Abstract
Xylanases from thermophilic fungi have a wide range of commercial applications in the bioconversion of lignocellulosic materials and biobleaching in the pulp and paper industry. In this study, an endoxylanase from the thermophilic fungus Rasamsonia composticola (XylRc) was produced using waste wheat bran and pretreated sugarcane bagasse (PSB) in solid-state fermentation. The enzyme was purified, biochemically characterized, and used for the saccharification of sugarcane bagasse. XylRc was purified 30.6-fold with a 22% yield. The analysis using sodium dodecyl sulphate–polyacrylamide gel electrophoresis revealed a molecular weight of 53 kDa, with optimal temperature and pH values of 80 °C and 5.5, respectively. Thin-layer chromatography suggests that the enzyme is an endoxylanase and belongs to the glycoside hydrolase 10 family. The enzyme was stimulated by the presence of K+, Ca2+, Mg2+, and Co2+ and remained stable in the presence of the surfactant Triton X-100. XylRc was also stimulated by organic solvents butanol (113%), ethanol (175%), isopropanol (176%), and acetone (185%). The Km and Vmax values for oat spelt and birchwood xylan were 6.7 ± 0.7 mg/mL, 2.3 ± 0.59 mg/mL, 446.7 ± 12.7 µmol/min/mg, and 173.7 ± 6.5 µmol/min/mg, respectively. XylRc was unaffected by different phenolic compounds: ferulic, tannic, cinnamic, benzoic, and coumaric acids at concentrations of 2.5–10 mg/mL. The results of saccharification of PSB showed that supplementation of a commercial enzymatic cocktail (Cellic® CTec2) with XylRc (1:1 w/v) led to an increase in the degree of synergism (DS) in total reducing sugar (1.28) and glucose released (1.05) compared to the control (Cellic® HTec2). In summary, XylRc demonstrated significant potential for applications in lignocellulosic biomass hydrolysis, making it an attractive alternative for producing xylooligosaccharides and xylose, which can serve as precursors for biofuel production.
Keywords: Biomass hydrolysis, Xylanase, Commercial enzyme, Reuse of agro-industrial residues
Introduction
Lignocellulosic biomass is an integral component of plant cell walls, comprising cellulose, hemicellulose, and lignin (Collard and Blin 2014). The continuous growth of agro-industrial activities in recent years has led to significant challenges associated with the improper disposal of lignocellulosic waste. Although these activities generate employment and stimulate the economy, the large-scale production in agro-industries results in the accumulation of residues. These residues require appropriate treatment before they can be returned to the environment or used as raw materials for the production of other valuable products (Anwar et al. 2014; Alokika et al. 2021). Agro-residues include sugarcane bagasse, corn cobs, straw, stems, husks, seeds, bran, pulp, and fibers (Yousuf et al. 2019; Castro-Ochoa et al. 2023). Nowadays, materials such as sugarcane bagasse are being utilized for electricity generation in the sugar and ethanol industry, thus contributing to energy savings (Carvalho et al. 2020).
The Brazilian economy relies mainly on farming and agricultural production, resulting in a substantial volume of agro-industrial waste, particularly sugarcane bagasse, originating from various sources across the country. Therefore, opportunities to add value to these by-products indirectly contribute to reducing the environmental effects of their improper disposal. These agro-residues primarily consist of lignocellulosic biomass, making them well-suited for sustainable biofuel, sugar, and high-value biochemical production (Rodrigues et al. 2022; Julio et al. 2017; Banerjee et al. 2017).
Sugarcane bagasse, owing to its high xylan content, is recognized as an excellent substrate for the cost-effective production of xylo-oligosaccharides (XOs) and xylose, both of which serve as valuable precursors for biofuel production (Ávila et al. 2020; Pinales-Márquez et al. 2021). Recent studies conducted by Kaushal et al (2022) also demonstrated the production of XOs using walnut shell waste as a xylan source. The molecular structure of xylans in sugarcane bagasse typically comprises 4-O-methyl-glucuronoarabinoxylans (4-O-MeGlcA-AX), characterized by a linear (1 → 4)-linked β-D-xylopyranosyl backbone with branches at O-2 and O-3, containing 4-O-methyl glucuronic acid and arabinofuranosyl units, respectively (Carvalho et al. 2017). Furthermore, sugarcane bagasse has drawn attention, especially in the context of second-generation bioethanol production (Bezerra and Ragauskas 2016; Jugwanth et al. 2020). However, the conversion of these residues into fermentable sugars is a challenge, and the development of cost-effective methods is essential. Technologies, including the production of fibrolytic enzymes, particularly xylanases and cellulases, play a crucial role in addressing this challenge (Sindhu et al. 2016).
Xylanases represent a group of enzymes responsible for the hydrolysis of xylan and can be categorized as endo-β-(1–4)-xylanases (EC 3.2.1.8), exo-β-(1–4)-xylanases (EC 3.2.1.37), β-xylosidases (EC 3.2.1.37), and various accessory enzymes such as α-L-arabinofuranosidase, α-glucuronidase, acetylxylan esterase, ferulic acid esterase, and p-coumaric acid esterase (Zanoelo et al. 2004; Almeida et al. 2022). Among these enzymes, endo-xylanases are the most abundant within the xylanolytic system.
Endo-xylanase cleaves the internal glycosidic bonds of the polymer, releasing XOs of various sizes, whereas exo-xylanases and β-xylosidases convert XOs into xylobiose and xylose, originating from the reducing and non-reducing ends, respectively (Silva et al. 2019).
Xylanases described in the literature belong to glycoside hydrolase (GH) families 5, 7, 8, 10, 11, and 43 (Lombard et al. 2014), with GH10 and GH11 being the most extensively explored for structural studies and industrial applications (Rashid and Sohail 2021). They are primarily produced by thermophilic fungi, bacteria, and archaea (Chadha et al. 2019). These enzymes exhibit differences in their sequences, physicochemical properties, modes of action, and substrate specificity (Gonçalves et al. 2012). GH10 and GH11 endo-xylanases demonstrate high catalytic specificity for polysaccharides composed of xylose, resulting in the production of short-chain XOs such as xylobiose (X2), xylotriose (X3), and xylotetraose (X4). In general, GH11 enzymes produce larger XOs and smaller amounts of free xylose, whereas GH10 enzymes liberate smaller XOs and larger amounts of free xylose (Poletto et al. 2020).
Thermostable xylanases have a wide range of applications in various industries, including cellulose and paper bio-bleaching, food production, textile manufacturing, juice clarification, and biofuel formulation. The estimated annual market for these enzymes is approximately USD 500 million (Sharma et al. 2019; Chadha et al. 2019; Dar and Dar 2021; Bhardwaj et al. 2021; Li et al. 2022). The paper industry has been modifying the bio-bleaching processes to reduce pollution. Thus, xylanases have been used in the paper industry as eco-friendly brighteners to promote environmental preservation (Singh et al. 2019). These enzymes offer an excellent alternative for the production of xylose, which can be chemically converted or fermented by microorganisms into valuable compounds, such as 1,4-butanediol, 1,2,4-butanetriol, ethylene glycol, xylitol, and second-generation ethanol (bioethanol) derived from lignocellulosic biomass (Zhao et al. 2020). Several thermophilic fungi, including Myceliophthora thermophila (Karnaouri et al. 2014), Humicola insolens (Du et al. 2013), Chrysosporiium lucknowenese (Ustinov et al. 2008), Remersonia thermophila (McPhillips et al. 2014), Thermomyces lanuginosus (Winger et al. 2014), and Thermoascus aurantiacus (Jain et al. 2015), produce multiple endo-xylanases as part of their strategy to hydrolyze xylan and lignocellulosic wastes. Rasamsonia composticola is a thermophilic fungus, and recent studies in our laboratory have demonstrated its excellent potential for the cost-effective production of thermotolerant xylanase using low-cost agro-industrial residues. Given these facts, the objectives of this work include the production of xylanase using agro-industrial residues as a carbon source, the biochemical purification of the enzyme, and the evaluation of the biotechnological potential of xylanase from R. composticola (XylRc) in the saccharification of pretreated sugarcane bagasse (PSB). We aim to compare its hydrolysis performance against the commercial cellulase cocktail Cellic®HTec2.
Material and methods
Maintenance of microorganisms
The strain R. composticola is deposited in the collection of the Laboratory of Biochemistry and Applied Microbiology, IBILCE, UNESP at São José do Rio Preto, SP, Brazil. It was generously provided for collaborative studies by Roberto Ruller PhD and Josiane Scarpassa PhD. To maintain the strain, repeated subcultures were carried out on potato dextrose agar at 40 °C for 5–10 days, and stored in the refrigerator for a maximum of 30 days.
Production of xylanase from R. composticola (XylRc) using solid-state fermentation
XylRc was produced with SSF using a suspension of conidia in sterile distilled water at a concentration of 1 × 108 spores/mL. This suspension was inoculated into 5 g of wheat bran mixed with exploded sugarcane bagasse (1:1; w/w) in a solid medium that had been previously autoclaved with 10 mL of deionized water. Cultivation occurred at 40 °C in 250 mL Erlenmeyer flasks.
After 5 days, 25 mL of cold distilled water was added to the culture and maintained under moderate agitation for 30 min. The mixture was then filtered and centrifuged at 10,000×g for 10 min at 4 ºC. Following the enzymatic assays, the supernatant, enriched with xylanase (XylRc), was stored at 4 °C.
Xylanase assay and protein quantification
Xylanase activity was assayed at 60 °C in 50 mmol/L−1 sodium acetate buffer, pH 5.0, containing 1% (w/v) birchwood xylan (Sigma-Aldrich, USA). The reaction was initiated by adding 10 μL of diluted enzyme and allowed to proceed for 10 min. To stop the reaction, 3,5-dinitrosalicylic acid (DNS) reagent was added to determine the reducing sugars released, following the method of Miller (1959), with xylose as a standard. The absorbance was measured at 540 nm using a Spectramax 384 plus microplate reader (Molecular Devices, USA). One unit of xylanase activity (1 U) was defined as the amount of enzyme capable of releasing 1 μmol of reducing sugars per minute under the assay conditions. Total protein was determined using the Bradford method (1976) with bovine serum albumin as the standard. The relationship between one unit of enzyme activity (1 U) and protein content (mg) was expressed as specific activity (U/mg of total protein or xylanase).
Purification of XylRc
The crude extract was lyophilized, resuspended in 10 mL of distilled water, and dialyzed against 50 mmol L−1 acetate buffer, pH 5.0, and 2.0 mmol L−1 NaCl. The sample was then applied to a phenyl-Sepharose (CL-4B) column (1.0 × 9.8 cm) at a flow rate of 0.2 mL/min (Cyntia, USA). The column was equilibrated with the same buffer conditions as described above. Elution was performed using an NaCl gradient ranging from 2.0 to 0 mol L−1. Xylanase-containing fractions were combined, dialyzed, and equilibrated with 50 mmol L−1 sodium acetate buffer, pH 5.0. The sample was subsequently applied to a Sephacryl-S200 column (3.0 × 65.5 cm) at a flow rate of 0.1 mL per min (Cyntia, USA). The column was pre-equilibrated under the same conditions as the filtration column. All protein detection was performed by measuring absorbance at 280 nm, and xylanase activity was measured as previously described. The active fractions were combined, dialyzed against distilled water, and used for purity analysis and biochemical characterization.
Polyacrylamide gel electrophoresis analysis
Sodium-dodecyl sulfate (SDS)-PAGE under denaturing conditions was conducted using 30 µg of purified XylRc in a 10% gel based on the method reported Laemmli (1970). A molecular weight standard from Sigma-Aldrich (C4861; Sigma-Aldrich, USA) was used. The gel was stained with Coomassie Brilliant Blue R.
Zymogram analysis was performed using a 7% polyacrylamide gel electrophoresis under non-denaturing conditions (PAGE), in accordance with Davis (1964). Following electrophoresis, the gel was sliced into two halves: one half was stained with Coomassie Brilliant Blue R, and the other was prewashed with 50 mmol L−1 sodium acetate buffer at pH 5.0, followed by incubation in 50 mmol/L sodium acetate containing 1% birchwood xylan. The gel was washed with distilled water, stained with Congo Red (Sigma-Aldrich, USA) and revealed with 1.0 mol L−1 NaCl solution.
Analysis of hydrolysis products using thin-layer chromatography (TLC)
The analysis of hydrolysis products was carried out using a preparation of XylRc (230 ± 5.0 U mg−1). XylRc activity was conducted at 60 °C in 50 mmol.L−1 sodium acetate buffer at pH 5.0, using 1% (w/v) birchwood xylan as a substrate. Aliquots were sampled at time intervals of 15, 30, and 60 min and then boiled for 3 min. After centrifugation at 5000×g for 10 min, the supernatant was employed for the analysis of hydrolysis products using TLC with silica gel. A 1% (w/v) xylose solution served as a standard. The mobile phase comprised a mixture of ethyl acetate, acetic acid, formic acid, and water (9:3:1:4 by volume). The formed compounds were detected by spraying the TLC with a solution of H2SO4 and methanol (1:9 by volume), supplemented with 0.2% (w/v) orcinol, followed by heating to 100 °C.
Biochemical characterization of the purified XylRc
Optimal pH, temperature, and thermostability of the enzyme
To determine the optimal temperature, XylRc activity was assessed over a range of temperatures from 30 to 90 °C in 50 mmol L−1 sodium acetate buffer at pH 5.0. The optimal pH was determined using the McIlvaine buffer (McIlvaine 1921), with pH levels 3.0–8.0, at 60 °C, employing 1% (w/v) birchwood xylan as a substrate.
To assess the thermal stability of XylRc, the enzyme was preincubated at temperatures between 40 and 85 °C. At each temperature, aliquots were sampled at time intervals of 15, 30, and 60 min, and XylRc activity was measured according to the XylRc assay under optimal conditions, using 1% (w/v) birchwood xylan as the substrate.
Effect of ions on XylRc activity
The effect of metal ions, including K+, Hg2+, Fe2+, Fe3+,Cu2+, Al3+, Ca2+, Zn2+, Mg2+, NH4+, Co2+, Mn2+, Li+, Sr2+ and ethylenediaminetetraacetic acid (EDTA), on XylRc activity was determined at concentrations of 1.0 and 5 mmol L−1, under optimal conditions of pH and temperature. Results were expressed as residual activity, with 100% representing the measurement in the absence of ions.
Effect of chemical compounds on XylRc stability
Purified XylRc was preincubated in solutions containing various organic solvents, such as ethanol, isopropanol, methanol, and dimethyl sulfoxide (DMSO), as well as acetone, each at a concentration of 10% (v/v). Additionally, surfactants including polyethylene glycol (PEG), cetyltrimethylammonium bromide (CTAB), SDS, and Triton X-100 (Sigma-Aldrich, USA) were used at a concentration of 10% (w/v). After 60 min incubation at room temperature (25 °C), XylRc activity was measured under optimal enzymatic conditions. All results were expressed as residual activity, with 100% corresponding to the measurement of xylanase in the absence of the mentioned chemical compounds.
XylRc stability in the presence of phenolic compounds
The effect of phenolic compounds on XylRc activity was evaluated using ferulic acid, tannic acid, cinnamic acid, benzoic acid, and coumaric acid (Sigma-Aldrich, USA). Each phenolic compound was prepared using 10% ethanol and incubated with the XylRc mixture at a 1:1 (v/v) ratio, at concentrations of 2.5, 5, and 10 mg/mL. The mixture was incubated for 8 h at room temperature (25 °C), and afterward, the residual xylanolytic activity was measured under optimal enzymatic assay conditions. All results were expressed as residual activity, with 100% corresponding to the measurement of xylanase in the absence of ethanol.
Substrate specificity and determination of kinetic parameters for XylRc activity
To assess substrate specificity, the xylanase activity of XylRc was measured using 1% (w/v) birchwood xylan, oat spelt xylan, carboxymethyl cellulose, starch, and pectin (Sigma-Aldrich, USA) in 50 mmol L−1 sodium acetate buffer at pH 5.5 and 80 °C.
The kinetic parameters for the xylanase activity of XylRc were determined using birchwood xylan or oat spelt xylan as substrates over a concentration range from 0.25 to 50 mg/mL. The maximal velocity (Vmax) and the constant of dissociation apparent of the substrate-enzyme complex (Km) were calculated using Origin 9.0 Software.
Saccharification of PSB using XylRc
For the enzymatic saccharification test, hydrothermally PSB was prepared following the method of Nakasu et al (2017). Combinations of cellulases Cellic®CTec2 (Novozymes, DNK) and XylRc enzymes were tested at the following ratios: (a) Cellic®CTec2 (5 mg/g of substrate); and (b) Cellic®CTec2 (2.5 mg/g of substrate) plus XylRc (2.5 mg/g of substrate). All assays were conducted in microtubes containing up to 3% PSB (w/v) in a final volume of 1.0 mL, with 50 mmol L−1 sodium acetate buffer at pH 5.0, according to Almeida et al (2022). The samples were incubated in an Accuterm™ thermomixer (Labnet, USA), at 50 ºC and 500 rpm, for 72 h (Alvira et al. 2011). Total glucose content was determined using the glucose oxidase method (Bergmeyer and Bernt 1974) with glucose as the standard. The measurement of total reducing sugars released was conducted using the DNS method, employing xylose as the standard. Enzyme mixture blanks were prepared simultaneously, and all experiments were performed in quintuplicate.
The synergy between individual enzyme preparations was assessed using the following equation described by Almeida et al (2022):
where DS is degree of synergism, PC mixture is the sum of the product concentrations obtained after using the enzymatic cocktail, and PC individual is the sum of the product concentrations of the individual enzymatic preparations.
Reproducibility and statistical analysis
All biochemical characterization assays were conducted in triplicate, using different purified XylRc samples, and the resulting values were presented as mean ± standard deviation. Enzymatic saccharification experiments were carried out in quintuplicate. Data were statistically analyzed using Student’s t-test or one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test, with the assistance of GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). Results were deemed significant when p ≤ 0.05.
Results and discussion
Production, purification, and molecular properties of XylRc
XylRc, the xylanase produced by R. composticola, was cultivated through SSF using a mixture of wheat bran and exploded sugarcane bagasse (1:1, w/w) at 50 °C for 5 days. The enzyme was then purified to near homogeneity from culture filtrates using a combination of chromatographic methods. The crude extract contained 2.350 total U of XylRc, which was primarily applied to the Phenyl-Sepharose column. The chromatogram revealed two peaks containing xylanase: the first peak had no interaction with the column and yielded very little, whereas the second peak of xylanase was eluted after applying a gradient of NaCl concentration, retaining a yield of 95.8% (2.252 U) and providing a 22-fold purification. This peak was collected, concentrated, and applied to the Sephacryl-S200 column. A single peak containing xylanase was obtained, with an activity of 528.0 U, a purification factor of 30.6-fold, and a yield of 22%. The purification steps are summarized in Table 1. Several fungal xylanases have been previously purified using different chromatographic methods. The endo-xylanase from Aspergillus awamori AFE1 was purified 6.86-fold with a yield of 5.20% using three chromatographic steps (Olopoda et al. 2022). An extracellular endo-xylanase produced by Thermomyces dupontii KKU–CLD–E2–3 was purified with a 27.92-fold purification and a recovery yield of 2.01% (Seemakram et al. 2020), which is comparable to our study. The xylanase from R. thermophila xylanase (RtXyl) exhibited a yield of 1.37% and 1.35-fold purification (McPhillips et al. 2014).
Table 1.
Purification of endoxylanase from R. composticola
| Step purification | Total Units (U) | Total Protein (mg) | Specific Activity (U mg−1) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude Extract | 2.350 | 314 | 7.5 | 100 | 1.0 |
| Phenyl-Sepharose (Peak II) | 2.252 | 13.6 | 165.5 | 95.8 | 22 |
| Sephacryl-S200 | 528 | 2.3 | 229 | 22 | 30.6 |
SDS-PAGE analysis (Figs. 1a and 2b) of the purified XylRc revealed a single band with an estimated molecular weight of 53 kDa. Under non-denaturing conditions (PAGE), the purified XylRc (Fig. 1c) exhibited a single band, consistent with the zymogram (Fig. 1d). The molecular weight of xylanases from thermophilic fungi varies widely, from 21 to 78 kDa (Maheshwari et al. 2000; Chadha et al. 2019), with most xylanases being single polypeptides. Some exceptions, such as Xyl I and Xyl II of Talaromyces emersoni, are dimeric (Tuohy et al. 1993). Xylanases of the GH10 family often have similar molecular weights, such as those from thermophilic fungi Myceliophthora sp. (53 kDa), C. lucknowenese (57 kDa), Sporotrichum thermophile (48 kDa), H. insolens (44 kDa), and R. thermophila (42 kDa) (Chada et al. 2004; Ustinov et al. 2008; Vafiadi et al. 2010; Du et al. 2013; McPhillips et al. 2014).
Fig. 1.

Electrophoretic profile of purified XylRc. Under denaturing conditions (SDS-PAGE): A molecular mass marker (Sigma-Aldrich C4861) stained with 0.1% (w/v) Coomassie Brilliant Blue R; B SDS-PAGE 10% (w/v) of purified XylRc (30 μg) stained with Coomassie Brilliant Blue R. C Non-denaturing conditions [PAGE 7% (w/v)] of purified XylRc (30 μg). D Zymogram in 7% (w/v) PAGE gel stained with 1.0% (w/v) Congo red
Fig. 2.

Thin-layer chromatography (TLC) of the hydrolysis products by XylRc using 1% (w/v) birchwood xylan substrate. Line A: xylose (5 mg/mL) Lines B–E: hydrolysis products using xylanase after 0, 15, 30, and 60 min of incubation, respectively. The TLC was sprayed using a solution of H2SO4 and methanol (1:9 per vol) added 0.2% (w/v) orcinol, followed by heating to 100 °C
The hydrolysis products of birchwood xylan by XylRc were analyzed using the TLC technique (Fig. 2). The hydrolysis products consisted of XOs of varying lengths at time intervals of 15, 30, and 60 min. Endo-xylanases randomly cleave β-1,4-glycosidic linkages within the xylan main chain, releasing XOs of different lengths (Zimbardi et al. 2013). Various endo-xylanases from H. brevis var thermoidea, T. aurantiacus, and A. japonicus have been observed to cause the hydrolysis of xylan and release of XOs as a product (Almeida et al. 2022; Chanwicha et al. 2015; Silva et al. 2019). The production of XOs, including X2, X3, and X4, through enzymatic hydrolysis, is a crucial strategy for the conversion of lignocellulosic biomass such as sugarcane bagasse. This method is also considered a cost-effective means of XO production and second-generation (2nd G) bioethanol production (Nascimento et al. 2022). Similar results were observed by Bhardwaj et al. (2021) and Almeida et al (2022). The results of TLC analysis, in conjunction with the estimated molecular weight by SDS-PAGE (53 kDa), suggest that XylRc belongs to the GH10 family (Pollet et al. 2010).
Biochemical characterization of XylRc
Determination of optimal pH, temperature, and thermostability
The effect of pH on the activity of purified XylRc is depicted in Fig. 3a. The endo-xylanase displayed a typical pH activity curve, with maximum activity at pH 5.5. It retained approximately 75% and 95% of its enzymatic activity at pH 5.0 and 6.0, respectively. Most filamentous fungal xylanases exhibit optimal pH values in the acidic range of 4.5–6.5 (Maheshwari et al. 2000; Polizeli et al. 2005; Sharma and Kumar 2013). Similar results were observed for xylanases from thermophilic fungi such as Rasamsonia emersonii (Zanoni et al. 2021) and Malbranchea pulchella xylanase expressed in Aspergillus nidulans (Ribeiro et al. 2014).
Fig. 3.
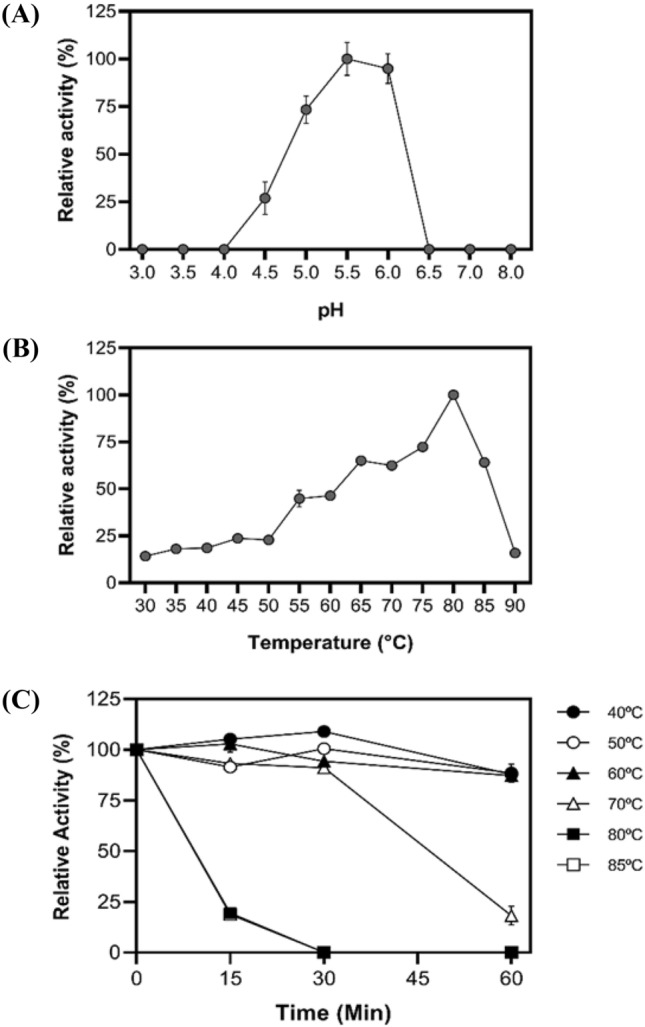
Effect of pH, temperature, and thermostability on XylRc. A Optimum pH estimated at 60 °C using 1% xylan birchwood in pH ranging from 3.0–8.0. B Optimal temperature was estimated by incubating the enzyme at different temperatures (30–90 °C) for 10 min. C Temperature stability determined by incubating the purified enzyme at temperatures ranging from 40 to 85 °C for 15, 30, and 60 min. The activity was measured in residual activity according to the test conditions. The XylRc activity that corresponds to 100% was 215 ± 5.0 U/mg
The optimum temperature for XylRc activity is displayed in Fig. 3b. The enzyme showed an increase in catalytic activity, reaching its maximal activity at 80 °C, with a decrease in xylanolytic activity at temperatures up to 90 °C. Other xylanases from thermophilic fungi, such as R. emersonii (Zanoni et al 2021), M. pulchella when expressed in A. nidulans (Ribeiro et al. 2014), and even the mesophilic fungi Crysoporthe cubensis exhibited their optimum activity at 80 °C.
The thermostability of XylRC is shown in Fig. 3c. The xylanase displayed remarkable thermostability in the temperature range of 40–60 °C after 60 min of incubation. At 70 °C, the enzyme remained stable, retaining 50% of its activity after 48.5 min (t1/2) of incubation. These results suggest that the enzyme maintains a stable conformation and lacks isoenzymes. Other thermophilic fungi, including Myceliophothora thermophila (Van de Brink et al. 2013), Chrysosporium lucknowense (Hinze et al. 2009), Rhizomucor pusillus (Hüttner et al. 2018), exhibit a broad range of xylanase thermostability.
Effect of ions on XylRc activity
Table 2 shows the effect of metal ions on XylRc activity. Most ions tested had a low influence on enzymatic activity. At a concentration of 1.0 mmol L−1, K+ (128.6% ± 2.9%) had the highest stimulatory effect, followed by Ca2+ (122.9% ± 3.5%), Mg2+ (120.1% ± 5.6%), NH4+ (113.8% ± 6.5%), and Co2+ (110.7% ± 6.8%), which also stimulated the endo-xylanase activity. Under concentrations of 5.0 mmol.L−1, only Mn2+ (112.0% ± 8.0%) showed an increase in XylRc activity compared to the salts at the lower concentration. Although some xylanases are inhibited by Mn2+ (Saha 2003), XylRc was stable in the presence of Mn2+ at 1.0 mmol L−1 and was even slightly stimulated at 5.0 mmol L−1 (12%). Similar results were reported for Xyl I from Trichoderma inhamatum (Silva et al. 2015a). XylRc was highly inhibited by Hg2+ at both concentrations tested and also inhibited at high concentrations of Cu2+, reducing enzymatic activity to 26%. Inhibition by Cu2+ and Hg2+ is common for xylanases and is indicative of the presence of thiol or carboxyl groups at the active site of the enzyme (Basit et al. 2018). Inhibition by Hg2+ and Cu2+ has been previously reported for other xylanases, such as those from A. awamori (Teixeira et al. 2010), Malbranchea cinnamomea (Basotra et al. 2018), and H. brevis var thermoidea (Almeida et al. 2022). The enzyme activity is not inhibited by the chelating agent EDTA. Therefore, the bioprospecting of thermostable xylanases resistant to metal ions is considered crucial for biotechnological applications.
Table 2.
Effect of metal ions and EDTA on the XylRc activity
| Additive | Relative activity (%) | |
|---|---|---|
| 1.0 mmol L−1 | 5.0 mmol L−1 | |
| Control | 100 ± 2.4 | 100 ± 2.4 |
| K+ | 128.6 ± 2.9* | 88.6 ± 6.7 |
| Ca2+ | 122.9 ± 3.5* | 104.8 ± 6.2 |
| Mg2+ | 120.1 ± 5.6* | 103.9 ± 1.8 |
| NH4+ | 113.8 ± 6.5* | 100.9 ± 2.3 |
| Co2+ | 110.7 ± 6.8 | 91 ± 6.3 |
| Li2+ | 107 ± 6.1 | 101 ± 0.2 |
| Mn2 | 101.8 ± 7.1 | 112 ± 8.3 |
| Sr2+ | 104 ± 4.2 | 87 ± 6.2 |
| Zn2+ | 91.1 ± 3.6 | 86.0 ± 3.6 |
| Al3+ | 54.9 ± 7,7* | 45 ± 6.2* |
| Cu2+ | 100.6 ± 1.7 | 26.1 ± 3,4* |
| Fe3+ | 64.4 ± 2.2* | 48.8 ± 4.9* |
| Fe2+ | 71.3 ± 9.8* | 0* |
| Hg2+ | 0* | 0* |
| EDTA | 119.9 ± 3.8* | 86.7 ± 4.5 |
Control Assay without addition of ions was considered as control (100%) corresponding 220 ± 6.0 U mg−1
*Significantly different from control group (p < 0.05)
Effect of organic solvents and surfactants on XylRc stability
Figure 4 illustrates the effect of various organic solvents and surfactants on the stability of XylRc. Following incubation in these substances, XylRc activity was stimulated by all organic solvents tested. Specifically, the enzyme was stimulated by the presence of butanol, ethanol, isopropanol, and acetone, with relative activities of 113%, 174%, 176%, and 185%, respectively. This tolerance of enzymatic activity to organic solvents can be attributed to the formation of a solvation layer that protects the enzyme from dehydration and aggregation (Cota et al. 2013). Xylanases with tolerance to organic acids, such as ethanol, are essential for various industries, including alcoholic beverages and brewing (Espejo 2021). This tolerance offers protection against microbial contamination and enhances the solubilization of hydrophobic substrates.
Fig. 4.
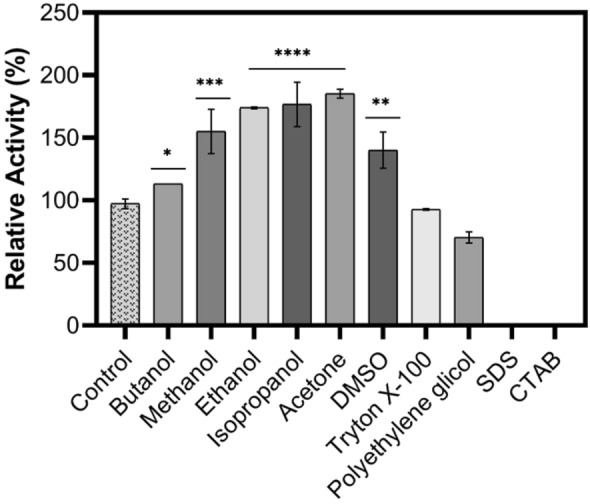
Effect of organic solvents and surfactants on the XylRc stability. Conditions: XylRc was incubated in the presence of 10% organic solvents or surfactants at room temperature for 1 h. The control (without addition of substance) was considered 100% corresponding 220 ± 6.0 U mg−1. The residual activity was measured according to the standard enzyme assay. Data was analyzed by ANOVA followed by Tukey's post hoc. P values are given as ****p < 0.0001, ***p < 0.001 and **p < 0.01. Results were considered significant when p < 0.05
XylRc also exhibited stability in the presence of DMSO, showing a stimulation of 140% in endo-xylanase activity. The enzyme was stable in the presence of the non-ionic surfactant Triton X-100 and was slightly inhibited by PEG at a concentration of 70% (w/v). Triton X-100 can promote protein disaggregation, improving enzymatic hydrolysis through enhanced exposure. However, SDS and CTAB significantly inhibited endo-xylanase activity. The inhibition of XylRc by SDS can be explained by the nature of the molecule's chemistry. SDS is an anionic surfactant and a potent protein denaturant (Manning and Colón 2004). It typically interacts with amino acid residues, causing protein unfolding and consequently a loss of enzymatic activity. Due to its anionic nature, SDS predominantly interacts with positively charged amino acid groups, such as histidine, arginine, and lysine, but it can also interact with hydrophobic groups through electrostatic interactions and Van der Waals attractions. On the other hand, the cationic surfactant CTAB interacts with negatively charged amino acid groups, such as glutamate and aspartate (Monica and Kapoor 2021). CTAB is a quaternary amine-based surfactant with a strong denaturing effect on catalytic sites (Monclaro et al. 2019). Similar results have been reported in other studies using Penicillium sclerotiorum (Knob and Carmona 2010) and A.japonicus (Silva et al. 2019).
Effect of phenolic compounds on XylRc stability
During the hydrolysis of lignocellulosic residues, various compounds are released that can interfere with the saccharification process. The composition of these inhibitory compounds depends on the pretreatment method (thermal, acid, or enzymatic), which can generate substances such as soluble sugars, furan derivatives, organic acids, and phenolic compounds (Duarte et al. 2012). Most enzymes involved in lignocellulosic degradation, including cellulases and hemicellulases, are typically inhibited by phenolic compounds. However, in some cases, these enzymes can exhibit resistance or even increased activity in the presence of phenolic compounds (Michelin et al. 2016; Amo et al. 2019; Silva et al. 2019; Almeida et al. 2022). Notably, XylRc demonstrated activation in the presence of most tested phenolic compounds at concentrations of 2.5, 5, and 10 mg/mL, as shown in Fig. 5.
Fig. 5.
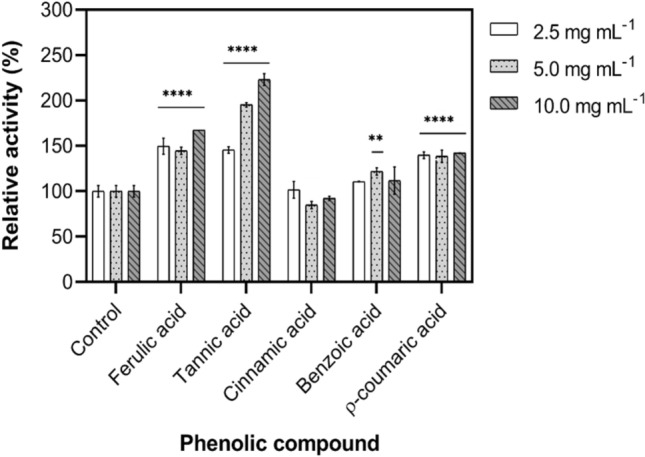
Effect of phenolic compounds on XylRc stability. XylRc was incubated in the presence of phenolic compounds at room temperature for 8 h. The control (without addition of substance) was considered 100% corresponding 220 ± 6.0 U mg−1. The residual activity was measured according to the standard enzyme assay Data were analyzed using ANOVA followed by Tukey's post hoc. P values are given as ****p < 0.0001 and **p < 0.01. Results were considered significant when p < 0.05
Upon preincubation of XylRc with tannic, ferulic, benzoic, and coumaric acids at various concentrations, the residual enzymatic activity remained unaffected and, in some cases, was stimulated by these phenolic compounds. Particularly, when XylRc was subjected to a concentration of 10 mg/mL of tannic acid, it exhibited a significant stimulation of enzyme activity, approximately 130%. At the same concentration, ferulic, benzoic, and coumaric acids did not adversely affect enzyme stability, and XylRc activity was either unaffected or activated, showing enhancements of 60%, 10%, and 40%, respectively. While endo-xylanases produced by T. lanuginosus (Mathibe et al. 2020), A. japonicus (Silva et al. 2019), and H. brevis var. thermoidea (Almeida et al. 2022) were inhibited by phenolic compounds, xylanases from Emericella nidulans (Silva et al. 2015b), and Aspergillus tamarii (Monclaro et al. 2019) were stimulated. These variations in enzymatic activity may be attributed to specific interactions, as phenolic compounds can promote the integrity of the catalytic site or enhance enzyme–substrate interactions during catalysis (Duarte et al. 2012). These results hold promise for various industrial applications, mainly in the hydrolysis of lignocellulosic residues for the production of XOs and bioethanol.
Specificity to substrates and kinetic parameters of XylRc
The enzyme demonstrated the ability to hydrolyze both birchwood and oat spelt xylan. However, it did not catalyze the hydrolysis of Avicel, citrus pectin, starch, and sucrose (data not shown). Similar results were reported for xylanase from A. tamarii, which exhibited higher activity toward oat spelt, birchwood and beechwood xylans (Monclaro et al. 2019). The kinetic parameters for birchwood and oat spelt xylan hydrolysis by XylRc are summarized in Table 3. The hydrolysis of both substrates by the enzyme exhibited a single curve of stimulation (data not shown) following Michaelian kinetics.
Table 3.
Kinetic Parameters of XylRc
| Substrates | Vmax (μmol min−1 mg−1) | Km (mg. mL−1) | Kcat (s−1) | Kcat/Km (mL s−1 mg−1) |
|---|---|---|---|---|
| Birchwood Xylan | 173.1 ± 8.8 | 2.3 ± 0.5 | 493.4 ± 6.9 | 211.3 ± 8.8 |
| Oat Spelt Xylan | 446.2 ± 6.7 | 6.7 ± 0.3 | 1269.5 ± 12.9 | 189.2 ± 10.2 |
In the case of birchwood xylan hydrolysis, XylRc displayed a Vmax of 173.7 ± 6.5 U mg−1and high substrate affinity with a Km of 2.3 ± 0.6 mg.mL−1. This resulted in a high turnover rate (Kcat = 493.4 ± 6.9/ s−1) and an efficient catalytic efficiency (Kcat/Km = 211.3 ± 8.8 s−1 mg−1 mL). In contrast, for oat spelt xylan hydrolysis, XylRc exhibited a higher Vmax of 446.7 ± 12.7 U mg−1 but a lower substrate affinity (Km = 6.7 ± 0.7 mg mL−1), resulting in a higher turnover rate (Kcat = 1269.5 ± 12.9 s1) but a lower catalytic efficiency (Kcat/Km = 189.2 ± 10.2 s−1 mg−1 mL). Fungal xylanases have Km values ranging from 0.14 to 14 mg mL−1 (Chadha et al. 2019). Xylanases from thermophilic fungi such as Malbranchea flava (Sharma et al. 2010), H. insolens (Du et al. 2013), R. thermophila (McPhillips et al. 2014), and Myceliophthora sp (Chadha et al. 2004) have Km values of 3.7, 2.8, 2.48, and 1.63 mg mL−1, respectively, all belonging to the GH10 family. These consistent substrate affinities indicate that XylRc has a high affinity for birchwood xylan.
Hydrolysis of PSB
Sugarcane bagasse is a valuable source of biopolymers that can be converted into fermentable sugars (Alokika et al. 2021). This biomass primarily consists of cellulose and hemicellulose, which are chemically linked to lignin. The presence of lignin poses a challenge to industrial bioprocessing as it hinders the effective action of carbohydrate-active enzymes (CAZymes), consequently reducing the efficiency of saccharification processes (Schmatz et al. 2020). Previous studies indicate that complete hydrolysis of lignocellulosic agro-waste cannot be achieved with a single type of enzyme (Bussamra et al. 2015; Lopes et al. 2018; Filiatrault-Chastel et al. 2021). Therefore, the combination of various CAZymes with different specificities and properties offers greater efficiency, higher hydrolysis yield, and reduced bioprocessing costs.
In this context, the potential of XylRc as a complementary enzyme to Cellic®CTec2 for saccharifying hydrothermally PSB was evaluated (Fig. 6). The results revealed a significant increase in glucose (16.60 ± 0.96 mg/mL) and total reducing sugar (25.10 ± 2.30 mg/mL) release in the saccharification of PSB when XylRc (1:1; w/w) was used in combination with Cellic®CTec2 after 72 h compared to using Cellic®CTec2 alone. Additionally, the degree of synergy (DS) was calculated as DS = 1.05 for glucose release and DS = 1.28 for total reducing sugars (Table 4). A DS greater than 1.0 indicates a synergistic interaction between enzymes, resulting in higher product yields compared to individual enzyme action (Hu et al. 2011; Almeida et al. 2022). Therefore, XylRc demonstrated a synergistic interaction with Cellic®CTec2.
Fig. 6.
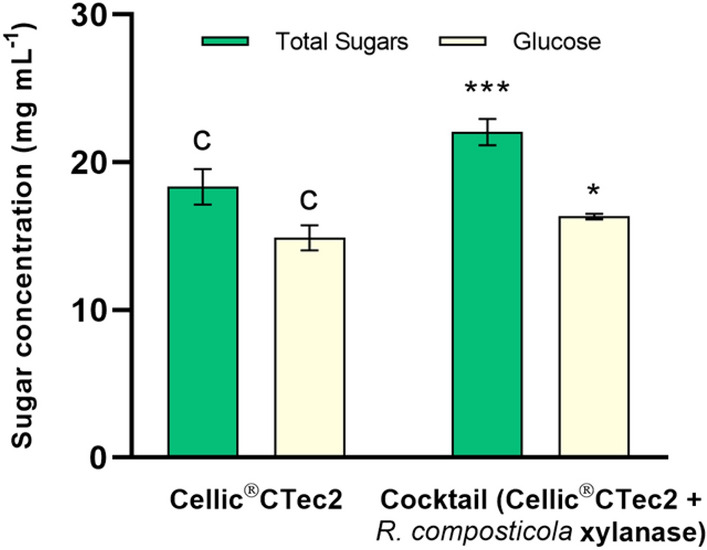
Saccharification assays of hydrothermally pretreated sugarcane bagasse using Cellic®CTec2 (5 mg/g of substrate) and Cellic®CTec2 (2.5 mg/g of substrate) plus XylRc (2.5 mg/g of substrate). The total glucose was determined using the glucose oxidase method (GOD). The total reducing sugars released were measured using the DNS method with xylose as the standard. Enzyme mixture blanks were prepared simultaneously, and all experiments were conducted in quintuplicates. Each value is presented as mean ± SD (n = 5). Statistical analysis was performed using two-way ANOVA followed by Tukey’s post hoc test. P-values are indicated as ***p < 0.001 and * p < 0.05. Results were considered significant when p < 0.05
Table 4.
Saccharification of hydrothermally pretreated sugarcane bagasse after 72 h
| Sample + Hydrolytic mixture | Glucose (mg mL−1) | DS for glucose | Total Sugar (mg mL−1) | DS for Total Sugars |
|---|---|---|---|---|
| PSB + Cellic®CTec2 | 15.75 ± 0.83 | – | 19.57 ± 2.10 | – |
| PSB + cocktail (Cellic®CTec2 + XylRc) | 16.6 ± 0.96 | 1.05 | 25.1 ± 2.30 | 1.28 |
Cellic®CTec2 (5 mg g−1 of substrate), Cocktail Cellic®CTec2 (2.5 mg g−1 of substrate) plus XylRc (2.5 mg g−1 of substrate). DS Degree of synergism
Each value is represented as mean ± SD (n = 5)
Statistical analysis by two-way ANOVA followed by Tukey's post hoc. P values are given as ***pʹ < 0.001, and *p < 0.05. Results were considered significant when p < 0.05
These results are in line with those reported for the commercial combination of Cellic®CTec2 and Cellic®HTec2 (1:1; w/w) in PSB hydrolysis bioprocesses after 72 h (Almeida et al. 2022). Likewise, the combination of an endo-xylanase from T. lanuginosus (XylTk) with Cellic®CTec2 led to a 16.5% increase in total reducing sugar release and reduced the amount of commercial cellulases required for PSB saccharification (Brar et al. 2020). However, the total reducing sugars released in this study were 114% higher than those obtained by using different combinations of recombinant hemicellulases with commercial cellulases for PSB saccharification after 48 h (Cintra et al. 2020). Therefore, supplementing of commercial cellulases with XylRc is proposed as a cost-effective and attractive alternative for formulating improved enzyme cocktails.
Supplementing cellulosic cocktails with xylanases improves the bioconversion of lignocellulosic residues. This increases the availability of cellulose fibers by hydrolyzing residual xylan fractions into xylo-oligosaccharides and xylose (Bhattacharya et al. 2015; Bajaj and Mahajan 2019; Pandey et al. 2023). It reduces the physical barriers posed by hemicellulose, thereby improving cellulase access to cellulose (Kostylev and Wilson 2012; Saini et al. 2022). Furthermore, the increased availability of cellulose prevents enzyme inhibition caused by adsorption to residual lignin fractions (Hu et al. 2011; Michelin et al. 2016). Additionally, the fermentable sugars generated, such as xylose and glucose, can be converted into high-value industrial products, including bioethanol, biohydrogen, xylitol, succinate, sorbitol, acetic acid, and hydroxymethylfurfural (Ajao et al. 2018; Ariffin et al. 2019; Haldar and Purkait 2020; Vries 2023).
Therefore, with its appealing characteristics, including synergistic interactions with commercial cocktail Cellic®CTec2, comparable saccharide to commercial preparations with Cellic®HTec2, stability in the face of elevated temperatures, ions, organic solvents, and phenolic compounds, proves to be an attractive candidate for formulating improved enzyme cocktails. Thus, it plays a crucial role in optimizing bioprocessing economics by reducing enzyme costs and increasing the yield of fermentable sugars for the production of valuable industrial products.
Conclusion
In the industrial production of enzymes, thermotolerance is a crucial characteristic. Xylanases, especially thermophilic enzymes, play a significant role in various industries, with numerous studies focusing on their production and purification. This study highlights XylRc as a promising enzyme for efficient enzymatic hydrolysis, converting biomass into fermentable sugars. XylRc was successfully produced using a combination of wheat bran and exploded sugarcane, and it exhibited remarkable stability at high temperatures. Through purification processes, XylRc demonstrated increased activity even in the presence of ions, solvents, and phenolic compounds. The findings presented in this study indicate that XylRc is well-suited for saccharifying lignocellulosic waste, such as sugarcane bagasse. Thus, the results demonstrate that XylRc has substantial potential for generating sugar-rich hydrolysate, which can be harnessed for bioethanol production.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001, in addition to providing scholarship to the first author. This work was supported by the Universidade Federal de Mato Grosso do Sul–UFMS/MEC Brazil. We are grateful for the Multicenter Graduate Program in Biochemistry and Molecular Biology (PMBqBM- SBBq) Brazil.
Data availability
The data are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Giovana Cristina Giannesi, Email: giannesigiovana@ufms.br.
Fabiana Fonseca Zanoelo, Email: fabiana.zanoelo@ufms.br.
References
- Ajao O, Marinova M, Savadogo O, Paris JJIC, Products Hemicellulose based integrated forest biorefineries: Implementation strategies. Ind Crops Prod. 2018;126:250–260. doi: 10.1016/j.indcrop.2018.10.025. [DOI] [Google Scholar]
- Almeida AP, Vargas IP, Marciano CL, Zanoelo FF, Giannesi GC, Polizeli MLTM, Jorge JA, Furriel RPM, Ruller R, Masui DC. Investigation of biochemical and biotechnological potential of a thermo-halo-alkali-tolerant endo-xylanase (GH11) from Humicola brevis var. thermoidea for lignocellulosic valorization of sugarcane biomass. Biocatal Agric Biotechnol. 2022;44:102424. doi: 10.1016/j.bcab.2022.102424. [DOI] [Google Scholar]
- Alokika A, Kumar A, Kumar V, Singh B. Cellulosic and hemicellulosic fractions of sugarcane bagasse: potential, challenges and future perspective. Int J Biol Macromol. 2021;169:564–582. doi: 10.1016/j.ijbiomac.2020.12.175. [DOI] [PubMed] [Google Scholar]
- Alvira P, Negro MJ, Ballesteros M. Effect of endoxylanase and α-L-arabinofuranosidase supplementation on the enzymatic hydrolysis of steam exploded wheat straw. Bioresour Technol. 2011;102(6):4552–4558. doi: 10.1016/j.biortech.2010.12.112. [DOI] [PubMed] [Google Scholar]
- Amo GS, Bezerra-Bussoli C, da Silva RR, Kishi LT, Ferreira H, Mariutti RB, Arni RK, Gomes E, Bonilla-Rodriguez GO. Heterologous expression, purification and biochemical characterization of a new xylanase from Myceliophthora heterothallica. Inter J Biol Macromol. 2019;131:798–805. doi: 10.1016/j.ijbiomac.2019.03.108. [DOI] [PubMed] [Google Scholar]
- Anwar Z, Gulfraz M, Irshad M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl. 2014;7(2):163–173. doi: 10.1016/j.jrras.2014.02.003. [DOI] [Google Scholar]
- Ariffin H, Sapuan, SM, Hassan, MA (2019) Lignocellulose for future bioeconomy. In: Lignocellulose for future bioeconomy. 10.1016/C2018-0-00037-7
- Ávila PF, Martins M, Costa FAA, Goldbeck R, Fibre D. Xylooligosaccharides production by commercial enzyme mixture from agricultural wastes and their prebiotic and antioxidant potential. Bioact Carbohyd Diet Fibre. 2020;24:100234. doi: 10.1016/j.bcdf.2020.100234. [DOI] [Google Scholar]
- Bajaj P, Mahajan R. Cellulase and xylanase synergism in industrial biotechnology. Appl Microbiol Biotechnol. 2019;103:8711–8724. doi: 10.1007/s00253-019-10146-0. [DOI] [PubMed] [Google Scholar]
- Banerjee J, Singh R, Vijayaraghavan R, MacFarlane D, Patti AF, Arora A. Bioactives from fruit processing wastes: green approaches to valuable chemicals. Food Chem. 2017;225(1):10–22. doi: 10.1016/j.foodchem.2016.12.093. [DOI] [PubMed] [Google Scholar]
- Basit A, Liu J, Rahim K, Jiang W, Lou H. Thermophilic xylanases: from bench to bottle. Crit Rev Biotechnol. 2018;38(7):989–1002. doi: 10.1080/07388551.2018.1425662. [DOI] [PubMed] [Google Scholar]
- Basotra N, Joshi S, Satyanarayana T, Pati PK, Tsang A, Chadha BS. Expression of catalytically efficient xylanases from thermophilic fungus Malbranchea cinnamomea for synergistically enhancing hydrolysis of lignocellulosics. Inter J Biol Macromol. 2018;108:185–192. doi: 10.1016/j.ijbiomac.2017.11.131. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Bernt E. Lactate dehydro-genase. UV-assay with pyruvate and NADH. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1974. pp. 574–578. [Google Scholar]
- Bezerra TL, Ragauskas AJ. A review of sugarcane bagasse for second-generation bioethanol and biopower production. Biofuels Bioprod Bioref. 2016;10(5):634–647. doi: 10.1002/bbb.1662. [DOI] [Google Scholar]
- Bhardwaj N, Agrawal K, Verma P. Xylanases: an overview of its Diverse Function in the Field of Biorefinery. In: Srivastava M, Srivastava N, Singh R, editors. Bioenergy Research: Commercial Opportunities & Challenges. Clean Energy Production Technologies. Singapore: Springer; 2021. [Google Scholar]
- Bhattacharya AS, Bhattacharya A, Pletschke BI. Synergism of fungal and bacterial cellulases and hemicellulases: a novel perspective for enhanced bio-ethanol production. Biotechnol Lett. 2015;37:1117–1129. doi: 10.1007/s10529-015-1779-3. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brar KK, Santo MCE, Pellegrini VO, Azevedo ER, Guimarães FEC, Polikarpov I, Chadha BS. Enhanced hydrolysis of hydrothermally and autohydrolytically treated sugarcane bagasse and understanding the structural changes leading to improved saccharification. Biomass Bioenerg. 2020;139:105639. doi: 10.1016/j.biombioe.2020.105639. [DOI] [Google Scholar]
- Bussamra BC, Freitas S, Costa ACJ. Improvement on sugar cane bagasse hydrolysis using enzymatic mixture designed cocktail. Bioresour Technol. 2015;187:173–181. doi: 10.1016/j.biortech.2015.03.117. [DOI] [PubMed] [Google Scholar]
- Carvalho DM, Martínez-Abad A, Evtuguin DV, Colodette JL, Lindström ME, Vilaplana F, Sevastyanova O. Isolation and characterization of acetylated glucuronoarabinoxylan from sugarcane bagasse and straw. Carb Polym. 2017;156:223–234. doi: 10.1016/j.carbpol.2016.09.022. [DOI] [PubMed] [Google Scholar]
- Carvalho AFA, Figueiredo FC, Campioni TS, Pastore GM, Neto PO. Improvement of some chemical and biological methods for the efficient production of xylanases, xylooligosaccharides and lignocellulose from sugar cane bagasse. Biomass Bioenerg. 2020;143:105851. doi: 10.1016/j.biombioe.2020.105851. [DOI] [Google Scholar]
- Castro-Ochoa LD, Hernández-Leyva SR, Medina-Godoy S, Gómez-Rodríguez J, Aguilar-Uscanga MG, Martínez CC. Integration of agricultural residues as biomass source to saccharification bioprocess and for the production of cellulases from filamentous fungi. Biotech. 2023;13:43. doi: 10.1007/s13205-022-03444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha BS, Ajay BK, Mellon F, Bhat MK Two endoxylanases active and stable at alkaline pH from the newly isolated thermophilic fungus, Myceliophthora sp. IMI 387099. J Biotechnol. 2004;109(3):227–237. doi: 10.1016/j.jbiotec.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Chadha BS, Kaur B, Basotra N, Tsang A, Pandey A. Thermostable xylanases from thermophilic fungi and bacteria: current perspective. Bioresour Technol. 2019;277:195–203. doi: 10.1016/j.biortech.2019.01.044. [DOI] [PubMed] [Google Scholar]
- Chanwicha N, Katekaew S, Aimi T, Boonlue S. Purification and characterization of alkaline xylanase from Thermoascus aurantiacus var. levisporus KKU-PN-I2–1 cultivated by solid-state fermentation. Mycoscience. 2015 doi: 10.1016/j.myc.2014.09.003. [DOI] [Google Scholar]
- Cintra LC, Costa IC, Oliveira ICM, Fernandes AG, Faria SP, Jesuíno RSA, Ravanal MC, Eyzaguirre J, Ramos LP, Faria FP, Ulhoa CJ. The boosting effect of recombinant hemicellulases on the enzymatic hydrolysis of steam-treated sugarcane bagasse. Enzyme Microb Technol. 2020;133:109447. doi: 10.1016/j.enzmictec.2019.109447. [DOI] [PubMed] [Google Scholar]
- Collard F-X, Blin J. A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sust Energ Ver. 2014;38:594–608. doi: 10.1016/j.rser.2014.06.013. [DOI] [Google Scholar]
- Cota J, Oliveira LC, Damásio ARL, Citadini AP, Hoffmam ZB, Alvarez TM, Codima CA, Leite VBP, Pastore G, Oliveira-Neto M, Murakami MT, Ruller R. Assembling a xylanase–lichenase chimera through all-atom molecular dynamics simulations. Biochim Biophys Acta. 2013;1834(8):1492–1500. doi: 10.1016/j.bbapap.2013.02.030. [DOI] [PubMed] [Google Scholar]
- Dar FM, Dar PM. Fungal xylanases for different industrial applications. In: Abdel-Azeem AM, Yadav AN, Yadav N, Sharma M, editors. Industrially important fungi for sustainable development. Fungal biology. Cham: Springer; 2021. [Google Scholar]
- Davis BJ. Method and application to human serum protein. Ann NY Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Shi P, Huang H, Zhang X, Luo H, Wang Y, Yao B. Characterization of three novel thermophilic xylanases from Humicola insolens Y1 with application potentials in the brewing industry. Bioresour Technol. 2013;130:161–167. doi: 10.1016/j.biortech.2012.12.067. [DOI] [PubMed] [Google Scholar]
- Duarte GC, Moreira LRS, Jaramillo PMD, Filho EXF. Biomass-derived inhibitors of holocellulases. Bioenerg Res. 2012;5:768–777. doi: 10.1007/s12155-012-9182-6. [DOI] [Google Scholar]
- Espejo F. Role of commercial enzymes in wine production: A critical review of recent research. J Food Sci Technol. 2021;58(1):9–21. doi: 10.1007/s13197-020-04489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiatrault-Chastel C, Heiss-Blanquet S, Margeot A, Berrin J-GJBA. From fungal secretomes to enzymes cocktails: the path forward to bioeconomy. Biotechnol Adv. 2021;52:10783. doi: 10.1016/j.biotechadv.2021.107833. [DOI] [PubMed] [Google Scholar]
- Gonçalves T, Damásio A, Segato F, Alvarez T, Bragatto J, Brenelli L, Citadini A, Murakami MT, Ruller R, Paes-Leme AP, Prade R, Squina FM. Functional characterization and synergic action of fungal xylanase and arabinofuranosidase for production of xylooligosaccharides. Bioresour Technol. 2012;119:293–299. doi: 10.1016/j.biortech.2012.05.062. [DOI] [PubMed] [Google Scholar]
- Haldar D, Purkait MK. Lignocellulosic conversion into value-added products: a review. Process Biochem. 2020;89:110–133. doi: 10.1016/j.procbio.2019.10.001. [DOI] [Google Scholar]
- Hinz SWA, Pouvreau LAM, Joosten R, Bartels J, Jonathan MC, Wery J, Schols HA. Hemicellulase production in Chrysosporium lucknowense C1. J Cereal Sci. 2009;50:318–323. doi: 10.1016/j.jcs.2009.07.005. [DOI] [Google Scholar]
- Hu J, Arantes V, Saddler JN. The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol Biofuels. 2011;4:36. doi: 10.1186/1754-6834-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttner S, Granchi Z, Nguyen TT, van Pelt S, Larsbrink J, Thanh VN, Olsson L. Genome sequence of Rhizomucor pusillus FCH 5.7, a thermophilic zygomycete involved in plant biomass degradation harbouring putative GH9 endoglucanases. Biotechnol Reports. 2018;20:e00279. doi: 10.1016/j.btre.2018.e00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain KK, Bhanja Dey T, Kumar S, Kuhad RC. Production of thermostable hydrolases (cellulases and xylanase) from Thermoascus aurantiacus RCKK: a potential fungus. Bioprocess Biosyst Eng. 2015;38(4):787–796. doi: 10.1007/s00449-014-1320-4. [DOI] [PubMed] [Google Scholar]
- Jugwanth Y, Sewsynker-Sukai Y, Kana E. Valorization of sugarcane bagasse for bioethanol production through simultaneous saccharification and fermentation: optimization and kinetic studies. Fuel. 2020;262:116552. doi: 10.1016/j.fuel.2019.116552. [DOI] [Google Scholar]
- Julio R, Albet J, Vialle C, Vaca-Garcia C, Sablayrolles C. Sustainable design of biorefinery processes: existing practices and new methodology. Biofuels Bioprod Biorefin. 2017;11(2):373–395. doi: 10.1002/bbb.1749. [DOI] [Google Scholar]
- Karnaouri A, Topakas E, Antonopoulou I, Christakopoulos P. Genomic insights into the fungal lignocellulolytic system of Myceliophthora thermophila. Front Microbiol. 2014;5:281. doi: 10.3389/fmicb.2014.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal J, Arya SK, Khatri M, Singh G, Nur NIW, Rajagopal R, Soon SW, Ravindran B, Awasthi MK. Efficacious bioconversion of waste walnut shells to xylotetrose and xylopentose by free xylanase (Xy) and MOF immobilized xylanase (Xy-Cu-BTC) Bioresour Technol. 2022;357:127374. doi: 10.1016/j.biortech.2022.127374. [DOI] [PubMed] [Google Scholar]
- Knob A, Carmona EC. Purification and characterization of two extracellular xylanases from Penicillium sclerotiorum: a novel acidophilic xylanase. Appl Biochem Biotechnol. 2010;162(2):429–443. doi: 10.1007/s12010-009-8731-8. [DOI] [PubMed] [Google Scholar]
- Kostylev M, Wilson DJB. Synergistic interactions in cellulose hydrolysis. Biofuels. 2012;3(1):61–70. doi: 10.4155/bfs.11.150. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li X, Dilokpimol A, Kabel MA, de Vries RP. Fungal xylanolytic enzymes: Diversity and applications. Bioresour Technol. 2022;344:126290. doi: 10.1016/j.biortech.2021.126290. [DOI] [PubMed] [Google Scholar]
- Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(D1):D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes AM, Ferreira Filho EX, Moreira LRS. An update on enzymatic cocktails for lignocellulose breakdown. J Appl Microbiol. 2018;125(3):632–645. doi: 10.1111/jam.13923. [DOI] [PubMed] [Google Scholar]
- Maheshwari R, Bharadwaj G, Bhat MK. Thermophilic fungi: their physiology and enzymes. Microbiol Mol Biol Rev. 2000;64(3):461–488. doi: 10.1128/mmbr.64.3.461-488.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Colón W. Structural basis of protein kinetic stability: resistance to sodium dodecyl sulfate suggests a central role for rigidity and a bias toward β-sheet structure. Biochem. 2004;43(35):11248–11254. doi: 10.1021/bi0491898. [DOI] [PubMed] [Google Scholar]
- Mathibe BN, Malgas S, Radosavljevic L, Kumar V, Shukla P, Pletschke BI. Lignocellulosic pretreatment-mediated phenolic by-products generation and their effect on the inhibition of an endo-1,4-β-xylanase from Thermomyces lanuginosus VAPS-24. 3Biotech. 2020;10(8):349. doi: 10.1007/s13205-020-02343-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvaine TC. A buffer solution for colorimetric comparison. J Biol Chem. 1921;49(1):183–186. doi: 10.1016/S0021-9258(18)86000-8. [DOI] [Google Scholar]
- McPhillips K, Waters DM, Parlet C, Walsh DJ, Arendt EK, Murray PG. Purification and Characterization of a β-1,4-Xylanase from Remersonia thermophila CBS 540.69 and Its Application in Bread Making. Appl Biochem Biotechnol. 2014;172:1747–1762. doi: 10.1007/s12010-013-0640-1. [DOI] [PubMed] [Google Scholar]
- Michelin M, Ximenes E, de Moraes MLT, Ladisch MR. Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour Technol. 2016;199:275–278. doi: 10.1016/j.biortech.2015.08.120. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Monclaro AV, Recalde GL, da Silva Jr FG, de Freitas SM, Ferreira Filho EX. Xylanase from Aspergillus tamarii shows different kinetic parameters and substrate specificity in the presence of ferulic acid. Enzyme Microb Technol. 2019;120:16–22. doi: 10.1016/j.enzmictec.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Monica P, Kapoor M. Alkali-stable GH11 endo-β-1,4 xylanase (XynB) from Bacillus subtilis strain CAM 21: application in hydrolysis of agro-industrial wastes, fruit/vegetable peels and weeds. Prep Biochem Biotechnol. 2021;51(5):475–487. doi: 10.1080/10826068.2020.1830416. [DOI] [PubMed] [Google Scholar]
- Nakasu PYS, Chagas MF, Costa AC, Rabelo SC. Kinetic study of the acid post-hydrolysis of xylooligosaccharides from hydrothermal pretreatment. Bioenerg Res. 2017;10:1045–1056. doi: 10.1007/s12155-017-9864-1. [DOI] [Google Scholar]
- Nascimento CEO, de Oliveira Simões LC, de Cassia PJ, da Silva RR, de Lima EA, de Almeida GC, Penna ALB, Boscolo M, Gomes E, da Silva R. Application of a recombinant GH10 endoxylanase from Thermoascus aurantiacus for xylooligosaccharide production from sugarcane bagasse and probiotic bacterial growth. J Biotechnol. 2022;347:1–8. doi: 10.1016/j.jbiotec.2022.02.003. [DOI] [PubMed] [Google Scholar]
- Olopoda IA, Lawal OT, Omotoyinbo OV, Kolawole AN, Sanni DM. Biochemical characterization of a thermally stable, acidophilic and surfactant-tolerant xylanase from Aspergillus awamori AFE1 and hydrolytic efficiency of its immobilized form. Process Biochem. 2022;121:45–55. doi: 10.1016/j.procbio.2022.06.030. [DOI] [Google Scholar]
- Pandey C, Sharma P, Gupta N. Engineering to enhance thermostability of xylanase: for the new era of biotechnology. J Appl Biol Biotechnol. 2023;11(2):41–54. doi: 10.7324/JABB.2023.110204. [DOI] [Google Scholar]
- Pinales-Márquez CD, Rodríguez-Jasso RM, Araújo RG, Loredo-Treviño A, Nabarlatz D, Gullón B, Ruiz HA. Circular bioeconomy and integrated biorefinery in the production of xylooligosaccharides from lignocellulosic biomass: a review. Ind Crops Prod. 2021;162:113274. doi: 10.1016/j.indcrop.2021.113274. [DOI] [Google Scholar]
- Poletto P, Pereira GN, Monteiro CR, Pereira MAF, Bordignon SE, de Oliveira D. Xylooligosaccharides: transforming the lignocellulosic biomasses into valuable 5-carbon sugar prebiotics. Process Biochem. 2020;91:352–363. doi: 10.1016/j.procbio.2020.01.005. [DOI] [Google Scholar]
- Polizeli MLTM, Rizzatti A, Monti R, Terenzi HF, Jorge JA, Amorim DS. Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol. 2005;67:577–591. doi: 10.1007/s00253-005-1904-7. [DOI] [PubMed] [Google Scholar]
- Pollet A, Delcour JA, Courtin CM. Structural determinants of the substrate specificities of xylanases from different glycoside hydrolase families. Crit Rev Biotechnol. 2010;30(3):176–191. doi: 10.3109/07388551003645599. [DOI] [PubMed] [Google Scholar]
- Rashid R, Sohail M. Xylanolytic Bacillus species for xylooligosaccharides production: a critical review. Bioresour Bioprocess. 2021;8(1):1–14. doi: 10.1186/s40643-021-00369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro LF, De Lucas RC, Vitcosque GL, Ribeiro LF, Ward RJ, Rubio MV, Damásio AR, Squina FM, Gregory RC, Walton PH, Jorge JA, Prade RA, Buckeridge MS, Polizeli MLTM. A novel thermostable xylanase GH10 from Malbranchea pulchella expressed in Aspergillus nidulans with potential applications in biotechnology. Biotechnol Biofuels. 2014;7:115. doi: 10.1186/1754-6834-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues PO, Moreira FS, Cardoso VC, Santos LD, Gurgel LVA, Pasquini D, Baffi MA. Combination of high solid load, on-site enzyme cocktails and surfactant in the hydrolysis of hydrothermally pretreated sugarcane bagasse and ethanol production. Waste Biomass Valor. 2022;13:3085–3094. doi: 10.1007/s12649-022-01685-1. [DOI] [Google Scholar]
- Saha BC. Hemicellulose bioconversion. J Ind Microbiol Biotechnol. 2003;30(5):279–291. doi: 10.1007/s10295-003-0049-x. [DOI] [PubMed] [Google Scholar]
- Saini JK, Kaur A, Mathur A. Strategies to enhance enzymatic hydrolysis of lignocellulosic biomass for biorefinery applications: A review. Bioresour Technol. 2022;360:127517. doi: 10.1016/j.biortech.2022.127517. [DOI] [PubMed] [Google Scholar]
- Schmatz AA, Tyhoda L, Brienzo M. Sugarcane biomass conversion influenced by lignin. Biofuels Bioprod Bioref. 2020;14(2):469–480. doi: 10.1002/bbb.2070. [DOI] [Google Scholar]
- Seemakram W, Boonrung S, Aimi T, Ekprasert J, Lumyong S, Boonlue S. Purification, characterization and partial amino acid sequences of thermo-alkali-stable and mercury ion-tolerant xylanase from Thermomyces dupontii KKU–CLD–E2–3. Sci Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-78670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Kumar A. Xylanases: an overview. Br Biotechnol J. 2013;3(1):1–28. doi: 10.9734/BBJ/2013/1784. [DOI] [Google Scholar]
- Sharma M, Chadha BS, Saini HS. Purification and characterization of two thermostable xylanases from Malbranchea flava active under alkaline conditions. Bioresour Technol. 2010;101(22):8834–8842. doi: 10.1016/j.biortech.2010.06.071. [DOI] [PubMed] [Google Scholar]
- Sharma A, Sharma A, Singh S, Kuhad RC, Nain L. Thermophilic fungi and their enzymes for biorefineries. In: Tiquia-Arashiro S, Grube M, editors. Fungi in extreme environments: ecological role and biotechnological significance. Springer Cham; 2019. [Google Scholar]
- Silva LAO, Terrasan CRF, Carmona EC. Purification and characterization of xylanases from Trichoderma inhamatum. Electron J Biotechnol. 2015;18(4):307–313. doi: 10.1016/j.ejbt.2015.06.00. [DOI] [Google Scholar]
- Silva COG, Aquino EN, Ricart CAO, Midorikawa GEO, Filho EXF. GH11 xylanase from Emericella nidulans with low sensitivity to inhibition by ethanol and lignocellulose-derived phenolic compounds. FEMS Microbiol Lett. 2015;362(13):fnv094. doi: 10.1093/femsle/fnv094. [DOI] [PubMed] [Google Scholar]
- Silva PO, Guimarães NCA, Serpa JDM, Masui DC, Marchetti CR, Verbisck NV, Zanoelo FF, Ruller R, Giannesi GC. Application of an endo-xylanase from Aspergillus japonicus in the fruit juice clarification and fruit peel waste hydrolysis. Biocatal Agric Biotechnol. 2019;21:101312. doi: 10.1016/j.bcab.2019.101312. [DOI] [Google Scholar]
- Sindhu R, Binod P, Pandey A. Biological pretreatment of lignocellulosic biomass—an overview. Bioresour Technol. 2016;199:76–82. doi: 10.1016/j.biortech.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Singh G, Kaur S, Khatri M, Arya SK. Biobleaching for pulp and paper industry in India: Emerging enzyme technology. Biocat Agric Biotechnol. 2019;17:558–565. doi: 10.1016/j.bcab.2019.01.019. [DOI] [Google Scholar]
- Teixeira RSS, Siqueira FG, Souza MVD, Filho EXF, Bon EPS. Purification and characterization studies of a thermostable β-xylanase from Aspergillus awamori. J Ind Microbiol Biotechnol. 2010;37(10):1041–1051. doi: 10.1007/s10295-010-0751-4. [DOI] [PubMed] [Google Scholar]
- Tuohy M, Puls J, Claeyssens M, Vršanská M, Coughlan MP. The xylan-degrading enzyme system of Talaromyces emersonii: novel enzymes with activity against aryl β-D-xylosides and unsubstituted xylans. Biochem J. 1993;290(2):515–523. doi: 10.1042/bj2900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinov BB, Gusakov AV, Antonov AI, Sinitsyn AP. Comparison of properties and mode of action of six secreted xylanases from Chrysosporium lucknowense. Enzyme Microb Technol. 2008;43(1):56–65. doi: 10.1016/j.enzmictec.2008.01.017. [DOI] [Google Scholar]
- Vafiadi C, Christakopoulos P, Topakas E. Purification, characterization and mass spectrometric identification of two thermophilic xylanases from Sporotrichum thermophile. Process Biochem. 2010;45(3):419–424. doi: 10.1016/j.procbio.2009.10.009. [DOI] [Google Scholar]
- Van den Brink J, van Muiswinkel GC, Theelen B, Hinz SW, de Vries RP. Efficient plant biomass degradation by thermophilic fungus Myceliophthora heterothallica. Appl Environ Microbiol. 2013;79(4):1316–1324. doi: 10.1128/AEM.02865-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vries JG. Industrial implementation of chemical biomass conversion. Curr Opin Green Sustain Chem. 2023;39:100715. doi: 10.1016/j.cogsc.2022.100715. [DOI] [Google Scholar]
- Winger A, Heazlewood J, Chan L, Petzold C, Permaul K, Singh S. Secretome analysis of the thermophilic xylanase hyper-producer Thermomyces lanuginosus SSBP cultivated on corn cobs. J Ind Microbiol Biotechnol. 2014;41(11):1687–1696. doi: 10.1007/s10295-014-1509-1. [DOI] [PubMed] [Google Scholar]
- Yousuf A, O Pirozzi D, Sannino F. Fundamentals of lignocellulosic biomass. In: Yousuf A, o Pirozzi D, Sannino F, A, editors. Lignocellulosic biomass to liquid biofuels. Massachusetts: Academic Press; 2019. pp. 1–15. [Google Scholar]
- Zanoelo FF, Polizeli MLTM, Terenzi HF, Jorge JA. Purification and biochemical properties of a thermostable xylose-tolerant β-D-xylosidase from Scytalidium thermophilum. J Ind Microbiol Biotechnol. 2004;31(4):170–176. doi: 10.1007/s10295-004-0129-6. [DOI] [PubMed] [Google Scholar]
- Zanoni JA, de Oliveira IB, Perrone OM, Ortega JO, Boscolo M, Gomes E, Bonilla-Rodriguez GO. Production and biochemical characterization of xylanases synthesized by the thermophilic fungus Rasamsonia emersonii S10 by solid-state cultivation. Ecletica Quim. 2021;46(1SI):53–67. doi: 10.26850/1678-4618eqj.v46.1SI. [DOI] [Google Scholar]
- Zhao Z, Xian M, Liu M, Zhao G. Biochemical routes for uptake and conversion of xylose by microorganisms. Biotechnol Biofuels. 2020;13:1–12. doi: 10.1186/s13068-020-1662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimbardi AL, Sehn C, Meleiro LP, Souza FH, Masui DC, Nozawa MS, Guimarães LH, Jorge JA, Furriel RPM. Optimization of β-glucosidase, β-xylosidase and xylanase production by Colletotrichum graminicola under solid-state fermentation and application in raw sugarcane trash saccharification. Int J Mol Sci. 2013;14(2):2875–2902. doi: 10.3390/ijms14022875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author on reasonable request.


