Abstract
This study aimed to evaluate the clinical efficacy of hepatic arterial infusion chemotherapy (HAIC) combined with lenvatinib and PD1 inhibitors vs. transarterial chemoembolization (TACE) combined with lenvatinib and PD1 inhibitors in the treatment of unresectable hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT) and artery-portal shunts (APFs). HCC Patients with PVTT and APFs who received HAIC in combination with PD1 inhibitor or TACE in combination with lenvatinib and PD1 inhibitor from March 2019 to May 2023 in Zhongshan People’s Hospital were included. The objective response rate (ORR), disease control rate (DCR), median overall survival (mOS), median progression-free survival (mPFS), median duration of response (mDOR), and adverse events (AEs) were assessed. A total of 95 patients were enrolled in this study, including 34 cases in the HAIC+L+P group and 61 cases in the TACE+L+P group. According to the RECIST1.1, the ORR was 52.9% and 27.9%, and the DCR was 100% and 88.5%, respectively (P values =0.03 and < 0.001, respectively). The mOS of HAIC+L+P group and TACE+L+P group were 25.00 and 19.30 months, respectively (P=0.035). The mPFS of the two groups were 21.74 and 8.74 months, respectively (P=0.0066). The mDOR of the two groups was 20.43 and 9.13 months, respectively (P=0.067). Compared with TACE in combination with lenvatinib and PD-1 inhibitors, HAIC (FOLFOX) in combination with lenvatinib and PD-1 inhibitors can improve tumor response and prolong OS, PFS, and DOR in HCC patients with PVTT and APFs.
Keywords: Hepatic arterial infusion chemotherapy, transarterial chemoembolization, hepatocellular carcinoma, targeted therapy, immunotherapy
Introduction
The global cancer incidence and mortality is increasing rapidly, reflecting the aging and growth of the population. China, the world’s most populous country, is also undisputedly the leading country in cancer incidence and cancer deaths. According to GLOBOCAN 2020 statistics, there are 910,000 new cases of liver cancer and 830,000 deaths worldwide, making it the seventh most common cancer and the third leading cause of tumor-related death worldwide. East Asia, especially China, Japan and Korea, are the regions with high incidence of liver cancer [1]. The proportion of patients with hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT) in China is 44%-62.2% [2]. PVTT has been considered as an independent risk factor for poor prognosis in HCC patients [3]. Hepatic artery-portal shunts (APFs) are present in 27.0-63.2% of patients with advanced HCC, which may be caused by PVTT [4]. APFs increase the risk of serious complications, such as esophageal varices, ascites, and hepatic encephalopathy [5], which severely affects the prognosis and survival of patients.
Transarterial chemoembolization (TACE) is an important guideline-recommended treatment for HCC [6]. TACE can block APFs with embolic materials (e.g., gelatin sponges, polyvinyl alcohol, microspheres). However, embolic material may block the portal vein branches through the fistula and impair liver function or cause ectopic embolism. Tumor tissue hypoxia after TACE may also induce tumor angiogenesis, leading to tumor recurrence and progression [7]. For advanced HCC with vascular invasion or multiple intrahepatic lesions, hepatic arterial infusion chemotherapy (HAIC) is recommended for its definite efficacy in clinical practice, and the Japan Society of Hepatology (JSH) directly recommend HAIC as the first-line treatment for HCC patients with PVTT [8]. A recent prospective non-randomized study showed that HAIC (FOLFOX) was more effective than TACE for advanced HCC [9]. However, both of these, as palliative treatments, are difficult to achieve complete tumor necrosis and are ineffective against extrahepatic metastases.
Treatments for advanced HCC have evolved rapidly with the development of systemic therapies. Lenvatinib is a first-line therapeutic agent for advanced HCC and has superior efficacy to sorafenib in terms of tumor response and progression-free survival (PFS) [6]. In recent years, immunotherapies, such as programmed death receptor 1 (PD-1) inhibitors, have received much attention from oncology researchers. In a phase Ib clinical trial, lenvatinib combined with PD-1 inhibitors was effective in treating advanced HCC, with a median overall survival (mOS) of 22.0 months [10].
Single treatment modality for advanced HCC brings unsatisfactory results, and local treatment combined with systemic treatment modality is the new trend for the treatment of advanced HCC in the future. Therefore, we believe that HAIC (FOLFOX) combined with lenvatinib and PD-1 inhibitors is expected to achieve better survival benefit in HCC patients with PVTT and APFs. In this study, we retrospectively analyzed and compared the efficacy and safety between HAIC (FOLFOX) + lenvatinib + PD-1 inhibitor and TACE + lenvatinib + PD-1 inhibitor in HCC patients with PVTT and APFs.
Materials and methods
Patient selection
We retrospectively analyzed patients treated at Zhongshan People’s Hospital for HCC with PVTT and APFs from March 2019 to May 2022, and the screening process is shown in Figure 1. A total of 95 patients were eventually enrolled in this study, stratified into 34 patients treated with HAIC (FOLFOX) + lenvatinib + PD-1 inhibitor (HAIC+L+P group) and 61 patients treated with TACE + lenvatinib + PD-1 inhibitor (TACE+L+P group).
Figure 1.
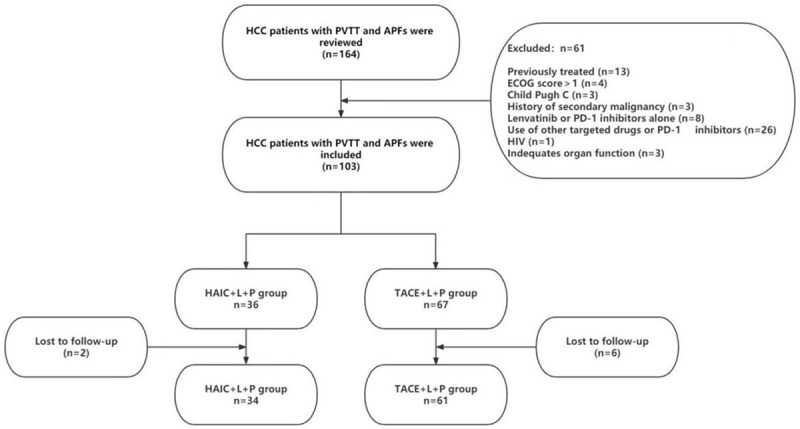
Flow diagram of HCC patients with PVTT and APFs who underwent HAIC combined with lenvatinib plus PD-1 inhibitors or TACE combined with lenvatinib plus PD-1 inhibitors. HCC: hepatocellular carcinoma; PVTT: portal vein tumor thrombosis; APFs: artery-portal shunts; HAIC: hepatic arterial infusion chemotherapy; TACE: transarterial chemoembolization.
Inclusion criteria: ① confirmed diagnosis of HCC with PVTT and APFs, and no previous treatment for HCC; ② age > 18 years; ③ Child-pugh grade A or B; ④ Eastern Cooperative Oncology Group (ECOG) score 0-1; ⑤ white blood cell count > 3×109/L, absolute neutrophil count > 1.5×109/L, platelet count > 30×109/L, and hemoglobin concentration > 85 g/L; ⑥ complete records with outcomes evaluation of HAIC or TACE treatment after surgery.
Exclusion criteria: ① contraindication to angiography or arterial puncture; ② uncontrolled infection around the lesion or systemic infection; ③ severe hepatic, renal, cardiopulmonary insufficiency; ④ history of other malignancies; ⑤ known history of human immunodeficiency virus (HIV) infection; ⑥ inability to tolerate or comply with treatment due to personal reasons.
The study was conducted in accordance with the Declaration of Helsinki and other ethical principles for medical research involving human subjects, and was approved by the Clinical Research and Application Ethics Committee of Zhongshan People’s Hospital.
Treatments
TACE treatment was performed as follows, using a modified Seldinger technique for percutaneous femoral artery puncture placement. Angiography of the celiac trunk, superior mesenteric artery, phrenic artery and other vessels was performed to find the blood vessels supplying the tumor. Depending on whether the microcatheter could pass through the APFs area, embolization was performed as follows: ① If the microcatheter could pass through the APFs area, the tumor supply artery was first embolized with an emulsion mixture of oxaliplatin 50 mg, pirarubicin 30 mg, and lipiodol ultra fluid (total amount < 20 ml) of chemotherapeutic drugs. After chemoembolization of the tumor was completed, the microcatheter was returned to the APFs area, and the fistula was embolized with microspheres or polyvinyl alcohol. ② If the microcatheter could not pass through the APFs area, simultaneous chemoembolization was performed after identifying the tumor blood supply vessels and APFs, and the chemoembolization drugs were the same as above. After embolization, the catheter was removed, and the wound was pressed to stop bleeding, and then bandaged. The periodicity of patients receiving TACE was determined by clinical need, with an interval of at least 3-4 weeks.
HAIC was carried out as follows, using a modified Seldinger technique for percutaneous femoral artery (radial artery or distal radial artery) puncture placement. ① If the intrahepatic tumor was supplied by a separate left or right hepatic artery, the microcatheter was inserted into the corresponding target vessel. ② If the intrahepatic tumor was supplied by the left and right hepatic arteries, the microcatheter was inserted into the proper hepatic artery. ③ If the intrahepatic tumor was supplied by the left and right hepatic arteries and cannot avoid the gastroduodenal artery, the gastroduodenal artery was embolized with a coil, and the microcatheter was inserted into the proper hepatic artery. In cases where a small portion of the blood supply to the tumor was derived some of the arteries (phrenic artery, intercostal artery, etc.), complete embolization of the target vessel was performed using embolic material. The FOLFOX regimen chemotherapy infusion doses were as follows: 85 mg/m2 of oxaliplatin arterial drip for 2 hours, 400 mg/m2 of calcium folinic acid intravenous drip for 1 hour, then combined with 5-fluorouracil 400 mg/m2 arterial drip for 1 hour and 2400 mg/m2 of 5-fluorouracil arterial drip for 23 hours. This was repeated every 3-4 weeks. If the tumor shrunk significantly after HAIC treatment, the dose of chemotherapy drugs could be reduced appropriately.
Lenvatinib was started on the third postoperative day and was administered 8 mg (when weight < 60 kg) or 12 mg (when weight ≥ 60 kg) orally once daily. If grade 3 or 4 treatment-related adverse events (TRAEs) occurred, the dose of lenvatinib was reduced to a dose of 8 mg per day (when weighing more than 60 kg) or 4 mg per day (when weighing less than 60 kg). PD-1 inhibitor was started on the third postoperative day. PD-1 inhibitors were administered with sintilimab injection, which was administered as 200 mg intravenously once every 3 weeks. Corticosteroids were used when severe TRAEs occurred. Lenvatinib and/or sintilimab injection was discontinued when grade 3 or 4 TRAEs still persisted. Dose may be resumed when toxicity diminished or when the treatment was tolerated by the patient (at the discretion of the investigator).
Data extraction
The follow-up termination date of this study was April 30, 2023. The follow-up interval was 3-6 weeks, and each follow-up visit included medical history, physical examination, CT/MRI enhanced scan of the abdomen, routine blood, routine urine, routine stool, liver and kidney function, coagulation function, thyroid function, blood glucose, and tumor-related markers. For the presence of extrahepatic metastasis, CT or MRI scan of the corresponding site was also required.
Efficacy and safety evaluation
The major study endpoint of this study was overall survival (OS), and the secondary study endpoints were progression-free survival (PFS), duration of response (DOR), objective response rate (ORR), disease control rate (DCR), and TRAEs. Tumor response was assessed independently by two physicians with more than 10 years of experience in diagnosing abdominal imaging, and consensus was reached through discussion if they had different views. Tumor response was assessed using RECIST1.1 and mRECIST [11,12], and was classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The ORR was defined as the proportion of patients who achieved CR and PR. The DCR was defined as the proportion of patients who did not show disease progression. OS was defined as the time from treatment initiation to death from any cause. DOR was defined as the time interval from the start of response (when CR or PR was first identified) to progression or death (whichever occurred first). PFS was defined as the time from the start of treatment to the first occurrence of PD or death (whichever occurred first). The TRAEs occurring during patient follow-up were recorded and evaluated using the Common Terminology Criteria of Adverse Events version 5.0 (CTCAE). Grade 1: mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated. Grade 2: moderate; requiring minor, local or non-invasive treatment, age-appropriate instrumental limitation of activities of daily living. Grade 3: severe or medically significant but not immediately life-threatening, resulting in hospitalization or prolonged hospitalization; disability; spontaneous limitation of activities of daily living. Grade 4: life-threatening consequences; urgent intervention indicated. Grade 5: death related to AE [13].
Statistical analysis
The statistical software used in this study was SPSS 20.0 and R 4.3.1. Normal distribution measurements were expressed as mean ± standard deviation, and counts were expressed as frequencies. The baseline data of patients and tumor response in the HAIC+L+P group were compared with those in the TACE+L+P group using the χ2 test or Fisher exact probability test. OS, PFS and DOR were compared between groups using the Kaplan-Meier method of log-rank test. The Cox’s proportional hazards regression model was used to analyze the possible influencing factors of OS and PFS, and the variables with P < 0.10 in the univariate analysis were included in the multivariate analysis. The results of the COX subgroup analysis were represented by forest plots. The differences were considered statistically significant at P < 0.05.
Results
Basic patient information
Baseline information of patients in both groups included gender, age, presence of hepatitis B, cirrhosis, ECOG score, Child Pugh grade, maximum tumor diameter, number of tumors, tumor capsule, alpha-fetoprotein (AFP), staging of PVTT [14], hepatic vein invasion, presence of extrahepatic metastases, and lymph node metastases (Table 1). The median follow-up time of patients in HAIC+L+P group was 24.60 months and received 4.43 (3.0-6.0) HAIC treatments; the median follow-up of patients in the TACE+L+P group was 38.50 months and received 3.58 (2.0-7.0) TACE treatments.
Table 1.
Baseline characteristics
| Characteristics | HAIC+L+P Group | TACE+L+P Group | X2 Value | P Value |
|---|---|---|---|---|
| Gender | - | 0.04 | ||
| Male | 27 | 58 | ||
| Female | 7 | 3 | ||
| Age | 4.04 | 0.04 | ||
| < 50 | 16 | 15 | ||
| ≥ 50 | 18 | 46 | ||
| HBsAg | - | 0.70 | ||
| Positive | 31 | 57 | ||
| Negative | 3 | 4 | ||
| Liver cirrhosis | 0.325 | 0.568 | ||
| Yes | 21 | 34 | ||
| No | 13 | 27 | ||
| ECOG | 1.83 | 0.18 | ||
| 0 | 10 | 28 | ||
| 1 | 24 | 33 | ||
| Child Pugh | 1.08 | 0.30 | ||
| A | 25 | 37 | ||
| B | 9 | 24 | ||
| Maximum tumor diameter | - | 0.65 | ||
| < 5 cm | 3 | 8 | ||
| 5-10 cm | 12 | 25 | ||
| > 10 cm | 19 | 28 | ||
| Tumor capsule | 5.81e-31 | 1.00 | ||
| Complete | 15 | 27 | ||
| Incomplete | 19 | 34 | ||
| AFP | 5.19 | 0.02 | ||
| < 400 ng/ml | 11 | 36 | ||
| ≥ 400 ng/ml | 23 | 25 | ||
| Number of tumors | 3.55e10-31 | 1.00 | ||
| ≤ 3 | 13 | 24 | ||
| > 3 | 21 | 37 | ||
| PVTT | 1.62 | 0.45 | ||
| Vp2 | 10 | 26 | ||
| Vp3 | 13 | 19 | ||
| Vp4 | 11 | 16 | ||
| Hepatic vein invasion | 0.26 | 0.61 | ||
| Yes | 5 | 13 | ||
| No | 29 | 48 | ||
| Extrahepatic metastases | 0.50 | 0.48 | ||
| Yes | 9 | 11 | ||
| No | 25 | 50 | ||
| Lymph node metastases | 0.49 | 0.49 | ||
| Yes | 6 | 16 | ||
| No | 28 | 45 |
ECOG: Eastern Cooperative Oncology Group; PVTT: portal vein tumor thrombus; Vp2: the presence of a PVTT in the second-order branches of the portal vein; Vp3: the presence of a PVTT in the first-order branches of the portal vein; Vp4: the presence of a PVTT in the main trunk of the portal vein or a contralateral portal vein branch or both.
Tumor response
The number of cases of CR, PR, SD and PD in the HAIC+L+P group was 14 (41.2%), 14 (41.2%), 6 (17.6%) and 0 cases, respectively, and the number of cases of CR, PR, SD and PD in the TACE+L+P group was 14 (22.9%), 28 (45.9%), 12 (19.7%) and 7 (11.5%) cases, respectively, when the tumor response was evaluated using mRECIST. The ORR was 82.4% and 68.8% (P=0.23), and DCR was 100% and 88.5% (P < 0.001) in the HAIC+L+P and TACE+L+P groups, respectively, and the difference in DCR was statistically significant. The number of cases of CR, PR, SD and PD in the HAIC+L+P group was 0, 18 (52.9%), 16 (47.1%) and 0 cases, respectively, and the number of cases of CR, PR, SD and PD in the TACE+L+P group was 0, 17 (27.9%), 37 (60.6%) and 7 (11.5%) cases, respectively, when the tumor response was evaluated using RECIST1.1. The ORR was 52.9% and 27.9% (P=0.03), and DCR was 100% and 88.5% (P < 0.001) in the HAIC+L+P and TACE+L+P groups, respectively, and the differences were statistically significant (Table 2).
Table 2.
Treatment response as assessed by imaging features according to the mRECIST and Recist1.1 criteria in two groups
| Treatment response | mRECIST | RECIST1.1 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Group. No. (%) | Group. No. (%) | |||||
|
|
|
|||||
| HAIC | TACE | P value | HAIC | TACE | P value | |
| CR | 14 | 14 | - | 0 | 0 | - |
| PR | 14 | 28 | - | 18 | 17 | - |
| SD | 6 | 12 | - | 16 | 37 | - |
| PD | 0 | 7 | - | 0 | 7 | - |
| ORR, % | 82.4 | 68.9 | 0.23 | 52.9 | 27.9 | 0.03 |
| DCR, % | 100.0 | 88.5 | < 0.001 | 100.0 | 88.5 | < 0.001 |
CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; ORR: objective response rate; DCR: disease control rate.
OS, PFS, DOR and factors influencing OS and PFS
By the end of follow-up, mOS was 25.00 (95% CI 15.30-not reached) and 19.30 (95% CI 9.87-20.60) months in the HAIC+L+P and TACE+L+P groups, respectively, and the difference was statistically significant (P=0.035) (Figure 2A). Subgrouping of PVTT was performed, in which patients with VP3 and VP4 had mOS of 15.78 (95% CI 10.7-not reached) and 8.33 (95% CI 7.20-19.40) months, respectively, with a statistically significant difference (P=0.017) (Figure 2B). The mPFS in the HAIC+L+P and TACE+L+P groups was 21.74 (95% CI 10.01-not reached) and 8.74 (95% CI 6.34-13.60) months, respectively, and the difference was statistically significant (P=0.0066) (Figure 2C). The mDOR in the HAIC+L+P and TACE+L+P groups was not reached (95% CI 13.00-not reached) and 7.57 (95% CI 5.93-22.50) months, respectively, as assessed by mRECIST, and the difference was statistically significant (P=0.013) (Figure 2D). The mDOR in the HAIC+L+P and TACE+L+P groups was 20.43 (95% CI 13.00-not reached) and 9.13 (95% CI 7.40-24.30) months, respectively, as assessed by RECIST1.1, and the difference was not statistically significant (P=0.067).
Figure 2.
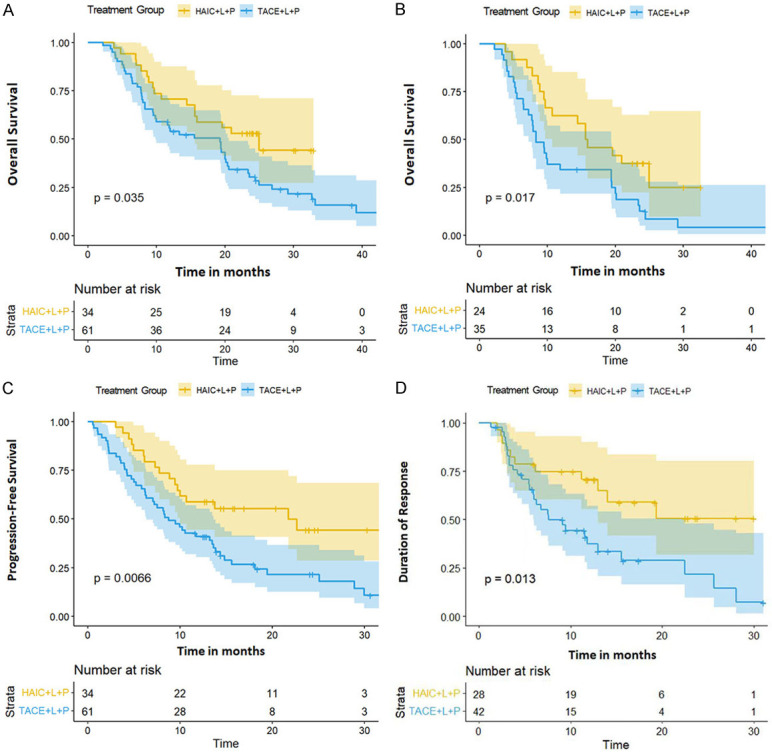
A. The mOS was 25.00 (95% CI 15.30-not reached) and 19.30 (95% CI 9.87-20.60) months; B. The mOS (VP3 and VP4) was 15.78 (95% CI 10.7-not reached) and 8.33 (95% CI 7.20-19.40) months; C. The mPFS was 21.74 (95% CI 10.01-not reached) and 8.74 (95% CI 6.34-13.60) months; D. The mDOR (mRecist) was not reached (95% CI 13.00-not reached) and 7.57 (95% CI 5.93-22.50) months. mOS: mean overall survival; mPFS: median progression-free survival; mDOR: median duration of response.
Univariate analysis of factors affecting OS: ECOG score 1 (HR 1.92, 95% Cl, 1.15-3.20, P=0.01), presence of extrahepatic metastases (HR 1.99, 95% Cl, 1.12-3.53, P=0.02), presence of cirrhosis (HR 1.86, 95% CI, 1.10-3.13, P=0.02), and PVTT (VP3 and VP4) (HR 2.14, 95% CI, 1.57-2.92, P < 0.001) were independent risk factors; multifactorial COX subgroup analysis is shown in Figure 3.
Figure 3.
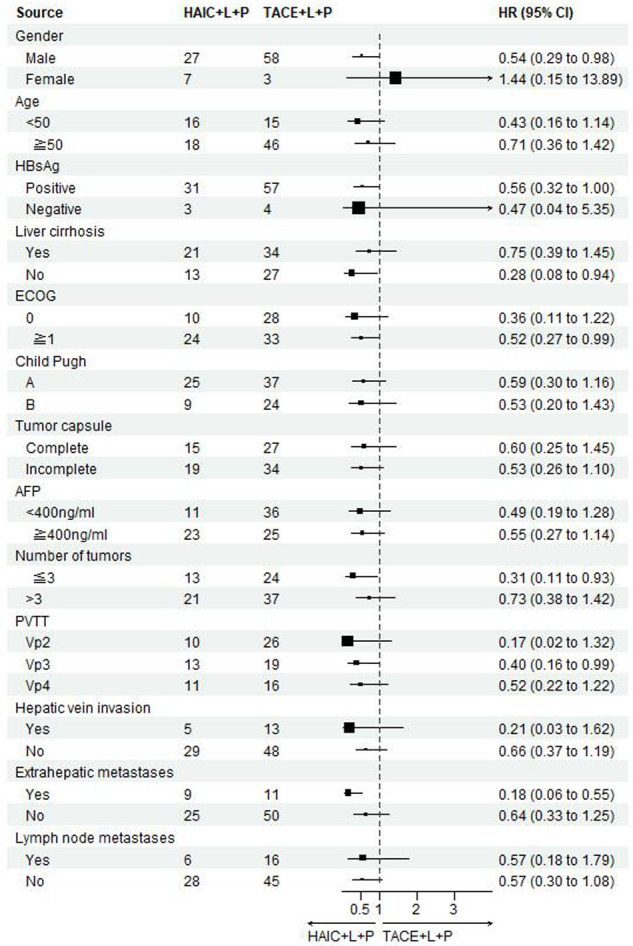
Forest plot of OS for subgroups in patients receiving HAIC combined with lenvatinib plus PD-1 inhibitors or TACE combined with lenvatinib plus PD-1 inhibitors. OS: overall survival; HAIC: hepatic arterial infusion chemotherapy; TACE: transarterial chemoembolization.
Univariate analysis of factors affecting PFS: ECOG score 1 (HR 1.93, 95% Cl, 1.16-3.23, P=0.01), presence of cirrhosis (HR 2.14, 95% CI, 1.27-3.61, P=0.003), and PVTT (VP3 and VP4) (HR 1.98, 95% CI, 1.46-2.70, P < 0.001) were independent risk factors; a multifactorial COX subgroup analysis is shown in Figure 4.
Figure 4.
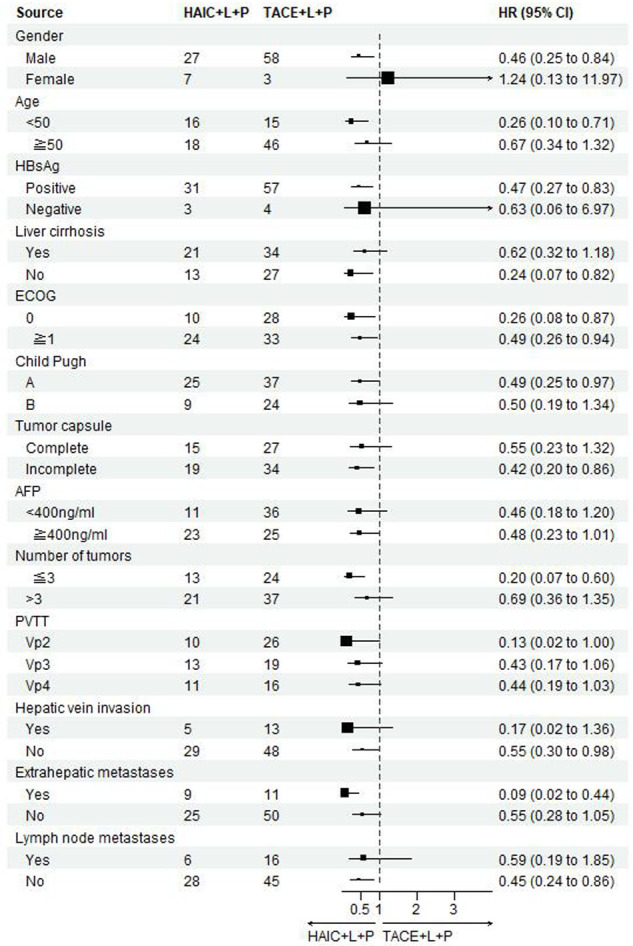
Forest plot of PFS for subgroups in patients receiving HAIC combined with lenvatinib plus PD-1 inhibitors or TACE combined with lenvatinib plus PD-1 inhibitors. PFS: progression-free survival; HAIC: hepatic arterial infusion chemotherapy; TACE: transarterial chemoembolization.
Treatment safety
The occurrence of relevant AEs in patients is shown in Table 3. None of the patients experienced treatment-related grade 4 or 5 AEs. Nausea and vomiting were more common in the HAIC+L+P group (χ2=4.612a, P=0.032) and leukopenia was more likely to occur (χ2=4.383a, P=0.036). In contrast, elevated bilirubin was more common in the TACE+L+P group (χ2=4.065a, P=0.044). The incidence of grade 3 adverse events between the two groups was 38.2% and 37.7%, respectively, with no statistically significant difference (χ2=0.003a, P=0.959). During the treatment period, three patients in the TACE+L+P group reduced their lenvatinib dose, compared with one patient in the HAIC+L+P group. No patients reduced or discontinued PD-1 inhibitors.
Table 3.
Patient treatment-related adverse events
| Adverse Events | Any level of adverse event | Grade 3 adverse events | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| TACE + Lenvatinib + PD-1 (n=61) | HAIC + Lenvatinib + PD1 (n=34) | X2 Value | P Value | TACE + Lenvatinib + PD-1 (n=61) | HAIC + Lenvatinib + PD-1 (n=34) | X2 Value | P Value | |
| Leukopenia | 10 (16.4%) | 12 (35.3%) | 4.383 | 0.036 | 0 | 2 (5.9%) | - | 0.126 |
| Thrombocytopenia | 6 (9.8%) | 7 (20.6%) | 2.137 | 0.144 | 0 | 2 (5.9%) | - | 0.126 |
| Rash | 8 (13.1%) | 5 (14.7%) | 0.047 | 0.829 | 0 | 0 | - | - |
| Itchy skin | 6 (9.8%) | 4 (11.8%) | - | 0.742 | 0 | 0 | - | - |
| Hand-foot syndrome | 20 (32.8%) | 10 (29.4%) | 0.115 | 0.734 | 2 (3.3%) | 0 | - | 0.535 |
| ALT and AST level increase | 38 (62.3%) | 15 (44.1%) | 2.619 | 0.106 | 0 | 0 | - | - |
| Serum bilirubin increase | 25 (41.0%) | 7 (20.6%) | 4.065 | 0.044 | 4 (6.6%) | 1 (2.9%) | - | 0.652 |
| Diarrhea | 8 (13.1%) | 5 (14.7%) | 0.047 | 0.829 | 2 (3.3%) | 1 (2.9%) | - | 1.000 |
| Nausea/Vomit | 14 (23.0%) | 15 (44.1%) | 4.612 | 0.032 | 0 | 0 | - | - |
| Proteinuria | 14 (23.0%) | 7 (20.6%) | 0.071 | 0.790 | 0 | 0 | - | - |
| Hypothyroidism | 15 (24.6%) | 9 (26.5%) | 0.041 | 0.840 | 1 (1.6%) | 0 | 1.000 | |
| Gastrointestinal bleeding | 6 (9.8%) | 2 (5.9%) | - | 0.707 | 6 (9.8%) | 2 (5.9%) | - | 0.707 |
| Stomach ache | 26 (42.6%) | 11 (32.4%) | 0.968 | 0.325 | 4 (6.6%) | 2 (5.9%) | - | 1.000 |
| Hair loss | 6 (9.8%) | 4 (11.8%) | - | 0.742 | 0 | 0 | - | - |
| Weight decreased | 10 (16.4%) | 5 (14.7%) | 0.047 | 0.829 | 0 | 0 | - | - |
| Decrease appetite | 24 (39.3%) | 16 (47.1%) | 0.533 | 0.465 | 0 | 0 | - | - |
| Fatigue | 26 (42.6%) | 13 (38.2%) | 0.174 | 0.677 | 0 | 0 | - | - |
| Hypertension | 23 (37.7%) | 14 (41.2%) | 0.111 | 0.739 | 4 (6.6%) | 3 (8.8%) | - | 0.698 |
ALT: Alanine aminotransferase; AST: Aspartate transaminase.
Discussion
PVTT is one of the common complications of HCC, and most patients with advanced HCC already have PVTT in the main trunk or branches of the portal vein at the time of initial diagnosis. HCC patients with PVTT usually have a poor prognosis and a high mortality rate. The mOS of HCC patients with PVTT under best supportive care (BSC) was only 2.7-4.0 months, compared to 10.0-24.0 months in HCC patients without PVTT [15]. APFs are mainly caused by HCC progression and direct invasion of portal vein branches, resulting in direct injection of blood from higher pressure hepatic arteries into lower pressure portal veins, which alters normal hemodynamics in the liver and increases the risk of tumor metastasis, portal hypertension, gastrointestinal bleeding, and intractable ascites, severely affecting the quality of patient survival [5]. Currently, there is still no international consensus on the treatment criteria for HCC combined with PVTT, and European and American guidelines for HCC use the Barcelona liver cancer staging (BCLC) as the standard, classifying HCC combined with PVTT as a progressive stage (BCLC stage C), and systemic therapy is recommended as the first-line treatment for patients in this stage [16]. In this regard, experts from China, Japan, and related Southeast Asian countries have different opinions and believe that local treatment (e.g., surgery, HAIC, TACE, and radiation therapy) combined with multiple systemic therapies can achieve more satisfactory results.
The results of this study showed that mOS and mPFS were significantly higher in the HAIC+L+P group than in the TACE+L+P group, and the ORR (Recist1.1 and mRecist) were also longer in the HAIC+L+P group than in the TACE+L+P group. These results suggest that HAIC+L+P is more effective in the treatment of HCC patients with PVTT and APFs, which improves patient response to oncologic therapy and survival prognosis.
TACE is a common method to treat unresectable HCC. For HCC patients with APFs, embolization materials can be used to embolize the fistula, and commonly used embolization agents include gelatin sponges, polyvinyl alcohol, microspheres, and coils. However, embolic materials may block the portal vein branches through the fistula and impair liver function or cause ectopic embolism, further aggravating liver function damage and affecting the survival prognosis of patients. Recent studies have shown that HAIC (FOLFOX) can result in a favorable tumor response in HCC patients with PVTT [17]. HAIC is a therapy with continuous infusion of chemotherapeutic agents via the hepatic artery, which allows high-dose chemotherapy to be delivered directly to the tumor through the hepatic artery to improve ORR while reducing systemic drug concentrations to minimize toxicity. HAIC does not use embolic material to embolize tumor vessels or APFs, mitigating the risk of hepatic impairment and ectopic embolism. The results of a prospective randomized controlled study mentioned above showed that HAIC (FOLFOX) in combination with sorafenib was significantly more effective than sorafenib. The OS was 13.37 months and 7.13 months (P < 0.001), PFS was 7.03 months and 2.60 months (P < 0.001), and ORR was 40.80% and 2.46% (P < 0.001) in the combination treatment group and sorafenib group, respectively. Sixteen patients in the combination treatment group underwent radical surgical resection, while only one patient in the sorafenib group underwent radical surgical resection. The surgical conversion success rates were 12.8% and 0.8% in the two groups, respectively (P < 0.001). The guidelines in Japan and the Consensus in Taiwan have recommended HAIC as one of the treatment options for HCC patients with PVTT of VP3 and VP4 [8,18].
In recent years, targeted therapy combined with immunotherapy has achieved good results in the treatment of advanced HCC. In a phase III IMBrave150 clinical trial of an atezolizumab in combination with bevacizumab versus sorafenib for advanced HCC [20], the ORR in the combination group reached 30%, much higher than that in the sorafenib group, while the risk of death and disease progression were reduced by 35% and 34%, respectively. In a recent phase III LEAP-002 clinical study of lenvatinib in combination with pembrolizumab versus lenvatinib for advanced HCC [19], the mOS was 21.1 months and 19.0 months (95% CI, 0.708-0.997), PFS was 8.2 months and 8.0 months (95% CI, 0.734-1.024) in the two groups, respectively. Although the improvement in OS and PFS did not reach the prespecified statistical difference in superiority, in the Asian population subgroup, OS and PFS were further prolonged, with mOS of 26.3 and 22.4 months (95% CI, 0.552-0.958), and PFS of 8.3 and 6.5 months (95% CI, 0.556-0.907), respectively. It was suggested that targeted therapy combination immunotherapy showed better anti-tumor effect and better survival benefit in Asian population. In a phase II/III clinical study of sindilizumab combined with bevacizumab versus sorafenib for unresectable HCC among Chinese population (ORIENT-32 study) [20], the efficacy in the combination group was significantly superior to that of the sorafenib group, with median OS of NE and 10.4 months (95% CI, not reached) in both groups, respectively, and a significant 44% reduction in the risk of death; the combination group showed significantly improved patient ORR versus sorafenib (24% vs. 4%).
For the good efficacy of HAIC combined with lenvatinib and PD1 inhibitors in the treatment of HCC patients with PVTT and APFs, we believe that it is related to these factors. First, chemotherapeutic agents can induce apoptosis through DNA damage and immunogenic cell death of tumor cells. This enhances the antitumor immune response and further promotes the efficacy of immunotherapy [21]. Second, TKIs (e.g., lenvatinib) can increase PD-L1 expression in tumors and promote the infiltration of immune cells into tumors [22]. Third, TKIs are multikinase inhibitors with anti-proliferative and anti-angiogenic activities that inhibit hypoxia-induced angiogenesis within the tumor after vascular embolization [23].
The AEs in this study were acceptable. Myelosuppression was more common in the HAIC+L+P group than in the TACE+L+P group. Patients undergoing HAIC should be closely monitored for fluctuations in leukocytes and platelets, and it is recommended to review the tests on postoperative days 3 and 7 for timely symptomatic support in patients with low leukocytes or low platelets.
There are limitations in this study. First, the selection of treatment options in this study was based on physician and patient preferences, which allowed for selection bias in the study population. Second, this is a single-center retrospective study with inherent drawbacks, which limit the ability to draw general conclusions. Third, this was a small sample study. Fourth, the mean follow-up time in the HAIC+L+P group was short, and longer follow-up time is needed to validate the effects of the combination treatment in future studies. Finally, more laboratory indicators needed to be analyzed in further studies.
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–491. e471. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 4.Park HC, Seong J, Tanaka M, Zeng ZC, Lim HY, Guan S, Bae SH, Tak WY. Multidisciplinary management of nonresectable hepatocellular carcinoma. Oncology. 2011;81(Suppl 1):134–140. doi: 10.1159/000333276. [DOI] [PubMed] [Google Scholar]
- 5.Zhou WZ, Shi HB, Liu S, Yang ZQ, Zhou CG, Xia JG, Zhao LB, Li LS. Arterioportal shunts in patients with hepatocellular carcinoma treated using ethanol-soaked gelatin sponge: therapeutic effects and prognostic factors. J Vasc Interv Radiol. 2015;26:223–230. doi: 10.1016/j.jvir.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, Choi JI, Kim HB, Lee WJ, Kim CM. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037–2044. doi: 10.1111/j.1349-7006.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, Toyoda H, Imai Y, Hiraoka A, Ikeda M, Izumi N, Moriguchi M, Ogasawara S, Minami Y, Ueshima K, Murakami T, Miyayama S, Nakashima O, Yano H, Sakamoto M, Hatano E, Shimada M, Kokudo N, Mochida S, Takehara T. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10:181–223. doi: 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W, Guo RP, Shi M. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017;36:83. doi: 10.1186/s40880-017-0251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72:288–306. doi: 10.1016/j.jhep.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 2021;112:90–92. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y, Monden M, Kudo M. Report of the 17th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2007;37:676–691. doi: 10.1111/j.1872-034X.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 16.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, Le Y, Zhou Z, Zhao M, Guo Y, Guo R, Chen M, Shi M. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5:953–960. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surveillance group; Diagnosis group; Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group; Target therapy/systemic therapy group; Radiotherapy group; Prevention group; Drafting group. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2018;117:381–403. doi: 10.1016/j.jfma.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Finn RS, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, Xu R, Edeline J, Ryoo BY, Ren Z, Cheng AL, Galle PR, Kaneko S, Kumada H, Wang A, Mody K, Dubrovsky L, Siegel AB, Llovet J. LBA34 primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2022;33:S1401. [Google Scholar]
- 20.Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Wu J, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Wang J, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 21.Cheu JW, Wong CC. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. 2021;74:2264–2276. doi: 10.1002/hep.31840. [DOI] [PubMed] [Google Scholar]
- 22.Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, Guo RP. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. 2021;11:618206. doi: 10.3389/fonc.2021.618206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, Zhou J, Lin L, Cao B, Chen Y, Zhou J, Zhu K. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi: 10.3389/fimmu.2022.848387. [DOI] [PMC free article] [PubMed] [Google Scholar]


