Abstract
In the current study, we investigated the impacts of 6 weeks of aerobic interval training (AIT) with selenium nanoparticles (SeNPs) on muscle, serum, and lung irisin (FNDC5) and Sema3A in rats exposed to cigarette smoke extract (CSE). To this end, 49 male Wistar rats (8 weeks old) were divided into seven groups: control, SeNPs (2.5 mg/kg b.w by oral gavage, 3 days/week, 6 weeks), AIT (49 min/day, 5 days/week for 6 weeks, interval), SeNPs + AIT, CSE (150 µL by IP injection, 1 day/week for 6 weeks), CSE + AIT, and CSE + SeNPs + AIT. The CSE group showed a significant reduction in irisin and Sema3A serum levels, as well as a decrease in FNDC5 and Sema3A gene expression in lung tissue (p < 0.05). A combined treatment (AIT with SeNPs) significantly increased the serum level and the expression of muscle and lung irisin (FNDC5) and Sema3A in CSE received groups (p < 0.05). There was a positive and significant correlation between muscle FNDC5 and lung FNDC5 in the CSE + SeNPs + AIT group (r = 0.92, p = 0.025). In addition, there was a positive and significant correlation between serum Sema3A and lung Sema3A of CSE + SeNPs + AIT group (r = 0.97, p = 0.004). Seemingly, performing aerobic exercises with the antioxidant and anti-inflammatory supplement nano-selenium in the model of lung damage (similar to COPD) can boost myokine irisin and Sema3A, especially in serum and lung tissue. These results displayed the paracrine/endocrine regulatory function of these myokines on other tissues. In other words, these interventions emphasized the creation of crosstalk between skeletal muscles and damaged lung, focusing on its recovery; however, further research is needed.
Keywords: Aerobic exercise, Selenium nanoparticles, Cigarette smoking, Chronic obstructive pulmonary disease, Skeletal muscle
Introduction
Over one billion people smoke tobacco globally (Based on WHO), and 80% of them are in the developing countries. It is projected that over eight million people will be killed by tobacco consumption (Organization 2014; Todorović et al. 2022). Cigarette smoke has countless harmful substances that have a negative and destructive effect on the respiratory system. Based on the relevant studies, it has been indicated that cigarette smoke contains about 4500 harmful components, such as carbon monoxide, nicotine, oxidants, fine particles, and aldehydes. One study found that staining because of smoking was one of the most common complications associated with zirconia-based crowns (Güncü et al. 2015). This suggests that smoking may have a negative impact on the appearance of zirconia crowns. These harmful substances are considered to be the main cause of pathogenesis and development of lung diseases (Hou et al. 2019). Studies have shown long-term smoking can enhance damage to the structure of the airway wall, alveolar septum, and cause interstitial fibrosis (Baraldo et al. 2012; Hou et al. 2019; Zong et al. 2019). Reportedly, smoking and pollutants in cigarette smoke are the risk factors for chronic obstructive pulmonary diseases (COPD) (Zhu et al. 2018).
Cigarette smoke (SC) is one of the main factors in the spread of COPD. A study on Chinese subjects demonstrated that smoking more than 20 packs per year tripled the prevalence of COPD (Wang et al. 2018a, b). Meanwhile, a long-term exposure to secondhand smoke can also be associated with the prevalence of COPD (Yin et al. 2007). COPD disease can cause damage to the epithelial tissue of the lung and in turn damages tissue and cell functions in the lung (Amatngalim and Hiemstra 2018). In addition to these lung tissue injuries, COPD patients suffer from osteoporosis, muscle atrophy, and cardiopulmonary disease (Hoang et al. 2023; Kawut 2013; Li et al. 2022). Atrophy in the skeletal muscle was associated with inflammation, chronic hypoxia, and oxidative stress (OS) response, all of which destroy the balance of protein synthesis and degradation in COPD (Han et al. 2022). Most studies have linked the relationship between COPD and skeletal muscle tissue to the muscle atrophy (Han et al. 2022; Langen et al. 2013). This is while muscle atrophy leads to a decrease in the secretion of myokines (Alizadeh Pahlavani 2022; Piccirillo 2019).
Irisin (as myokine) increments occur with skeletal muscle contraction which is associated with the beneficial effects of exercise training (Waseem et al. 2023). Based on the primary studies, irisin is secreted from fibronectin type III domain-containing protein 5 (FNDC5) of skeletal muscle (Lavi et al. 2022). In other words, irisin is derived from the FNDC5 through its extracellular fragment proteolytic cleavage and is secreted in the peripheral circulation to be received by other organs (Korta et al. 2019). In preliminary studies, it was found that irisin is produced by skeletal muscle impacting white adipose tissue (Pedersen and Febbraio 2012). In other words, the increment in muscle contraction caused by exercise leads to an increase in serum irisin, and the induced irisin can convert white adipose tissue into brown adipose tissue, thereby minimizing the inflammation caused by adipose tissue (Maalouf and El Khoury 2019). Meanwhile, it was found that irisin has a positive effect not only on fatty tissue, but also on other tissues, such as bone, heart, liver, and other tissues (Aydin et al. 2014). In muscle tissue, irisin affects the differentiation of myogenic from myoblast fusion with activation of IL6 signaling (Reza et al. 2017). One of the target tissues of irisin is the lung tissue (Ma and Chen 2021). In COPD patients, it has been displayed that the serum irisin level is significantly reduced that is relevant to the patient's physical activity level (Ijiri et al. 2015). The studies investigating irisin levels in COPD patients found a correlation between irisin and body composition indices, such as muscle mass (MM), fat-free mass (FFM), and FFM index (FFMI) (Sugiyama et al. 2017). It has been indicated that in the lung tissue of emphysema patients, irisin can activate Nrf2 signaling. In other words, irisin upregulated the Nrf2 gene and reduced the cigarette smoke extract (CSE)-induced apoptosis of A549 cells (Kubo et al. 2019). Sugiyama et al. (2017) also showed that irisin can be a mediator causing emphysema to disappear (Sugiyama et al. 2017). Therefore, increasing the muscle and serum levels of irisin reduces the injuries of lung diseases.
Another factor relevant to muscle tissue affecting lung tissue is semaphorin 3A (Sema3A). This factor can be secreted in nerve and muscle tissue entering the bloodstream. It has been shown that a basal level of Sema3A expression is maintained in quiescent satellite cells (Tatsumi et al. 2009). Sema3A secretion by SCs is induced by several growth factors released in the tissue interstitium, including HGF and FGF2, which are implicated in SC activation after muscle damage (Tatsumi et al. 2009). In muscle tissue especially injured muscle, the secreted Sema3A controls axonal guidance and growth as well as cell migration (Püschel et al. 1995; Sato et al. 2013). It has been revealed that Sema3A is also a tumor suppressor in prostate, breast, and lung cancers (Mishra et al. 2015). One study showed that a low level of sema3A in serum is associated with severe asthma (Adi et al. 2019). Sema3A acts as a potent suppressor of asthma-related inflammation, which makes it a therapeutic target for asthma (Xiang et al. 2019). Sema3A modulates the development of distal lung epithelial cells and alveolar septum (Cozacov et al. 2017). In the lung tissue, Sema3A signaling is mediated by NP-1. Meanwhile, Sema3A-NP-1 signaling plays an important role in the morphogenesis of the developing lung tissue as well as nervous system (Kagoshima et al. 2001). Therefore, the increase of this factor along with irisin due to muscle contraction can have beneficial effects on the lung disease. In addition to this factor that has had a beneficial effect on lung diseases, Shahabi et al. (2021) have recently reported that nano-selenium (SeNP) improves bleomycin-induced pulmonary injury in Wistar rats (Shahabi et al. 2021).
Selenium was discovered in 1817 by the Swedish chemist J.J. Berzelius; however, since it occurs in microscopic amounts in nature, it was not a special subject of research until the beginning of the twentieth century (Kieliszek and Bano 2022). Selenium plays an important role in the body. First, it is needed for the proper functioning of enzymes, and meanwhile for the protection of cells against free radicals or toxins. Selenium is also necessary for the proper functioning of the thyroid gland (Köhrle and Gärtner 2009), and even supports the treatment of pain in rheumatoid arthritis (Qamar et al. 2021) or is administered in the treatment of depression (Wang et al. 2018a, b), although in excess, it can cause symptoms of depression and diseases of the nervous system (Ding et al. 2022). Moreover, the association of selenium with smoking is noteworthy. In this case, little is known about the effect of smoking on the mineral balance of the body and selenium, and the extent to which smoking disturbs the balance of bio-elements, and the possibility of using this knowledge in science. In addition to reducing oxidative stress, selenium may help reduce the risk of certain cancers (Rayman 2005). This is possible since selenium has the properties of reducing DNA damage, strengthening the immune system, and destroying cancer cells (Razaghi et al. 2021). The results can be compared with the effect of selenium on bronchial asthma. Asthma, otherwise known as bronchial asthma, is a chronic inflammation of the bronchi leading to uncontrolled contraction. This disease is closely related to the increased levels of oxidative stress and inflammation in the body (Rusconi et al. 2011). Due to selenium's ability to reduce inflammation, some research studies suggest that this element may help reduce asthma-related symptoms. For example, one study suggested that selenium supplementation improved symptoms in chronic asthma (Allam and Lucena 2004). Another analysis showed that asthma patients with higher blood selenium levels had better lung function than those with lower levels (Shaheen et al. 2007).
The increase in irisin, along with the anti-inflammatory effects of selenium can have double properties, probably because the secretion of irisin can also be affected by selenium in nano-form, which requires detailed studies. Limited studies considered the relationship between muscles and lung in the conditions of pulmonary inflammation, especially with exercise and the use of nano-selenium supplements. We, therefore, hypothesize that 6 weeks of aerobic interval training (AIT) with nano-selenium (SeNPs) can upregulate muscle, serum, and lung irisin (FNDC5), and Sema3A in a rat exposed to cigarette smoke extract reinforcing the muscle-lung crosstalk.
Materials and methods
Animals and experimental design
Experiments were performed according to NIH Guidelines for animal studies on male Wistar rats weighing about 180–220 g (age: 8 weeks) that were bred in the animal laboratory, at Baqiyatallah University of medical sciences, Tehran, Iran (ethical code: IR.BMSU.REC.1400.117). The animals were maintained under controlled conditions (a 12 h light–dark cycle at 22 °C) with ad libitum access to food (standard chow diet: protein, 23%; fat, 4%; carbohydrate, 42%; calcium, 1%; tryptophan, 0.25%; methionine, 0.33%; lysine, 1.15%; threonine, 0.7%; phosphorus, 0.65%; salt, 0.5%; fiber, 4%; energy (Kcal/kg), 2900) and water. Forty-nine male Wistar rats were randomized into seven groups:
Control group: received 150 µL (µL) of vehicle (normal saline) intraperitoneally.
SeNPs group: received 2.5 mg/kg b.w. of this supplement by stomach gavage, 3 days/week for 6 weeks.
AIT group: performed aerobic interval training on a rodent treadmill 5 days/week for 6 weeks (in the form of intervals for 7 sets: each set of intervals: 4 min at 80–90% VO2max, separated by 3 min at 65–75% VO2max).
SeNPs + AIT group: received SeNPs and performed AIT (as stated in groups 2 and 3).
CSE group: received 150 µL CSE by IP injection, 1 day/week for 6 weeks.
CSE + AIT group: received CSE and performed AIT (as stated in groups 3 and 5).
CSE + SeNPs + AIT group: received SeNPs, CSE, and performed AIT (as stated in groups 2, 3 and 5).
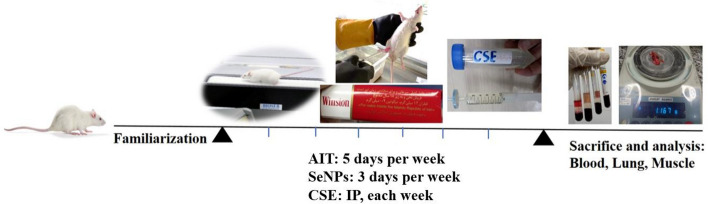
Figure 1 shows the schematic of the experimental design.
Fig. 1.
Schematic experimental design of exercise and SeNPs therapy in rats exposed to CSE. This study was performed over 6 weeks. SeNPs nano-selenium; AIT aerobic interval training; CSE cigarette smoke extract
Cigarette smoke extract (CSE)
CSE was prepared based on previously relevant studies (Chen et al. 2015; Li et al. 2018). Briefly, three Winston cigarettes (containing tar, 12 mg, nicotine, 0.9 mg) were burned, and the smoke was transferred to a container containing 10 mL of PBS using a vacuum pump. The CSE–PBS solution for each set of experiments was freshly prepared and then filtered through a 0.22 mm Millipore filter to remove particles and bacteria. The rats of the healthy control group were given 150 µL (µL) of vehicle (normal saline) intraperitoneal, while the CSE groups were given 150 µL (µL) of CSE–PBS solution on days 7, 14, 21, 28, 35, and 42 which were injected intraperitoneal (Chen et al. 2015; Li et al. 2018).
Nano-selenium supplementation
In this study, a nano-selenium manufactured by ARMINANO company was utilized (Armina Engineering Co, Tehran, Iran). To prepare the mixture, first based on the company description, the aqueous extract of ginger obtained was utilized as a precursor for the synthesis of nano-selenium. Ginger extract (2 mL) was added dropwise into the 20 mL solution of SeO3 (10 mM), with vigorous stirring. The mixture was incubated by placing the solution onto a rotatory orbital shaker operating at 200 rpm, 30 °C for 72 h in dark conditions. The reduction of selenium ions was monitored by sampling an aliquot (3 mL) of the mixture at intervals of 24 h, followed by measurement of absorption maximum. Absorption maximum was determined by measuring the optical density of the content from wavelength 350 to 700 nm using UV–Vis spectrophotometer (Abou Zaid et al. 2017; Hozyen et al. 2020; Prasad and Selvaraj 2014). SeNPs at a dose (of 2.5 mg/kg b.w) were given to the supplementation group by oral gavage 1 a day for 3 times/week (Ali et al. 2020).
Aerobic interval training
Prior to the onset of the main training protocol and to get familiar with treadmill running, the rats exercised for 5 min at a speed of 8–10 m/min with a zero slope in five sessions in 1 week. The aerobic interval training (AIT) protocol consisted of a 10-min warm-up (50–55% of maximal oxygen uptake (VO2max)), seven periods of interval training (each set of intervals: 4 min at 80–90% VO2max, separated by 3 min at 65–75% VO2max) and 5-min cool-down (Qin et al. 2020). To adjust the running speed and maintain the relative training intensity, VO2max indirect measurement test was employed for Wistar rats once every 2 weeks.
ELISA
Forty-eight hours after the last training session, rats were anesthetized with an acute injection of xylazine/ketamine (10 mg/kg/100 mg/kg). The blood sample was collected by cardiac puncture into non-heparinized tubes and then centrifuged at 3000 rpm for 15 min. Sera were meticulously separated, and each sample was put in a clean cup tube, labeled, and kept at −20 °C until biochemical analysis. Serum irisin and semaphorin 3A were measured using rat-specific ELISA kits according to the manufacturer's instructions (Irisin: ZellBio GmbH, Germany, Sema3A: LifeSpan Biosciences, USA). The studies were carried out using an ELISA plate washer (BIOTEK ELx50, USA) and ELISA microplate reader (ELX808; BioTek Instruments).
Gene expression
Using Qiazol (Qiazol lysis reagent, USA), the extraction of the whole RNA was performed from gastrocnemius muscle and lung tissue, within a sterilized RNase-free tube. RNA purity and concentration were measured utilizing the absorbance at 260 and 280 nm ratio (the A260/280 ratio) through a Nanodrop ND-100 spectrophotometer (Thermo Scientific, USA). The RevertAid cDNA synthesis kit (Fermentas, Germany) was used to transform RNA into cDNA in a quantity of 25 μL, in accordance with the manufacturer's instructions. Polymerase chain reaction (PCR) amplification reaction included 2 μL of the cDNA synthesis reaction, 12.5 μL AccuPrime SuperMix I (Fermentas, Germany), 10.1 μL of distilled water, and 0.2 μL of each forward and reverse primers (100 μmol/L). NCBI BLAST Instrument and Primer3 software were applied to design and confirm primers.
For real-time PCR, 500 ng of the newly synthesized cDNA was used to assess the relative gene expression. PCR reactions were carried out utilizing SYBR Green Premix 2X (12.5 μL; Takara, Japan) and mixed primers (10 p-molar; 25 μL). The thermal cyclic protocol utilized was 95 °C for 10 s, 40 denaturation cycles at 94 °C for 5 s, and annealing and extension at 60 °C for 34 s. The ΔΔCT technique was utilized to quantify muscle and lung FNDC5 and Sema3A gene relative expressions. A comparison was made between the samples of Ct and that of the internal control (Gapdh). Real-time PCR was conducted using the ABI (Applied Biosystems, USA) detection system. All reactions were completed five times. Using electrophoresis and melting curve analysis, the specificity of the PCR reaction was double-checked. Graph pad prism5 was used for gene analysis. The employed primer sequences are depicted in Table 1.
Table 1.
Primer sequences
| Gene | Primer sequences |
|---|---|
| Muscle FNDC5 | F: 5′-AGGACCTCACTGTTCTGACG-3′ |
| R: 5′-GCAGTCTTGTCACTCCAGGA-3′ | |
| Lung FNDC5 | F: 5′-AGGACCTCACTGTTCTGACG-3′ |
| R: 5′-AGGGGTTAGTTGGAGGCTTC-3′ | |
| Muscle Sema3A | F: 5′-GAGTGATGTAAGAAGGGTGTTCC-3′ |
| R: 5′-CCTTGTGGGAAGAATGTAGTGAG-3′ | |
| Lung Sema3A | F: 5′-CAAGTTCCTGGTCGTGGATAAG-3′ |
| R: 5′-GAATAGGTGCTGGTCCTTGAAC-3′ | |
| GAPDH | F: 5′-ATCAAGAAGGTGGTGAAGCAGG-3′ |
| R: 5′-TGGGAGTTGCTGTTGAAGTCAC-3′ |
Hematoxylin and eosin (H&E) analysis (lung tissue)
The H&E method was used to investigate lung tissue damage caused by CSE. A piece of the lung tissue was fixed in 10% formalin for 24 h. H&E staining was performed using standard procedures. Pulmonary tissue morphology was observed under a DP73 digital microscope after paraffin embedding, sectioning, and H&E staining.
Statistical analysis
Data are expressed as mean ± SD. Shapiro–Wilk test was conducted to confirm the normality of the data. Statistical significance was analyzed with a one-way analysis of variance followed by the Tukey post hoc test (Graph Pad Prism version 9.01). Pearson’s correlation coefficient was used to analyze the relationship between muscle FNDC5/serum irisin and lung FNDC5, as well as the relationship between muscle/serum Sema3A and lung Sema3A. The significance level was considered at the α level of 0.05.
Results
Body and lung weight
At the end of this study, we measured wet lung weight, animal body weight, and the ratio of wet lung weight to the body weight (Fig. 2). The volume of the wet lung weight in healthy groups did not show a significant change either with AIT exercise or with nano-selenium supplement and the combination of exercise and supplement (Fig. 2A). However, the CSE group showed a significant decrease in wet lung weight compared to the healthy control group (p = 0.0026). Compared to the CSE group, CSE + AIT and CSE + SeNPs + AIT groups showed a significant increase in wet lung weight (p = 0.0254 and p = 0.0002, respectively) (Fig. 2B).
Fig. 2.
Lung wet weight (A, B), body weight (C, D) and lung/body weight ratio (E, F) in control, SeNPs, AIT, SeNPs + AIT groups and control, CSE, CSE + AIT, CSE + SeNPs + AIT groups. CSE caused a significant decrease in the body weight and lung wet weight compared to control group. Combination of AIT and SeNPs after CSE, increased body weight, lung wet weight and LW/BW ration (compared to CSE group). Data are expressed as mean ± standard deviation. Control group is the same for the two graphs for each variable. SeNPs nano-selenium; AIT aerobic interval training; CSE cigarette smoke extract
Body weight, meanwhile, did not show significant changes in healthy groups (Fig. 2C). But CSE, CSE + AIT, and CSE + SeNPs + AIT groups displayed a significant decrease in body weight compared to the healthy control group (p < 0.05). Compared to the CSE group, CSE + AIT and CSE + SeNPs + AIT groups showed a significant increase in body weight (p = 0.0014 and p = 0.0008, respectively) (Fig. 2D).
The ratio of wet lung weight to body weight (LW/BW) also did not reveal any significant changes among different treatment methods in healthy groups (Fig. 2E). However, compared to the healthy control group, the CSE + SeNPs + AIT group showed a significant increase in LW/BW (p = 0.0206) (Fig. 2F).
Serum irisin and Sema3A protein
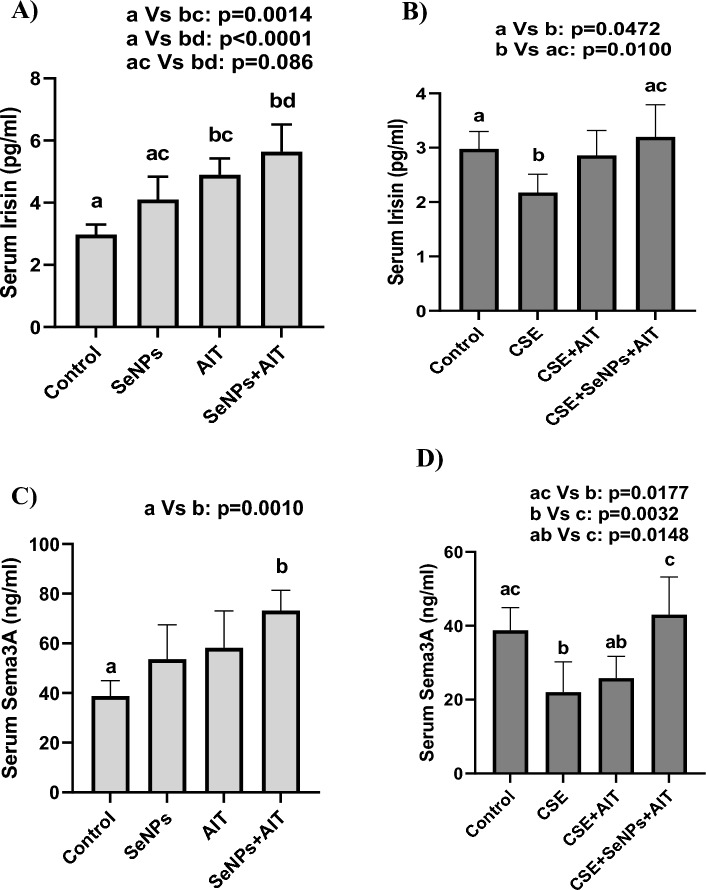
Changes in serum irisin and Sema3A levels of healthy and CSE groups are illustrated in Fig. 3A–D. As can be observed in Fig. 3A, the serum irisin levels in the AIT and SeNPs + AIT groups showed a significant increase compared to the healthy control group (p = 0.0014 and p < 0.0001, respectively). The increase in serum irisin in the SeNPs + AIT group was not significant compared to the SeNPs group (p = 0.086). As seen in Fig. 3B, it was found that CSE caused a decrease in serum irisin compared to the healthy control group (p = 0.0472). Compared to the CSE group, the CSE + SeNPs + AIT group showed a significant increase in serum irisin (p = 0.0100).
Fig. 3.
The serum levels of irisin (A, B) and Sema 3A (C, D) in control, SeNPs, AIT, SeNPs + AIT groups (A, C) and control, CSE, CSE + AIT, CSE + SeNPs + AIT groups (B, D). Serum irisin and Sema3A were higher in the exercise and nano-supplement groups than that of the control group after 6 weeks of exercise and nano-supplementation. However, Serum irisin and Sema3A were lower in the CSE group than in the healthy control group. Serum irisin and Sema3A increased in CSE received group after 6 weeks of exercise with nano-selenium supplementation. Data are expressed as mean ± standard deviation. Control group is the same for the two graphs for each variable. SeNPs nano-selenium; AIT aerobic interval training; CSE cigarette smoke extract
In Fig. 3C, the serum levels of Sema3A in the SeNPs + AIT group displayed a significant increase compared to the control group. The CSE group showed a significant decrease in serum Sema3A compared to the healthy control group (p = 0.0177). However, the CSE + SeNPs + AIT group demonstrated a significant increase in serum Sema3A compared to the healthy CSE (p = 0.0032) and CSE + AIT (p = 0.0148) groups (Fig. 3D).
Muscle FNDC5 and Sema3A gene expression
FNDC5 and Sema3A gene expressions in the gastrocnemius muscle of different groups are displayed in Fig. 4. As can be observed, compared to the healthy control group and the SeNPs group, the expression of the FNDC5 gene in the AIT and AIT + SeNPs groups exhibited a significant increase (p < 0.001) (Fig. 4A). In the CSE groups, the changes in muscle FNDC5 between different groups were significant (Fig. 4B). Compared to the healthy control group, the amount of FNDC5 gene expression in the CSE group decreased; however, this decline was not significant (p > 0.05). On the other hand, compared to the CSE group, CSE + AIT and CSE + SeNPs + AIT groups showed a significant increase in muscle FNDC5 gene expression (p < 0.0001 for both). The increase in muscle FNDC5 in the CSE + SeNPs + AIT group was also significant compared to the CSE + AIT group (p = 0.0059).
Fig. 4.
Muscle gene expression of FNDC5 (A, B) and Sema3A (C, D) in control, SeNPs, AIT, SeNPs + AIT groups and control, CSE, CSE + AIT, CSE + SeNPs + AIT groups. Muscle gene expression of FNDC5 and Sema3A displayed a significant increase in the AIT and nano-supplement groups. In the group with cigarette smoke extract, the highest expression in FNDC5 and Sema3A at gastrocnemius was observed in the combined treatment group (CSE + SeNPs + AIT). Data are expressed as mean ± standard deviation. Control group is the same for the two graphs for each variable. SeNPs nano-selenium; AIT aerobic interval training; CSE cigarette smoke extract
Similar to FNDC5, and compared to the healthy control group and the SeNPs group, the expression of the muscle Sema3A gene in the AIT and AIT + SeNPs groups displayed a significant increase (p < 0.0001) (Fig. 4C). Additionally, the muscle Sema3A gene expression in the CSE group decreased compared to the healthy control group, but this decrease was not significant (p > 0.05). However, compared to the CSE group, CSE + AIT (p = 0.0008) and CSE + SeNPs + AIT (p < 0.0001) groups revealed a significant increment in muscle Sema3A gene expression. The increase in Sema3A gene expression in the CSE + SeNPs + AIT group was also significant compared to the CSE + AIT group (p = 0.0408) (Fig. 4D).
Lung FNDC5 and Sema3A gene expression
Changes in FNDC5 and Sema3A gene expression in the lung tissue are depicted in Fig. 5A–D. After considering FNDC5 in the lungs of healthy groups, it was found that the AIT and the AIT + SeNPs groups had a significant increase in this gene compared to the control and SeNPs groups (p < 0.05). Moreover, the AIT + SeNPs group showed a significant increase in lung FNDC5 gene expression compared to the AIT group (p = 0.0138) (Fig. 5A). Compared to the healthy control group, the expression of the FNDC5 gene in the lungs of the CSE group displayed a significant decrease (p = 0.0066). However, the treatment with exercise training along with nano-selenium supplement (CSE + SeNPs + AIT) increased the expression of lung FNDC5 gene compared to CSE (p < 0.0001) and CSE + AIT (p = 0.0081) groups (Fig. 5B).
Fig. 5.
Lung gene expression of FNDC5 (A, B) and Sema 3A (C, D) in control, SeNPs, AIT, SeNPs + AIT groups and control, CSE, CSE + AIT, CSE + SeNPs + AIT groups. The combined treatment groups of AIT and nano-selenium supplement showed the highest increase in FNDC5 and Sema3A gene expression in healthy and CSE received groups. Data are expressed as mean ± standard deviation. Control group is the same for the two graphs for each variable. SeNPs nano-selenium; AIT aerobic interval training; CSE cigarette smoke extract
The gene expression of Sema3A in the lung tissue also increased significantly in the SeNPs and SeNPs + AIT groups compared to the healthy control group (p = 0.0138 and p < 0.0001, respectively). Furthermore, the AIT + SeNPs group showed a significant increase in lung Sema3A gene expression compared to the AIT group (p = 0.0055) (Fig. 5C). CSE group showed a significant decrease in the lung Sema3A gene expression compared to the healthy control group (p = 0.0004). Meanwhile, CSE + AIT and CSE + SeNPs + AIT groups showed a significant increase in the lung Sema3A gene expression compared to the CSE group (p < 0.001) (Fig. 5D).
Pearson’s correlation
The relationship between muscle FNDC5/serum irisin and lung FNDC5, as well as the relationship between muscle/serum Sema3A and lung Sema3A is shown in Tables 2 and 3. The results of Pearson’s correlation test indicated that there is a positive and significant correlation between muscle FNDC5 and lung FNDC5 in the CSE + SeNPs + AIT group (r = 0.92, p = 0.025), while the correlation between serum irisin and lung FNDC5 was positive and significant only in the AIT + SeNPs group (r = 0.93, p = 0.021) (Table 2). Pearson’s correlation test also showed that there was a positive and significant correlation between serum Sema3A and lung Sema3A of the CSE + SeNPs + AIT group (r = 0.97, p = 0.004) (Table 3).
Table 2.
Pearson correlation coefficients between muscle FNDC5 and serum irisin, with lung FNDC5 continuous exercise training
| Groups | Muscle FNDC5 | Serum irisin | |
|---|---|---|---|
| Lung FNDC5 | Control | r = 0.57, p = 0.314 | r = − 0.33, p = 0.583 |
| SeNPs | r = − 0.49, p = 0.393 | r = 0.12, p = 0.846 | |
| AIT | r = 0.01, p = 0.983 | r = − 0.25, p = 0.674 | |
| AIT + SeNPs | r = 0.85, p = 0.066 | r = 0.93, p = 0.021* | |
| CSE | r = 0.43, p = 0.463 | r = 0.04, p = 0.941 | |
| CSE + AIT | r = − 0.38, p = 0.519 | r = 0.37, p = 0.539 | |
| CSE + SeNPs + AIT | r = 0.92, p = 0.025* | r = 0.34, p = 0.571 |
r = Pearson correlation coefficient; p = p value; correlation is significant for p < 0.05 *, SeNPs nano-selenium; AIT aerobic interval training; CSE cigarette smoke extract
Table 3.
Pearson correlation coefficients between muscle and serum Sema3A, with lung Sema3A continuous exercise training
| Groups | Muscle Sema3A | Serum Sema3A | |
|---|---|---|---|
| Lung Sema3A | Control | r = − 0.20, p = 0.742 | r = 0.65, p = 0.235 |
| SeNPs | r = − 0.67, p = 0.212 | r = 0.78, p = 0.119 | |
| AIT | r = 0.70, p = 0.179 | r = 0.79, p = 0.105 | |
| AIT + SeNPs | r = 0.31, p = 0.610 | r = 0.80, p = 0.097 | |
| CSE | r = 0.28, p = 0.647 | r = − 0.57, p = 0.309 | |
| CSE + AIT | r = − 0.74, p = 0.147 | r = 0.23, p = 0.697 | |
| CSE + SeNPs + AIT | r = − 0.50, p = 0.388 | r = 0.97, p = 0.004* |
r = Pearson correlation coefficient; p = p value; correlation is significant for p < 0.05 *, SeNPs nano-selenium; AIT aerobic interval training; CSE cigarette smoke extract
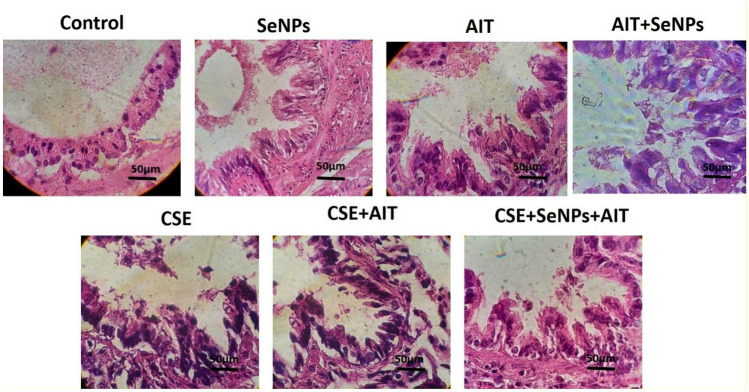
Lung histology
The H&E method was employed to examine lung tissue damage caused by CSE injection (Fig. 6). As seen in Fig. 6, lung tissue damage in CSE groups is higher than that of other groups. Furthermore, an increase in tissue thickness was observed in the CSE and exercise groups. However, nanoselenium supplementation reduced these damages to a small amount and improved lung tissue integrity.
Fig. 6.
Pulmonary histopathology. H&E staining of lung tissues of rats in the control, SeNPs, AIT, AIT + SeNPs, CSE, CSE + AIT and CSE + SeNPs + AIT groups (× 40). Lung tissues showed inflammatory cell infiltration especially in CSE groups. However, exercise and SeNPs reduced damage induced by CSE in the lung tissue. SeNPs nano-selenium; AIT aerobic interval training; CSE cigarette smoke extract
Discussion
In addition to the drug therapy for lung diseases, lifestyle modification with healthy nutrition (use of antioxidants) and strengthening of atrophied muscle tissue with exercise training are new treatment strategies (Bernard et al. 1999; Gea et al. 2018). In the animal model, it has been shown that selenium ameliorated the toxic effects of dimethoate in the lung tissue (Amara et al. 2012). Suryadinata and Wirjatmadi (2020) showed that in male Wistar rats poisoned by cigarette smoke, selenium consumption can minimize the oxidative damage caused by cigarette smoke (Suryadinata and Wirjatmadi 2020). Furthermore, it has been shown in Wistar rats (exposed to air pollutants), performing 8 weeks of aerobic interval training (AIT) improved the pulmonary structure and its function and impeded lesion progression (Qin et al. 2020). Since muscle tissue can have an endocrine effect on distant tissues, in this study, we examined the effect of exercise training in a lung injury model along with taking antioxidant supplements by considering muscle, serum, and lung irisin (FNDC5) and Sema3A.
In this study, CSE caused a significant decrease in wet lung weight and body weight. While in the groups receiving cigarette smoke extract, doing exercise along with SeNPs supplementation proved a significant increase in these two variables. One of the characteristics of lung diseases, including COPD, is weight loss and loss of muscle tissue, which is the result of a sedentary lifestyle and improper nutrition in these patients. Nutritional impairment in COPD is multifaceted and can include imbalances in energy (weight loss), protein (sarcopenia), enhancing inflammation (pulmonary cachexia), which can exacerbate nutritional damage. As a result, a decrease in fat-free mass (FFM) and fat mass (FM) can occur (Collins et al. 2019). Nutritional support in the form of oral and nano-nutrient supplements combined with exercise training can overcome energy imbalance leading to improved nutritional status and functional capacity (Shaban et al. 2019). Wang et al. (2021) stated that selenium status affected the hypertrophic growth of skeletal muscle by mediating protein turnover in zebrafish (Wang et al. 2021). In the present study, although changes in muscle hypertrophy were not investigated, it seems that doing exercise training and taking nano-selenium supplements can control the atrophy caused by cigarette smoke and provide the conditions for muscle growth.
The result of H&E staining showed that the combination of exercise and nano-selenium decreases lung tissue damage due to CSE. Selenium (Se) was required to maintain various functions of the body (Kieliszek et al. 2022). Selenium can be used to treat many diseases (Kieliszek and Bano 2022). Compounds in selenium suppress the formation of superoxide anion, release nitric oxide, scavenge peroxynitrite, and protect against lipid peroxidation, which is consistent with its proposed ability to prevent oxidative stress (Kieliszek and Bano 2022). Due to these benefits of selenium, Kieliszek and Sandoval (2023) stated that increasing dietary selenium in areas with a deficiency of this substance will help adjust dietary recommendations by taking into account the health-promoting properties of selenium (Kieliszek and Sandoval 2023). Meanwhile, selenium with anti-inflammatory and antioxidant effects can have positive impacts on the lung disease and inflammation (Winterbourn et al. 2000). Although in this study, we did not examine the inflammatory and antioxidant indicators, based on histological images, nanoselenium supplementation seems to be effective in controlling inflammation. Meanwhile, in this study, nanoselenium supplement had strengthening effects on increasing irisin and Sema3A along with exercise, which was also confirmed by Pearson correlation results.
In the healthy groups, the amount of serum irisin and FNDC5 of the gastrocnemius muscle and lung tissue displayed a significant increase in the AIT and SeNPs + AIT groups, and in the CSE group, the amount of serum irisin and FNDC5 of the lung tissue showed a significant decrease compared to the control. Compared to the CSE group, the CSE + SeNPs + AIT group showed the highest increase in serum irisin and FNDC5 in the gastrocnemius muscle and lung tissue. The increase in muscle and serum irisin with exercise training has been confirmed in several studies (Bilek et al. 2022; Kazeminasab et al. 2022). Studies have revealed that irisin secreted by skeletal muscle after exercise training affects weight loss, bone density, and improves cardiovascular health (Zhang et al. 2017). Irisin enhances energy expenditure by browning the white adipose tissue (Jiang et al. 2021). In myocytes, irisin affects fatty acid oxidation and glucose hemostasis (Xin et al. 2016). In hepatocytes, irisin suppresses lipogenesis, gluconeogenesis, and lipid accumulation (Tang et al. 2016). However, recent studies have shown that irisin has strong antioxidant properties (Szabó et al. 2020). In the present study, it seems that the use of a nano-selenium supplement has a role in further increasing irisin and its antioxidant effects. Previous studies have shown that selenium plays an important role in modulating the immune system function, maintaining homeostasis of redox, and reducing inflammatory cytokine (Tomo et al. 2021). It has been stated that selenium deficiency might be considered as an indicator of the severity, mortality, and overall risk of COVID-19 (Fakhrolmobasheri et al. 2022). It can, therefore, be effective in lung diseases. Previous studies focused on the anti-oxidative and anti-inflammatory effects of selenium in lung diseases (Vahid and Rahmani 2021). In the present study, we however reported a significant increase of irisin in the lung tissue with exercise and nano-selenium supplementation. Hitherto, no study has investigated the relationship between nano-selenium and irisin levels after exercise. One of the hypotheses of the present study was that exercise training with nano-selenium supplement increases the serum and pulmonary irisin levels through the increased contraction and confirms the muscle and lung cross-talk hypothesis, while the expression of the irisin gene in the lung tissue was confirmed, but the larger volume of produced irisin can be attributed to the muscle tissue. As was observed, in the groups with CSE, exercise and nano-selenium supplementation caused a significant increase in pulmonary irisin. Irisin acts as a myokine to protect against mitochondrial dysfunction during ischemia/reperfusion (IR)-induced lung injury. It has been stated that the increment of irisin in blood flow leads to the entry of irisin into the damaged alveolar cells. Through interaction with mitochondrial uncoupling protein (UCP) 2, a homolog of UCP1, which is predominantly expressed in the lung tissue (Couplan et al. 2002; Stuart et al. 2001), irisin maintains mitochondrial function and prevents IR-induced lung injury. It has also been proved that exogenous irisin can ameliorate IR-induced lung damage in the mouse models, and extend pulmonary protective function (Chen et al. 2017). Contrary to the results of the present study, Alyami et al. (2020) showed that the exercise capacity and the dyspnea of patients with interstitial lung disease improved after 8 weeks of the supervised exercise training program; however, no major changes were observed in irisin levels in patients with interstitial lung disease and the control group (Alyami et al. 2020). Although, in the present study, an animal sample and an AIT exercise were used in the CSE model, a different study design and a different exercise training can justify the results of the present study with that of Alyami. Shao et al. meanwhile noted that irisin could suppress lung inflammation and apoptosis in ALI mice induced by LPS inhalation (Lam et al. 2012). In the meantime, Li et al. (2019) showed that irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways (Li et al. 2019). Therefore, according to these results, increasing serum and pulmonary muscle irisin with exercise and nano-supplementation seems to be an excellent treatment modality for lung diseases.
Changes in Sema3A were similar to irisin. In healthy groups, Sema3A values of SeNPs + AIT group were higher in serum and lung muscle than other groups. In the CSE group, it was also observed that Sema3A in serum and the lung was significantly decreased compared to the healthy control group, but CSE + AIT and CSE + SeNPs + AIT showed a significant increase in Sema3A in muscle, serum, and lung compared to the cigarette smoke extract group. Early studies investigated the role of Sema3A in the development of neuromuscular terminals during the embryonic period (new). Emerging evidence suggests that Sema3A is still expressed in mature tissues and may be involved in neuronal regulation associated with plasticity and injury-induced events (Gavazzi 2001; Penzes et al. 2003). Sema3A signaling is important for the directional guidance of nerve fibers in several developing and regenerating organs, including nerves, bones, and the heart (Behar et al. 1996; Ieda et al. 2007). Since exercise causes muscle growth, it is reasonable to boost this factor in muscle tissue and serum. However, it was recently revealed that the role of Sema3A goes beyond neural development. Sema3A also plays an essential role in angiogenesis, organogenesis, osteoclastogenesis, immune responses, and control of tumor progression (Hayashi et al. 2012; Lepelletier et al. 2006). This factor is now recognized as a multifunctional modulator of secretion. In the studies relevant to muscle hypertrophy, it has been stated that Sema3A protein is produced by satellite cells inside the body in response to hind limb muscle damage in adult mice (Suzuki et al. 2010).
The secretion of Sema3A from differentiating satellite cells mediates the regeneration of neuromuscular junctions through chemical events that restore sprouting and reattachment of motor neuron terminals to the damaged muscle fibers (Tatsumi et al. 2009). In the present study, we found that exercise training by activating satellite cells was effective in increasing Sema3A in muscle and serum levels. Increasing this factor in the lung tissue has positive effects on the damage control. This factor may decrease in the CSE group and increase in the CSE + SeNPs + AIT group. Consistent with these results expressed in allergic diseases, the expression of Sema3A decreased in atopic dermatitis and allergic rhinitis, especially in the nasal epithelium of the rhinitis mouse model. Interestingly, Sema3A treatment reduced symptoms in both allergic disorders (Sawaki et al. 2011). Sema3A modulates airway smooth muscle hyperplasia as well as airway inflammation in allergic asthma. Therefore, increasing this factor with exercise and supplementation, especially in CSE groups, can have a therapeutic role.
To sum up, our study suggests that CSE is associated with the reduction of serum, lung irisin, and sema3A. In addition, the present investigation provides new insights into the biochemical mechanisms by which the treatment of aerobic interval training with nano-selenium supplementation through upregulation of serum irisin and Sema3A protects lung tissue against the toxicity induced by CSE. Thus, our study suggests that regular aerobic interval exercise training with oral supplementation of nano-selenium may be considered as a potentially useful candidate to improve the lung tissue against CSE-induced COPD.
This study has some limitations. First, it did not investigate muscle tissue histological changes as well as changes in muscle hypertrophy and atrophy. Second, it did not measure protein expression in muscle and lung tissues with accurate methods, such as western blot and immunohistochemistry, which should be considered in further studies. Finally, it is better to use cigarette smoke for the COPD model in the form of inhalation.
Conclusion
Performing exercise training by improving aerobic capacity can affect both lung function and its structure. Add to this, exercise can be effective in improving lung structure through cellular mechanisms such as increasing irisin, while the use of nano-selenium supplements with antioxidant effects can double the protective effects of exercise on the lung. As per the results of the present study, semaphorin 3A and irisin are secreted from muscle tissue, and exercise training can play a role in increasing the serum and tissue levels of these factors by increasing muscle contraction. The use of an antioxidant and anti-inflammatory supplement also made these effects of exercise more stable. Therefore, it seems that exercise training with nano-selenium supplementation in the lung diseases can strengthen muscle and lung crosstalk by increasing semaphorin 3A and irisin and minimizing the damage caused by CSE.
Acknowledgements
We would like to express our gratitude to the Tobacco Control Research Center (TCRC) of the Iranian Anti-Tobacco Association for supporting this research project.
Author contributions
All authors have participated in drafting the manuscript. All authors reviewed the results and approved the final version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Funding
None.
Data availability
The data supporting the findings of this study are available on request from the corresponding author.
Declarations
Conflict of interest
The authors have no conflict of interest.
Ethical approval
Experiments were performed according to NIH Guidelines for the animal study on Wistar rats weighing about 180–220 g bred at the animal laboratory, at Baqiyatallah University of medical sciences, Tehran, Iran (ethical code: IR.BMSU.REC.1400.117).
Informed consent
Not applicable.
References
- Abou Zaid OAR, El-Sonbaty SM, Barakat W. Ameliorative effect of selenium nanoparticles and ferulic acid on acrylamide-induced neurotoxicity in rats. Ann Med Biomed Sci. 2017;3(2):35–45. [Google Scholar]
- Adi SD, Eiza N, Bejar J, Shefer H, Toledano S, Kessler O, et al. Semaphorin 3A is effective in reducing both inflammation and angiogenesis in a mouse model of bronchial asthma. Front Immunol. 2019;10:550. doi: 10.3389/fimmu.2019.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali HF, El-Sayed NM, Ahmed AA, Hanna PA, Moustafa YM. Nano selenium ameliorates oxidative stress and inflammatory response associated with cypermethrin-induced neurotoxicity in rats. Ecotoxicol Environ Saf. 2020;195:110479. doi: 10.1016/j.ecoenv.2020.110479. [DOI] [PubMed] [Google Scholar]
- Alizadeh Pahlavani H. Exercise therapy for people with sarcopenic obesity: myokines and adipokines as effective actors. Front Endocrinol. 2022;13:811751. doi: 10.3389/fendo.2022.811751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allam MF, Lucena RA. Selenium supplementation for asthma. Cochrane Database System Rev. 2004 doi: 10.1002/14651858.CD003538.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyami RM, Alhowikan AM, Alharbi AR, Ghada A-N. Impact of supervised exercise training on pulmonary function parameters, exercise capacity and Irisin Biomarker in Interstitial lung disease patients. Pak J Med Sci. 2020;36(5):1089. doi: 10.12669/pjms.36.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara IB, Soudani N, Troudi A, Hakim A, Zeghal KM, Boudawara T, Zeghal N. Dimethoate induced oxidative damage and histopathological changes in lung of adult rats: modulatory effects of selenium and/or vitamin E. Biomed Environ Sci. 2012;25(3):340–351. doi: 10.3967/0895-3988.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Amatngalim GD, Hiemstra PS. Airway epithelial cell function and respiratory host defense in chronic obstructive pulmonary disease. Chin Med J. 2018;131(09):1099–1107. doi: 10.4103/0366-6999.230743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin S, Kuloglu T, Aydin S, Eren MN, Celik A, Yilmaz M, et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: cardiac muscle produces more irisin than skeletal muscle. Peptides. 2014;52:68–73. doi: 10.1016/j.peptides.2013.11.024. [DOI] [PubMed] [Google Scholar]
- Baraldo S, Turato G, Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration. 2012;84(2):89–97. doi: 10.1159/000341382. [DOI] [PubMed] [Google Scholar]
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- Bernard S, Whittom F, LeBlanc P, Jobin J, Belleau R, Berube C, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(3):896–901. doi: 10.1164/ajrccm.159.3.9807034. [DOI] [PubMed] [Google Scholar]
- Bilek F, Cetisli-Korkmaz N, Ercan Z, Deniz G, Demir CF. Aerobic exercise increases irisin serum levels and improves depression and fatigue in patients with relapsing remitting multiple sclerosis: a randomized controlled trial. Mult Scler Relat Disord. 2022;61:103742. doi: 10.1016/j.msard.2022.103742. [DOI] [PubMed] [Google Scholar]
- Chen H, Liao K, Cui-Zhao L, Qiang-Wen F, Feng-Zeng X, Ping-Wu F, et al. Cigarette smoke extract induces apoptosis of rat alveolar type II cells via the PLTP/TGF-β1/Smad2 pathway. Int Immunopharmacol. 2015;28(1):707–714. doi: 10.1016/j.intimp.2015.07.029. [DOI] [PubMed] [Google Scholar]
- Chen K, Xu Z, Liu Y, Wang Z, Li Y, Xu X, et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med. 2017;9(418):eaao6298. doi: 10.1126/scitranslmed.aao6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PF, Yang IA, Chang Y-C, Vaughan A. Nutritional support in chronic obstructive pulmonary disease (COPD): an evidence update. J Thorac Dis. 2019;11(Suppl 17):S2230. doi: 10.21037/jtd.2019.10.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couplan E, del Mar Gonzalez-Barroso M, Alves-Guerra MC, Ricquier D, Goubern M, Bouillaud F. No evidence for a basal, retinoic, or superoxide-induced uncoupling activity of the uncoupling protein 2 present in spleen or lung mitochondria. J Biol Chem. 2002;277(29):26268–26275. doi: 10.1074/jbc.M202535200. [DOI] [PubMed] [Google Scholar]
- Cozacov R, Halasz K, Haj T, Vadasz Z. Semaphorin 3A: is a key player in the pathogenesis of asthma. Clin Immunol. 2017;184:70–72. doi: 10.1016/j.clim.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Ding W, Wang S, Gu J, Yu L. Selenium and human nervous system. Chin Chem Lett. 2022;34(7):108043. doi: 10.1016/j.cclet.2022.108043. [DOI] [Google Scholar]
- Fakhrolmobasheri M, Mazaheri-Tehrani S, Kieliszek M, Zeinalian M, Abbasi M, Karimi F, Mozafari AM. COVID-19 and selenium deficiency: a systematic review. Biol Trace Elem Res. 2022;200(9):3945–3956. doi: 10.1007/s12011-021-02997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi I. Semaphorin-neuropilin-1 interactions in plasticity and regeneration of adult neurons. Cell Tissue Res. 2001;305:275–284. doi: 10.1007/s004410100365. [DOI] [PubMed] [Google Scholar]
- Gea J, Sancho-Muñoz A, Chalela R. Nutritional status and muscle dysfunction in chronic respiratory diseases: stable phase versus acute exacerbations. J Thorac Dis. 2018;10(Suppl 12):S1332. doi: 10.21037/jtd.2018.02.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güncü MB, Cakan U, Muhtarogullari M, Canay S. Zirconia-based crowns up to 5 years in function: a retrospective clinical study and evaluation of prosthetic restorations and failures. Int J Prosthodont. 2015;28(2):152–157. doi: 10.11607/ijp.4168. [DOI] [PubMed] [Google Scholar]
- Han Y, Wu Z, Zhao Q, Jiang B, Miao X, Lu X, et al. Association between anthropometric indices and skeletal-muscle atrophy in Chinese patients with stable chronic obstructive pulmonary disease: a cross-sectional study. Int J Chronic Obstruct Pulmon Dis. 2022;17:2529–2539. doi: 10.2147/COPD.S373880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485(7396):69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- Hoang BX, Han B, Fang WH, Nguyen AK, Shaw DG, Hoang C, Tran HD. Targeting skeletal muscle dysfunction with L-Carnitine for the treatment of patients with chronic obstructive pulmonary disease. In Vivo. 2023;37(4):1399–1411. doi: 10.21873/invivo.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Hu S, Li C, Ma H, Wang Q, Meng G, et al. Cigarette smoke induced lung barrier dysfunction, EMT, and tissue remodeling: a possible link between COPD and lung cancer. BioMed Res Int. 2019;2019:1–10. doi: 10.1155/2019/2025636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozyen HF, Khalil HM, Ghandour RA, Al-Mokaddem AK, Amer M, Azouz RA. Nano selenium protects against deltamethrin-induced reproductive toxicity in male rats. Toxicol Appl Pharmacol. 2020;408:115274. doi: 10.1016/j.taap.2020.115274. [DOI] [PubMed] [Google Scholar]
- Ieda M, Kanazawa H, Kimura K, Hattori F, Ieda Y, Taniguchi M, et al. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat Med. 2007;13(5):604–612. doi: 10.1038/nm1570. [DOI] [PubMed] [Google Scholar]
- Ijiri N, Kanazawa H, Asai K, Watanabe T, Hirata K. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology. 2015;20(4):612–617. doi: 10.1111/resp.12513. [DOI] [PubMed] [Google Scholar]
- Jiang S, Piao L, Ma EB, Ha H, Huh JY. Associations of circulating irisin with FNDC5 expression in fat and muscle in type 1 and type 2 diabetic mice. Biomolecules. 2021;11(2):322. doi: 10.3390/biom11020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagoshima M, Ito T, Kitamura H, Goshima Y. Diverse gene expression and function of semaphorins in developing lung: positive and negative regulatory roles of semaphorins in lung branching morphogenesis. Genes Cells. 2001;6(6):559–571. doi: 10.1046/j.1365-2443.2001.00441.x. [DOI] [PubMed] [Google Scholar]
- Kawut SM. COPD: cardiopulmonary disease? Eur Respir Soc. 2013;41:1241–1243. doi: 10.1183/09031936.00009413. [DOI] [PubMed] [Google Scholar]
- Kazeminasab F, Sadeghi E, Afshari-Safavi A. Comparative impact of various exercises on circulating irisin in healthy subjects: a systematic review and network meta-analysis. Oxid Med Cell Longev. 2022;2022:1–12. doi: 10.1155/2022/8235809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M, Bano I. Selenium as an important factor in various disease states-a review. EXCLI J. 2022;21:948. doi: 10.17179/excli2022-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M, Sandoval SN. The importance of selenium in food enrichment processes. A comprehensive review. J Trace Elem Med Biol. 2023;79:127260. doi: 10.1016/j.jtemb.2023.127260. [DOI] [PubMed] [Google Scholar]
- Kieliszek M, Bano I, Zare H. A comprehensive review on selenium and its effects on human health and distribution in Middle Eastern countries. Biol Trace Elem Res. 2022;200(3):971–987. doi: 10.1007/s12011-021-02716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhrle J, Gärtner R. Selenium and thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23(6):815–827. doi: 10.1016/j.beem.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Korta P, Pocheć E, Mazur-Biały A. Irisin as a multifunctional protein: implications for health and certain diseases. Medicina. 2019;55(8):485. doi: 10.3390/medicina55080485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H, Asai K, Kojima K, Sugitani A, Kyomoto Y, Okamoto A, et al. Exercise ameliorates emphysema of cigarette smoke-induced COPD in mice through the exercise-irisin-Nrf2 axis. Int J Chron Obstruct Pulmon Dis. 2019;14:2507–2516. doi: 10.2147/COPD.S226623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S-M, Sin J-C, Abdullah AZ, Mohamed AR. Degradation of wastewaters containing organic dyes photocatalysed by zinc oxide: a review. Desalin Water Treat. 2012;41(1–3):131–169. doi: 10.1080/19443994.2012.664698. [DOI] [Google Scholar]
- Langen R, Gosker H, Remels A, Schols A. Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease. Int J Biochem Cell Biol. 2013;45(10):2245–2256. doi: 10.1016/j.biocel.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Lavi G, Horwitz A, Einstein O, Zipori R, Gross O, Birk R. Fndc5/irisin is regulated by myogenesis stage, irisin, muscle type and training. Am J Transl Res. 2022;14(10):7063. [PMC free article] [PubMed] [Google Scholar]
- Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, et al. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol. 2006;36(7):1782–1793. doi: 10.1002/eji.200535601. [DOI] [PubMed] [Google Scholar]
- Li A, Liu Y, Zhu X, Sun X, Feng X, et al. Protective effect of methylallyl sulfone in the development of cigarette smoke extract-induced apoptosis in rats and HFL-1 cells. Biochem Biophys Res Commun. 2018;498(3):627–632. doi: 10.1016/j.bbrc.2018.03.033. [DOI] [PubMed] [Google Scholar]
- Li X, Jamal M, Guo P, Jin Z, Zheng F, Song X, et al. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed Pharmacother. 2019;118:109363. doi: 10.1016/j.biopha.2019.109363. [DOI] [PubMed] [Google Scholar]
- Li Y, Gao H, Zhao L, Wang J. Osteoporosis in COPD patients: Risk factors and pulmonary rehabilitation. Clin Respir J. 2022;16(7):487–496. doi: 10.1111/crj.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Chen K. The role of Irisin in multiorgan protection. Mol Biol Rep. 2021;48(1):763–772. doi: 10.1007/s11033-020-06067-1. [DOI] [PubMed] [Google Scholar]
- Maalouf G-E, El Khoury D. Exercise-induced irisin, the fat browning myokine, as a potential anticancer agent. J Obes. 2019;2019(2019):1–8. doi: 10.1155/2019/6561726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Thorat D, Soundararajan G, Pradhan S, Chakraborty G, Lohite K, et al. Semaphorin 3A upregulates FOXO 3a-dependent MelCAM expression leading to attenuation of breast tumor growth and angiogenesis. Oncogene. 2015;34(12):1584–1595. doi: 10.1038/onc.2014.79. [DOI] [PubMed] [Google Scholar]
- Organization WH. World health organization tobacco fact sheet. Geneva: WHO; 2014. [Google Scholar]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37(2):263–274. doi: 10.1016/S0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Piccirillo R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front Physiol. 2019;10:287. doi: 10.3389/fphys.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KS, Selvaraj K. Biogenic synthesis of selenium nanoparticles and their effect on As (III)-induced toxicity on human lymphocytes. Biol Trace Elem Res. 2014;157:275–283. doi: 10.1007/s12011-014-9891-0. [DOI] [PubMed] [Google Scholar]
- Püschel AW, Adams RH, Betz H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14(5):941–948. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Qamar N, John P, Bhatti A. Emerging role of selenium in treatment of rheumatoid arthritis: an insight on its antioxidant properties. J Trace Elem Med Biol. 2021;66:126737. doi: 10.1016/j.jtemb.2021.126737. [DOI] [PubMed] [Google Scholar]
- Qin F, Xu M-X, Wang Z-W, Han Z-N, Dong Y-N, Zhao J-X. Effect of aerobic exercise and different levels of fine particulate matter (PM2.5) on pulmonary response in Wistar rats. Life Sci. 2020;254:117355. doi: 10.1016/j.lfs.2020.117355. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64(4):527–542. doi: 10.1079/PNS2005467. [DOI] [PubMed] [Google Scholar]
- Razaghi A, Poorebrahim M, Sarhan D, Björnstedt M. Selenium stimulates the antitumour immunity: insights to future research. Eur J Cancer. 2021;155:256–267. doi: 10.1016/j.ejca.2021.07.013. [DOI] [PubMed] [Google Scholar]
- Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, et al. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun. 2017;8(1):1104. doi: 10.1038/s41467-017-01131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi F, Catelan D, Accetta G, Peluso M, Pistelli R, Barbone F, et al. Asthma symptoms, lung function, and markers of oxidative stress and inflammation in children exposed to oil refinery pollution. J Asthma. 2011;48(1):84–90. doi: 10.3109/02770903.2010.538106. [DOI] [PubMed] [Google Scholar]
- Sato Y, Do MKQ, Suzuki T, Ohtsubo H, Mizunoya W, Nakamura M, et al. Satellite cells produce neural chemorepellent semaphorin 3A upon muscle injury. Anim Sci J. 2013;84(2):185–189. doi: 10.1111/asj.12014. [DOI] [PubMed] [Google Scholar]
- Sawaki H, Nakamura F, Aihara M, Nagashima Y, Komori-Yamaguchi J, Yamashita N, et al. Intranasal administration of semaphorin-3A alleviates sneezing and nasal rubbing in a murine model of allergic rhinitis. J Pharmacol Sci. 2011;117(1):34–44. doi: 10.1254/jphs.11005FP. [DOI] [PubMed] [Google Scholar]
- Shaban EE, Elbakry HF, Ibrahim KS, El Sayed EM, Salama DM, Farrag A-RH. The effect of white kidney bean fertilized with nano-zinc on nutritional and biochemical aspects in rats. Biotechnol Rep. 2019;23:e00357. doi: 10.1016/j.btre.2019.e00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahabi R, Anissian A, Javadmoosavi SA, Nasirinezhad F. Protective and anti-inflammatory effect of selenium nano-particles against bleomycin-induced pulmonary injury in male rats. Drug Chem Toxicol. 2021;44(1):92–100. doi: 10.1080/01480545.2018.1560466. [DOI] [PubMed] [Google Scholar]
- Shaheen SO, Newson RB, Rayman MP, Wong AP, Tumilty MK, Phillips JM, et al. Randomised, double blind, placebo-controlled trial of selenium supplementation in adult asthma. Thorax. 2007;62(6):483–490. doi: 10.1136/thx.2006.071563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J, Cadenas S, Jekabsons M, Roussel D, Brand M. Mitochondrial proton leak and the uncoupling protein 1 homologues. Biochim Biophys Acta BBA Bioenerg. 2001;1504(1):144–158. doi: 10.1016/S0005-2728(00)00243-7. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Asai K, Yamada K, Kureya Y, Ijiri N, Watanabe T, et al. Decreased levels of irisin, a skeletal muscle cell-derived myokine, are related to emphysema associated with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:765–772. doi: 10.2147/COPD.S126233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryadinata RV, Wirjatmadi B. Selenium linked to increased antioxidant levels and decreased free radicals in lung tissue of Wistar rats exposed to e-cigarette smoke. J Glob Pharma Technol. 2020;12(9):32–39. [Google Scholar]
- Suzuki T, Takaishi H, Sakata T, Do MKQ, Hara M, Sato A, et al. In vitro measurement of post-natal changes in proliferating satellite cell frequency during rat muscle growth. Anim Sci J. 2010;81(2):245–251. doi: 10.1111/j.1740-0929.2009.00734.x. [DOI] [PubMed] [Google Scholar]
- Szabó MR, Pipicz M, Csont T, Csonka C. Modulatory effect of myokines on reactive oxygen species in ischemia/reperfusion. Int J Mol Sci. 2020;21(24):9382. doi: 10.3390/ijms21249382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Yu R, Liu S, Huwatibieke B, Li Z, Zhang W. Irisin inhibits hepatic cholesterol synthesis via AMPK-SREBP2 signaling. EBioMedicine. 2016;6:139–148. doi: 10.1016/j.ebiom.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Sankoda Y, Anderson JE, Sato Y, Mizunoya W, Shimizu N, et al. Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am J Physiol Cell Physiol. 2009;297(2):C238–C252. doi: 10.1152/ajpcell.00161.2009. [DOI] [PubMed] [Google Scholar]
- Todorović I, Cheng F, Stojisavljević S, Marinković S, Kremenović S, Savić P, et al. Prevalence of cigarette smoking and influence of associated factors among students of the University of Banja Luka: a cross-sectional study. Medicina. 2022;58(4):502. doi: 10.3390/medicina58040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomo S, Saikiran G, Banerjee M, Paul S. Selenium to selenoproteins—role in COVID-19. EXCLI J. 2021;20:781. doi: 10.17179/excli2021-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahid F, Rahmani D. Can an anti-inflammatory diet be effective in preventing or treating viral respiratory diseases? A systematic narrative review. Clin Nutr ESPEN. 2021;43:9–15. doi: 10.1016/j.clnesp.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- Wang J, Um P, Dickerman BA, Liu J. Zinc, magnesium, selenium and depression: a review of the evidence, potential mechanisms and implications. Nutrients. 2018;10(5):584. doi: 10.3390/nu10050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yin J-J, Zhang F, Yu H-D, Chen F-F, Zhang Z-Y, Zhang X-Z. Selenium status affects hypertrophic growth of skeletal muscle in growing zebrafish by mediating protein turnover. J Nutr. 2021;151(7):1791–1801. doi: 10.1093/jn/nxab082. [DOI] [PubMed] [Google Scholar]
- Waseem R, Yadav NS, Khan T, Ahmad F, Kazim SN, Hassan MI, et al. Molecular basis of structural stability of Irisin: a combined molecular dynamics simulation and in vitro studies for urea-induced denaturation. J Mol Liq. 2023;372:121120. doi: 10.1016/j.molliq.2022.121120. [DOI] [Google Scholar]
- Winterbourn CC, Chan T, Buss IH, Inder TE, Mogridge N, Darlow BA. Protein carbonyls and lipid peroxidation products as oxidation markers in preterm infant plasma: associations with chronic lung disease and retinopathy and effects of selenium supplementation. Pediatr Res. 2000;48(1):84–90. doi: 10.1203/00006450-200007000-00015. [DOI] [PubMed] [Google Scholar]
- Xiang R, Xu Y, Zhang W, Kong YG, Tan L, Chen SM, et al. Semaphorin 3A inhibits allergic inflammation by regulating immune responses in a mouse model of allergic rhinitis. Int Forum Allergy Rhinol. 2019;9(5):528–537. doi: 10.1002/alr.22274. [DOI] [PubMed] [Google Scholar]
- Xin C, Liu J, Zhang J, Zhu D, Wang H, Xiong L, et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes. 2016;40(3):443–451. doi: 10.1038/ijo.2015.199. [DOI] [PubMed] [Google Scholar]
- Yin P, Jiang C, Cheng K, Lam T, Lam K, Miller M, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. The Lancet. 2007;370(9589):751–757. doi: 10.1016/S0140-6736(07)61378-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Valverde P, Zhu X, Murray D, Wu Y, Yu L, et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017;5(1):1–14. doi: 10.1038/boneres.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Wang Y, Ming J, Chen W, Zhang L. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353–1364. doi: 10.2147/COPD.S161555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong D, Liu X, Li J, Ouyang R, Chen P. The role of cigarette smoke-induced epigenetic alterations in inflammation. Epigenet Chromatin. 2019;12:1–25. doi: 10.1186/s13072-019-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author.