FIG. 9.
Blood clearance of Ad vectors. hCD46Ge mice were injected in the tail vein with 1011 VP of Ad5-CMV-GFP, Ad5/11-CMV-GFP, and Ad11-CMV-GFP. (a) Presence of vector genomes in serum. Serum from blood extracted at 3, 15, and 120 min postinjection was analyzed for the presence of vector genomes by quantitative PCR by using primers specific for GFP and the BGH poly(A) signal. (b) Association of vector genomes with blood cells. Total blood cells were isolated from blood drawn at 3, 15, and 120 min postinjection, and total DNA was extracted. Vector genomes associated with blood cells were measured by quantitative PCR by using primers specific for GFP and the BGH poly(A) signal.
DISCUSSION
Ad5-based vectors containing the fibers of Ad serotypes 11 and 35 have shown great promise, as they are able to transduce many human cell types, including human hematopoietic progenitor cells, human DCs, and primary human tumor cells, with greater efficiency than parental unmodified Ad5 vectors (4, 52, 54, 57, 61). While chimeric Ad5/35 or Ad5/11 vectors are beneficial for ex vivo transduction, their in vivo application is limited by Ad5 antibodies, which are found in most humans (23). Anti-Ad antibodies interfere with the infection of target cells and increase the toxicity of systemically applied Ad5 vectors (67, 68). A large portion of anti-Ad5 antibodies are directed against the hexon protein (66), which is still present in Ad5/35 and Ad5/11 vectors. This problem can be addressed by the construction of vectors completely derived from Ad35 or Ad11. While other investigators have reported on the development of Ad35 vectors (14, 48, 51, 69), we focused on Ad11 as a basis for a new vector system. Our work on Ad11 vectors was initiated based on the following two findings. First, the prevalence of Ad11-neutralizing antibodies in serum from nonimmunocompromised donors and cancer patients was much lower than the prevalence of Ad5-neutralizing antibodies, indicating that Ad11 vectors may be more applicable for widespread use in humans than Ad5 vectors. In agreement with our observations, low prevalence of neutralizing antibodies to other B group Ad serotypes, including Ad11 and Ad35, in humans has been seen in other studies (10, 44, 51, 69). Second, vectors containing Ad11 fibers were more efficient than Ad5/35 vectors in transduction of HSCs (61), indicating the existence of differences in tropism between these vectors, which could be beneficial for gene therapy applications. This prompted us to study in more detail the receptor usage of Ad5/11 and Ad5/35 vectors. We discovered that Ad5/11 appeared to use CD46 as well as a yet unidentified, new receptor(s) and that this receptor(s) cannot be used by vectors containing the fiber from serotype Ad35. It is unlikely that the differences in infection of HeLa and A549 cells by Ad5/11-CMV-GFP and Ad5/35-CMV-GFP in the presence of Ad11p and Ad35 fiber knobs are due to differences in the affinities of Ad11p and Ad35 fibers for CD46, because infection of CHO-C2 cells (which express CD46) by Ad5/11-CMV-GFP and Ad5/35-CMV-GFP is inhibited at similar levels by Ad11p and Ad35 fiber knobs (Fig. 5). Other lines of evidence also support the existence of an additional receptor(s) for Ad11, in particular, Ad11p, which, unlike CD46, cannot be used by all B group Ads. First, the amino acid sequences of B group Ad fiber knobs are highly divergent (63). Second, although Ad11 is typically associated with kidney and urinary tract infections and is commonly found in immunocompromised patients (18, 19, 72), unlike Ad35, it has also been associated with both respiratory infections and conjunctivitis (1, 24). Third, radiolabeled Ad11a and Ad11p demonstrate different levels of attachment to a number of human cell lines (35). Fourth, radiolabeled Ad11p is able to bind to human CD34-positive cells with greater efficiency than Ad3 and Ad35 (61). Fifth, antiserum to Ad11p fiber, but not Ad11a or Ad35 fiber, can completely block Ad11p binding to A549 cells (35). Sixth, Ad11p fiber knob can completely block binding of wild-type Ad35 to A549 cells, while recombinant Ad35 fiber knob cannot completely block Ad11p binding (34). These data support our observations with Ad5/11 and Ad5/35 vectors and provided a rationale to initiate development of Ad vectors based entirely on Ad11p with the aim of developing a vector system with a more beneficial cell tropism than vectors targeted through Ad5 or Ad35 fibers.
Using either eukaryotic homologous recombination or E. coli recombination, we were able to successfully rescue E1-deleted Ad11 vector genomes that are able to replicate in cells expressing Ad5 E1 and E4 proteins or cells expressing Ad5 E1 proteins and the Ad11-E1B55K protein. Replication of E1-deleted vectors based on the B group serotype Ad35, which shows >98% homology in all ORFs except hexon and fiber (63), has also been seen in similar cell lines (48, 69). Since no regions of homology are present between Ad11 vector genomes and the complementing Ad11-E1B55K sequence in 293-Ad11-E1B55K cells, it is unlikely that replication-competent Ad11 vectors would emerge in culture. Although Ad5 E1 sequences from 293-Ad11-E1B55K cells could theoretically be rescued into the E1-deleted Ad11 backbone, it is doubtful that an Ad11(Ad5E1+) vector would emerge in culture since the Ad5 and Ad11 E1 regions show less than 60% DNA homology and since 293 cells expressing Ad5 E1 are not able to support efficient replication of E1-deleted Ad11 vectors. By using the plasmids described in this report, it should be possible to make vectors containing inserts up to ∼4.4 kb based on the observation that Ad capsids can package genomes of ∼105% the wild-type length. We are currently developing the Ad11 vector system to introduce deletions in E3 that will enable the insertion of larger cassettes. To date, the largest vector genome we have rescued is ∼101% the wild-type Ad11 genome length. In 293-Ad11-E1B55K cells the genome-to-PFU ratio of Ad11 vectors was considerably higher than for Ad5 and Ad5/11 vectors, which have genome-to-PFU ratios of ∼20:1, which is consistent with previous observations for Ad35 vectors (51). Although the reason for this higher ratio is not known, it may be a cell-specific observation caused by differences in trafficking of Ad5, Ad5/11, and Ad11 vectors.
One of our concerns over the use of Ad11 as a gene transfer vector was the mild oncogenicity of wild-type B group Ads previously seen in rodents (15, 16, 22, 30). Although no link between human cancer and Ad7 or Ad11 DNA was found in a previous study with radiolabeled Ad7 and Ad11 DNA and normal and tumor DNA from multiple organs (71), we wanted to compare the transforming activity of Ad11 with Ad5, which is known to be non oncogenic in rodents (7). In our studies no transforming activity was seen with E4+ or E1+E4+ Ad11 genome plasmids. (For human Ads the E1 and E4 regions are known to contain transforming genes.) Furthermore, the highest levels of transforming activity were seen with E1+E4+ genome plasmids from nononcogenic Ad5. These observations allay any concerns over the potential oncogenicity of Ad11-based vectors.
A currently accepted paradigm in Ad biology is that the interaction between the Ad fiber and the primary attachment receptor determine the tropism and infection efficiency of Ads. Like all wild-type B group Ads (12, 58), Ad5/11 and Ad11 vectors are able to utilize CD46 as a cellular receptor; however, although Ad11 and Ad5/11 possess the same fiber, they transduce K562 cells and CD34-positive hematopoietic progenitor cells with different efficiencies and also show different biodistributions upon intravenous injection into CD46 transgenic mice. We speculate that capsid proteins other than fiber (which are different for Ad5/11 and Ad11 vectors) influence virus attachment, internalization, and/or intracellular trafficking in a cell type-specific manner. Alternatively, differences in capsid net charge between Ad5 and Ad11 hexon or penton proteins may affect the interaction between the Ad11 fiber and cellular receptors (56).
In order to characterize the in vivo characteristics of Ad11 vectors, biodistribution studies were carried out in transgenic mice that express human CD46 in a similar pattern to humans (25). These mice express both CAR and CD46 and enable the comparison of Ad5, Ad5/11, and Ad11 vectors following tail vein infusion. Thirty minutes after intravenous delivery, Ad genomes were found in the liver for all three vectors. Although higher levels of Ad5/11 genomes are shown in Fig. 8, this is not representative of all animals. Similar levels of Ad5, Ad5/11, and Ad11 genomes were found at this time point in other animals (data not shown), and this corroborates our previous observation that similar levels of long-shafted CAR-interacting Ad5/9L and short-shafted CD46 interacting-Ad5/35 genomes are present in liver at 30 min postinjection (53). At 72 h postinfection, fewer Ad5/11 than Ad5 vector genomes were present in the liver and, as the GFP expression data suggest, these genomes represent vector particles that have transduced hepatocytes. This finding is in agreement with studies in wild-type mice with Ad5/35 vectors (53). Neither Ad11 genomes nor Ad11-mediated GFP expression was found in livers at 72 h postinfection, indicating that Ad11 particles are efficiently cleared from the liver and do not transduce hepatocytes. A potential explanation for the absence of hepatocyte transduction might be the rapid clearance of Ad11. Alternatively, Ad11 vectors might not be able to transduce hepatocytes via blood factors, as was recently shown for Ad5 and Ad5/35 vectors (57a). In the lung and kidney, more Ad11 genomes than Ad5 and Ad5/11 genomes were found at 30 min postinfection; however, all vectors were cleared from these organs by 72 h. Notably, it is unlikely that vector signals in Southern blots originated from contaminating blood cells because blood was flushed from all organs before harvesting.
Ad particle trapping in liver, lung, and spleen and subsequent degradation are apparently more pronounced for Ad11 vectors than Ad5 or Ad5/11 vectors. The mechanism of Ad11 vector clearance from these tissues is unclear. We speculate that it involves resident macrophages. Differences between Ad5/11 and Ad11 indicate a role for Ad11 capsid proteins other than fiber in these processes.
While CD46 is a major receptor that interacts with the Ad11 fiber, our in vitro studies suggest the existence of an additional Ad11 receptor. The influence of this yet unknown receptor on in vivo Ad11 tropism remains unclear. Notably, preliminary studies between Ad5/11 and Ad5/35 (Ad35 fiber cannot interact with the new Ad11 receptor) in CD46 transgenic mice (11) and baboons (S. Ni, K. Bernt, A. Gaggar, and A. Lieber, unpublished data) (baboons express CD46 at similar levels to humans [21]) did not reveal marked differences in the biodistributions of these two vectors, thus raising a question about the dominant influence of the new Ad11 receptor on in vivo tropism of Ad11 vectors.
Analysis of vector genomes in blood indicates that a large fraction (10 to 20%) of incoming Ad11 particles bind to blood cells (data not shown) and that this phenomenon is more pronounced than for Ad5 and Ad5/11 vectors. Blood cell binding is a problem for all Ad vectors (9), and the higher levels seen with Ad11 vectors give us an easier model to study this phenomenon. We are currently investigating the blood cell types involved in Ad11 binding, which apparently represent a major trap responsible for clearance not only of Ad11 particles but also Ad5 and Ad5/11 particles.
We have successfully developed a system for generating E1-deleted Ad11 vectors. These vectors are nononcogenic, are less likely to be neutralized in the presence of human serum, and efficiently transduce important gene therapy target cells. Our study, particularly the comparison of Ad11 and Ad5/11 tropism, has also uncovered important differences in in vitro and in vivo transduction between these vectors. Our data indicate that differences in Ad5 and Ad11 capsid proteins other than fiber can indirectly (for example, through electrostatic repulsion) influence the interaction between Ad11 fiber attachment receptors and/or that capsid proteins other than fiber can mediate Ad11 attachment and infection. Our data suggest that the currently accepted model that postulates that the interaction between the Ad fiber and a primary attachment receptor determines Ad infectivity and tropism should be revised. This study also points towards the necessity of careful evaluation of tropism-modified Ad vectors in adequate animal models before their clinical application can be considered.
Acknowledgments
We thank Dmitry Shayakhmetov for critical discussion of the manuscript.
This work was supported by funding from NIH grants HL-00-008 and P01 HL53750 and a grant from the Cystic Fibrosis Foundation.
REFERENCES
- 1.Aoki, K., and Y. Tagawa. 2002. A twenty-one year surveillance of adenoviral conjunctivitis in Sapporo, Japan. Int. Ophthalmol. Clin. 42:49-54. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, J. F., L. H. Butterfield, M. D. Roth, L. A. Bui, S. M. Kiertscher, R. Lau, S. Dubinett, J. Glaspy, W. H. McBride, and J. S. Economou. 1997. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther. 4:17-25. [PubMed] [Google Scholar]
- 3.Berkner, K. L., and P. A. Sharp. 1983. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 11:6003-6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernt, K. M., S. Ni, Z. Y. Li, D. M. Shayakhmetov, and A. Lieber. 2003. The effect of sequestration by nontarget tissues on anti-tumor efficacy of systemically applied, conditionally replicating adenovirus vectors. Mol. Ther. 8:746-755. [DOI] [PubMed] [Google Scholar]
- 5.Caravokyri, C., and K. N. Leppard. 1995. Constitutive episomal expression of polypeptide IX (pIX) in a 293-based cell line complements the deficiency of pIX mutant adenovirus type 5. J. Virol. 69:6627-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson, C. A., D. M. Shayakhmetov, and A. Lieber. 2002. An adenoviral expression system for AAV rep78 using homologous recombination. Mol. Ther. 6:91-98. [DOI] [PubMed] [Google Scholar]
- 7.Chanock, R. M., W. Ludwig, R. J. Heubner, T. R. Cate, and L. W. Chu. 1966. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue cultures. I. Safety and lack of oncogenicity and tests for potency in volunteers. JAMA 195:445-452. [PubMed] [Google Scholar]
- 8.Chartier, C., E. Degryse, M. Gantzer, A. Dieterle, A. Pavirani, and M. Mehtali. 1996. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J. Virol. 70:4805-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cichon, G., S. Boeckh-Herwig, D. Kuemin, C. Hoffmann, H. H. Schmidt, E. Wehnes, W. Haensch, U. Schneider, U. Eckhardt, R. Burger, and P. Pring-Akerblom. 2003. Titer determination of Ad5 in blood: a cautionary note. Gene Ther. 10:1012-1017. [DOI] [PubMed] [Google Scholar]
- 10.D'Ambrosio, E., N. Del Grosso, A. Chicca, and M. Midulla. 1982. Neutralizing antibodies against 33 human adenoviruses in normal children in Rome. J. Hyg. 89:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaggar, A., S. Ni, and A. Lieber. 2004. Vector host interactions of group B adenoviruses in CD46 transgenic mice. Mol. Ther. 9:S53. [Google Scholar]
- 12.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 13.Gall, J., A. Kass-Eisler, L. Leinwand, and E. Falck-Pedersen. 1996. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J. Virol. 70:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao, W., P. D. Robbins, and A. Gambotto. 2003. Human adenovirus type 35: nucleotide sequence and vector development. Gene Ther. 10:1941-1949. [DOI] [PubMed] [Google Scholar]
- 15.Girardi, A. J., M. R. Hilleman, and R. E. Zwickey. 1964. Tests in hamsters for oncogenic quality of ordinary viruses including adenovirus type 7. Proc. Soc. Exp. Biol. Med. 115:1141-1150. [DOI] [PubMed] [Google Scholar]
- 16.Green, M. 1970. Oncogenic viruses. Annu. Rev. Biochem. 39:701-756. [DOI] [PubMed] [Google Scholar]
- 17.Havenga, M. J., A. A. Lemckert, O. J. Ophorst, M. van Meijer, W. T. Germeraad, J. Grimbergen, M. A. van Den Doel, R. Vogels, J. van Deutekom, A. A. Janson, J. D. de Bruijn, F. Uytdehaag, P. H. Quax, T. Logtenberg, M. Mehtali, and A. Bout. 2002. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J. Virol. 76:4612-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hierholzer, J. C., T. Adrian, L. J. Anderson, R. Wigand, and J. W. Gold. 1988. Analysis of antigenically intermediate strains of subgenus B and D adenoviruses from AIDS patients. Arch. Virol. 103:99-115. [DOI] [PubMed] [Google Scholar]
- 19.Hierholzer, J. C., R. Wigand, L. J. Anderson, T. Adrian, and J. W. Gold. 1988. Adenoviruses from patients with AIDS: a plethora of serotypes and a description of five new serotypes of subgenus D (types 43-47). J. Infect. Dis. 158:804-813. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann, C., P. Loser, G. Cichon, W. Arnold, G. W. Both, and M. Strauss. 1999. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J. Virol. 73:6930-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu, E. C., R. E. Dorig, F. Sarangi, A. Marcil, C. Iorio, and C. D. Richardson. 1997. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J. Virol. 71:6144-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huebner, R. J., M. J. Casey, R. M. Chanock, and K. Schell. 1965. Tumors induced in hamsters by a strain of adenovirus type 3: sharing of tumor antigens and “neoantigens” with those produced by adenovirus type 7 tumors. Proc. Natl. Acad. Sci. USA 54:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imperiale, M. J., and S. Kochanek. 2004. Adenovirus vectors: biology, design, and production. Curr. Top. Microbiol. Immunol. 273:335-357. [DOI] [PubMed] [Google Scholar]
- 24.Kajon, A. E., A. S. Mistchenko, C. Videla, M. Hortal, G. Wadell, and L. F. Avendano. 1996. Molecular epidemiology of adenovirus acute lower respiratory infections of children in the south cone of South America (1991-1994). J. Med. Virol. 48:151-156. [DOI] [PubMed] [Google Scholar]
- 25.Kemper, C., M. Leung, C. B. Stephensen, C. A. Pinkert, M. K. Liszewski, R. Cattaneo, and J. P. Atkinson. 2001. Membrane cofactor protein (MCP; CD46) expression in transgenic mice. Clin. Exp. Immunol. 124:180-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knaan-Shanzer, S., I. Van Der Velde, M. J. Havenga, A. A. Lemckert, A. A. De Vries, and D. Valerio. 2001. Highly efficient targeted transduction of undifferentiated human hematopoietic cells by adenoviral vectors displaying fiber knobs of subgroup B. Hum. Gene Ther. 12:1989-2005. [DOI] [PubMed] [Google Scholar]
- 27.Krasnykh, V. N., G. V. Mikheeva, J. T. Douglas, and D. T. Curiel. 1996. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J. Virol. 70:6839-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kremer, E. J., S. Boutin, M. Chillon, and O. Danos. 2000. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J. Virol. 74:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krougliak, V., and F. L. Graham. 1995. Development of cell lines capable of complementing E1, E4, and protein IX defective adenovirus type 5 mutants. Hum. Gene Ther. 6:1575-1586. [DOI] [PubMed] [Google Scholar]
- 30.Larson, V. M., A. J. Girardi, M. R. Hilleman, and R. E. Zwickey. 1965. Studies of oncogenicity of adenovirus type 7 virus in hamsters. Proc. Soc. Exp. Biol. Med. 118:15-24. [DOI] [PubMed] [Google Scholar]
- 31.Lieber, A., C. Y. He, I. Kirillova, and M. A. Kay. 1996. Recombinant adenoviruses with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J. Virol. 70:8944-8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mack, C. A., W. R. Song, H. Carpenter, T. J. Wickham, I. Kovesdi, B. G. Harvey, C. J. Magovern, O. W. Isom, T. Rosengart, E. Falck-Pedersen, N. R. Hackett, R. G. Crystal, and A. Mastrangeli. 1997. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum. Gene Ther. 8:99-109. [DOI] [PubMed] [Google Scholar]
- 34.Mei, Y. F., K. Lindman, and G. Wadell. 2002. Human adenoviruses of subgenera B, C, and E with various tropisms differ in both binding to and replication in the epithelial A549 and 293 cells. Virology 295:30-43. [DOI] [PubMed] [Google Scholar]
- 35.Mei, Y. F., K. Lindman, and G. Wadell. 1998. Two closely related adenovirus genome types with kidney or respiratory tract tropism differ in their binding to epithelial cells of various origins. Virology 240:254-266. [DOI] [PubMed] [Google Scholar]
- 36.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizuguchi, H., and M. A. Kay. 1998. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum. Gene Ther. 9:2577-2583. [DOI] [PubMed] [Google Scholar]
- 38.Morral, N., W. O'Neal, K. Rice, M. Leland, J. Kaplan, P. A. Piedra, H. Zhou, R. J. Parks, R. Velji, E. Aguilar-Cordova, S. Wadsworth, F. L. Graham, S. Kochanek, K. D. Carey, and A. L. Beaudet. 1999. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl. Acad. Sci. USA 96:12816-12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison, M. D., D. Reid, D. Onions, N. Spibey, and L. Nicolson. 2002. Generation of E3-deleted canine adenoviruses expressing canine parvovirus capsid by homologous recombination in bacteria. Virology 293:26-30. [DOI] [PubMed] [Google Scholar]
- 40.Nakabayashi, H., K. Taketa, K. Miyano, T. Yamane, and J. Sato. 1982. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 42:3858-3863. [PubMed] [Google Scholar]
- 41.Nan, X., B. Peng, T. W. Hahn, E. Richardson, A. Lizonova, I. Kovesdi, and M. Robert-Guroff. 2003. Development of an Ad7 cosmid system and generation of an Ad7ΔE1ΔE3HIVMN env/rev recombinant virus. Gene Ther. 10:326-336. [DOI] [PubMed] [Google Scholar]
- 42.Neering, S. J., S. F. Hardy, D. Minamoto, S. K. Spratt, and C. T. Jordan. 1996. Transduction of primitive human hematopoietic cells with recombinant adenovirus vectors. Blood 88:1147-1155. [PubMed] [Google Scholar]
- 43.Nevels, M., S. Rubenwolf, T. Spruss, H. Wolf, and T. Dobner. 1997. The adenovirus E4orf6 protein can promote E1A/E1B-induced focus formation by interfering with p53 tumor suppressor function. Proc. Natl. Acad. Sci. USA 94:1206-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nwanegbo, E., E. Vardas, W. Gao, H. Whittle, H. Sun, D. Rowe, P. D. Robbins, and A. Gambotto. 2004. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 11:351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostapchuk, P., and P. Hearing. 2001. Pseudopackaging of adenovirus type 5 genomes into capsids containing the hexon proteins of adenovirus serotypes B, D, or E. J. Virol. 75:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rea, D., M. J. Havenga, M. van Den Assem, R. P. Sutmuller, A. Lemckert, R. C. Hoeben, A. Bout, C. J. Melief, and R. Offringa. 2001. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J. Immunol. 166:5236-5244. [DOI] [PubMed] [Google Scholar]
- 47.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakurai, F., H. Mizuguchi, and T. Hayakawa. 2003. Efficient gene transfer into human CD34+ cells by an adenovirus type 35 vector. Gene Ther. 10:1041-1048. [DOI] [PubMed] [Google Scholar]
- 49.Sakurai, F., H. Mizuguchi, T. Yamaguchi, and T. Hayakawa. 2003. Characterization of in vitro and in vivo gene transfer properties of adenovirus serotype 35 vector. Mol. Ther. 8:813-821. [DOI] [PubMed] [Google Scholar]
- 50.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seshidhar Reddy, P., S. Ganesh, M. P. Limbach, T. Brann, A. Pinkstaff, M. Kaloss, M. Kaleko, and S. Connelly. 2003. Development of adenovirus serotype 35 as a gene transfer vector. Virology 311:384-393. [DOI] [PubMed] [Google Scholar]
- 52.Shayakhmetov, D. M., C. A. Carlson, H. Stecher, Q. Li, G. Stamatoyannopoulos, and A. Lieber. 2002. A high-capacity, capsid-modified hybrid adenovirus/adeno-associated virus vector for stable transduction of human hematopoietic cells. J. Virol. 76:1135-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shayakhmetov, D. M., Z. Y. Li, S. Ni, and A. Lieber. 2004. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J. Virol. 78:5368-5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shayakhmetov, D. M., Z. Y. Li, S. Ni, and A. Lieber. 2002. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 62:1063-1068. [PubMed] [Google Scholar]
- 55.Shayakhmetov, D. M., Z. Y. Li, V. Ternovoi, A. Gaggar, H. Gharwan, and A. Lieber. 2003. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J. Virol. 77:3712-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shayakhmetov, D. M., and A. Lieber. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 74:10274-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shayakhmetov, D. M., T. Papayannopoulou, G. Stamatoyannopoulos, and A. Lieber. 2000. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 74:2567-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57a.Shayakhmetov, D., A. Gaggar, S. Ni, Z.-Y. Li, and A. Lieber. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity, J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 58.Sirena, D., B. Lilienfeld, M. Eisenhut, S. Kalin, K. Boucke, R. R. Beerli, L. Vogt, C. Ruedl, M. F. Bachmann, U. F. Greber, and S. Hemmi. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 78:4454-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skog, J., Y. F. Mei, and G. Wadell. 2002. Human adenovirus serotypes 4p and 11p are efficiently expressed in cell lines of neural tumour origin. J. Gen. Virol. 83:1299-1309. [DOI] [PubMed] [Google Scholar]
- 60.Sova, P., X. W. Ren, S. Ni, K. M. Bernt, J. Mi, N. Kiviat, and A. Lieber. 2004. A tumor-targeted and conditionally replicating oncolytic adenovirus vector expressing TRAIL for treatment of liver metastases. Mol. Ther. 9:496-509. [DOI] [PubMed] [Google Scholar]
- 61.Stecher, H., D. M. Shayakhmetov, G. Stamatoyannopoulos, and A. Lieber. 2001. A capsid-modified adenovirus vector devoid of all viral genes: assessment of transduction and toxicity in human hematopoietic cells. Mol. Ther. 4:36-44. [DOI] [PubMed] [Google Scholar]
- 62.Stevenson, S. C., M. Rollence, J. Marshall-Neff, and A. McClelland. 1997. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J. Virol. 71:4782-4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stone, D., A. Furthmann, V. Sandig, and A. Lieber. 2003. The complete nucleotide sequence, genome organization, and origin of human adenovirus type 11. Virology 309:152-165. [DOI] [PubMed] [Google Scholar]
- 64.Tsujimura, A., K. Shida, M. Kitamura, M. Nomura, J. Takeda, H. Tanaka, M. Matsumoto, K. Matsumiya, A. Okuyama, Y. Nishimune, M. Okabe, and T. Seya. 1998. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem. J. 330:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Eb, A. J., and F. L. Graham. 1980. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 65:826-839. [DOI] [PubMed] [Google Scholar]
- 66.Varghese, R., Y. Mikyas, P. L. Stewart, and R. Ralston. 2004. Postentry neutralization of adenovirus type 5 by an antihexon antibody. J. Virol. 78:12320-12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varnavski, A. N., Y. Zhang, M. Schnell, J. Tazelaar, J. P. Louboutin, Q. C. Yu, A. Bagg, G. P. Gao, and J. M. Wilson. 2002. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J. Virol. 76:5711-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vlachaki, M. T., A. Hernandez-Garcia, M. Ittmann, M. Chhikara, L. K. Aguilar, X. Zhu, B. S. The, E. B. Butler, S. Woo, T. C. Thompson, H. Barrera-Saldana, and E. Aguilar-Cordova. 2002. Impact of preimmunization on adenoviral vector expression and toxicity in a subcutaneous mouse cancer model. Mol. Ther. 6:342-348. [DOI] [PubMed] [Google Scholar]
- 69.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watanabe, T., C. Kuszynski, K. Ino, D. G. Heimann, H. M. Shepard, Y. Yasui, D. C. Maneval, and J. E. Talmadge. 1996. Gene transfer into human bone marrow hematopoietic cells mediated by adenovirus vectors. Blood 87:5032-5039. [PubMed] [Google Scholar]
- 71.Wold, W. S., J. K. Mackey, P. Rigden, and M. Green. 1979. Analysis of human cancer DNA's for DNA sequence of human adenovirus serotypes 3, 7, 11, 14, 16, and 21 in group B1. Cancer Res. 39:3479-3484. [PubMed] [Google Scholar]
- 72.Zahradnik, J. M., M. J. Spencer, and D. D. Porter. 1980. Adenovirus infection in the immunocompromised patient. Am. J. Med. 68:725-732. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, L. Q., Y. F. Mei, and G. Wadell. 2003. Human adenovirus serotypes 4 and 11 show higher binding affinity and infectivity for endothelial and carcinoma cell lines than serotype 5. J. Gen. Virol. 84:687-695. [DOI] [PubMed] [Google Scholar]