Abstract
The immediate-early ie0-ie1 gene complex expresses the only baculovirus spliced gene that produces an alternate protein product. Autographa californica multiple nucleopolyhedrovirus (AcMNPV) IE1 is a potent transcriptional transactivator that is essential for viral replication in transient assays. IE1 contains 582 amino acids that are arranged into different domains, including an acidic activation domain at the N terminus, a DNA binding domain, and an oligomerization domain at the C terminus. IE0 is a 52-amino-acid N-terminally elongated form of IE1. We investigated the functions of IE0 and IE1 in virus-infected cells by constructing the first ie1 open reading frame knockout virus. An infectious AcMNPV bacmid was used to generate the ie1 knockout, and the resulting virus, AcBacIE1KO, effectively deletes both ie0 and ie1. AcBacIE1KO does not infect Spodoptera frugiperda cells, showing that the ie0-ie1 gene complex is essential for viral infection. Rescue viruses of AcBacIE1KO were constructed that express only IE1, IE1 and IE0, or only IE0. Our results show that both IE0 and IE1 can function independently, but not equivalently, to support replication, producing infectious virus. Viruses expressing predominately, or only, IE0 produced significantly fewer cells with polyhedra than either the IE1 counterpart or wild-type virus. In addition, DNA replication was prolonged and budded virus and late gene expression were delayed. Viruses expressing only IE1 also produced fewer polyhedra, but replication was slightly faster and achieved higher levels than that of the wild-type virus. Both IE0 and IE1 are therefore required and must be expressed in the correct quantitative ratios to achieve a wild-type infection.
Autographa californica multiple nucleopolyhedrovirus (AcMNPV) infection of insect cells occurs in a cascading fashion punctuated by three distinct transcriptional phases: early, late, and very late gene expression. One of the critical elements of this cascade is the immediate-early protein IE1, which is a potent transcriptional regulator of both early and late genes (1, 3-5, 11, 14, 17, 18, 26). Previous studies have also shown that ie1 is also one of six required genes for viral DNA replication in transient assays (15, 27). It is therefore not surprising that ie1 is found in nearly all of the baculovirus genomes in the family Baculoviridae sequenced to date (12).
AcMNPV IE1 is a 66.9-kDa protein composed of a number of functionally distinct domains. Present in the N terminus are two acidic transcriptional activation domains, separated by basic domain I, which is required for hr binding (10, 25, 27, 36). Located in the C terminus are basic domain II and a helix-loop-helix domain. These domains are involved in DNA binding, nuclear import, and dimerization (10, 23-25, 27, 34, 38).
ie0 is unique as it is the only known baculovirus spliced gene that produces an alternate protein product (2, 16, 29, 38). AcMNPV IE0 is a 72.6-kDa 636-amino-acid (aa) protein composed of 38 aa encoded by orf141 (exon0), 16 aa encoded by the upstream nontranslated leader of ie1, and the entire 582-aa IE1 protein The final product is therefore identical to IE1 except for the additional 54 aa fused to the N terminus (2). Although a great deal of attention has been paid to the role that IE1 plays during infection, less has been given to the possible roles of IE0. Previous studies have revealed similarities, as well as differences, in functionality between these two related proteins. IE0 and IE1 share the abilities to enhance transcription via hr binding, to activate very late gene expression in transient assays, and to form either homodimers or heterodimers (17, 18, 24, 25). Unlike IE1, IE0 is not capable of hr-independent transactivation, and no examples of negative gene regulation have been reported (17). However, IE0 from the baculovirus Orgyia pseudotsugata multiple nucleopolyhedrovirus (OpMNPV) is able to transactivate genes in an hr-independent manner (38).
A prominent difference between IE0 and IE1 is their expression profiles (4, 14). Both proteins can be detected by 2 h postinfection (hpi), and IE0 peaks at 4 hpi and is expressed at higher levels than IE1 prior to the initiation of replication but then slowly declines (14). IE0 is therefore the predominate protein at early times postinfection (p.i.) IE1, on the other hand, continues to increase after replication and obtains relatively high steady-state levels at very late times p.i. The variability in expression pattern suggests that these two proteins play competitive roles during infection, with IE0 functioning primarily during early times p.i. and IE1 functioning primarily at late times p.i. In support of this hypothesis, we recently showed that when IE0 is coexpressed with IE1 very-late gene expression is reduced in transient expression assays (14). Changing the regulation of IE0 can also have significant effects on viral replication. This was demonstrated in a recent study that showed that down regulation of IE0 resulted in the ability of AcMNPV to replicate to high levels in cells that are normally nonpermissive (21). In addition, a comparative analysis of the functions of OpMNPV IE0 and IE1 has shown that IE0 appears to have greater specificity for some early promoters (38).
IE0 and IE1 are both present throughout infection, and to date it has not been possible to determine the function of each protein independently of that of the other. The objective of this study was to determine if both IE0 and IE1 are essential for infection of cultured insect cells or if either protein can support viral replication independently. To test this, we constructed an AcMNPV ie0-ie1 double-knockout virus and determined that it could not replicate in insect cells. However, rescue of the ie0-ie1 knockout with either ie0 or ie1 produced infectious virus, which indicates that this gene complex is essential but that either gene can support virus replication. Expression of either protein by itself, however, did not result in WT levels of replication or polyhedron production.
MATERIALS AND METHODS
Viruses and cells.
Spodoptera frugiperda Sf9 cells were maintained in 10% fetal bovine serum-supplemented TC100 medium at 27°C. AcMNPV recombinant bacmids were derived from commercially available bacmid bMON14272 (Invitrogen Life Technologies) and propagated in Escherichia coli strain DH10B.
Construction of IE0-IE1 AcMNPV knockout bacmid.
To knock out the IE1-encoding gene of AcMNPV, we first created a recombination vector composed of the Zeocin resistance gene flanked by 575 and 743 bp of the 3′ and 5′ untranslated ie1 gene regions, respectively. The backbone and flanking regions were taken from a plasmid containing the AcMNPV ie1 gene (pAcIE1-DT1) by PCR with primers 401 (5′-GGGTCTGGTACCGGTGTCCGACGCTATA-3′) and 396 (5′-GTTCTCGAGCTTGTCGCCGCCAGTGT-3′). A second PCR with primers 393 (5′-GGACTCGAGCTGCAGCACGTGTTGAC-3′) and 394 (5′-GCCGTCGGACCGGTGGTGTTGGTC-3′) amplified the EM7 promoter and Zeocin resistance gene from plasmid pZeoSV (Invitrogen Life Technologies). XhoI and AgeI sites included in the primers were used to clone these PCR fragments together, resulting in recombination vector pAcIE1(fr)-Zeo.
To construct the IE0-IE1 knockout bacmid, the λ phage Red recombinase method described by Datsenko and Wanner (7) was used. Initially, a linear 1.8-kbp fragment composed of the IE1 flanking regions and the Zeocin resistance gene was gel purified after digestion of pAcIE1(fr)-Zeo with BamHI and HindIII. One microgram of this fragment and 1 μg of bacmid DNA were cotransformed into E. coli strain BW25113/pKD46. Cells were allowed to recover for 4 h in 1.5 ml of SOC medium (7) and then selected on Luria-Bertani (LB) agar plates containing 50 μg of kanamycin per ml and 30 μg of Zeocin per ml. Plates were incubated overnight at 37°C, and resistant colonies were screened by PCR for recombination. Verification of a selected IE0-IE1 knockout bacmid was confirmed with the primer pairs 429 (5′-CTGTACGCCGAGTGGTCGGAGGTCG-3′)-430 (5′-CATACGCGGAGTATTTCAGGGCATTTCA-3′) and 526 (5′-CTGTTGCTGACCGTCGAACATGCCT-3′)-527 (5′-GCACCGGAACGGCACTGGTCAA-3′), which amplified the 3′ and 5′ crossover events, respectively. As the original bacmid bMON14272 is polyhedrin negative, the ie1 knockout bacmid was named AcBacIE1KO-P− (Fig. 1A).
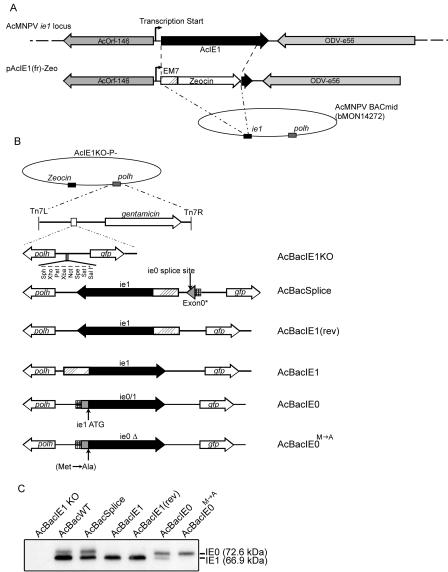
FIG. 1.
Construction of ie1 knockout, rescue, and WT AcMNPV bacmids. (A) Schematic maps of the AcMNPV ie1 gene locus showing the relative positions and orientations of the orf146, ie1, and odv-e56 ORFs (top) and recombination vector plasmid pAcIE1(fr)-Zeo (bottom). pAcIE1(fr)-Zeo contains the ie1 ORF replaced by the Zeocin resistance-encoding gene and was used to generate the ie1 knockout bacmid by recombination in E. coli. (B) Schematic diagrams of rescue viruses AcBacIE1KO, AcBacSplice, AcBacIE1, AcBacIE1(rev), AcBacIE0, and AcBacIE0 showing the genes inserted into the polyhedrin (polh) locus by Tn7-mediated transposition. (C) Western blot analysis for the presence or absence of IE1 or IE0 protein expressed from bacmids AcBacIE1KO, AcBacWT, AcBacSplice, AcBacIE1, AcBacIE1(rev), AcBacIE0, and AcBacIE0. Locations of the IE0- and IE1-specific bands are shown on the right.
Construction of rescue transfer vectors.
To introduce IE1 and IE0 constructs into AcBacIE1KO-P−, rescue transfer vectors were constructed in the plasmid backbone pFAcT-GFP (6). pFAcT-GFP contains two Tn7 transposition excision sites that allow the transposition of genes cloned between the sites to be transposed into the miniATT region located in AcBacIE1KO-P− (22). In addition to the cloned gene of interest, the pFAcT-GFP backbone contains the AcMNPV polyhedrin-encoding gene and a green fluorescent protein (GFP)-encoding gene, both of which are included in the transposed DNA cassette (Fig. 1B).
Rescue vectors containing AcMNPV IE1 were constructed as follows. Plasmid pAcIE1-DT1 (37) was digested with XbaI, and the 2,651-bp fragment was gel purified and ligated into XbaI-digested pFAcT-GFP. Colonies were screened for orientation, and two clones containing inserts in opposite orientations were selected and named pFAcT-AcIE1-GFP and pFAcT-AcIE1(rev)-GFP. pFAcT-AcIE1-GFP inserts the gene for IE1 in the same orientation as the upstream exon0 gene upon transposition, while pFAcT-AcIE1(rev)-GFP inserts it in the reverse orientation.
Two vectors containing a prespliced IE0-encoding gene, pFAcT-AcIE0-GFP and pFAcT-AcIE0M→A, were also constructed. The first construct results in expression of the genes for both IE0 and IE1 (via internal translation), and the second construct contains a substitution of an alanine for a methionine, preventing the translation of IE1 (14). Primer pair 338 (5′-TGGAGCTCCGTATTACCGCCTTTGAGTGA-3′)-432 (5′-CAAAAGCTCTGCAGGCGCTGGCAAAGATT-3′) was used to amplify both ie0 and ie0M→A from plasmids pAc-IE01 and pAc-IE0Δ, respectively (14). These PCR products were cloned into pFAcT-GFP with PstI and SstI restriction sites to form pFAcT-AcIE0-GFP and pFAcT-AcIE0M→A.
As the IE0 construct contains IE0 under the control of its native promoter, expression of IE1 from internal translation was not expected to be equivalent to that of the WT. Therefore, a final construct, pFAcT-Splice, was constructed to mimic the WT virus, where ie0 is generated from a splicing event. Profiles of IE0 and IE1 expression from this construct were expected to be more similar to WT levels and ratios. To create this vector, a 517-bp fragment was amplified from AcMNPV with primers 559 (5′-AAAGATTGAGCTCGACCCTTGCCCCCGG-3′) and 560 (5′-TTTGTATGGCGGCCGCGTACATGTGAAAGA-3′). This PCR product was digested with SstI and NotI and subsequently ligated to SstI-NotI-digested and gel-purified pFAcT-AcIE1(rev)-GFP. The resulting construct, pFAcT-Splice, consists of the ie0 portion of exon0 under the regulatory control of the ie0 promoter, followed by the ie1 gene under control of the ie1 promoter. As the splice donor and acceptor sites are still present, splicing occurs in vivo to create ie0.
Generation of AcBacIE1KO rescue bacmids.
To create AcBacIE1KO rescue bacmids, Tn7-mediated transposition was used as previously described by Luckow et al. (22). Transposition of pFAcT-GFP, pFAcT-AcIE1-GFP, pFAcT-AcIE1(rev), pFAcT-IE0-GFP, pFAcT-IE0M→A, and pFAcT-Splice into our AcBacIE1KO-P− bacmid yielded polyhedrin- and GFP-positive AcBacIE1KO bacmids, herein referred to as AcBacIE1KO, AcBacIE1, AcBacIE1(rev), AcBacIE0, AcBacIE0M→A, and AcBacSplice, respectively. For each bacmid, approximately 100 ng of rescue transfer vector was transformed into competent DH10Br that contained bacmid AcBacIE1KO-P− plus helper plasmid pMON7124 (Invitrogen Life Technologies). Transformations were allowed to recover for 4 h at 37°C in low-salt LB medium, after which they were plated onto LB agar plates containing 7 μg of gentamicin per ml, 10 μg of tetracycline per ml, 30 μg of Zeocin per ml, 50 μg of kanamycin per ml, 40 μg of isopropyl-β-d-thiogalactopyranoside (IPTG) per ml, and 100 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml. Plates were incubated for 48 h at 37°C, and white resistant colonies were replica plated in order to select a colony that harbored the rescue bacmid in the absence of both the rescue transfer vector and the helper plasmid. Selected colonies were resistant to gentamicin, kanamycin, and Zeocin and sensitive to ampicillin and tetracycline. Putative rescue bacmids were then confirmed by visualization of GFP and occlusion body formation upon transfection into Sf9 cells. To verify correct expression of the IE1 and IE0 proteins, a Western blot analysis was performed on transfected Sf9 cell pellets.
In addition, a control bacmid, AcBacIE1KO, that is polyhedrin and GFP positive was generated by transposition of pFAcT-GFP into AcBacIE1KO-P−. A control WT bacmid (AcBacWT) containing GFP and polyhedrin was also generated by transposition of pFAcT-GFP into AcBacP− (bMON14272) as described by Dai et al. (6).
Time course analysis of viral infections.
Two time course studies were performed to compare viral DNA replication, budded-virus (BV) production, and protein synthesis of AcBacIE1KO, AcBacWT, AcBacIE1, AcBacIE1(rev), AcBacIE0, AcBacIE0M→A, and AcBacSplice. Sf9 cells, plated at 106 per well (six-well plate), were transfected with 1 μg of AcBacIE1KO or AcBacWT DNA and collected at 6, 12, 24, 30, 48, 72, or 96 h posttransfection. A time course study with BV at a multiplicity of infection (MOI) of 5 was also performed with Sf9 cells in suspension at a density of 2 × 106/ml. Cells were infected with AcBacWT, AcBacIE1, AcBacIE1(rev), AcBacIE0, AcBacIE0M→A, or AcBacSplice, and 2-ml samples were collected at 6, 12, 18, 24, 30, 36, 48, 72, 96, 120, and 168 hpi. Cells were pelleted by centrifugation at 5,000 × g for 10 min, and the supernatant containing BV was removed and stored at 4°C. The cell pellet was washed with 1× phosphate-buffered saline (PBS) and stored at −80°C.
Slot blot analysis of viral DNA replication.
Sf9 cell pellets collected during the time course study were resuspended in 0.4 M NaOH-0.125 mM EDTA to a cell density of 1,000/μl and boiled for 10 min to ensure cell lysis and nucleic acid denaturation. A Schleicher & Schuell slot blot apparatus was used to apply 30 μl of sample under vacuum to Zeta-Probe GT membrane (Bio-Rad). Membranes were hybridized to an α-32P-labeled AcMNPV EcoRI fragment T probe (RadPrime; Invitrogen Life Technologies) in accordance with the Zeta-Probe membrane protocol (Bio-Rad). A Storm PhosphorImager was used to visualize membranes exposed on Kodak PhosphorScreens. Subsequent quantification of each slot blot was performed with ImageQuant TL v2003.02 software (Molecular Dynamics).
Titration of BV.
Titration of BV stocks was done with a modified plaque assay outlined previously by Volkman and Goldsmith (39). Briefly, Sf9 cells were seeded at 2 × 104/well on a 12-well slide. Cells were incubated with 10 μl of BV diluted in TC100 medium for 1 h, after which the medium was removed and wells were overlaid with 0.6% methyl cellulose in Grace's medium and 10% fetal bovine serum. Slides were incubated at 28°C for 48 h, and plaques were counted by GFP visualization under UV light.
Western blot analysis.
Sf9 cell pellets from each time point were lysed by two freeze-thaw cycles and resuspended in 1× PBS with 6 μl of protease inhibitor cocktail. An equal volume of 2× sodium dodecyl sulfate loading buffer (19) was added, and samples were incubated at 100°C for 5 min and snap-cooled. Samples were then sheared with a 1-ml syringe and a 22-gauge needle. Protein samples were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (19) and transferred to Millipore Immobilon-P membrane with the Bio-Rad Mini-Protean II and Liquid Transfer apparatuses, respectively, in accordance with the manufacturers' recommended protocols. Western blots were probed with one of the following five primary antibodies: (i) AcMNPV IE1 mouse monoclonal IE1-4B7 at 1:2,500 (35), (ii) OpMNPV V39 mouse monoclonal α-39K OpMNPV at 1:2,000 (30), (iii) AcMNPV GP64 mouse monoclonal AcV5 at 1:1,000 (13), (iv) OpMNPV polyhedron mouse monoclonal at 1:10,000 (33), and (v) AcMNPV P35 rabbit polyclonal at 1:1,000. Horseradish peroxidase-conjugated rabbit anti-mouse secondary antibody was used at 1:15,000 or horseradish peroxidase-conjugated goat anti-rabbit secondary antibody was used at 1:10,000 and detected with the enhanced chemiluminescence system (Amersham). Immunohybridizations to Western blots were performed in accordance with standard protocols. The relative levels of protein expression were analyzed by densitometry with NIH Image software.
RESULTS
Replication analysis of AcMNPV ie0-ie1 double-knockout bacmid AcBacIE1KO.
Because of the requirement of IE1 for DNA replication in transient assays, previous studies have implicated ie1 as essential for AcMNPV infection of insect cells. However, to date the ie1 gene has not been knocked out directly. With bacmids and recombination in E. coli, the ie1 open reading frame (ORF) was replaced with the Zeocin resistance gene to create AcBacIE1KO-P− (Fig. 1A and B). As the ie1 ORF is required for the transcription of not just ie1 but also ie0, AcBacIE1KO-P− actually represents a double knockout. The AcMNPV polyhedrin and gfp genes were inserted into AcBacIE1KO-P− via site-specific transposition to create AcBacIE1KO (Fig. 1B). Addition of gfp allowed the use of a simple visual method of monitoring the progression of infection following bacmid transfection. The knockout of the IE1 ORF was verified by PCR and Western blot analysis of transfected Sf9 cells, which confirmed that neither IE1 nor IE0 was expressed (Fig. 1C).
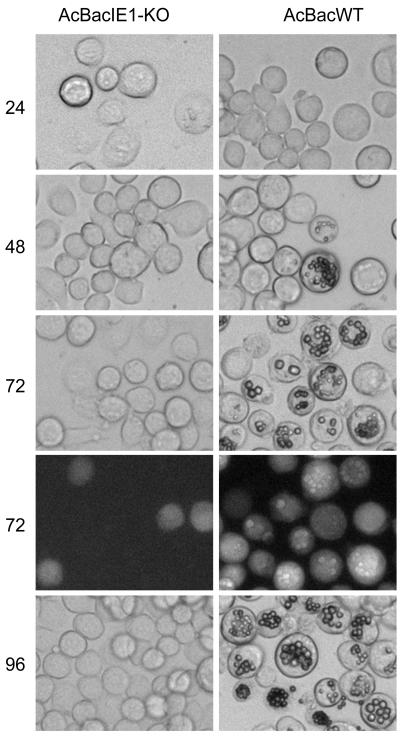
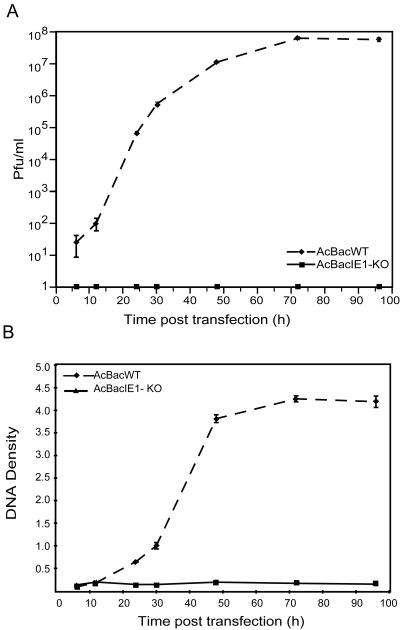
To characterize the effects of deleting IE0 and IE1 on AcMNPV infection, the replication of AcBacIE1KO and that of the wild-type (WT) bacmid (AcBacWT) in transfected Sf9 cells were compared. Microscopic analysis showed no obvious signs of infection by AcBacIE1KO (Fig. 2). Nuclear morphology and cell growth were similar to those of mock-infected cells. GFP expression in AcBacIE1KO-transfected cells was restricted to single cells and exhibited no evidence of virus spread over the entire time course (Fig. 2, 72 hpi). AcBacWT, on the other hand, exhibited widespread GFP expression and the formation of polyhedra in some cells by 48 hpi; 100% of the cells contained polyhedra by 96 hpi (Fig. 2). Supernatants of AcBacIE1KO and AcBacWT were also assayed for BV production. Quantification of BV production by plaque assay revealed that while AcBacWT produced levels of up to 108 PFU/ml, AcBacIE1KO did not produce a single detectable BV (Fig. 3A). To determine if the lack of infection by AcBacIE1KO was due to the inability to replicate, a slot blot assay was performed with a virus-specific probe. As expected, AcBacIE1KO showed no DNA replication (Fig. 3B). These results confirm that the presence of IE1 or IE0 is required for viral replication; however, further studies were needed to determine if either IE0 or IE1 could independently support viral infection.
FIG. 2.
Analysis of viral replication and polyhedron development by AcBacIE1KO and AcBacWT in bacmid DNA-transfected Sf9 cells. Cells were transfected with 2.0 μg of bacmid DNA from each virus and analyzed by light microscopy at 24, 48, 72, and 96 h posttransfection. Fluorescence microscopy images of cells at 72 h posttransfection are also presented to demonstrate AcBacIE1KO transfection by constitutive expression of GFP.
FIG. 3.
Virus growth curves and DNA replication of AcBacIE1KO and AcBacWT in Sf9 cells. (A) Sf9 cells were transfected with 2.0 μg of DNA from each virus. Cell culture supernatants were harvested at the indicated time points posttransfection and assayed for the production of infectious virus by plaque assay. Each data point represents the average titer derived from multiple plaque assays. Error bars represent standard errors. (B) Slot blot analysis of AcBacIE0-KO and AcBacWT viral DNA replication. Sf9 cells were transfected with 2.0 μg of DNA from AcBacIE0-KO and AcBacWT. At the designated times posttransfection, cells were harvested and cell lysates were prepared and subjected to slot blot analysis. EcoRI fragment T of AcMNPV was labeled with [32P]dCTP and used as a hybridization probe. DNA levels were quantified by phosphorimager analysis. Error bars represent standard errors.
Analysis of AcBacIE1KO rescue bacmids.
A total of five different rescue constructs were introduced into the AcBacIE1KO-P− bacmid (Fig. 1B). ie1 was introduced in both the same and reverse orientations as the upstream exon0 (orf141) gene [AcBacIE1 and AcBacIE1(rev), respectively]. AcBacIE1(rev) controlled for the unlikely possibility of splicing occurring from exon0 located more than 15 kbp upstream. Two constructs that express both IE0 and IE1 were also constructed. The first, AcBacIE0, contained the entire ie0 ORF as a cDNA under the control of the ie0 promoter. This construct also expresses IE1, as we have previously shown, via internal translation initiation (14, 38). AcBacSplice consisted of the ie0 promoter and the first 182 bp of the ie0 ORF, followed by the ie1 promoter and the ie1 ORF. This construct, which maintained the splice site of ie0, was expected to produce IE0 and IE1 levels that were similar to those of the WT virus. The final rescue construct (AcBacIE0M→A) is identical to AcBacIE0 except that the internal ATG of the ie1 ORF was mutated to GCG and thus only IE0 is translated. Each of the above constructs was cloned into transposition vector pFAcT-GFP, which contains the polyhedrin and gfp genes. Thus, upon site-specific transposition into AcBacIE1KO-P−, all rescue constructs were also made polyhedrin and GFP positive (Fig. 1B).
To confirm the expression of the correct proteins by the rescue bacmids, a Western blot analysis was performed on infected Sf9 cells (Fig. 1C). The Western blot shows that AcBacSplice expresses both IE0 and IE1 as expected but the level of IE1 relative to IE0 was reduced compared to that produced by AcBacWT. Both AcBacIE1 and AcBacIE1(rev) produced only IE1, as expected. AcBacIE0 is translated predominately as IE0, but a low level of IE1 is produced from internal translation initiation and AcBacIE0M→A was translated only as IE0.
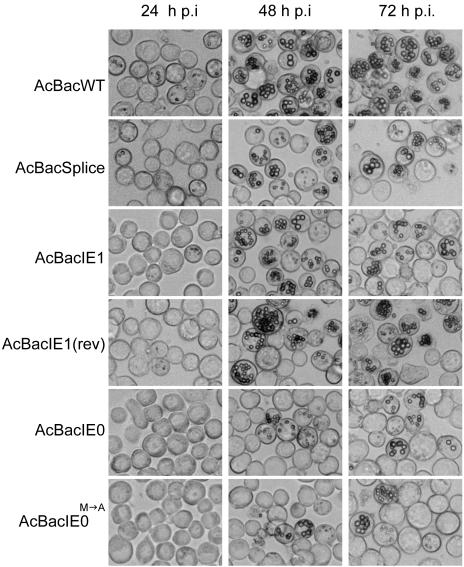
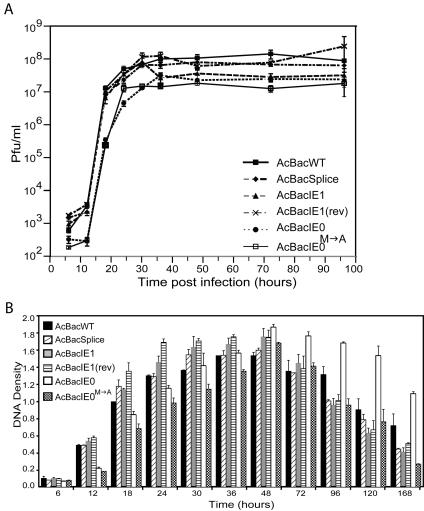
To evaluate the ability of the above bacmids to rescue the ie0-ie1 knockout, AcBacIE1KO, each was transfected into Sf9 cells and BV stocks were produced. With BV, viral-infection time course studies were performed at equal MOIs to compare and contrast the replication of each of the rescue viruses. The progression of infection was initially monitored by the production of polyhedra in each of the six infections (Fig. 4). By 24 hpi, polyhedra could be observed in approximately 10% of AcBacWT-infected cells and 5% of AcBacSplice-, AcBacIE1-, and AcBacIE1(rev)-infected cells. This value increased to nearly 100% for AcBacWT-infected cells by 48 hpi but only 70% for AcBacSplice-, AcBacIE1-, and AcBacIE1(rev)-infected cells. Although polyhedron development in AcBacSplice-, AcBacIE1-, and AcBacIE1(rev)-infected cells was slower than that in AcBacWT-infected cells, it did reach near-AcBacWT levels by 96 hpi. AcBacIE0 and AcBacIE0M→A, on the other hand, not only showed a 24-h delay relative to AcBacWT in the appearance of polyhedra but also produced much fewer cells containing polyhedra throughout the entire time course. By 120 hpi, AcBacIE0 had produced polyhedra in approximately 50% of the cells while AcBacIE0M→A had done so in only approximately 15% of the cells. The general morphology of the occlusion bodies in AcBacSplice-, AcBacIE0-, and AcBacIE0M→A-infected cells was similar to that of the occlusion bodies in AcBacWT-infected cells. However, in AcBacIE1- and AcBacIE1(rev)-infected cells they appeared to be individually smaller and aggregated together to a greater extent. These results confirm that both IE1 and IE0 alone can support viral replication in cell culture. Given the severe delay in the production and low numbers of polyhedra in AcBacIE0- and AcBacIE0M→A-infected cells, we wanted to determine if this effect was restricted to polyhedra or was reflective of more global effects, including BV production, DNA replication, and protein synthesis.
FIG. 4.
Analysis of viral replication and polyhedron development by light microscopy at 24, 48, and 72 hpi in Sf9 cells infected with AcBacWT, AcBacSplice, AcBacIE1, AcBacIE1(rev), AcBacIE0, or AcBacIE0. Sf9 cells were infected at an MOI of 5.
BV supernatants were collected from the viral infection time course and quantified by plaque assay (Fig. 5A). The onset of BV production by AcBacWT was between 6 and 12 hpi, with an exponential increase to 107 PFU/ml by 18 hpi and a leveling off to a maximum of approximately 1.0 × 108 PFU/ml by 30 hpi. AcBacSplice, AcBacIE1, and AcBacIE1(rev) produced BV at nearly identical rates and times p.i. relative to AcBacWT. AcBacIE0 and AcBacIE0M→A, on the other hand, showed a delayed onset between 12 and 18 hpi, followed by an exponential increase up to 24 hpi. AcBacIE0M→A peaked at 107 PFU/ml by 24 hpi, and AcBacIE0 gradually increased to peak levels of 2.5 × 107 PFU/ml by 36 hpi. The BV levels produced by AcBacIE0 and AcBacIE0M→A were therefore significantly lower than those produced by all of the other constructs.
FIG. 5.
Viral growth curves and slot blot analysis of viral DNA replication of AcBacWT, AcBacSplice, AcBacIE1, AcBacIE1(rev), AcBacIE0, and AcBacIE0 in Sf9 cells. (A) Sf9 cells were infected at an MOI of 5 for each virus, and supernatants were harvested at the indicated times p.i. and assayed for the production of infectious BV by plaque assay. Each data point represents the average titer derived from multiple assays. Error bars represent standard errors. (B). DNA levels in Sf9 cells infected at an MOI of 5 for each virus. At the designated times p.i., cells were harvested and cell lysates were prepared and subjected to slot blot analysis. EcoRI fragment T of AcMNPV was labeled with [32P]dCTP and used as a hybridization probe. DNA was quantified by phosphorimager analysis, and each column represents the average from two or three independent infections. Samples have been normalized relative to AcBacWT at 18 hpi. Error bars represent standard errors.
Analysis of AcBacIE0M→A revealed that in addition to only 15% of the cells containing polyhedra, it also only produced 10% of the BV made by either AcBacWT or AcBacIE1. These reduced numbers may have been a result of a decreased rate of DNA replication. To investigate this possibility, a DNA slot blot assay was performed with a virus-specific probe. As AcBacIE0 and AcBacIE0M→A showed delays in polyhedron and BV production, the time course was continued to 168 hpi. DNA replication in AcBacWT-infected cells was detectable by 12 hpi, with peak levels occurring at 36 hpi and a gradual decline to 168 hpi (Fig. 5B). AcBacSplice followed a similar course but exhibited peak levels at approximately 30 hpi, 6 h earlier than AcBacWT. AcBacIE1 and AcBacIE1(rev) also reached earlier (24 to 30 hpi) and higher peak levels than AcBacWT. Surprisingly, both AcBacIE0 and AcBacIE0M→A showed onset of DNA replication by 12 hpi, but peak levels were not reached until 48 hpi, 12 h after AcBacWT. DNA replication of both AcBacIE0 and AcBacIE0M→A was lower in level up to 24 hpi and did not increase as rapidly as that of AcBacWT. However, these constructs did attain higher steady-state levels of viral DNA than AcBacWT did. AcBacIE0 in particular reached higher levels of DNA replication than any of the other viruses from 48 to 68 hpi.
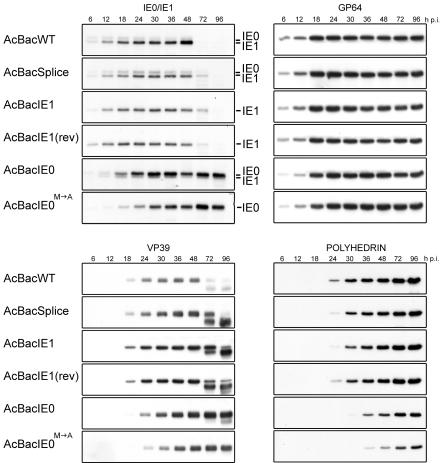
Western blot analyses were performed on specific early and late genes to determine if changes in their expression could be observed in any of the rescue viruses (Fig. 6). Initially, IE0 and IE1 expression by each of the rescue viruses was characterized. In the AcBacWT infection, IE1 and IE0 were detected at 6 hpi. IE0 levels peaked between 18 to 24 hpi, followed by declining levels up to 48 hpi, whereas IE1 levels increased continuously up to 48 hpi, similar to previous analyses (4, 14). AcBacSplice showed similar intensities of IE1 and IE0 at 6 and 12 hpi; however, unlike those produced by AcBacWT, IE0 levels increased until 18 hpi and IE1 levels increase until 24 hpi. Although the intensity of IE1 is still greater than that of IE0, the relative ratio of IE0 to IE1 was higher in AcBacSplice than in AcBacWT. AcBacIE1 and AcBacIE1(rev) also produced protein ratios different from those produced by AcBacWT. The strength of the IE1 signal early in infection was comparable to that produced by AcBacWT; however, increasing levels were only observed up to 36 hpi, after which levels declined. AcBacIE0 expressed IE0 levels comparable to those produced by AcBacWT at 6 and 12 hpi, but unlike AcBacWT, IE0 continued to increase until 30 hpi and maintained steady-state levels to 96 hpi. IE1 expression, which originates from internal initiation, did not increase in AcBacIE0 infection but instead remained at low constant levels from 6 to 96 hpi. Thus, the ratio of IE0 to IE1 produced by AcBacIE0 was very high, in contrast to those produced by both AcBacWT and AcBacSplice. In AcBacIE0M→A infection, expression of IE0 was very low at 6 hpi but continued to increase throughout infection, reaching levels that were much greater than those observed with AcBacWT. Interestingly, the IE0 and IE1 proteins expressed from AcBacWT, AcBacSplice, AcBacIE1, and AcBacIE1(rev) all showed significant amounts of degradation by 72 hpi, presumably because of cell death and lysis. However, this was not observed with IE0 and IE0M→A produced by AcBacIE0 and AcBacIE0 M→A. Even at 96 hpi, IE0 exhibited only minimal degradation while IE0M→A showed none.
FIG. 6.
Western blot time course analysis of IE0, IE1, GP64, VP39, and polyhedrin synthesis in Sf9 cells infected with AcBacWT, AcBacSplice, AcBacIE1, AcBacIE1(rev), AcBacIE0, or AcBacIE0M→A. Each protein was analyzed with a monoclonal antibody specific to that protein. Sf9 cells were infected at an MOI of 5, and at the designated times p.i., cells were harvested and cell lysates were prepared for Western blot analysis. The locations of the IE0- and IE1-specific bands are indicated on the right.
GP64 is a viral fusion protein that is sequestered in the insect cell membrane for subsequent inclusion in the BV envelope. Western blot analysis revealed different GP64 expression profiles for the six viruses. AcBacWT, AcBacSplice, AcBacIE1, and AcBacIE1(rev) had comparable increasing levels from 6 to 18 hpi. After 18 hpi, GP64 remained at approximately the same steady-state levels for AcBacWT and AcBacSplice. AcBacIE1 and AcBacIE1(rev), however, appeared to peak from 18 to 24 hpi and then declined slowly. AcBacIE0 was similar to AcBacWT and reached a peak steady-state level by 24 hpi. In contrast, the level of GP64 produced by AcBacIE0M→A continued to increase throughout the time course. This suggests that IE1 must be present to down regulate or moderate the expression of GP64. The levels of GP64 expression for each of the viruses, however, did not differ as significantly as those of the other proteins analyzed.
Western blot assays were performed with antibodies against nucleocapsid protein VP39 and polyhedrin to determine if late and very late gene expression was affected by the presence or absence of IE0 and IE1 (Fig. 6). VP39 expression was very similar for AcBacWT, AcBacSplice, AcBacIE1, and AcBacIE1(rev). Specifically, VP39 was detected by 18 hpi, after which levels increased until 48 hpi and by 72 hpi degradation of the protein was observed. Although these four viruses gave the same trend, AcBacSplice appears to express VP39 at higher levels than AcBacWT but lower levels than AcBacIE1 and AcBacIE1(rev). VP39 expression in AcBacIE0-infected cells was detected by 18 hpi, but the levels were much lower than those produced by AcBacWT. However, the levels of VP39 continued to increase up to 96 hpi, resulting in higher levels than those produced by AcBacWT. AcBacIE0M→A was similar to AcBacIE0, but the overall levels were lower. This was especially evident at 18 hpi when a longer exposure time is necessary to detect the presence of a band (data not shown). These results demonstrate that expression of only IE1 results in higher levels of VP39 expression with less degradation at late times p.i. and no temporal change in expression. However, when only IE0 is expressed or is the dominant protein, high level expression of VP39 is delayed and little or no degradation is observed at late times p.i.
Analysis of polyhedrin expression showed that in AcBacWT-infected cells polyhedrin could be detected by 24 hpi and levels increased steadily until 96 hpi. The viruses AcBacSplice, AcBacIE1, and AcBacIE1(rev) were essentially equivalent to AcBacWT. AcBacIE0 and AcBacIE0M→A, on the other hand, showed a 6-h delay in the onset of polyhedrin expression. Further, although the levels of polyhedrin did increase over time, they were significantly lower than those produced by AcBacWT. For example, the intensity seen at 24 hpi for AcBacWT was comparable to that seen at 36 hpi for IE0 and at 48 hpi for IE0M→A. This result suggests that IE1, but not IE0, is required for high-level and temporally correct expression of the hyperexpressed very late gene polyhedrin.
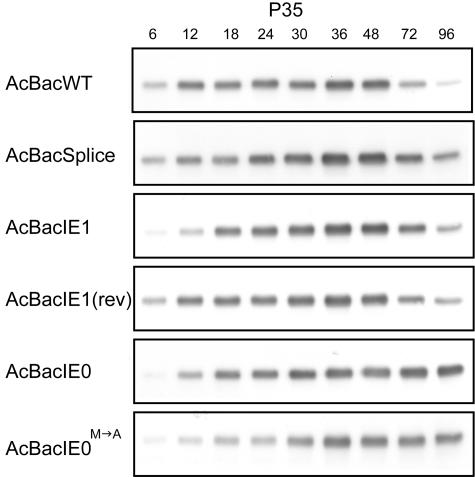
IE1 has been implicated as an inducer of apoptosis in infected cells (31, 32). Altering the expression of IE0 or IE1 could potentially have significant effects on the expression of the AcMNPV apoptosis inhibitor P35. We examined P35 expression for each of the recombinant viruses by Western blot assay (Fig. 7). No significant differences in P35 expression were observed among AcBacWT, AcBacSplice, AcBacIE1, and AcBacIE1(rev). Unlike those in AcBacWT-infected cells, the P35 levels in AcBacIE0- and AcBacIE0M→A-infected cells did not decrease at 72 and 96 hpi. In addition, the overall level of P35 expression was lower in AcBacIE0M→A-infected cells. This suggests that the presence of IE1 may be required for WT levels of P35 expression.
FIG. 7.
Time course analysis of the apoptosis inhibitor P35 by Western blot assay of Sf9 cells infected with AcBacWT, AcBacSplice, AcBacIE1, AcBacIE1(rev), AcBacIE0, or AcBacIE0M→A. P35 was analyzed with a specific monoclonal antibody. Sf9 cells were infected at an MOI of 5, and at the designated times p.i., cells were harvested and cell lysates were prepared for Western blot analysis.
DISCUSSION
Previous studies investigating the genes required for AcMNPV replication have identified immediate-early protein IE1 as being essential for transient DNA replication (15, 20). However, the role of the highly similar spliced gene product IE0 was not taken into account during these assays even though both proteins are always expressed during the infection cycle. A recent study by Huijskens et al. (14) showed that IE0 is the dominate protein at early times p.i. and is capable of activating late gene expression. It was also observed that IE0 and IE1 were mutually antagonistic when coexpressed in transient late gene assays, suggesting that these proteins have significantly different roles during the infection cycle. In addition, it was unknown if either protein alone could support viral replication in virus-infected cells. To address this question and further delineate the roles of IE0 and IE1, we used AcMNPV infectious bacmids to create an ie0-ie1 double knockout (AcBacIE1KO) by deleting the ie1 ORF. We investigated the ability of IE0 or IE1 to individually support AcMNPV infection by reinserting ie0 or ie1 separately into bacmid AcBacIE1KO. AcBacIE1KO did not replicate in insect cells; however, the presence of either IE1 or IE0 supported viral replication and produced both BV and occlusion-derived virus. These results show that the ie0/ie1 gene complex is essential but that either IE0 or IE1 can support viral replication.
Although viruses expressing only IE0 or IE1 produced an infection, they did not do so equally. The infection produced by AcBacIE0M→A, which only expressed IE0, was significantly impaired relative to that produced by AcBacWT. AcBacIE0, which expressed IE0 and very small amounts of IE1 via internal translation initiation, showed reduced levels of polyhedra and BV, but they were intermediate between those of AcBacIE0M→A and AcBacWT. Overall, there was a correlation observed between up regulated levels of IE0 and slow progression to peak levels of DNA replication and late gene protein synthesis and reduced BV and polyhedra.
Viruses expressing only IE1 [AcBacIE1 and AcBacIE1(rev)] produced BV levels similar to those of AcBacWT, but the percentage of cells that produced polyhedra was reduced. The onset of DNA replication and protein synthesis of specific proteins (IE1, GP64, and VP39) in AcBacIE1-infected cells occurred at the same time p.i. as with AcBacWT, but the time required to reach peak levels was approximately 6 h shorter (Fig. 5B) (8). Taken together, these data suggest that rapid expression of IE1 leads to enhanced DNA replication and protein expression but inappropriate conditions for optimal polyhedron production. It has been reported that in vivo, specific tissues such as the midgut, produce little or no polyhedra (8). It will be interesting to determine if the ratio of IE0 to IE1 in these tissues differs relative to that in other tissues that produce high levels of polyhedra.
The results of this study show that there is an intimate relationship between the levels of IE0 and IE1 that is essential for a successful WT infection. Prior to 4 hpi, IE0 levels are twofold higher than those of IE1; however, beyond 4 hpi IE1 becomes the dominant protein, reaching levels 12 times that of IE0 by 48 hpi (14). Even minor changes in the ratio of IE0 to IE1, as was observed with AcBacSplice, can alter the infection process relative to that of the WT virus. Lu et al. (21) recently reported that changes in the IE0-IE1 ratio can also affect the viral host range. They showed that down regulation of the ie0 promoter enables AcMNPV to replicate in the normally nonpermissive SL2 cell line. SL2 cells normally undergo rapid apoptosis upon AcMNPV infection, but in the ie0 down regulated virus, levels of the apoptosis inhibitor P35 were apparently increased. It was therefore surprising for us to observe in this study that viruses that expressed only IE1 did not exhibit significant differences in P35 expression in Sf9 cells (Fig. 7). However, in cells expressing predominately or only IE0 (AcBacIE0 and AcBacIE0M→A) it was observed that levels of P35 expression were decreased at early times but increased continuously throughout infection. These results suggest that IE1 is more important for the early rapid up regulation of this critical gene and that IE0 may inhibit that process. In the study by Lu et al. (21) WT IE0 expression was higher than IE1 expression in SL2 cells, which on the basis of our results would result in lower early P35 expression and potentially increased apoptosis.
Expression of only IE0 prolonged the replication cycle of the virus; that is, DNA replication continued over a longer time period, late gene expression was delayed, and the cells did not die as quickly as determined by trypan blue exclusion (data not shown). Higher levels of IE0 during early times p.i. may provide adequate time for the virus to transcribe IE0-specific genes before IE1 up regulates viral DNA replication and late gene expression. The adenovirus regulatory gene E1A produces two splice variants, 12S and 13S. The 13S protein contains an additional 46-aa domain known as CR1. It has been shown that this unique segment of E1A 13S is necessary for the efficient transcription of viral early genes (for a review, see reference 9). Similarly, IE0 may be efficiently up regulating early genes via its unique 54-aa N-terminal domain.
It is not known if the prolonged-infection phenotype of AcBacIE0M→A translates into an inability to produce a successful systemic infection and subsequent vertical transmission in vivo. However, studies of the function of ie0-ie1 of Lymatria dispar multiple nucleopolyhedrovirus (LdMNPV) suggested that ie0 is the main transcriptional activator and not ie1 (28). LdMNPV has a comparatively slow infection rate in vivo, similar to that of AcBacIE0M→A. It will be interesting to determine if there are higher levels of IE0 than IE1 present during LdMNPV infections and if this is characteristic of other relatively slowly replicating baculoviruses.
IE0 and IE1 are known to form dimers and heterodimers (18, 24, 25). It is possible that IE0 moderates IE1 activity early in infection via dimerization. Therefore, when the ratio of IE0 to IE1 is greater than 1, IE0-IE0 dimers will be more prevalent and IE0-specific transcription will occur. An IE0-to-IE1 ratio of less than 1 would mean that IE1-IE1 dimers would be more prevalent and that IE1-driven transcription would dominate. Therefore, at late times this would also suggest that very few IE0-IE0 dimers would be formed, which could account for the differences seen between AcBacSplice and AcBacIE0. Although both of these viruses expressed IE1 and IE0, the ratios of IE0 to IE1 were significantly different. AcBacSplice, like AcBacWT, maintained an IE0-to-IE1 ratio of less than 1 from 6 hpi, while AcBacIE0 always displayed a ratio of greater than 1. Thus, the infections generated by AcBacIE0 and AcBacSplice more closely resembled those generated by AcBacIE0M→A and AcBacWT, respectively.
In conclusion, this study has shown that both IE0 and IE1 are required for a WT infection, although either protein can support viral replication. In addition, the relative levels of these two proteins are critical to enable WT viral replication to occur. Further studies involving the oral infectivity of our IE1- and IE0M→A-producing constructs are needed to determine the impact of these two proteins during insect infections. Insect studies will provide further insight into the requirement of having both IE1 and IE0 present during AcMNPV infection.
Acknowledgments
We thank Xiaojiang Dai for critical reading of the manuscript and Paul Friesen for the P35 antibody.
Funding for this study was provided by the Biocontrol Network, a Natural Sciences and Engineering Research Council network grant.
REFERENCES
- 1.Blissard, G. W. 1996. Baculovirus-insect cell interactions. Cytotechnology 20:73-93. [DOI] [PubMed] [Google Scholar]
- 2.Chisholm, G. E., and D. J. Henner. 1988. Multiple early transcripts and splicing of the Autographa californica nuclear polyhedrosis virus IE-1 gene. J. Virol. 62:3193-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi, J., and L. A. Guarino. 1995. The baculovirus transactivator IE1 binds to viral enhancer elements in the absence of insect cell factors. J. Virol. 69:4548-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, J., and L. A. Guarino. 1995. Expression of the IE1 transactivator of Autographa californica nuclear polyhedrosis virus during viral infection. Virology 209:99-107. [DOI] [PubMed] [Google Scholar]
- 5.Choi, J., and L. A. Guarino. 1995. A temperature-sensitive IE1 protein of Autographa californica nuclear polyhedrosis virus has altered transactivation and DNA binding activities. Virology 209:90-98. [DOI] [PubMed] [Google Scholar]
- 6.Dai, X., T. M. Stewart, J. A. Pathakamuri, Q. Li, and D. A. Theilmann. 2004. Autographa californica multiple nucleopolyhedrovirus exon0 (orf141), which encodes a RING finger protein, is required for efficient production of budded virus. J. Virol. 78:9633-9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federici, B. A. 1997. Baculovirus pathogenesis, p. 33-60. In L. K. Miller (ed.), Baculovirus. Plenum Publishing Corporation, New York, N.Y.
- 9.Flint, J., and T. Shenk. 1997. Viral transactivating proteins. Annu. Rev. Genet. 31:177-212. [DOI] [PubMed] [Google Scholar]
- 10.Forsythe, I. J., C. E. Shippam, L. G. Willis, S. Stewart, T. Grigliatti, and D. A. Theilmann. 1998. Characterization of the acidic domain of the IE1 regulatory protein from Orgyia pseudotsugata multicapsid nucleopolyhedrovirus. Virology 252:65-81. [DOI] [PubMed] [Google Scholar]
- 11.Friesen, P. D. 1997. Regulation of baculovirus early gene expression, p. 141-166. In L. K. Miller (ed.), Baculovirus. Plenum Publishing Corporation, New York, N.Y.
- 12.Herniou, E. A., J. A. Olszewski, J. S. Cory, and D. R. O'Reilly. 2003. The genome sequence and evolution of baculoviruses. Annu. Rev. Entomol. 48:211-234. [DOI] [PubMed] [Google Scholar]
- 13.Hohmann, A. W., and P. Faulkner. 1983. Monoclonal antibodies to baculovirus structural proteins: determination of specificities by Western blot analysis. Virology 125:432-444. [DOI] [PubMed] [Google Scholar]
- 14.Huijskens, I., L. Li, L. G. Willis, and D. A. Theilmann. 2004. Role of AcMNPV IE0 in baculovirus very late gene activation. Virology 323:120-130. [DOI] [PubMed] [Google Scholar]
- 15.Kool, M., C. H. Ahrens, R. W. Goldbach, G. F. Rohrmann, and J. M. Vlak. 1994. Identification of genes involved in DNA replication of the Autographa californica baculovirus. Proc. Natl. Acad. Sci. USA 91:11212-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovacs, G. R., L. A. Guarino, B. L. Graham, and M. D. Summers. 1991. Identification of spliced baculovirus RNAs expressed late in infection. Virology 185:633-643. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs, G. R., L. A. Guarino, and M. D. Summers. 1991. Novel regulatory properties of the IE1 and IE0 transactivators encoded by the baculovirus Autographa californica multicapsid nuclear polyhedrosis virus. J. Virol. 65:5281-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer, A., and D. Knebel-Morsdorf. 1998. The early baculovirus he65 promoter: on the mechanism of transcriptional activation by IE1. Virology 249:336-351. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Lu, A., P. J. Krell, J. M. Vlak, and G. F. Rohrmann. 1997. Baculovirus DNA replication, p. 171-192, In L. K. Miller (ed.), The baculoviruses. Plenum Press, New York, N.Y.
- 21.Lu, L., Q. Du, and N. Chejanovsky. 2003. Reduced expression of the immediate-early protein IE0 enables efficient replication of Autographa californica multiple nucleopolyhedrovirus in poorly permissive Spodoptera littoralis cells. J. Virol. 77:535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luckow, V. A., S. C. Lee, G. F. Barry, and P. O. Olins. 1993. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 67:4566-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okano, K., V. S. Mikhailov, and S. Maeda. 1999. Colocalization of baculovirus IE-1 and two DNA-binding proteins, DBP and LEF-3, to viral replication factories. J. Virol. 73:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson, V. A., J. A. Wetter, and P. D. Friesen. 2002. Baculovirus transregulator IE1 requires a dimeric nuclear localization element for nuclear import and promoter activation. J. Virol. 76:9505-9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson, V. A., J. A. Wetter, and P. D. Friesen. 2003. The highly conserved basic domain I of baculovirus IE1 is required for hr enhancer DNA binding and hr-dependent transactivation. J. Virol. 77:5668-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passarelli, A. L., and L. K. Miller. 1993. Three baculovirus genes involved in late and very late gene expression: ie-1, ie-n, and lef-2. J. Virol. 67:2149-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathakamuri, J. A., and D. A. Theilmann. 2002. The acidic activation domain of the baculovirus transactivator IE1 contains a virus-specific domain essential for DNA replication. J. Virol. 76:5598-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson, M. N., and G. F. Rohrmann. 1997. Splicing is required for transactivation by the immediate early gene 1 of the Lymantria dispar multinucleocapsid nuclear polyhedrosis virus. Virology 235:153-165. [DOI] [PubMed] [Google Scholar]
- 29.Pearson, M. N., R. L. Russell, and G. F. Rohrmann. 2002. Transcriptional mapping of two genes encoding baculovirus envelope-associated proteins. J. Gen. Virol. 83:937-943. [DOI] [PubMed] [Google Scholar]
- 30.Pearson, M. N., R. L. Russell, G. F. Rohrmann, and G. S. Beaudreau. 1988. p39, a major baculovirus structural protein: immunocytochemical characterization and genetic location. Virology 167:407-413. [PubMed] [Google Scholar]
- 31.Prikhod'ko, E. A., and L. K. Miller. 1999. The baculovirus PE38 protein augments apoptosis induced by transactivator IE1. J. Virol. 73:6691-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prikhod'ko, E. A., and L. K. Miller. 1996. Induction of apoptosis by baculovirus transactivator IE1. J. Virol. 70:7116-7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quant, R. L., M. N. Pearson, G. F. Rohrmann, and G. S. Beaudreau. 1984. Production of polyhedrin monoclonal antibodies for distinguishing two Orgyia pseudotsugata baculoviruses. Appl. Environ. Microbiol. 48:732-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodems, S. M., S. S. Pullen, and P. D. Friesen. 1997. DNA-dependent transregulation by IE1 of Autographa californica nuclear polyhedrosis virus: IE1 domains required for transactivation and DNA binding. J. Virol. 71:9270-9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross, L., and L. A. Guarino. 1997. Cycloheximide inhibition of delayed early gene expression in baculovirus-infected cells. Virology 232:105-113. [DOI] [PubMed] [Google Scholar]
- 36.Slack, J. M., and G. W. Blissard. 1997. Identification of two independent transcriptional activation domains in the Autographa californica multicapsid nuclear polyhedrosis virus IE1 protein. J. Virol. 71:9579-9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theilmann, D. A., and S. Stewart. 1991. Identification and characterization of the IE-1 gene of Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. Virology 180:492-508. [DOI] [PubMed] [Google Scholar]
- 38.Theilmann, D. A., L. G. Willis, B. J. Bosch, I. J. Forsythe, and Q. Li. 2001. The baculovirus transcriptional transactivator ie0 produces multiple products by internal initiation of translation. Virology 290:211-223. [DOI] [PubMed] [Google Scholar]
- 39.Volkman, L. E., and P. A. Goldsmith. 1982. Generalized immunoassay for Autographa californica nuclear polyhedrosis virus infectivity in vitro. Appl. Environ. Microbiol. 44:227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]