Abstract
Infection of cultured cells with Kaposi's sarcoma associated herpesvirus (KSHV) typically establishes a latent infection, in which only a few viral genes are expressed. Recently, it has been reported that a subset of lytic genes are transiently expressed very early after viral entry but that this burst of abortive lytic gene expression is terminated with the supervention of latency (H. H. Krishnan, P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran, J. Virol. 78:3601-3620, 2004). To identify molecules imported into cells by KSHV that might influence this gene expression program, we have examined the protein composition of the KSHV particle. Immunoblotting of virus particles demonstrated that RTA, the lytic switch protein, and RAP, a viral protein that is a transcriptional and cell cycle modulator, were both incorporated into virus particles. In a second approach, polypeptides isolated from purified virions were identified by mass-spectrometric analysis of their constituent tryptic peptides. With this approach we were able to identify 18 major virion proteins, including structural, regulatory, and signaling proteins of both viral and cellular origin.
Herpesviruses comprise a family of large DNA viruses that share several structural characteristics. The herpesvirus virion is composed of three distinct parts: an icosahedral capsid containing the double-stranded DNA viral genome, a lipid envelope studded with virally encoded glycoproteins, and an amorphous layer of protein termed the tegument, which resides between the capsid and the envelope. The tegument is composed of both cellular and viral proteins that are encapsidated during viral egress. Upon virus entry into a cell, the tegument proteins are released and are able to affect the cell, preparing it for viral replication. Tegument proteins are thought to play important roles in the virus life cycle and exhibit diverse functions, including cell cycle modulation (30, 43), transcriptional activation of viral genes (14, 32, 42, 73), shutoff of host gene expression (57) and translocation of the viral capsid to the nucleus (59, 74).
Kaposi's sarcoma-associated herpesvirus (KSHV; also called human herpesvirus 8) is associated with the endothelial neoplasm Kaposi's sarcoma (KS) as well as with two B-cell lymphoproliferative disorders, primary effusion lymphoma and multicentric Castleman's disease (8-10). Fragments of the KSHV genome were first identified in 1994, and subsequent sequencing of the entire viral genome placed it into the gammaherpesvirus subfamily (10, 56). Like Epstein-Barr virus, the prototype human gammaherpesvirus, KSHV establishes a latent infection upon entry into cultured cells; only a few viral genes are expressed, and no viral progeny are produced (1-3, 12, 21, 35, 50, 65, 68). Latently infected cells display a low level of spontaneous lytic replication, characterized by a temporally regulated cascade of viral gene expression, replication of the viral DNA, and the release of virus particles. Lytic replication can be induced in latently infected cells by the addition of phorbol esters or butyrate or by overexpression of the viral switch protein, RTA (3, 7, 8, 22, 25, 31, 45, 47, 48, 63, 67). The physiological triggers controlling RTA expression (and thus, the shift between lytic and latent infection) are unknown, however (44). Although de novo infection typically results in latency by 24 h postinfection, a recent study by Chandran and colleagues has shown that immediately after infection there is a burst of viral gene expression that includes several markers traditionally expressed only during lytic replication (34). The full lytic program is not induced, and viral DNA replication is not triggered. Instead, this initial burst of lytic gene expression is followed by a rapid decline, giving way to a more stable state in which mostly latent transcripts are detected (34). The lytic transcripts identified included transcriptional regulators, immunomodulatory and antiapoptotic molecules, and it has been proposed that these may play important roles in establishing KSHV infection (34). How this burst of aberrant lytic expression comes about is unknown, as is the mechanism by which it is extinguished.
One possibility is that virion proteins imported into the cell during infection may influence viral gene expression immediately upon entry. Little is known of KSHV virion proteins, aside from the identities of the four predominant capsid proteins; in particular, very little is known about the KSHV tegument, where many important regulatory activities are likely to reside (52). Zhu and Yuan demonstrated that ORF45, a viral inhibitor of type I interferon induction via IRF7, was present in KSHV virions (most likely in the tegument), but there has been no systematic assessment of the components of the virus particle (75). Accordingly, we employed gel fractionation, mass spectrometry, and immunoblotting to identify components of purified KSHV virions. Here we describe the most abundant cellular and viral proteins packaged into the virus particle and discuss their potential implications in the virus life cycle.
MATERIALS AND METHODS
Virus and cells.
BCBL-1 cells were grown as previously described (3). KSHV replication was induced by addition of 0.3 mM sodium butyrate. Virus was isolated from the supernatant of BCBL-1 cells 6 to 7 days postinduction as previously described (3). KSHV virions were isolated from crude stocks by gradient centrifugation as described previously (75). In brief, concentrated KSHV was layered onto 9 ml of 20 to 35% Histodenz gradients and centrifuged for 2 h at 71,000 × g in an SW41 rotor, and then fractions were collected from the bottom by puncturing a hole in the tube.
Immunoblotting.
Protein samples were separated on 7.5 or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). Membranes were blocked in 5% nonfat dry milk in TBST (10 mM Tris [pH 8], 150 mM NaCl, 0.05% Tween 20), and all antibody incubations were done in 1% milk in TBST. Primary antibodies were used at the following concentrations: LANA (latency-associated nuclear antigen), 1:10,000; RTA, 1:5,000; RAP, 1:5,000; K8.1, 1:5,000; SOX, 1:3,000; kaposin, 1:5,000; ezrin and moesin (Santa Cruz), 1:250; enolase (Santa Cruz), 1:250; EF-2 (Santa Cruz), 1:250; actin and tubulin (Sigma), 1:20,000; Hsc70 (Stressgen), 1:1,000. Secondary antibodies were conjugated to horseradish peroxidase and used at the following concentrations: mouse (Sigma) and rat (Jackson ImmunoResearch), 1:10,000; rabbit and goat (Santa Cruz), 1:5,000.
Dot blot.
A total of 25 μl of each fraction was lysed by addition of 2× lysis buffer (20 mM Tris [pH 8], 50 mM EDTA, 200 mM NaCl, 1.2% SDS) containing 200 μg of proteinase K/ml. Samples were incubated for 2 h at 37°C, extracted twice with phenol:chloroform:isoamyl alcohol (25:24:1), and precipitated by addition of 100% ethanol. The DNA was resuspended in water and then adjusted to 10 mM EDTA and 400 mM NaOH and boiled for 10 min and applied to a nylon membrane (Amersham Pharmacia Biotech) by use of a dot blotter. The DNA was fixed by rinsing wells with 0.4 M NaOH. The nylon was then probed with 32P-radiolabeled DNA corresponding to the locus of PAN, a polyadenylated nuclear RNA.
Trypsin and Triton X-100treatment of KSHV virions.
Gradient-purified virions (isolated from 5 liters of nonconcentrated induced BCBL-1 cell supernatants) were treated with 170 U of trypsin (Promega) in the presence or absence of 1% Triton X-100 (Sigma) for 30 min at 37°C in 1 mM CaCl2-100 mM NaCl-50 mM Tris (pH 7.4). The reaction was quenched by the addition of soybean trypsin inhibitor and phenylmethylsulfonyl fluoride (Sigma). Samples were then mixed with an equal volume of 2× Laemmli sample buffer containing 20% 2-mercaptoethanol and boiled for 5 min.
Protein separation and Coomassie blue staining.
Protein samples were separated on 7.5% SDS-PAGE gels at 70 V for at least 16 h. The gels were then stained in Coomassie blue (0.05% Coomassie brilliant blue, 50% methanol, 10% acetic acid) for 1 h and then destained (50% methanol, 10% acetic acid) for 3 h. Coomassie blue-stained bands were excised from gels rinsed 20 times in distilled water and placed in an Eppendorf tube. The gel slices were dehydrated for 1 to 2 days and then stored at room temperature.
In-gel tryptic digest and MALDI-MS.
45 mM dithiothreitol (Sigma) in 10 mM ammonium bicarbonate (pH 7.8) was added to polyacrylamide gel slices and then incubated for 30 min at 55°C. The solution was changed to 100 mM acrylamide (Bio-Rad) in 10 mM ammonium bicarbonate and incubated 1 h at room temperature. The previous solution was removed, and 0.5 ml of 10 mM ammonium bicarbonate and 50% acetonitrile was added to the gel and incubated for 30 min. The gel slice was then dried to completion. A small volume (2 to 10 μl) of 10 mM ammonium bicarbonate containing 4 to 20 pmol of trypsin (Promega) was added to the slice. The gel was then saturated with 10 mM ammonium bicarbonate buffer. Buffer was added continuously over 2 h until the gel was swollen and covered with buffer. The gel was then incubated with trypsin overnight at 37°C. Peptides were extracted onto Ziptips (Millipore) from an aliquot of the solution and then washed with 0.1% trifluoroacetic acid (Applied Biosystems) and eluted directly to a matrix-assisted laser desorption ionization (MALDI) plate with 0.5 μl of 50% acetonitrile and 0.1% trifluoroacetic acid. The eluate was partially dried, and then 0.5 μl of alpha-cyano-4-hydrozycinnamic acid (Agilent) (5 mg/ml) was added. MALDI-mass spectrometry (MS) was performed using the reflector mode to obtain monoisotopic peptide masses and tandem MS. The results were then used to search protein and genomic databases with Mascot software.
RESULTS
Virion purification.
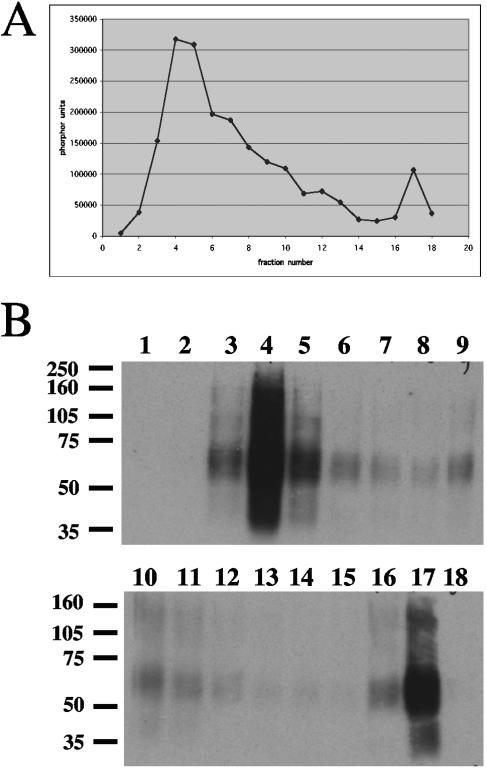
The goal of our study was to identify the protein components of the KSHV virion by mass spectrometry and immunoblotting. Since both infectious virions and noninfectious particles are released from infected cells, it was important to separate virions from other particle types. Virions were isolated by separating the released particles on 20 to 35% Histodenz gradients, followed by assaying each fraction for the presence of viral DNA (by blot hybridization) and the KSHV glycoprotein K8.1 (by immunoblotting). DNA extracted from each fraction was spotted onto nylon membranes and then probed for the KSHV gene encoding PAN, a polyadenylated nuclear RNA. Phosphorimager quantitation of the signal from each spot was then plotted, and a peak of viral DNA was observed in fractions 4 and 5 (Fig. 1A). Protein from each fraction was separated on SDS-PAGE gels, blotted to PVDF, and then probed with a monoclonal antibody specific for K8.1. We found that the K8.1 glycoprotein was also enriched in fractions 4 to 5 (Fig. 1B). These fractions were pooled, diluted with PBS, and reconcentrated by centrifugation. There was also a small peak of viral DNA at fraction 17 that additionally displayed K8.1 staining. We suspect that this fraction contains disrupted, defective, or incomplete particles, and these were not further examined.
FIG. 1.
Gradient purification of KSHV virions. Concentrated KSHV virus stocks were separated on a 20 to 35% Histodenz gradient. Fractions were collected from the bottom (fraction 1) and analyzed for viral DNA and glycoprotein content as described in Materials and Methods. (A) Plot of phosphorimager quantitation of KSHV viral DNA isolated from each fraction, spotted onto nylon membranes, and probed for the PAN locus. (B) Immunoblots of proteins isolated from each fraction, separated on 10% SDS-PAGE gels, transferred to PVDF, and probed for K8.1.
Analysis of candidate KSHV regulatory proteins.
To determine the localization of individual proteins within virions, we examined the sensitivity of those proteins to proteolysis under a variety of conditions, each chosen to digest one or another virion compartment. KSHV virions are composed of three distinct compartments: the capsid, the tegument, and the envelope. Trypsin treatment of intact virions results in the removal of contaminating proteins that adhere to the outside of the particles (as well as degrading the external domains of the viral glycoproteins), while the tegument and capsid proteins are protected from digestion by the lipid bilayer. Addition of the detergent Triton X-100 to the trypsin digestion solubilizes the lipid bilayer and thus exposes all of the virion proteins to trypsin digestion; this should leave only proteins that are intrinsically resistant to trypsin digestion (and, of course, trypsin-resistant limit-digested products).
We first examined virions for the presence of candidate proteins with likely roles in the regulation of viral and host gene expression, since one of our goals was to look for factors that might shed light on the burst of aberrant lytic gene expression that accompanies viral entry (34). We focused on regulatory proteins whose synthesis is upregulated during lytic replication (when virions are formed) and for which we had available antisera for detection. This list included RTA, RAP (K-bZIP), SOX (ORF37, alkaline exonuclease), kaposin B, and LANA (latency-associated nuclear antigen). RTA is the major viral transcription factor controlling the switch to lytic gene expression; as such, it is an obvious candidate for regulation of early lytic gene expression (44, 63). RAP, which functions importantly in viral DNA synthesis, has recently been shown to bind to RTA and downregulate RTA action on selected lytic promoters (29, 41). SOX is a protein that promotes global degradation of host mRNA (24); in herpes simplex virus (HSV), the vhs protein, which is structurally unrelated to SOX but has an analogous function, was one of the first regulatory functions assigned to the tegument (57). Kaposin B, a latent protein whose expression is strongly upregulated by RTA during the lytic cycle, has recently been found to be an activator of the p38/MK2 mitogen-activated protein kinase pathway (46a). This pathway regulates the degradation of transcripts that contain AU-rich elements (AREs) in their 3′ noncoding regions; such elements are often found in cytokine and growth factor mRNAs and in other regulators of signaling and gene expression. In the ground state, such transcripts are rapidly degraded but are strongly stabilized by activators of MK2, including kaposin B. LANA, which is responsible for maintenance of the viral genome in latency, is also a transcriptional modulator and can affect both gene expression and cell proliferation by interacting with p53, phosphorylated Rb, and glycogen synthase kinase 3β (17, 18, 19, 23). LANA also antagonizes the positive-feedback loop of RTA on the RTA promoter, causing levels of RTA to diminish and thereby potentially impacting its ability to cause a switch to lytic replication (37). As such, if delivered in virions it could play a role in assuring that any early RTA action is aborted before full lytic reactivation can proceed.
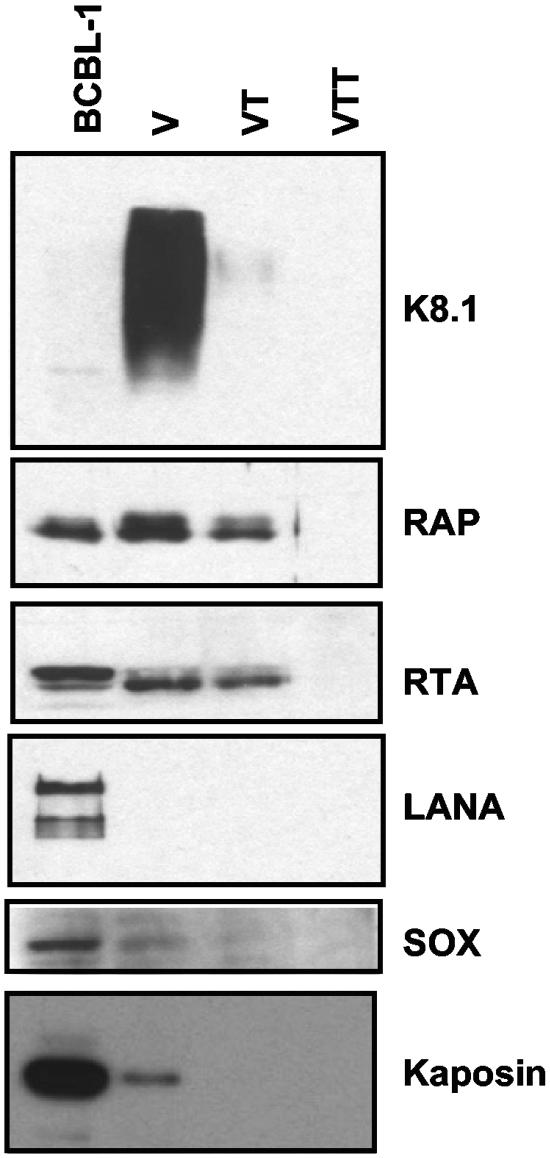
Gradient-purified virions were either mock treated or trypsin treated in the presence or absence of Triton X-100; products were then separated on 10% SDS-PAGE gels, transferred to PVDF, and probed for the aforementioned KSHV proteins. The blots were also probed for the K8.1 glycoprotein as a control for the trypsin treatment. (The monoclonal antibody against K8.1 recognizes an epitope in the external domain of the protein, exposed on the virion surface. Upon trypsin treatment of the particles, the epitope should be degraded and monoclonal antibody binding should be abolished.) As expected, K8.1 was present on virions but not on virions that were treated with trypsin (Fig. 2; K8.1 panel), confirming that the trypsin treatment had successfully removed the proteins external to the particle. We found that both RTA and RAP proteins are present in the virions, as they are found in both intact and trypsin-treated virions but not in particles treated with trypsin plus Triton X-100 (Fig. 2; RTA and RAP panels). Note that RTA and RAP exist in several different isoforms in infected cells (Fig. 2; RAP and RTA panels, BCBL-1 lane). Both RAP and RTA isoforms were incorporated into KSHV virions, but we observed that the higher-mobility isoform of RTA was present in significantly higher levels than the slower-mobility form, even though it is less abundant at late times of infection (Fig. 2; RAP and RTA panels). This suggests specificity in packaging, although the biological meaning of the preferential incorporation of the lower RTA isoform is unclear. Such preferential incorporation is not without precedent, however; in human cytomegalovirus (HCMV), only one of the phosphorylated isoforms of UL69 protein produced during infection is incorporated in the virus particle (72).
FIG. 2.
Immunoblots for viral components. Induced BCBL-1 cells and mock-treated (V), trypsin-treated (VT), and trypsin- and Triton X-100-treated (VTT) gradient-purified virions were separated on 10% SDS-PAGE gels, transferred to PVDF, and then probed for various KSHV proteins.
By contrast, we found that LANA was not present in KSHV virions, even though we could detect significant levels of the protein in lysates prepared at late times of infection (Fig. 2; LANA panel). We also probed mock- and trypsin-treated virions for SOX and kaposin proteins. We found that although both of these proteins were found in induced BCBL-1 lysates and at low levels in gradient-purified virions, neither was detected in trypsin-treated virions, indicating that both proteins were merely contaminants adhering to the outside of the virus particle (Fig. 2; SOX and Kaposin panels). These results again reveal specificity in KSHV packaging, since not all proteins abundantly expressed at late times of infection get incorporated into virus particles.
Mass spectrometric analysis of virion polypeptides.
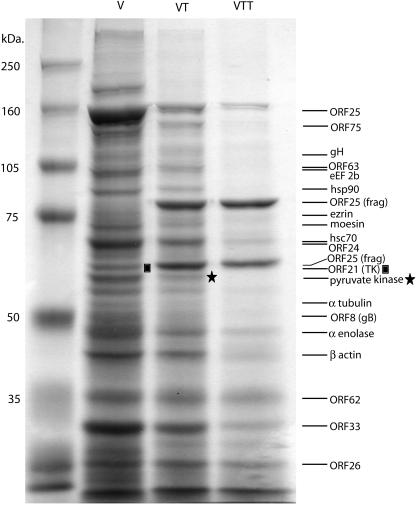
Next, we used gel fractionation followed by mass spectrometric analysis to perform a more systematic analysis of the virion protein composition. Gradient-purified virions (approximately 5 liters of induced BCBL-1 cell supernatants per condition) were mock treated or treated with trypsin in the presence or absence of Triton X-100 and then separated on large, SDS-7.5% polyacrylamide gels and stained with Coomassie blue. As shown in Fig. 3, a series of discrete bands is observed in the mock-treated samples (Fig. 3; V lane). The addition of trypsin affected the migration of several bands (Fig. 3; VT lane), suggesting that these proteins (or domains of them) were external to the virus particle. Several bands disappeared (including a very prominent band of ca. 150 kDa), and at least two new, very prominent, protein bands appeared at around 60 and 80 kDa, likely representing proteolytic cleavage products of the missing 150-kDa band. The majority of virion proteins, however, were unaffected by the trypsin treatment, demonstrating their presence on the inside of the lipid bilayer. We observed that the intensity of staining for some of these bands was decreased in the trypsin-treated lane versus the mock-treated lane. We hypothesize that some of the virions may have been damaged during the isolation and purification procedure, rendering a fraction of the internal proteins now subject to a low level of cleavage. The addition of trypsin and Triton X-100 to the virions results in the degradation of most of the proteins, although a significant number of trypsin-resistant bands were present, especially at the lower molecular weights (Fig. 3; VTT lane). We interpret these bands as displaying intrinsic trypsin resistance.
FIG. 3.
Coomassie blue staining of treated virions. Mock-treated (V), trypsin-treated (VT), and trypsin- and Triton X-100-treated (VTT) gradient-purified virions were separated on a SDS-7.5% PAGE gel and then stained with Coomassie blue. The destained gel is shown. Each lane represents material from ∼5 liters of induced BCBL-1 cells. Protein identifications to the right of the gel are the identifications resulting from mass spectrometric analysis of the designated bands. Bands designated as fragments represent the tryptic fragments of the protein.
Individual protein bands were excised from lanes corresponding to either virions or trypsin-treated virions, eluted, and subjected to proteolysis with trypsin. The resulting proteolytic fragments were then analyzed by mass spectrometry. We were able to positively identify 18 of the proteins composing the KSHV virions by aligning peptide sequences with those in the database of known gene products. These proteins were of both cellular and viral origin (Table 1). As expected, we identified several known KSHV structural proteins. Three of the four capsid proteins, ORF25 (MCP), ORF26 (TRI-2), and ORF62 (TRI-1), as well as two glycoproteins, ORF8 (gB) and ORF22 (gH), were identified in this fashion. ORF25 encodes the KSHV major capsid protein, and we observed both the full-length protein and two tryptic fragments in the virions treated with trypsin (Fig. 3; VT and VTT lanes). The significant level of the ORF25 fragments present in the samples treated with trypsin alone suggested that this internal component of the virion was subject to digestion even without the detergent, supporting our hypothesis that the virions were slightly damaged during purification. The mass spectrometric analysis also identified several viral polypeptides not previously annotated as being structural proteins, including the products of ORF21, ORF24, ORF33, ORF63, and ORF75 (Fig. 3 and Table 1). With the exception of ORF21 protein, the viral thymidine kinase (TK), none of the other proteins have been ascribed catalytic, regulatory, or structural functions.
TABLE 1.
Distinct peptides identified by mass spectrometry of KSHV virions
| Gene product | Accession no. | Size (aa) | Distinct peptides identified |
|---|---|---|---|
| ORF8 (gB) | AAC83368 | 845 | 470-481/DGINQVLEELSR |
| 482-485/AWCR | |||
| 606-611/DYAYLR | |||
| 650-662/LASSVFDLETMFR | |||
| 663-670/EYNYYTHR | |||
| 692-705/DLSEIVADLGGIGK | |||
| 775-884/APPSGGAPTR | |||
| 775-888/APPSGGAPTREEIK | |||
| 789-802/NILLGMMQLQQEER | |||
| 811-818/STPSVFQR | |||
| ORF21 (TK) | gil18845987NP_572073 | 580 | 90-97/FPPRPLIR |
| 126-132/FAFQSPR | |||
| 195-205/LROGGFAFSPR | |||
| 197-205/DGGFAFSPR | |||
| 244-251/TPVTOYR | |||
| 267-281/STLVNAVGCILPQER | |||
| 282-294/VTSFPEPMVYWTR | |||
| 295-301/AFTDCYK | |||
| 328-333/FSLPFR | |||
| 334-341/TNATAILR | |||
| 396-414/ATEGOVVAILTLSSAESLR | |||
| 422-433/KNOGTVEQNYIR | |||
| 423-433/NOGTVEQNYIR | |||
| 547-557/QILSNPAIKPR | |||
| ORF22 (gH) | gil1136819AABO8395 | 730 | 234-245/GHATYDELTFAR |
| 341-349/QYAELFLR | |||
| 391-405/SSQETVLAMVQLGAR | |||
| 487-498/FSKPOSLNIYR | |||
| 498-507/AFSPCFLGLA | |||
| ORF24 | gil10140946 | 752 | 155-166/VSWTLMQIISR |
| 331-339/TALCMAVAK | |||
| 331-343/TALCMAVAKHICR | |||
| 355-367/QQLSLARALVANFEK | |||
| 446-453/LVNNFVIK | |||
| 566-573/IHGSOLTK | |||
| 666-672/VCNSIPK | |||
| 698-706/GAPHMINTK | |||
| 721-729/SYHFAGCAK | |||
| 721-731/SYHFAGCAKAK | |||
| ORF25 (MCP) | gil2246481AAB62606 | 1,376 | 95-106/ISMPTIAHGDGR |
| 158-172/TTTSALQFGMOALER | |||
| 185-193/HAPPVFILK | |||
| 194-203/TLGOPVYSER | |||
| 260-284/GVSTYTTASGQQVAGVLETTDSVMR | |||
| 286-309/LMNLLGQVESAMSGPAAYASYVVR | |||
| 310-321/GANLVTAVSYGR | |||
| 325-332/NFEQFMAR | |||
| 333-347/IVDHPNALPSVEGDK | |||
| 348-359/AALAOGHDEIQR | |||
| 360-381/IDGKFVAIESLQR | |||
| 373-381/FVAIESLQR | |||
| 413-420/YSTSVSVR | |||
| 421-437/GVESPAIQSTETWVVNK | |||
| 458-484/NHNPTQSAQALNQALNQAFPDPDGGHGYGLR | |||
| 485-495/YEQTPNMNLFR | |||
| 496-504/TFHQYYMGK | |||
| 516-530/ALVTTEDLLHPTSHR | |||
| 560-572/TMVGNIPQPLAPR | |||
| 648-658/LAFVNSYHMVR | |||
| 672-679/EAHGHYRK | |||
| 694-702/LAGHETVGR | |||
| 742-753/IGDQNDNPQNR | |||
| 754-760/ATFINLR | |||
| 761-777/GRMEDLVNHVNIYQTR | |||
| 763-777/MEDLVNNVNIYQTR | |||
| 861-876/DILQAGDIRPTVOMIR | |||
| 898-911/RDPAQSFATHEYGK | |||
| 912-930/DVAQTVLVNGFGAFAVADR | |||
| 968-972/LPNQR | |||
| 1030-1042/HRMHPGFAMTVVR | |||
| 1032-1042/MHPGFAMTVVR | |||
| 1043-1056/TDEVLAEHILYCSR | |||
| 1057-1070/ASTSMFVGLPSVVR | |||
| 1057-1071/ASTSMFVGLPSVVRR | |||
| 1138-1150/MKAGVQTGSPGNR | |||
| 1140-1150/AGVQTGSPGNR | |||
| 1151-1162/MOHVGYTAGVPR | |||
| 1201-1217/AACVVSCDAYSNESAER | |||
| 1218-1232/LLTDHSIPOPAYECR | |||
| 1233-1242/STNNPWASQR | |||
| 1243-1255/GSLGDVLYNITFR | |||
| 1268-1277/QFFHKEDIMR | |||
| 1281-1292/GLYTVNEYSAR | |||
| 1346-1365/QTHAPVHMGQYLJEEVAPMK | |||
| ORF26 (TRI-2) | gil11086047A55371 | 305 | 6-14/SIVVNFTSR |
| 15-26/LFADELAALQSK | |||
| 27-38/IGSVLPLGDCHR | |||
| 39-53/LQNIQALGLGCVCSR | |||
| 69-83/CTAVLEEVRPDSLR | |||
| 225-231/LLTALVR | |||
| 323-251/HDRHPLTEVFEGVVPDEVTR | |||
| 235-251/HPITEVFEGVVPDEVTR | |||
| ORF33 | gil2246501AAB62626 | 334 | 10-14/NFLNX |
| 117-126/FPYIAPPPSR | |||
| 135-145/QELVHTSQVVR | |||
| 197-203/VAALLTR | |||
| 217-203/GHVNVFR | |||
| 257-270/NFLGLLFDPIVQSR | |||
| 276-303/ITSHPIPTHVENVLTGVLDDGTLVPSSK | |||
| 304-310/APWVLLR | |||
| 318-324/LLIYECK | |||
| ORF62 (TR1-1) | gil16846032NP_572118 | 331 | 14-25/QVLGLLPPPTHR |
| 45-61/YAASTRPTVGSLHEALR | |||
| 235-250/VNQRPSMALTFFQSGK | |||
| 251-260/GFAEVVAMIK | |||
| 261-268/DHFTDVIR | |||
| 271-275/YIQLR | |||
| 276-282/HELYINA | |||
| ORF63 | gil18846033NP_572119 | 928 | 26-33/LLMEFQLR |
| 69-74/VFALVR | |||
| 75-92/AAYFLEPPSSIOPLEAAR | |||
| 129-141/LLAHYADQIAGFK | |||
| 219-231/TLVTEHHELFVSR | |||
| 248-253/ALAIYR | |||
| 306-312/LESFLSR | |||
| 430-439/HPGISDIPLR | |||
| 445-460/ALAFFVPPAPINTLQR | |||
| 461-471/VYAALPSQLMR | |||
| 480-492/TTWGGAVPANLAR | |||
| 523-534/VGDTEYDEDIVR | |||
| 535-544/SPLFADAFTK | |||
| 562-569/NRALFQIR | |||
| 564-569/ALFQIR | |||
| 592-603/AYFHIMDILEER | |||
| 683-695/LEFEYDSEGDFVR | |||
| 715-724/TIQTTEQATR | |||
| 725-743/EATVAMTTIAKPIYPAYIR | |||
| 748-753/LEYLNR | |||
| 754-760/LNHHILR | |||
| 761-781/IPFPQDALSELQETYLAAFAR | |||
| 800-817/YFGVLFQHQLVPTAIVKK | |||
| 818-844/LLHIDEAXDTTEAFLQSLAQPVVQGQR | |||
| 826-844/DTTEAFLQSLAQPVVQGQR | |||
| 865-882/INPQFTDAQANIPPSIKA | |||
| 888-902/YDVPEVSVDWETYSR | |||
| 903-913/SAFEAPDDELR | |||
| 914-923/FVPLTLAGLR | |||
| ORF75 | gil5669897AAD46504 | 1,296 | 63-84/DVEIQTVLAVLSPLLGYPHVIR |
| 120-128/ELGLQEWAR | |||
| 139-153/ITQTLLEPHPPQFIR | |||
| 216-222/YFVIPGR | |||
| 235-252/QAFGIHGAYTHVHSSVQR | |||
| 256-289/GLGNLLFHSTLFPGGQTQGALTGLYATE | |||
| 301-321/GVQQAEMLQGAGVPTLGGFLK | |||
| 365-379/FEPTOGPTYPNLYR | |||
| 401-409/GPIFSGLNR | |||
| 419-426/HLQALAPR | |||
| 510-517/TSEQPGIR | |||
| 518-528/IVDDLTGETTR | |||
| 529-551/VFSVDQPSSTPPSPWLALSDGVR | |||
| 552-575/VSGHPEDVDWGLFATGSTIHLQLLR | |||
| 655-667/ALPQELLPVPAWR | |||
| 741-760/ELGVALSISSAASSPTLSER | |||
| 792-799/YRVTPDVK | |||
| 991-999/LEHHLGSLR | |||
| 1009-1017/LFSCPTSPR | |||
| 1055-1069/GQSLSGFSGLITCLR | |||
| 1127-1141/SESSPYTYGPTPPQR | |||
| 1157-1161/SVFLR | |||
| 1193-1203/YEQDALEYILR | |||
| 1204-1224/QRGEITLTYHGNAADETLPAR | |||
| 1229-1245/NPTGNSTVAGLTSSDGR | |||
| Actin (beta) | gil14250401AAH08633 | 368 | 12-21/AGFAGDDAPR |
| 22-32/AVFPSIVGRPR | |||
| 89-106/VAPEEHPVLLTEAPLNPK | |||
| 171-176/LALAGR | |||
| 185-189/ILTER | |||
| 190-199/GYSFTTTAER | |||
| 232-247/SYELPDGQVITIGNER | |||
| 278-283/COVDIR | |||
| 322-328/IIAPPER | |||
| 322-329/IIAPPERK | |||
| 353-365/QEYDESGPSIVHR | |||
| α-Enolase | gil4503571NP_001419 | 434 | 1-9/MSILKIHAR |
| 10-15/EIFFDSR | |||
| 16-28/GNPTVEVDLFTSK | |||
| 33-50/AAVPSGASTGIYEALELT | |||
| 82-92/LNVTEQEKIDK | |||
| 93-103/LMIEMDGTENK | |||
| 127-132/GVPLVR | |||
| 163-179/LAMQEFMILPVGAANFR | |||
| 184-193/IGAEVYHNLK | |||
| 203-221/DATNVGDEGGFAPNILENK | |||
| 240-253/VVIGMDVAASEFFR | |||
| 270-281/YISPDQLADLYK | |||
| 307-326/FTASAGIQVVGDDLTVTNPK | |||
| 407-412/YNQLLR | |||
| EF-2B | AAH24689 | 517 | 51-59/GPLMMYISK |
| 69-74/FYAFGR | |||
| 75-85/VFSGLVSTGLK | |||
| 98-108/KEDLYLKPIQR | |||
| 99-108/EDLYLKPIQR | |||
| 158-165/FSVSPVVR | |||
| 179-185/LVEGLKA | |||
| 219-231/DLEEDHACIPIKK | |||
| 265-284/ARPFDPGLAEDIDKGEVSAR | |||
| 307-335/KIWCFGPOGTGPNILTDITK | |||
| 327-335/GVQYLNEIK | |||
| 376-385/GGGQIIPTAR | |||
| 427-444/GHVFEESQVAGTPMFVVK | |||
| 445-460/AYLPVNESFGFTADLR | |||
| 505-517/EGIPALDNFLDKL | |||
| Ezrin (villin 2) | gil21614499NP_003370 | 586 | 152-162/LIPQRVMDQHK |
| 238-246/IGFPWSEIR | |||
| 263-273/KAPDFVFYAPR | |||
| 264-273/APDFVFYAPR | |||
| 361-371/AERELSEQIQR | |||
| 364-371/ELSEQIQR | |||
| 372-379/ALQLEEER | |||
| 382-393/AQEEAERLEADR | |||
| 413-427/SQEQLAAELAEYTAK | |||
| 428-436/IALLEEARR | |||
| 530-542/QLLTLSSELSQAR | |||
| 578-586/QRIDEFEAL | |||
| Hsc70 | S11456a27077 | 350 | 26-36/VEIIANDQGNR |
| 37-49/TTPSYVAFTDTER | |||
| 138-155/TVTNAVVTVPAYFNDSQR | |||
| 160-171/DAGTIAGLNVLR | |||
| 221-236/STAGDTHLGGEDFDNR | |||
| 300-311/ARFEELNADLFR | |||
| 302-311/FEELNAOLFR | |||
| 424-447/QTQTFTTYSONQPGVLIQVYEGER | |||
| 459-469/FELTGIPPAPR | |||
| Hsp90 | HS9A_HUMANP07900 | 732 | 41-45/EIFLR |
| 74-86/ELHINLIPNKQDR | |||
| 185-200/VILHLKEDQTEYLEER | |||
| 292-298/TKIPWTR | |||
| 327-337/HFSVEGQLEFR | |||
| 338-344/ALLFVPR | |||
| 345-354/RAPFDLFENR | |||
| 346-355/APFDLFENR | |||
| 346-355/APFDLFENRK | |||
| 386-399/GVVDSEDLPLNISR | |||
| 458-463/LSELLR | |||
| 489-498/HIYYITGETX | |||
| 567-575/FENLCKIMK | |||
| 632-646/HLEINPOHSIIETLR | |||
| Moesin | AAH01112 | 329 | 28-35/QLFDQVVR |
| 41-53/EVWFFGLQYQDTK | |||
| 54-60/GFSTWLK | |||
| 54-63/GFSTWLKLNK | |||
| 72-79/KESPLLFK | |||
| 73-79/ESPLLFK | |||
| 84-100/FYPEDVSEELIQDITQR | |||
| 101-107/LFFLQVK | |||
| 181-193/GMLREDAVLEYLK | |||
| 185-193/EDALEYLK | |||
| 238-246/IGFPWSEIR | |||
| 255-262/FVIKPIDK | |||
| 263-273/KAPDFVFYAPR | |||
| 264-273/APDFVFYAPR | |||
| Pyruvate kinase | gil20178296P14618 | 531 | 32-42/LDIDSPPITAR |
| 33-43/LDIDSPPITAR | |||
| 44-56/NTGIICTIGPASR | |||
| 93-115/TATESFASDPILYRPVAVALDTK | |||
| 174-186/IYDOGLISLQVK | |||
| 231-246/FGVEQDVDMFASFIR | |||
| 271-278/IENHEGVR | |||
| 279-294/RFDEILEASDGIMVAR | |||
| 280-294/FDEILEASDGIMVAR | |||
| 295-311/GDLGIEIPAEKVFLAQK | |||
| 368-376/GDYPLEAVR | |||
| 377-383/MQHLIAR | |||
| 384-400/EAEAAIYHLQLFEELPR | |||
| 401-422/LAPITSOPTEATAVGAVEASFK | |||
| 448-455/APILAVTR | |||
| 462-467/QAHLYR | |||
| Tubulin | 1TUBA | 440 | 65-79/AVFVDLEPTVIDEVR |
| 85-96/QLFHPEQLITGK | |||
| 113-121/EIIDLVLDR | |||
| 216-229/NLDIERPTYTNLVPYPR | |||
| 244-264/FDGALNVDLTEFQTNLVPYPR |
We also identified several cellular proteins that were specifically incorporated into the virus particle. These include actin and tubulin, major components of the cytoskeleton, several housekeeping metabolic proteins (e.g., enolase and pyruvate kinase), two cellular chaperones (Hsp90 and Hsc70), and a translational repressor (EF-2b). In addition, ezrin and moesin, two members of the ERM (for “ezrin-radixin-moesin”) family of proteins, which are involved in cytoskeletal rearrangements, were identified (Fig. 3 and Table 1). Ezrin has previously been suggested to perform important functions early during KSHV infection (58). The implications of the inclusion of these proteins in the virus particle will be discussed below.
Immunoblot confirmation of cellular proteins in virions.
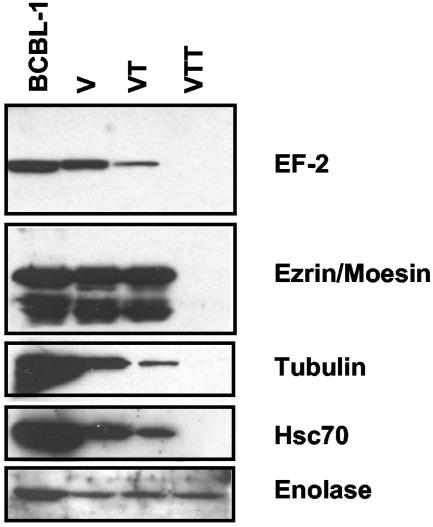
To confirm the presence of cellular proteins in the KSHV virions, we performed immunoblotting experiments analogous to those of Fig. 2. Gradient-purified virions, either mock treated or trypsin treated in the presence or absence of 1% Triton X-100, were separated on 10% SDS-PAGE gels, transferred to PVDF membranes, and probed with the appropriate antibodies. As shown in Fig. 4, EF-2, α-tubulin, ezrin, moesin, and hsc70 were all found in the mock-treated and trypsin-treated virions but not in virions treated with trypsin plus Triton X-100—confirming their presence within the virus particle. Ezrin and moesin proteins migrate at approximately 75 kDa. We detected several bands around 75 kDa, consistent with the fact that the antibody recognizes ezrin, moesin, and radixin. Moesin and radixin are 74 and 77% identical to ezrin, respectively (20, 38).
FIG. 4.
Immunoblots of cellular virion components. Induced BCBL-1 cells, mock-treated virions (V), trypsin-treated virions (VT), and trypsin- and Triton X-100-treated virions (VTT) were separated on 10% SDS-PAGE gels, transferred to PVDF, and then probed with antibodies for the indicated cellular proteins.
We detected the α-enolase protein in trypsin-treated virus regardless of the presence or absence of Triton X-100, suggesting the protein is both incorporated into virions and intrinsically resistant to the trypsin digestion. Upon review of our Coomassie blue-stained gel (Fig. 3), it is similarly apparent the 48-kDa band identified by mass spectrometric analysis as α-enolase is resistant to trypsin digestion in that experiment as well, since it is present in all three lanes (Fig. 3; ∼48 kDa). Analysis of the protein sequence for α-enolase revealed many potential sites for trypsin digestion (a fact confirmed by the trypsin digestion of the excised, denatured protein described in Table 1). This indicates that either these sites are inaccessible to the enzyme when the protein is folded or that they are shielded from the enzyme by interactions with other proteins in the particle.
DISCUSSION
Here we have used a combination of (i) immunoblotting for candidate proteins and (ii) protein isolation followed by proteolytic fragmentation and mass spectrometry to identify components of the KSHV virion. The immunoblotting approach identified both RTA and RAP in the virus tegument. RTA, the product of the ORF50 locus, plays a critical role in the KSHV life cycle. RTA is composed of an N-terminal DNA binding domain and a C-terminal transactivation domain (44). RTA transactivates various viral promoters, either directly (60-62) or by recruitment of cofactors like CSL (39, 40) or C/EBP (69, 70). It is required for the switch between lytic and latent replication: ectopic expression of wild-type RTA in latently infected cells induces the lytic cycle, while expression of dominant-negative mutant RTA suppresses this switch (44, 45). Our finding of RTA in the virion is surprising, both in light of earlier negative results of attempts of others to detect it there (75) and in view of the fact that de novo KSHV infection generally results in latency (3, 21, 35, 67, 68). (RTA is a minor component of the KSHV virion and we suspect the previous attempt [75] to probe for RTA in KSHV virions may have failed because not enough virions were utilized for the assay.) However, this finding may shed light on the recent observations of Chandran and colleagues (34), who reported that immediately following viral entry, a burst of aberrant lytic-cycle gene expression can transiently occur. The delivery of RTA with the tegument could certainly jumpstart such expression. But this raises another question: why does such expression not trigger the full program of lytic gene expression?
Here, we can suggest at least two possibilities, neither of which is exclusive of the other. The first model derives from the fact that RTA undergoes extensive posttranslational modification during infection, especially by phosphorylation (44). But Fig. 2 shows that it is the undermodified isoforms of RTA that appear to be preferentially encapsidated (alternatively, RTA may be actively dephosphorylated following encapsidation). Perhaps these underphosphorylated isoforms of RTA are incapable of activating key promoters required for lytic progression. A second possibility is suggested by the incorporation of RAP into the particles. Recent work of Kung and colleagues (29, 41) demonstrated that RAP can bind to RTA, repressing transactivation of several RTA-responsive viral promoters. This suggests a model in which incoming RTA from the virus tegument may drive initial lytic gene expression, while RAP released into the cell would temper this transactivation and blunt progression of the lytic cycle.
In any case, it is likely that additional mechanisms are also at work to modulate this very-early-expression program. Certainly, virus binding to cell surface integrin receptors can trigger signaling cascades that may affect expression of viral genes. Recent work of Sharma-Walia and colleagues, who have shown that recombinant gB can activate FAK kinase (via integrin-mediated signaling), leading to activation and relocalization of ezrin, is interesting in this connection (58). Our finding that ezrin is present in substantial quantities in virions raises the possibility that some of this substrate ezrin may be supplied by incoming virions, to amplify such signaling. For example, virion-delivered ezrin may traffic preferentially to sites of activation. Clearly, further experiments are required to examine these possibilities.
In addition, newly released virion DNA may not be chromatinized at early times postinfection, and this may release certain lytic genes from negative regulation by chromatin—which presumably must be reassembled upon circularization of the incoming viral DNA as latency is established. Moreover, we have recently discovered that some of the RNAs found by Krishnan et al. to be present in cells immediately after KSHV entry are also encapsidated into KSHV virions, and so are most likely passively transferred to the cytoplasm upon infection (J. T. Bechtel and D. Ganem, unpublished data). Finally, LANA protein produced early after infection would also affect RTA's activities by limiting its ability to transactivate its own promoter. LANA transcripts are detected within 30 min of infection and peak at 24 h postinfection, so there is potential for LANA to play a critical role in the latent-lytic switch even though it is not deposited in the cell with the virus particle. This is the first report of a herpesvirus TK being localized to the virus tegument. In other herpesviruses, TK is involved in DNA replication, phosphorylating deoxynucleoside triphosphates for incorporation into replicating DNA (55). Given the limited role TK plays during virus infection, it is surprising to find it specifically incorporated into the virus tegument. It is possible that the KSHV TK has evolved a new function that is important during the initial stages of virus infection. Further work will be required to examine this possibility, but this postulated evolution would not be without precedent in KSHV. Another KSHV protein, SOX (ORF37 gene product), is the homolog of the herpesvirus alkaline exonuclease, a protein conserved in all herpesvirus that is involved in the resolution of replicating DNA structures prior to packaging (46). In KSHV, SOX is also responsible for host shutoff, a novel function for this conserved gene (24).
ORF24 and ORF75 have been identified as a virion components for murine gammaherpesvirus 68, another gammaherpesvirus (5). ORF75 is the homolog of the FGARAT gene involved in purine biosynthesis, though why this function should be encapsidated is obscure (53, 56). ORF33 is homologous to HSV UL16 and HCMV UL94. HSV UL16 is a tegument component thought to be involved in capsid maturation, while HCMV UL94 has been identified as a tegument component but has not been ascribed a function (51, 66, 71). ORF63 is conserved among all of the herpesviruses and has been characterized in HSV, pseudorabies virus, and HCMV. Its homologs in HSV and pseudorabies virus (UL37 proteins) are involved in egress and reenvelopment, while a HCMV mutant lacking the ORF63 homolog (UL47) exhibits an entry defect potentially at the step of capsid disassembly (4, 13, 33).
Several chaperones have been found in the tegument of HCMV (66), another herpesviruses, and play critical roles in both cellular and viral processes. In the cell, they are involved in assisting protein folding and preventing aggregation of improperly folded chains. Heat shock cognate protein 70, or hsc70, is a cellular chaperone that has been found in association with virion components of several different virus families. Hsc70 is specifically incorporated into HIV virions (26). Hsc70 binds posttranslationally to simian virus 40 VP1, colocalizing with it to the nucleus, where it is thought to prevent premature capsid assembly (11). During papillomavirus infection, hsc70 is required for the nuclear translocation of the L2 protein (16). Hsc70 also is responsible for the posttranslational translocation of the hepatitis B large envelope protein into the endoplasmic reticulum (36) and is found in virions and subviral particles of avian hepatitis B virus (64). The HSV regulatory protein ICP0 causes hsc70 relocalization to distinct nuclear domains (6). Proteosomal machinery is also localized to these sites, suggesting a role in quality control, perhaps specifically acting during the formation of the capsid portal, a dodecamer of the HSV UL6 protein.
In addition to being directly incorporated into the virions of several viruses, the chaperones are also intimately involved in diverse events of virus replication. Heat shock protein 90, hsp90, is considered a specialized chaperone, binding proteins at a near-native state and controlling their activities (54). Known targets of hsp90 are involved in signal transduction, cell cycle modulation, and transcriptional regulation. The protein has diverse roles in the replication of different viruses. During influenza virus infection, hsp90 is relocalized to the nucleus and binds to PB2 to stimulate polymerase activity (49). Hsp90 is required for reverse transcription in duck hepatitis B virus, where it assists in the formation of a ribonucleoprotein complex and in protein priming of replication (27). Inhibition of hsp90 by geldanamycin reduces vaccinia virus titers 1 to 3 logs (28). The chaperone is not packaged into the virus, although it binds to the vaccinia virus core protein, and it is speculated to function during viral uncoating. So it is conceivable that delivery of hsp90 by KSHV virions may play a role in influencing biochemical processes on the next round of infection. In KSHV, the v-FLIP protein was found in a complex with IKKγ and hsp90, an interaction that is crucial for v-FLIP's ability to activate NFκB (15).
However, it is equally possible that some of these cellular proteins are present in virions merely because of their high abundance in the cytosol at the time of envelopment and may play no active role in KSHV biology. Certainly, actin and tubulin are likely to fall into this category; the chaperones and ERM proteins are also highly abundant. Clearly, additional work will be required to address both the mechanisms by which such proteins are incorporated into particles and the biological consequences of that incorporation.
Acknowledgments
We thank Chris Sullivan, Adam Grundhoff, and Craig McCormick for critical reading of the manuscript.
J.T.B. was supported by a training grant from the National Cancer Institute (T32CA09043).
REFERENCES
- 1.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 77:7978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 3.Bechtel, J. T., Y. Liang, J. Hvidding, and D. Ganem. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechtel, J. T., and T. Shenk. 2002. Human cytomegalovirus UL47 tegument protein functions after entry and before immediate-early gene expression. J. Virol. 76:1043-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bortz, E., J. P. Whitelegge, Q. Jia, Z. H. Zhou, J. P. Stewart, T. T. Wu, and R. Sun. 2003. Identification of proteins associated with murine gammaherpesvirus 68 virions. J. Virol. 77:13425-13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burch, A. D., and S. K. Weller. 2004. Nuclear sequestration of cellular chaperone and proteasomal machinery during herpes simplex virus type 1 infection. J. Virol. 78:7175-7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon, J. S., D. Ciufo, A. L. Hawkins, C. A. Griffin, M. J. Borowitz, G. S. Hayward, and R. F. Ambinder. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J. Virol. 74:10187-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 9.Cesarman, E., P. S. Moore, P. H. Rao, G. Inghirami, D. M. Knowles, and Y. Chang. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708-2714. [PubMed] [Google Scholar]
- 10.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 11.Chromy, L. R., J. M. Pipas, and R. L. Garcea. 2003. Chaperone-mediated in vitro assembly of Polyomavirus capsids. Proc. Natl. Acad. Sci. USA 100:10477-10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 75:5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai, P., G. L. Sexton, J. M. McCaffery, and S. Person. 2001. A null mutation in the gene encoding the herpes simplex virus type 1 UL37 polypeptide abrogates virus maturation. J. Virol. 75:10259-10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estridge, J. K., L. M. Kemp, and D. S. Latchman. 1990. The herpes simplex virus protein Vmw65 can trans-activate both viral and cellular promoters in neuronal cells. Biochem. J. 271:273-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field, N., W. Low, M. Daniels, S. Howell, L. Daviet, C. Boshoff, and M. Collins. 2003. KSHV vFLIP binds to IKK-gamma to activate IKK. J. Cell Sci. 116:3721-3728. [DOI] [PubMed] [Google Scholar]
- 16.Florin, L., K. A. Becker, C. Sapp, C. Lambert, H. Sirma, M. Muller, R. E. Streeck, and M. Sapp. 2004. Nuclear translocation of papillomavirus minor capsid protein L2 requires Hsc70. J. Virol. 78:5546-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 18.Fujimuro, M., and S. D. Hayward. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3β. J. Virol. 77:8019-8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimuro, M., F. Y. Wu, C. ApRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9:300-306. [DOI] [PubMed] [Google Scholar]
- 20.Funayama, N., A. Nagafuchi, N. Sato, and S. Tsukita. 1991. Radixin is a novel member of the band 4.1 family. J. Cell Biol. 115:1039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, S. J., J. H. Deng, and F. C. Zhou. 2003. Productive lytic replication of a recombinant Kaposi's sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 77:9738-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao, S. J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 23.Garber, A. C., M. A. Shu, J. Hu, and R. Renne. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glaunsinger, B., and D. Ganem. 2004. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol. Cell 13:713-723. [DOI] [PubMed] [Google Scholar]
- 25.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurer, C., A. Cimarelli, and J. Luban. 2002. Specific incorporation of heat shock protein 70 family members into primate lentiviral virions. J. Virol. 76:4666-4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu, J., and D. Anselmo. 2000. In vitro reconstitution of a functional duck hepatitis B virus reverse transcriptase: posttranslational activation by Hsp90. J. Virol. 74:11447-11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung, J. J., C. S. Chung, and W. Chang. 2002. Molecular chaperone Hsp90 is important for vaccinia virus growth in cells. J. Virol. 76:1379-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izumiya, Y., S. F. Lin, T. Ellison, L. Y. Chen, C. Izumiya, P. Luciw, and H. J. Kung. 2003. Kaposi's sarcoma-associated herpesvirus K-bZIP is a coregulator of K-Rta: physical association and promoter-dependent transcriptional repression. J. Virol. 77:1441-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalejta, R. F., J. T. Bechtel, and T. Shenk. 2003. Human cytomegalovirus pp71 stimulates cell cycle progression by inducing the proteasome-dependent degradation of the retinoblastoma family of tumor suppressors. Mol. Cell. Biol. 23:1885-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kedes, D. H., E. Operskalski, M. Busch, R. Kohn, J. Flood, and D. Ganem. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2:918-924. [DOI] [PubMed] [Google Scholar]
- 32.Kinchington, P. R., D. Bookey, and S. E. Turse. 1995. The transcriptional regulatory proteins encoded by varicella-zoster virus open reading frames (ORFs) 4 and 63, but not ORF61, are associated with purified virus particles. J. Virol. 69:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 75:8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan, H. H., P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 78:3601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert, C., and R. Prange. 2003. Chaperone action in the posttranslational topological reorientation of the hepatitis B virus large envelope protein: implications for translocational regulation. Proc. Natl. Acad. Sci. USA 100:5199-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lan, K., D. A. Kuppers, S. C. Verma, and E. S. Robertson. 2004. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J. Virol. 78:6585-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lankes, W. T., and H. Furthmayr. 1991. Moesin: a member of the protein 4.1-talin-ezrin family of proteins. Proc. Natl. Acad. Sci. USA 88:8297-8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang, Y., J. Chang, S. J. Lynch, D. M. Lukac, and D. Ganem. 2002. The lytic switch protein of KSHV activates gene expression via functional interaction with RBP-Jkappa (CSL), the target of the Notch signaling pathway. Genes Dev. 16:1977-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang, Y., and D. Ganem. 2004. RBP-J (CSL) is essential for activation of the K14/vGPCR promoter of Kaposi's sarcoma-associated herpesvirus by the lytic switch protein RTA. J. Virol. 78:6818-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao, W., Y. Tang, S. F. Lin, H. J. Kung, and C. Z. Giam. 2003. K-bZIP of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) binds KSHV/HHV-8 Rta and represses Rta-mediated transactivation. J. Virol. 77:3809-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu, B., and M. F. Stinski. 1992. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J. Virol. 66:4434-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu, M., and T. Shenk. 1999. Human cytomegalovirus UL69 protein induces cells to accumulate in G1 phase of the cell cycle. J. Virol. 73:676-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 46.Martinez, R., R. T. Sarisky, P. C. Weber, and S. K. Weller. 1996. Herpes simplex virus type 1 alkaline nuclease is required for efficient processing of viral DNA replication intermediates. J. Virol. 70:2075-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.McCormick, C., and D. Ganem. 2005. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307:739-741. [DOI] [PubMed] [Google Scholar]
- 47.Mesri, E. A., E. Cesarman, L. Arvanitakis, S. Rafii, M. A. Moore, D. N. Posnett, D. M. Knowles, and A. S. Asch. 1996. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J. Exp. Med. 183:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller, G., M. O. Rigsby, L. Heston, E. Grogan, R. Sun, C. Metroka, J. A. Levy, S. J. Gao, Y. Chang, and P. Moore. 1996. Antibodies to butyrate-inducible antigens of Kaposi's sarcoma-associated herpesvirus in patients with HIV-1 infection. N. Engl. J. Med. 334:1292-1297. [DOI] [PubMed] [Google Scholar]
- 49.Momose, F., T. Naito, K. Yano, S. Sugimoto, Y. Morikawa, and K. Nagata. 2002. Identification of Hsp90 as a stimulatory host factor involved in influenza virus RNA synthesis. J. Biol. Chem. 277:45306-45314. [DOI] [PubMed] [Google Scholar]
- 50.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nalwanga, D., S. Rempel, B. Roizman, and J. D. Baines. 1996. The UL 16 gene product of herpes simplex virus 1 is a virion protein that colocalizes with intranuclear capsid proteins. Virology 226:236-242. [DOI] [PubMed] [Google Scholar]
- 52.Nealon, K., W. W. Newcomb, T. R. Pray, C. S. Craik, J. C. Brown, and D. H. Kedes. 2001. Lytic replication of Kaposi's sarcoma-associated herpesvirus results in the formation of multiple capsid species: isolation and molecular characterization of A, B, and C capsids from a gammaherpesvirus. J. Virol. 75:2866-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patterson, D., J. Bleskan, K. Gardiner, and J. Bowersox. 1999. Human phosphoribosylformylglycineamide amidotransferase (FGARAT): regional mapping, complete coding sequence, isolation of a functional genomic clone, and DNA sequence analysis. Gene 239:381-391. [DOI] [PubMed] [Google Scholar]
- 54.Picard, D. 2002. Heat-shock protein 90, a chaperone for folding and regulation. Cell. Mol. Life Sci. 59:1640-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roizman, B., and A. Sears. 1996. Herpes simplex viruses and their replication, p. 1043-1107. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fundamental virology. Lippincott-Raven, Philadelphia, Pa.
- 56.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schek, N., and S. L. Bachenheimer. 1985. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J. Virol. 55:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma-Walia, N., P. P. Naranatt, H. H. Krishnan, L. Zeng, and B. Chandran. 2004. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J. Virol. 78:4207-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song, M. J., H. J. Brown, T. T. Wu, and R. Sun. 2001. Transcription activation of polyadenylated nuclear RNA by Rta in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:3129-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song, M. J., H. Deng, and R. Sun. 2003. Comparative study of regulation of RTA-responsive genes in Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 77:9451-9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song, M. J., X. Li, H. J. Brown, and R. Sun. 2002. Characterization of interactions between RTA and the promoter of polyadenylated nuclear RNA in Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 76:5000-5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swameye, I., and H. Schaller. 1997. Dual topology of the large envelope protein of duck hepatitis B virus: determinants preventing pre-S translocation and glycosylation. J. Virol. 71:9434-9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomescu, C., W. K. Law, and D. H. Kedes. 2003. Surface downregulation of major histocompatibility complex class I, PE-CAM, and ICAM-1 following de novo infection of endothelial cells with Kaposi's sarcoma-associated herpesvirus. J. Virol. 77:9669-9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp, 2nd, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vieira, J., M. L. Huang, D. M. Koelle, and L. Corey. 1997. Transmissible Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi's sarcoma. J. Virol. 71:7083-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vieira, J., P. O'Hearn, L. Kimball, B. Chandran, and L. Corey. 2001. Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J. Virol. 75:1378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang, S. E., F. Y. Wu, M. Fujimuro, J. Zong, S. D. Hayward, and G. S. Hayward. 2003. Role of CCAAT/enhancer-binding protein alpha (C/EBPα) in activation of the Kaposi's sarcoma-associated herpesvirus (KSHV) lytic-cycle replication-associated protein (RAP) promoter in cooperation with the KSHV replication and transcription activator (RTA) and RAP. J. Virol. 77:600-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang, S. E., F. Y. Wu, Y. Yu, and G. S. Hayward. 2003. CCAAT/enhancer-binding protein-α is induced during the early stages of Kaposi's sarcoma-associated herpesvirus (KSHV) lytic cycle reactivation and together with the KSHV replication and transcription activator (RTA) cooperatively stimulates the viral RTA, MTA, and PAN promoters. J. Virol. 77:9590-9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wing, B. A., G. C. Lee, and E. S. Huang. 1996. The human cytomegalovirus UL94 open reading frame encodes a conserved herpesvirus capsid/tegument-associated virion protein that is expressed with true late kinetics. J. Virol. 70:3339-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winkler, M., and T. Stamminger. 1996. A specific subform of the human cytomegalovirus transactivator protein pUL69 is contained within the tegument of virus particles. J. Virol. 70:8984-8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao, F., and R. J. Courtney. 1989. A major transcriptional regulatory protein (ICP4) of herpes simplex virus type 1 is associated with purified virions. J. Virol. 63:3338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye, G.-J., K. T. Vaughan, R. B. Vallee, and B. Roizman. 2000. The herpes simplex virus 1 UL34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J. Virol. 74:1355-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu, F. X., and Y. Yuan. 2003. The ORF45 protein of Kaposi's sarcoma-associated herpesvirus is associated with purified virions. J. Virol. 77:4221-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]