Abstract
Autoinduction plays an important role in intercellular communication among symbiotic and pathogenic gram-negative bacteria. We report here that a nitrogen-fixing symbiont of Phaseolus vulgaris, Rhizobium etli CNPAF512, produces at least seven different autoinducer molecules. One of them exhibits a growth-inhibitory effect like that of the bacteriocin small [N-(3R-hydroxy-7-cis-tetradecanoyl)-l-homoserine lactone]. At least two of the other autoinducers are synthesized by a LuxI-homologous autoinducer synthase. The corresponding luxI homologous gene (raiI) and a luxR homolog (raiR) have been identified and characterized. Enhanced expression of raiI is dependent on cell density and on the presence of one or more autoinducer molecules synthesized by R. etli CNPAF512. A raiI mutant was shown to release only three different autoinducer molecules; a raiR mutant releases four different autoinducer molecules. Examination of different mutants for nodulation of beans showed that raiI is involved in the restriction of nodule number, whereas nitrogen-fixing activity in terms of acetylene reduction per nodule was not affected.
Autoinduction is a highly conserved mechanism of differential gene expression in many gram-negative bacteria (20, 48). The key trigger of this system is the concentration of small diffusible molecules, termed autoinducers for their biological activity. All autoinducers so far identified are N-acyl homoserine lactones (AHLs) (4, 8, 13, 43, 50, 56, 63). They are synthesized by an autoinducer synthase, the product of a luxI-homologous gene, and are thought to bind to a protein belonging to the LuxR family of transcriptional activators. This autoinducer-protein complex activates the expression of defined genes or sets of genes. As this gene activation occurs only when a required threshold concentration of AHLs is attained, the onset of specific genes is dependent on the cell density of bacteria. Consequently, autoinduction allows bacteria to monitor their own population density and to discriminate between high and low cell density. It can also be understood as a cell-cell communication system (48).
The physiological processes regulated by autoinduction are diverse, as exemplified by the following systems: bioluminescence in Vibrio fischeri (10, 13, 14), plasmid conjugal transfer in Agrobacterium tumefaciens (18, 30, 44, 63), antibiotic production in Erwinia carotovora (1, 2, 45, 55), and synthesis of exoenzymes in plant and animal pathogens such as E. carotovora (32) and Pseudomonas aeruginosa (21, 41, 42). In Escherichia coli, a LuxR homolog is involved in the regulation of cell division (22). Rhizobium leguminosarum bv. viciae contains a transcriptional-activator protein, RhiR, belonging to the LuxR family (11). RhiR is encoded by the symbiotic plasmid pRL1JI and regulates transcription of an operon of three rhizosphere-expressed genes of unknown function (the rhiABC operon). The bacteriocin small, produced by fast-growing R. leguminosarum strains (60), has been shown to be an AHL [N-(3R-hydroxy-7-cis-tetradecanoyl)-l-homoserine lactone] (50). Gray (24) has reported that R. leguminosarum produces two additional autoinducer compounds, activating rhiABC together with RhiR. small increases production of these signals independently from RhiR. A gene encoding an autoinducer synthase has so far not been identified.
Rhizobium etli CNPAF512 (formerly classified as R. leguminosarum bv. phaseoli CNPAF512) forms nitrogen-fixing nodules on the roots of the common bean. Within this structure bacteria are densely packed and differentiate into their symbiotic state, that of the bacteroids, able to reduce atmospheric dinitrogen into ammonia. In view of the high cell density during Rhizobium-plant interaction, autoinduction may be involved at some stage of symbiosis.
We demonstrate in this study that R. etli CNPAF512 produces at least seven different autoinducer molecules. We have identified luxI- and luxR-homologous genes and demonstrate their contribution to the synthesis of autoinducers and to the nodulation of Phaseolus vulgaris.
(Parts of the results described here have been presented on posters at the 8th International Congress on Molecular Plant-Microbe Interactions, Knoxville, Tenn., 14 to 19 July 1996, at the Euroconference on Microbial Response to Stress, Sesimbra, Portugal, 15 to 18 March 1997, at the International Congress on Marker/Reporter Genes in Microbial Ecology, Stockholm, Sweden, 14 to 17 June 1997, and at the 11th International Congress on Nitrogen Fixation, Paris, France, 20 to 25 July 1997.)
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Antibiotic concentrations were as follows (in micrograms per milliliter): nalidixic acid, 30; ampicillin, 100; kanamycin, 25; neomycin, 60; tetracycline, 10; gentamicin, 25; carbenicillin, 100; and rifampin, 100. E. coli DH5α was grown in Luria-Bertani medium (49) at 37°C, R. etli CNPAF512 was grown in TY medium (6), and A. tumefaciens NT1(pJM749, pSVB33) was grown in AB medium (9) at 28°C.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F− φ80DlacZΔM15 endA1 recA1 hsdR17(rK− mK−) supE44 thi-1 gyrA96 Δ(lacZYA-argF) | 49 |
| HB101 | F−hsd20
(rK− mK−)
supE44 ara-14 galK2 lacY1 proA2 rpsL20 Smrxyl-5 leuB6 mtl-1 lambda−recA13 thy thi |
49 |
| FAJ1323 | DH5α containing pFAJ1323; Apr | This study |
| A. tumefaciens NT1(pJM749, pSVB33) | Indicator strain for detecting AHLs; Kmr Cbr | 44 |
| R. leguminosarum bv. viciae | ||
| 248 | Sensitive to bacteriocin small; Rifr | |
| RBL1309 | small producing | 57 |
| RBL1376 | RBL1309 Tn5 mutant; small | 57 |
| R. etli | ||
| CNPAF512 | Wild-type isolate; Nalr | Embrapa, Brazil |
| FAJ1328 | raiI::gusA-Km mutant, convergently oriented; Nalr Kmr | This study |
| FAJ1329 | raiR mutant with gusA-Km in reverse orientation; Nalr Kmr | This study |
| CNPAF512::pFAJ1328 | cis merodiploid raiI::gusA-Km; Gmr Nalr Kmr | This study |
| CNPAF512::pFAJ1334 | cis merodiploid ORF1::gusA-Km; two copies of raiI/raiR; Gmr Nalr Kmr | This study |
| Plasmids | ||
| pHV200 | 8.8-kb SalI fragment with V. fischeri ES184 lux regulon; hybridization probe | 25 |
| pUC18 | ColE1 origin; cloning vehicle; Apr | 62 |
| pLAFR1 | IncP1 cos oriT; Tcr | 17 |
| pCR™2.1 TA | ColE1 origin, F1 origin; cloning vehicle for PCR products; Apr Kmr | Invitrogen |
| pWM6 | Source of gusA-Kmr cassette; Apr Kmr | 37 |
| pJQ200uc1 | Suicide vector, sacB; Kmr Gmr | 47 |
| pRK2013 | ColE1 replicon with RK2 transfer genes; Kmr | 15 |
| pFAJ1322 | Cosmid clone, pLAFR1 derivative, AHL+; Tcr | This study |
| pFAJ1323 | SalI fragment from pFAJ1322 in pUC18; AHL+; Apr | This study |
| pFAJ1326 | PCR fragment (raiI raiR ORF1) in pCR2.1 TA; Apr | This study |
| pFAJ1327 | PCR fragment (raiI raiR ORF1) in pJQ200uc1 via NotI; Gmr | This study |
| pFAJ1328 | pFAJ1327; raiI::gusA-Kmr in XhoI; Kmr Gmr | This study |
| pFAJ1329 | pFAJ1327; raiR with gusA-Kmr insertion in NdeI; Kmr Gmr | This study |
| pFAJ1334 | pFAJ1327; ORF1::gusA-Kmr in BamHI; Kmr Gmr | This study |
Nalr, nalidixic acid resistant; Apr, ampicillin resistant; Kmr, kanamycin-neomycin resistant; Tcr, tetracycline resistant; Gmr, gentamicin resistant; Cbr, carbenicillin resistant; Rifr, rifampin resistant.
Extraction and detection of autoinducers.
Rhizobium was grown for 48 h and E. coli was grown for 24 h in 500 ml of medium to stationary phase. After centrifugation, the supernatant was extracted twice with an equal volume of ethyl acetate containing 1.5 ml of acetic acid per liter. The extract was evaporated to dryness by vacuum rotation at 42°C and redissolved in a small volume of ethyl acetate. One microliter of this suspension was spotted on a C18 reversed-phase thin-layer chromatography (TLC) plate (RP-18F254S; Merck), which was then developed with 60% methanol. The air-dried plate was overlaid with AB soft agar (9) containing 5-bromo-4-chloro-3-indolyl-β-galactopyranoside (X-Gal) and A. tumefaciens NT1(pJM749, pSVB33) indicator cells (44, 52). This strain contains a Tn3HoHo1-generated lacZ fusion to a tra gene, the expression of which is dependent on TraR and autoinducer. As the clone lacks the Ti plasmid, it does not produce an autoinducer detectable with this system. Consequently, the lacZ reporter fusion is expressed only upon exogenous supply of autoinducer molecules (44). Incubation of the TLC plates at 28°C allowed the visualization of compounds activating the A. tumefaciens tra system expression. For comparison, we used purified autoinducer molecules from V. fischeri (VAI) [N-(3-oxohexanoyl)-l-homoserine lactone] and from P. aeruginosa (PAI) [N-(3-oxododecanoyl)-l-homoserine lactone], kindly provided by E. P. Greenberg.
For fractionation, the ethyl acetate extract has been diluted 1:10 with 30% methanol in water, loaded on a C18 reversed-phase high-performance liquid chromatography column (Bondclone 10 C18; Phenomenex), and eluted isocratically with 30% methanol. The fractions were tested for activation of the A. tumefaciens tra system and for bacteriostatic activity towards the sensitive strain R. leguminosarum bv. viciae 248 as described by Schripsema et al. (50).
Screening of an R. etli CNPAF512 gene library in E. coli HB101 comprising 5,000 clones was performed in microtiter plates. Each 100 μl of AB medium supplemented with 0.003% leucine, 0.023% proline, 0.004% thymine, and 0.0017% thiamine, containing A. tumefaciens indicator cells and X-Gal, was pipetted into the wells of a microtiter plate. The clones of the gene library were added, and the plates were incubated at 28°C with shaking.
DNA techniques and nucleotide sequencing.
Standard techniques were used for isolation and manipulation of DNA (49). Enzymes were purchased from Boehringer Mannheim. Hybridization experiments were carried out with digoxigenin-labeled probes according to the instructions of the manufacturer (Boehringer Mannheim). High-stringency hybridization was performed at 68°C, and low-stringency hybridization was performed at 40°C. Nucleotide sequencing was accomplished by using the A.L.F. sequencer (Pharmacia Biotech). Sequences were compiled and compared with the aid of the PCGENE software package (IntelliGenetics Inc.).
Construction of raiI and raiR mutants.
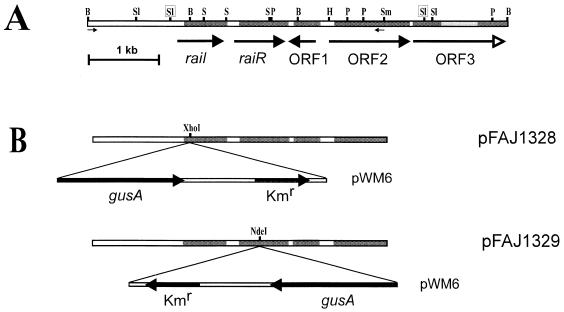
A 4-kb region from pFAJ1322 comprising raiI, raiR, and open reading frame 1 (ORF1) has been amplified by PCR. NotI sites were introduced by designing appropriate primers (5′-CGCGCGGCCGCCATAGCCATCGCTGGTGATGTTGC-3′ and 5′-CGCGCGGCCGCATGATAGGCATCGCCGAGAAAGAGG-3′). The product was cloned into the pCR2.1 TA cloning vector (pFAJ1326). For verification, the terminal regions were sequenced. Subsequently, the fragment was excised with NotI and ligated into pJQ200uc1 (pFAJ1327) (47). For mutagenesis of raiI, this construct was linearized with XhoI, allowing the insertion of a promoterless glucuronidase (gusA) gene coupled to a kanamycin resistance gene from pWM6 (37) 28 bp downstream of the predicted raiI translation start, resulting in a nonpolar mutation (pFAJ1328). The same cassette was inserted in reverse orientation into raiR via a unique NdeI site, 126 bp downstream of the predicted translation start (pFAJ1329) (see Fig. 2B). These plasmids were conjugated into R. etli CNPAF512 with the helper E. coli HB101/pRK2013, yielding cis merodiploid recombinants with a raiI::gusA fusion (CNPAF512::pFAJ1328) or a gusA-Km insertion in raiR. Selection for double homologous recombination using the sacRB-based positive selection system on sucrose (38) led to a raiI mutant with a raiI::gusA fusion (FAJ1328) or a raiR mutant (FAJ1329), respectively. The genotypes of the mutants were verified by hybridization of a raiI-raiR probe and a probe of the pWM6 cassette to the same R. etli EcoRI fragment.
FIG. 2.
(A) Physical and genetic map of the region containing raiI and raiR from R. etli CNPAF512. Coding regions are shaded. Arrows indicate directions of transcription. The open arrow represents the beginning of an open reading frame. Light shading illustrates a part of ORF3 that has not been sequenced. The boxed SalI sites have been used for construction of pFAJ1323. The small arrows indicate the primers with NotI sites used for amplification of a 4-kb fragment. Restriction sites: B, BamHI; H, HindIII; P, PstI; S, SphI; Sl, SalI; Sm, SmaI. (B) Construction of the raiI (FAJ1328) and raiR (FAJ1329) mutant. The PCR product was cloned into pCR2.1 TA, excised with NotI, and inserted into pJQ200uc1. A cassette with a promoterless gusA and kanamycin resistance from pWM6 was introduced in the XhoI (raiI mutant) or NdeI (raiR mutant) site by blunt-end ligation.
Expression analyses.
Cell density-dependent expression of raiI was monitored by using a raiI::gusA fusion. Each 5 ml of TY medium was inoculated with a different volume of a stationary-phase culture and grown for 12 h. Prior to inoculation, the cells were washed twice with 10 mM MgSO4 to remove autoinducers. Quantitative analysis of GusA activity was carried out with p-nitrophenyl-β-d-glucuronide as the substrate by the method of Miller (39) in microtiter plates.
Plant experiments.
Surface-sterilized and germinated bean seedlings (58) were planted under sterile conditions in 0.5× Jenssen medium (59) and were subsequently inoculated with 200 μl of stationary-phase bacterial cultures. The plants were grown at 26°C for 18 days with a 12-h day length. Nitrogen fixation was measured in terms of acetylene reduction by gas chromatography (5890A; Hewlett-Packard).
Nucleotide sequence accession numbers.
The nucleotide sequences of raiI and raiR have been deposited under accession no. U92712 and U92713, respectively, in the GenBank Sequence Data Library.
RESULTS AND DISCUSSION
Detection of autoinducers.
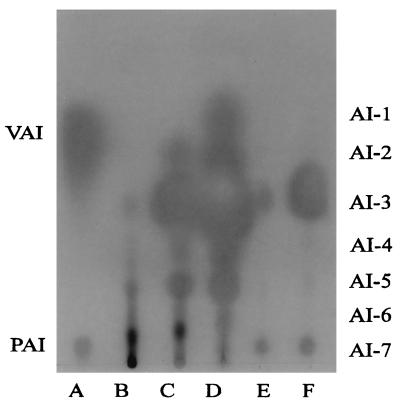
Among several reporter systems available for detection of autoinducers, such as activation of lasB (26), the lux operon (56), or the tra system (44), the last recognized the broadest spectrum of different molecules in our hands. With the exception of N-butanoyl-, N-(3-hydroxyoctanoyl)-, and N-(3-hydroxydecanoyl)-l-homoserine lactone, activation of tra gene expression has been shown with 3-oxo-, 3-hydroxy-, and 3-unsubstituted side chains with even chain lengths of C4 up to C12 (52). R. etli CNPAF512 was grown in 500 ml of TY medium for 48 h to stationary phase. The ethyl acetate extract of the cell-free spent medium was analyzed on a TLC plate for compounds activating the A. tumefaciens tra system expression (Fig. 1, lane D). Seven distinct spots could be detected, indicating that there are at least seven different autoinducer types (AI-1 through AI-7) present in R. etli CNPAF512. P. aeruginosa has been shown to produce six different autoinducers (52, 61). The existence of seven autoinducers in one species is described here for Rhizobium for the first time. Purified PAI and VAI were used for comparison (Fig. 1, lane A). VAI migrated between AI-1 and AI-2, and PAI exhibited chromatographic features similar to those of AI-7. The amount of autoinducer molecules necessary to activate the A. tumefaciens test system depends on the recognition of autoinducers by TraR and thus on the type of molecule. In Fig. 1, lane A, 10 ng of PAI and 20 pg of VAI were applied. Although about 3 orders of magnitude more PAI than VAI has been spotted on the plate, VAI evokes a bigger spot. This demonstrates that the amounts of different autoinducers are incomparable, as the assay used is based on biological activity. Therefore, absolute quantification of unknown molecules by measuring spot intensity is not possible.
FIG. 1.
Purified VAI and PAI (A) and autoinducers produced by E. coli DH5α (B), E. coli DH5α containing the R. etli CNPAF512 raiI and raiR in pUC18 (FAJ1323) (C), wild-type R. etli CNPAF512 (D), the R. etli CNPAF512 mutant with disrupted raiI (FAJ1328) (E), and the R. etli CNPAF512 mutant with disrupted raiR (FAJ1329) (F). Ethyl acetate extracts (lanes B to E) were spotted on a C18 reversed-phase TLC plate, and 60% methanol was used as the liquid phase. Molecules with autoinducer activity were visualized with a soft agar overlayer containing X-Gal and A. tumefaciens indicator cells.
The R. etli CNPAF512 ethyl acetate extract has been fractionated on a C18 reversed-phase high-performance liquid chromatography column. Assaying the fractions for tra activation confirmed the presence of at least seven different autoinducer molecules, as expected from the results of the TLC plates (data not shown). In addition to the list of bacteriocin-producing rhizobia published by Hirsch (29), we observed that R. etli CNPAF512 inhibits the growth of R. leguminosarum bv. viciae 248, too. Therefore, the fractions from high-performance liquid chromatography were tested for bacteriostatic activity toward this sensitive strain. Only the fractions containing AI-7 exhibited a growth-inhibitory effect like that of the bacteriocin small (27, 50). Although we cannot exclude a coelution of two different molecules, one showing the features of a bacteriocin and the other activating tra expression in A. tumefaciens, we have evidence that the A. tumefaciens system also recognizes small: TLC analysis of an extract from the small-producing strain R. leguminosarum bv. viciae RBL1309 (57) revealed the presence of three autoinducer molecules. The molecule with the highest hydrophobicity, which was missing in the small mutant R. leguminosarum bv. viciae RBL1376, comigrated with AI-7 (data not shown).
Identification and characterization of luxI- and luxR-homologous genes.
An R. etli CNPAF512 gene library in E. coli HB101, previously constructed in the cosmid pLAFR1 by partial EcoRI digestion of genomic R. etli CNPAF512 DNA, was screened for clones activating the A. tumefaciens tra system in microtiter plates. After 12 h, eight wells were blue. Restriction analysis of the corresponding clones and hybridization with a V. fischeri luxI probe from pHV200 (25) revealed that they all contained an identical 3.8-kb SalI-fragment. E. coli DH5α containing this SalI fragment in the pUC18 vector (FAJ1323) could still induce the A. tumefaciens test system to the same extent as the positive E. coli HB101 clones from the library. Sequencing of pFAJ1323 revealed two open reading frames, the deduced amino acid sequences of which showed similarity to the autoinducer synthases of the LuxI family and to transcriptional activators of the LuxR family. Downstream of these genes are two complete open reading frames and the beginning of a third. The deduced ORF1 protein shows homology to members of the Lrp family of transcriptional regulators (46). The deduced ORF2 protein shows homology to a catabolic alanine racemase (DadX), and the deduced ORF3 protein shows homology to the small subunit of d-amino acid dehydrogenase (DadA) from E. coli (34). The racemase converts l-alanine to the d isomer, which is then oxidatively deaminated by the d-amino acid dehydrogenase to pyruvate and ammonia (16). In E. coli, dadA and dadX form an operon of which Lrp is a direct repressor (36). In Pseudomonas putida, the lrp homolog bkdR is required for the expression of the bkd operon, which is necessary for the metabolism of the branched-chain amino acids (35). Comparable to the gene order in R. etli CNPAF512, bkdR is located immediately upstream of the bkd operon in the opposite orientation. In Bacillus subtilis, the lrp homolog azlB is the first gene of an operon involved in branched-chain amino acid transport (5). Figure 2A shows the physical map of this region, including the two BamHI fragments that overlap the 3.8-kb SalI fragment.
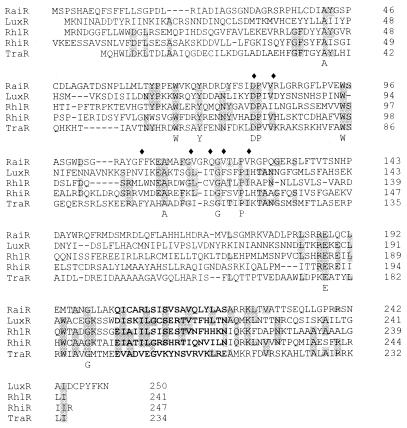
The luxI-homologous gene, termed raiI (Rhizobium autoinducer synthase), consists of a 639-bp open reading frame that possibly codes for an autoinducer synthase with 212 amino acids. Similarities of the deduced amino acid sequence with those of TraI, RhlI, and LuxI are relatively low (Table 2). The highest similarities are clustered in the N-terminal region, especially from residue 22 to 36 and from residue 67 to 84 (referring to RaiI), which is a characteristic feature of LuxI homologs described so far (Fig. 3). Amino acid residues found to be conserved in 12 LuxI homologs (28) are also present in RaiI (Arg-24, Phe-28, Trp-34, Asp-48, Arg-70, and Arg-104). However, instead of a cysteine at position 68 of LuxI, which is thought to be the active site of the autoinducer synthase, RaiI contains a serine. Autoinducer synthesis is proposed to involve transfer of the fatty acyl substrate from the acyl carrier protein to a cysteine residue on the LuxI homolog, forming a covalent bond with the sulfhydryl group (40). Given the structural and chemical similarity of serine and cysteine, it can be speculated that the hydroxyl group of the serine at the active site could also form a covalent bond with the fatty acyl group, as proposed by Hanzelka et al. (28). raiI is followed by the luxR homolog raiR, separated by 144 bp. raiR consists of a 729-bp open reading frame and codes for a protein of 242 amino acids. Identities and similarities of RaiR with RhlR, TraR, and RhiR are given in Table 2. The LuxR-related transcriptional activators consist of an N-terminal regulator domain and a C-terminal activator domain. In spite of the overall low similarity, the regions for autoinducer binding and the helix-turn-helix motif for DNA binding are conserved (Fig. 4). The organization of raiI and raiR differs from that of other species, as both are transcribed unidirectionally and the luxI homolog precedes the luxR homolog. In P. aeruginosa the gene order of the two autoinduction systems (las and rhl) is reversed, and in A. tumefaciens traI and traR are not clustered. In all other identified autoinduction systems, both genes are either convergently or divergently transcribed (48).
TABLE 2.
Identities and similarities of LuxI and LuxR homologsa
FIG. 3.
Alignments of members of the LuxI family. Shaded amino acid residues are identical in at least three of the aligned sequences. Amino acid residues that are conserved in all the homologs aligned are shown below the sequences. The sequences for RhlI (accession no. U40458) and RaiI (U92712) are in the GenBank Sequence Data Library; those for LuxI (accession no. P12747) and TraI (P33907) are in the Swiss-Prot Protein Sequence Database.
FIG. 4.
Alignments of members of the LuxR family. Shaded amino acid residues are identical in at least three of the aligned sequences. Amino acid residues that are conserved in all the homologs aligned are shown below the sequences. Diamonds indicate LuxR positions at which mutations affect interaction with VAI (51, 54). The helix-turn-helix motif (19) is given in bold letters. Accession numbers are S25491 (PIR Protein Database) for TraR, P12746 (Swiss-Prot Protein Sequence Database) for LuxR, and, in the GenBank Sequence Data Library, U40458 for RhlR, U92713 for RaiR, and M98835 for RhiR.
E. coli DH5α containing the 3.8-kb SalI fragment in pUC18 (FAJ1323) was grown in 500 ml for 24 h to stationary phase. Cell-free culture supernatant was extracted with ethyl acetate. Analysis on TLC plates revealed the presence of four different molecules (Fig. 1, lane C). The extract from E. coli DH5α evoked two spots on the TLC plate (Fig. 1, lane B), indicating that this strain produced molecules able to activate tra expression in A. tumefaciens. The other spots in Fig. 1, lanes B and C, are due to brownish contaminating material in the extract. There have been indications that E. coli produces extracellular factors, which are involved in cell-to-cell signalling and transcriptional regulation (22, 53). We demonstrate here for the first time that these molecules trigger the autoinducer-dependent activation of tra genes in A. tumefaciens.
The pattern of autoinducers produced by FAJ1323 (Fig. 1, lane C) indicates that RaiI directly catalyzes the synthesis of AI-2, AI-3, AI-4, and AI-5. It has been described for V. fischeri that one autoinducer synthase (LuxI) can direct the synthesis of two different autoinducer molecules [N-hexanoyl- and N-(3-oxohexanoyl)-l-homoserine lactone] (33). Comparison with the autoinducers synthesized by an raiI mutant (Fig. 1, lane E) (see below) suggests that the spots of AI-3 and AI-5 consist of comigrating molecules. Thus, AI-3 and AI-5 produced by FAJ1323 could be synthesized by RaiI, and the corresponding autoinducers produced by the raiI mutant could be synthesized by an autoinducer synthase(s) other than RaiI. However, at this stage we cannot exclude the possibility that the increased intensities of AI-3 and AI-5 from FAJ1323 in comparison to E. coli DH5α could be due to enhanced synthesis of E. coli DH5α molecules with autoinducer activity.
The 3.8-kb SalI fragment was inserted downstream of the lac promoter into the pUC18 vector, which is induced in the presence of isopropyl-β-d-thiogalactopyranoside. However, the synthesis of AI-3 and AI-5 also occurs in the absence of the inducer. It can therefore be concluded that the cloned fragment contained all necessary promoter elements.
Hybridization of total R. etli CNPAF512 DNA in a Southern blot with a raiI probe at low stringency gave rise to only one band. This indicates that the homology between raiI and the gene(s) encoding the other autoinducer synthase(s) is less than the homology shared by raiI and luxI (Table 2), as these are cross-hybridizing (see above). It is also possible that the other autoinducer synthase gene(s) does not belong to the luxI family, as is the case for ainS in V. fischeri (23) or luxL and luxM in V. harveyi (3).
Analysis of raiI and raiR mutants.
In order to examine the phenotypic relevance of raiI and raiR in R. etli, mutants have been constructed as described in Materials and Methods.
Spent culture supernatants of the mutants FAJ1328 and FAJ1329 were extracted and analyzed on TLC plates. The chromatogram revealed that the raiI mutant does not produce AI-1, AI-2, AI-4, and AI-6 in amounts detectable with the assay used (Fig. 1, lane E). The absence of AI-2 and AI-4 confirmed that raiI is directly responsible for the synthesis of these two molecules. The failure to detect AI-1 and AI-6 suggests that their synthesis by an autoinducer synthase(s) other than RaiI is dependent on autoinducers made by RaiI. However, we cannot exclude the possibility that the synthesis of AI-1 and AI-6 is directly dependent on raiI and that in E. coli these compounds are not produced. The intensities of the spots representing AI-3 and AI-5 are clearly lower than in the wild type. This supports the above-mentioned hypothesis that the AI-3 and AI-5 spots consist of comigrating molecules. In the raiR mutant, four molecules could be detected (Fig. 1, lane F): AI-7 and AI-5 in about the same concentration as in the raiI mutant, AI-3 in a concentration higher than that in the raiI mutant, and AI-4, which could not be detected in the raiI mutant. AI-1, AI-2, and AI-6 could not be detected, indicating that RaiR is necessary for their synthesis.
Expression studies of raiI.
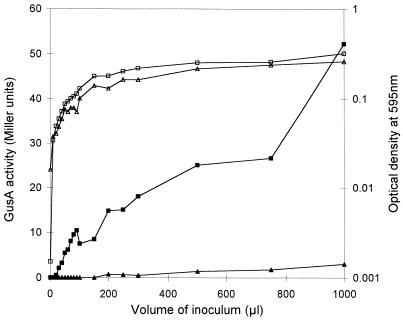
raiI expression in a wild-type (CNPAF512::pFAJ1328) and a mutant (FAJ1328) background was examined quantitatively in a cell density-dependent way. As shown in Fig. 5, expression of raiI in a wild-type background increased with cell density. In the mutant, raiI expression was nearly negligible, indicating that at least one of the autoinducers AI-1, AI-2, AI-4, and AI-6 is necessary for direct or indirect activation of raiI. raiI expression in FAJ1328 was restored in filter-sterilized spent medium from R. etli CNPAF512. At low cell densities, expression levels under these conditions exceeded those of the wild type in fresh medium (data not shown).
FIG. 5.
Comparison of cell density-dependent expression of raiI in wild-type and raiI mutant backgrounds. Expression of the raiI::gusA fusions was monitored by using p-nitrophenyl-β-d-glucuronide as a substrate. Values for optical density and Miller units were based on measurement in microtiter plates. Curves show averages from three separate experiments. Squares, CNPAF512::pFAJ1328 (raiI::gusA in a wild-type background); triangles, FAJ1328 (raiI knockout mutant); open symbols, optical density at 595 nm; solid symbols, GusA activity in Miller units.
Mutation of raiI affects number of nodules of inoculated bean plants.
The phenotypic relevance of the rai genes for symbiosis with beans and for nitrogen fixation was examined. Germinated bean seedlings were planted under sterile conditions and subsequently inoculated with 200 μl of dense cultures (optical density, >1) of R. etli CNPAF512, FAJ1328 (raiI mutant), FAJ1329 (raiR mutant), or CNPAF512::pFAJ1334 (merodiploid with respect to raiI and raiR). The plants were grown at 26°C for 18 days. While no significant differences in delay of the appearance of the first nodules and dry weight of the shoot were observed (Table 3), the number of nodules per plant inoculated with the raiI mutant, FAJ1328, was about twice as high as that for the plants inoculated with the wild type. The raiR mutant, FAJ1329, did not differ significantly from the wild type. Inoculation with the merodiploid CNPAF512::pFAJ1334 resulted in significantly lower acetylene reduction per plant and a reduced number of nodules, although a relatively high variation was observed. Values for dry weight of nodules and for per-plant nitrogen fixation illustrate this observation. However, nitrogen-fixing activity in terms of acetylene reduction per nodule remained unchanged. We therefore conclude that raiI, but not raiR, is involved in the restriction of the number of nodules, whereas nitrogen-fixing activity is not affected. We further conclude by comparison of the patterns of autoinducers produced by the raiI and raiR mutants (Fig. 1, lanes E and F) that the molecules representing AI-3, which is synthesized at wild-type levels in the raiR mutant but is present only in a lower amount in the raiI mutant, and/or AI-4, which could not be detected in the raiI mutant, are involved in the regulation of the number of nodules. This observation adds a new element in the signalling pathways between Rhizobium and the host plant and is currently being further explored.
TABLE 3.
Phenotypic characterization of mutants under symbiotic conditionsa
| Strain | Appearance of 1st nodules (day) | Dry wt of shoot (mg) | No. of nodules | Dry wt of nodules (mg) | Acetylene reduction (μmol h−1)/plant | Acetylene reduction (nmol h−1)/nodule |
|---|---|---|---|---|---|---|
| CNPAF512 | 9.1 (1.5)A | 217 (55)A | 59.5 (15.3)A | 18.7 (5.6)A | 3.1 (0.4)A | 52.1 (6.7)A |
| FAJ1328 | 10.6 (1.8)A | 270 (63)A | 118.4 (26.2)B | 31.6 (6.9)B | 5.1 (0.9)B | 43.1 (7.6)A |
| FAJ1329 | 10.0 (1.2)A | 218 (58)A | 62.8 (17.4)A | 18.1 (4.9)A | 3.0 (0.4)A | 47.8 (6.4)A |
| CNPAF512::pFAJ1334 | 11.6 (1.6)A | 196 (45)A | 44.9 (12.3)A | 10.9 (4.1)A | 1.9 (0.3)C | 42.3 (6.7)A |
Each value is based on examination of at least 10 plants. The 95% confidence interval is given in parentheses. Strains with the same letter are not significantly different for the tested parameter.
ACKNOWLEDGMENTS
We thank Stephen Farrand for the gift of the A. tumefaciens autoinducer detection system; Anton van Brussel for the small-sensitive strain R. leguminosarum bv. viciae 248, the small-producing strain R. leguminosarum bv. viciae RBL1309, and the small mutant R. leguminosarum bv. viciae RBL1376; and E. Peter Greenberg for purified PAI and VAI and for the V. fischeri ES184 lux regulon.
V.R. is a fellow of the Training and Mobility of Researchers Program (no. ERBFMBICT 950142), which is financed by the European Commission. J.M. is a postdoctoral fellow of the Fund for Scientific Research—Flanders.
REFERENCES
- 1.Bainton N J, Bycroft B W, Chhabra S R, Stead P, Gledhill L, Hill P J, Rees C E D, Winson M K, Salmond G P C, Stewart G S A B, Williams P A. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene. 1992;116:87–91. doi: 10.1016/0378-1119(92)90633-z. [DOI] [PubMed] [Google Scholar]
- 2.Bainton N J, Stead P, Chhabra S R, Bycroft B W, Salmond G P C, Stewart G S A B, Williams P. N-(3-oxohexanoyl)-l-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem J. 1992;288:997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassler B L, Wright M, Showalter R E, Silverman M R. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 4.Beck von Bodman S, Farrand S K. Capsular polysaccharide biosynthesis and pathogenicity in Erwinia stewartii require induction by an N-acylhomoserine lactone autoinducer. J Bacteriol. 1995;177:5000–5008. doi: 10.1128/jb.177.17.5000-5008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belitsky B R, Gustafsson M C U, Sonenshein A L, von Wachenfeldt C. An lrp-like gene of Bacillus subtilisinvolved in branched-chain amino acid transport. J Bacteriol. 1997;179:5448–5457. doi: 10.1128/jb.179.17.5448-5457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beringer J E. R-factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;120:421–429. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- 7.Brint J M, Ohman D E. Synthesis of multiple exoproducts in Pseudomonas aeruginosais under control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J-G, Meighen E A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989;264:21670–21676. [PubMed] [Google Scholar]
- 9.Chilton M-D, Currier T C, Farrand S K, Bendich A J, Gordon M P, Nester E W. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA. 1974;71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi S H, Greenberg E P. Genetic dissection of DNA binding and luminescence gene activation by the Vibrio fischeriLuxR protein. J Bacteriol. 1992;174:4064–4069. doi: 10.1128/jb.174.12.4064-4069.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cubo M T, Economou A, Murphy G, Johnston A W B, Downie J A. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation of Rhizobium leguminosarum biovar viciae. J Bacteriol. 1992;174:4026–4035. doi: 10.1128/jb.174.12.4026-4035.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devine J H, Countryman C, Baldwin T O. Nucleotide sequence of the luxR and luxI genes and the structure of the primary regulatory region of the lux regulon of Vibrio fischeriATCC 7744. Biochemistry. 1988;27:837–842. [Google Scholar]
- 13.Eberhard A, Burlingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer N J. Structural identification of autoinducer of Photobacterium fischeriluciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 14.Engebrecht J, Nealson K, Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 15.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin F C H, Venables W A. Biochemical, genetic and regulatory studies of alanine catabolism in Escherichia coliK12. Mol Gen Genet. 1976;149:229–237. doi: 10.1007/BF00332894. [DOI] [PubMed] [Google Scholar]
- 17.Friedman A M, Long S R, Brown S E, Buikema W J, Ausubel F M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobiummutants. Gene. 1982;18:289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- 18.Fuqua W C, Winans S C. A LuxR-LuxI type regulatory system activates AgrobacteriumTi plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuqua W C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 21.Gambello M J, Iglewski B H. Cloning and characterization of the Pseudomonas aeruginosa lasRgene, a transcriptional activator of elastase expression. J Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García-Lara J, Shang L H, Rothfield L I. An extracellular factor regulates expression of sdiA, a transcriptional activator of cell division genes in Escherichia coli. J Bacteriol. 1996;178:2742–2748. doi: 10.1128/jb.178.10.2742-2748.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilson L, Kuo A, Dunlap P V. AinS and a new family of autoinducer synthesis proteins. J Bacteriol. 1995;177:6946–6951. doi: 10.1128/jb.177.23.6946-6951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray, K. M. 1996. Identification of multiple autoinducer signals in Rhizobium leguminosarum. Presented as poster X15 at the 8th International Congress on Molecular Plant-Microbe Interactions, Knoxville, Tenn., 14 to 19 July 1996.
- 25.Gray K M, Greenberg E P. Physical and functional maps of the luminescence gene cluster in an autoinducer-deficient Vibrio fischeristrain isolated from a squid light organ. J Bacteriol. 1992;174:4384–4390. doi: 10.1128/jb.174.13.4384-4390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray K M, Passador L, Iglewski B H, Greenberg E P. Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. J Bacteriol. 1994;176:3076–3080. doi: 10.1128/jb.176.10.3076-3080.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray K M, Pearson J P, Downie J A, Boboye B E A, Greenberg E P. Cell-to-cell signaling in the symbiotic nitrogen-fixing bacterium Rhizobium leguminosarum: autoinduction of a stationary phase and rhizosphere-expressed genes. J Bacteriol. 1996;178:372–376. doi: 10.1128/jb.178.2.372-376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanzelka B L, Stevens A M, Parsek M R, Crone T J, Greenberg E P. Mutational analysis of the Vibrio fischeriLuxI polypeptide: critical regions of an autoinducer synthase. J Bacteriol. 1997;179:4882–4887. doi: 10.1128/jb.179.15.4882-4887.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch P R. Plasmid-determined bacteriocin production by Rhizobium leguminosarum. J Gen Microbiol. 1979;113:219–228. [Google Scholar]
- 30.Hwang I, Cook D M, Farrand S K. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J Bacteriol. 1995;177:449–458. doi: 10.1128/jb.177.2.449-458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang J, Li P-L, Zhang L, Piper K R, Cook D M, Tate M E, Farrand S K. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acyl homoserine lactone autoinducer. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones S, Yu B, Bainton N J, Birdsall M, Bycroft B W, Chhabra S R, Cox A J R, Golby P, Reeves P J, Stephens S, Winson M K, Salmond G P C, Stewart G S A B, Williams P. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo A, Blough N V, Dunlap P V. Multiple N-acyl-l-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1994;176:7558–7565. doi: 10.1128/jb.176.24.7558-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobocka M, Hennig J, Wild J, Klopotowski T. Organization and expression of the Escherichia coli K-12 dad operon encoding the smaller subunit of d-amino acid dehydrogenase and the catabolic alanine racemase. J Bacteriol. 1994;176:1500–1510. doi: 10.1128/jb.176.5.1500-1510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madhusudhan K T, Lorenz D, Sokatch J R. The bkdR gene of Pseudomonas putida is required for expression of the bkd operon and encodes a protein related to Lrp of Escherichia coli. J Bacteriol. 1993;175:3934–3940. doi: 10.1128/jb.175.13.3934-3940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathew E, Zhi J, Freundlich M. Lrp is a direct repressor of the dad operon in Escherichia coli. J Bacteriol. 1996;178:7234–7240. doi: 10.1128/jb.178.24.7234-7240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metcalf W W, Wanner B L. Construction of new β-glucuronidase cassettes for making transcriptional fusions and their use with new methods for allele replacements. Gene. 1993;129:17–25. doi: 10.1016/0378-1119(93)90691-u. [DOI] [PubMed] [Google Scholar]
- 38.Michiels J. Regulation of nitrogen fixation in Rhizobium leguminosarum biovar phaseoli CNPAF512. Dissertationes de agricultura. Vol. 244. Leuven, Belgium: K. U. Leuven; 1993. [Google Scholar]
- 39.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 354–358. [Google Scholar]
- 40.Moré M I, Finger D, Stryker J L, Fuqua C, Eberhard A, Winans S C. Enzymatic synthesis of a quorum-sensing autoinducer through the use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 41.Ochsner U A, Reiser J. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:6424–6428. doi: 10.1073/pnas.92.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosavirulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 43.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of Pseudomonas aeruginosavirulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciensregulates Ti plasmid transfer by autoinduction. Nature. 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 45.Pirhonen M, Flego D, Heikinheimo R, Palva E T. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993;12:2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Platko J V, Willins D A, Calvo J M. The ilvIH operon of Escherichia coliis positively regulated. J Bacteriol. 1990;172:4563–4570. doi: 10.1128/jb.172.8.4563-4570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quandt J, Hynes M F. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 48.Salmond G P C, Bycroft B W, Stewart G S A B, Williams P. The bacterial ‘enigma’: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Schripsema J, de Rudder K E E, van Vliet T B, Lankhorst P P, de Vroom E, Kijne J W, van Brussel A A N. Bacteriocin small of Rhizobium leguminosarum belongs to the class of N-acyl-l-homoserine lactone molecules, known as autoinducers and as quorum sensing co-transcription factors. J Bacteriol. 1996;178:366–371. doi: 10.1128/jb.178.2.366-371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shadel G S, Young R, Baldwin T O. Use of regulated cell lysis in a lethal genetic selection in Escherichia coli: identification of the autoinducer-binding region of the LuxR protein from Vibrio fischeriATCC 7744. J Bacteriol. 1990;172:3980–3987. doi: 10.1128/jb.172.7.3980-3987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaw P D, Ping G, Daly S L, Cha C, Cronan J E, Jr, Rinehart K L, Farrand S K. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sitnikov D M, Schineller J B, Baldwin T O. Control of cell division in Escherichia coli: regulation of transcription of ftsQA involves both rpoSand SdiA-mediated autoinduction. Proc Natl Acad Sci USA. 1996;93:336–341. doi: 10.1073/pnas.93.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slock J, VanRiet D, Kolibachuk D, Greenberg E P. Critical regions of the Vibrio fischeriLuxR protein defined by mutational analysis. J Bacteriol. 1990;172:3974–3979. doi: 10.1128/jb.172.7.3974-3979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swift S, Winson M K, Chan P F, Bainton N J, Birdsall M, Reeves P J, Rees C E D, Chhabra S R, Hill P J, Throup J P, Bycroft B W, Salmond G P C, Williams P, Stewart G S A B. A novel strategy for the isolation of luxIhomologues: evidence for widespread distribution of a LuxR:LuxI superfamily in enteric bacteria. Mol Microbiol. 1993;10:511–520. doi: 10.1111/j.1365-2958.1993.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 56.Throup J P, Camara M, Briggs G S, Winson M K, Chhabra S R, Bycroft B W, Williams P, Stewart G S A B. Characterisation of the yenI/yenR locus from Yersinia enterocoliticamediating the synthesis of two N-acylhomoserine lactone signal molecules. Mol Microbiol. 1995;17:345–356. doi: 10.1111/j.1365-2958.1995.mmi_17020345.x. [DOI] [PubMed] [Google Scholar]
- 57.van Brussel A A N, Zaat S A J, Wijffelman C A, Pees E, Lugtenberg B J J. Bacteriocin smallof fast-growing rhizobia is chloroform soluble and is not required for effective nodulation. J Bacteriol. 1985;162:1079–1082. doi: 10.1128/jb.162.3.1079-1082.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Rhijn P J S, Feys B, Verreth C, Vanderleyden J. Multiple copies of nodO in Rhizobium tropiciCIAT899 and BR816. J Bacteriol. 1993;175:438–447. doi: 10.1128/jb.175.2.438-447.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vincent J M. A manual for the practical study of the root-nodule bacteria. Oxford, United Kingdom: Blackwell Scientific Publications; 1970. [Google Scholar]
- 60.Wijffelman C A, Pees E, van Brussel A A N, Hooykaas P J J. Repression of small bacteriocin excretion in Rhizobium leguminosarum and Rhizobium trifoliiby transmissible plasmids. Mol Gen Genet. 1983;192:171–176. [Google Scholar]
- 61.Winson M K, Camara M, Latifi A, Foglino M, Chhabra S R, Daykin M, Bally M, Chapon V, Salmond G P C, Bycroft B W, Lazdunski A, Stewart G S A B, Williams P. Multiple N-acyl-l-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 63.Zhang L, Murphy P J, Kerr A, Tate M E. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature. 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]