Abstract
Ubiquitin-dependent degradation of Cdc25A is a major mechanism for damage-induced S-phase checkpoint. Two ubiquitin ligases, the Skp1-cullin-β-TrCP (SCFβ-TrCP) complex and the anaphase-promoting complex (APCCdh1), are involved in Cdc25A degradation. Here we demonstrate that the transforming growth factor β (TGF-β)-Smad3 pathway promotes SCFβ-TrCP-mediated Cdc25A ubiquitination. Cells treated with TGF-β, as well as cells transfected with Smad3 or a constitutively active type I TGF-β receptor, exhibit increased ubiquitination and markedly shortened half-lives of Cdc25A. Furthermore, Cdc25A is stabilized in cells transfected with Smad3 small interfering RNA (siRNA) and cells from Smad3-null mice. TGF-β-induced ubiquitination is associated with Cdc25A phosphorylation at the β-TrCP docking site (DS82G motif) and physical association of Cdc25A with Smad3 and β-TrCP. Cdc25A mutant proteins deficient in DS82G phosphorylation are resistant to TGF-β-Smad3-induced degradation, whereas a Cdc25A mutant protein defective in APCCdh1 recognition undergoes efficient degradation. Smad3 siRNA inhibits β-TrCP-Cdc25A interaction and Cdc25A degradation in response to TGF-β. β-TrCP2 siRNA also inhibits Smad3-induced Cdc25A degradation. In contrast, Cdh1 siRNA had no effect on Cdc25A down-regulation by Smad3. These data suggest that Smad3 plays a key role in the regulation of Cdc25A ubiquitination by SCFβ-TrCP and that Cdc25A stabilization observed in various cancers could be associated with defects in the TGF-β-Smad3 pathway.
Cdc25 phosphatases promote cell cycle progression by dephosphorylating and activating cyclin-dependent kinases (Cdks), which form the major driving force of cell cycle progression (16, 40). Cdc25A activates cyclin E (A)-Cdk2 during G1 through S and also seems to be involved in activation of Cdk1 at the G2/M boundary (4, 8, 20, 33, 55). The other members of the Cdc25 family, Cdc25B and Cdc25C, collaborate for timely activation of cyclin B-Cdk1 at the G2/M boundary. Ectopic expression of Cdc25A shortens the passage of human breast carcinoma MCF-7 cells and HeLa cells through G1 with Cdk2 activation (4, 45), whereas antisense Cdc25A oligonucleotides reduce Cdk2 activity in MCF-7 cells and inhibit entry into the S phase (7). The expression of Cdc25A is increased by estrogen treatment in MCF-7 mammary carcinoma cells (17) and decreased during senescence-associated growth arrest of human mammary epithelial cells (45). Overexpression of Cdc25A protein is frequently observed in various types of cancers, and Cdc25A has been implied to be an oncogene (7, 21, 22, 53). Promoting cell cycle progression via Cdk2 and Cdk1 activation could be part of the mechanisms for the oncogenic action of Cdc25A, while Cdc25A overexpression also can inhibit apoptosis in some cellular contexts (18, 35, 36, 58).
In normal cells Cdc25A levels are controlled precisely by multilayered mechanisms. While the transcription of the Cdc25A gene is regulated in an E2F-dependent manner (50), the protein levels are controlled more dynamically by ubiquitin-dependent proteasomal degradation. The significance of ubiquitination has been extensively investigated in a variety of biological processes, including cell cycle control (43). Two types of ubiquitin ligase (E3) complexes are known to mediate ubiquitination of Cdc25A: the anaphase-promoting/cyclosome complex (APC) and the Skp-cullin-F-box (SCF) complex (6, 14, 15, 32). The APCCdh1 complex mediates degradation of Cdc25A from the exit of mitosis throughout the G1 phase of the cell cycle. APCCdh1 recognizes specific KEN box sequences of Cdc25A, and Cdc25A KEN mutants are resistant to APC-mediated degradation. At the beginning of the S phase, Emi1 accumulates in an E2F-dependent manner and inhibits APCCdh1 activity, which contributes to up-regulation of Cdc25A during S phase (27, 44). Independently, the SCFβ-TrCP complex plays a critical role in Cdc25A degradation during proliferation and also in response to DNA damage (6, 32). The F-box protein β-TrCP binds to a DSG consensus sequence of Cdc25A in a manner dependent on phosphorylation of Ser82 within the motif (6, 32). Suppression of β-TrCP1 and β-TrCP2 by small interfering RNA (siRNA) results in stabilization and accumulation of Cdc25A during S and G2 phases and also eliminates DNA damage-induced Cdc25A degradation. These data suggest that SCFβ-TrCP is competent to regulate Cdc25A in both unperturbed S/G2 progression and damage-induced S-phase checkpoint. Cdc25A phosphorylation at multiple serine residues surrounding the DS82G motif is required for recognition by SCFβ-TrCP. Ser76 phosphorylation is a prerequisite event for subsequent phosphorylation of Ser82, which is critical for β-TrCP binding (1, 6, 13). While the checkpoint kinase Chk1 has been demonstrated to phosphorylate Ser76 (32), another undefined kinase(s) appears to be involved in phosphorylation of the “degron” sequence of Cdc25A. Thus, complex signaling pathways seem to converge on the Cdc25A ubiquitination system.
The Smad family proteins mediate the intracellular signal transduction elicited by the transforming growth factor β (TGF-β), which plays a critical role in development and tumor suppression (11, 46). Upon ligand association, the type II TGF-β receptor, TβRII, transphosphorylates and activates interacting type I receptor, TβRI. TβRI phosphorylates receptor Smad (R-Smad) proteins, such as Smad2 and Smad3. Activated R-Smad heterodimerizes with a common shared partner, Smad4, and then translocates into the nucleus, where the complex participates in transcriptional regulation of target genes, by recruitment of coactivator or corepressor proteins. In the present study, we have demonstrated that TGF-β signals facilitate β-TrCP-mediated ubiquitination of Cdc25A and identified Smad3 as a rate-limiting factor for this process. Our data suggest that SCFβ-TrCP-dependent control of Cdc25A degradation, as well as E2F-mediated repression of Cdc25A transcription (29, 30), is an important target for TGF-β signaling and a potential tumor-suppressive mechanism.
MATERIALS AND METHODS
Cell culture.
Human osteosarcoma U2OS cells and mammary epithelial MCF-10A cells were obtained from the American Type Culture Collection. Human diploid fibroblasts (HDF) used for the study were previously described (9). Mouse embryonic fibroblasts (MEFs) were isolated from day 12.5 mouse embryos, as described previously (49). Smad3−/− MEFs were described previously (54). U2OS cells were cultured in Dulbecco's minimum essential medium supplemented with 10% fetal bovine serum, and MCF-10A cells were cultured in Dulbecco's minimum essential medium-F-12 mixed (1:1) medium supplemented with 5% horse serum. Cells were treated with 10 ng (400 pM) of human recombinant TGF-β1 (R&D Systems)/ml for 24 h unless otherwise indicated. For complete inhibition of the action of residual TGF-β in culture medium, cells were incubated with 20 μg of neutralizing anti-TGF-β1,2,3 monoclonal antibody (1D11)/ml, obtained from R&D Systems. To inhibit proteasome-dependent degradation of proteins, U2OS cells were treated with 1 μM MG132 for 15 h. To inhibit translation and determine the stability of Cdc25A, cells were incubated with 50 μg of cycloheximide/ml.
Plasmid construction and transfection.
Site-directed mutagenesis of cDNAs was performed using the QuikChange kit (Stratagene), followed by verification of the altered sequence at the W. M. Keck Center of Biotechnologies, the University of Illinois at Urbana-Champaign. Cells were transfected with plasmids, with the use of the Superfect lipofection reagent (Qiagen) according to the manufacturer's instructions.
siRNA.
siRNAs were custom synthesized by Dharmacon. The sequence of anti-Smad3 siRNA was 5′-AAUGGUGCGAGAAGGCGGUCAdTdT-3′. The sequences of anti-β-TrCP1/β-TrCP2 and anti-Cdh1 siRNAs were described previously (15). Cells were transfected with 200 nM specific siRNAs or randomized cocktails of double-stranded RNA (dsRNA) as a control, with the use of the Oligofectamine reagent (Gibco/Life Technologies). At 24 h posttransfection, cells were transfected again with the same RNA preparations to ensure efficient silencing effects.
RT-PCR.
Total RNA was isolated from cells with the Trizol reagent (Invitrogen). Total RNA (1 μg) was subjected to reverse transcriptase (RT) reaction with the use of the SuperScript first-strand synthesis system (Invitrogen). After the RT reaction, RNase H was added to remove the RNA template from the reaction mixture. Subsequently, PCR was performed in a total volume of 50 μl with 1 μl of the RT product or 1 μl of 10-fold-diluted product in the case of cells with Cdc25A overexpression. To confirm that the amounts of target mRNAs were within the semiquantitative linear range of the reaction, increasing amounts of RNA samples were examined in pilot experiments. The primers used for human Cdc25A mRNA were 5′-GGCAGACCGAGATGAATCCTCA-3′ and 5′-CCGGTAGCTAGGGGGCTCACA-3′ for amplification of a 570-bp product (7). The primers used for coamplification (143 kb) of the control RPS14 ribosomal mRNA were 5′-GGCAGACCGAGATGAATCCTCA-3′ and 5′-CAGGTCCAGGGGTCTTGGTCC-3′. The reaction was performed in the PTC-200 Peltier thermal cycler (MJ Research), at 94°C for 2 min, followed by 23 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. Amplified DNAs were analyzed by agarose gel electrophoresis, and signals in ethidium bromide-stained gels were quantified using the EDAS-290 imaging system (Kodak).
Antibodies, immunoblotting, and immunoprecipitation.
Monoclonal anti-Cdc25A antibodies, Ab-3 and F6, were obtained from Neomarkers and Santa Cruz Biotechnology, respectively. Anti-phospho-S82/S88 polyclonal antibody was generated and described previously (6). Antiubiquitin monoclonal antibody (P4D1) and anti-β-TrCP (N-15) goat polyclonal antibody were from Santa Cruz Biotechnology, and anti-Cdh1 (monoclonal, CC43) was from Calbiochem. Antihemagglutinin (12CA5) antibody was from Roche Applied Science, and anti-V5 and anti-Myc monoclonal antibodies were from Invitrogen. Anti-Flag (M2) and anti-β-actin (AC-15) monoclonal antibodies were purchased from Sigma. Anti-Smad3 polyclonal antibody (LPC3) was from Zymed. Horseradish peroxidase-conjugated anti-mouse and rabbit immunoglobulin secondary antibodies, as well as recombinant protein A-agarose beads and the Supersignal West Pico chemiluminescence reagent, were obtained from Pierce. To prepare cell lysates, cells were scraped off and lysed by sonication in a buffer including 50 mM HEPES-KOH (pH 7.5), 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 1 mM dithiothreitol, 10 mM β-glycerophosphate, 1 mM NaF, 0.1 mM sodium orthovanadate, ×100-diluted protease inhibitor cocktail (P8340; Sigma), 10% glycerol, and 0.1% NP-40. All the chemicals were purchased from Sigma. Immunoblotting and immunohistochemistry were performed as described previously (57, 58). The signals on films were quantified by densitometric scanning with the GS-700 imaging system and Molecular Analyst software (Bio-Rad Laboratories).
RESULTS
TGF-β signals enhance ubiquitin-dependent degradation of Cdc25A.
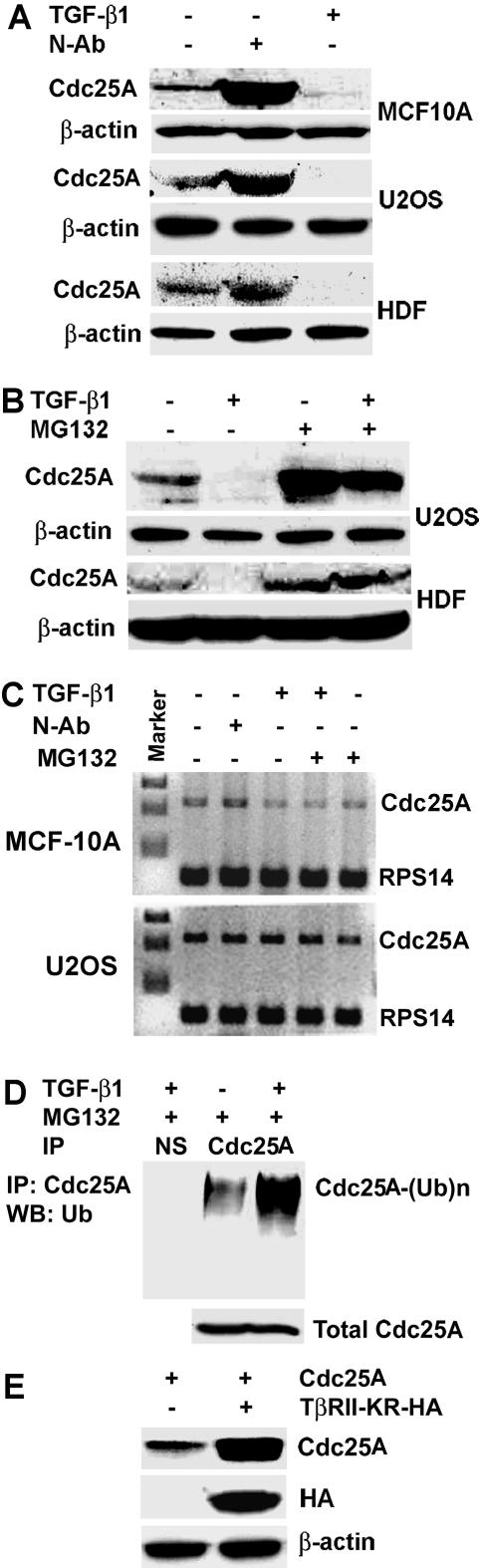
We first examined effects of TGF-β signals on Cdc25A protein levels in MCF-10A mammary epithelial cells, U2OS osteosarcoma cells, and primary HDF. Treatment with TGF-β1 significantly decreased Cdc25A levels in all cells tested (Fig. 1A). In contrast, treatment with a neutralizing anti-TGF-β1,2,3 antibody increased Cdc25A levels. This effect is probably attributed to the basal signaling activity of TGF-β contained in the sera for culture. To determine whether proteasomal degradation is involved in TGF-β regulation of Cdc25A, cells were treated with TGF-β in the presence of the proteasome inhibitor MG132. MG132 increased Cdc25A levels in both TGF-β-treated and untreated cells (Fig. 1B). TGF-β down-regulated Cdc25A by 80% in the absence of MG132. However, in the presence of MG132, TGF-β decreased Cdc25A levels in U2OS cells by only 20 to 30% and minimally affected those in HDF. Similar results were obtained in MCF-10A cells (data not shown). It has been demonstrated that TGF-β signals repress the transcription of the Cdc25A gene in an E2F-dependent manner (29, 30). We determined cellular levels of Cdc25A mRNA in TGF-β-treated MCF10A and U2OS cells (Fig. 1C). TGF-β treatment for 24 h decreased Cdc25A mRNA by 30% in MCF10A cells, while it minimally (<10%) decreased Cdc25A mRNA in U2OS cells. MG132 did not significantly alter Cdc25A mRNA levels. These data suggest that TGF-β-mediated Cdc25A down-regulation is attributable largely to proteasome-dependent degradation. We further showed that TGF-β treatment increases ubiquitin-associated forms of Cdc25A in U2OS cells (Fig. 1D), suggesting that TGF-β signals promote ubiquitination with Cdc25A, which should facilitate proteasomal degradation. Cotransfection of a dominant-negative mutant of the TGF-β type II receptor [TβRII(KR)] and Cdc25A resulted in significant increase in Cdc25A protein levels (Fig. 1E). These data support the idea that TGF-β signals control Cdc25A mainly at the posttranscriptional level, since Cdc25A is expressed from the exogenous plasmid carrying the constitutive cytomegalovirus (CMV) promoter in this experiment.
FIG. 1.
TGF-β1 promotes Cdc25A ubiquitination and degradation. (A) Exponentially proliferating MCF-10A and U2OS cells and HDF were either left untreated (−) or treated (+) with either TGF-β1 (10 ng/ml or 400 pM for 24 h) or anti-TGF-β1,2,3 neutralizing antibody (N-Ab; 20 μg/ml for 48 h). Cdc25A steady-state levels were determined by immunoblotting. (B) The decrease in Cdc25A protein levels upon TGF-β1 (10 ng/ml) treatment was significantly abolished by cotreatment with MG132 (1 μM for 15 h). (C) TGF-β1 treatment (10 ng/ml for 24 h) modestly down-regulated Cdc25A mRNA in MCF-10Acells, but not in U2OS cells, while N-Ab minimally affected Cdc25A mRNA. mRNA levels were determined by RT-PCR. RPS14, rRNA for internal control. (D) Ubiquitination of Cdc25A was induced by TGF-β1 treatment (10 ng/ml). U2OS cells were treated either with 1 μM MG132 for 15 h or with TGF-β1 (for 24 h) plus 1 μM MG132 (for 15 h prior to lysis). Lysates were subjected to anti-Cdc25A immunoprecipitation followed by immunoblotting with anti-Ub antibody. Polyubiquitinated species of Cdc25A are indicated as CDC25A-(Ub)n. (E) Cotransfection of dominant-negative TβRII-KR-HA significantly increased the steady-state level of exogenously expressed Cdc25A. U2OS cells were transfected with the indicated expression vectors. Cell lysates were prepared 24 h posttransfection, and Cdc25A level was detected by immunoblotting.
Smad3 is rate limiting for Cdc25A degradation.
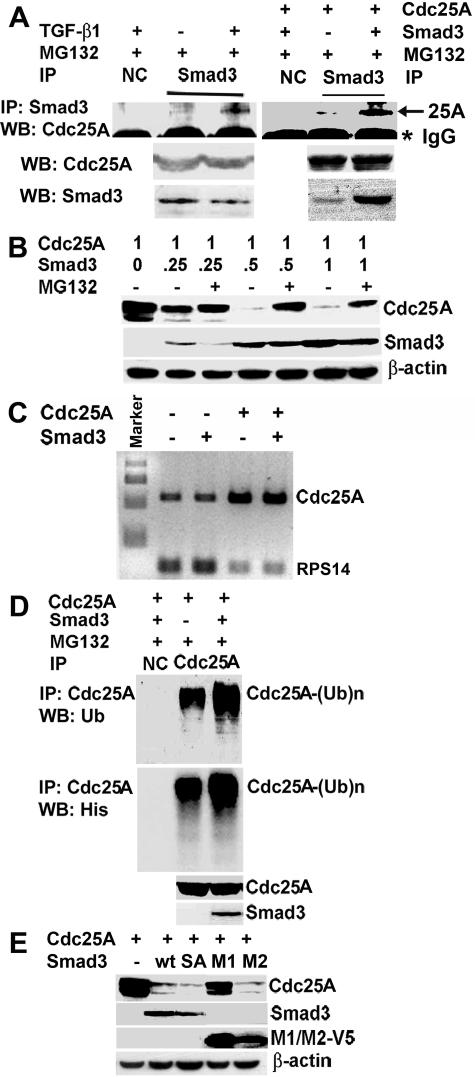
Next, we examined possible involvement of Smad proteins in Cdc25A degradation. We found that a small amount of endogenous Cdc25A coimmunoprecipitated with endogenous Smad3 in nontransfected MG132-treated U2OS cells (Fig. 2A, left panels). TGF-β treatment significantly increased Smad3 proteins in complex with Cdc25A. We also observed that overexpression of Smad3 by transfection markedly increased the amount of Smad3-Cdc25A complexes even without TGF-β (Fig. 2A, right panels). These data suggest that Cdc25A binds to Smad3 prior to proteasomal degradation. Consistent with this notion, cotransfection of the Cdc25A plasmid with increasing amounts of a Smad3 expression plasmid resulted in decreasing levels of Cdc25A protein, and this down-regulation was eliminated by MG132 (Fig. 2B). Smad3 overexpression did not affect Cdc25A mRNA levels in either nontransfected cells or cells transfected with Cdc25A (Fig. 2C), confirming that Cdc25A down-regulation by Smad3 is not at the transcription level. In contrast to Smad3, Smad2 showed minimum effects on Cdc25A levels (data not shown). Analyses with flow cytometry showed that Smad3 overexpression did not significantly alter cell cycle distributions at 24 h posttransfection (data not shown). Ubiquitination of exogenously expressed Cdc25A was enhanced by coexpression of Smad3 (Fig. 2D), similarly to the TGF-β effect on endogenous Cdc25A (Fig. 1C). Coexpression experiments with Cdc25A and deletion mutants of Smad3 (Fig. 2E) showed that the C-terminal MH2 domain of Smad3, which is involved in receptor-mediated phosphorylation, oligomerization with Smad4, and transactivation (26), is sufficient to down-regulate Cdc25A. In contrast, the N-terminal MH1 domain cannot affect Cdc25A levels. It is known that TβRI directly phosphorylates Smad3 at the carboxyl-terminal SSVS motif (46). Interestingly, we found that a Smad3 mutant defective in SSVS phosphorylation (Smad3-SA) was as effective as the wild type in down-regulating Cdc25A (Fig. 2E). Smad3 has been reported to enhance degradation of HEF1 (human enhancer of filamentation 1) similarly in a manner independent of SSVS phosphorylation (37).
FIG. 2.
Smad3 regulates Cdc25A degradation in a ubiquitination-dependent manner. (A) TGF-β1 treatment enhances physical interaction of Cdc25A and Smad3. U2OS cells without transfection were treated for 15 h with either 1 μM MG132 or 10 ng of TGF-β1/ml for 24 h plus 1 μM MG132 for the last 15 h (left panels). U2OS cells were also cotransfected with Cdc25A and Smad3 and then treated with MG132 between 9 and 24 h posttransfection (right panels). Cell lysates were subjected to immunoprecipitation by Smad3 antibody or normal control immunoglobulin G (NC), followed by immunoblotting with Cdc25A antibody. Asterisk, immunoglobulin heavy chain. (B) Smad3 can trigger proteasomal degradation of Cdc25A in a dose-dependent manner. U2OS cells were transfected with a constant amount of a Cdc25A plasmid with the CMV promoter and increasing amounts of a Smad3 plasmid with the CMV promoter. The amounts of each plasmid are shown as micrograms. Cells were treated with or without 1 μMMG132 between 9 and 24 h posttransfection, and cell lysates were prepared and immunoblotted for Cdc25A. (C) Smad3 overexpression does not affect Cdc25A mRNA levels. Cells at 24 h posttransfection were analyzed by RT-PCR. cDNA samples from cells with Cdc25A transfection (lanes 4 and 5) were diluted 1:10 for PCR amplification, to ensure semiquantitative analyses. RPS14, rRNA for internal control. (D) Overexpression of Smad3 increases ubiquitination of Cdc25A. U2OS cells were transfected as indicated and were treated with 1 μM MG132 between 9 and 24 h posttransfection. Cdc25A was immunoprecipitated and then immunoblotted with anti-His or anti-Ub antibody to detect ubiquitinated forms [Cdc25A-(Ub)n]. The expression level of transfected Cdc25A was examined by immunoblotting (bottom panel). (E) The MH2 domain of Smad3 is sufficient to trigger Cdc25A degradation. U2OS cells were either transfected with Cdc25A alone or cotransfected with either wt (wild type), SA (SSVS->AAVA mutant), or MH1- or MH2-domain-only Smad3 mutants. Levels of transfected proteins were determined by immunoblotting.
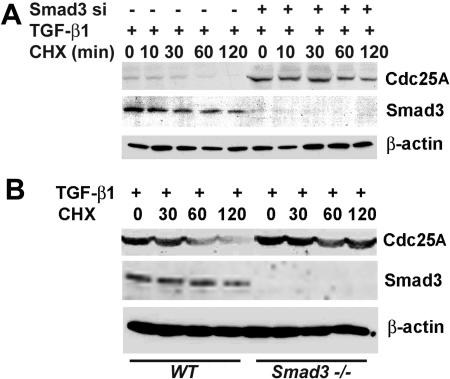
To further address the significance of Smad3 regulation in Cdc25A stability, Smad3 expression is suppressed in U2OS cells by siRNA transfection (Fig. 3A). Cycloheximide treatment demonstrated that siRNA-mediated depletion of Smad3 resulted in increased stability of Cdc25A protein in cells cultured with TGF-β. Moreover, Smad3 siRNA significantly stabilized Cdc25A protein in cells without TGF-β treatment (data not shown), suggesting that Smad3 controls Cdc25A during unperturbed proliferation. Consistently, we observed increased stability of Cdc25A protein in TGF-β-treated Smad3−/− MEFs from Smad3-knockout mice (54), compared with that in wild-type MEFs (Fig. 3B). We observed similar stabilization of Cdc25A in MEFs from another strain of Smad3-null mice (10) (data not shown). These data suggest that Smad3 expression is a rate-limiting factor for ubiquitination of Cdc25A.
FIG. 3.
Smad3 expression levels determine Cdc25A stability in TGF-β treated cells. (A) Smad3 knockdown by siRNA increases the stability of Cdc25A in TGF-β-treated cells. U2OS cells were transfected twice with Smad3 siRNA, with a 24-h interval. At 24 h after second transfection, cells were treated with 10 ng of TGF-β1/ml for 22 h, followed by incubation with 50 μg of cycloheximide/ml for the times indicated. The levels of the listed proteins were examined by immunoblotting. (B) Cdc25A is stabilized in Smad3-null MEFs. MEFs from wild-type (WT) and Smad3−/− mice were treated with 10 ng of TGF-β1/ml for 22 h, followed by incubation with 50 μg of cycloheximide/ml for the times indicated in minutes, and analyzed by immunoblotting for the proteins indicated.
Cdc25A phosphorylation is critical for TGF-β-Smad3-induced ubiquitination.
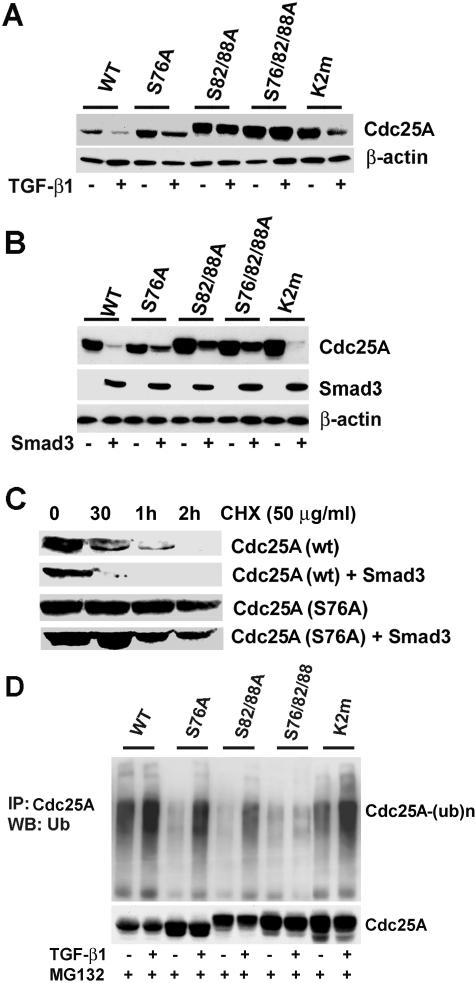
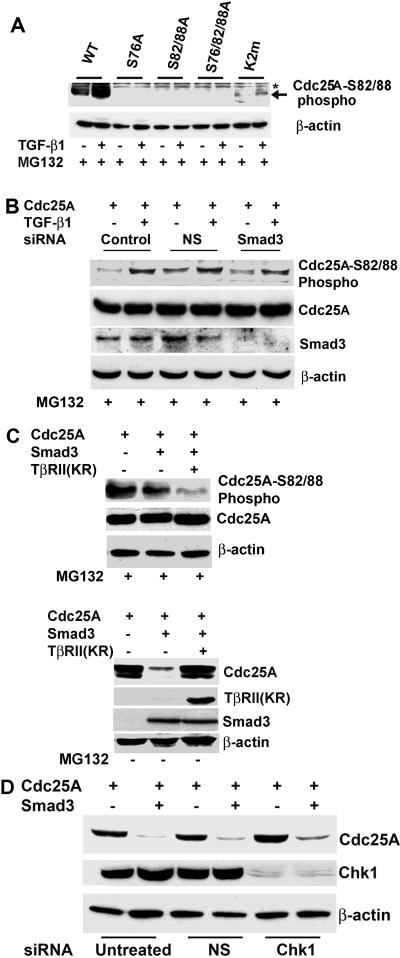
With the finding that Smad3 association facilitates ubiquitination of Cdc25A, we examined possible involvement of Cdc25A phosphorylation. Ser76 phosphorylation is involved in Chk1-mediated Cdc25A degradation, which appears to be a “priming” event for Ser82 phosphorylation (14). Ser82 is located at the center of the DS82G motif, which is critical for β-TrCP association (13). Phosphorylation of the Ser73 residue of Xenopus laevis Cdc25A, the counterpart of Ser76 of human Cdc25A, is critical for Cdc25A degradation at midblastula transition during development (47). Expression of a nonphosphorylatable S73A mutant of Cdc25A results in embryonic lethality in Xenopus. Thus, we speculated that developmental signals, such as TGF-β and activin, might regulate Ser76 phosphorylation of Cdc25A in mammals. Indeed, the Cdc25A(S76A) mutant was relatively refractory to down-regulation induced by TGF-β treatment (Fig. 4A) or Smad3 overexpression (Fig. 4B). The Cdc25A(S82A/S88A) mutant defective in β-TrCP association also exhibited resistance to TGF-β- or Smad3-induced down-regulation, which was more prominent with a triple mutant, Cdc25A(S76A/S82A/S88A). In contrast, the K2m mutant defective in APC-mediated ubiquitination (15) was degraded quite efficiently by TGF-β treatment (Fig. 4A) or Smad3 overexpression (Fig. 4B). These observations suggest that APC does not play a major role in TGF-β-Smad3-mediated Cdc25A ubiquitination, unlike its function in G1-specific ubiquitination of the phosphatase (15). Treatment with cycloheximide demonstrated that Smad3 expression markedly decreased the stability of wild-type Cdc25A but poorly affected that of Cdc25A(S76A) (Fig. 4C). Cdc25A(S76A), Cdc25A(S82A/S88A), and Cdc25A(S76A/S82A/S88A) were also refractory to down-regulation by a constitutively active TGF-β type I receptor (TβRI), while Cdc25A(K2m) was rapidly down-regulated (data not shown). Treatment with MG132 eliminated TGF-β-induced down-regulation of all these mutants, as expected (Fig. 4D, lower panel). Ubiquitination of Cdc25A(S76A) in the presence of TGF-β and MG132 was decreased compared with that of wild-type Cdc25A and Cdc25A(K2m) (Fig. 4D, upper panel). Ubiquitination of Cdc25A(S82A/S88A) was further diminished, and that of Cdc25A(S76A/S82A/S88A) was almost insensitive to TGF-β treatment (Fig. 4D). Thus, ubiquitination of the Cdc25A mutants correlates with TGF-β-induced down-regulation of the proteins. Similar results were obtained in cells cotransfected with Smad3 (data not shown). We then examined phosphorylation at the DS82G motif for β-TrCP docking, using the antibody specific for Cdc25A phosphorylated at Ser82/Ser88 (anti-phospho-S82/S88) (6). TGF-β treatment in the presence of MG132 significantly stimulated phosphorylation at the DSG site of wild-type Cdc25A and Cdc25A(K2m), but such stimulation was not detectable with the S76A, S82A/S88A, or S76A/S82A/S88A mutant (Fig. 5A). To examine whether altered expression of Smad3 affects phosphorylation of the DS82G site, U2OS cells were transfected with Smad3 siRNA or nonspecific (NS) dsRNA, followed by treatment with MG132 and TGF-β (Fig. 5B). Smad3 siRNA down-regulated Smad3 protein to barely detectable levels but did not significantly affect TGF-β-induced increases in S82/S88 phosphorylation, despite the prolonged half-life of the Cdc25A protein (Fig. 3A). We also examined effects of Smad3 overexpression on S82/S88 phosphorylation. Cotransfection of Smad3 did not alter S82/S88 phosphorylation in the presence of MG132; however, addition of the dominant-negative TβRII to transfection clearly diminished S82/S88 phosphorylation (Fig. 5C, upper panels). In the absence of MG132, cotransfection of TβRII completely eliminated Smad3-induced Cdc25A down-regulation (Fig. 5C, lower panels). We also observed that cellular treatment of the neutralizing TGF-β antibody or SB431542, a chemical inhibitor of TβRI kinase, diminished S82/S88 phosphorylation and restored Cdc25A levels in cells transfected with Smad3 (data not shown). We further examined whether Chk1 plays a role in TGF-β-induced Cdc25A degradation (Fig. 5D). U2OS cells were transfected with Chk1 siRNA or NS dsRNA, followed by cotransfection with Smad3 and Cdc25A. Although Chk1 protein levels were suppressed over 90%, Smad3 down-regulated Cdc25A as effectively, suggesting that Chk1 does not play a major role in Smad3-mediated Cdc25A degradation. Taken together, these data suggest that signals from TGF-β receptors play a key role in Cdc25A phosphorylation at the DS82G site, while cellular levels of Smad3 may play another role in Cdc25A ubiquitination.
FIG. 4.
Phosphorylation around the DSG motif is involved in TGF-β-Smad3-mediated Cdc25A degradation. (A) Cdc25A mutants defective in phosphorylation around the DSG motif are refractory to TGF-β-mediated degradation. U2OS cells were transfected with wild type (WT) or the mutants of Cdc25A indicated in the panel and then treated with 10 ng of TGF-β1/ml between 9 and 24 h posttransfection. K2m, a K141A/E142A/N143A mutant defective in APC recognition. Cell lysates were analyzed by immunoblotting for the proteins indicated. (B) The DSG phosphorylation mutants of Cdc25A are refractory to Smad3-mediated degradation. U2OS cells were cotransfected with Smad3 and either wild type or the mutants of Cdc25A. Cell lysates at 24 h posttransfection were analyzed by immunoblotting. (C) Smad3 overexpression decreases stability of wild-type (wt) Cdc25A, whereas it affects the half-life of Cdc25A (S76A) only modestly. At 22 h posttransfection, cycloheximide (50 μg/ml) was added to the culture medium, and cells were further incubated for indicated times, followed by immunoblotting. (D) Phosphorylation around the DSG motif is critical for TGF-β1-mediated ubiquitination. U2OS cells were cotransfected with the indicated expression vectors and treated with or without TGF-β1 for 24 h in the presence of 1 μM MG132 between 9 and 24 h. Ubiquitinated Cdc25A [Cdc25A-(ub)n] was detected by immunoprecipitation (IP) followed by immunoblotting (WB).
FIG. 5.
Cdc25A phosphorylation at the DSG motif is regulated by TGF-β signals independently of Smad3 levels. (A) TGF-β up-regulates phosphorylation at the DSG motif. U2OS cells were transfected with wild type (WT) and the mutants of Cdc25A and then treated with 10 ng of TGF-β1/ml for 24 h and with 1 μM MG132 between 9 and 24 h posttransfection, followed by immunoblotting with a polyclonal antibody specifically for Cdc25A phosphorylated at Ser82/Ser88. (B) Smad3 knockdown does not affect TGF-β-induced DSG phosphorylation. U2OS cells were transfected with Smad3 siRNA or NS dsRNA (NS), followed by transfection with Cdc25A and treatment with TGF-β and MG132 at 9 to 24 h posttransfection. Cells were then analyzed by immunoblotting with the indicated antibodies. (C) TGF-β receptor signaling is critical for DSG phosphorylation. (Upper panels) U2OS cells were transfected with indicated plasmids, followed by treatment with MG132 at 9 to 24 h posttransfection. TβRII(KR), a dominant-negative type II TGF-β receptor mutant. (Lower panels) The above experiment was performed in the absence of MG132. (D) Chk1 kinase is not critical for Smad3-induced Cdc25A degradation. U2OS cells were transfected with Chk1 siRNA or NS dsRNA, followed by transfection of Cdc25A in the presence or absence of Smad3. After 24 h, cells were subjected to immunoblotting for the indicated proteins.
Physical association of β-TrCP with Cdc25A and Smad3.
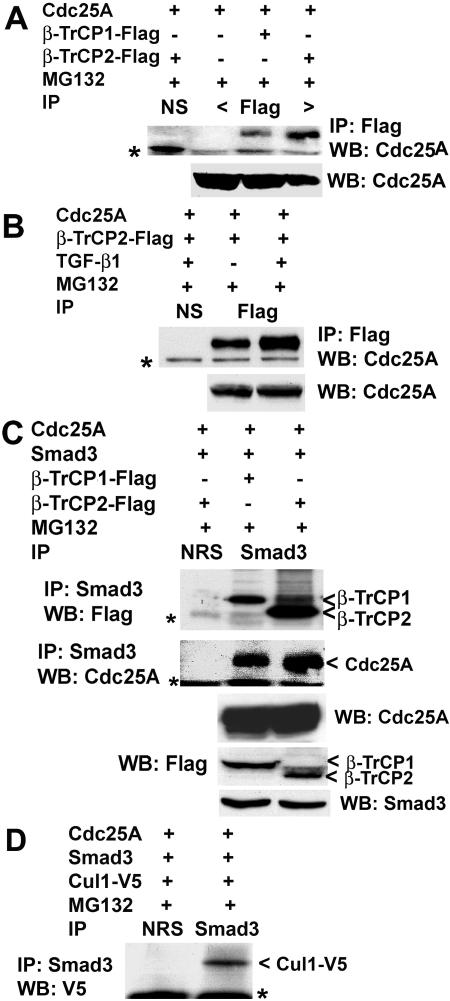
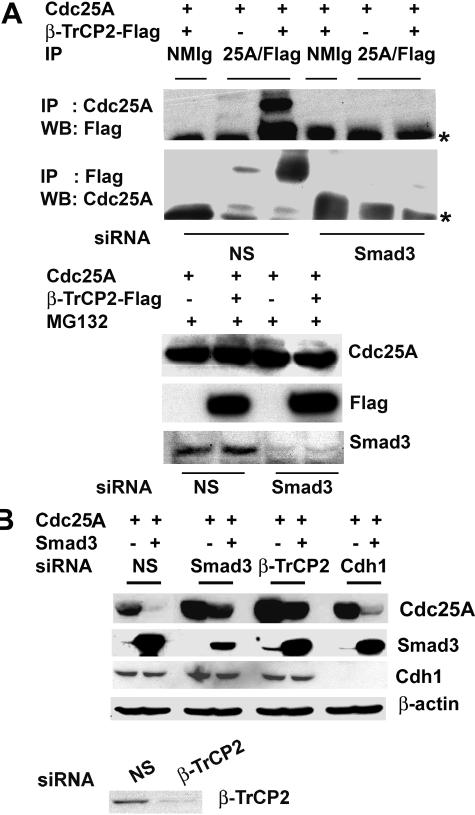
The importance of DS82G phosphorylation in TGF-β-induced Cdc25A ubiquitination prompted us to directly examine the involvement of SCFβ-TrCP. We observed that Cdc25A is physically associated with β-TrCP1 and β-TrCP2 when cotransfected in U2OS cells (Fig. 6A), and TGF-β treatment increased Cdc25A-β-TrCP complexes (Fig. 6B). Recent reports (19, 51) demonstrated that Smad3 can associate with SCFβ-TrCP. Thus, we examined Smad3 interaction with β-TrCP and cullin-1 in MG132-treated U2OS cells after cotransfection. It was readily observed that Smad3 coimmunoprecipitated with β-TrCP1 or β-TrCP2 (Fig. 6C) and with cullin-1 (Fig. 6D). Smad3 appeared to bind more efficiently to β-TrCP2 than to β-TrCP1. In these experiments Smad3-Cdc25A association was also detected, as expected. Next, we examined effects of Smad3 knockdown upon Cdc25A-β-TrCP interaction. Transfection with Smad3 siRNA almost eliminated Cdc25A-β-TrCP interaction in coimmunoprecipitation assays (Fig. 7A), indicating that Smad3 is required for β-TrCP docking to Cdc25A. To further assess the significance of β-TrCP in Smad3-induced Cdc25A degradation, U2OS cells were transfected with NS dsRNA or β-TrCP1, β-TrCP2, or Cdh1 siRNA, followed by cotransfection with Cdc25A and Smad3 (Fig. 7B). Each of the specific siRNAs significantly down-regulated the target protein. The β-TrCP2 siRNA significantly prevented Cdc25A from undergoing Smad3-induced down-regulation, and the β-TrCP1 siRNA showed a similar tendency to a lesser extent (data not shown). In contrast, Cdh1 siRNA did not affect Cdc25A down-regulation. These results suggest that Smad3, in collaboration with TβR signals, facilitates Cdc25A interaction with SCFβ-TrCP and subsequent ubiquitination and proteasomal degradation.
FIG. 6.
Association of β-TrCP with Cdc25A and Smad3. (A) Cdc25A physically associates with the F-box proteins β-TrCP1 and β-TrCP2 in cotransfected U2OS cells. Cells were transfected with Cdc25A and Flag-tagged β-TrCP1 or β-TrCP2 and treated with 2 μM MG132 at 16 to 24 h posttransfection. Lysates were immunoprecipitated (IP) with an anti-Flag monoclonal antibody, followed by immunoblotting (WB) for Cdc25A. Asterisk, immunoglobulin heavy chain. The lower panel shows direct WB. (B) TGF-β up-regulates Cdc25A association with β-TrCP. Cells were transfected as described for panel A, followed by treatment with 10 ng of TGF-β/ml1 for 24 h. MG132 (1 μM) was added for the last 15 h of incubation. Asterisk, immunoglobulin heavy chain. The lower panel shows direct WB. (C) Smad3 physically associates with β-TrCP. Cells were transfected with the indicated plasmids, followed by treatment with 2 μM MG132 at 16 to 24 h posttransfection. Asterisks, immunoglobulin heavy chain. The lower three panels show direct WB. (D) Smad3 interacts with cullin-1 (Cul1). Cells were transfected with Cdc25A, Smad3, and Cul1-V5 as indicated, followed by the treatment with 2 μM MG132 at 16 to 24 h posttransfection. Lysates were immunoprecipitated with anti-Smad3 antibody followed by immunoblotting with V5 antibody for cullin-1. Asterisk, immunoglobulin heavy chain.
FIG. 7.
β-TrCP mediates Smad3-induced Cdc25A ubiquitination. (A) Smad3 knockdown diminishes Cdc25A interaction with β-TrCP. U2OS cells were transfected with Smad3 siRNA or NS dsRNA, followed by transfection with Flag-tagged β-TrCP2 and/or Cdc25A. Cells were treated with 2 μM MG132 at 16 to 24 h posttransfection and subjected to immunoprecipitation (IP) followed by immunoblotting (WB), as indicated. Direct WB data are shown in lower panels. Asterisks, immunoglobulin heavy chain. (B) siRNA-mediated down-regulation of β-TrCP protects Cdc25A from Smad3-mediated degradation. U2OS cells were transfected twice with NS, Smad3, β-TrCP2, or Cdh1 siRNA. At 24 h posttransfection, cells were further transfected with either Cdc25A alone or Cdc25A and Smad3, and 24 h later, protein levels were determined by immunoblotting.
DISCUSSION
Proteasomal degradation of Cdc25A is regulated by at least two different ubiquitin ligases, APCCdh1 and SCFβ-TrCP (6, 15, 32). Previous studies showed that APCCdh1 actively ubiquitinates Cdc25A at the end of mitosis through G1 (15), which is counteracted by the S-phase-specific induction of the APC inhibitor Emi1 (23, 44). During S and G2 phases of unperturbed cell cycle progression, SCFβ-TrCP is involved in the control of Cdc25A stability (6). Moreover, SCFβ-TrCP plays a critical role in active Cdc25A degradation in response to DNA damage, which has been defined as a Chk1-dependent S-phase checkpoint (6, 32). The present study demonstrated that the TGF-β signaling regulates the SCFβ-TrCP-mediated ubiquitination of Cdc25A, in a Smad3-dependent fashion. This regulation is quite specific and efficient for Cdc25A but not for Cdc25B and Cdc25C (data not shown). TGF-β treatment markedly decreases the stability of both endogenous and exogenously expressed Cdc25A. TGF-β signals up-regulate Cdc25A phosphorylation at the DS82G motif, which is critical for β-TrCP docking (13). Consistently, Cdc25A mutants defective in DS82G phosphorylation are refractory to TGF-β- or Smad3-induced ubiquitination and degradation. Furthermore, siRNA-mediated knockdown of β-TrCP2, but not that of Cdh1, inhibits Smad3-induced Cdc25A degradation. These data indicate that Cdc25A phosphorylation at the DS82G motif is a previously undefined target of TGF-β signaling.
It has been shown that Ser76 phosphorylation is a step requisite for Ser82 phosphorylation (6, 32). The exact mechanism of the hierarchical order of phosphorylation is unknown. It also remains elusive what kinases are responsible for Ser76 and Ser82 phosphorylation. Although a previous study showed that purified Chk1 can efficiently phosphorylate Ser76 in vitro (25, 32), some other kinases may phosphorylate the site in concert with TGF-β signaling. In addition, Chk1 cannot phosphorylate Ser82 in vitro. The present study has shown that Chk1 siRNA does not affect Smad3-induced down-regulation of Cdc25A, suggesting that Chk1 does not play a major role in the TGF-β-Smad3 regulation of Cdc25A. A previous study of Xenopus Cdc25A suggested that an unidentified kinase phosphorylates Ser73, the Xenopus counterpart of Ser76, at the midblastula transition during development (47). Xenopus Chk1 cannot phosphorylate Ser73 in this system. It is well known that TGF-β and activin signals are important during embryogenesis, especially the midblastula transition stage. Mammalian non-Chk1 protein kinases responsible for TGF-β-induced Ser76 and Ser82 phosphorylation remain to be identified.
Smad3 is obviously required for Cdc25A ubiquitination induced by TGF-β signals. Cdc25A is stabilized in cells transfected with Smad3 siRNA or in cells from Smad3-knockout mice. It is noteworthy that Cdc25A is more stable in those cells with reduced Smad3 expression, even in culture medium without TGF-β addition (data not shown). Also, Smad3 overexpression destabilizes Cdc25A quite effectively without exogenous TGF-β. The dominant-negative type II TGF-β receptor, neutralizing TGF-β antibodies, or the type I receptor inhibitor SB431542 can eliminate Smad3-induced Cdc25A degradation, suggesting that Smad3 overexpression is not sufficient for execution of Cdc25A ubiquitination and that receptor signals are still required. In cells cultured without exogenous TGF-β, basal signaling activities from TGF-β receptors, probably induced by TGF-β in fetal bovine serum, may cooperate with altered Smad3 levels to determine the rate of Cdc25A ubiquitination. The exact mechanism of the rate-limiting role for Smad3 remains to be determined. Association of β-TrCP with Cdc25A is almost eliminated by siRNA-mediated knockdown of Smad3. However, neither Smad3 knockdown nor overexpression significantly alters Ser82-Ser88 phosphorylation. One of the possible explanations for these observations is that Smad3-dependent signals activate a kinase responsible for Cdc25A phosphorylation at another site(s) that is more critical for β-TrCP association. Another possibility is that Smad3 may facilitate β-TrCP association with Cdc25A more directly, forming a regulatory layer downstream of DS82G phosphorylation. We have demonstrated that Smad3 can bind to Cdc25A, β-TrCP, and cullin-1. Fukuchi et al. previously showed that the C-terminal MH2 domain of Smad3 physically binds to ROC1 (also termed Rbx1 or Hrt1) in yeast two-hybrid assays and Smad3 associates with SCFβ-TrCP complex in mammalian cells (19). ROC1 functions as a linker between cullin and an E2 enzyme. They showed that Smad2 does not bind to SCFβ-TrCP, which is consistent with our observation that Smad2 expression does not down-regulate Cdc25A. Another report showed that β-TrCP1 binds weakly to Smad3 and strongly to Smad4 in cotransfected mammalian cells (51), which may be relevant to our data showing that β-TrCP2 binds to Smad3 more efficiently than β-TrCP1. Thus, Smad3 might participate in SCFβ-TrCP complexes, via interaction with ROC1, and help assembly with Cdc25A phosphorylated at the DS82G site. Interestingly, we also found that Cdc25A mutants defective in DS82G phosphorylation cannot bind to Smad3 in cotransfected U2OS cells (data not shown). Smad3 also has been shown to recruit the APCCdh1 complex to SnoN, a negative regulator of the TGF-β signaling (48), and the human enhancer of filamentation 1 (HEF1), a Cas family cytoplasmic docking protein (37, 42). In both cases, Smad3 seems to facilitate ubiquitination by physical association with the substrate and APC components. However, Cdh1 siRNA does not protect Cdc25A from Smad3-induced down-regulation, indicating that the APCCdh1 complex is not involved in the Smad3-mediated ubiquitination of Cdc25A. Consistently, a dominant-negative mutant of cullin-1 effectively eliminates Smad3-induced Cdc25A degradation, while Emi1, a specific inhibitor of APC, has minimum effects (D. Ray and H. Kiyokawa, unpublished observations). Further studies are necessary to clarify how Smad3 controls ubiquitination of multiple substrates by SCFβ-TrCP and APC.
Previous reports demonstrated that TGF-β down-regulates Cdc25A at the level of transcription (29, 30). TGF-β signals result in recruitment of the E2F4-p130 repressor complex onto an E2F binding site of the Cdc25A promoter. Cdc25A down-regulation seems to collaborate with transactivation of the Cdk inhibitor p15INK4b and p21Cip1/Waf1 and repression of c-myc, in inducing G1 cell cycle arrest in response to TGF-β (46). In addition, Cdc25A phosphatase activity is also a target of TGF-β signals. TGF-β activates p160ROCK in a RhoA-dependent manner, and p160ROCK diminishes Cdc25A phosphatase activity by phosphorylation (3). Thus, the TGF-β signaling pathway controls Cdc25A at multiple levels, i.e., transcriptional repression, proteasomal degradation, and enzymatic inhibition, suggesting the importance of Cdc25A as a target of developmental and tumor-suppressive functions of the TGF-β signaling pathway. Accumulating evidence suggests that Smad3 is a tumor suppressor gene. Most intriguingly, Smad3−/− mice develop metastatic colorectal carcinoma (56). Furthermore, a recent study showed that Smad3 protein was undetectable in all of 10 T-cell acute lymphocytic leukemia samples examined, despite intact expression of Smad3 mRNA without mutations (52). Smad3+/− p27Kip1−/− mice exhibit lymphocytic infiltration in multiple organs, and 10% of those animals develop T-cell leukemia (52). Loss of Smad3 protein expression is also observed in a fraction of gastric cancer tissues (24). Smad3 could play an important role in tumor suppression by controlling transcription of TGF-β target genes. Another critical role for Smad3 is to regulate ubiquitination of various proteins, such as SnoN, HEF1, and Cdc25A. Our studies with Smad3 overexpression, Smad3 siRNA, and Smad3-null cells clearly indicate that the expression level of Smad3 is a determining factor for stability and steady-state levels of Cdc25A. Cdc25A protein is overexpressed in various types of malignancies, including colon, esophagus, breast, and ovarian cancers and lymphoma (5, 7, 12, 31, 41). Increased Cdc25A levels may lead to not only unrestricted proliferation by Cdk activation but also suppressed apoptosis (58), either of which can contribute to tumor initiation and progression. Recently it was shown that Cdc25A overexpression in several breast cancer cell lines results from impaired protein degradation (38). We observed that breast cancer cells without detectable Smad3 expression, e.g., MCF-7 cells, display stable Cdc25A protein, whereas cancer cells with high-level Smad3 expression, such as MDA-MB231 cells, exhibit active degradation of Cdc25A at the basal state (D. Ray and H. Kiyokawa, unpublished observations). Moreover, Cdc25A degradation is also enhanced when murine leukemia M1 cells undergo interleukin-6-induced differentiation (2). It is known that TGF-β-Smad3 signaling plays a role in myeloid differentiation (28, 34). These observations are consistent with the reciprocal relationship between Smad3 and Cdc25A established in the present study. Smad3 deficiency or other defects in the pathway regulating SCFβ-TrCP-mediated ubiquitination should lead to Cdc25A stabilization. As a consequence, Cdks will become hyperactivated and promote cell cycle progression, while Cdk4 and Cdk2 will in turn phosphorylate Smad3 and down-regulate its activity (39), creating a feedback loop of regulation. The significance of Smad3-dependent Cdc25A regulation in development and carcinogenesis awaits further investigations.
Acknowledgments
We thank Michele Pagano for sharing the sequences of β-TrCP1/2 siRNAs prior to publication; Chuxia Deng for Smad3−/− MEFs; Peter Jackson for the Emi1 and Cdh1 plasmids; Pradip Raychaudhuri for cullin plasmids and technical suggestions; and Helen Piwnica-Worms, Antonio Iavarone, Siwanon Jirawatnotai, and Evan Osmundson for helpful discussions.
This work was supported in part by funds provided to H.K. by the National Institutes of Health (R01HD38085 and R01CA100204) and the Department of Defense (DAMD 17-02-1-0413).
REFERENCES
- 1.Bartek, J., and J. Lukas. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3:421-429. [DOI] [PubMed] [Google Scholar]
- 2.Bernardi, R., D. A. Liebermann, and B. Hoffman. 2000. Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene 19:2447-2454. [DOI] [PubMed] [Google Scholar]
- 3.Bhowmick, N. A., M. Ghiassi, M. Aakre, K. Brown, V. Singh, and H. L. Moses. 2003. TGF-beta-induced RhoA and p160ROCK activation is involved in the inhibition of Cdc25A with resultant cell-cycle arrest. Proc. Natl. Acad. Sci. USA 100:15548-15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomberg, I., and I. Hoffmann. 1999. Ectopic expression of Cdc25A accelerates the G1/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol. Cell. Biol. 19:6183-6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broggini, M., G. Buraggi, A. Brenna, L. Riva, A. M. Codegoni, V. Torri, A. A. Lissoni, C. Mangioni, and M. D'Incalci. 2000. Cell cycle-related phosphatases CDC25A and B expression correlates with survival in ovarian cancer patients. Anticancer Res. 20:4835-4840. [PubMed] [Google Scholar]
- 6.Busino, L., M. Donzelli, M. Chiesa, D. Guardavaccaro, D. Ganoth, N. V. Dorrello, A. Hershko, M. Pagano, and G. F. Draetta. 2003. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature 426:87-91. [DOI] [PubMed] [Google Scholar]
- 7.Cangi, M. G., B. Cukor, P. Soung, S. Signoretti, G. Moreira, Jr., M. Ranashinge, B. Cady, M. Pagano, and M. Loda. 2000. Role of the Cdc25A phosphatase in human breast cancer. J. Clin. Investig. 106:753-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, M. S., C. E. Ryan, and H. Piwnica-Worms. 2003. Chk1 kinase negatively regulates mitotic function of Cdc25A phosphatase through 14-3-3 binding. Mol. Cell. Biol. 23:7488-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, S. J., W. Yuan, Y. Mori, A. Levenson, M. Trojanowska, and J. Varga. 1999. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-beta: involvement of Smad 3. J. Investig. Dermatol. 112:49-57. [DOI] [PubMed] [Google Scholar]
- 10.Datto, M. B., J. P. Frederick, L. Pan, A. J. Borton, Y. Zhuang, and X. F. Wang. 1999. Targeted disruption of Smad3 reveals an essential role in transforming growth factor β-mediated signal transduction. Mol. Cell. Biol. 19:2495-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derynck, R., and Y. E. Zhang. 2003. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577-584. [DOI] [PubMed] [Google Scholar]
- 12.Dixon, D., T. Moyana, and M. J. King. 1998. Elevated expression of the cdc25A protein phosphatase in colon cancer. Exp. Cell Res. 240:236-243. [DOI] [PubMed] [Google Scholar]
- 13.Donzelli, M., L. Busino, M. Chiesa, D. Ganoth, A. Hershko, and G. F. Draetta. 2004. Hierarchical order of phosphorylation events commits Cdc25A to βTrCP-dependent degradation. Cell Cycle 3:469-471. [PubMed] [Google Scholar]
- 14.Donzelli, M., and G. F. Draetta. 2003. Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep. 4:671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donzelli, M., M. Squatrito, D. Ganoth, A. Hershko, M. Pagano, and G. F. Draetta. 2002. Dual mode of degradation of Cdc25 A phosphatase. EMBO J. 21:4875-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draetta, G., and J. Eckstein. 1997. Cdc25 protein phosphatases in cell proliferation. Biochim. Biophys. Acta 1332:M53-M63. [DOI] [PubMed] [Google Scholar]
- 17.Foster, J. S., D. C. Henley, A. Bukovsky, P. Seth, and J. Wimalasena. 2001. Multifaceted regulation of cell cycle progression by estrogen: regulation of Cdk inhibitors and Cdc25A independent of cyclin D1-Cdk4 function. Mol. Cell. Biol. 21:794-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuhrmann, G., C. Leisser, G. Rosenberger, M. Grusch, S. Huettenbrenner, T. Halama, I. Mosberger, S. Sasgary, C. Cerni, and G. Krupitza. 2001. Cdc25A phosphatase suppresses apoptosis induced by serum deprivation. Oncogene 20:4542-4553. [DOI] [PubMed] [Google Scholar]
- 19.Fukuchi, M., T. Imamura, T. Chiba, T. Ebisawa, M. Kawabata, K. Tanaka, and K. Miyazono. 2001. Ligand-dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol. Biol. Cell 12:1431-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galaktionov, K., and D. Beach. 1991. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell 67:1181-1194. [DOI] [PubMed] [Google Scholar]
- 21.Galaktionov, K., A. K. Lee, J. Eckstein, G. Draetta, J. Meckler, M. Loda, and D. Beach. 1995. CDC25 phosphatases as potential human oncogenes. Science 269:1575-1577. [DOI] [PubMed] [Google Scholar]
- 22.Gasparotto, D., R. Maestro, S. Piccinin, T. Vukosavljevic, L. Barzan, S. Sulfaro, and M. Boiocchi. 1997. Overexpression of CDC25A and CDC25B in head and neck cancers. Cancer Res. 57:2366-2368. [PubMed] [Google Scholar]
- 23.Guardavaccaro, D., Y. Kudo, J. Boulaire, M. Barchi, L. Busino, M. Donzelli, F. Margottin-Goguet, P. K. Jackson, L. Yamasaki, and M. Pagano. 2003. Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev. Cell 4:799-812. [DOI] [PubMed] [Google Scholar]
- 24.Han, S. U., H. T. Kim, D. H. Seong, Y. S. Kim, Y. S. Park, Y. J. Bang, H. K. Yang, and S. J. Kim. 2004. Loss of the Smad3 expression increases susceptibility to tumorigenicity in human gastric cancer. Oncogene 23:1333-1341. [DOI] [PubMed] [Google Scholar]
- 25.Hassepass, I., R. Voit, and I. Hoffmann. 2003. Phosphorylation at serine 75 is required for UV-mediated degradation of human Cdc25A phosphatase at the S-phase checkpoint. J. Biol. Chem. 278:29824-29829. [DOI] [PubMed] [Google Scholar]
- 26.Heldin, C. H., K. Miyazono, and P. ten Dijke. 1997. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390:465-471. [DOI] [PubMed] [Google Scholar]
- 27.Hsu, J. Y., J. D. Reimann, C. S. Sorensen, J. Lukas, and P. K. Jackson. 2002. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat. Cell Biol. 4:358-366. [DOI] [PubMed] [Google Scholar]
- 28.Hu, X., and K. S. Zuckerman. 2001. Transforming growth factor: signal transduction pathways, cell cycle mediation, and effects on hematopoiesis. J. Hematother. Stem Cell Res. 10:67-74. [DOI] [PubMed] [Google Scholar]
- 29.Iavarone, A., and J. Massague. 1997. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature 387:417-422. [DOI] [PubMed] [Google Scholar]
- 30.Iavarone, A., and J. Massague. 1999. E2F and histone deacetylase mediate transforming growth factor β repression of cdc25A during keratinocyte cell cycle arrest. Mol. Cell. Biol. 19:916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito, Y., H. Yoshida, F. Matsuzuka, N. Matsuura, Y. Nakamura, H. Nakamine, K. Kakudo, K. Kuma, and A. Miyauchi. 2004. Cdc25A and cdc25B expression in malignant lymphoma of the thyroid: correlation with histological subtypes and cell proliferation. Int. J. Mol. Med. 13:431-435. [PubMed] [Google Scholar]
- 32.Jin, J., T. Shirogane, L. Xu, G. Nalepa, J. Qin, S. J. Elledge, and J. W. Harper. 2003. SCFβ-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 17:3062-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jinno, S., K. Suto, A. Nagata, M. Igarashi, Y. Kanaoka, H. Nojima, and H. Okayama. 1994. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 13:1549-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, S. J., and J. Letterio. 2003. Transforming growth factor-beta signaling in normal and malignant hematopoiesis. Leukemia 17:1731-1737. [DOI] [PubMed] [Google Scholar]
- 35.Leisser, C., G. Fuhrmann, G. Rosenberger, M. Grusch, T. Halama, S. Sasgary, C. Cerni, and G. Krupitza. 2001. Cdc25a mediates survival by activating akt kinase. Sci. World J. 1:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leisser, C., G. Rosenberger, S. Maier, G. Fuhrmann, M. Grusch, S. Strasser, S. Huettenbrenner, S. Fassl, D. Polgar, S. Krieger, C. Cerni, R. Hofer-Warbinek, R. DeMartin, and G. Krupitza. 2004. Subcellular localisation of Cdc25A determines cell fate. Cell Death Differ. 11:80-89. [DOI] [PubMed] [Google Scholar]
- 37.Liu, X., A. E. Elia, S. F. Law, E. A. Golemis, J. Farley, and T. Wang. 2000. A novel ability of Smad3 to regulate proteasomal degradation of a Cas family member HEF1. EMBO J. 19:6759-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loffler, H., R. G. Syljuasen, J. Bartkova, J. Worm, J. Lukas, and J. Bartek. 2003. Distinct modes of deregulation of the proto-oncogenic Cdc25A phosphatase in human breast cancer cell lines. Oncogene 22:8063-8071. [DOI] [PubMed] [Google Scholar]
- 39.Matsuura, I., N. G. Denissova, G. Wang, D. He, J. Long, and F. Liu. 2004. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 430:226-231. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson, I., and I. Hoffmann. 2000. Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res. 4:107-114. [DOI] [PubMed] [Google Scholar]
- 41.Nishioka, K., Y. Doki, H. Shiozaki, H. Yamamoto, S. Tamura, T. Yasuda, Y. Fujiwara, M. Yano, H. Miyata, K. Kishi, H. Nakagawa, A. Shamma, and M. Monden. 2001. Clinical significance of CDC25A and CDC25B expression in squamous cell carcinomas of the oesophagus. Br. J. Cancer 85:412-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nourry, C., L. Maksumova, M. Pang, X. Liu, and T. Wang. 2004. Direct interaction between Smad3, APC10, CDH1 and HEF1 in proteasomal degradation of HEF1. BMC Cell Biol. 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagano, M., and R. Benmaamar. 2003. When protein destruction runs amok, malignancy is on the loose. Cancer Cell 4:251-256. [DOI] [PubMed] [Google Scholar]
- 44.Reimann, J. D., E. Freed, J. Y. Hsu, E. R. Kramer, J. M. Peters, and P. K. Jackson. 2001. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105:645-655. [DOI] [PubMed] [Google Scholar]
- 45.Sandhu, C., J. Donovan, N. Bhattacharya, M. Stampfer, P. Worland, and J. Slingerland. 2000. Reduction of Cdc25A contributes to cyclin E1-Cdk2 inhibition at senescence in human mammary epithelial cells. Oncogene 19:5314-5323. [DOI] [PubMed] [Google Scholar]
- 46.Shi, Y., and J. Massague. 2003. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685-700. [DOI] [PubMed] [Google Scholar]
- 47.Shimuta, K., N. Nakajo, K. Uto, Y. Hayano, K. Okazaki, and N. Sagata. 2002. Chk1 is activated transiently and targets Cdc25A for degradation at the Xenopus midblastula transition. EMBO J. 21:3694-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stroschein, S. L., S. Bonni, J. L. Wrana, and K. Luo. 2001. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev. 15:2822-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsutsui, T., B. Hesabi, D. S. Moons, P. P. Pandolfi, K. S. Hansel, A. Koff, and H. Kiyokawa. 1999. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27Kip1 activity. Mol. Cell. Biol. 19:7011-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vigo, E., H. Muller, E. Prosperini, G. Hateboer, P. Cartwright, M. C. Moroni, and K. Helin. 1999. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol. Cell. Biol. 19:6379-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan, M., Y. Tang, E. M. Tytler, C. Lu, B. Jin, S. M. Vickers, L. Yang, X. Shi, and X. Cao. 2004. Smad4 protein stability is regulated by ubiquitin ligase SCF beta-TrCP1. J. Biol. Chem. 279:14484-14487. [DOI] [PubMed] [Google Scholar]
- 52.Wolfraim, L. A., T. M. Fernandez, M. Mamura, W. L. Fuller, R. Kumar, D. E. Cole, S. Byfield, A. Felici, K. C. Flanders, T. M. Walz, A. B. Roberts, P. D. Aplan, F. M. Balis, and J. J. Letterio. 2004. Loss of Smad3 in acute T-cell lymphoblastic leukemia. N. Engl. J. Med. 351:552-559. [DOI] [PubMed] [Google Scholar]
- 53.Wu, W., Y. H. Fan, B. L. Kemp, G. Walsh, and L. Mao. 1998. Overexpression of cdc25A and cdc25B is frequent in primary non-small cell lung cancer but is not associated with overexpression of c-myc. Cancer Res. 58:4082-4085. [PubMed] [Google Scholar]
- 54.Yang, X., J. J. Letterio, R. J. Lechleider, L. Chen, R. Hayman, H. Gu, A. B. Roberts, and C. Deng. 1999. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 18:1280-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao, H., J. L. Watkins, and H. Piwnica-Worms. 2002. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc. Natl. Acad. Sci. USA 99:14795-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu, Y., J. A. Richardson, L. F. Parada, and J. M. Graff. 1998. Smad3 mutant mice develop metastatic colorectal cancer. Cell 94:703-714. [DOI] [PubMed] [Google Scholar]
- 57.Zou, X., D. Ray, A. Aziyu, K. Christov, A. D. Boiko, A. V. Gudkov, and H. Kiyokawa. 2002. Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence. Genes Dev. 16:2923-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zou, X., T. Tsutsui, D. Ray, J. F. Blomquist, H. Ichijo, D. S. Ucker, and H. Kiyokawa. 2001. The cell cycle-regulatory CDC25A phosphatase inhibits apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21:4818-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]