Abstract
Purpose
Sheep are used as a large-animal model for otology research and can be used to study implantable hearing devices. However, a method for temporal bone extraction in sheep, which enables various experiments, has not been described, and literature on middle ear access is limited. We describe a method for temporal bone extraction and an extended facial recess surgical approach to the middle ear in sheep.
Methods
Ten temporal bones from five Hampshire sheep head cadavers were extracted using an oscillating saw. After craniotomy and removal of the brain, a coronal cut was made at the posterior aspect of the orbit followed by a midsagittal cut of the occipital bone and disarticulation of the atlanto-occipital joint. Temporal bones were surgically prepared with an extended facial recess approach. Micro-CT scans of each temporal bone were obtained, and anatomic dimensions were measured.
Results
Temporal bone extraction was successful in 10/10 temporal bones. Extended facial recess approach exposed the malleus, incus, stapes, and round window while preserving the facial nerve, with the following surgical considerations: minimally pneumatized mastoid; tegmen (superior limit of mastoid cavity) is low-lying and sits below temporal artery; chorda tympani sacrificed to optimize middle ear exposure; incus buttress does not obscure view of middle ear. Distance between the superior aspect of external auditory canal and tegmen was 2.7 (SD 0.9) mm.
Conclusion
We identified anatomic landmarks for temporal bone extraction and describe an extended facial recess approach in sheep that exposes the ossicles and round window. This approach is feasible for studying implantable hearing devices.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10162-023-00907-0.
Keywords: Sheep, Temporal bone, Middle ear, Implantable hearing prosthetics, Facial recess
Introduction
Small-animal models are often used for auditory research, most commonly gerbils, guinea pigs, mice, and chinchillas. However, there are limitations to small-animal models, particularly when studying implantable hearing devices of a similar size used in humans. Therefore, a practical large-animal model is needed with similar characteristics to the human ear.
Sheep have been proposed as a suitable large-animal model for auditory research and otologic surgical training due to similarities between sheep and human temporal bone anatomy [1, 2]. The structures of the Corriedale-Texel sheep middle and external ear are at least two-thirds the size of their equivalent human structures, except for the horizontal diameter of the tympanic membrane being 62% the respective size in humans [3]. Many of the dimensions of the Corriedale-Texel sheep inner ear are also at least two-thirds their size in humans [4], and the length of the cochlea (34.1 mm in sheep) and number of turns (2.25 in sheep) are similar [5]. Additionally, the round window membrane (RWM) of sheep is anatomically similar to humans, suggesting that a sheep model would be appropriate for studying RWM permeability and inner ear drug delivery [6, 7].
Sheep also have a relatively similar auditory spectrum to humans, but with better sensitivity at higher frequencies [8]. Péus et al. measured middle ear ossicular velocities and intracochlear sound pressure in White-Alpine sheep and concluded that sheep can be an appropriate model for implantable hearing devices [9]. Péus et al. studied the anatomy of dissected sheep ossicles using micro-CT scans, noting several differences from humans, including a more anteroinferior orientation of the manubrium of the malleus and a smaller incus with a shorter distance to the lenticular process [10].
Distortion-product and click-evoked otoacoustic emissions have been measured in sheep [11, 12], including before and after hyperinsulinemia induction [13, 14]. Additionally, evoked auditory potential changes have been measured via transtympanic electrocochleography in response to hyperinsulinemia in sheep [15]. Sheep have been used as an animal model for studying the effects of acoustic trauma on perilymph [16], and tympanometry has been demonstrated in conscious sheep [17].
Given the relative anatomic similarities of the sheep ear to humans, sheep have been used as a model for otologic surgical training, particularly endoscopic ear surgery training [18–24]. Sheep heads have been used to practice canalplasty [21, 23], myringoplasty [21, 23], ossiculoplasty [21, 23], and stapedotomy [22]. Sheep have been studied as a model for surgical training in cochlear implantation [1, 25]. Mantokoudis et al. noted that the minimally pneumatized mastoid and smaller cochlea in sheep can help prepare surgeons for difficult cochlear implantation in humans [25].
The feasibility of sheep as a large-animal model for studying implantable hearing devices has been established. Cochlear implant electrode insertion has been demonstrated in cadaveric sheep temporal bones [5, 25–27], and Schnabl et al. performed round window and incus vibroplasty in cadaveric sheep temporal bones [5].
Sheep have also been used for in vivo otology studies. Lavinksy et al. used sheep to test utriculostomy as a proposed surgical treatment of Meniere’s disease [28], and Neudert et al. studied osseointegration of titanium stapes footplate prostheses in live sheep [29]. Sheep have been used to study intratympanic dexamethasone injection in vivo [30, 31]. Larsson et al. conducted in vivo studies of bone-anchored hearing implants in sheep [32, 33], and sheep have been used to study bone conduction implants in vivo [34]. Kaufmann et al. performed the first in vivo sheep cochlear implantation in Suffolk-Dorset sheep [35], and sheep have been used for in vivo studies of minimally invasive robotic cochlear implantation [36–40]. Additionally, sheep have been used to test novel totally implantable microphones in vivo [41, 42].
Cadaveric sheep temporal bones can be a useful starting point for adapting an implantable hearing device to sheep anatomy prior to in vivo testing. Despite the increased use of sheep as an animal model for auditory research and otologic surgery training, a detailed method for temporal bone extraction from sheep heads has not previously been described, and the existing literature on middle ear access via a mastoidectomy and facial recess approach in sheep is limited. Here we present a method for temporal bone extraction from sheep heads that preserves the external, middle, and inner ear and yields a specimen that can be stored easily for repeat testing and experimentation. We also present a surgical approach for middle ear access via a transmastoid extended facial recess approach in sheep, including pertinent surgical observations and anatomic measurements from micro-CT scans.
Materials and Methods
Materials
Five fresh-frozen Hampshire sheep heads (four female adults and one male lamb) were thawed in a refrigerator prior to temporal bone extraction. Heads were byproducts obtained from sheep slaughtered for human consumption. Sheep were pre-screened for Coxiella burnetii. A scalpel, Bosch StarlockPlus® oscillating saw, mallet, chisel, and periosteal elevator were used for the temporal bone extraction and preparation. An otologic drill and surgical microscope were used for the transmastoid facial recess surgical approach.
Temporal Bone Extraction
A superior midline sagittal incision was made along the skin, and the skin was peeled laterally to each side to expose the superior cranium sufficiently for craniotomy and removal of the brain. The boundaries of the craniotomy were as follows (Fig. 1):
Anterior border: halfway between the anterior and posterior aspect of the orbit
Posterior border: halfway between the posterior orbit and the occiput
Medial borders: medial aspects of the orbits bilaterally (sawing any further laterally or too deep/inferior can damage the inner/middle ear)
Fig. 1.
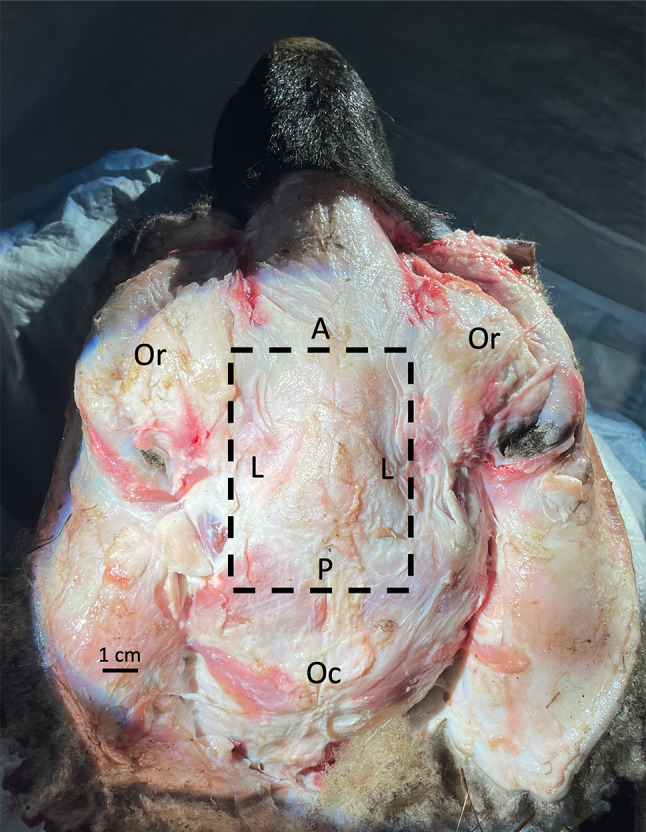
Superior view of sheep head, skin removed, with dashed lines depicting boundaries of craniotomy. A, anterior border of craniotomy; L, lateral borders of craniotomy (medial aspect of orbit); Oc, occiput; Or, orbits; P, posterior border of craniotomy
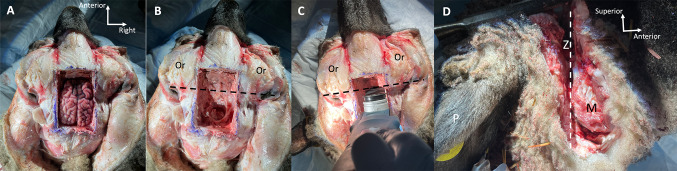
The oscillating saw, mallet, and chisel were used to perform the craniotomy. After the removal of the superior cranium, the brain was removed (Fig. 2A, B). The cranial nerves separate easily, but the vestibulocochlear and facial nerves can be specifically severed.
Fig. 2.
Steps of sheep temporal bone removal. A Sheep head after craniotomy, superior view. B Sheep head after removal of the brain, dashed line depicting coronal plane at posterior aspect of orbits (Or) where sawing occurs. C Coronal cut with oscillating saw at the level of the posterior aspect of the orbit (Or). D Right lateral view of sheep head with coronal cut through zygomatic arch (Z), posterior to the mandible (M). P, pinna
Next, the anterior portion of the head was removed by sawing in a coronal plane at the level of the posterior aspect of the orbit (Fig. 2C). Skin and soft tissue were also sufficiently removed to identify and cut through the zygomatic arch at this level using the saw (Fig. 2D), and if necessary, through the posterior aspect of the mandible. The hyoid bone was also cut at this level, as the sheep hyoid bone extends posteriorly bilaterally and attaches to the temporal bone via its tympanohyoid segment. Because the saw blade was unable to reach the medial aspects of the skull, the mallet and chisel were used to finish separating the anterior and posterior portions of the head from each other. The anterior portion of the head was discarded.
The cervical vertebrae and neck were disarticulated from the head at the atlanto-occipital joint (Fig. 3) and discarded. Any remaining brain/brainstem tissue in the skull was removed from the foramen magnum inferiorly. The saw was used to cut the remaining posterior portion of the skull in half in a midsagittal plane, resulting in two temporal bones. The remaining tympanohyoid segment of the hyoid bone was removed from each temporal bone. Scalpel and periosteal elevator were used to remove as much remaining soft tissue as possible, including the pinnae if not already severed.
Fig. 3.
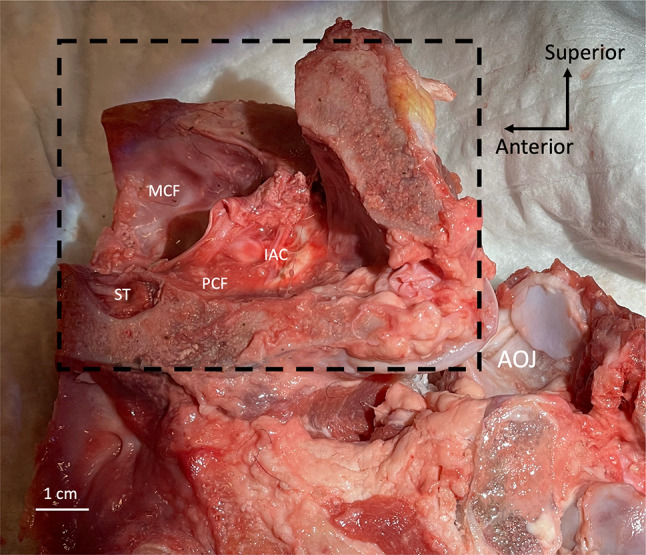
View from the medial side of right sheep temporal bone (dashed box) and neck showing severed right atlanto-occipital joint (AOJ), lateral surface of temporal bone resting on cloth. IAC, internal auditory canal; MCF, middle cranial fossa; PCF, posterior cranial fossa; ST, sella turcica
For the first sheep head, we initially removed the skin from the entire head and then removed the mandible, vertebrae, and neck before performing the craniotomy and severing the anterior portion of the head. We found that this approach allowed us to better visualize the anatomy to determine where to saw. We adopted the above-described approach for the remaining sheep heads, as we found that approach to be more time efficient.
Transmastoid Extended Facial Recess Approach
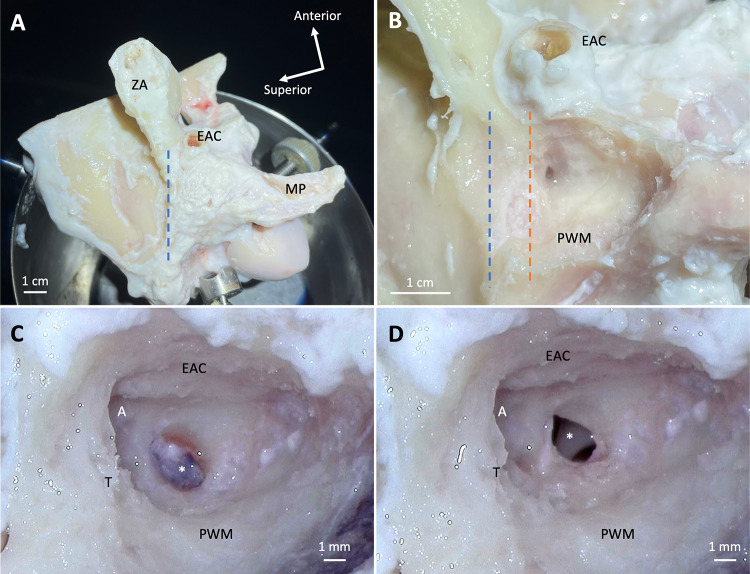
Each extracted temporal bone was secured in a temporal bone holder (Fig. 4A). Any remaining soft tissue inferior to the temporal line and posterior to the external auditory canal (EAC) was removed sharply or with a cutting burr. Mastoidectomy was started in standard fashion, and care was taken to identify but not violate the tegmen (the superior aspect of the mastoid cavity); its level approximated the superior aspect of the EAC in sheep (Fig. 4B).
Fig. 4.
Steps of surgical approach in sheep right temporal bone (different specimen in C and D). All photos depict surgical view. A Temporal bone secured in a temporal bone holder. B Temporal bone during mastoidectomy; dashed orange line depicts the level of the tegmen relative to the temporal line (dashed blue line). C Mastoid cavity with antrum (A) and visible middle ear mucosa (asterisk). D Mastoid cavity with incus (asterisk) visible deep to surgical opening to middle ear cavity after lysing the mucosa. EAC, external auditory canal; MP, mastoid process; PWM, posterior wall of mastoid; T, tegmen; ZA, zygomatic arch
Because the sheep mastoid cavity is poorly pneumatized and typical anatomic landmarks are difficult to identify, we adopted an approach of identifying and following the tegmen medially until identifying the antrum, which usually appeared to be a large air cell. It is difficult to appreciate the antrum in Fig. 4C due to the overhanging bone. Another view of the antrum is shown in Supplemental Fig. 1. Unlike in humans, the incus could not be identified readily upon identifying the antrum and performing mastoid antrostomy, even after widening the antrum and optimizing the microscopic view. We instead found that by drilling the bone about 2–3 mm inferior to the antrum (i.e., where the incus buttress would be in a human temporal bone), we could expose the thick, hyperplastic mucosa of the sheep middle ear (Fig. 4C), which we lysed using a curved (e.g., Rosen) needle. Deep to this space, the incus was visible (Fig. 4D). This surgical opening was the beginning of the facial recess, which could then be extended inferiorly. Care was taken not to drill the facial nerve; the tympanic segment lies behind the incus from our surgical perspective. The course of the facial nerve could then be determined by skeletonizing the mastoid segment of the facial nerve, the posterior boundary of the facial recess. The tympanic segment could be identified superior and posterior to the stapes, as well as medial to the short process of the incus before it enters the mastoid cavity. The chorda tympani was sacrificed upon extending the facial recess inferiorly to optimize middle ear exposure, creating an extended facial recess.
Micro-CT Scans
Each temporal bone was imaged using a micro-CT scanner (Quantum FX, PerkinElmer, Waltham, MA) at a voltage of 90 kV and current of 180 µA with a field of view (FOV) diameter of 40 × 40 mm focused on the inner, middle, and external ear and an isotropic voxel size of 80 µm. Each temporal bone was also imaged at a voltage of 90 kV and current of 180 µA with a FOV diameter of 73 × 57 mm and isotropic voxel size of 148 µm to ensure that the entire temporal bone was captured. The temporal bones from the first two sheep heads were imaged after performing the mastoidectomy, and the temporal bones from the third, fourth, and fifth sheep heads were imaged both before and after the mastoidectomy.
The open-source software 3D Slicer was used to measure anatomic dimensions of each temporal bone using multiplanar reconstruction [43]. The length of the bony EAC was measured as the distance between the head of the malleus and the lateral edge of the bony canal (Supplemental Figs. 2–3). The wide and narrow diameters of the EAC (i.e., the major and minor axes, respectively, if considering the canal to be an ellipse) were measured at the midpoint between the head of the malleus and lateral aspect of the bony EAC on a CT scan slice perpendicular to the canal (Supplemental Figs. 2–3). The circumference and area of the EAC were also measured using this slice (Supplemental Figs. 2–3). The perpendicular distance between the plane tangent to the superior aspect of the EAC and the roof of the mastoid cavity (the tegmen) was measured at the level of the posterior EAC as a measurement of the height of the tegmen (Supplemental Fig. 4). The shortest distance between the manubrium of the malleus and the cochlear promontory was measured using the 3D Slicer volume rendering (Supplemental Fig. 5).
Results
Temporal Bone Extraction
Temporal bone extraction was successful with the preservation of the inner, middle, and external ear in 10/10 temporal bones. Temporal bones could be secured in a standard temporal bone holder.
Transmastoid Extended Facial Recess Approach
The transmastoid extended facial recess surgical approach provides exposure of the malleus (including the manubrium), incus, and stapes, as well as the round window without obstruction by the facial nerve or sacrificing the facial nerve (Fig. 5). (The focus of the present study is on temporal bone extraction and a surgical approach to the middle ear and ossicles in sheep. We will detail round window access and consideration of this breed of sheep as a model for cochlear implantation in a subsequent publication.) The bony buttress, where the posterior incudal ligament attaches, does not obscure the view of the body of the incus and the manubrium of the malleus. The manubrium is long and extends anteroinferiorly deep into the middle ear cavity from the surgical view (Fig. 5B).
Fig. 5.
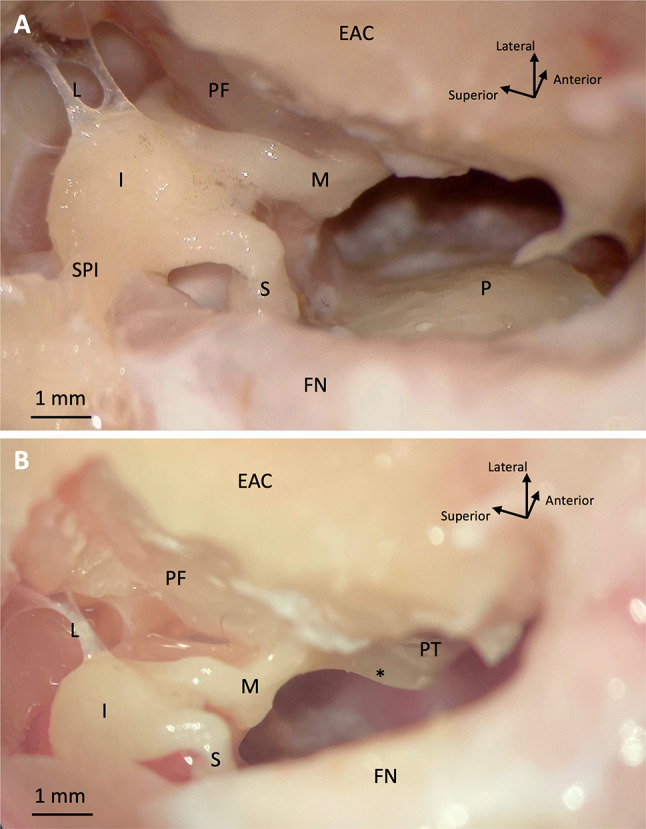
Microscopic views (A and B) of sheep right middle ear through extended facial recess surgical approach. EAC, external auditory canal; I, incus; FN, facial nerve; L, superior ligaments of incus; M, malleus; P, cochlear promontory; PF, pars flaccida; PT, pars tensa; S, stapes; SPI, incus short process; *, manubrium of malleus
The mastoid of all specimens was poorly pneumatized; as such, identification of standard anatomic landmarks typically visualized during human mastoid surgery was difficult. The semicircular canals in sheep are located further medially and deeper in the mastoid cavity compared to humans, so they are not in the vicinity of the surgical approach and are not available as an anatomic landmark. The roof of the sheep mastoid, the tegmen, is located inferior to the level of the temporal line and close to the level of the superior EAC (Fig. 4B). Unlike in humans, the tegmen in sheep sits below a soft tissue-filled space that contains the sheep temporal artery, rather than the middle cranial fossa and brain. The middle ear of the sheep has a thick, blue-purple mucosa that was apparent in most specimens and served as a landmark for entering the middle ear (Fig. 4C). In some specimens, the middle ear was entered without first seeing the middle ear mucosa.
The incus short process points in a more posteromedial direction in sheep compared to humans, so there is no need to surgically preserve an incus buttress that spans from the posterior canal wall to the posterior border of the facial recess in order to protect the posterior incudal ligament, as is done in human temporal bone surgery (Fig. 5A). Therefore, a larger portion of the ossicles, including the body of the incus and the incudomalleolar joint, was safely exposed. Compared to humans, the incus also lies deeper into the facial recess relative to the bony wall of the middle ear (Figs. 4D and 5). The sheep pars flaccida is large and friable (Fig. 5). The facial nerve was difficult to identify initially given the minimally pneumatized mastoid; however, it could be identified in all specimens by first identifying the incus and carefully skeletonizing the mastoid segment of the nerve (Fig. 5). The facial nerve was exposed in 5/10 specimens; it was noted to be thick compared to a human facial nerve. The chorda tympani was sacrificed in all specimens to optimize middle ear exposure.
Micro-CT Scans
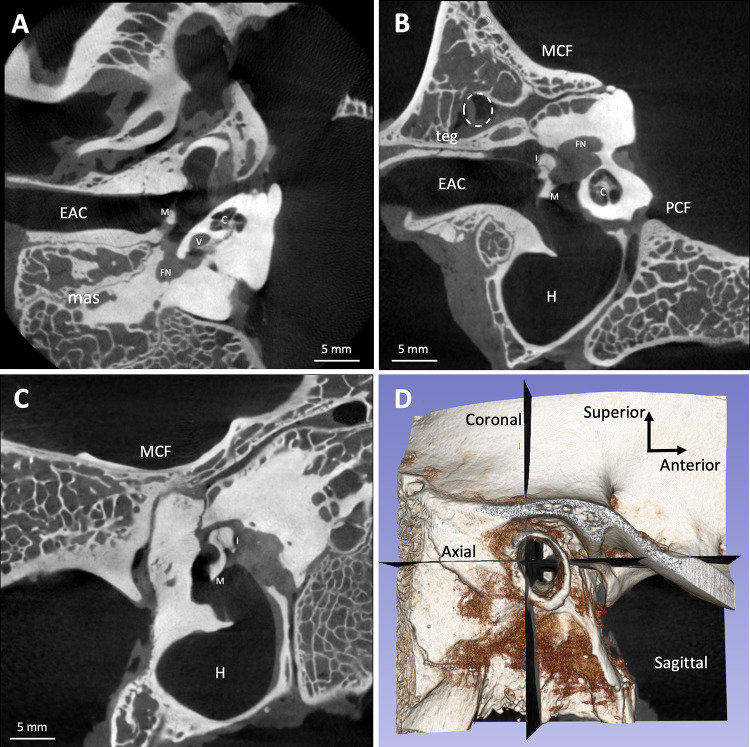
Micro-CT scans are available upon request. Presurgical CT scan slices of specimen 3R are shown in Fig. 6A-D and postsurgical CT scan slices are shown in Supplemental Fig. 6. Videos scrolling through the axial, coronal, and sagittal CT scans of specimen 3R (both presurgical and postsurgical) are also included as Supplemental Movies 1–6. Across all 10 temporal bones, the mean wide diameter of the EAC at its midpoint was 6.7 (SD 0.8) mm. The mean narrow diameter was 4.7 (SD 0.4) mm (Table 1). The mean area of the EAC was 23.9 (SD 5.5) mm2, and the mean circumference was 17.9 (SD 2.0) mm. The mean distance between the tegmen and roof of the EAC was 2.3 (SD 0.8) mm at the posterior aspect of the EAC. The mean distance between the manubrium and cochlear promontory was 1.7 (SD 0.2) mm.
Fig. 6.
Right sheep temporal bone CT scan slices (A axial, B coronal, C sagittal) with right lateral view of 3-dimensional volume rendering depicting the orientation of the slices (D). C, cochlea; EAC, external auditory canal; FN, facial nerve; H, hypotympanum; I, incus; M, malleus; mas, mastoid; MCF, middle cranial fossa; PCF, posterior cranial fossa; teg, tegmen; V, vestibule; white dashed circle, temporal artery
Table 1.
Sex, age, and anatomic measurements of each sheep temporal bone
| Temporal bone | Sex | Age (years) | EAC length (mm) | EAC wide diameter (mm) | EAC narrow diameter (mm) | EAC circumference (mm) | EAC area (mm2) | Tegmen heighta (mm) | Manubrium-promontory distance (mm) |
|---|---|---|---|---|---|---|---|---|---|
| 1L | F | 5 | 15.8 | 7.1 | 4.4 | 18.5 | 24.6 | 2.0 | 1.3 |
| 1R | F | 5 | 15.1 | 7.0 | 4.5 | 18.8 | 25.5 | 2.7 | 1.5 |
| 2L | F | 3 | 13.0 | 5.5 | 4.6 | 16.1 | 20.0 | 1.5 | 2.1 |
| 2R | F | 3 | 12.2 | 6.1 | 5.3 | 18.0 | 24.8 | 1.2 | 2.1 |
| 3L | F | 8 | 15.0 | 6.1 | 3.8 | 15.6 | 16.8 | 2.3 | 1.6 |
| 3R | F | 8 | 16.0 | 7.5 | 4.8 | 16.5 | 20.2 | 3.4 | 1.6 |
| 4L | M | 0.75 | 14.7 | 6.0 | 4.5 | 16.7 | 21.2 | 4.0 | 1.6 |
| 4R | M | 0.75 | 15.0 | 6.1 | 4.5 | 16.7 | 21.0 | 3.2 | 1.7 |
| 5L | F | 5 | 15.2 | 8.1 | 5.3 | 22.1 | 35.8 | 3.3 | 1.7 |
| 5R | F | 5 | 16.5 | 7.5 | 4.9 | 20.2 | 29.4 | 3.6 | 1.7 |
| Mean | 14.9 | 6.7 | 4.7 | 17.9 | 23.9 | 2.7 | 1.7 | ||
| SD | 1.3 | 0.8 | 0.4 | 2.0 | 5.5 | 0.9 | 0.2 | ||
| Meanf | 14.9 | 6.9 | 4.7 | 18.2 | 24.6 | 2.5 | 1.7 | ||
| SDf | 1.5 | 0.9 | 0.5 | 2.2 | 6.0 | 0.9 | 0.3 |
EAC, external auditory canal, SD, standard deviation, R, right; L, left
aMeasured from the superior aspect of EAC at the posterior aspect of EAC
fThis denotes the mean and standard deviation of only the adult female sheep measurements (excluding 4L and 4R)
Discussion
This study determined a method for temporal bone extraction from Hampshire sheep heads, which will aid in otology research using sheep. We identified appropriate anatomic landmarks for temporal bone extraction using an oscillating saw while preserving the inner, middle, and external ear. Our method is similar to the skull base block method for human cadaveric temporal bone removal described by Dinh et al. [44]. Similar to their coronal cut at the level of the optic foramen in humans, we make a coronal cut at the level of the posterior aspect of the orbit in sheep. We also similarly make a midsagittal cut through the foramen magnum and occipital bone to separate the posterior skull into two temporal bones. The exact order in which some of these steps are carried out for temporal bone extraction in sheep is not particularly important. Although for our first sheep head, we found that completely removing the skin from the sheep head and first removing the mandible and neck allowed us to better identify the landmarks and determine where to cut, the approach we ultimately adopted for subsequent sheep was more time efficient.
Péus et al. also extracted sheep temporal bones using an oscillating saw; however, they do not describe their approach in detail [9]. They do mention that they precisely cut the cranial nerves prior to the removal of the brain and brainstem. We found that the cranial nerves separated easily upon removal of the brain, but they can be specifically cut beforehand, particularly the facial nerve and vestibulocochlear nerve, which run through the inner ear. In some cases, it is possible to make the coronal cut at the level of the posterior aspect of the orbit completely posterior to the mandible and avoid sawing into the mandible; however, this was only the case in two of the sheep heads, perhaps owing to anatomic differences across sheep in addition to taking care to avoid sawing into the ear. The extracted temporal bones can be stored in a freezer after wetting with saline and wrapping in plastic wrap and aluminum foil. They can alternatively be stored in the fridge in a solution of saline with several drops of betadine, changing the solution every several days; however, we found that the facial nerve tends to swell after prolonged soaking.
We also determined a facial recess (i.e., a posterior tympanotomy) approach for surgical access to the middle ear in sheep without sacrifice of the facial nerve or canal-wall-down mastoidectomy. The chorda tympani was sacrificed to optimize middle ear exposure, resulting in an extended facial recess approach. This surgical approach is especially applicable to live-animal trials of hearing devices that are implanted via a facial recess approach to increase generalizability to implantation in humans. Prior studies have required a canal-wall-down mastoidectomy and/or sacrifice of the facial nerve to optimize middle ear exposure in sheep [25, 26]. Kaufmann et al. adopted a retrofacial approach to access the round window membrane in their in vivo cochlear implantation survival study in Suffolk-Dorset sheep owing to sheep developing facial paralysis after attempting a facial recess approach, which required decompression and mobilization of the facial nerve [35]. A retrofacial approach involves exposing the round window via an approach posterior to the facial nerve rather than anterior to it. However, this approach would not achieve exposure of the ossicles. A combination of our surgical approach and the anatomy of the Hampshire sheep breed apparently allows for an extended facial recess approach. The facial nerve was exposed in 5/10 of the temporal bones; we intentionally decompressed the facial nerve in the first temporal bone to delineate its course in the mastoid cavity.
A notable difference between sheep and human is a low-lying mastoid cavity roof (tegmen) in sheep. Superior to the roof of the mastoid in sheep is a soft tissue-filled space, whereas in humans, the tegmen sits under the middle cranial fossa and brain. Per our micro-CT scans, this space in sheep contains a blood vessel running anteriorly (Fig. 6B), identified as the temporal artery in Fig. 2 of Gurr et al. [1]. The low-lying sheep tegmen is therefore an important consideration for in vivo sheep surgery, as violating this space may result in hemorrhage of the temporal artery. The temporal artery also descends as it approaches the coronal plane of the ear canal from posterior to anterior. We found the sheep tegmen to be grossly inferior to the level of the temporal line (Fig. 4B) and 2.7 (SD 0.9) mm superior to the roof of the ear canal per the micro-CT scan measurements (Fig. 6B). For comparison, a retrospective study of high-resolution CT scans of 105 human patients with chronic suppurative otitis media found the average tegmen height relative to the ear canal roof to be 8.4 (SD 3.1) mm laterally and 9.7 (SD 1.5) mm medially [45]. Notably, this is a patient population that tends to have a lower tegmen than the average cochlear implant recipient without chronic ear disease. Thus, surgeons must take extra care to avoid drilling too far superiorly in the sheep mastoid.
Because of the minimally pneumatized mastoid cavity and absence of typical anatomic landmarks, we adopted an approach of identifying and closely following the tegmen medially until identifying the antrum, which was apparent in most specimens. In contrast to Gurr et al. who state that the antrum cannot be identified, we found that many of the specimens had a small identifiable antrum just inferior to the tegmen once the drilling was extended sufficiently medially. Unlike in humans, the incus could not be identified readily after performing mastoid antrostomy, even after widening the antrum and optimizing the microscopic view. However, after identifying the antrum, the blue-purple, thick middle ear mucosa of the sheep could typically be identified by drilling the bone inferior to the antrum (Fig. 4C), and upon lysing the mucosa, the incus was visible deep to the bony wall of the middle ear (Fig. 4D). Another notable difference from human anatomy is that the incus short process in sheep points in a more posteromedial direction compared to humans (Fig. 5A), so there is no need to surgically preserve an incus buttress that spans from the posterior canal wall to the posterior border of the facial recess in order to protect the posterior incudal ligament. This has implications for the insertion of implantable middle ear hearing devices in sheep, as it allows for improved visibility and maneuverability.
To our knowledge, the thick, hyperplastic middle ear mucosa in sheep, which serves as a useful anatomic landmark delineating the middle ear, has not been reported in sheep literature. When we noticed it, we initially mistook this for the pars flaccida, which is large and friable in sheep. Because we used postmortem specimens, it is possible that the hyperplastic middle ear mucosa was due to postmortem artifact. However, it is notable that Yildiz et al. similarly report a hyperplastic middle ear mucosa in live pigs. They sometimes mistook this for the round window membrane [46]. Our surgical approach also involves sacrifice of the chorda tympani nerve, technically making this an extended facial recess approach. Other studies do not mention the chorda tympani other than Schnabl et al., who state that the sheep chorda tympani has a similar course to humans [5].
We also found that the sheep semicircular canals are located further medially compared to humans and are therefore not in the vicinity of our surgical approach. Kaufmann et al. did observe cases of intraoperative violation of the semicircular canals in their in vivo survival sheep cochlear implantation, which may have been a result of their retrofacial approach [35]. Additionally, because the sheep mastoid cavity is further inferior to the skull base than in humans (Fig. 6B), the sigmoid sinus does not run through the mastoid cavity and is not an available anatomic landmark.
Overall, we found the mastoidectomy and facial recess approach in sheep to be more technically challenging than in human temporal bones. The small, poorly pneumatized mastoid cavity and low-lying tegmen make the surgery more similar to a human patient with chronic ear disease. Although Fermi et al. claim that sheep are not a suitable candidate for transmastoid surgical training [47], these technical challenges could improve otologic surgical technique in trainees by simulating a human temporal bone affected by chronic ear disease.
Our measurements of the EAC dimensions (wide diameter of 6.7 [SD 0.8] mm and narrow diameter of 4.7 [SD 0.4] mm) are similar to other measurements reported in the literature. Seibel et al. report a mean horizontal diameter of the EAC in sheep of 3.9 mm (measured in an axial plane) and a mean EAC length of 14.1 mm (measured in a coronal plane) in 6-year-old adult Corriedale-Texel cross sheep. However, they did not use multiplanar reconstruction, which could result in overestimates. Schnabl et al. found a mean EAC minimal diameter of 4.9 and mean EAC length of 11 mm in 4–8-month-old lambs using multiplanar reconstruction of CT scans with an effective resolution of 0.7 mm. To our knowledge, we are the only study to measure these dimensions using multiplanar reconstruction and a slice thickness of 80 µm. We recognize that we have a heterogenous sample of temporal bones given the inclusion of temporal bones from one male lamb and four adult females, so we have also included the mean and standard deviation of only the adult female sheep measurements in Table 1.
When procuring sheep for auditory research, it is recommended to take precautions around Q fever because sheep can be a reservoir for the bacterium Coxiella burnetii, the causative organism of Q fever. Transmission occurs via aerosols, so any sawing or drilling can result in exposure. Therefore, we ensured that our specimens came from sheep that had negative Q fever antibody testing.
Our study is limited by a sample size of five sheep heads (10 temporal bones). Due to irregularities in the shape of an individual EAC along its length, the dimensions at the midpoint (width, area, and circumference) do not necessarily reflect the dimensions along its entire length. As mentioned previously, we had a heterogenous sample of specimens, given the inclusion of two male lamb temporal bones with eight adult female sheep temporal bones. There may also be differences in sheep breed such that our surgical approach and findings in Hampshire sheep are not generalizable to other breeds. In order to translate our approach to live sheep, in future studies, we will demonstrate this surgical approach in whole-head cadavers. It is notable that the transmastoid facial recess surgical approach for our first two specimens was performed successfully in a whole head prior to temporal bone extraction, suggesting that the approach described here is translatable.
Conclusion
Cadaveric sheep temporal bones are a useful starting point for determining and practicing a surgical approach for adapting an implantable hearing device to sheep anatomy for the purposes of live-animal trials. Extracted sheep temporal bones are easy to store and secure in a temporal bone holder for drilling, and they are suitable for improving otologic surgical technique in trainees by simulating a human temporal bone affected by chronic ear disease. Our extended facial recess surgical approach provides exposure of the malleus (including the manubrium), incus, and stapes, as well as the round window without sacrificing the facial nerve in Hampshire sheep. Further research can continue to explore differences across breeds of sheep and the application of this surgical approach to live-animal trials. Our future work will involve using this cadaveric approach to test middle ear implants and cochlear implants in preparation for in vivo studies in sheep.
In summary, our method for temporal bone extraction is as follows:
Peel skin laterally to expose superior cranium for craniotomy and removal of brain
Saw in a coronal plane at posterior aspect of orbits and discard anterior portion of head
Disarticulate cervical vertebrae at atlanto-occipital joint and discard neck
Saw posterior skull in midsagittal plane and remove remaining soft tissue
Our extended facial recess surgical approach is as follows:
Begin mastoidectomy in standard fashion posterior to ear canal and inferior to temporal line
Follow tegmen medially until identifying antrum
Drill the bone 2–3 mm inferior to the antrum to expose middle ear mucosa and lyse middle ear mucosa to expose and identify incus
Extend facial recess inferiorly, skeletonizing facial nerve and sacrificing chorda tympani to optimize middle ear exposure
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1. Video scrolling through the axial CT scan of presurgical specimen 3R from inferior to superior. (MOV 82236 KB)
Supplementary file2. Video scrolling through the coronal CT scan of presurgical specimen 3R from posterior to anterior. (MOV 92701 KB)
Supplementary file3. Video scrolling through the sagittal CT scan of presurgical specimen 3R from right (lateral) to left (medial). (MOV 105851 KB)
Supplementary file4. Video scrolling through the axial CT scan of postsurgical specimen 3R from inferior to superior. (MOV 75986 KB)
Supplementary file5. Video scrolling through the coronal CT scan of postsurgical specimen 3R from posterior to anterior. (MOV 91274 KB)
Supplementary file6. Video scrolling through the sagittal CT scan of postsurgical specimen 3R from right (lateral) to left (medial). (MOV 67198 KB)
Acknowledgements
We acknowledge Jonathan May of May Family Enterprises Inc. for supplying the sheep specimens. We also acknowledge Christopher B. Damoci, Manager of the Oncology Precision Therapeutics and Imaging Core (OPTIC) at Columbia University Medical Center, for his assistance with the micro-CT scans. The Columbia University Medical Center Cancer Center Support Grant (CCSG), NIH grant #P30 CA013696 (National Cancer Institute) partially funds this shared resource. We also acknowledge Yew Song Cheng, MD, for his comments and suggestions on the paper.
Funding
This work was supported by funding from the National Institute on Deafness and Other Communication Disorders (NIDCD) R01 DC016874.
Data Availability
Micro-CT scan files are available upon request.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alexander Chern and Brandon J. Vilarello contributed equally to this work.
References
- 1.Gurr A, Pearson MD, Dazert S. Lambs' temporal bone anatomy under didactic aspects. Braz J Otorhinolaryngol. 2011;77(1):51–57. doi: 10.1590/s1808-86942011000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soares HB, Lavinsky L. Histology of sheep temporal bone. Braz J Otorhinolaryngol. 2011;77(3):285–292. doi: 10.1590/s1808-86942011000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seibel VA, Lavinsky L, De Oliveira JA. Morphometric study of the external and middle ear anatomy in sheep: a possible model for ear experiments. Clin Anat. 2006;19(6):503–509. doi: 10.1002/ca.20218. [DOI] [PubMed] [Google Scholar]
- 4.Seibel VA, Lavinsky L, Irion K. CT-Scan sheep and human inner ear morphometric comparison. Braz J Otorhinolaryngol. 2006;72(3):370–376. doi: 10.1016/s1808-8694(15)30971-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnabl J, Glueckert R, Feuchtner G, Recheis W, Potrusil T, Kuhn V, Wolf-Magele A, Riechelmann H, Sprinzl GM. Sheep as a large animal model for middle and inner ear implantable hearing devices: a feasibility study in cadavers. Otol Neurotol. 2012;33(3):481–489. doi: 10.1097/MAO.0b013e318248ee3a. [DOI] [PubMed] [Google Scholar]
- 6.Han S, Suzuki-Kerr H, Suwantika M, Telang RS, Gerneke DA, Anekal PV, Bird P, Vlajkovic SM, Thorne PR. Characterization of the sheep round window membrane. J Assoc Res Otolaryngol. 2021;22(1):1–17. doi: 10.1007/s10162-020-00778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerneis S, Escoffre J-M, Galvin JJ, Bouakaz A, Presset A, Alix C, Oujagir E, Lefèvre A, Emond P, Blasco H, Bakhos D (2023) Sonoporation of the round window membrane on a sheep model: a safety study. Pharmaceutics 15(2). 10.3390/pharmaceutics15020442 [DOI] [PMC free article] [PubMed]
- 8.Ames DR, Arehart LA. Physiological response of lambs to auditory stimuli. J Anim Sci. 1972;34(6):994–998. doi: 10.2527/jas1972.346994x. [DOI] [PubMed] [Google Scholar]
- 9.Peus D, Dobrev I, Prochazka L, Thoele K, Dalbert A, Boss A, Newcomb N, Probst R, Roosli C, Sim JH, Huber A, Pfiffner F. Sheep as a large animal ear model: middle-ear ossicular velocities and intracochlear sound pressure. Hear Res. 2017;351:88–97. doi: 10.1016/j.heares.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Peus D, Dobrev I, Pfiffner F, Sim JH. Comparison of sheep and human middle-ear ossicles: anatomy and inertial properties. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020;206(5):683–700. doi: 10.1007/s00359-020-01430-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFadden D, Pasanen EG, Valero MD, Roberts EK, Lee TM. Dissociation between distortion-product and click-evoked otoacoustic emissions in sheep (Ovis aries) J Acoust Soc Am. 2008;124(6):3730–3738. doi: 10.1121/1.2982402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFadden D, Pasanen EG, Valero MD, Roberts EK, Lee TM. Effect of prenatal androgens on click-evoked otoacoustic emissions in male and female sheep (Ovis aries) Horm Behav. 2009;55(1):98–105. doi: 10.1016/j.yhbeh.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maia FC, Lavinsky L, Mollerke RO, Duarte ME, Pereira DP, Maia JE. Distortion product otoacoustic emissions in sheep before and after hyperinsulinemia induction. Braz J Otorhinolaryngol. 2008;74(2):181–187. doi: 10.1016/s1808-8694(15)31086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuma e Maia FC, Lavinsky L (2006) Distortion product otoacoustic emissions in an animal model of induced hyperinsulinemia. Int Tinnitus J 12(2):133–9 [PubMed]
- 15.Angeli RD, Lavinsky L, Dolganov A. Alterations in cochlear function during induced acute hyperinsulinemia in an animal model. Braz J Otorhinolaryngol. 2009;75(5):760–764. doi: 10.1016/s1808-8694(15)30530-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boullaud L, Blasco H, Caillaud E, Emond P, Bakhos D (2022) Immediate-early modifications to the metabolomic profile of the perilymph following an acoustic trauma in a sheep model. J Clin Med 11(16). 10.3390/jcm11164668 [DOI] [PMC free article] [PubMed]
- 17.Pohl F, Paasche G, Lenarz T, Schuon R. Tympanometric measurements in conscious sheep - a diagnostic tool for pre-clinical middle ear implant studies. Int J Audiol. 2017;56(1):53–61. doi: 10.1080/14992027.2016.1227480. [DOI] [PubMed] [Google Scholar]
- 18.Gocer C, Eryilmaz A, Genc U, Dagli M, Karabulut H, Iriz A. An alternative model for stapedectomy training in residency program: sheep cadaver ear. Eur Arch Otorhinolaryngol. 2007;264(12):1409–1412. doi: 10.1007/s00405-007-0437-3. [DOI] [PubMed] [Google Scholar]
- 19.Cordero A, del mar Medina M, Alonso A, Labatut T (2011) Stapedectomy in sheep: an animal model for surgical training. Otol Neurotol 32(5):742–7. 10.1097/MAO.0b013e31821ddbc2 [DOI] [PubMed]
- 20.Cordero A, Benitez S, Reyes P, Vaca M, Polo R, Perez C, Alonso A, Cobeta I. Ovine ear model for fully endoscopic stapedectomy training. Eur Arch Otorhinolaryngol. 2015;272(9):2167–2174. doi: 10.1007/s00405-014-3114-3. [DOI] [PubMed] [Google Scholar]
- 21.Anschuetz L, Bonali M, Ghirelli M, Mattioli F, Villari D, Caversaccio M, Presutti L. An ovine model for exclusive endoscopic ear surgery. JAMA Otolaryngol Head Neck Surg. 2017;143(3):247–252. doi: 10.1001/jamaoto.2016.3315. [DOI] [PubMed] [Google Scholar]
- 22.Beckmann S, Yacoub A, Fernandez IJ, Niederhauser L, Fermi M, Caversaccio M, Bonali M, Anschuetz L. Exclusive endoscopic laser-stapedotomy: feasibility of an ovine training model. Otol Neurotol. 2021;42(7):994–1000. doi: 10.1097/MAO.0000000000003168. [DOI] [PubMed] [Google Scholar]
- 23.Shrivastava T, Khan MM, Parab SR. Learning Curve of Two Handed Endoscopic Ear Surgery on Sheep Temporal Bone: A Fellow's Perspective. Indian J Otolaryngol Head Neck Surg. 2022;74(Suppl 1):550–558. doi: 10.1007/s12070-021-02388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okhovat S, Milner TD, Iyer A. Feasibility of ovine and synthetic temporal bone models for simulation training in endoscopic ear surgery. J Laryngol Otol. 2019;133(11):966–973. doi: 10.1017/S0022215119002135. [DOI] [PubMed] [Google Scholar]
- 25.Mantokoudis G, Huth ME, Weisstanner C, Friedrich HM, Nauer C, Candreia C, Caversaccio MD, Senn P. Lamb temporal bone as a surgical training model of round window cochlear implant electrode insertion. Otol Neurotol. 2016;37(1):52–56. doi: 10.1097/MAO.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 26.Trinh TT, Cohen C, Boullaud L, Cottier JP, Bakhos D. Sheep as a large animal model for cochlear implantation. Braz J Otorhinolaryngol. 2021 doi: 10.1016/j.bjorl.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisstanner C, Mantokoudis G, Huth M, Verma RK, Nauer C, Senn P, Caversaccio MD, Wagner F. Radiation dose reduction in postoperative computed position control of cochlear implant electrodes in lambs - an experimental study. Int J Pediatr Otorhinolaryngol. 2015;79(12):2348–2354. doi: 10.1016/j.ijporl.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 28.Lavinsky L, Goycoolea M, Gananca MM, Zwetsch Y. Surgical treatment of vertigo by utriculostomy: an experimental study in sheep. Acta Otolaryngol. 1999;119(5):522–527. doi: 10.1080/00016489950180739. [DOI] [PubMed] [Google Scholar]
- 29.Neudert M, Beleites T, Ney M, Kluge A, Lasurashvili N, Bornitz M, Scharnweber D, Zahnert T. Osseointegration of titanium prostheses on the stapes footplate. J Assoc Res Otolaryngol. 2010;11(2):161–171. doi: 10.1007/s10162-009-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piu F, Wang X, Fernandez R, Dellamary L, Harrop A, Ye Q, Sweet J, Tapp R, Dolan DF, Altschuler RA, Lichter J, LeBel C. OTO-104: a sustained-release dexamethasone hydrogel for the treatment of otic disorders. Otol Neurotol. 2011;32(1):171–179. doi: 10.1097/MAO.0b013e3182009d29. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Fernandez R, Dellamary L, Harrop A, Ye Q, Lichter J, Lau D, Lebel C, Piu F. Pharmacokinetics of dexamethasone solution following intratympanic injection in guinea pig and sheep. Audiol Neurootol. 2011;16(4):233–241. doi: 10.1159/000320611. [DOI] [PubMed] [Google Scholar]
- 32.Larsson A, Andersson M, Wigren S, Pivodic A, Flynn M, Nannmark U. Soft tissue integration of hydroxyapatite-coated abutments for bone conduction implants. Clin Implant Dent Relat Res. 2015;17(Suppl 2):e730–e735. doi: 10.1111/cid.12304. [DOI] [PubMed] [Google Scholar]
- 33.Larsson A, Wigren S, Andersson M, Ekeroth G, Flynn M, Nannmark U. Histologic evaluation of soft tissue integration of experimental abutments for bone anchored hearing implants using surgery without soft tissue reduction. Otol Neurotol. 2012;33(8):1445–1451. doi: 10.1097/MAO.0b013e318268d4e0. [DOI] [PubMed] [Google Scholar]
- 34.Taghavi H, Hakansson B, Eeg-Olofsson M, Johansson CB, Tjellstrom A, Reinfeldt S, Bergqvist T, Olsson J. A vibration investigation of a flat surface contact to skull bone for direct bone conduction transmission in sheep skulls in vivo. Otol Neurotol. 2013;34(4):690–698. doi: 10.1097/MAO.0b013e3182877aee. [DOI] [PubMed] [Google Scholar]
- 35.Kaufmann CR, Tejani VD, Fredericks DC, Henslee AM, Sun DQ, Abbas PJ, Hansen MR. Pilot evaluation of sheep as in vivo model for cochlear implantation. Otol Neurotol. 2020;41(5):596–604. doi: 10.1097/MAO.0000000000002587. [DOI] [PubMed] [Google Scholar]
- 36.Anso J, Stahl C, Gerber N, Williamson T, Gavaghan K, Rosler KM, Caversaccio MD, Weber S, Bell B. Feasibility of using EMG for early detection of the facial nerve during robotic direct cochlear access. Otol Neurotol. 2014;35(3):545–554. doi: 10.1097/MAO.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 37.Wyss Balmer T, Anso J, Muntane E, Gavaghan K, Weber S, Stahel A, Buchler P. In-vivo electrical impedance measurement in mastoid bone. Ann Biomed Eng. 2017;45(4):1122–1132. doi: 10.1007/s10439-016-1758-4. [DOI] [PubMed] [Google Scholar]
- 38.Feldmann A, Anso J, Bell B, Williamson T, Gavaghan K, Gerber N, Rohrbach H, Weber S, Zysset P. Temperature prediction model for bone drilling based on density distribution and in vivo experiments for minimally invasive robotic cochlear implantation. Ann Biomed Eng. 2016;44(5):1576–1586. doi: 10.1007/s10439-015-1450-0. [DOI] [PubMed] [Google Scholar]
- 39.Anso J, Balmer TW, Jegge Y, Kalvoy H, Bell BJ, Dur C, Calvo EM, Williamson TM, Gerber N, Ferrario D, Forterre F, Buchler P, Stahel A, Caversaccio MD, Weber S, Gavaghan KA. Electrical impedance to assess facial nerve proximity during robotic cochlear implantation. IEEE Trans Biomed Eng. 2019;66(1):237–245. doi: 10.1109/TBME.2018.2830303. [DOI] [PubMed] [Google Scholar]
- 40.Henslee AM, Kaufmann CR, Andrick MD, Reineke PT, Tejani VD, Hansen MR. Development and characterization of an electrocochleography-guided robotics-assisted cochlear implant array insertion system. Otolaryngol Head Neck Surg. 2022;167(2):334–340. doi: 10.1177/01945998211049210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Djinovic Z, Pavelka R, Tomic M, Sprinzl G, Plenk H, Losert U, Bergmeister H, Plasenzotti R. In-vitro and in-vivo measurement of the animal’s middle ear acoustical response by partially implantable fiber-optic sensing system. Biosens Bioelectron. 2018;103:176–181. doi: 10.1016/j.bios.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Pfiffner F, Prochazka L, Dobrev I, Klein K, Sulser P, Péus D, Sim JH, Dalbert A, Röösli C, Obrist D, Huber A (2018) Proof of concept for an intracochlear acoustic receiver for use in acute large animal experiments. Sensors (Basel) 18(10). 10.3390/s18103565 [DOI] [PMC free article] [PubMed]
- 43.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinh C, Szczupak M, Moon S, Angeli S, Eshraghi A, Telischi FF. Human temporal bone removal: the skull base block method. J Neurol Surg B Skull Base. 2015;76(4):278–280. doi: 10.1055/s-0034-1543972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makki FM, Amoodi HA, van Wijhe RG, Bance M. Anatomic analysis of the mastoid tegmen: slopes and tegmen shape variances. Otol Neurotol. 2011;32(4):581–588. doi: 10.1097/MAO.0b013e31820e75f7. [DOI] [PubMed] [Google Scholar]
- 46.Yildiz E, Gerlitz M, Gadenstaetter AJ, Landegger LD, Nieratschker M, Schum D, Schmied M, Haase A, Kanz F, Kramer AM, Glueckert R, Staecker H, Honeder C, Arnoldner C (2022) Single-incision cochlear implantation and hearing evaluation in piglets and minipigs. Hear Res 426:108644. 10.1016/j.heares.2022.108644 [DOI] [PubMed]
- 47.Fermi M, Chiari F, Mattioli F, Bonali M, Molinari G, Alicandri-Ciufelli M, Anschuetz L, Fernandez IJ, Presutti L (2022) Surgical training on ex vivo ovine model in otolaryngology head and neck surgery: a comprehensive review. Int J Environ Res Public Health 19(6). 10.3390/ijerph19063657 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1. Video scrolling through the axial CT scan of presurgical specimen 3R from inferior to superior. (MOV 82236 KB)
Supplementary file2. Video scrolling through the coronal CT scan of presurgical specimen 3R from posterior to anterior. (MOV 92701 KB)
Supplementary file3. Video scrolling through the sagittal CT scan of presurgical specimen 3R from right (lateral) to left (medial). (MOV 105851 KB)
Supplementary file4. Video scrolling through the axial CT scan of postsurgical specimen 3R from inferior to superior. (MOV 75986 KB)
Supplementary file5. Video scrolling through the coronal CT scan of postsurgical specimen 3R from posterior to anterior. (MOV 91274 KB)
Supplementary file6. Video scrolling through the sagittal CT scan of postsurgical specimen 3R from right (lateral) to left (medial). (MOV 67198 KB)
Data Availability Statement
Micro-CT scan files are available upon request.