Abstract
Abelmoschus manihot (A.manihot) is a herbaceous flowering medicinal plant and flavonoids are its main pharmacological active ingredients. A.manihot is listed in the 2020 edition of the Chinese Pharmacopoeia for the treatment of chronic kidney disease (CKD). A.manihot significantly reduces proteinuria in CKD, and the effectiveness and safety of A.manihot in the treatment including primary glomerulonephropathy and diabetic kidney disease (DKD) have been proved by several randomized controlled trials (RCT). Emerging pharmacological studies have explored the potential active small molecules and the underlying mechanisms in A.manihot. The active constituents of A.manihot are mainly seven flavonoids, including hibifolin, hyperoside, isoquercetin, rutin, quercetin, myricetin, and quercetin-3-O-robinobioside. The mechanisms of action mainly include alleviating renal fibrosis, reducing the inflammatory response and decreasing the apoptosis of podocytes. In this review, we summarize the updated information of active components and molecular mechanisms of A.manihot on chronic kidney disease.
Keywords: Abelmoschus manihot, Chronic kidney disease, Molecular mechanism, Active components, Diabetic kidney disease
1. Introduction
Abelmoschus manihot is an annual flowering plant of the Malvaceae family, widely distributed throughout the temperate and subtropical regions of Eastern Europe and Asia. During the Northern Song Dynasty, seven Chinese medicine literatures, including Zhang Yuxi, made the earliest record of the medicinal use of A.manihot flowers in “Jiayou Materia Medica. The Compendium of Materia Medica, written by Li Shizhen in the later Ming Dynasty, gave a more systematic introduction to the A.manihot. The book wrote that A.manihot are used for damp-heat suppression, stranguria, and edema; externally, it was used to treat carbuncle and swollen poison, and fire scald. Huangkui capsule is preparation made from the extract of A.manihot. According to the implementation standard of the National Drug Standard of the State Food and Drug Administration, Huangkui capsule (HKC) is composed of 80 % ethanol extract of A.manihot, 17 % calcium hydrogen phosphate composition and 3 % magnesium stearate [1]. In 1999, Chinese State Food and Drug Administration approved huangkui capsule for clinical use in the treatment of chronic kidney diseases, such as chronic glomerulonephritis, diabetic nephropathy, membranous nephropathy, as well as other inflammatory diseases (Z19990040) [2,3]. Amount of CKD patients have undesirable prognosis and progress to end stage renal disease (ESRD) under the guideline such as Renin-angiotensin system (RAS) inhibitors, SGLT2i, nsMRAs, GLP1R antagonists, glucocorticoids, and immunosuppressants. Novel treatments to reverse the progression of chronic kidney disease is an unmet need. Several clinical studies have confirmed the efficacy and safety of A.manihot in treating chronic kidney disease [4,5]. A randomized controlled clinical trial enrolled 417 patients with primary glomerulonephropathy into A.manihot, losartan, and combined treatment groups. Duration of treatment is 24 weeks. The results of the study showed a significant reduction in 24-h proteinuria level in each treatment group (P<0.001), and the reduction in proteinuria level were significantly higher in the combined treatment and A.manihot groups than that in the losartan group (P<0.05). However, the values of change in estimated GFR and serum creatinine from baseline to the end of treatment were not statistically significant among the three groups [2]. In addition, a systematic evaluation and meta-analysis with 850 participants showed that the combination of A.manihot and RAS inhibitors significantly improved proteinuria compared to RAS inhibitors, that A.manihot was generally well tolerated and did not increase the incidence of adverse events [6]. A multicenter randomized, double-blind, parallel-controlled clinical trial with 413 participants showed that the combination of A.manihot and irbesartan apparently reduced the excretion of albuminuria and proteinuria in type 2 diabetic patients. The main results were changed values of ACR from baseline to the end of the 24-week follow-up period in the three groups. The adjusted mean difference in ACR was - 99.52 (95 % CI - 213.64–14.60, P = 0.087) in the A.manihot group and - 197.94 (-311.63 ∼ - 84.25; P < 0.001) in the combined therapy group, compared to the irbesartan group. However, the values of change in estimated glomerular filtration rate, HbA1c, and hs-CRP from baseline to after treatment were no difference on statistics in the treatment groups [7]. In this paper, we summarize the molecular mechanism of A.manihot in treating chronic kidney disease, hoping to provide a new therapeutic strategy for chronic kidney disease.
2. Abelmoschus manihot and its active components
The composition of A.manihot flowers are significantly different from other parts of A.manihot. Most of the amino acids and flavonoids of A.manihot are concentrated on the flowers of A.manihot, and the chemical marker that distinguishes the flowers of A.manihot from other parts in A.manihot included L-serine, L-valine, L-threonine, hyperoside, quercetin-3-O-robinobioside, isoquercetin, hibifolin, quercetin 3′-O-glucoside [8]. Phytochemical studies have found that flavonoids, polysaccharides, amino acids, organic acids, nucleosides, volatile oils, and steroids are the main compositions of A.manihot. Flavonoids are regarded as active ingredients and are officially used as markers for monitoring the quality of herbal medicines and preparations containing A.manihot extract in the 2020 edition of the Chinese Pharmacopoeia [9]. The active ingredients of A.manihot are mainly seven flavonoid glycosides, including hibifolin, hyperoside, isoquercetin, rutin, quercetin, myricetin, and quercetin-3-O-robinobioside [10]. These chemical constituents and their molecular structure are represented in Table 1.
Table 1.
Chemical structure of the main active components of A.manihot.
| Active components | Chemical structure |
|---|---|
| Hyperoside | 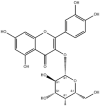 |
| Myricetin | 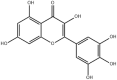 |
| Quercetin | 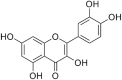 |
| Rutin | 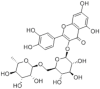 |
| Isoquercetin | 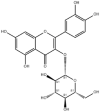 |
| Hibifolin | 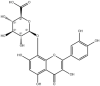 |
| quercetin-3-O-robinobioside | 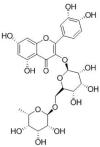 |
3. Pharmacological mechanisms of A.manihot in different chronic kidney diseases
3.1. Diabetic kidney disease
Diabetic kidney disease (DKD) is a common chronic kidney disease clinically characterized by proteinuria and decreased glomerular filtration rate. Early renal pathological changes of DKD include glomerular hypertrophy, mesangial matrix expansion, and basement membrane thickening; advanced DKD manifests with glomerular nodular sclerosis, mesangial lysis, and tubulointerstitial fibrosis [11].
HKC are widely used in clinical practice to treat early diabetic nephropathy. It has also shown protective effects against kidney disease in preclinical models. HKC reduced the expression of inflammatory genes and improved lipid metabolism in the kidney of DKD rats by activating the transcriptional activity of PPARα and PPARγ. In addition, HKC reduced endoplasmic reticulum (ER) stress and c-Jun NH2-terminal kinase activation in the kidney of DKD rats to improve renal injury [12]. Recent studies have shown that HKC can reduce proteinuria and alleviate early glomerular lesions, including glomerular hypertrophy, glomerular basement membrane (GBM) thickening, and mild mesangial matrix expansion in DKD rats, probably by inhibiting Akt/mTOR/p70S6K signaling in vitro and in vivo [13]. A recent study showed that 2 g/kg/d of HKC significantly reduced renal tubular EMT in DKD rats by inhibiting NLRP3 inflammasome activation and TLR4/NF-κB signaling in the kidney [14]. In addition, total flavones of Abelmoschus manihot (TFA) inhibited the expression of NIMA-related kinase 3, NLRP3, ASC, cystein-7 and PTEN, and increased the expression of p-PI3K and p-Akt in MPC-5 cells under high glucose, and further mechanism exploration confirms that TFA may alleviate podocyte pyroptosis and injury by targeting m6A modification-mediated NLRP3-inflammasome activation and PTEN/PI3K/Akt signaling [15].
Not only can A.manihot improve DKD from different aspects, but also the main active ingredients of A.manihot alone can delay the progress of DKD. Hyperoside can reduce fasting blood glucose (FBG) levels and improve glomerular mesangial matrix dilation and hyperlipidemia in dbdb mice. Pre-treatment with hyperoside reduces the expression of MCP-1, iNOS, and TNF-α and increases the expression of anti-inflammatory cytokines Arg-1 and CD163/CD206 surface molecules both in vitro and in vivo. In addition, hyperoside pretreatment inhibits the proliferation of CD4+ T cells. It also promotes CD4+ T cells differentiation into CD4+Foxp3+ Treg and CD4+IL-4+ Th2 cells, indicating that hyperoside inhibits renal inflammation in db/db mice by promoting macrophage transition to anti-inflammatory M2 phenotype and regulating the balance of Th1/Th2 and Th17/Treg [16]. In vitro studies have shown that hyperoside inhibits HG-induced HK-2 cell apoptosis and inflammatory response by inducing upregulation of miR-499a-5p [17]. In addition, hyperoside can inhibit the expression of the heparin enzyme in podocytes caused by high glucose and reactive oxygen species (ROS) and restore the Heparan sulfate content in the GBM of diabetic nephropathy (DN) mice, thereby reducing proteinuria in DN mice [18]. In vitro, studies have shown that hyperoside inhibits the proliferation of mouse mesangial cell lines (SV40-MES13) induced by high glucose by inhibiting the activation of the ERK pathway and phosphorylation of downstream transcription factor CREB and expression of miRNA-34a [19]. Hyperoside inhibits monocyte adhesion, cell adhesion molecule (CAM) expression, ROS formation, and NF-κB activation, thus alleviates HG-induced vascular inflammation [20]. Hyperoside also restrains apoptosis in diabetes models. Hyperoside treatment reduced the expression of caspase-3, caspase, Bax, and other apoptotic proteins in the retinal vascular endothelial cells (RVECs) of diabetes rats. It increased the expression of antiapoptotic protein Bcl-2 to protect against diabetes retinopathy [21].
Both oxidative stress and inflammation lead to TGF-β/Smad pathway activation and fibrosis, and in a streptozotocin (STZ)-induced diabetic mouse model, myricetin inhibited oxidative stress by increasing Nrf2 and suppressed inflammation by inhibiting IκBα phosphorylation and degradation, suggesting that myricetin may inhibit fibrosis by suppressing the TGF-β/Smad pathway [22]. Myricetin protects human glomerular endothelial cells (HUVECs) from high-glucose (HG)-induced oxidative stress by decreasing cleaved cystein-3 protein expression, increasing total cellular antioxidant capacity (TAC), and declining Bax/Bcl-2 protein ratio and cystein-3 expression [23]. Myricetin treatment also inhibited the expression of HG-induced ER stress markers (including P-PERK, P-eIF2α, ATF4, and CHOP) and protected β-cells from HG-induced apoptosis, probably by inhibiting the activity of cyclin-dependent kinase 5 (CDK5) and by increasing the expression of pancreatic duodenal homeobox 1 (PDX1) and sarcoendoplasmic reticulum calcium ATPase (SERCA2b) expression [24].
Quercetin improved renal function in DKD rats by inhibiting the overexpression of TGF-β1 and CTGF in the kidney [25]. Another study showed that 8 weeks of quercetin treatment delayed mesangial cells (MCs) proliferation in glomerular mesangial cells and alleviated renal function in db/db mice, and in vitro also demonstrated that quercetin effectively inhibited MCs proliferation induced by high glucose, possibly by activating the Hippo pathway [26]. In type 2 diabetic mice, quercetin-treated groups showed significantly lower FBG levels and hepatic and renal marker enzymes, increased the levels of glutathione, SOD, catalase, glutathione-S-transferase and increased the expression of GLUT4, suggesting that quercetin ameliorates hyperglycemia and oxidative stress by attenuating free radical-induced toxicity [27].
Rutin, the active component of A.manihot significantly reduced FBG, serum creatinine (Scr), blood urea nitrogen (BUN), and urinary protein, inhibited mesangial cell proliferation, and decreased glomerular basement membrane thickness in rats with DKD. In addition, rutin significantly inhibited the expression of AGEs, type IV collagen, laminin, TGF-β1, p-Smad 2/3, and CTGF, suggesting that rutin may alleviate DKD progression by inhibiting TGF-β1/Smad/ECM and TGF-β1/CTGF/ECM signaling pathways [28]. In addition, rutin inhibited the RhoA/ROCK signaling pathway by reducing reactive oxygen species mediated by Nrf2 activation, thereby significantly preventing the renal endothelial barrier function in human glomerular endothelial cells disrupted by hyperglycemia [29].
In a diabetic rat model established by STZ, isoquercetin was administered intrathecally to 6-week-old diabetic rats at 10 μM and 30 μM for 3 d. The results showed that isoquercetin significantly improved altered behavioral pain thresholds and functional parameters in diabetic rats both intrathecally (10 and 30 μM) and intraperitoneally (10 mg/kg), through inhibition of the Wnt/β-catenin signaling pathway [30]. In a high-fat diet and STZ-induced type 2 diabetic mouse model, isoquercetin enhanced glucagon-like peptide (GLP-1) and insulin secretion by inhibiting dipeptidyl peptidase IV (DPP-IV), thereby reduced blood glucose and alleviated body weight [31]. In high-glucose-stimulated HUVECs, administration of isoquercetin significantly inhibited the expression of the pro-apoptotic proteins p53, Bax, and cleaved (C)-caspase3. It increased the expression of the anti-apoptotic protein Bcl-2 in HUVECs by inhibiting HG-induced phosphorylation of p53 at Ser15 and increasing nuclear translocation of USP10 [32]. The molecular mechanism of the active ingredients of A. manihot in the treatment of different kinds of chronic kidney diseases are summerized in Table 2.
Table 2.
The molecular mechanism of the active ingredients of A.manihot in the treatment of chronic kidney disease.
| Active ingredient | Molecular mechanism | Experiment | Intervention method |
|---|---|---|---|
| Huangkui Capsules (Tu et al., 2013) | TGF-β1↓, p-p38MAPK↓ | Adriamycin-induced nephropathy in SD rats | HKC (0.5, 2 g/kg/d, i.g.) for 4 weeks |
| Huangkui Capsules (Ge et al., 2016) | PPARα↑, PPARγ↑ | STZ-induced DN in SD rats | HKC (75, 175, 300 mg/kg/d, p.o.) for 12 weeks |
| Huangkui Capsules (Cai et al., 2017) | NOX1↓, NOX2↓, NOX4↓, α-SMA↓, p-ERK↓ | Adenine-induced CRF in SD rat, HG-induced EMT in HK-2 |
HKC (150 mg/kg/d, p.o.) quercetin, isoquercitrin quercetin-3′-O-β-D-glucoside, gossypetin-8-O-β-D-glucuronide, hyperoside (5, 10, 25, 50, 100 μM) |
| Huangkui Capsules (Wu et al., 2018) | p-Akt↓, p-mTOR↓, p-p70S6K↓ | STZ + HFD-induced DN in SD rats | HKC (2 g/kg/d, p.o.) for 4 weeks |
| Huangkui Capsules (Han et al., 2019) | NLRP3↓, TLR4↓, p-IKK↓, p65↓ | STZ-induced DN in SD rats | HKC (2 g/kg/d, p.o.) for 4 weeks |
| Huangkui Capsules (Gu et al., 2020) | TRPC6↓ | UUO in C57BL/6 mice | HKC (0.15, 0.5, 1.5 g/kg, i.g.) for 7d |
| Huangkui Capsules (Zhao et al., 2023) | Nephrin↓, Podocin↓, ZO-1↓, p-JAK2↓, p-STAT3↓, p-PI3K↓, p-Akt↓ | Doxorubicin (DOX) induced CKD in mice | HKC (0.15, 0.5, 1.5 g/kg, p.o.) |
| Total flavonoids(Li et al., 2019) | NLRP3 inflammasome↓, ERK1/2↓, ROS↓ | Adriamycin-induced nephropathy in SD rats, Adriamycin-induced injury in NRK-52E |
TFA (1.5 g/kg/d, p.o.) for 14d, TFA (100 μg/mL) for 24h |
| Total flavonoids(Tu et al., 2020) | IL1β↓, TNF-α↓, NF-κB ↓, AMPK↑, SIRT1↑ |
Uninephrectomy, potassium oxonate, and proinflammatory diet induced CRF in SD rats, LPS in RAW 264.7 cells |
HKC (2 g/kg/d, p.o.) for 10weeks, TFA (20 μg/mL) for 24h |
| Total flavonoids (Liu et al., 2021) | NEK7↓, NLRP3↓, ASC↓, caspase-1↓, p-PI3K↑, p-Akt↑ | HG in MPC-5 cells | TFA (20 μg/ml) |
| Hyperoside(Ku et al., 2014) | ICAM-1, VCAM-1↓, ROS↓, NF-κB↓ | HG-induced vascular inflammation in HUVECs | Hyperoside (25 mM) for 24 h |
| Hyperoside(An et al., 2017) | HS contents↑, heparanase↓ | STZ-induced diabetic in C57BL6 mice | Hyperoside (10, 30 mg/kg/d, p.o.) for 4weeks |
| Hyperoside(Liu et al., 2018) | p-AMPK↓, p-ULK1↓ | D- gal induced aging and injury in SD rats, NRK-52E cells exposed to D-gal |
Hyperoside (20, 30 mg/kg/d, p.o.) for 4weeks (5, 10 μg/mL) for 24h |
| Hyperoside (Zhang et al., 2020) | p-ERK↓, p-CREB↓, miRNA-34a↓ | HG-induced cell proliferation in SV40-MES13 cells | Hyperoside (200 μM) for 48h |
| Hyperoside (Wu et al., 2020) | Caspase-3↓, caspase↓, Bax↓, Bcl-2↑ | HG in RVECs | Hyperoside (10 μg/mL) for 72 h |
| Hyperoside (Zhou et al., 2021) | miR-499a-5p↑ | HG-induced apoptosis in HK-2 cells | Hyperoside (50 nM) |
| Hyperoside(Liu et al., 2021) | MCP-1↓, TNF-α↓, iNOS↓Arg-1↑, CD163/CD206↑ | db/db mice, HG in BMDMs |
Hyperoside (40, 80mg/kg/d, p.o) for 12weeks, (12.5, 25, 50 μM) |
| Myricetin (Yang et al., 2019) | Nrf2↓, HO-1↓, p-IκB↓, p-p65↓ | STZ-induced diabetes mellitus in C57BL/6mice | Myricetin (100 mg/kg/d, p.o.) for 6months |
| Myricetin (Karunakaran et al., 2019) | P-PERK↓, P-eIF2α↓, ATF4↓, CHOP↓, GRP78↑, CDK5↓, PDX1↑, SERCA2b↑ | HG-induced apoptosis in INS-1 cells | Myricetin (20 μM) for 7h |
| Myricetin (Aminzadeh et al., 2020) | Bax/Bcl-2↓, caspase-3↓ | HG-induced oxidative stress in HUVECs | Myricetin (0.5, 1 μM) for 24h |
| Quercetin (Lai et al., 2012) | TGF-β1↓, CTGF↓ | STZ-induced diabetic in SD rats | Quercetin (50 mg/kg/d, p.o.) for 12weeks |
| Quercetin (Alam et al., 2014) | Glutathione↑, SOD↑, catalase↑, glutathione-S-transferase↑, GLUT4↑ | Alloxan-induced diabetic in Swiss albino mice | Quercetin (20 mg/kg/d, p.o.) for 3weeks |
| Quercetin (Lu et al., 2018) | iNOS↓, IL-12↓, F4/80 + /CD11b + /CD86 +↓, NF-κB p65↓, IRF5↓ |
UUO in ICR/JCL mice, LPS in RAW264.7 |
Quercetin (20 mg/kg/d, p.o.) for 3d (10, 100 μM/mL) for 24h |
| Quercetin (Dos et al., 2018) | IL-6↓, TNF-α↓, TGF-β1↓, Bax↓, TBARS↓, CAT↑, SOD1↑ |
Pristane-induced LN mice | Quercetin (50 mg/kg/d, p.o.) for 5months |
| Quercetin (Cao et al., 2018) | miR-21↓, PTEN↑, TIMP3↑ | TGF-β-induced fibrosis in HK-2 cell | Quercetin (7.5, 15, 30 mg/mL) for 72h |
| Quercetin (Liu et al., 2019) | TGF-β1↓, TGFBR1/2↓, Smad2/3↓, Smad4↓, GSK-3β↓, β-catenin, α-SMA↓, FSP-1, Smad7↑ | Uninephrectomy of the left kidney induced GS in SD rats | Quercetin (25, 50, 100 mg/kg/d, p.o.) for 8weeks |
| Quercetin (Lei et al., 2019) | p- MST1↑, p-Lats1↑, nuclear YAP↓ | C57BL/KSJ db/db mice, HG-treated mouse mesangial cells |
Quercetin (50, 100, 150 mg/kg/d) for 8 weeks, (10, 20, 40 μM) for 24h |
| Quercetin (Wang et al., 2020) | IL-1β↓, IL-6↓, TNF-α↓, ATF3↓ | AKI in C57BL/6J mice, NRK-52E cells and HK-2 cells |
Quercetin (25 mg/kg, 3times/day, i.p.) for 1d, (10 μM) for 24h |
| Quercetin (Liu et al., 2020) | SIRT1↑, PINK1↑, Parkin↑ | UUO in SD rats, AngII in NRK-52E |
Quercetin (20 mg/kg/d, p.o) for 14d (20 μM) for 2h |
| Quercetin (Tan et al., 2020) | Mincle↓, p-Syk↓, NF-κB↓ | AKI in C57BL/6 mice | Quercetin (50, 100 mg/kg/d, p.o) for 3d |
| Rutin (Korkmaz et al., 2010) | ROS↓, MDA↓, GSH↓, MnSOD↑ | Right nephrectomy + ischemia/reperfusion in Wistar rats | Rutin (1 g/kg i.p.) |
| Rutin (Hao et al., 2012) | p-Smad 7↓, AGEs↓, collagen IV↓, laminin↓, TGF-β (1) ↓, p-Smad 2/3↓, CTGF↓ | STZ-induced diabetic in SD rats | Rutin (10, 30, 90 mg/kg/d, p.o) for 10weeks |
| Rutin (Han et al., 2015) | TGF-β1↓, p-smad2↓ | 5/6 nephrectomy in Wistar rats | Rutin (15, 45 mg/kg) for 20weeks |
| Rutin (Wang et al., 2016) | ROS↓, RhoA↓, ROCK↓, Nrf2↑ | HG-induced barrier dysfunction in HRGECs | Rutin (25 μM) for 24h |
| Rutin (Qu et al., 2019) | Nrf2↑, HO-1↑, NF-κB↓ | Vancomycin-induced nephrotoxicity in Wistar rats | Rutin (150 mg/kg, p.o.) for 7d |
| Rutin (Qu et al., 2019) | caspase‐3/7↓, caspase‐9↓, ROS ↓, SOD↑, CAT↑ | Vancomycin-induced apoptosis in LLC-PK1 cells | Rutin (5, 10, 20 μM) for 2h |
| Isoquercetin (Zhang et al., 2018) | Glucagon-like peptide (GLP)-1↑, dipeptidyl peptidase IV (DPP-IV) ↓ |
High-fat diet and STZ-induced type 2 diabetic in Chinese Kunming mice | Isoquercetin (20, 40, 80 mg/kg/d, p.o.) for 8weeks |
| Isoquercetin (Resham et al., 2020) | c-myc↓, MMP2↓, β-catenin↓ | STZ-induced diabetic SD rats | Isoquercetin (10, 30 μM) for 3d |
| Isoquercetin (Wang et al., 2020) | IL-6↓, TNF-α↓, COX-2↓, p-ERK↓, cleaved caspase-3/total caspase-3↓ | Cisplatin-induced nephrotoxicity in C57BL/6J mice, Cisplatin-induced cell injury in mPTCs and HK2 cells |
Isoquercetin (50 mg/kg/day, p.o) for 4d (80 μM for mPTCs and 120 μM for HK2) for 2 h |
| Isoquercetin (Liu et al., 2021) | p53↓, Bax↓, C-caspase3↓, Bcl-2↑ | HG-induced apoptosis in HUVECs | Isoquercetin (100 μM) for 24 h |
3.2. Renal fibrosis
A.manihot ameliorates kidney fibrosis in chronic renal failure (CRF) and unilateral ureteral obstruction (UUO) models. In the adenine-induced rat CRF model, treatment with HKC significantly reduced Scr, BUN, NADPH oxidase 1, NADPH oxidase 2 and NADPH oxidase 4, α-smooth muscle actin (α-SMA) and phosphorylated-extracellular signal-regulated kinase (p-ERK1/2). In vitro, the main active components of A.manihot including quercetin, hyperoside, isoquercetin, hibifolin, and quercetin-3′-O-glucoside inhibited EMT in HG-induced HK-2 cells.The results showed that these five active ingredients could significantly inhibit the expression of NADPH oxidase 1, NADPH oxidase 2, NADPH oxidase 4, α-SMA and p-ERK1/2 under high glucose, indicating that HKC and their active ingredients protect CRF tubular interstitial fibrosis by inhibiting the NADPH oxidase/ROS/ERK pathway [33]. A CRF rat model was established by right nephrectomy, potassium oxonate, and proinflammatory diet in another study. Total flavones of Abelmoschus manihot inhibited IL1β, TNF-α, and NF-κB protein expression in CRF rats. In vitro, treatment of RAW 264.7 cells exposed to LPS with TFA indicates that TFA promotes macrophage polarized into M2 functional phenotype, and further studies show that it may regulate autophagy-mediated macrophage polarization by inhibiting the AMPK-SIRT1 pathway to attenuate the inflammation of CRF rats [34]. HKC reduces renal tubular interstitial injury scores in a dose-dependent manner in UUO mice, reduces protein expression of the renal fibrosis marker α-SMA and increases expression of the anti-fibrotic protein E-calcine mucin in a dose-dependent way. HKC significantly inhibits UUO-induced expression of TRPC6, CnA, and NFAT, implying that HKC may prevent renal fibrosis by inhibiting the TRPC6/CnA/NFAT signaling pathway [35].
In animal models of kidney disease, the active components of A.manihot can also alleviate renal fibrosis. Quercetin reduces Scr and BUN levels, inflammatory cell infiltration, and inflammatory factor expression in UUO mice and inhibits the expression of TGF-β1R, p-Smad2, p-Smad3, and Smad2/3 in kidney tissues of UUO mice. The possible molecular mechanism is that quercetin inhibits TLR4/MyD88 signaling pathway, downregulates NF-κB and IRF5 activity, inhibits M1 macrophage polarization, reduces renal inflammatory injury, and inhibits the polarization of M2 macrophages to reduce renal fibrosis [36]. It has been shown that quercetin can improve Scr and BUN, reduce proteinuria, improve lipid metabolism, and alleviate pathological changes such as basement membrane widening and increased mesangial matrix in glomerulosclerosis (GS) rats. Further studies showed that quercetin down-regulated transforming growth factor (TGF)-β signaling proteins (TGF-β1, TGFBR1/2, Smad2/3, Smad4, GSK-3β, and β-catenin) and EMT proteins (α-SMA and FSP-1) as well as upregulated proteins such as Smad7, suggesting that quercetin may protect the kidney by inhibiting the TGF-β signaling pathway [37]. Consistent with the in vivo findings, quercetin treatment reduced TGF-β-induced expression of collagen I, fibronectin, α-SMA, and E-calcineurin and increased expression of the anti-fibrotic factors PTEN and TIMP3 in human renal tubular epithelial HK-2 cells, possibly by inhibiting miR-21 to upregulate anti-fibrotic gene expression and thus inhibit renal fibrosis [38]. In addition, quercetin may alleviate renal fibrosis by activating mitosis and reducing renal tubular epithelial cell(RTEC)senescence through a possible mechanism of activating SIRT1/PINK1/Parkin-mediated mitochondrial autophagy [39].
3.3. Other kidney diseases
The Adriamycin nephropathy (ADRN) model mainly presents with proteinuria and loss of renal function, and it has been widely used in laboratory studies to simulate CKD in humans [40]. A study demonstrated that 2 g/kg/d of HKC improved proteinuria and glomerulosclerosis in ADRN rats, significantly reducing the infiltration of ED1+ and ED3+ macrophages in the glomeruli and the expression of renal TNF-α protein, and inhibiting the activity of p38MAPK signaling pathway [41]. Similarly, continuous 5-week treatment with HKC can improve renal function in mice with doxorubicin-induced nephropathy and reduce the expression of podocyte damage markers nephrin and podocin. In vitro, results showed that the active component of HKC, hibifolin, can significantly inhibit the expressions of nephrin, Podocin, and ZO-1 proteins in the mouse podocyte clone 5 (MPC-5) injury model. Mechanism exploration suggests that HKC may inhibit the protection of podocytes through the JAK2/STAT3 and PI3K/Akt pathways [42]. Interestingly, recent studies have confirmed that the total extract of A.manihot can also protect renal tubular cells from doxorubicin-induced renal tubular injury by inhibiting ROS-ERK1/2-NLRP3 inflammasomes [43].
Similarly, the active ingredients of A.manihot can also exert therapeutic effects in other kidney diseases. Lupus nephritis (LN) is a common and severe manifestation of systemic lupus erythematosus. The ultimate goal of LN treatment is to prevent nephron loss and prevent CKD [44]. Quercetin can also reduce proteinuria levels in LN mice and significantly reduce IL-6, TNF-a, and TGF- β1. The tissue expression of Bax and TBARS indicates that quercetin can reduce inflammatory cell infiltration, oxidative stress, apoptosis, and fibrosis in LN mice [45]. Pretreatment of porcine renal tubular LLC-PK1 cells with 2,10 and 20 μM rutin for 24 h protects cells from vancomycin-induced cysteinase activation, mitochondrial membrane depolarization, and subsequent apoptosis [46],Consistent with the in vivo findings, rutin achieves renal recovery from vancomycin (VCM) injury by increasing Nrf2 and HO-1 and reducing NF-κB expression and isoquercetin reduced cisplatin-induced nephrotoxicity by ameliorating renal injury by improving oxidative stress, inflammation, and apoptosis [47,48]. In addition, hyperoside attenuates D-gal-induced renal senescence and injury by inhibiting AMPK-ULK1 signaling-mediated autophagy [49].
Acute kidney injury (AKI) is with high morbidity, mortality and also a significant cause of CKD. Studies have shown that quercetin ameliorates AKI by inhibiting ferroptosis and reducing macrophage infiltration by inhibiting ATF3 expression or by reducing macrophage inflammatory factor expression by inhibiting Mincle and its downstream signaling [50,51]. Similarly, rutin attenuated renal injury in rats with renal ischemia-reperfusion, and the mechanism may be related to the antioxidant activity of rutin [52]. In addition, rutin treatment in 5/6 nephrectomy rats significantly increased the mean activity of antioxidant enzymes in the model group, thereby reducing renal damage [53].
4. Discussion
As a significant traditional medicine, A.manihot has been applied in the treatment of chronic kidney diseases in clinical practice. A large number of trials have verified the efficacy and safety of A.manihot among patients with IgA nephropathy, diabetic nephropathy and other CKD. Also animal studies have shown that A.manihot and its active ingredients protected the kidney function in multiple models of CKD, CRF and AKI. Its therapeutic efficacy is mainly exerted by relieving inflammatory response, decreasing oxidative stress and ameliorating renal fibrosis, etc. In conclusion, these studies exemplify the medicinal value and medicinal prospects of A.manihot. However, there are some shortcomings that need further improvement and research, mainly including the following aspects. (1) A.manihot has been widely used in clinical practice in the form of huangkui capsule, but studies involving the use of its active ingredient alone are still at the animal level, and whether it has the same therapeutic effect on human beings is still unknown. Therefore, large-sample clinical trials are needed to obtain high-quality clinical evidence, uncover the mechanism of action of each active ingredient, and identify more specific active ingredients for further development and optimization to ensure their safety and efficacy in clinical applications. (2) The current clinical dose of HKC (7.5 g/d) is relatively high, which may affect the compliance of some patients. Therefore, there is a need to develop more advanced and efficient purification techniques to purify and extract flavonoids to ensure that the same therapeutic effect can be achieved with a reduced dose. In addition, more systematic studies should be carried out to combine different active ingredients, study the interactions and patterns of action of these chemical components on renal diseases, and screen for low-dose combinations of ingredients with fewer adverse effects and better therapeutic efficacy. (3) Liver and kidney are the main organs for drug metabolism, although clinical studies have shown that A.manihot treatment of kidney diseases hardly occurs serious adverse events such as hepatorenal toxicity, and has a certain degree of safety. However, there are fewer toxicity studies on A.manihot and its main active ingredients, so it is necessary to carry out pharmacokinetic studies on A.manihot and its active ingredients before developing drug reagents to ensure the effectiveness and safety of the developed drugs.
Funding
This work was supported by the National Natural Science Foundation of China (No. 32141005, 82274327) and the National Key Technology R&D Program (No.2022YFC3602005 and No. 2021YFC3002203)
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Cuiting Wei: Writing – original draft. Chao Wang: Data curation. Run Li: Data curation. Yunfeng Bai: Investigation. Xue Wang: Data curation. Qingyun Fang: Writing – review & editing. Xiangmei Chen: Supervision. Ping Li: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Xiangmei Chen, Email: xmchen301@126.com.
Ping Li, Email: liping.8@163.com.
References
- 1.Li N., et al. Chemical constituents, clinical efficacy and molecular mechanisms of the ethanol extract of Abelmoschus manihot flowers in treatment of kidney diseases. Phytother Res. 2021;35(1):198–206. doi: 10.1002/ptr.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L., et al. Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. Am. J. Kidney Dis. 2014;64(1):57–65. doi: 10.1053/j.ajkd.2014.01.431. [DOI] [PubMed] [Google Scholar]
- 3.Du L.Y., et al. Comparative characterization of nucleotides, nucleosides and nucleobases in Abelmoschus manihot roots, stems, leaves and flowers during different growth periods by UPLC-TQ-MS/MS. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2015;1006:130–137. doi: 10.1016/j.jchromb.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Li P., et al. Efficacy and safety of Abelmoschus manihot for IgA nephropathy: a multicenter randomized clinical trial. Phytomedicine. 2020;76 doi: 10.1016/j.phymed.2020.153231. [DOI] [PubMed] [Google Scholar]
- 5.Sun X., et al. Efficacy and safety of Abelmoschus manihot in treating chronic kidney diseases: a multicentre, open-label and single-arm clinical trial. Phytomedicine. 2022;99 doi: 10.1016/j.phymed.2022.154011. [DOI] [PubMed] [Google Scholar]
- 6.Jia Q., et al. The effectiveness and safety of Abelmoschus manihot in treating IgA nephropathy: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/9730753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J., et al. Efficacy of combined Abelmoschus manihot and irbesartan for reduction of albuminuria in patients with type 2 diabetes and diabetic kidney disease: a multicenter randomized double-blind parallel controlled clinical trial. Diabetes Care. 2022;45(7):e113–e115. doi: 10.2337/dc22-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin S., et al. Comparison of multiple bioactive constituents in the corolla and other parts of Abelmoschus manihot. Molecules. 2021;26(7) doi: 10.3390/molecules26071864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luan F., et al. Traditional uses, chemical constituents, biological properties, clinical settings, and toxicities of Abelmoschus manihot L.: a comprehensive review. Front. Pharmacol. 2020;11:1068. doi: 10.3389/fphar.2020.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo J.M., et al. Metabolite identification strategy of non-targeted metabolomics and its application for the identification of components in Chinese multicomponent medicine Abelmoschus manihot L. Phytomedicine. 2015;22(5):579–587. doi: 10.1016/j.phymed.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Jefferson J.A., Shankland S.J., Pichler R.H. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74(1):22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 12.Ge J., et al. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-α/γ and attenuating endoplasmic reticulum stress in rats. J. Ethnopharmacol. 2016;189:238–249. doi: 10.1016/j.jep.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Wu W., et al. Inhibition of akt/mTOR/p70S6K signaling activity with huangkui capsule alleviates the early glomerular pathological changes in diabetic nephropathy. Front. Pharmacol. 2018;9:443. doi: 10.3389/fphar.2018.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han W., et al. Huangkui capsule alleviates renal tubular epithelial-mesenchymal transition in diabetic nephropathy via inhibiting NLRP3 inflammasome activation and TLR4/NF-κB signaling. Phytomedicine. 2019;57:203–214. doi: 10.1016/j.phymed.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Liu B.H., et al. Total flavones of Abelmoschus manihot ameliorates podocyte pyroptosis and injury in high glucose conditions by targeting METTL3-dependent m(6)A modification-mediated NLRP3-inflammasome activation and PTEN/PI3K/Akt signaling. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.667644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J., et al. Hyperoside suppresses renal inflammation by regulating macrophage polarization in mice with type 2 diabetes mellitus. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.733808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J., et al. Hyperoside protects HK-2 cells against high glucose-induced apoptosis and inflammation via the miR-499a-5p/NRIP1 pathway. Pathol. Oncol. Res. 2021;27 doi: 10.3389/pore.2021.629829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An X., et al. Hyperoside pre-treatment prevents glomerular basement membrane damage in diabetic nephropathy by inhibiting podocyte heparanase expression. Sci. Rep. 2017;7(1):6413. doi: 10.1038/s41598-017-06844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L., et al. Hyperoside alleviates high glucose-induced proliferation of mesangial cells through the inhibition of the ERK/CREB/miRNA-34a signaling pathway. Internet J. Endocrinol. 2020;2020 doi: 10.1155/2020/1361924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ku S.K., et al. Hyperoside inhibits high-glucose-induced vascular inflammation in vitro and in vivo. Inflammation. 2014;37(5):1389–1400. doi: 10.1007/s10753-014-9863-8. [DOI] [PubMed] [Google Scholar]
- 21.Wu W., et al. Hyperoside ameliorates diabetic retinopathy via anti-oxidation, inhibiting cell damage and apoptosis induced by high glucose. Front. Pharmacol. 2020;11:797. doi: 10.3389/fphar.2020.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z.J., et al. Myricetin attenuated diabetes-associated kidney injuries and dysfunction via regulating nuclear factor (erythroid derived 2)-like 2 and nuclear factor-κb signaling. Front. Pharmacol. 2019;10:647. doi: 10.3389/fphar.2019.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aminzadeh A., Bashiri H. Myricetin ameliorates high glucose-induced endothelial dysfunction in human umbilical vein endothelial cells. Cell Biochem. Funct. 2020;38(1):12–20. doi: 10.1002/cbf.3442. [DOI] [PubMed] [Google Scholar]
- 24.Karunakaran U., et al. Myricetin protects against high glucose-induced β-cell apoptosis by attenuating endoplasmic reticulum stress via inactivation of cyclin-dependent kinase 5. Diabetes Metab. J. 2019;43(2):192–205. doi: 10.4093/dmj.2018.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai P.B., Zhang L., Yang L.Y. Quercetin ameliorates diabetic nephropathy by reducing the expressions of transforming growth factor-β1 and connective tissue growth factor in streptozotocin-induced diabetic rats. Ren. Fail. 2012;34(1):83–87. doi: 10.3109/0886022X.2011.623564. [DOI] [PubMed] [Google Scholar]
- 26.Lei D., et al. Quercetin inhibited mesangial cell proliferation of early diabetic nephropathy through the Hippo pathway. Pharmacol. Res. 2019;146 doi: 10.1016/j.phrs.2019.104320. [DOI] [PubMed] [Google Scholar]
- 27.Alam M.M., Meerza D., Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014;109(1):8–14. doi: 10.1016/j.lfs.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Hao H.H., et al. Preventive effects of rutin on the development of experimental diabetic nephropathy in rats. Life Sci. 2012;91(19–20):959–967. doi: 10.1016/j.lfs.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Wang X., et al. Rutin prevents high glucose-induced renal glomerular endothelial hyperpermeability by inhibiting the ROS/rhoa/ROCK signaling pathway. Planta Med. 2016;82(14):1252–1257. doi: 10.1055/s-0042-110859. [DOI] [PubMed] [Google Scholar]
- 30.Resham K., et al. Neuroprotective effects of isoquercitrin in diabetic neuropathy via Wnt/β-catenin signaling pathway inhibition. Biofactors. 2020;46(3):411–420. doi: 10.1002/biof.1615. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L., et al. Hypoglycemic effect and mechanism of isoquercitrin as an inhibitor of dipeptidyl peptidase-4 in type 2 diabetic mice. RSC Adv. 2018;8(27):14967–14974. doi: 10.1039/c8ra00675j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L., et al. Isoquercitrin protects HUVECs against high glucose-induced apoptosis through regulating p53 proteasomal degradation. Int. J. Mol. Med. 2021;48(1) doi: 10.3892/ijmm.2021.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai H.D., et al. Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. J. Ethnopharmacol. 2017;206:152–159. doi: 10.1016/j.jep.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 34.Tu Y., et al. Total flavones of Abelmoschus manihot remodels gut microbiota and inhibits microinflammation in chronic renal failure progression by targeting autophagy-mediated macrophage polarization. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.566611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu L.F., et al. Huangkui capsule ameliorates renal fibrosis in a unilateral ureteral obstruction mouse model through TRPC6 dependent signaling pathways. Front. Pharmacol. 2020;11:996. doi: 10.3389/fphar.2020.00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu H., et al. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem. Pharmacol. 2018;154:203–212. doi: 10.1016/j.bcp.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Dai E., Yang J. Quercetin suppresses glomerulosclerosis and TGF-β signaling in a rat model. Mol. Med. Rep. 2019;19(6):4589–4596. doi: 10.3892/mmr.2019.10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao Y., et al. Quercetin is able to alleviate TGF-β-induced fibrosis in renal tubular epithelial cells by suppressing miR-21. Exp. Ther. Med. 2018;16(3):2442–2448. doi: 10.3892/etm.2018.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu T., et al. Quercetin alleviates kidney fibrosis by reducing renal tubular epithelial cell senescence through the SIRT1/PINK1/mitophagy axis. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118116. [DOI] [PubMed] [Google Scholar]
- 40.Lee V.W., Harris D.C. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology. 2011;16(1):30–38. doi: 10.1111/j.1440-1797.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- 41.Tu Y., et al. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, ameliorates adriamycin-induced renal inflammation and glomerular injury via inhibiting p38MAPK signaling pathway activity in rats. J. Ethnopharmacol. 2013;147(2):311–320. doi: 10.1016/j.jep.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Zhao L., Han S., Chai C. Huangkui capsule alleviates doxorubicin-induced proteinuria via protecting against podocyte damage and inhibiting JAK/STAT signaling. J. Ethnopharmacol. 2023;306 doi: 10.1016/j.jep.2023.116150. [DOI] [PubMed] [Google Scholar]
- 43.Li W., et al. Total extracts of Abelmoschus manihot L. Attenuates adriamycin-induced renal tubule injury via suppression of ROS-ERK1/2-mediated NLRP3 inflammasome activation. Front. Pharmacol. 2019;10:567. doi: 10.3389/fphar.2019.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamirou F., Houssiau F.A. Management of lupus nephritis. J. Clin. Med. 2021;10(4) doi: 10.3390/jcm10040670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dos Santos M., et al. Protective effects of quercetin treatment in a pristane-induced mouse model of lupus nephritis. Autoimmunity. 2018;51(2):69–80. doi: 10.1080/08916934.2018.1442828. [DOI] [PubMed] [Google Scholar]
- 46.Qu S., et al. Rutin attenuates vancomycin-induced renal tubular cell apoptosis via suppression of apoptosis, mitochondrial dysfunction, and oxidative stress. Phytother Res. 2019;33(8):2056–2063. doi: 10.1002/ptr.6391. [DOI] [PubMed] [Google Scholar]
- 47.Qu S., et al. Rutin attenuates vancomycin-induced nephrotoxicity by ameliorating oxidative stress, apoptosis, and inflammation in rats. Antimicrob. Agents Chemother. 2019;63(1) doi: 10.1128/AAC.01545-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H., et al. Isoquercitrin ameliorates cisplatin-induced nephrotoxicity via the inhibition of apoptosis, inflammation, and oxidative stress. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.599416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu B., et al. Hyperoside attenuates renal aging and injury induced by D-galactose via inhibiting AMPK-ULK1 signaling-mediated autophagy. Aging (Albany NY) 2018;10(12):4197–4212. doi: 10.18632/aging.101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan R.Z., et al. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation. Phytother Res. 2020;34(1):139–152. doi: 10.1002/ptr.6507. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021;28:231–243. doi: 10.1016/j.jare.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korkmaz A., Kolankaya D. Protective effect of rutin on the ischemia/reperfusion induced damage in rat kidney. J. Surg. Res. 2010;164(2):309–315. doi: 10.1016/j.jss.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 53.Han Y., et al. Rutin ameliorates renal fibrosis and proteinuria in 5/6-nephrectomized rats by anti-oxidation and inhibiting activation of TGFβ1-smad signaling. Int. J. Clin. Exp. Pathol. 2015;8(5):4725–4734. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


