Abstract
Human U1 and U6 snRNA genes are transcribed by RNA polymerases II and III, respectively. While the p53 tumor suppressor protein is a general repressor of RNA polymerase III transcription, whether p53 regulates snRNA gene transcription by RNA polymerase II is uncertain. The data presented herein indicate that p53 is an effective repressor of snRNA gene transcription by both polymerases. Both U1 and U6 transcription in vitro is repressed by recombinant p53, and endogenous p53 occupancy at these promoters is stimulated by UV light. In response to UV light, U1 and U6 transcription is strongly repressed. Human U1 genes, but not U6 genes, contain a high-affinity p53 response element located within the core promoter region. Nonetheless, this element is not required for p53 repression and mutant p53 molecules that do not bind DNA can maintain repression, suggesting a reliance on protein interactions for p53 promoter recruitment. Recruitment may be mediated by the general transcription factors TATA-box binding protein and snRNA-activating protein complex, which interact well with p53 and function for both RNA polymerase II and III transcription.
The p53 tumor suppressor protein plays a critical role in preventing unwarranted cellular proliferation by activating transcription of key target genes that influence cell growth and apoptosis (reviewed in references 28, 31, 35, and 64). Though p53 can enable both pathways, the switch controlling which cellular outcome is enacted is uncertain (reviewed in references 65 and 66), but both the p53 level and the nature of the DNA damage can influence apoptotic response (8). Altogether, p53 activity serves to prevent passage of mutations to daughter cells after DNA damage.
Recent evidence suggests that p53 regulates transcription of genes that are not obviously involved in controlling cell cycle arrest or apoptosis. Indeed, p53 can repress RNA polymerase I (3, 72) and III (5, 9) transcription of genes encoding a variety of nontranslated RNAs that play critical roles at numerous points during global gene expression. RNA polymerase III activity is elevated in p53−/− knockout fibroblasts (5) and in a variety of cancer-derived cell lines that lack p53 function (57). However, the mechanism for p53 regulation of RNA polymerase III transcription is controversial. A kinetic analysis of RNA polymerase III repression using p53 expressed from a stably integrated inducible p53 gene suggested that RNA polymerase III repression is mediated indirectly through p53-dependent degradation of TFIIIB (11). In contrast, recombinant p53 can repress in vitro transcription from a variety of RNA polymerase III-specific promoters and can interact with components of the general transcription machinery required for RNA polymerase III transcription (5, 9, 10, 58), indicating that p53 might directly repress transcription by RNA polymerase III.
Within the group of genes transcribed by RNA polymerase III, the human snRNA gene family is intriguing because these genes contain similar sets of promoter elements, and yet only some genes are transcribed by RNA polymerase III while others are transcribed by RNA polymerase II (see references 19, 23, 24, and 42 for review). Regardless of polymerase specificity, human snRNA genes contain a distal sequence element in the upstream promoter region that serves as the recognition element for activator proteins, including Oct-1, STAF, and Sp1 (33, 54). These factors activate transcription from the core promoters that commonly contain a proximal sequence element (PSE). The PSE is directly recognized by a general transcription factor called the snRNA activating protein complex (SNAPC) (52), which is also known as the PSE transcription factor (49). SNAPC is involved in human snRNA gene transcription by both RNA polymerases II and III (20-22, 51, 69). RNA polymerase III-transcribed snRNA genes also contain a TATA box that serves to recruit the TATA-box binding protein (TBP) as part of an snRNA-specific TFIIIB complex (45, 55, 60).
The conservation of important promoter elements among human snRNA genes suggests that transcription of these genes by RNA polymerases II and III may be coordinately regulated. However, it is not known whether p53 can regulate human snRNA gene transcription by RNA polymerase II. A role for p53 in this process is suggested from two sources. Firstly, in response to UV light treatment, human U1 and U2 snRNA genes exhibit a delayed and prolonged reduction in transcription by RNA polymerase II (14, 47, 48). In part, this reduction may be attributable to increased hyperphosphorylation of the carboxy-terminal domain of the RNA polymerase II largest subunit in response to UV light (27). However, in normal human diploid fibroblasts, the balance of hyper- and hypophosphorylated RNA polymerase II is restored by 6 h after UV light treatment (46), suggesting additional cellular mechanisms that enable snRNA gene repression after UV light exposure. Potentially, p53 activation by DNA damage might play a direct role in the prolonged repression of these genes.
Secondly, infection of human cells by adenovirus serotype 12 causes metaphase fragility at four chromosomal sites, including the U1 snRNA (RNU1) and U2 snRNA (RNU2) loci (1, 36), in a process that requires p53 (38, 39). It was postulated that fragile site formation occurs during viral infection, because RNA polymerase II stalls at these genes and interferes with chromosome condensation during metaphase (37). Interestingly, p53 that harbors mutations in the DNA binding domain supports fragile site formation (39), and overexpression of the C-terminal domain of p53 alone, which lacks the DNA binding domain, induces fragility during transient transfection (71). Together, these data indicate that p53 is important for generation of fragile sites at the U1 and U2 snRNA gene loci and may play a role in regulation of these genes in a fashion that does not require sequence-specific binding of p53 to DNA.
In this study, the role of p53 in governing human snRNA gene transcription by RNA polymerase II and III was examined. We show that recombinant p53 represses both U1 and U6 transcription by RNA polymerase II and III, respectively. Repression is supported by the C-terminal region of p53 alone, indicating that sequence-specific DNA binding by p53 is not critical for repression. Both the full-length and C terminus of p53 alone can associate with the U1 and U6 promoters during repression, and promoter recruitment may be assisted through interactions with the general transcription factors SNAPC and TBP, which are commonly required for transcription of both U1 and U6 snRNA genes. In vivo, p53 can bind to both U1 and U6 snRNA genes in untreated human MCF-7 cells, and promoter occupancy is stimulated after UV light treatment. These results further indicate that p53 contributes to snRNA gene regulation in response to DNA damage.
MATERIALS AND METHODS
Cell culture and UV irradiation.
Human MCF-7 and HeLa cells were maintained in Dulbecco's modified Eagle's medium supplemented with penicillin-streptomycin and 10% (MCF-7) or 5% (HeLa) fetal bovine serum. Cells grown to 70 to 80% confluence were washed with phosphate-buffered saline and irradiated with 50 J of UV light (254-nm peak)/m2 by using a UV Stratalinker (Stratagene). After irradiation, growth medium was added and cells were incubated at 37°C under 5% CO2 for the indicated times. Additionally, HeLa cells were grown to 50% confluence in 150-mm plates and were then transiently transfected with 2.5 μg of the pRc/RSV or pRc/RSV-p53-Flag.wt plasmids by using Lipofectin reagent (Invitrogen) for 6 h. Subsequently, the medium was replaced and cells were incubated for 48 h for further analysis in nuclear run-on assays.
Nuclear run-on assays.
Nuclear run-on assays were performed as described elsewhere (6) in the presence of [α-32P]UTP using approximately 107 nuclei that were isolated from MCF-7 or HeLa cells before or 8 h after exposure to UV light. Additional assays were performed using HeLa cells transiently transfected with pRc/RSV or pRc/RSV-p53-Flag.wt as described. Labeled RNA was recovered and hybridized to a nitrocellulose membrane containing approximately 7 μg of U1, U6, and 5S rRNA target gene DNAs or 10 μg of pUC119 plasmid DNA, as a negative control. Target gene DNAs corresponding to the coding regions of the indicated genes were generated by PCR and were immobilized on a nylon membrane at levels calculated to be in excess relative to the corresponding snRNA population in the nuclei. Hybridizations were performed for 16 h at 42°C in hybridization buffer containing 50% formamide. Membranes were then washed extensively in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate (SDS) at 50°C. Hybridized RNA transcripts were visualized by autoradiography for 7 days. Similar results were observed when signals were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression by varying the exposure time (data not shown).
RNA isolation and RT-PCR.
RNA was isolated using the TRIzol reagent as recommended by the manufacturer (Gibco-BRL). RNA preparations were quantified by UV spectrometry and examined for integrity by agarose formaldehyde morpholinepropanesulfonic acid gel electrophoresis. Reverse transcription-PCR (RT-PCR) was performed by a two-step procedure using U1-, U6-, and GAPDH-specific primers. The primers used for amplification of each gene were the following: U1 forward, 5′-ATACTTACCTGGCAGGGGAG-3′; U1 reverse, 5′-CAGGGGGAAAGCGCGAACGCA-3′; U6 forward, 5′-GGAATCTAGAACATATACTAAAATTGGAAC-3′; U6 reverse, 5′-GGAACTCGAGTTTGCGTGTCATCCTTGCGC-3′; GAPDH forward, 5′-AGGTCATCCCTGAGCTGAAC-3′; and GAPDH reverse, 5′-GCAATGCCAGCCCCAGCGTC-3′.
Expression and purification of recombinant proteins.
Glutathione S-transferase (GST), GST-tagged full-length human p53, and a GST-tagged C terminus of human p53 (amino acids 301 to 393) [p53 (301-393)] were expressed in Escherichia coli BL21(DE3) codon+ cells (Stratagene) and were affinity purified by binding to glutathione agarose beads (Sigma). GST and GST-p53 were then eluted from beads in HEMGT-150 buffer containing 50 mM glutathione for 4 h at 4°C or, alternatively, untagged p53 was obtained by digestion with thrombin. Proteins were further purified by chromatography using a Mono-Q (HR5/5) column (Pharmacia) and were concentrated by centrifugation using a Centricon YM-30 spin column (Millipore) in HEMGT-80 buffer (20 mM HEPES [pH 7.9], 0.1 mM EDTA, 10 mM MgCl2, 10% glycerol [vol/vol], 0.1% Tween 20, 80 mM KCl) containing protease inhibitors and 1 mM dithiothreitol.
In vitro transcription assays.
In vitro transcription assays were performed as described previously (21, 43) using 18, 2, 2, and 10 μl of HeLa cell nuclear extract for the U1 snRNA, U6 snRNA, 5S rRNA, and adenovirus major late promoter (AdML) transcription reactions, respectively. The pU1-4.0 (1 μg), pU6/Hae/RA.2 (250 ng), pH5Ssa (250 ng), and M13-AdML (250 ng) templates were used for the U1 snRNA, U6 snRNA, 5S rRNA, and AdML transcription reactions, respectively. Purified p53 or GST-tagged p53 proteins were added in the amounts indicated in the figure legends. Transcription reactions were performed for 1 h at 30°C. Transcripts were separated by denaturing 6% polyacrylamide gel electrophoresis (PAGE) and visualized by autoradiography.
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation assays were performed as described previously (25). Human MCF-7 cells were grown to 60 to 80% confluence and were then cross-linked with 1% formaldehyde for 30 min at room temperature. After cell lysis and sonication, immunoprecipitation reactions were performed overnight at 4°C using chromatin from approximately 107 cells per reaction mixture and 1 μg of each antibody. The anti-p53 antibodies used were the following: anti-p53 (21-25) (Ab-6; Oncogene), anti-p53 (371-380) (Ab-1; Oncogene), anti-p53 (213-217) (Ab240; Pharmingen), anti-acetyl-p53 (373 + 382) (Upstate), and anti-acetyl-p53 (320) (Upstate). Recovered chromatin was suspended in 50 μl of H2O, and PCR analysis was performed using 5 μl of immunoprecipitated chromatin or input chromatin. The primers used for amplification of each gene were the following: U1 forward, 5′-CACGAAGGAGTTCCCGTG-3′; U1 reverse, 5′-CCCTGCCAGGTAAGTATG-3′; U2 forward, 5′-AGGGCGTCAATAGCGCTGTGG-3′; U2 reverse, 5′-TGCGCTCGCCTTCGCGCCCGCCG-3′; U6 forward, 5′-GTACAAAATACGTGACGTAGAAAG-3′; U6 reverse, 5′-GGTGTTTCGTCCTTTCCAC-3′; GAPDH forward, 5′-AGGTCATCCCTGAGCTGAAC-3′; GAPDH reverse, 5′-GCAATGCCAGCCCCAGCGTC-3′; U1 upstream forward, 5′-GAACTTACTGGGATCTGG-3′; U1 upstream reverse, 5′-GAGACAACTGAGCCACTTG-3′; p21 upstream forward, 5′-CCGCTCGAGCCCTGTCGCAAGGATCC-3′; p21 upstream reverse, 5′-GGGAGGAAGGGGATGGTAG-3′.
PCR products were separated by 2% agarose electrophoresis in Tris-borate-EDTA buffer and were stained with ethidium bromide.
Immunoprecipitations from in vitro transcription reactions.
In vitro transcription assay mixtures containing U1 or U6 promoter plasmids and equal molar amounts of pUC119 were performed as described previously (25) in the absence or presence of full-length GST-p53 (wild-type or R175H), GST-p53 (301-393), or GST. Five microliters of each transcription reaction mixture was diluted to 500 μl and was cross-linked in 1% formaldehyde for 10 min at room temperature, quenched with 125 mM glycine for 10 min at room temperature, and immunoprecipitated with immunoglobulin G (IgG), anti-SNAP43 (CS48), or anti-p53 (Ab-1; Oncogene) antibodies. Recovered plasmid DNA was analyzed by PCR using primers specific to the U1 and U6 promoter regions or to pUC119 as a negative control.
DNase I footprinting.
Footprinting assays were generally performed as described elsewhere (4). Linear DNA encompassing the human U1 promoter from −151 to +13 was generated by PCR using primers that were end labeled with [γ-32P]ATP by using T4 polynucleotide kinase (New England BioLabs). U1 promoter probes were incubated with increasing amounts of recombinant GST-p53 for 40 min at room temperature and were then digested with 0.04 U of DNase I (Roche) for 2 min at room temperature. The resultant fragments were purified and separated by 8% denaturing PAGE. Footprints were visualized by autoradiography and were mapped relative to sequencing ladders generated from the same labeled primers used to generate the U1 promoter probes.
EMSA.
Electrophoretic mobility shift assays (EMSA) were performed as described elsewhere (7). The amounts of p53 used are indicated in the figure legends. Reaction mixtures were incubated at room temperature for 20 min prior to addition of radiolabeled probes. Unless otherwise noted, the U1 probe encompasses −312 to +13 and the U6 probe encompasses −267 to +1. DNA binding reactions were carried out at room temperature for 20 min, and resulting DNA-protein complexes were separated on a 4% polyacrylamide gel in 0.5× Tris-borate-EDTA running buffer at 150 V. Complexes were visualized by autoradiography.
Coimmunoprecipitation and GST pull-down experiments.
GST pull-down assays were performed as previously described (25). Coimmunoprecipitation assays were performed using 5 mg of total protein contained in MCF-7 nuclear extracts from untreated or UV-treated cells and 2 μg of rabbit anti-SNAP43 antibodies (CS48) (22). Western blot analyses of recovered proteins were performed using anti-SNAP43 (CS48), anti-p53 (Ab-6; Oncogene), and anti-galectin-3 (Mac2) antibodies. The reciprocal immunoprecipitation reactions were performed using 2 μg of anti-p53 antibody (Ab-6) with approximately 1.6 or 5 mg of MCF-7 extract, followed by anti-SNAP43 Western blot analysis.
RESULTS
p53 represses human snRNA gene transcription by both RNA polymerases II and III.
To determine whether p53 can repress U1 transcription by RNA polymerase II, as has been previously shown for U6 transcription by RNA polymerase III (5, 9), the effect of recombinant p53 on human U1 in vitro transcription was tested. The recombinant full-length wild-type p53 and the GST proteins used for these experiments are shown in Fig. 1A. As shown in Fig. 1B, p53 effectively repressed correctly initiated U1 transcription (labeled U1 5′) by RNA polymerase II, and this repressive effect was specific, because concomitant RNA polymerase II transcription of an mRNA read-through transcript derived from the same plasmid was unaffected in these reactions. As a positive control for p53 activity, human U6 snRNA gene transcription was tested. Indeed, the same amounts of p53 effectively repressed U6 snRNA transcription by RNA polymerase III, while RNA polymerase II transcription from the AdML was unaffected. Therefore, p53 can repress human snRNA gene transcription by both RNA polymerases II and III.
FIG. 1.
p53 represses human snRNA gene transcription by both RNA polymerases II and III in vitro. (A) Recombinant full-length wild-type p53 and GST proteins were separated by SDS-12.5% PAGE and were stained with Coomassie blue. (B) In vitro transcription from U1, U6, and AdML promoter constructs was tested using HeLa nuclear extracts containing 0, 50, 200, and 800 ng of p53 (lanes 1 to 4) or 800 ng of GST (lane 5). Fifty nanograms of p53 represents an approximate 2:1 molar ratio of monomeric p53 to U1 promoter template DNA and an approximate 8:1 molar ratio to the U6 and AdML promoter plasmids. p53 effectively repressed correctly initiated U1 transcription (U1 5′) and U6 transcription (U6 5′), but it didn't affect read-through (RT) transcription from the U1 reporter plasmid or transcription from the AdML promoter. (C) U1, U6, and 5S rRNA invitro transcription reaction mixtures were supplemented with 800 ng of active or heat-inactivated p53 (lanes 2 to 4 and lanes 5 to 7, respectively) at different times, as indicated. Transcription was allowed to proceed for an additional 60 min. Recombinant p53 repressed U6 gene transcription both prior to and after preinitiation complex assembly but did not repress U1 and 5S rRNA gene transcription after the formation of a preinitiation complex.
As a first step towards understanding the mechanism for p53 repression of U1 and U6 transcription, a time course for p53 repression was performed (Fig. 1C). As a positive control, p53 repression of 5S rRNA gene transcription was also examined. To ensure maximal repression, an excess of p53 was used, because 5S rRNA gene transcription appears less sensitive to p53 repression (9) (data not shown). As was demonstrated previously (5), p53 can repress 5S rRNA transcription when added to reactions concomitantly with nuclear extract (lane 2) or nuclear extract plus template DNA (lane 3) prior to initiation of transcription by nucleotide addition. However, p53 did not repress 5S rRNA transcription when the nuclear extract was preincubated with template DNA prior to p53 addition (lane 4). Presumably, p53 cannot repress 5S rRNA gene transcription after the formation of a preinitiation complex. For all time points, repression was specific for functional p53, because repression was disabled by heat inactivation of p53 (lanes 5 to 7). In contrast, p53 could effectively repress U6 transcription by RNA polymerase III even after the nuclear extract had been preincubated with the template DNA. This result suggests that the U6 preinitiation complex is not recalcitrant to p53 repression. Surprisingly, the pattern for p53 repression of U1 transcription by RNA polymerase II was similar to that of 5S rRNA rather than the U6 repression pattern. This observation suggests that formation of a preinitiation complex could render U1 snRNA genes refractory to p53 repression. This result also suggests that p53 represses U1 and U6 snRNA gene transcription by different mechanisms.
As UV light exposure activates p53, this treatment was used here to determine whether p53 is involved in human snRNA transcriptional regulation in vivo. The majority of in vivo studies presented herein were performed with human MCF-7 breast adenocarcinoma cells, because these cells exhibit a robust increase in p53 levels in response to UV light treatment (Fig. 2A, lanes 4 to 6) compared to human HeLa cervical carcinoma cells, wherein p53 levels are low and remain unchanged after UV light exposure (lanes 1 to 3).
FIG. 2.
UV light inhibits snRNA gene transcription and stimulates p53 binding to human snRNA gene promoters. (A) Whole-cell extracts from untreated and UV light-treated HeLa (lanes 1 to 3) and MCF-7 (lanes 4 to 6) cells were analyzed by SDS-12.5% PAGE and Western blot analysis of endogenous p53 and actin. MCF-7 cells exhibited robust accumulation of endogenous p53 in response to UV light treatment, whereas no changewas observed in HeLa cells. (B) UV light represses transcription of endogenous human U1 and U6 snRNA genes in MCF-7 cells, but not in HeLa cells. Nuclear run-on assays measuring polymerase density at U1 snRNA, U6 snRNA, and 5S rRNA genes in nuclei from untreated HeLa cells (lane 1) or MCF-7 cells (lane 3) were compared to results with nuclei harvested 8 h after UV light treatment (lanes 2 and 4). After hybridization, membranes were exposed to film for 7 days. Similar trends were also obtained when exposure times were varied to normalize to GAPDH gene transcription, which was unaffected by UV light treatment in these assays (data not shown). (C) Transiently transfected p53 represses U1 snRNA gene transcription in HeLa cells. Nuclear run-on assays were performed on HeLa cells (lane 1) or HeLa cells transiently transfected with either the empty vector pRC/RSV (lane 2) or pRC-RSV expressing wild-type full-length Flag-tagged p53 (pRc/RSV-p53-Flag) (lane 3). Levels of p53 expression were determined by Western blotting (bottom panel). (D) Endogenous p53 associates with human snRNA gene promoters. Chromatin immunoprecipitation experiments were performed using chromatin harvested from MCF-7 cells prior to or 8 h after UV light treatment and using antibodies directed against SNAP43 (lane 3), various epitopes within p53 (lanes 4 to 7), and nonspecific IgG (lane 8) as a negative control. Enrichment of U1 and U6 promoter regions was measured by PCR and was compared to the p21 promoter (−1.4 kb site), as a positive control, and the U1 upstream region and GAPDH exon 2, as negative controls. (E) Endogenous p53 was not detected at human snRNA gene promoters in untreated or UV light-treated HeLa cells. Chromatin immunoprecipitation experiments were performed using HeLa cell chromatin with the indicated antibodies. (F) UV light causes a decrease in steady-state U1 snRNA levels. (Top panel) Total RNA was isolated from untreated MCF-7 cells and was titrated (0, 0.1, 0.3, 1, 3, and 10 ng [lanes 2 through 7, respectively]) into RT-PCRs performed using U1 gene-specific primers. The amount of U1 cDNA amplification is proportional to the amount of total RNA used for RT-PCR. (Bottom panel) Steady-state levels of U1, U6, and GAPDH RNA were measured by RT-PCR using 2 ng, 10 ng, and 1 μg of total RNA, respectively, harvested before (lane 1) or after (lanes 2 to 7) UV light treatment.
To determine the effect of UV light on snRNA gene transcription, nuclear run-on experiments were performed using nuclei harvested from HeLa and MCF-7 cells before and 8 h after UV light exposure. As shown in Fig. 2B, UV light treatment of HeLa cells did not substantially affect U1 transcription by RNA polymerase II compared to cells that did not receive UV light exposure. Similarly, U6 snRNA and 5S rRNA transcription by RNA polymerase III was unaffected, suggesting that the transcription of these genes is insensitive to UV light. These results for U1 transcription are in contrast with that previously described wherein U1 transcription in nuclear run-on assays was markedly reduced 2 h after UV light treatment of HeLa cells (48). In the present study, the 8-h posttreatment time point was selected because RNA polymerase II is ubiquitylated and degraded in response to UV light but normal levels are restored by 6 h after UV light treatment (46). Additionally, a longer recovery period after UV light treatment was desirable to allow sufficient time for DNA damage repair.
In contrast with HeLa cells, MCF-7 cells exhibited a marked reduction in U1 transcription 8 h after UV light treatment. Additional studies revealed that repression was already established by 4 h posttreatment (data not shown). Interestingly, UV light elicited different effects on RNA polymerase III-transcribed genes, causing reduced U6 transcription while stimulating 5S rRNA transcription. In all cases, the signals detected for these transcripts are specific, because no hybridization to pUC119 was detected in any of these experiments. Therefore, U1 and U6 transcription exhibits cell type-specific responses to UV light treatment, with UV light invoking a prolonged repressive effect in MCF-7 cells but not in HeLa cells. In two independent replicates of this experiment, U1 and U6 transcription levels were reduced to 42 and 39%, respectively, of the untreated sample levels, whereas 5S RNA transcription rates were increased to 170%. Stimulated 5S rRNA gene transcription under these conditions was unexpected but does indicate that the 5S rRNA transcriptional response to DNA damage depends upon cellular p53 status.
To determine whether p53 contributes to regulation of endogenous snRNA genes, p53 was overexpressed in HeLa cells and the effect on endogenous U1, U6, and 5S rRNA gene transcription was again measured by nuclear run-on assays. As shown in Fig. 2C, increased p53 expression was correlated with diminished U1 transcription. In contrast, U6 transcription was unaffected, whereas 5S rRNA transcription was stimulated. The reason for the unresponsiveness of U6 transcription to p53 expression is unknown, but p53 may require additional UV light-stimulated modification for activity at this gene. Interestingly, the expression pattern for U1 snRNA and 5S rRNA transcription in response to p53 expression is similar to that observed with UV light treatment of MCF-7 cells, consistent with the idea that p53 regulates these genes in response to DNA damage.
Endogenous p53 associates with human snRNA gene promoters.
Chromatin immunoprecipitation experiments were then performed to determine whether p53 is directly involved in the regulation of endogenous snRNA genes in response to UV light. As shown in Fig. 2D, substantial enrichment of U1 and U2 snRNA promoter DNA was observed in anti-p53-immunoprecipitated samples (lanes 4 and 5) with chromatin harvested from MCF-7 cells prior to UV light treatment, and these levels were markedly enriched by using chromatin harvested from cells 8 h after UV light treatment. In contrast, only low levels of U6 promoter DNA were enriched in the anti-p53-immunoprecipitated samples prior to UV light treatment, but promoter recovery was noticeably enhanced after treatment.
Previously, it was shown that p53 is acetylated within its C terminus in response to DNA damage, which may stimulate DNA binding by p53 (18, 44) and increase recruitment of coregulatory proteins (2). Furthermore, acetylation but not phosphorylation of p53 within the C-terminal domain contributes to fragile site formation at the U2 snRNA gene loci, indicating that p53 acetylation may be important for p53 function at snRNA genes (71). Therefore, immunoprecipitation reactions were also performed with antibodies that specifically recognize p53 acetylated at K320 or at K372 and K382 (Fig. 2D). Interestingly, significant levels of U1 and U2 promoter enrichment were observed with antibodies that recognize acetylated p53 (lanes 5 and 6), but UV light treatment either did not affect promoter enrichment or caused a modest reduction. In contrast, no significant recovery of U6 promoter DNA was obtained with antibodies that recognize acetylated p53. Enrichment of the p21 promoter (−1.4 kb site) in the anti-p53-immunoprecipitated samples was low prior to UV light treatment, but recovery increased significantly after treatment, as has been previously demonstrated (30). UV light treatment also resulted in increased p21 promoter enrichment for those reactions performed using anti-acetylated p53 antibodies. Together, these data indicate that p53 associates with the endogenous U1 and U2 snRNA gene promoters prior to genotypic stress and UV light stimulates p53 association with these promoters, although the apparent proportion of p53 that is acetylated decreases. Second, low levels of p53 associate with the U6 promoter prior to stress and UV light stimulates p53 promoter association, but this p53 is not acetylated to a significant degree. These observations are in contrast to those seen with the p21 promoter, where p53 association is low but the total p53 level and proportion that is acetylated increase in response to UV light treatment. The data in Fig. 2E show that neither U1 nor p21 promoter association by p53 was observed in HeLa cells (lane 5) using an anti-p53 antibody that efficiently recovered these DNA segments from MCF-7 cells (lane 6). As previously observed for MCF-7 cells, UV light also did not affect SNAPC occupancy at the U1 promoter in HeLa cells (lane 3).
Human snRNA molecules are abundant and very stable (13, 17, 68) and, thus, it is not clear what effect diminished U1 transcription would have on overall U1 snRNA levels. Therefore, RT-PCR assays were employed to determine the effect of UV light on steady-state U1 and U6 snRNA levels. As shown in Fig. 2F (top panel), addition of increasing amounts of total cellular RNA harvested from untreated MCF-7 cells resulted in a linear amplification of U1 sequences (lanes 3 to 7), thus demonstrating that this assay is suitable for measuring changes in steady-state U1 snRNA levels. Similar preliminary experiments were performed to determine the range for linear amplification of both U6 snRNA and GAPDH mRNA (data not shown). Under these conditions, U1 steady-state levels were noticeably reduced 8 h after UV light treatment, whereas U6 snRNA and GAPDH mRNA levels remained relatively stable before and after treatment. In three independent replicates of this experiment, the steady-state level of U1 snRNA at 8 h posttreatment was 43% of levels in untreated cells (data not shown). The reduction in U1 steady-state levels in response to UV light could be attributable to increased degradation of this RNA, decreased transcription from U1 snRNA genes, or a combination of both factors. U1 snRNA is traditionally viewed as extremely stable, with a half-life greater than 24 h and, thus, little change was expected in steady-state U1 levels by 8 h, even if transcription were completely repressed. The kinetics of the decrease in U1 steady-state levels and the results shown in Fig. 2B suggest that both a reduction in U1 transcription and an increase in U1 snRNA degradation contribute to reduced U1 snRNA levels after UV light treatment. Together, these results indicate that UV light initiates a complicated network of control governing expression of human snRNA genes.
Human U1 snRNA gene core promoters contain a high-affinity p53 binding site.
As a first step towards understanding the mechanism for p53 repression, EMSA were performed to determine whether p53 could bind directly to human U1 snRNA gene promoters. Indeed, recombinant p53 bound extremely well to a U1 probe encompassing the region from −422 to +13 of the promoter (Fig. 3A, lanes 2 to 4). Competition experiments suggest that p53 affinity for the U1 promoter is comparable to the p53 binding element contained within the GADD45 promoter (data not shown). Interestingly, two different complexes formed on the U1 promoter probe, suggesting that the U1 promoter may contain two p53 binding elements. The protein-DNA complexes formed on these probes are due to p53, because inclusion of anti-p53 antibodies in the DNA binding reactions retarded the migration of these complexes (data not shown). To determine the location of the high-affinity p53 binding element, EMSA was also performed with probes containing different regions of the promoter. Strong binding of p53 to DNA was observed in reactions with equivalently labeled probes containing the −312 to +13 and −150 to +13 regions of the promoter. Consistently, low-affinity binding was observed in probes containing the −422 to −152 and −312 to −152 promoter regions. Together, these data indicate that the high-affinity p53 binding element is contained between −150 and +13 of the U1 core promoter.
FIG. 3.
Human U1 snRNA gene core promoters contain a high-affinity p53 binding site. (A) EMSA with increasing amounts of recombinant p53 (0, 80, 160, and 320 ng) were performed with double-stranded DNA probes encompassing various regions of the core U1 promoter, as indicated. At higher p53 concentrations, two p53-dependent complexes were formed on those probes that exhibited high-affinity p53 binding. (B) DNase I footprinting reactions were performed using labeled template stand (lanes 1 to 5) or nontemplate stand (lanes 6 to 10) U1 promoter probes encompassing −150 to +13 with increasing amounts of GST-p53 (0, 100, 200, and 400 ng). Digestion of the probe DNA without added p53 is shown in lanes 1, 5, 6, and 10. The relative positions of the PSE and transcription start site are indicated. Two protected regions within the template strand (labeled F1 and F2) overlap with the three protected regions (labeled F1′, F2′, and F3′) from the nontemplate strand.
DNase I footprinting experiments were then performed to further map the location of the high-affinity p53 binding element. As shown in Fig. 3B, those reactions in which the template strand was end labeled exhibited two sites of protection (labeled F1 and F2) in the presence of GST-p53 (left panel), whereas three regions (F1′, F2′, and F3′) were protected in reactions with the labeled nontemplate strand (right panel). The F1′ and F3′ regions correspond to the same region as the F1 footprint (hereafter referred to as p53 footprint 1), whereas the F2 and F2′ regions map to the same region (hereafter referred to as p53 footprint 2). Interestingly, the regions protected by p53 flank the PSE, which is required for DNA binding by SNAPC and is an essential promoter element for high-level expression of human snRNA genes. The juxtaposition of p53 footprints1 and 2 with the PSE raises the possibility that p53 represses U1 transcription by occluding SNAPC promoter binding. However, to date we have not observed any effect of p53 on promoter recognition by SNAPC (data not shown).
p53 repression and sequence-specific DNA binding are separable activities.
As a transcription factor, p53 can either activate or repress transcription, depending upon the structure of the target gene promoter. Notably, p53 can activate transcription from gene promoters that contain consensus p53 binding elements (12). A few examples have been described where p53 represses target genes that contain consensus p53 binding elements (reviewed in reference 26), but it is generally believed that p53 can repress transcription of other target genes whose promoters lack specific p53 recognition elements or contain a noncanonical p53 binding element (29). A comparison of p53 binding elements from target genes that are activated by p53 revealed that a consensus p53 binding site contains two half sites separated by 0 to 13 bp (50). Each half site contains two quarter sites containing the sequence PuPuPuC(A/T) arranged in a head-to-head orientation. Interestingly, the p53 footprint 1 region contains a sequence that is similar to the consensus p53 binding element usually associated with transcriptional activation by p53 (Fig. 4A).
FIG.4.
The high-affinity p53 element in the U1 promoter is not essential for p53 repression in vitro. (A) Primary sequence of the U1 core promoter region and the location of the p53 footprints. The plasmid pU1-4.0 contains a wild-type promoter sequence from −151 to +13. Scanning mutagenesis across this region was performed, and the introduced mutations and plasmid identity are indicated. Dots represent positions of identity to this wild-type sequence. (B) Mutations in the p53 footprint 1 region disrupt p53 binding. EMSA was performed using the various U1 promoter probes and either 200 or 400 ng of p53, as indicated (lanes 2 to 37). Lane 1 contains the wild-type U1 promoter probe and no added p53. Mutations within the p53 footprint 1 region caused a marked reduction in p53 binding to the U1 promoter (probes 4.8, 4.9, 4.10, and 4.11). (C) Mutations within the p53 footprint 1 region do not affect p53 repression in vitro. Selected mutations were incorporated into a U1 G-less reporter plasmid for in vitro U1 transcription assays (lanes 1 to 20) in the absence or presence of p53 (800 ng). Lane 21 shows transcription from the wild-type U1 reporter in the presence of GST (800 ng). The read-through (RT) and correctly initiated transcripts (U1 5′) are indicated. (D) p53 represses transcription of the wild-type and mutant U1 reporter constructs to similar extents. (Left panel) An extensive titration of p53 (0, 20, 40, 80, and 160 ng) into U1 transcription assay mixtures was performed using the wild-type (U1 4.0; lanes 1 to 4) and mutant (U1 4.9 + 4.10; lanes 6 to 9) U1 reporter. Lanes 5 and 10 show transcription from the wild-type U1 plasmid in the presence of 160 ng of GST, as a negative control. (Right panel) Transcription levels for two independent experiments were normalized to the signals from transcription reactions containing no added p53, and the average dose-response curves are shown.
To investigate the contribution of the high-affinity p53 binding element for U1 promoter recognition and transcriptional repression, a scanning mutagenesis of the U1 core promoter was performed. The sequence of the U1 core promoter region and the location of the p53 footprints are shown in Fig. 4A. This figure also shows the location and identity of the mutations introduced into the U1 core promoter. The ability of recombinant p53 to bind DNA probes harboring these mutations was tested by EMSA (Fig. 4B). The majority of mutations throughout the U1 core promoter, including mutations within the region corresponding to p53 footprint 2 (U1-4.5 and U1-4.6 probes), had no significant effect on DNA binding by p53. A slight reduction for p53-DNA complex 2 formation for the U1-4.5 and U1-4.6 probes seen in this figure was not observed in replicates of this experiment. In contrast, mutations within the p53 footprint 1 region caused a marked reduction in p53 binding to the U1 promoter. The strongest effect was seen with the U1-4.9, U1-4.10, and U1-4.11 probes, whereas the U1-4.8 probe exhibited only a modest reduction in p53 binding. Formation of both p53-DNA complex 1 and p53-DNA complex 2 was affected by these mutations. These data indicate that the region adjacent to the U1 transcriptional start site contains the high-affinity p53 binding element.
Next, the requirement of the high-affinity p53 binding element for p53 repression was tested. As shown in Fig. 4C, recombinant p53 effectively repressed transcription from all U1 reporter constructs tested. Neither single nor double sets of mutations within the p53 footprint 1 region or within both p53 footprint 1 and 2 regions had a major effect on p53 repression ability. In some cases, mutation of the U1 core promoter caused a slight reduction in transcription in the absence of p53, which suggests that factors binding to these regions may have a positive role in U1 transcription in the absence of added p53. To more carefully examine the contribution of the high-affinity p53 binding element to p53 transcriptional repression, a titration of p53 was performed on the wild-type U1 reporter (U1-4.0) and mutant U1 reporter (U1-4.9 + 4.10), which harbors two sets of mutations within the high-affinity p53 binding element (Fig. 4D, left panel). Transcription initiated from the U1 promoter was quantified, and levels were normalized to levels in transcription reactions containing no added p53 for each U1 reporter construct. The average U1-specific response from two replicates of this experiment is shown in Fig. 4D (right panel). This analysis revealed that both U1 reporter constructs exhibited similar repression responses to increasing amounts of p53. Together these data indicate that the high-affinity p53 binding element contained within the human U1 core promoter region is not required for in vitro transcriptional repression by p53.
The p53 C terminus contributes to both RNA polymerase II and III repression.
Our previous data suggested that p53 is an effective repressor of U6 transcription by RNA polymerase III. Therefore, p53 binding to the U6 promoter was next investigated to determine the contribution of sequence-specific DNA binding by p53 to the repression of RNA polymerase III transcription. As shown in Fig. 5A, p53 binds well to the U1 promoter probe in EMSA, as expected, but approximately 10- to 20-fold less well to the U6 promoter probes (lanes 6 to 8). The weak binding by p53 to the U6 promoter probe suggests that p53 may not rely on direct promoter recognition to mediate RNA polymerase III repression. However, it is possible that p53 binds DNA specifically elsewhere in the reporter constructs during repression.
FIG. 5.
The p53 C terminus is sufficient for transcriptional repression and promoter association. (A) The U6 core promoter does not contain a high-affinity p53 binding element. EMSA was performed with increasing amounts of recombinant p53 (0, 80, 160, and 320 ng) and equivalently labeled double-stranded DNA probes encompassing the U1 core promoter (lanes 1 to 4) and U6 core promoter (lanes 5 to 8). (B) Direct U1 promoter recognition requires the p53 DNA binding domain. EMSA were performed with a wild-type U1 promoter probe (−151 to +13) using 250, 500, and 1,000 ng of full-length wild-type or mutant (R175H) GST-p53 (lanes 2 to 4 and 5 to 7, respectively). Reactions containing truncated GST-p53 (301-393) or GST alone are shown in lanes 8 to 10 and 11 to 13, respectively. Lane 1 contains no added protein. (C) Wild-type and mutant GST-p53 repress transcription similarly. Approximately 100, 200, and 400 ng of wild-type GST-p53 (lanes 2 to 4), GST-p53 (R175H) (lanes 5 to 7), GST-p53 (301-393) (lanes 8 to 10), or 400 ng of GST (lane 11) were titrated into U1, U6, and AdML in vitro transcription reaction mixtures. Both wild-type and mutant p53 molecules repressed U1 and U6 transcription, whereas AdML transcription was unaffected. (D) The p53 DNA binding domain is not required for U1 and U6 promoter association during repression. A portion of untreated or p53 repressed U1 and U6 in vitro transcription reaction mixtures (lanes 1, 4, 7, 10, and 11 in panel C) was cross-linked with formaldehyde and subjected to immunoprecipitation with anti-SNAP43 (lanes 3 and 8), IgG (lanes 4 and 9), or anti-p53 (lanes 5 and 10) antibody. Enrichment of the U1 and U6 reporter plasmids or negative control pUC119 DNA was compared by PCR using promoter-specific or pUC-specific primers. Lanes 1, 2, 6, and 7 show the amplification directly from the input DNA (10 and 1% in lanes 1 and 2 for U1 and lanes 6 and 7 for U6, respectively) contained in the transcription assay mixtures prior to immunoprecipitation. The results of PCRs using the pUC-specific primers under all conditions were indistinguishable and are shown only for the reactions containing added wild-type GST-p53.
To determine whether DNA binding by p53 is important for repression of U1 and U6 transcription, a point mutation in the p53 DNA binding domain was introduced to eliminate sequence-specific DNA binding. In particular, the arginine at position 175 was changed to a histidine (R175H) to mimic a hot spot mutation commonly found in human cancers. Additionally, the C-terminal region of p53 (301-393) was tested for activity, because this region is sufficient for chromosome fragile site formation at the multicopy U1 and U2 snRNA gene loci during adenovirus infection (38, 71). First, the wild-type and mutant GST-p53 proteins were tested for DNA binding activity in EMSA using probes encompassing the U1 core promoter region. As shown in Fig. 5B, only the wild-type recombinant GST-p53 bound effectively to the U1 promoter DNA while the GST-p53 (R175H) and GST-p53 (301-393) proteins were completely inactive in this assay. Next, the ability of these proteins to regulate in vitro U1 and U6 transcription by RNA polymerases II and III, respectively, were tested (Fig. 5C). Interestingly, GST-p53 (R175H) repressed U1 transcription as effectively as wild-type GST-p53, whereas GST-p53 (301-393) was approximately twofold less effective, but still capable of repression. Both mutant GST-p53 proteins repressed U6 transcription, but GST-p53 (R175H) was less effective than either wild-type GST-p53 or GST-p53 (301-393), which were equivalently active in these assays. In these assays, the repression of RNA polymerase III transcription by p53 (R175H) is in contrast with previous experiments, wherein the introduction of the R-to-H mutation switched p53 from a repressor to an activator of general RNA polymerase III transcription (57). These data herein indicate that DNA binding by p53 is not essential for repression of U1 and U6 snRNA gene transcription by RNA polymerases II and III, respectively, and the C-terminal region encompassing amino acids 301 to 393 is sufficient for p53 repression activity.
Next, recruitment of the mutant p53 proteins to the U1 and U6 promoters during repression was examined. Portions of in vitro transcription reaction mixtures containing wild-type or mutant GST-p53 proteins were cross-linked with formaldehyde prior to immunoprecipitation by using antibodies directed against SNAP43 or p53. Transcription reaction mixtures containing GST or no added proteins were also tested. The specific recoveries of the U1 and U6 promoter-containing plasmids were compared to recovery of an irrelevant plasmid (pUC119) included in the original transcription assay mixtures. As shown in Fig. 5D, significant U1 and U6 reporter plasmid recovery by anti-SNAP43 immunoprecipitation was obtained from the untreated transcription reaction mixture (lanes 3 and 8). Neither wild-type nor mutant p53 affected reporter enrichment by anti-SNAP43 immunoprecipitation, suggesting that p53 may not disrupt SNAPC promoter binding during repression. However, it is difficult to eliminate the possibility that SNAPC can bind to both actively transcribed and inactive templates, whereas p53 may be specifically bound to only those active templates containing a complete preinitiation complex. When the untreated U1 and U6 transcription assays were used for anti-p53 immunoprecipitation, only background levels of reporter plasmid enrichment were observed (lanes 5 and 10). This result was expected, because these assays were performed using HeLa cell nuclear extracts, which contain very low levels of endogenous p53. However, significant recovery of the U1 and U6 promoter plasmids was observed in reactions containing additional wild-type p53. Interestingly, U1 and U6 reporter DNA enrichment was also observed in reactions containing the mutant p53 (R175H) and p53 (301-393) proteins. In all cases, recovery of the reporter-containing plasmids was substantially greater than that observed for the irrelevant pUC119 plasmid (Fig. 5D, bottom panel, and data not shown). These results indicate that p53 can associate with the U1 and U6 promoters during repression. The preferential association of the mutant p53 proteins with the promoter-containing plasmids suggests that during repression p53 is actively recruited to U1 and U6 promoters independently of sequence-specific DNA binding by p53. Furthermore, the C-terminal domain of p53 is likely critical for promoter recruitment.
p53 associates with general transcription factor SNAPC.
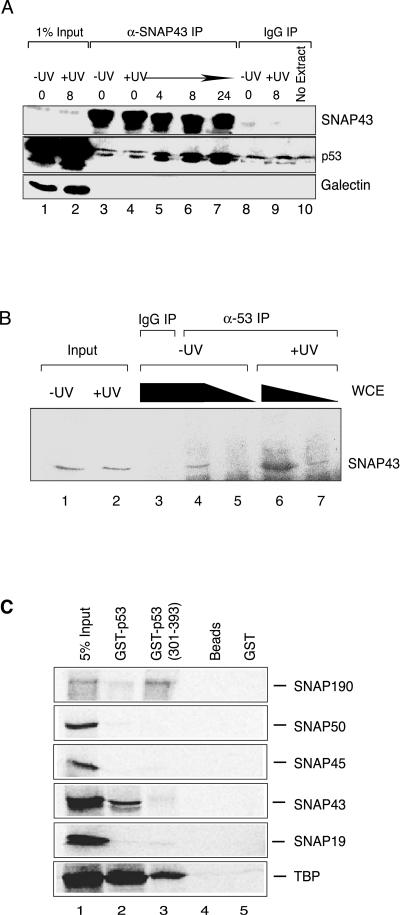
Promoter association by p53 in the absence of direct DNA binding suggests that protein-protein interactions are critical for p53 recruitment. Furthermore, p53 was capable of repressing both U1 and U6 transcription by RNA polymerases II and III, raising the possibility that a factor commonly used for these genes could be targeted by p53. Both the general transcription factors SNAPC and TBP are utilized during transcription of these genes and are thus candidate p53 targets. Indeed, TBP has been shown to interact strongly with p53 within the N-terminal activation domain and the C-terminal oligomerization domain (16, 41, 61). To determine whether p53 could potentially target SNAPC, coimmunoprecipitation assays were performed using antibodies directed against the SNAP43 subunit of SNAPC. The association of endogenous p53 with SNAP43 was measured by Western analysis before and after UV light treatment. As shown in Fig. 6A, p53 was not detected in anti-SNAP43 immunoprecipitation reactions prior to UV treatment, but p53 recovery increased by 4 h and continued to increase by 24 h after UV light treatment. No recovery of the splicing factor galectin-3 was observed in these reactions. Reciprocal anti-p53 immunoprecipitation assays were performed using extracts prepared from MCF-7 cells that were untreated or UV light treated (8 h posttreatment). As shown in Fig. 6B, UV light treatment had little effect on the levels of SNAP43 present in the extract (lanes 1 and 2) but caused increased SNAP43 association with p53 (compare lanes 4 and 5 to 6 and 7). Together, these data indicate that UV light modulates the association between endogenous p53 and SNAPC.
FIG. 6.
Endogenous p53 associates with the general transcription factor SNAPC. (A) Immunoprecipitation with anti-SNAP43 antibodies was performed from whole-cell extracts prepared from untreated and UV-treated MCF-7 cells at different time points after UV light treatment. Western analysis revealed significant differences in recovered p53 between the negative control IgG immunoprecipitations (lanes 8 to 10) and anti-SNAP43 immunoprecipitation by the 4-h time point (lane 5). The amount of coimmunoprecipitated p53 continued to increase up to 24 h after UV light treatment (lanes 6 and 7), whereas the amount of precipitated SNAP43 did not change from 0 to 24 h after UV exposure. No galectin-3 was observed in any of the immunopre-cipitations. Lanes 1 and 2 contain 1% of the extract used for immunoprecipitation reactions. (B) Immunoprecipitation with anti-p53 (Ab-6) antibodies was performed from whole-cell extracts (5 mg, lanes 3, 4, and 6; 1.7 mg, lanes 5 and 7) prepared from untreated and UV-treated MCF-7 cells 8 h after UV light treatment, as indicated. Lanes 1 and 2 contain 150 μg of extract that was used as input for immunoprecipitation reactions. Western analysis with anti-SNAP43 antibodies revealed increased SNAP43 association with p53 by 8 h after UV light exposure. (C) p53 interacts with SNAP43, SNAP190, and TBP. GST pull-down experiments were performed with 1 μg of recombinant full-length GST-p53 (lane 2), GST-p53 (301-393) (lane 3), GST (lane 5), or beads alone (lane 4) with individual SNAPC subunits and TBP that were translated in vitro and labeled with [35S]methionine. Lane 1 contains 5% of radiolabeled proteins added to each pull-down reaction mixture.
GST pull-down experiments were then performed to determine whether p53 could interact with TBP and individual components of SNAPC (Fig. 6C). In these assays, full-length GST-p53 interacted well with TBP and SNAP43 while SNAP190 interacted only weakly in this assay. No significant interactions were observed for GST-p53 with SNAP50, SNAP45, and SNAP19. Interestingly, truncating p53 to include only the C-terminal domain abolished interactions with SNAP43 but stimulated interactions with SNAP190. These observations suggest that p53 may interact with TBP and SNAPC to correctly target human snRNA genes for repression.
DISCUSSION
The p53 tumor suppressor protein acts as a transcriptional activator in response to cellular stress, but it is also competent for concomitant repression of other sets of target genes. The data presented herein demonstrate that endogenous p53 associates with human snRNA gene promoters and that UV light exposure stimulates p53 association with both U1 and U6 snRNA genes. These in vivo data indicate that p53 is directly involved in the regulation of human snRNA gene transcription by both RNA polymerases II and III. That recombinant p53 effectively repressed both U1 and U6 snRNA gene transcription in vitro suggests that these genes similarly could be repressed by p53 in response to DNA damage in vivo. Indeed, cellular U1 and U6 snRNA gene transcription is down regulated in p53-positive MCF-7 cells after UV light exposure. In contrast, transcriptional repression was not observed in p53-deficient HeLa cells after UV light exposure.
The data presented herein also suggest that p53 uses different mechanisms to repress U1 and U6 transcription. First, U1 snRNA genes contain a high-affinity p53 binding element located with the core promoter region adjacent to the PSE. Second, U6 snRNA genes remain sensitive to p53 repression after preinitiation complex assembly, whereas U1 snRNA genes become refractory to p53 influence, suggesting that the potential targets required for RNA polymerase II repression may be unavailable for p53 interaction after preinitiation complex assembly. Thus, it is tempting to speculate that p53 interferes with SNAPC or TBP promoter occupancy during repression, which for U1 genes could involve direct competition between p53 and SNAPC for adjacent promoter elements. However, the high-affinity p53 binding element is clearly not required for U1 repression in vitro, and the levels of SNAPC detected on U1 and U6 snRNA gene promoters by chromatin immunoprecipitation do not substantively change in response to UV light, even though there is a substantial increase in p53 association. Consistently, we have not observed any effect of full-length p53 on PSE binding by SNAPC in EMSA (data not shown). We currently favor the hypothesis that p53 interferes with snRNA gene transcription at steps occurring after promoter association by SNAPC.
As U1 and U6 snRNA gene transcription relies on different assemblies of transcription factors, it is likely that to enact repression p53 targets different factors specifically required for RNA polymerase II or III transcription. For RNA polymerase II transcription, one candidate is TFIIH, which can interact with p53 (67, 70). TFIIH may be utilized during transcription of human U1 snRNA genes, as removal of TFIIH from extracts impairs U1 snRNA gene transcription (32). However, U1 transcription was not reconstituted in the depleted extracts by addition of purified TFIIH, suggesting that additional factors may complement the purified TFIIH for activity (32). A second candidate coregulator is the Cockayne syndrome group B (CSB) protein, which also can interact with p53 (67). CSB was originally suggested to play a role in DNA repair and, more recently, was shown to interact with RNA polymerase II (63). CSB may be an elongation factor (34, 56, 59, 62) which could mediate transcription of genes encoding RNA products with significant secondary structure, such as human U1 and U2 snRNA genes. As previously described for p53, mutations in CSB also cause chromosomal fragile site formation at U1 and U2 snRNA genes (71), suggesting that CSB may play a role in transcription of these genes by RNA polymerase II. It was hypothesized that activated p53 interferes with CSB-mediated elongation to cause RNA polymerase II stalling at U1 and U2 snRNA genes (71). The data presented herein support a role for p53 in mediating repression of U1 snRNA gene transcription by RNA polymerase II, but whether CSB is involved in this process is not yet known.
During genomic threats, p53 is stabilized from destruction and activated by a cascade of posttranslational modifications, including phosphorylation and acetylation. Acetylation of p53 has been suggested to increase sequence-specific DNA binding by p53 (18, 44) and to stimulate cofactor recruitment, such as the p300 histone acetyltransferase, during activated transcription of p53 target genes (2, 15). Thus, it is interesting that while acetylated p53 was detected at human U1 genes prior to UV light exposure, the level of acetylated p53 at these genes was not stimulated by UV light even though a substantial increase in the total p53 levels at these genes was observed. Furthermore, little to no acetylated p53 was observed at a U6 snRNA gene promoter after UV light treatment, indicating that most of the p53 associated with this gene is not acetylated. One possibility is that p53 recruits a histone deacetylase to enact repression, and this enzyme deacetylates p53. Another possibility is that p53 acetylation is not compatible with transcriptional repression. For example, the sites of p53 acetylation are all located in the C-terminal region (18, 40, 53), and this region is sufficient for transcriptional repression of human U1 and U6 snRNA gene transcription. Presumably, the p53 C terminus makes key protein-protein contacts to enact repression and, as we have shown, this region interacts with SNAP190 and TBP. Acetylation of p53 may disrupt these contacts and prevent p53 from being recruited to these snRNA promoters. However, it is difficult to exclude the possibility that p53 acetylation is important for p53 repression where the sites of acetylation are intimately involved in protein-protein contacts and are not available for antibody recognition during chromatin immunoprecipitation. Nonetheless, p53 acetylation is unlikely to be essential for repression, because the recombinant wild-type and mutant p53 used for the U1 and U6 repression assays were not substantially acetylated. The presence of acetylated p53 at the U1 snRNA gene promoters in untreated MCF-7 cells suggests that activated p53 can bind to these genes, possibly through direct recognition of the high-affinity p53 binding element contained within the U1 snRNA gene core promoter, even though this element is not required for U1 repression in vitro. An intriguing possibility is that p53 may have dual roles at human U1 snRNA genes, with direct promoter recognition and activation functions prior to substantial DNA damage but repressive activity mediated via a different promoter-targeting mechanism after DNA damage, such as that caused by UV light.
Acknowledgments
The anti-galectin-3 antibody (Mac2) was a gift from John Wang (Michigan State University). The pRc/RSV and pRc/RSV-p53-Flag.wt plasmids were a gift from Roland Kwok (University of Michigan). We thank Liping Gu, Gauri Jawdekar, Min-Hao Kuo, and Jim Geiger for critical assessment of the manuscript and appreciate the technical assistance provided by Craig Hinkley, Brandon LaMere, Xianzhou Song, and Tharakeswari Selvakumar. We acknowledge the National Cell Culture Center for preparation of HeLa cells.
This work was supported by an NIH grant (GM59805) to R.W.H.
REFERENCES
- 1.Bailey, A. D., Z. Li, T. Pavelitz, and A. M. Weiner. 1995. Adenovirus type 12-induced fragility of the human RNU2 locus requires U2 small nuclear RNA transcriptional regulatory elements. Mol. Cell. Biol. 15:6246-6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlev, N. A., L. Liu, N. H. Chehab, K. Mansfield, K. G. Harris, T. D. Halazonetis, and S. L. Berger. 2001. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 8:1243-1254. [DOI] [PubMed] [Google Scholar]
- 3.Budde, A., and I. Grummt. 1999. p53 represses ribosomal gene transcription. Oncogene 18:1119-1124. [DOI] [PubMed] [Google Scholar]
- 4.Cain, C., S. Miller, J. Ahn, and C. Prives. 2000. The N terminus of p53 regulates its dissociation from DNA. J. Biol. Chem. 275:39944-39953. [DOI] [PubMed] [Google Scholar]
- 5.Cairns, C. A., and R. J. White. 1998. p53 is a general repressor of RNA polymerase III transcription. EMBO J. 17:3112-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey, M., and S. T. Smale. 2000. Transcriptional regulation in eukaryotes. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 7.Chen, X., G. Farmer, H. Zhu, R. Prywes, and C. Prives. 1993. Cooperative DNA binding of p53 with TFIID (TBP): a possible mechanism for transcriptional activation. Genes Dev. 7:1837-1849. [Erratum, 7:2652.] [DOI] [PubMed] [Google Scholar]
- 8.Chen, X., L. J. Ko, L. Jayaraman, and C. Prives. 1996. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10:2438-2451. [DOI] [PubMed] [Google Scholar]
- 9.Chesnokov, I., W. M. Chu, M. R. Botchan, and C. W. Schmid. 1996. p53 inhibits RNA polymerase III-directed transcription in a promoter- dependent manner. Mol. Cell. Biol. 16:7084-7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crighton, D., A. Woiwode, C. Zhang, N. Mandavia, J. P. Morton, L. J. Warnock, J. Milner, R. J. White, and D. L. Johnson. 2003. p53 represses RNA polymerase III transcription by targeting TBP and inhibiting promoter occupancy by TFIIIB. EMBO J. 22:2810-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichhorn, K., and S. P. Jackson. 2001. A role for TAF3B2 in the repression of human RNA polymerase III transcription in nonproliferating cells. J. Biol. Chem. 276:21158-21165. [DOI] [PubMed] [Google Scholar]
- 12.el-Deiry, W. S., S. E. Kern, J. A. Pietenpol, K. W. Kinzler, and B. Vogelstein. 1992. Definition of a consensus binding site for p53. Nat. Genet. 1:45-49. [DOI] [PubMed] [Google Scholar]
- 13.Eliceiri, G. L. 1974. Short-lived, small RNAs in the cytoplasm of HeLa cells. Cell 3:11-14. [DOI] [PubMed] [Google Scholar]
- 14.Eliceiri, G. L., and J. H. Smith. 1983. Sensitivity to UV radiation of small nuclear RNA synthesis in mammalian cells. Mol. Cell. Biol. 3:2151-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinosa, J. M., and B. M. Emerson. 2001. Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol. Cell 8:57-69. [DOI] [PubMed] [Google Scholar]
- 16.Farmer, G., J. Colgan, Y. Nakatani, J. L. Manley, and C. Prives. 1996. Functional interaction between p53, the TATA-binding protein (TBP), and TBP-associated factors in vivo. Mol. Cell. Biol. 16:4295-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frederiksen, S., I. R. Pedersen, P. Hellung-Larsen, and J. Engberg. 1974. Metabolic studies of small molecular weight nuclear RNA components in BHK-21 cells. Biochim. Biophys. Acta 340:64-76. [DOI] [PubMed] [Google Scholar]
- 18.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595-606. [DOI] [PubMed] [Google Scholar]
- 19.Henry, R. W., E. Ford, R. Mital, V. Mittal, and N. Hernandez. 1998. Crossing the line between RNA polymerases: transcription of human snRNA genes by RNA polymerases II and III. Cold Spring Harbor Symp. Quant. Biol. 63:111-120. [DOI] [PubMed] [Google Scholar]
- 20.Henry, R. W., B. Ma, C. L. Sadowski, R. Kobayashi, and N. Hernandez. 1996. Cloning and characterization of SNAP50, a subunit of the snRNA-activating protein complex SNAPc. EMBO J. 15:7129-7136. [PMC free article] [PubMed] [Google Scholar]
- 21.Henry, R. W., V. Mittal, B. Ma, R. Kobayashi, and N. Hernandez. 1998. SNAP19 mediates the assembly of a functional core promoter complex (SNAPc) shared by RNA polymerases II and III. Genes Dev. 12:2664-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry, R. W., C. L. Sadowski, R. Kobayashi, and N. Hernandez. 1995. A TBP-TAF complex required for transcription of human snRNA genes by RNA polymerase II and III. Nature 374:653-656. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez, N. 2001. Small nuclear RNA genes: a model system to study fundamental mechanisms of transcription. J. Biol. Chem. 276:26733-26736. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez, N. 1992. Transcription of vertebrate snRNA genes and related genes, p. 281-313. In S. McKnight and K. Yamamoto (ed.), Transcriptional regulation. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Hirsch, H. A., G. W. Jawdekar, K. A. Lee, L. Gu, and R. W. Henry. 2004. Distinct mechanisms for repression of RNA polymerase III transcription by the retinoblastoma tumor suppressor protein. Mol. Cell. Biol. 24:5989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho, J., and S. Benchimol. 2003. Transcriptional repression mediated by the p53 tumour suppressor. Cell Death Differ. 10:404-408. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs, E. Y., I. Ogiwara, and A. M. Weiner. 2004. Role of the C-terminal domain of RNA polymerase II in U2 snRNA transcription and 3′ processing. Mol. Cell. Biol. 24:846-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin, S., and A. J. Levine. 2001. The p53 functional circuit. J. Cell Sci. 114:4139-4140. [DOI] [PubMed] [Google Scholar]
- 29.Johnson, R. A., T. A. Ince, and K. W. Scotto. 2001. Transcriptional repression by p53 through direct binding to a novel DNA element. J. Biol. Chem. 276:27716-27720. [DOI] [PubMed] [Google Scholar]
- 30.Kaeser, M. D., and R. D. Iggo. 2002. Chromatin immunoprecipitation analysis fails to support the latency model for regulation of p53 DNA binding activity in vivo. Proc. Natl. Acad. Sci. USA 99:95-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko, L. J., and C. Prives. 1996. p53: puzzle and paradigm. Genes Dev. 10:1054-1072. [DOI] [PubMed] [Google Scholar]
- 32.Kuhlman, T. C., H. Cho, D. Reinberg, and N. Hernandez. 1999. The general transcription factors IIA, IIB, IIF, and IIE are required for RNA polymerase II transcription from the human U1 small nuclear RNA promoter. Mol. Cell. Biol. 19:2130-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunkel, G. R., and J. D. Hixson. 1998. The distal elements, OCT and SPH, stimulate the formation of preinitiation complexes on a human U6 snRNA gene promoter in vitro. Nucleic Acids Res. 26:1536-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, S. K., S. L. Yu, L. Prakash, and S. Prakash. 2001. Requirement for yeast RAD26, a homolog of the human CSB gene, in elongation by RNA polymerase II. Mol. Cell. Biol. 21:8651-8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 36.Li, Y. P., R. Tomanin, J. R. Smiley, and S. Bacchetti. 1993. Generation of a new adenovirus type 12-inducible fragile site by insertion of an artificial U2 locus in the human genome. Mol. Cell. Biol. 13:6064-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, Z., A. D. Bailey, J. Buchowski, and A. M. Weiner. 1998. A tandem array of minimal U1 small nuclear RNA genes is sufficient to generate a new adenovirus type 12-inducible chromosome fragile site. J. Virol. 72:4205-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, Z., A. Yu, and A. M. Weiner. 1998. Adenovirus type 12-induced fragility of the human RNU2 locus requires p53 function. J. Virol. 72:4183-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao, D., A. Yu, and A. M. Weiner. 1999. Coexpression of the adenovirus 12 E1B 55 kDa oncoprotein and cellular tumor suppressor p53 is sufficient to induce metaphase fragility of the human RNU2 locus. Virology 254:11-23. [DOI] [PubMed] [Google Scholar]
- 40.Liu, L., D. M. Scolnick, R. C. Trievel, H. B. Zhang, R. Marmorstein, T. D. Halazonetis, and S. L. Berger. 1999. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol. 19:1202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, X., C. W. Miller, P. H. Koeffler, and A. J. Berk. 1993. The p53 activation domain binds the TATA box-binding polypeptide in Holo-TFIID, and a neighboring p53 domain inhibits transcription. Mol. Cell. Biol. 13:3291-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lobo, S. M., and N. T. Hernandez. 1994. Transcription of snRNA genes by RNA polymerases II and III, p. 127-159. In R. C. Conaway and J. W. Conaway (ed.), Transcription: mechanisms and regulation. Raven Press, Ltd., New York, N.Y.
- 43.Lobo, S. M., M. Tanaka, M. L. Sullivan, and N. Hernandez. 1992. A TBP complex essential for transcription from TATA-less but not TATA-containing RNA polymerase III promoters is part of the TFIIIB fraction. Cell 71:1029-1040. [DOI] [PubMed] [Google Scholar]
- 44.Luo, J., M. Li, Y. Tang, M. Laszkowska, R. G. Roeder, and W. Gu. 2004. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc. Natl. Acad. Sci. USA 101:2259-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCulloch, V., P. Hardin, W. Peng, J. M. Ruppert, and S. M. Lobo-Ruppert. 2000. Alternatively spliced hBRF variants function at different RNA polymerase III promoters. EMBO J. 19:4134-4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKay, B. C., F. Chen, S. T. Clarke, H. E. Wiggin, L. M. Harley, and M. Ljungman. 2001. UV light-induced degradation of RNA polymerase II is dependent on the Cockayne's syndrome A and B proteins but not p53 or MLH1. Mutat. Res. 485:93-105. [DOI] [PubMed] [Google Scholar]
- 47.Morra, D. S., B. P. Eliceiri, and G. L. Eliceiri. 1986. Effect of UV light on small nuclear RNA synthesis: increased inhibition during postirradiation cell incubation. Mol. Cell. Biol. 6:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morra, D. S., S. H. Lawler, B. P. Eliceiri, and G. L. Eliceiri. 1986. Inhibition of small nuclear RNA synthesis by ultraviolet radiation. J. Biol. Chem. 261:3142-3146. [PubMed] [Google Scholar]
- 49.Murphy, S., J. B. Yoon, T. Gerster, and R. G. Roeder. 1992. Oct-1 and Oct-2 potentiate functional interactions of a transcription factor with the proximal sequence element of small nuclear RNA genes. Mol. Cell. Biol. 12:3247-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qian, H., T. Wang, L. Naumovski, C. D. Lopez, and R. K. Brachmann. 2002. Groups of p53 target genes involved in specific p53 downstream effects cluster into different classes of DNA binding sites. Oncogene 21:7901-7911. [DOI] [PubMed] [Google Scholar]
- 51.Sadowski, C. L., R. W. Henry, R. Kobayashi, and N. Hernandez. 1996. The SNAP45 subunit of the small nuclear RNA (snRNA) activating protein complex is required for RNA polymerase II and III snRNA gene transcription and interacts with the TATA box binding protein. Proc. Natl. Acad. Sci. USA 93:4289-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadowski, C. L., R. W. Henry, S. M. Lobo, and N. Hernandez. 1993. Targeting TBP to a non-TATA box cis-regulatory element: a TBP-containing complex activates transcription from snRNA promoters through the PSE. Genes Dev. 7:1535-1548. [DOI] [PubMed] [Google Scholar]
- 53.Sakaguchi, K., J. E. Herrera, S. Saito, T. Miki, M. Bustin, A. Vassilev, C. W. Anderson, and E. Appella. 1998. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 12:2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaub, M., E. Myslinski, C. Schuster, A. Krol, and P. Carbon. 1997. Staf, a promiscuous activator for enhanced transcription by RNA polymerases II and III. EMBO J. 16:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schramm, L., P. S. Pendergrast, Y. Sun, and N. Hernandez. 2000. Different human TFIIIB activities direct RNA polymerase III transcription from TATA-containing and TATA-less promoters. Genes Dev. 14:2650-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selby, C. P., and A. Sancar. 1997. Cockayne syndrome group B protein enhances elongation by RNA polymerase II. Proc. Natl. Acad. Sci. USA 94:11205-11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein, T., D. Crighton, J. M. Boyle, J. M. Varley, and R. J. White. 2002. RNA polymerase III transcription can be derepressed by oncogenes or mutations that compromise p53 function in tumours and Li-Fraumeni syndrome. Oncogene 21:2961-2970. [DOI] [PubMed] [Google Scholar]
- 58.Stein, T., D. Crighton, L. J. Warnock, J. Milner, and R. J. White. 2002. Several regions of p53 are involved in repression of RNA polymerase III transcription. Oncogene 21:5540-5547. [DOI] [PubMed] [Google Scholar]
- 59.Tantin, D., A. Kansal, and M. Carey. 1997. Recruitment of the putative transcription-repair coupling factor CSB/ERCC6 to RNA polymerase II elongation complexes. Mol. Cell. Biol. 17:6803-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teichmann, M., Z. Wang, and R. G. Roeder. 2000. A stable complex of a novel transcription factor IIB-related factor, human TFIIIB50, and associated proteins mediate selective transcription by RNA polymerase III of genes with upstream promoter elements. Proc. Natl. Acad. Sci. USA 97:14200-14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Truant, R., H. Xiao, C. J. Ingles, and J. Greenblatt. 1993. Direct interaction between the transcriptional activation domain of human p53 and the TATA box-binding protein. J. Biol. Chem. 268:2284-2287. [PubMed] [Google Scholar]
- 62.Van Den Boom, V., E. Citterio, D. Hoogstraten, A. Zotter, J. M. Egly, W. A. Van Cappellen, J. H. Hoeijmakers, A. B. Houtsmuller, and W. Vermeulen. 2004. DNA damage stabilizes interaction of CSB with the transcription elongation machinery. J. Cell Biol. 166:27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Gool, A. J., E. Citterio, S. Rademakers, R. van Os, W. Vermeulen, A. Constantinou, J. M. Egly, D. Bootsma, and J. H. Hoeijmakers. 1997. The Cockayne syndrome B protein, involved in transcription-coupled DNA repair, resides in an RNA polymerase II-containing complex. EMBO J. 16:5955-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogelstein, B., D. Lane, and A. J. Levine. 2000. Surfing the p53 network. Nature 408:307-310. [DOI] [PubMed] [Google Scholar]
- 65.Vousden, K. H. 2000. p53: death star. Cell 103:691-694. [DOI] [PubMed] [Google Scholar]
- 66.Vousden, K. H., and X. Lu. 2002. Live or let die: the cell's response to p53. Nat. Rev. Cancer. 2:594-604. [DOI] [PubMed] [Google Scholar]
- 67.Wang, X. W., H. Yeh, L. Schaeffer, R. Roy, V. Moncollin, J. M. Egly, Z. Wang, E. C. Freidberg, M. K. Evans, B. G. Taffe, et al. 1995. p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat. Genet. 10:188-195. [DOI] [PubMed] [Google Scholar]
- 68.Weinberg, R., and S. Penman. 1969. Metabolism of small molecular weight monodisperse nuclear RNA. Biochim. Biophys. Acta 190:10-29. [DOI] [PubMed] [Google Scholar]
- 69.Wong, M. W., R. W. Henry, B. Ma, R. Kobayashi, N. Klages, P. Matthias, M. Strubin, and N. Hernandez. 1998. The large subunit of basal transcription factor SNAPc is a Myb domain protein that interacts with Oct-1. Mol. Cell. Biol. 18:368-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao, H., A. Pearson, B. Coulombe, R. Truant, S. Zhang, J. L. Regier, S. J. Triezenberg, D. Reinberg, O. Flores, C. J. Ingles, et al. 1994. Binding of basal transcription factor TFIIH to the acidic activation domains of VP16 and p53. Mol. Cell. Biol. 14:7013-7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu, A., H. Y. Fan, D. Liao, A. D. Bailey, and A. M. Weiner. 2000. Activation of p53 or loss of the Cockayne syndrome group B repair protein causes metaphase fragility of human U1, U2, and 5S genes. Mol. Cell 5:801-810. [DOI] [PubMed] [Google Scholar]
- 72.Zhai, W., and L. Comai. 2000. Repression of RNA polymerase I transcription by the tumor suppressor p53. Mol. Cell. Biol. 20:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]