Abstract
Bergenia purpurascens (B. purpurascens, Saxifragaceae) has been used to treat several diseases in different countries, such as lung diseases, stomach problems, rheumatic pains, boosting immunity etc. However, the information on phytochemistry, pharmacology and toxicology of this plant has rarely been comprehensively and critically reported. This paper aims to study and evaluate its therapeutic potential, including the traditional uses and all the latest information of phytochemistry, pharmacology and toxicology. The main components of this plant are phenols compounds and the characteristic substance is bergenin.The results about modern pharmacology have shown that its pharmacological effects include antibacterial, antiviral, cough relieving, anti-inflammatory and so on. In addition, it could inhibit diabetic neuropathy, restore insulin secretion, treat cancer, protect liver and prevent Alzheimer's disease (AD). Thus, its therapeutic fields may be cancer, diabetic and AD in the future. The information will help to further update and study pharmacologic effect and action mechanism of this herb, which is more widely, effectively, and safely used in clinic.
Keywords: Bergenia purparescens, Phytochemistry, Pharmacology, Toxicology, Traditional uses
1. Introduction
Bergenia purpurascens (B.purpurascens, Saxifragaceae) is herb of the Bergenia (Saxifraga family), which grows in the jungle, alpine meadows and rock crevices. It is distributed mainly in some regions of Asia, including China, Mongolia, Siberia, Altai, the Himalayan region [1,2]. The species from China were previously called B.purpurascens and included in the Pharmacopoeia of the People's Republic in China (2020) [3,4]. It has also been applied according to the traditional folk medicine in China. Due to herbal research, the common names of B.purpurascens include Yanbaicai, Shiyancai, Yanqi, and Xuetoukaihua in Yunnan Province. Yanbaicai was firstly recorded in a book named “Classification of Chinese Herbal Medicine" in the Qing Dynasty, which reported its haemostatic and anti-tussive effects [5,6]. In addition, it has long been used as a Tibetan medicine to exert tonic, haemostatic and antitussive effects [7]. Commonly, its dried roots and stems are used except for the leaves. In the concept of TCM, the rhizome of this herb has been used to relieve cough and asthma [5]. The flavor and nature is bitter, astringent and flat, being belonged to the lung, liver and spleen meridians. Its functions are curing diarrhea, bleeding and cough, relaxing muscles and calming wind to activate collaterals in TCM, Nepalese and Indian medicine.
At present, pharmacological studies have shown that compounds or extracts from B.purpurascens exhibit pharmacological effects which are listed in Table 2. However, great majority of pharmacological studies still lack depth and breadth without systematic and theoretical study, impacting on application and promotion of this plant in clinic. Thus, the studies are needed to investigate the clinical and pharmacological evidences. The review manages research progress of this plant with sharp critical analysis. Based on the pharmacological effects and traditional functions of this herb, this paper first gives a brief summary of the discoveries and significance obtained from the research, the problems or limitations in this research of B.purpurascens are explored by the authors. Further, the authors suggest the future research orientation are proposed to solve the questions. In the end, it is hoped to utilize this plant better in clinic in the future.
Table 2.
| Pharmacological effects of B.purpurascens.
| Effects | Active components/compounds | Source | Reference(s) |
|---|---|---|---|
| Anticancer activity | bergenin(1) | rhizome; root | [22] |
| Myricetin (22) | leaf | [28,37] | |
| Quercetin (24) | rhizome | [37] | |
| Kaempferol (23) | rhizome, leaf | [37] | |
| gallic acid (17) | root, rhizome | [37] | |
| 1-O-β-d- glucopyranosyl-2-methoxy-3-hydroxyl-phenylethene (16) | leaf | [22] | |
| 4-O-Galloyl-bergenin (2) | root, rhizome | [38] | |
| Cough-relieving effect | Bergenin(1) | rhizome; root | [22] |
| Antibacterial and Anti-inflammatory effects | catechin-3-O-gallate (49) | root | [30] |
| Bergenin (1) | rhizome, root | [30,39] | |
| Ethanol extracts | rhizome, root | [28,[40], [41], [42]] | |
| Ethyl acetate extracts | rhizome, root | [43] | |
| 11-O-galloylbergenin (3) | root, rhizome, leaf | [29,44] |
2. Traditional uses
Traditionally, B.purpurascens has been used to cure some diseases in China. In the concept of TCM, it displays antidiarrheal and haemostatic effects and relieving cough, relaxing muscles and activating collateral [4,8]. It has been widely used in clinic to treat diarrhea, dysentery, internal and external traumatic bleeding, tuberculosis cough, bronchitis cough and other diseases. In China, bergenin has been made into antitussive and expectorant agents [8]. In Sichuan and Guizhou provinces, B.purpurascens can treat cough [9]. Additionally, it is used to treat vomit, dizziness and cough in Xinjiang [10]. This plant can treat dysentery and diarrhea in Tibet and bronchitis in Xinjiang and Yunnan. Further, its preparations have been prepared to treat diseases in Tibet and Yunnan [11]. The chemical analysis on this hurb found that the main contents were arbutin, liquiritin, isoliquiritigenin, gallic acid, bergenin and glycyrrhizic acid. Due to the lack of in-depth research on the active ingredients of different parts of this plant in China, its rhizome is usually used in clinic.
In addition, the fresh leaf-paste is applied on skin to protect from harmful ultraviolet radiation in Tibet [12]. Some studies show that the leaf or leaf-juice relieves constipation and reduces earaches. The roots of Bergenia can be used to prevent venereal diseases [13]. The leaves of the Bergenia are commonly used in Russian, blackened leaves in Altai, fresh leaves in Buryats and Mongols. In India, Bergenia species have been made to ayurvedic preparations to dissolve bladder and kidney stones. However, the pharmacological effects in different countries or regions vary greatly due to various use habits in the folks. Therefore, it is necessary to carry out modern pharmacological research based on the traditional uses of this plant.
3. Botany
B. purpurascens is an evergreen, perennial, drought-resistant plant in Southwest China, Belonging to the genus Bergenia (Saxifragaceae) [4,7]. The height is 45 cm, and its leaves are very big and plump (see Fig. 1A), and it earns some interesting nicknames such as “pig squeak” and “elephant ears” [7,14]. It has dark green foliage and red flowers and grows in tufts or clumps. The flowers are loose racemes of 6 or 7 rose-red flowers (see Fig. 1B) [7]. Usually, the flowering period is between May and June. This plant is often used in rock garden or timber for the planting flowers [15,16]. The roots and stems of B.purpurascens are used for medical use (see Fig. 1C) [4,7]. The fruit period from May to October is capsule globose and 2-lobed, and numerous tiny seeds (see Fig. 1D) [7].Today, the study has been not reported on the components or pharmacological effects of the flowers or fruits.
Fig. 1.
Images of B.purpurascens: (A)leaves; (B) flowers; (C) Root and rhizome; (D)Fruits.
4. Phytochemistry
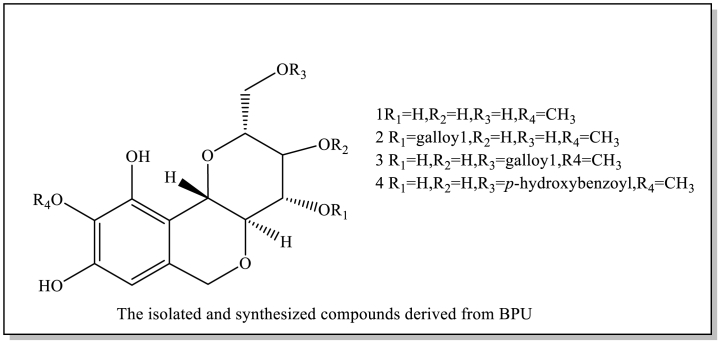
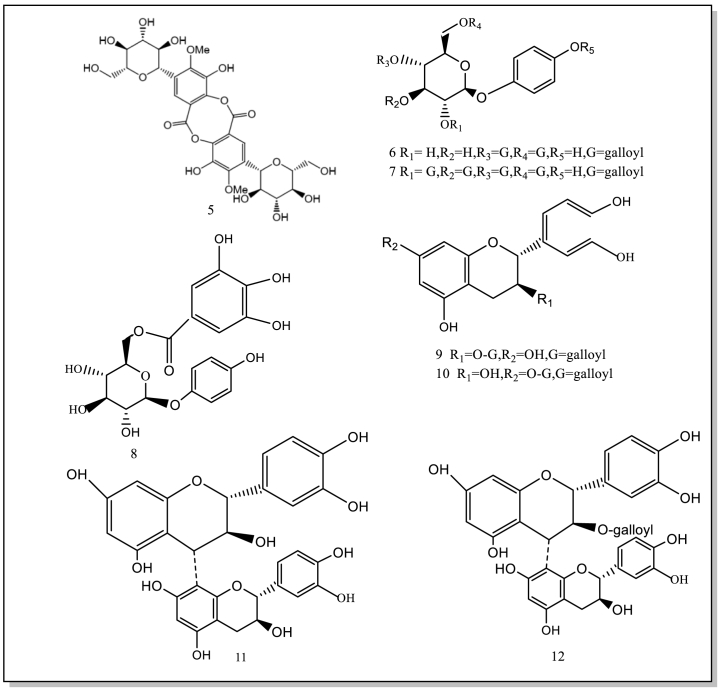
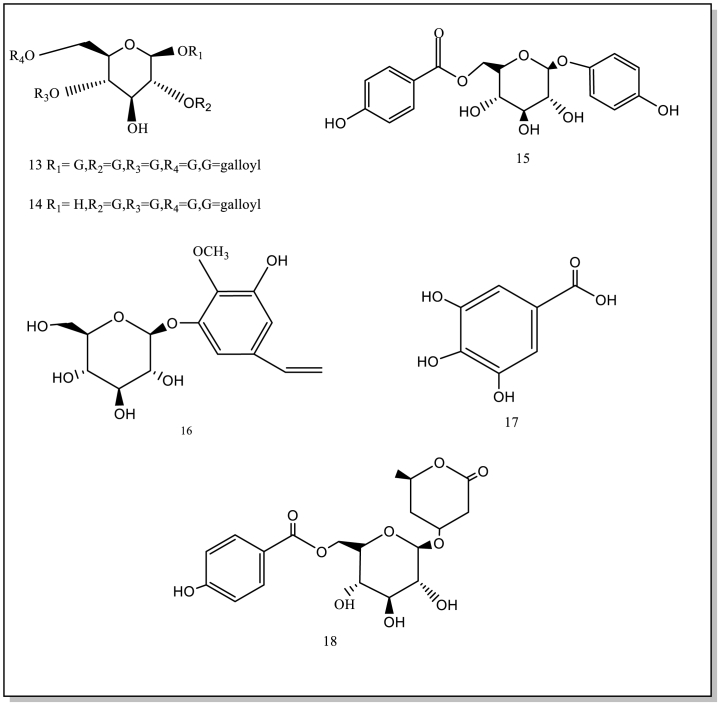
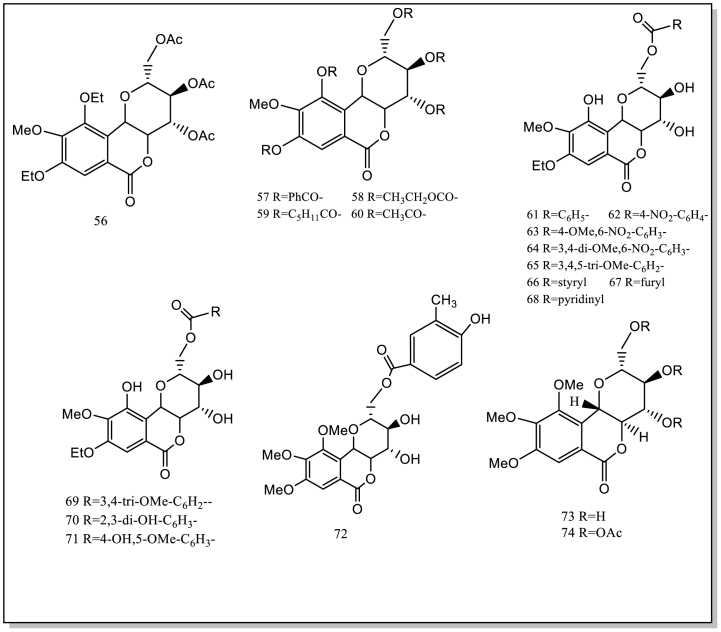
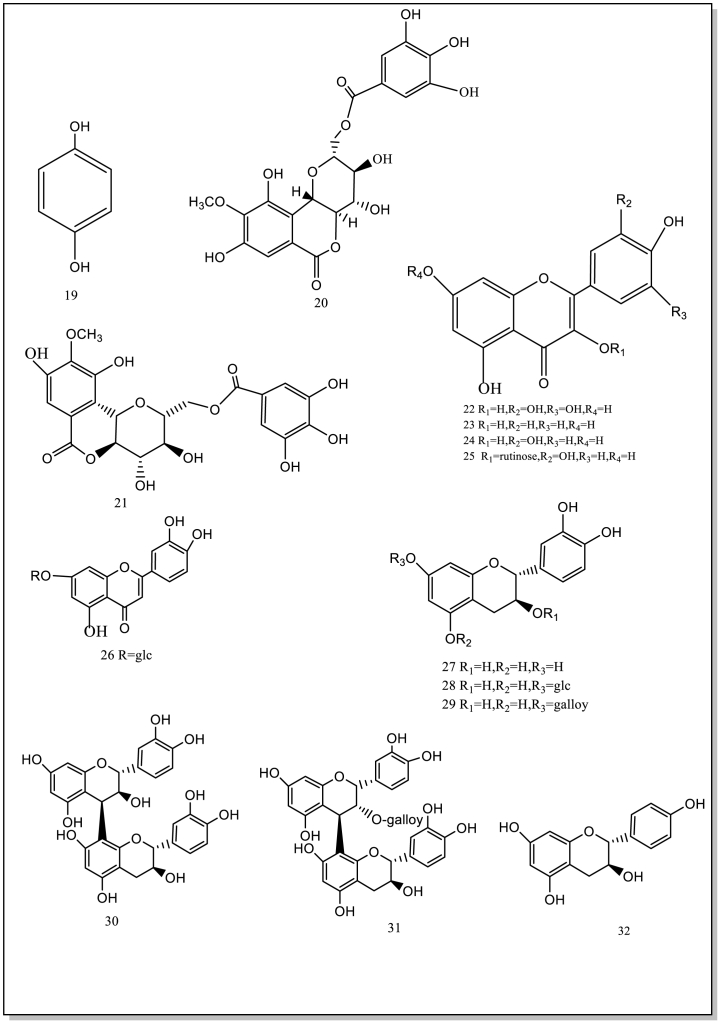
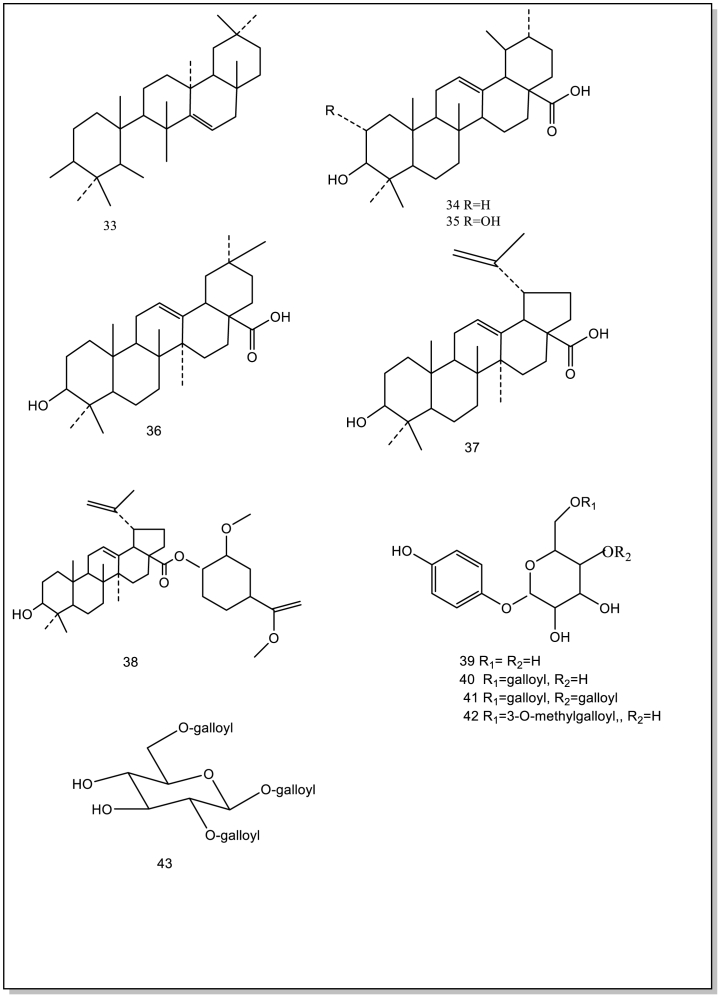
Current studies have showed/indicated that B. purpurascens contain 74 isolated or synthesized chemical compounds, including 21 Phenols (1–21) and 19 derivatives of bergenin and derivative compounds (56–74), 11 flavonoids (22–32), 11 terpenoids (33–43), 6 organic acids (44–49), 6 other compounds (50–55). Currently, no studies have been conducted on the components in the flowers and fruits. Therefore, it will be strengthened chemical studies on these components of the flowers and fruits. These compound structures and names (1–74) are as follows (Table 1; Fig. 2).
Table 1.
The isolated and synthesized compounds derived from B.purpurascens.
| No | compound name | Parts of plant | Source | References(s) |
|---|---|---|---|---|
| 1 | bergenin | root, rhizome | Ethanol extracts | [17,18] |
| 2 | 4-O-Galloyl-bergenin | root, rhizome | Ethanol extracts | [[18], [19], [20]] |
| 3 | 11-O-Galloyl-bergenin | root, rhizome, | Ethanol extracts | [19,21] |
| leaf | ||||
| 4 | 11-O-P-Hydroxybenzoyl-bergenin | root, rhizome, leaf | Ethanol extracts | [19,22] |
| 5 | ardimerin | leaf | Ethanol extracts | [22] |
| 6 | 4′,6′-di-O-galloylarbutin | root, rhizome | Ethanol extracts | [17] |
| 7 | 2′,3′,4′,6′-tetra-O-galloylarbutin | root, rhizome | Ethanol extracts | [17] |
| 8 | 6′-O-galloylarbutin | root, rhizome | Ethanol extracts | [17] |
| 9 | 3-O-galloyl- (+)-catechin | root, rhizome | Ethanol extracts | [23] |
| 10 | 7-O-galloyl- (+)-catechin | root, rhizome | Ethanol extracts | [17] |
| 11 | Procyanidin-B-3 | root, rhizome | Ethanol extracts | [17] |
| 12 | 3-O-galloylprocyanidin-B-1 | root, rhizome | Ethanol extracts | [17] |
| 13 | 1,2,4,6-tetra-O-galloyl-β-d-glucose | root, rhizome | Ethanol extracts | [17] |
| 14 | 2,4,6-tri-O-galloyl- β-d-glucose | root, rhizome | Ethanol extracts | [17] |
| 15 | Breynioside A | the whole plant | Ethanol extracts | [24] |
| 16 | 1-O-β-d-glucopyranosyl-2-methoxy-3-hydroxyl-phenylethene | leaf | Ethanol extracts | [25] |
| 17 | gallic acid | root, rhizome | Ethanol extracts | [17] |
| 18 | 6′-O-p-hydroxybenzoyl-parasorboside | root, rhizome | Ethanol extracts | [17] |
| 19 | hydroquinone | root | Based on bergenin | [26] |
| 20 | 11-O-(4′-O-methylgalloyl)-bergenin | root | Based on bergenin | [27] |
| 21 | 11-O-(4′-hydroxybenzoyl) bergenin | leaf | Ethanol extracts | [25] |
| 22 | Myricetin | leaf | Ethanol extracts | [28] |
| 23 | Kaempferol | rhizome, leaf | Ethanol extracts | [25,29] |
| 24 | Quercetin | rhizome | Ethanol extracts | [30,31] |
| 25 | rutin | leaf | Ethanol extracts | [25] |
| 26 | Elycohol-7-β-d-glucopyranoside | rhizome | Ethanol extracts | [28] |
| 27 | Catechin | rhizome | Ethanol extracts | [32] |
| 28 | Catechin-7-O-β-d-glucopyranoside | rhizome | Ethanol extracts | [32] |
| 29 | 7-O-Gallocatechin | rhizome | Ethanol extracts | [32] |
| 30 | Procyanidin-B-3 | rhizome | Ethanol extracts | [32] |
| 31 | 3-O-galloylprocyanidin-B-1 | rhizome | Ethanol extracts | [32] |
| 32 | Aphrodite | rhizome | Ethanol extracts | [21] |
| 33 | β-taraxerol) | leaf | Ethanol extracts | [22,25] |
| 34 | Ursolic acid | leaf | Ethanol extracts | [22,25] |
| 35 | 2α-hydroxy ursolic acid | leaf | Ethanol extracts | [22,25] |
| 36 | Oleanolic acid | leaf | Ethanol extracts | [22,25] |
| 37 | Betulinic acid | leaf | Ethanol extracts | [22,25] |
| 38 | ocimol | leaf | Ethanol extracts | [22,25] |
| 39 | Arbutin | leaf | Ethanol extracts | [25] |
| 40 | 6-O-Galloyl arbutin | leaf | Ethanol extracts | [22,33] |
| 41 | 4,6-O-Digalloyl arbutin | leaf | Ethanol extracts | [25] |
| 42 | 6′-O-(3″-O-methylgalloyl) arbutin | leaf | Ethanol extracts | [25] |
| 43 | 1,2,6-O-trigalloyl glucose | leaf | Ethanol extracts | [25] |
| 44 | 3,4,5-Trihydroxybenzoic acid | root | Ethanol extracts | [34] |
| 45 | Protocatechuic acid | root | Ethanol extracts | [30,34,35] |
| 46 | Methyl gallate | root | Ethanol extracts | [34,36] |
| 47 | Syringic acid | root | Ethanol extracts | [36] |
| 48 | Vanillic acid | root | Ethanol extracts | [36] |
| 49 | (+)-catechin-3-O-gallate | root | Ethanol extracts | [27] |
| 50 | β-sitosterol | root | Ethanol extracts | [27] |
| 51 | β-sitosterol-palmitate | root | Ethanol extracts | [27] |
| 52 | Carotene | root | Ethanol extracts | [27] |
| 53 | 12E-diene | root | Ethanol extracts | [27] |
| 54 | Diethyl disulfide | root | Ethanol extracts | [27] |
| 55 | β-daucosterol | root | Ethanol extracts | [27] |
| 56 | bergenin diethyl ether triacetate | root | Based on bergenin | [27] |
| 57 | bergenin pentaacylate A | root | Based on bergenin | [1] |
| 58 | bergenin pentaacylate B | root | Based on bergenin | [1] |
| 59 | bergenin pentaacylate C | root | Based on bergenin | [1] |
| 60 | bergenin pentaacylate D | root | Based on bergenin | [1] |
| 61–68 | bergenin ester A | root | Based on bergenin | [1] |
| 61–68 | bergenin ester B | root | Based on bergenin | [1] |
| 61–68 | bergenin ester B | root | Based on bergenin | [1] |
| 61–68 | bergenin ester B | root | Based on bergenin | [1] |
| 61–68 | bergenin ester B | root | Based on bergenin | [1] |
| 69 | bergenin monoacylate A | root | Based on bergenin | [1] |
| 70 | bergenin monoacylate B | root | Based on bergenin | [1] |
| 71 | bergenin monoacylate C | root | Based on bergenin | [1] |
| 72 | di-O-methylbergenin esters | root | Based on bergenin | [1] |
| 73 | 8,10-di-O-methylbergenin | root | Based on bergenin | [1] |
| 74 | 3,4,11-triacetate derivative | root | Based on bergenin | [1] |
Fig. 2.
The isolated and synthesized compounds derived from BPU.
4.1. Phenols
Bergenin (C-glucoside of 4-O-methyl gallic acid) is a key phenol compound. It is a characteristic substance isolated from B.purpurascens [9]. Most of bergenin is from the root and rhizome of this plant, little in stem and leaf organs. Recently, some researchers have separated and identified some chemical components of this herb by the high-resolution performance of mass spectrometry, rapid matching and powerful characterization of the UNIFI screening platform. The purification of bergenin from the rhizome extract of B.purpurascens is performed by column chromatography. The required quality standard of bergenin was not less than 8.2 % in China [4]. However, no studies have been conducted on bergenin from different production regions with respect to season and location. At the same time, a lot of derivatives are 21 phenolic compounds (1–21) and 19 bergenin derivatives (56–74) [1]. Some isolated and/or synthesized drugs have been found to have pharmacological activity. In phytochemical analysis, the characteristic compounds of B.purpurascens through LC-MS analysis to identify the phytochemical components of the extractLi, Wu [38]. These methods provide a good reference for controlling the quality by detecting characteristic substances in this plant, such as bergenin.
4.2. Flavonoids
Eleven flavonoid compounds (22–32) have been isolated from B.purpurascens. It has been confirmed that flavonoids scavenge hydroxyl radicals and resists oxidation. One of these active compounds is catechin (27), being used to reduce potentially damaging and associated local immune responses. In addition, it can prevent cognitive decline caused by HIV and prevent neurotoxic oxidative stress. Therefore, the compound may be strong therapeutic candidates.
4.3. Terpenoids
Terpenoids (33–43) are compounds derived from mevalonic acid. Their molecular skeletons have isoprene units (C5 units) as the basic structures. Terpenoids are widely present in this plant, and they are main constituents of fragrances, resins, and pigments. Currently, these compounds were isolated from the whole plant of B.purpurascens and identified by infrared spectrum, mass spectrum.
Terpene compounds (33–43) are derived from valproic acid. The basic structure their molecular skeleton are isoprene units (C5 unit). Terpenes are found in many plants and the main components are spices, resins, and pigments. Arbutine (39) has a high content and more potential source to use for infectious diseases in the plant [45].
4.4. Organic acids
There are 6 organic acids (44–49) in B.purpurascens. The gallic acid was distributed in the cells of leaf, including vacuoles and chloroplastsTaneyama and Yoshida [46]. Gallic acid(3,4,5-trihydroxybenzoic acid) is a type of organic acid [47]. Gallic acid exterts has antioxidant, antiviral and antifungal activities. In addition, gallic acid is an inhibitor of weak carbonic anhydrase [48]. Besides, 3,4,5-Trihydroxybenzoic acid (44) by LC-MS and evaluated its antibacterial activity [29].
4.5. Other compounds
Other compounds (50–55) have been separated from B.purpurascens. The studies that the quantity and type of extracts from this plant by different solvents are different. Therefore, it is necessary to conduct a systematic study on the extraction and separation of the components from B.purpurascens. Further, this is also important to strengthen investigations on chemical structures and biological of these components.
5. Pharmacology
Pharmacological actions have been performed in the extracts and components of B.purpurascens. These pharmacological actions are lighted in Table 2.
5.1. Anticancer activity
In the vivo experiment, it has been reported that bergenin possesses anti-tumor activity [49]. the model of skin carcinogenesis induced by 7,12-dimethylbenzo [a] anthracene (DMBA) and 12-O-tetradecanoyl camphor 13 acetate (TPA). The drug groups were treated with bergenin; the control group was treated with TPA alone. The results showed that the formation papillomas of the mouse skin was delayed [49]. However, the action mechanism on skin cancer is unknown and the positive control group is confused with the model group, which has not also the number of mice and drug dosage in the animal experiment. Secondly, the effects of compounds (1) and (17) on α- Melanocyte stimulating hormone and stimulated melanogenesis inhibitory activity in B16 melanoma cells [22]. In vitro, the compound (16) has antiproliferative activity of five human cancer cell lines (MGC-803, T24, HepG2, NCI–H460 and HL-7702) with MTT [22]. Compared with other six compounds, the compound (16) showed good inhibitory activity and significantly reduced NCI–H460 cells (IC50 = 17.69 μm) and T24 cells (IC50 = 14.36 μM). At the same time, the bergenin against the murine breast cancer cell line in vitro, but it did not set suitable parameters and process according to the result of the experiment [37].
In summary, bergenin, glucopyranosyl-2-methoxy-3-hydroxyl-phenylethene, and other compounds from this plant significantly possessed the strongest anti-tumor effect, inhibiting melanoma cells, breast cancer cell, prostate cancer cell and other cells, becoming the potential compound of anticancer drugs in vivo or vitro. However, most studies of this drug are used to treat malignancy in vitro, lacked also positive controls and more evidences on the animal and clinic experiments. Based on the above related research, it is necessary and comprehensive to understand the efficacy and mechanism of B.purpurascens extracts in vivo by anti-tumor animal models through RT-PCR, and Western blot analysis in the future.
5.2. Cough-relieving effect
In China, bergenin from B.purpurascens in this herbal medicine has remarkable antitussive effect caused by electrical stimulation of laryngeal nerve and ammonia spray [10]. Some researchers evaluated that 4-O-Galloyl-bergenin (2) from this herb is naturally antitussive and expectorant agent in the total antioxidant phosphomolybdate assay [50].
Due to its selective inhibition of the cough center, the tolerance was not found after 23-days continuous administration of bergenin [51,52].
5.3. Antibacterial and anti-inflammatory activity
Bacteria and viruses are one of the most prevalent infectious diseases in the world, which is a major public health problem [53,54]. The natural antioxidants and antimicrobial drugs are demanding gradually [55].
In vitro, the bacteriostasis and sterilization actions of B.purpurascens extract on SA, MRSA, and ESBLs-SA [42]. In the experiments, 50 mice were randomly divided into five groups (n = 10), namely B.purpurascens extract high group (67.6 g/kg), medium group (33.8 g/kg) and low-dose group (16.9 g/kg), positive control indomethacin group (0.65 g/kg) and blank control group (equivalent volume of physiological saline). B.purpurascens had inhibitory actions on SA, MRSA, and ESBLs SA, and could inhibit xylene induced ear edema in mice, cotton ball granular tissue proliferation, and increase capillary permeability. The effect mechanisms were associated with inhibiting inflammatory cytokines that might be serotonin and histamine, identified the main components of the extract by LC-MS analysis [42]. These components were bergenin (1), catechin (9), myricetin (22) and gallic acid (17). The results of the study showed that the extract has certain bactericidal effect and could inhibited xylene-induced ear edema, agar granuloma and increased peritoneal capillary permeability of 0.6 % acetic acid-induced mice models [42]. But the mechanisms and active components from the extract were not confirmed. Thus, it is need to do some works for in vivo the antibacterial and anti-inflammatory mechanisms for some compounds from B.purpurascens in clinical application. At the same time, these extracts have the lowest MIC for strains and the highest MIC for Streptococcus pneumoniae, ranging from 75 to 150 μg/ml (see Table 3) [42]. Therefore, these extracts (50 and 100 mg/kg) improve the survival rate of newborn rats infected with S.aureus. Although this study provides some evidence for the anti-inflammatory and antioxidant effects of these extracts on Staphylococcus aureus from in vitro and in vivo experiments, its inhibitory effects on other bacteria, as well as the active components in the extracts and their mechanisms of action are still unclear.
Table 3.
Antibacterial activity and MBCs of methanolic extract of B.purpurascens.
| Bacterial strain | Inhibition Zone (mm) | MIC (g/ml) | MBC (μg/ml) |
|---|---|---|---|
| Escherichia coli | 22 ± 2 | 85 ± 3 | 170 ± 6 |
| Streptococcus pneumonia | 14 ± 2 | 150 ± 3 | 280 ± 6 |
| Haemophilus influenzae | 23 ± 2 | 85 ± 4 | 160 ± 4 |
| Klebsiella pneumoniae | 24 ± 3 | 80 ± 3 | 140 ± 3 |
| Staphylococcus aureus | 28 ± 3 | 75 ± 3 | 140 ± 2 |
| Enterobacter cloacae. | 16 ± 3 | 125 ± 3 | 245 ± 4 |
| Streptomycin | 30 ± 3 | 14.5 ± 2 | 27 2 |
In vitro, the bergenin and its derivatives had anti-inflammatory properties that could inhibit inflammatory mediators, such as TNF-α and NO [44]. Bergenin and its derivatives may be cytotoxic activity and potent inhibitors of NO through the MTT method. Thus as, the studies on anti-inflammatory are needed to systemically evaluate the bergenin and its derivatives from this plant comprehensively in various experimental animal.
Combined with network pharmacology, B.purpurascens could treat arthritis and osteoarthritis [30]. Bergenin and its derivatives might be potent inhibitors of NO and cytotoxic activity through MTT assay, which might inhibit MAPK signaling pathways and induce inflammatory factors. B.purpurascens slowed the inflammatory response of osteoarthritis. The results showed that these components were closely related to the MAPK pathway, including bergenin (1), arbutin (39), 11-O-gallocatechin (3), and catechin-3-O-gallate (9), directly related to caspase-3 and MAPK1 [30]. Nowadays, the study is a preliminary study on treating osteoarthritis effect of the extracts from B.purpurascens. So, studies on various experimental cell and animal will be necessary to study the action mechanisms of these components from B.purpurascens.
The phenols from this herb were detected for extracted with obtainresidues of ethyl acetate extracts, especially these extracts had the strong antiradical activity with TAU734/mg values of 10.7 ± 1.03 [39]. Moreover, the result partially confirms some antibacterial properties of phenolic compounds, but hydroquinone (19) was main antibacterial substance. However, the mechanisms of antibacterial and anti-inflammatory activity on the component are still unknown. Therefore, studies on various experimental cell and animal are necessary to study on action mechanisms of these compounds, and the different effect mechanism of the two components. In vitro and vivo, bergenin was potently antifungal against S. sclerotiorum. These extracts of B.purpurascens exhibited the strongest antibacterial activity [56]. But these extracts on the pharmacological mechanism are not investigated, which seriously influenced clinical application and drug innovation.
The potential mechanisms of Srolo Bzhtang (SBT, consisted of B.purpurascens.) on airway inflammation and mucus secretion in a rat model of chronic bronchitis (CB) induced by cigarette smoke (CS) [57]. In the experiment, 60 male rats were randomly divided to six groups including control group (room air exposure), model group (CS exposure), dexamethasone group (0.2 mg/kg/day), and three SBT groups (1.67, 2.50, and 3.34 g/kg/day). The results showed that SBT significantly downregulated the mRNA and protein expressions of IL-13, STAT6/p-STAT6, and MUC5AC [57]. Therefore, these researchers thought that bergenin and other compounds may be the protective effects of SBT on MUC5AC hypersecretion and CS induced airway inflammation.These compounds could suppress the tobacco smoke-induced infiltration of inflammatory cells and reduce the secretion of mucus in the airway of a rat CB model.
In summary, these studies show B.purpurascens are antibacterial and anti-inflammatory herb. The anti-inflammatory and antibacterial effects are strong activity on Staphylococcus aureus. But the mechanisms for antibacterial and anti-inflammatory of bergenin and its derivatives are unclear. Therefore, it is need to investigate the active compositions of B.purpurascens and effect mechanism between these different compounds by the pharmacological studies, especially animal experiments and clinical trials on the chemical constituents of this plant. However, these were only experimental studies of animal pharmacology and lacked of clinical trials. So, the clinical trials will provide important references for clinical applications of this plant.
5.4. Antiviral activity
As COVID-19 continues to invade the world, In addition to the guidelines issued by the National Health Commission of China, the autonomous region has also issued clinical practice guidelines for recommending Chinese herbal medicines [58].The important focus on COVID-19 is the modern medicine of new substances and strategies to combat disease. The Chinese traditional medicine is widely used in clinical treatment, such as treating cough, sore throat, and runny nose et al. Therefore, it is a necessary to confirm active substance of this plant.
By enzyme-linked immunosorbent assay, bergenin was the reason for the anti HCV formation of Streptomyces melanogenesis. The experimental results showed that the polypeptide ester of d-glucose had the strongest inhibitory effect on HCV NS3 serine protease, with a small cytotoxic effect [59]. The polyphenolic ethyl acetate fraction in herbal extracts was the most active, 1,2,3,4,6-penta-O-galloyl-β-D-glucoside (13). The compound would be potential to be used to design and develop drugs for treatment viral infection (IC50 = 0.68 ± 0.05). These researchers suggested that the compound 13 might be nature for the antiviral effects, but the action mechanisms of the component are still unknown. Therefore, it is necessary to investigate the antiviral mechanisms of the compound in vivo.
5.5. Pharmacological effects of diabetic neuropathy and restored insulin secretory
Firstly, it was reported that the bergenin ameliorated the mice(C57/BL6).The mice model of streptozotocin (STZ) were induced painful diabetic neuropathy (DN) [2]. Briefly, the animal experiments had six groups including a control groups (n = 6, non-diabetic, accepted only citrate buffer) and 5 DN groups (n = 6). The mice of 5 DN groups were treated with bergenin (intraperitoneal, 3.125–25 mg/kg) or vehicle (2 % dimethyl sulfoxide in saline; VEH), including STZ + VEH groups, STZ + bergenin groups (25 mg/kg, 12.5 mg/kg, 6.25 mg/kg, 3.125 mg/kg). The experimental results showed that bergenin (intraperitoneal injection, 3.125–25 mg/kg) could completely restore mechanical abnormal pain in DN mice (p < 0.05).
In the second experiment [2], multi dose bergenin treated diabetes sensory neuropathy. These groups include the bergenin group (n = 6; bergenin, 25 mg/kg, intraperitoneal injection), the VEH group, the gabapentin (GABA) group (gold standard drug, 40 mg/kg, oral), and multiple (25 mg/kg; twice daily, for 14 days). The results showed that the treatment threshold gradually increased daily in the bergenin treated group compared to the VEH group. The results indicated that the previous day's treatment had a lasting analgesic effect. However, GABA group did not induce sustained therapeutic effect of bergenin treatment. Moreover, compared with the GABA group, the bergenin group had greater efficacy and increased antinociceptive effects over time (p < 0.05).
|
Table 2 (continued) | |||
|---|---|---|---|
| Effects | Active components/compounds | Source | Reference(s) |
| Antiviral activity | the polyphenolic ethyl acetate part | root, rhizome | [59] |
| gallatedesters of d-glucose (14) | root, rhizome | [59] | |
| 1,2,3,4,6-penta-O-galloyl-β-D-glucoside (13) | root, rhizome | [59] | |
| Rutin (25) | leaf | [59] | |
| catechin-3-O-gallate (49) | root | [30] | |
| Bergenin (1) | rhizome, root | [22,30,39,59] | |
| Liver protection | Ethanol extracts | leaf, Rhizome, root | [[60], [61], [62]] |
| Bergenin (1) | root, rhizome | [60] | |
| Pharmacological effects of diabetic neuropathy and restored insulin secretory | bergenin(1) | root, rhizome | [2,63,64] |
These researchers detected cytokines, antioxidant genes, and markers of oxidative stress in neural tissue by ELISA, RT-qPCR, and biochemical analysis. The results showed that bergenin can reduce the production of nitric oxide in vitro and the content of malondialdehyde/nitrite in vivo. In addition, bergenin regulated the production of pro-inflammatory, anti-inflammatory cytokines and induced long-lasting analgesic effects in DN groups. Therefore, the mechanisms were associated with a shift of the cytokine balance toward anti-inflammatory predominance and upregulation of antioxidant pathways, favoring the reestablishment of redox and immune homeostasis in the nervous system [2]. This study systemically investigated the therapeutic potential of bergenin in the treatment of painful diabetic neuropathy. The experiment should be designed completely, which was efficient to illuminate the efficacy and mechanism of bergenin. Therefore, it is needed to study applications of gene knock-out animals in vivo. B.purpurascens potentially treated some diseases associated with diabetic. Based on beta cells in the presence of cytokines, the effects of bergenin were assayed by cellular ATP levels of INS-1E cells, mitochondrial physiological parameters, and flow cytometry methods [64,65].
Bergenin has significantly treating diabetes and hypolipidemic effects on type 2 diabetes rats [63]. In this study, 36 rats were divided into 6 groups, including normal group, diabetes control group, three bergenin groups (2.5, 5 and 10 mg/kg; oral), and glibenclamide group (normal rats treated with glibenclamide; 10 mg/kg; p.o.). The diabetes model rats fasted overnight, induced by streptozotocin (STZ), and administered at 2.5, 5, and 10 mg/kg doses. The rats were treated at same dose level. During the experiment, blood samples were collected through the posterior orbital plexus under light ether anesthesia. Compared with the rats in the diabetes control group, the liver glycogen level of the three bergenin groups and glibenclamide group were significantly increased. In addition, histopathological studies have confirmed the effect of bergenin on the pancreas β Cell regeneration. Although they provided some experimental evidences for Type 2 diabetic rat models, the action mechanisms of the component are still unknown in clinic.
In summary, B.purpurascens significantly treated diabetic, avoided redox and reconstitution of immune homeostasis. These studies showed the bergenin could prevent diabetic neuropathy and restore insulin secretory. However, it will be highlight as a promising antiapoptotic agent for diabetes. B.purpurascens could help protecting body functions and treat some diseases related to diabetes, such as neuropathy, hyperlipidemia, inflammation et al. However, some problems were listed, including 1) The dose-efficacy relationship and mechanism of B.purpurascens or extract also needed to be clarified; 2) There were no positive controls in all of these experiments, and metformin might be a suitable option; 3) These studies were only animal and cell experiments, lacking on the mechanisms of clinical research.
5.6. Preventive and ameliorative Alzheimer's disease
Multiple benefits of bergenin for Alzheimer disease (AD) were evaluated in vitro and vivo [66]. In vitro experiment, the effect of bergenin on the activity of SH-SY5Y was tested, acetylcholinesterase and butyrylcholinesterase inhibition assay, and resazurin reduction assays. In 96 well plates, the cells were treated with 100 μL solution of bergenin solubilized and incubated for 48 h. Each test was conducted three times to ensure statistical significance. Then, the preventive effect of bergenin on the cytotoxicity of SHSY5Y induced by N-methyl-d-aspartate (NMDA) was evaluated. In vivo experiment [66], rats were also induced by streptozotocin and formation of AD rat model (3 mg/kg, intracerebral ventricular, unilateral).The AD mice were divided into seven groups(n = 6 per group), including normal control group (no surgery, 28 days, dosed orally 0.3 % sodium carboxymethylcellulose, NaCMC), pseudo control group (ICV injection; oral NaCMC; 28 days), standard control group (DPZ, donepezil hydrochloride, 5 mg/kg), disease control group (ICV injection with STZ, oral NaCMC suspension), bergenin group (oral 20 mg/kg, 40 mg/kg, 80 mg/kg). Treatment with 80 mg/kg provided the greatest benefit, with a significant (p < 0.01) decrease in MEL and a significant (p < 0.001) increase in MPA; 6) Compared with NC group rats, the number of neurons in the hippocampus of DC group rats was significantly reduced (p < 0.05). Compared with DPZ, the average neuron count (n = 3) in the bergenin (80 mg/kg) group was the most significantly improved (p < 0.05). These results showed that the bergenin had inhibitory activity in vitro. Moreover, the extract could prevent neuronal death. In addition, bergenin was considered to be a positive regulator of neuroinflammation. Although evaluation of bergenin for its activity on multiple targets on AD, these studies are still animal and cell experimental level. Hence, there is no positive control in the experiment, and rivastatin may be a suitable option.
5.7. Liver protection
In the light of studies, B.purpurascens might possess potential of liver protection. The protective effect and possible mechanism of bergenin on primary cultured rat hepatocytes induced by galactosamine poisoning [60]. The release of alanine aminotransferase and sorbitol dehydrogenase at 100 mM bergenin was blocked by 50.9 % and 45.1 %, respectively. Compared with control hepatocytes, it was not attacked with galactosamine which reduced RNA synthesis by a factor of 2.5. Therefore, these researchers thought that bergenin had the protective effect on liver cells in rats with galactosamine poisoning by inhibiting the release of glutamic pyruvate transaminase and sorbitol dehydrogenase and increasing RNA synthesis. This study evaluated the protective effect of B.purpurascens on liver cells, providing some experimental evidence for the treatment of liver injury related diseases with bergenin from B.purpurascens. However, this study is lacking of positive control and animal experiment. It is necessary to study the relationship between effect and dose of the extracts in liver injury models.
|
Table 2 (continued) | |||
|---|---|---|---|
| Effects | Active components/compounds | Source | Reference(s) |
| Antioxidant and Antimalarial activity | bergenin(1) | root, rhizome | [1,65,67] |
| 11-O-Galloylbergenin (3) | root, rhizome, leaf | [29] | |
| Gallic acid (17) | root, rhizome | [29] | |
| Quercetin (24) | root, rhizome | [29] | |
| Preventive and ameliorative Alzheimer's disease | Bergenin (1) | root, rhizome | [66] |
5.8. Antioxidant and antimalarial activity
The contents of B.purpurascens are a preliminary study on antioxidant and antimalarial effects. Bergenin and 11-O-galloylbergenin of the plant were useful for various pathological conditions related to the destructive effects of reactive oxygen species [29].Two compounds exhibited significant activity at low concentrations with IC50 value below 2.5 μg/mL, while the IC50 value of chloroquine as a positive control was 0.028 μM. Especially from the analysis of total antioxidant phosphomolybdic acid as ascorbic acid equivalent (CAHT), 11-O-gallic acid based cabbagenin was an effective antioxidant in the model of antioxidant activity in vitro. At the same time, the antimicrobial activity of methanolic extract of B.purpurascens in neonatal rats were evaluated in vitro [68]. (+)-catechin 3-O-gallate (49) were the radical scavenging and anti-α-glucosidase principles. Compound 49 and the crude extract were weak acetylcholinesterase inhibitors, indicating an inhibitory effect on α- Glucosidase had high selectivity. Compared to compound 49, the main components of this plant were poor free radical scavengers and enzyme inhibitors, including bergenin and arbutin. Ultimately, although 11-O-galloylbergenin, bergenin and its synthetic derivatives showed excellent antioxidant activities, the studies did not study on action mechanism of these active components. Finally, the antioxidant activity of B.purpurascens was measured by FRAP, NBT, and DPPH and positive control was ascorbic acid [28]. The experiment showed that the absorbance increased as the concentration of the extract increased from 12.5 to 200 μg/ml of the extract. Similarly, the superoxide scavenging activity of B.purpurascens extract(200 μg/mL) evaluated by NBT assay was 66.26 %, and the scavenging activity of ascorbic acid was 87.66 %. The antioxidant activity of B.purpurascens was also positively correlated with antioxidant activity.
6. Toxicology
Due to its diverse pharmacological activities, B.purpurascens may become the main herbal medicine for anticancer drugs. Apart from bergenin, few studies have been conducted on the toxicity of these extracts or compounds. Bergenin inhibited 8 human liver CYP450 isoforms, using human liver microsomesenzyme kinetics and time-dependent inhibition (HLMs) in vitro [69]. Bergenin and its natural derivatives had no significant cytotoxicity, which allowed them to be used as a lead compound in the synthesis of new therapeutic agents that might be more effective and specific treatment of various ulcers without harmful side effects [1,12]. In the light of micronucleus, chromosomal aberration and sister chromatid exchange frequency tests, some researchers investigated toxicity of this plant, which neither induces micro nucleus formation nor chronosomal aberration and SCE [70]. Also, Bergenin led to dose-dependent prevention inducing toxicity in the cells [66]. However, it is not enough to fully understand the toxicity on this herb. In the safety evaluation of future studies, the toxicological effects of B.purpurascens are also considered on the central nervous system, cardiovascular system, respiratory system, urinary system, gastrointestinal system et al. However, the point could not be proved on the experiment evidences. Therefore, it is necessary to study the toxicity of bergenin and its derivatives.
7. Applications
After extensive clinical validation, the efficacy of compound preparations or B.purpurascens alone is relatively stable and improved, used as an antitussive expectorant in People's Republic of China Pharmacopoeia. The pharmaceutical formulations of the extracts or compounds from B.purpurascens are usually compound bergenin tablets, qingjin syrup, compound Hu ear tablets, qingfei antitussive syrup, liver poison net particles, kechuanping oral solution, and silicone lung oral solution. The arbutin of B.purpurascens contained beneficial for diuresis and antibiosis and inhibited degradation of insulin [12]. In addition, bergenin or the extract from the plant was used to treat painful diabetic neuropathy, arthritis and osteoarthritis by multi-component, multi-target, multi-channel collaborative [2]. B.purpurascens tablets was used to treat patients with esophageal epithelial cell proliferation and inflammation for five years in Lang Zhong and Yan Ting Counties, Sichuan Province, the cure rate of 88.7 % was achieved [70]. However, most of the related application mechanisms still are not known. Thus as, it is need to research these application and action mechanisms of B.purpurascens.
8. Conclusions
After reviewing the traditional uses, chemistry, pharmacology, and applied research of B.purpurascens, some challenges are proposed for the future research of this plant. In chemistry study, UPLC-Q/TOF-MS is only used to identify the structure of phytochemical components, and NMR is not used. In addition, by different solvents and methods, the studies that the quantity and type of extracts from this plant are different. Most chemical research focuses on the main components, while systematic research on other components is rare. Further, the anticancer mechanism analysis and new activity of bergenin and its derivatives have not also been clarified. Although many studies have provided some experimental evidence which are the anti-inflammatory and antibacterial activities of B.purpurascens, the mechanism of the active components is still unclear in clinical studies.
Limited data showed that antioxidant and antimalarial activity activities were relative with bergenin and 11-O-galloylbergenin. Compared to bergenin and its synthetic derivatives, 11-O-galloyl bergenin exhibits excellent antioxidant and antimalarial activities. Interestingly, the bergenin showed currently good and new activities, such as restored insulin secretory activity, liver protection, preventive and ameliorative AD. However, the experiments are animal and cell experiments, seldom systematic clinical data elevate these activities. In addition, bergenin is used to treat painful diabetes neuropathy, which is a mouse model of streptozotocin induced painful diabetes neuropathy. Despite the activity of bergenin against multiple targets of AD in vitro and in vivo, the action mechanisms of bergenin are still animal and cell experimental level, no positive control in the experiment. Clinical research is not also clear about the mechanism of action of the active components in the extract. Hence, it is required to investigate these pharmacology activities of this plant by multi-component, multi-target and multi-channel collaboration.
In summary, this review also proposes ideas for the research and development of this herbal medicine and shortcomings in new pharmacological research. In addition, new pharmacology has also been discovered for bergenin and its derivatives. We also summarized and analyzed the importance of these pharmacology and provided some new research directions for the ethnic medicine. Although the analysis described above, further investigation of the B.purpurascens is still required, most chemical studies have focused on the roots and stems of this plant. However, the chemical composition and pharmacological activity of the flowers or fruits have not been recorded in these reports. At the same time, there has been no research report on the differences between the main production regions and seasons of the same component. Nowadays, B.purpurascens has been widely used in the treatment of cough diseases, such as antibacterial and anti-inflammatory effects. Additionally, the pharmacological action of the bergenin was found restoring insulin secretory activity, liver protection, preventive and ameliorative AD. Bergenin has various pharmacological activities and may become a leading compound of anticancer drugs. Most of the related mechanisms still remain unknown, it is necessary to furtherly research the pharmacological action of B.purpurascens, which is more efficient and safer in clinic.
Funding
This work is funded by the fifth batch of national excellent clinical talents of traditional chinese medicine research and training program of the state Administration of traditional chinese medicine (No.: G.Z.Y.J.H. [2022] No. 1). At the same time, this work is also funded by national and provincial science technology innovation talent teams cultivation project of Guizhou university of traditional Chinese medicine (No. Gui Traditional Chinese Medicine TD He Zi [2022] No. 005).
Data availability statement
Data included in article/supp. material/referenced in article.Data associated with the study been deposited into a publicly available repository.All the available data on this paper was retrieved from various databases including PubMed, Embase, CINAHL, Chinese Biomedical Literature Database, VIP Database for Chinese Technical Periodicals, China National Knowledge Infrastructure (CNKI), and Wan fang.
CRediT authorship contribution statement
Yi Liu: Writing – review & editing, Writing – original draft, Data curation. Zhenxiang An: Resources, Project administration, Methodology, Funding acquisition. Yuanli He: Writing – review & editing, Methodology, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22249.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
References
- 1.Bajracharya G.B. Diversity, pharmacology and synthesis of bergenin and its derivatives: potential materials for therapeutic usages. Fitoterapia. 2015;101:133–152. doi: 10.1016/j.fitote.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Villarreal C.F., Santos D.S., Lauria P.S., Gama K.B., Espírito S.R., Juiz P.J. Bergenin reduces experimental painful diabetic neuropathy by restoring redox and immune homeostasis in the nervous system. Inter.J.Mol.Sci. 2020;21:4850. doi: 10.3390/ijms21144850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan J., Gu C., Huang S. First, Beijing Science PressBei Jing; 2001. Flora of China. [Google Scholar]
- 4.Commission S.P. China Medico-Pharmaceutical Science & Technology Publishing House; Bei Jing: 2020. Pharmacopoeia of the People's Republic of China 2020, Forth. [Google Scholar]
- 5.Lv X.M., Wang J.X. Research progress in genus Bergenia plants. J. Chin. Med. Mater. 2003;26:58–60. [PubMed] [Google Scholar]
- 6.Medica Y.N. First, Yunnan Science and Technology Press; Kunming: 2006. Important Natural Medicine in Yunnan. [Google Scholar]
- 7.Plants of the World Online. 2023. http://www.plantsoftheworldonline.org/ [Google Scholar]
- 8.Meng W., You H.N., Yan F.W. Research progress on the anti-tumor activity of bergenin and its derivatives. J. Pharm. Res. 2018;37:308–412. [Google Scholar]
- 9.Encyclopedia of Traditional Chinese Medicines. 2023. www.springer.com [Google Scholar]
- 10.Xu L.J., Liu A.L., Du G.H. 2018. Bergenin, Natural Small Molecule Drugs from Plants; pp. 379–384. [Google Scholar]
- 11.Ou Z.L.J., Dawa T., Luo B., Zha X.C. Development of a sunscreen skin care product based on the formula of Tibetan folk sunscreen cream. J. Tibet. Uni. 2011;26:36–38. [Google Scholar]
- 12.Li W.C., Gou F.G., Zhang L.M., Yu H.M., Li X., Lin C. The situation and prospect of research on Bergenia purpurascens. J. Yunnan. Agri. Uni. 2006;21:845. [Google Scholar]
- 13.Pokhrel P., Parajuli R.R., Tiwari A.K., Banerjee J. A short glimpse on promising pharmacological effects of Begenia ciliata. J. Appl. Pharm. Res. 2014;2:1–6. [Google Scholar]
- 14.Liu S.J., Yu B., Hu C.H. The Variation of POD activities in Bergenla Tianquanensis in tissue culture progress. Adv.Mat.Res. 2011:196–200. [Google Scholar]
- 15.Popov S., Ovodova R., Popova G.Y., Nikitina I., Ovodov Y.S. Inhibition of neutrophil adhesion by pectic galacturonans. Russ. J. Bioorg. Chem. 2007;33:175–180. doi: 10.1134/s1068162007010219. [DOI] [PubMed] [Google Scholar]
- 16.Golovchenko V., Bushneva O., Ovodova R., Shashkov A., Chizhov A., Ovodov Y.S. Structural study of bergenan, a pectin from Bergenia crassifolia. Russ.J.Bioorg.Chem. 2007;33:47–56. doi: 10.1134/s1068162007010050. [DOI] [PubMed] [Google Scholar]
- 17.Xin M.C., Yoshida T., Hatano T., Fukushima M., Okuda T. Galloylarbutin and other polyphenols from Bergenia purpurascens. Phytochemistry. 1987;26:515–517. [Google Scholar]
- 18.Siddiq F., Fatima I., Malik A., Afza N., Iqbal L., Lateef M. Biologically active bergenin derivatives from Bergenia stracheyi. Chem. Biodivers. 2012;9:91–98. doi: 10.1002/cbdv.201100003. [DOI] [PubMed] [Google Scholar]
- 19.Banzragchgarav O., Murata T., Tuvshintulga B., Suganuma K., Igarashi I., Inoue N. Chemical constituents of Bergenia crassifolia roots and their growth inhibitory activity against Babesia bovis and B. bigemina. Phytoche. Lett. 2019;29:79–83. [Google Scholar]
- 20.Chen W.D., Nie M.H. HPLC determination of bergenin in Astilbe chinensis (Maxim.) Franch. et Sav. and Bergenia purpurascens (Hook. F. et Thoms.) Engl. Yao Xue Xue Bao. 1988;23:606–609. [PubMed] [Google Scholar]
- 21.Chandrareddy U.D., Chawla A.S., Mundkinajeddu D., Maurya R., Handa S.S. Paashaanolactone from Bergenia ligulata. Phytochemistry. 1998;47:907–909. [Google Scholar]
- 22.Zhang S.S., Liao Z.X., Huang R.Z., Gong C.C., Ji L.J., Sun H.F. A new aromatic glycoside and its anti-proliferative activities from the leaves of Bergenia purpurascens. Nat. Prod. Res. 2018;32:668–675. doi: 10.1080/14786419.2017.1338278. [DOI] [PubMed] [Google Scholar]
- 23.Bhat C., Murari R., Parthasarathy M., Seshadri T. Components of Bergenia strecheyi and B. ligulata. Ind. J. Chem. 1974;12:1038–1039. [Google Scholar]
- 24.Xiao L.S., Mao Z.W., Zuo A.X., Rao G.X. Studies on chemical constituents from the Bergenia pururascens. J. Yunnan Uni. Trad. Chin. Med. 2014;37 [Google Scholar]
- 25.Zhang S.S. Southeast university; 2017. Studies on Chemical Constituents of the Leaves of Bergenia purpurascens. [Google Scholar]
- 26.Song Y.I., Feng Z.B., Cheng Y.X., Gao J.M. Chemical components of Chaenomeles speciosa (Sweet) Nakai. Acta. Bot. Bor. Occ. Sin. 2007;27:831. [Google Scholar]
- 27.Jiang W. Southeast university; 2018. Studies on Chemical Constituents of the Roots of Bergenia Purpurascens and the Structural Modification of its Effective Constituent Ursolic Acid. [Google Scholar]
- 28.Liu B., Wang M., Wang X.N. Phytochemical analysis and anti-bacterial activity of methanolic extract of Bergenia purpurascens against common respiratory infection causing bacterial species in vitro and in neonatal rats. Microb.pathogenesis. 2018;117:315–319. doi: 10.1016/j.micpath.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 29.Uddin G., Sadat A., Siddiqui B. Comparative antioxidant and antiplasmodial activities of 11-O-galloylbergenin and bergenin isolated from Bergenia ligulata. Trop. Biomed. 2014;31:143–148. [PubMed] [Google Scholar]
- 30.Qu Y., Zhang C., Liu R., Wu H., Sun Y., Zhang N. Rapid characterization the chemical constituents of Bergenia purpurascens and explore potential mechanism in treating osteoarthritis by ultra high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry combined with network pharmacology. J.Sep.Sci. 2020;43:3333–3348. doi: 10.1002/jssc.201901284. [DOI] [PubMed] [Google Scholar]
- 31.Yu K.Y., Gao W., Li S.Z., Wu W., Li P., Dou L.L. Qualitative and quantitative analysis of chemical constituents in Ardisiae Japonicae Herba. J.Sep.Sci. 2017;40:4347–4356. doi: 10.1002/jssc.201700667. [DOI] [PubMed] [Google Scholar]
- 32.Chen X.M., Takashi Y., Tsutomu H., Makoto F., Takuo O. Galloylarbutin and other polyphenols from Bergenia purpurascens. Phytochemistry. 1987:26. [Google Scholar]
- 33.Xing G.S., Wen H.H., Miao M., Bao L.G., Gui Y.W. Comparative studies on content of arbutin, bergenin and catechin in different part of Bergenia purpurascens and B.crassifolia. J.Chin.Mater. Med. 2010;35:2079–2082. [PubMed] [Google Scholar]
- 34.Ivanov S.A., Nomura K., Malfanov L.L., Sklyar L.V., Ptitsyn L.R. Isolation of a novel catechin from Bergenia rhizomes that has pronounced lipase-inhibiting and antioxidative properties. Fitoterapia. 2011;82:212–218. doi: 10.1016/j.fitote.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava S., Rawat A.K.S. Botanical and phytochemical comparison of three Bergenia species. J.Sci.Ind. Res. 2008;67:65–72. [Google Scholar]
- 36.Srivastava N., Srivastava A., Srivastava S., Rawat A.K.S., Khan A.R. HPTLC-densitometric determination and kinetic studies on antioxidant potential of monomeric phenolic acids (MPAs) from Bergenia species. Rsc. Adv. 2014;4 [Google Scholar]
- 37.Sumino M., Sekine T., Ruangrungsi N., Igarashi K., Ikegami F. Ardisiphenols and other antioxidant principles from the fruits of Ardisia colorata. Chem. Pharm. Bull. 2002;50:1484–1487. doi: 10.1248/cpb.50.1484. [DOI] [PubMed] [Google Scholar]
- 38.Li B.H., Wu J.D., Li X.L. LC-MS/MS determination and pharmacokinetic study of bergenin, the main bioactive component of Bergenia purpurascens after oral administration in rats. J.Pharm.Anal. 2013;3:229–234. doi: 10.1016/j.jpha.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zbikowska B., Franiczek R., Sowa A., Polukord G., Krzyzanowska B., Sroka Z. Antimicrobial and antiradical activity of extracts obtained from leaves of five species of the genus Bergenia: identification of antimicrobial compounds. Microb. Drug Resist. 2017;23:771–780. doi: 10.1089/mdr.2016.0251. [DOI] [PubMed] [Google Scholar]
- 40.Khan A., Jan G., Khan A., Jan F.G., Danish M. Evaluation of antioxidant and antimicrobial activities of Bergenia ciliata Sternb (Rhizome) crude extract and fractions. Pak. J. Pharm. Sci. 2018;31:31–35. [PubMed] [Google Scholar]
- 41.Kokoska L., Polesny Z., Rada V., Nepovim A., Vanek T. Screening of some Siberian medicinal plants for antimicrobial activity. J. Ethnopharmacol. 2002;82:51–53. doi: 10.1016/s0378-8741(02)00143-5. [DOI] [PubMed] [Google Scholar]
- 42.Shi X., Li X., He J., Han Y., Li S., Zou M. Study on the antibacterial activity of Bergenia purpurascens extract. Afr. J. Tradit., Complementary Altern. Med. : AJTCAM. 2014;11:464–468. doi: 10.4314/ajtcam.v11i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rauf A., Patel S., Uddin G., Siddiqui B.S., Ahmad B., Muhammad N. Phytochemical, ethnomedicinal uses and pharmacological profile of genus Pistacia. Biomed. Pharmacother. 2017;86:393–404. doi: 10.1016/j.biopha.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Shah M.R., Arfan M., Amin H., Hussain Z., Qadir M.I., Choudhary M.I. Synthesis of new bergenin derivatives as potent inhibitors of inflammatory mediators NO and TNF-α, Bioor. Med.Chem.Lett. 2012;22:2744–2747. doi: 10.1016/j.bmcl.2012.02.096. [DOI] [PubMed] [Google Scholar]
- 45.Żbikowska B., Franiczek R., Sowa A., Połukord G., Krzyżanowska B., Sroka Z. Antimicrobial and antiradical activity of extracts obtained from leaves of five species of the genus Bergenia: identification of antimicrobial compounds. Microb. Drug Resist. 2017;23:771–780. doi: 10.1089/mdr.2016.0251. [DOI] [PubMed] [Google Scholar]
- 46.Taneyama M., Yoshida S. Studies on C-Glycosides in Higher Plants,(2). Incorporation Of/sup 14/C-Glucose into Bergenin and Arbutin in Saxifraga Stolonifera. Shokubutsugaku Zasshi; 1979. p. 92. (Japan) [Google Scholar]
- 47.Fiuza S.M., Gomes C., Teixeira L.J., Dacruz M.G., Cordeiro M.N.D., Milhazes N. Phenolic acid derivatives with potential anticancer properties––a structure–activity relationship study. Part 1: methyl, propyl and octyl esters of caffeic and gallic acids. Bioor.Med.Chem. 2004;12:3581–3589. doi: 10.1016/j.bmc.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 48.Chauhan S.K., Singh B., Agrawal S. Simultaneous determination of bergenin and gallic acid in Bergenia ligulata wall by high-performance thin-layer chromatography. J. AOAC Int. 2000;83:1480–1483. [PubMed] [Google Scholar]
- 49.Jie Z., Yu K.N., Harukuni T., Nobutaka S., Ken Y., Worapong K. Cancer chemopreventive effect of bergenin from Peltophorum pterocarpum wood. Chem. Biodivers. 2013;10:1866–1875. doi: 10.1002/cbdv.201300182. [DOI] [PubMed] [Google Scholar]
- 50.Jing W.L., Ying Y., Jing F.C., Zhi B.Z., Yong X.Q., Tie T.D. Third. People's Health Publishing House; Shanghai: 2020. Dictionary of Chinese Medicine. [Google Scholar]
- 51.Hua Z., Cao B. vol. 11. Shanghai Scientific and Techincal Publishers; 1999. pp. 48–49. (Committee of State Traditional Chinese Medicine Administration). [Google Scholar]
- 52.Nigel W., Feng Y. paradigm publications; Sixth: 1998. A Practical Dictionary of Chinese Medicine. [Google Scholar]
- 53.Zhang R.F., Eggleston K., Rotimi V., Zeckhauser R.J. Antibiotic resistance as a global threat: evidence from China, Kuwait and the United States, Globalization. health. 2006;2:1–14. doi: 10.1186/1744-8603-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paterson D.L. Impact of antibiotic resistance in gram-negative bacilli on empirical and definitive antibiotic therapy. Clin. Infect. Dis. 2008;47:S14–S20. doi: 10.1086/590062. [DOI] [PubMed] [Google Scholar]
- 55.Shan B., Cai Y.Z., Brooks J.D., Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007;117:112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y.X., Yan J.W., Yan F.L., Yin Y.Y., Zhuang F.F. Synthesis and anti-tumour activity evaluation of bergenin derivatives. J.Chem.Res. 2015;39 [Google Scholar]
- 57.Jing L., Su S., Zhang D., Li Z., Lu D., Ge R. Srolo Bzhtang, a traditional Tibetan medicine formula, inhibits cigarette smoke induced airway inflammation and muc5ac hypersecretion via suppressing IL-13/STAT6 signaling pathway in rats. J. Ethnopharmacol. 2019;235:424–434. doi: 10.1016/j.jep.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Liu C.X. Pay attention to situation of SARS-CoV-2 and TCM advantages in treatment of novel coronavirus infection. Chin. Herb. Med. 2020;12:97–103. doi: 10.1016/j.chmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuo G.Y., Li Z.Q., Chen L.R., Xu X.J. In vitro anti-HCV activities of Saxifraga melanocentra and its related polyphenolic compounds. Antivir. Chem. Chemother. 2005;16:393–398. doi: 10.1177/095632020501600606. [DOI] [PubMed] [Google Scholar]
- 60.Lim H.K., Kim H.S., Chung M.W., Kim Y.C. Protective effects of bergenin the major constituent of Mallotus japonicus on D-galactosamine-intoxicated rat hepatocytes. J. Ethnopharmacol. 2000;70 doi: 10.1016/s0378-8741(99)00138-5. [DOI] [PubMed] [Google Scholar]
- 61.Mansoor M., Bhagyarao D., Rao D.S. Photochemical analysis and hepatoprotective activity of Saxifraga ligulat leaves extract. J. Sci. Res. Pharm. 2015;4:93–97. [Google Scholar]
- 62.Shutov D. Hepatoprotective effect of Bergenia crassifolia extract and silymarin at experimental inhibition of (3-oxidation of fatty acids caused by 4-pentenioc acid. Bull. Sib. Med. 2007;7:64–70. [Google Scholar]
- 63.Kumar R., Patel D.K., Prasad S.K., Laloo D., Krishnamurthy S., Hemalatha S. Type 2 antidiabetic activity of bergenin from the roots of Caesalpinia digyna Rottler. Fitoterapia. 2012;83:395–401. doi: 10.1016/j.fitote.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Rajput S.A., Mirza M.R., Choudhary M.I. Bergenin protects pancreatic beta cells against cytokine-induced apoptosis in INS-1E cells, PloS. One. 2020;15 doi: 10.1371/journal.pone.0241349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim M.H., Ha S.Y., Oh M.H., Kim H.H., Kim S.R., Lee M.W. Anti-oxidative and anti-proliferative activity on human prostate cancer cells lines of the phenolic compounds from Corylopsis coreana. Uyeki.Molecules. 2013;18:4876–4886. doi: 10.3390/molecules18054876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barai P., Raval N., Acharya S., Borisa A., Bhatt H., Acharya N. Neuroprotective effects of bergenin in Alzheimer's disease: investigation through molecular docking, in vitro and in vivo studies. Behav. Brain Res. 2019;356:18–40. doi: 10.1016/j.bbr.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Nazir N., Koul S., Qurishi M.A., Taneja S.C., Ahmad S.F., Bani S. Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis-a flow cytometric study. J.Ethnopharmacology. 2007;112:401–405. doi: 10.1016/j.jep.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 68.Roselli M., Lentini G., Habtemariam S. Phytochemical, antioxidant and anti-α-glucosidase activity evaluations of Bergenia cordifolia. Phytother Res. 2012;26:908–914. doi: 10.1002/ptr.3655. [DOI] [PubMed] [Google Scholar]
- 69.Dong G., Zhou Y., Song X.S. In vitro inhibitory effects of bergenin on human liver cytochrome P450 enzymes. Pharm. Biol. 2018;56:620–625. doi: 10.1080/13880209.2018.1525413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai Y. Experimental studies with Bergenia purpurascens, the frequency of micronucleus in NIH mice, the frequency of SCE, and chromosome aberration in human lymphocytes. Acta. Academ. Med. Sin. 1991;13:73–77. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.Data associated with the study been deposited into a publicly available repository.All the available data on this paper was retrieved from various databases including PubMed, Embase, CINAHL, Chinese Biomedical Literature Database, VIP Database for Chinese Technical Periodicals, China National Knowledge Infrastructure (CNKI), and Wan fang.