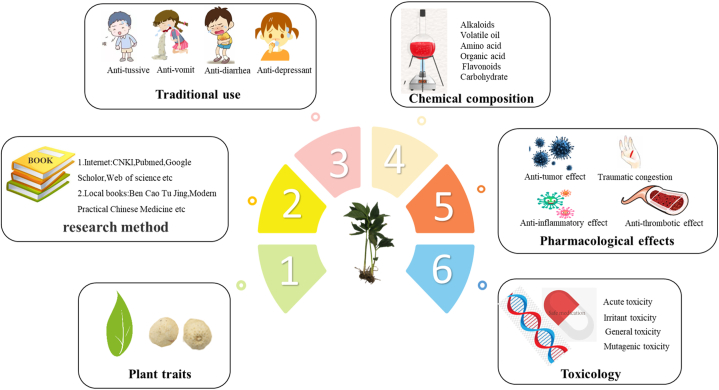
Abstract
Ethnopharmacological relevance
Pinellia ternata (Thunb.) Breit. is a well-known perennial herb that is used in traditional medicine in China, Japan and Korea. It's drawing worldwide interests in medicinal applications owing such as anti-diarrhea, lipid-lowering, anti-tumor, anti-cough, expectorant, anti-gastric ulcer, etc.
Aim of the study
This review aims to provide useful information on the botany, traditional uses, phytochemistry, pharmacology, toxicity and quality control of Pinellia ternata to help increase its efficiency. In addition, this review will discuss the future research trends and development prospects of this plant.
Materials and methods
Data was obtained through a systematic search of published literature and online databases such as Google Scholar, Web of Science, PubMed, Science Direct, and Sci-Finder. The botanical names were confirmed using the World Flora Online and chemical structures were drawn using the ChemBio Draw Ultra Version 19.0 Software.
Results
Pinellia ternata is distributed in regions of China and other areas. Pinellia ternata and its compound preparations can be used for cough, vomiting, gastric ulcer and other diseases. Approximately 212 chemical constituents have been isolated from Pinellia ternata, including alkaloids, volatile oils, amino acids, organic acids, flavonoids, cerebrosides, phenylpropanoids and other compounds. Considerable pharmacological experiments in vitro and in vivo have demonstrated that Pinellia ternata possessed antitumor effect, antitussive effects, antiasthmatic effects, increasing resistance to gastric ulcer, and antidiarrheal effect. However, these extracts can also lead to various toxicities such as irritant toxicity, cardiotoxicity, hepatotoxicity and embryonic toxicity. Considerable experiments have demonstrated that different processing methods and suitable compatibility with other herbs can effectively reduce the toxicities and increase the efficiency of Pinellia ternata.
Conclusions
Pinellia ternata is an ancient herbal medicine with a broad spectrum of pharmacological activities that has been used for thousands of years in China. Future studies should perform an in-depth analyses of the pharmacokinetics and mechanisms of toxicity of Pinellia ternata. Quality standards should be developed to correspond to the various application methods to ensure the efficacy of drugs in actual treatment.
Keywords: Pinellia ternata (Thunb.) breit, Botany, Traditional uses, Phytochemistry, Pharmacology, Toxicity, Quality control
1. Introduction
Pinelliae Rhizoma (PR), also named Shoutian (守田), Shuiyu (水玉), Diwen (地文), and Xiezicao (蝎子草), is a tuber of Pinellia ternata (Thunb.) Breit. from the family Araceae, which comprises approximately 115 genera and more than 2000 species distributed worldwide, with more than 92 % of them produced in the tropics. PR was first recorded as a herbal medicine in the Shennong Herbal Classic. It is acrid in flavor, warm and toxic, and acts on the spleen, stomach, and lung channels, where it can promote the circulation of Qi and eliminate dampness. In the spleen and lung, it can eliminate dampness and activate the spleen to clear phlegm; thus, it is applicable to the treatment of cough and asthma due to damp phlegm, cold phlegm, retention of dampness, and stasis in middle-jiao (中焦). As PR can also regulate the stomach and lower the adverse Qi to stop vomiting and eliminate stasis, it is indicated for vomiting due to the adverse flow of Qi caused by failure of the descending of stomach-Qi, stasis, and distention due to cold and heat.
To date, phytochemical studies have shown that PR is rich in various ingredients, including alkaloids, volatile oils, amino acids, organic acids, flavonoids, cerebrosides, phenylpropanoids and other chemical components. With increasing interest in research on the pharmacological activities of PR, researchers have revealed the significant pharmacological effects of PR, including as a cough suppressant and expectorant, as well as its antiasthmatic, antivomiting, anti-gastric ulcer, antidiarrheal, hypolipidemic, and antitumor effects. PR is one of 28 clinically toxic herbs [1], it causes violent irritation of the mucous membranes of the mouth and laryngopharynx when taken incorrectly, and its toxicity can be effectively minimized by proper handling. The common processing products of PR include Pinelliae Rhizoma Praeparatum Cum Alumine (PRPCA, Qing-Banxia, 清半夏), Pinelliae Rhizoma Praeparatum Cum Zingibere et Alumine (PRPZA, Jiang-Banxia, 姜半夏), and Pinelliae Rhizoma Praeparatum (PRP, Fa-Banxia, 法半夏). For its pharmacological effects, toxicity mechanism of action, has become a hot spot for researchers. The aim of this paper is to provide up-to-date and comprehensive information on botanicals, traditional uses, processing, phytochemistry, quality control, pharmacology and toxicity in order to lay the foundation for further development and utilization of Pinellia ternata. (Fig. 1).
Fig. 1.
Graphical abstract.
2. Materials and methods
To cultivate a comprehensive understanding of the current research status of PR, we performed a no language restrictions the use of database search, including China National Knowledge Infrastructure (CNKI), Google Scholar, PubMed, Web of Science, SpringerLink, Wiley, Wanfang Data and Baidu Academic to retrieve articles on the botany, phytochemistry, extraction methods, and pharmacology of PR, for information about PR. We also searched for articles on the botany and traditional uses of PR from PhD and MS dissertations and books such as Ben Cao Tu Jing, A Textual Research on the Name and Reality of Plants and Modern Practical Chinese Medicine. By summarizing and organizing this review, this review covers extraction methods as well as botanical, phytochemical, pharmacological, and toxicological research on PR from 1975 to 2023. Publications on unrelated topics and non-SCI indexed journal issues were excluded. In total, we found more than 300 articles, as well as books. We here cite a total of 165 sources, mostly phytochemical and pharmacological studies.
3. Botany
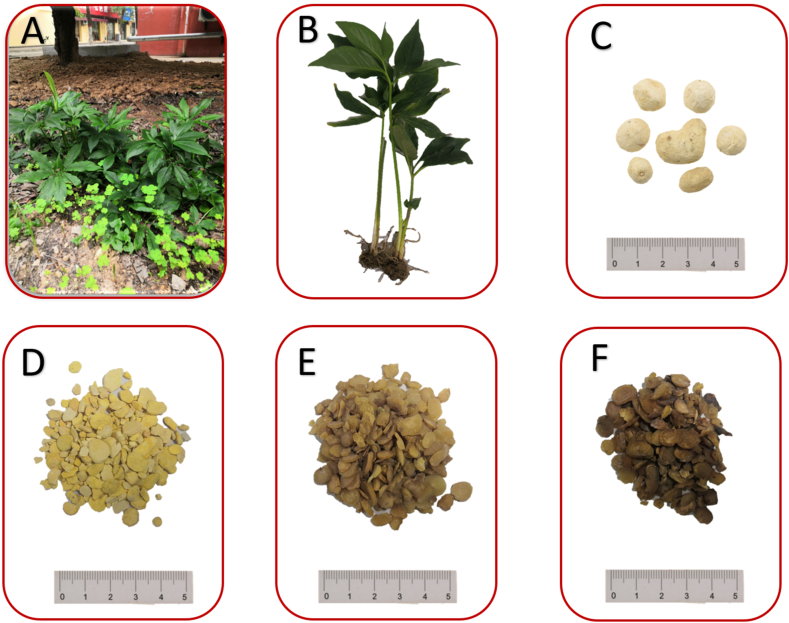
PR is a perennial herb of Araceae (Fig. 2A), with a plant height of 10–35 cm. The metamorphic stems of PR are mostly spherical with a diameter of 0.5–4 cm, and many fibrous roots under the tuber; the pearl buds are 0.5–1 cm, and the lower fibrous roots are few and thin. The seedlings of pearl buds or small tubers are mainly single-leaved, ovoid; seedlings born from tubers of ≥2 years have 2–7 leaves, usually three compound leaves, with a larger one in the middle (Fig. 2B). The flowering period of PR is from May to July, and the fruit period is August [2].
Fig. 2.
Photograph of Pinellia ternata (Thunb.) Breit. Note: (A) The morphology of the Pinellia ternata (Thunb.) Breit; (B) the whole plant or overground part of Pinellia ternata (Thunb.) Breit; (C) Pinellia ternata decoction pieces; (D) Pinelliae Rhizoma Praeparatum (Fa-Banxia, 法半夏); (E) Pinelliae Rhizoma Praeparatum Cum Zingibere et Alumine (Jiang-Banxia, 姜半夏); (F) Pinelliae Rhizoma Praeparatum Cum Alumine (Qing-Banxia清半夏).
PR is spherical in shape, some slightly inclined, with a diameter of 0.7–1.6 cm. The surface is white or light yellow, with sunken stem scars at the top and pitted root scars around; the lower layer is blunt and smooth. Oral administration of PR will cause numbness of the tongue and tingling of the throat (Fig. 2C).
Fa-Banxia are spherical or broken into irregular granules. The surface is yellowish white, yellow, or brownish yellow. The texture is more flimsy or hard and brittle, yellow or pale yellow in cross-section, and the granules are slightly hard and brittle. Fa-Banxia have a mild odor, a light and slightly sweet taste, and a slight numbing sensation on the tongue (Fig. 2D).
Jiang-Banxia are characterized as flaky, irregularly granular, or sphere-like, with a brown to tan surface. The texture is hard and brittle, yellowish brown at the cross-section, often with a horny luster. The smell is slightly fragrant, the taste is light, and it causes slight numbness of the tongue and slightly sticky teeth when chewing (Fig. 2E).
Qing-Banxia decoction pieces are characterized by oval, round or irregular pieces. The section is grayish to grayish white or yellowish white to yellowish brown, with grayish white dotted or short linear vascular bundle traces, and some residual embolisms showing light purplish-red markings below. Qing-Banxia decoction pieces are brittle and easily broken, and its sections are slightly powdery or horny. The smell is slight, while the taste is slightly astringent and leaves a slightly numb tongue feeling (Fig. 2F).
4. Traditional uses
4.1. Processing drugs
Raw PR is a toxic traditional Chinese medicine (TCM) that has been described as having strong irritant toxicity [3]. The toxicity of PR has been recorded in the Shennong Herbal Classic. In ancient times, water, ginger juice, licorice, tofu, alum, and rice vinegar were the common ingredients used in the processing of PR. In modern research, licorice, lime, ginger, and alum are more effective than the previously used substances in reducing toxicity and improving efficiency. However, only three kinds of processed products were included in the Chinese pharmacopoeia, including Fa-Banxia, Jiang-Banxia, and Qing-Banxia [4].
Fa-Banxia is made by long-term soaking of PR, licorice extract, and lime, which has the effects of drying dampness and resolving phlegm. Licorice can enhance the role of removing the phlegm while reducing toxicity; in contrast, lime can ensure that PR will not rot by long-term soaking. Jiang-Banxia is made by boiling PR, ginger, and alum, which has the effects of resolving phlegm and stopping vomiting. Ginger and alum can enhance the effect of resolving phlegm and reduce toxicity. Additionally, ginger can also enhance the effect of stopping vomiting. Qing-Banxia is made by long-term soaking in PR and lime, which has the effects of drying dampness and resolving phlegm. Alum can enhance the role of resolving phlegm while reducing toxicity and ensuring that the PR will not rot by long-term soaking. Modern research has found that researchers using mice ammonia cough method, tracheal phenol red excretion method, electrical stimulation of the cat supraglottic nerve due to cough model, ammonia fumigation cough model mice, have confirmed that the PR has the efficacy of analgesic cough expectorant. The antiemetic effect of PR was confirmed using a model of mink locomotor vomiting induced by various vomitogenic agents such as digitalis, apomorphine and copper sulfate. Using a mouse model of colon adenocarcinoma under the intervention of chemotherapeutic drugs, as well as Ehrlich ascites carcinoma cells, gastric cancer BGC823 cells, and chronic myelogenous leukemia cells (5K62), the anticancer effect of PR was confirmed. The anti-inflammatory and analgesic effects of PR were confirmed using the xylene-induced auricular swelling model in mice, and the pain model in mice caused by the hot plate method [1,5].
In summary, although modern studies have shown that processing PR reduces its toxicity and enhances its efficacy, there have been relatively few studies on the changes in its components before and after processing. In future research work, comparisons of different compositions will be key for clarifying the processing principles and will provide a more accurate scientific basis for clinical use.
4.2. Prescription application
PR, as a TCM, has a history of more than 2000 years in China. It was first published in the Shennong Herbal Classic (Han Dynasty), which states that it was used to treat cough, excessive phlegm, nausea and vomiting, and soreness and swelling due to poison. The Liu juan zi gui yi fang (Jin Dynasty) states that it was used to treat carbuncles and gangrene; the Bei ji qian jin yao fang (Tang Dynasty) recorded that was used for treating kidney yang deficiency; the Wai tai mi yao (Tang Dynasty) states that it was used for the treatment of cough and asthma; the Taiping Huimin Mixture (Song Dynasty) recorded that it was used to treat wind phlegm; and the Yao lei fa Xiang (Yuan Dynasty) recorded its efficacy in treating headache. Modern studies have shown that Huang et al. [6] found that Pinellia ternata alkaloids can improve the immune function of tumor model mice, improve the immune microenvironment, and have a certain inhibitory effect on tumor cells. Cheng et al. [7] used the total alkaloids of PR in mice by gavage for 13 d and found that the total alkaloids of PR prolonged the latency period of epilepsy and attenuated the extent of seizures in rats with chronic ignition of penicillin. Huang et al. [8] found that PR polysaccharide can inhibit mucus secretion in lung tissue and reduce sputum production in allergic asthma model rats, etc.
PR has become an important herbal medicine, which is commonly used in the clinical treatment of tumor, gastric ulcer, diarrhea, epilepsy, and other diseases. Simultaneously, various forms of formulations have been developed, including powders, pills, and tablets, to better meet the clinical needs (Table 1)
Table 1.
The traditional and clinical uses of Pinellia ternata (Thunb.) Breit. in China.
| Preparation name | Main compositions | Formulation | Traditional and clinical uses | References |
|---|---|---|---|---|
| Xiaoqinglong soup | Ephedra sinica Stapf 9 g, Paeonia lactiflora Pall. 9 g, Asarum sieboldii Miq 6 g, Zingiber officinale Rosc. 6 g, Glycyrrhiza uralensis Fisch processing with honey 6 g, Cinnamomum cassia Presl 9 g, Schisandra chinensis (Turcz.) Bail. 6 g, Pinellia ternata (Thunb.) Breit. 9 g | Decoction | Dispersing cold from the exterior of the body, warming the lung to dissolve drinks. | Treatise On Febrile Disease⟪伤寒论⟫ |
| Shegan Mahuang soup | Belamcanda chinensis (L.) DC. 9 g, Ephedra sinica 9 g, Zingiber officinale 6 g, Asarum sieboldii 6 g, Aster tataricus L. f. 6 g, Ziziphus jujuba Mil. 3 piece, Pinellia ternata 9 g, Schisandra chinensis (Turcz.) 3 g | Decoction | Expelling phlegm from the lung, relieving cough by lowering the Qi. | Jin Kui Yao Lue⟪金匮要略⟫ |
| Jinfeicao powder | Inula japonica Thunb. 90 g, Ephedra sinica 90 g, Peucedanum praeruptorum Dunn 90 g, Schizonepeta tenuisfolia Briq. 120 g, Glycyrrhiza uralensis process with honey 30 g, Pinellia ternata 30 g, Paeonia ladiflora Pall. 30 g | Powder | Dispersing wind and cold, lowering Qi and resolving phlegm | Bo Ji Fang⟪博济方⟫ |
| Dachaihu soup | Bupleurum chinense DC. 15 g, Scutellaria baicalensis Georgi 9 g, Paeonia lactiflora. 9 g, Pinellia ternata 9 g, Zingiber officinale 15 g, Citrus aurantium 9 g, Ziziphus jujuba 4 piece, Rheum palmatum L. 6 g | Decoction | Harmonizing Shao Yang, Internal diarrhea of hot knots | Jin Kui Yao Lue⟪金匮要略⟫ |
| Shensu decoction | Panax ginseng C. A. Mey. 6 g, Perilla frutescens (L.) Brit. 6 g, Pueraria lobata (Willd.) Ohwi 6 g, Pinellia ternata 6 g, Peucedanum praeruptorum 6 g, Poria cocos (Schw.) Wolf 6 g, Citrus aurantium L. 4 g, Platycodon grandiflorum (Jacq.) A. DC. 4 g, Aucklandia lappa Decne. 4 g, Citrus reticulata Blanco 4 g, Glycyrrhiza uralensis processing with honey 4 g | Decoction | Benefiting Qi and relieving symptoms, regulating Qi and resolving phlegm | Taiping Huimin Heji Ju Fang⟪太平惠民和剂局方⟫ |
| Xiaochaihu soup | Bupleurum chinense 12 g, Scutellaria baicalensis 9 g, Panax ginseng 6 g, Pinellia ternata 9 g, Glycyrrhiza uralensis 5 g, Zingiber officinale 9 g, Ziziphus jujuba 4 piece | Decoction | Harmonizing Shaoyang, harmonizing the stomach and lowering rebelliousness | Treatise On Febrile Disease⟪伤寒论⟫ |
| Zhuye Shigao soup | Lophatherum gracile Brongn 6 g, Gypsum Fibrosum 50 g, Pinellia ternata 9 g, Ophiopogon japonicus (L.f) Ker-Gawl. 20 g, Panax ginseng 6 g, Oryzae semen 10 g, Glycyrrhiza uralensis 6 g | Decoction | Clearing heat and promoting the production of body fluid, benefiting Qi and harmonizing the stomach | Treatise On Febrile Disease⟪伤寒论⟫ |
| Xiangsha Liujunzi soup | Panax ginseng 3 g, Atractylodes macrocephala 6 g, Glycyrrhiza uralensis 2 g, Citrus reticulata 2.5 g, Pinellia ternata 3 g, Amomum kravanh Pierre ex Gagnep. 2.5 g, Aucklandia lappa 2 g | Decoction | Benefiting Qi and strengthening the spleen, moving Qi and resolving phlegm | Gu Jin Ming Yi Fang Lun⟪古今名医方论⟫ |
| Huiyang Jiuji soup | Aconitum carmichaelii Debx. processing with digestion 9 g, Zingiber officinale 6 g, Panax ginseng 6 g, Glycyrrhiza uralensis 6 g, Atractylodes macrocephala Koidz. processing with bran fried 9 g, Cinnamomum cassia 3 g, Citrus reticulata 6 g, Schisandra chinensis 3 g, Poria cocos 9 g, Pinellia ternata 9 g | Decoction | Returning Yang to consolidate detachment, benefiting Qi and generating pulse | Shang Han Liu Shu⟪伤寒六书⟫ |
| Sixjunzi soup | Panax ginseng 9 g, Atractylodes macrocephala 9 g, Poria cocos 9 g, Glycyrrhiza uralensis 6 g, Citrus reticulata 3 g, Pinellia ternata 4.5 g | Decoction | Benefiting Qi and strengthening the spleen, drying dampness and resolving phlegm | Yi Xue Zheng Chuan⟪医学正传⟫ |
| Binglang powder | Areca catechu 30 g, Pinellia ternata 30 g, Prunus armeniaca 30 g, Platycodon grandiflorum 30 g, Citrus reticulata 30 g, Inula Japonica 30 g, Zingiber officinale 30 g, Atractylodes macrocephala 30 g, Panax ginseng 15 g, Glycyrrhiza uralensis 15 g | Powder | Phlegm and phlegm in the chest and diaphragm, and a deficient sound in the abdomen | Chong Ding Yan Shi Ji Sheng Fang⟪重订严氏济生方⟫ |
| Buqi Huatan soup | Astragalus membranaceus 45 g, Platycodon grandiflorum 9 g, Adenophora Stricta Miq 24 g, Prunus armeniaca 9 g, Aster tataricus 9 g, Poria cocos 10 g, Lilium brownii var. viridulum Baker 12 g, Pinellia ternata 12 g, Glycyrrhiza uralensis 9 g | Decoction | Tonifying Qi and calming asthma, relieving cough and resolving phlegm | Li Shao Nan Fang ⟪李绍南方⟫ |
| Shengyang Yiwei soup | Astragalus membranaceus (Fisch.) Bge. 30 g, Pinellia ternata 5 g, Panax ginseng 15 g, Glycyrrhiza uralensis 15 g, Angelica pubescens Maxim. f. biserrata Shan et Yuan 9 g, Saposhnikovia divaricata (Turcz.) Schischk. 9 g, Paeonia lactiflora. 9 g, Notopterygium incisum Ting ex H. T. Chang 9 g, Citrus reticulata 6 g, Poria cocos 5 g, Bupleurum chinense 5 g, Alisma orientate (Sam.) Juzep. 5 g, Atractylodes macrocephala 5 g, Coptis chinensis Franch. 1.5 g | Decoction | Benefiting Qi and raising Yang, clearing heat and removing dampness | Nei Wai Shang Bian Huo Lun⟪内外伤辨惑论⟫ |
| Anzhong Tiaoqi pill | Citrus reticulata 60 g, Pinellia ternata 30 g, Poria cocos (Schw.) Wolf. 30 g, Atractylodes macrocephala 60 g, Citrus aurantium 30 g, Perilla frutescens 18 g, Ligusticum chuanxiong 15 g, Angelica sinensis (Oliv.) Diels 15 g, Paeonia lactiflora 24 g, Aucklandia lappa 3 g, Glycyrrhiza uralensis 9 g, Cyperus rotundus L. 90 g, Massa Medicata Fermentata 30 g, Coptis chinensis 30 g, Amomum kravanh 15 g, Raphanus sativus L. 15 g | Pills | Turning the stomach and phlegm diaphragm | Gu Jin Yi Jian⟪古今医鉴⟫ |
| Banfu soup | Aconitum carmichaelii 7.5 g, Pinellia ternata 7.5 g, Zingiber officinale 10 piece | Decoction | Warming the stomach and resolving phlegm | Yi Xue Ru Men⟪医学入门⟫ |
| Babao Huichun soup | Aconitum carmichaelii 30 g, Panax ginseng 30 g, Ephedra sinica 30 g, Scutellaria baicalensis 30 g, Stephania tetrandra S. Moore 30 g, Cyperus rotundus 30 g, Prunus armeniaca L. var. ansu Maxim. 30 g, Ligusticum chuanxiong 30 g, Angelica sinensis 30 g, Poria cocos 45 g, Citrus reticulata 30 g, Saposhnikovia divaricata 30 g, Paeonia lactiflora 150 g, Aquilaria sinensis (Lour.) Gilg 15 g, Pinellia ternata 45 g, Aconitum carmichaelii 15 g, Cinnamomum cassia 30 g, Atractylodes macrocephala 60 g, Lindera aggregate (Sims) Kosterm. 15 g, Zingiber officinale 30 g, Astragalus membranaceus 90 g, Glycyrrhiza uralensis 30 g, Rehmannia glutinosa Libosch. processing with wine 30 g, Rehmannia glutinosa 30 g | Decoction | Expelling wind, harmonizing Qi, invigorating blood | Zhu Shi Ji Yan Fang⟪朱氏集验方⟫ |
| Basheng yinzi | Aconitum carmichaelii 60 g, Arisaema erubescens (Wall.) Schott 30 g, Typhonium giganteum 30 g, Gastrodia elata 30 g, Atractylodes macrocephala 30 g, Ligusticum chuanxiong 15 g, Aucklandia lappa 15 g, Buthus martensii Karsch 15 g, Pinellia ternata 15 g | Decoction | Yang Qi does not converge, wind and evil invade outside | Zhu Shi Ji Yan Fang⟪朱氏集验方⟫ |
| Bajunzi soup | Panax ginseng 3 g, Poria cocos 3 g, Atractylodes macrocephala 3 g, Glycyrrhiza uralensis 1.5 g, Pinellia ternata 3 g, Citrus reticulata 2.4 g, Atractylodes lancea (Thunb.) DC. 2.4 g, Angelica sinensis 7.5 g | Decoction | Dampness and phlegm in the spleen and stomach | Chen Nang An Fu Ke Bu Xie⟪陈囊庵妇科补解⟫ |
| Bafeng nord | Talcum 30 g, Gastrodia elata Bl. 30 g, Cinnamomum camphora (L.) Presl 0.3 g, Moschus berezovskii Flerov 0.3 g, Bombyx mori Linnaeus 15 g, Typhonium giganteum Engl. 9 g, Pinellia ternata 60 g, Calcitum 250 g | Pills | Phlegm-heat attack | Taiping Huimin Heji Ju Fang⟪太平惠民和剂局方⟫ |
| Bawu soup | Rehmannia glutinosa processing with wine 6 g, Ligusticum chuanxiong 6 g, Paeonia lactiflora 6 g, Angelica sinensis 6 g, Panax ginseng 4.5 g, Atractylodes macrocephala 9 g, Citrus reticulata 3 g, Pinellia ternata 6 g | Decoction | Enrich the Qi and the blood | Nu Ke Qie Yao⟪女科切要⟫ |
| Bailuo pill | Atractylodes lancea (Thunb.) DC. 60 g, Gardenia jasminoides Ellis 60 g, Cyperus rotundus 60 g, Arisaema erubescens 60 g, Citrus aurantium 15 g, Citrus reticulata 15 g, Aucklandia lappa 15 g, Pinellia ternata 15 g, Amomum kravanh 15 g | Pills | Accumulation of phlegm and beverages, pain in the stomach and epigastric region | Dan Xi Xin Fa⟪丹溪心法⟫ |
| Banxia soup | Pinellia ternata 10 g, Setaria italica (L.) Beauv. 15 g | Decoction | Relieving phlegm and harmonizing the stomach | Ling Shu⟪灵枢⟫ |
| Baiziren soup | Platycladus orientalist (L.) Franco 15 g, Codonopsis pilosula (Franch.) Nannf. 15 g, Atractylodes macrocephala 10 g, Pinellia ternata 6 g, Schisandra chinensis 10 g, Ostrea gigas Thunberg 20 g, Ephedra sinica 10 g, Triticum aestivum L 15 g, Ziziphus jujuba Mill. 5 piece | Decoction | Calming the mind and tranquilizing the soul, nourishing Yin and astringing sweat | Zhou Mi Dao Fang⟪周明道方⟫ |
| Baiziren pill | Platycladus orientalist 60 g, Rhizoma Pinelliae Fermentata 60 g, Ostrea gigas 30 g, Panax ginseng 30 g, Atractylodes macrocephala 30 g, Ephedra sinica 30 g, Schisandra chinensis 30 g, Triticum sativum 15 g | Pills | Deficiency of heart yang, heart twitching and night sweating | Yi Zong Bi Du⟪医宗必读⟫ |
| Banxia Baizhu Tianma soup | Pinellia ternata 4.5 g, Atractylodes macrocephala 6 g, Gastrodia elata 4.5 g | Decoction | Strengthening the spleen and resolving phlegm, calming the liver and calming the wind | Gu Jin Yi Jian⟪古今医鉴⟫ |
| Baiqian soup | Cynanchum glaucescens (Decne.) Hand.-Mazz. 6 g, Aster tataricus 9 g, Pinellia ternata 9 g, Cirsium japonicum Fisch. ex DC. 3 g | Decoction | Treatment of cough and upper air, body swelling | Wai Tai Mi Yao⟪外台秘要⟫ |
| Bangua pill | Pinellia ternata 150 g, Trichosanthes kirilowii Maxim. 150 g, Platycodon grandiflorum 60 g, Citrus aurantium 45 g, Dioscorea opposita Thunb. 30 g | Pills | Relieve phlegm and cough | Yi Xue Ru Men⟪医学入门⟫ |
| Banjie soup | Paeonia lactiflora 30 g, Bupleurum chinense 6 g, Angelica sinensis 9 g, Ligusticum chuanxiong 15 g, Glycyrrhiza uralensis 3 g, Vitex trifolia L. 3 g, Pinellia ternata 3 g | Decoction | Clearing the liver and relieving depression, invigorating the blood and dispelling wind | Bian Zheng Lu⟪辨证录⟫ |
| Banbei pill | Pinellia ternata 9 g, Fritillaria cirrhosa D. Don 9 g | Pills | Truncated malaria | Chong Ding Tong Shu Treatise on Febrile Disease⟪重订通俗伤寒论⟫ |
| Banxia Buxin soup | Pinellia ternata 12 g, Zingiber officinale 10 g, Poria cocos 6 g, Cinnamomum cassia 6 g, Citrus aurantium 6 g, Citrus reticulata 6 g, Atractylodes macrocephala 8 g, Saposhnikovia divaricata 4 g, Polygala tenuifolia Willd. 4 g | Decoction | Warming the stomach and invigorating the spleen, regulating Qi and resolving drinks | Bei Ji Qian Jin Yao Fang⟪备急千金要方) |
| Banxia Lige pill | Atractylodes macrocephala 30 g, Panax ginseng 30 g, Poria cocos 30 g, Alumen 30 g, Talcum 30 g, Fritillaria cirrhosa 30 g, Arisaema erubescens 45 g, Typhonium giganteum 60 g, Pinellia ternata 90 g | Pills | Expelling wind and resolving phlegm, benefiting Qi and strengthening the spleen | Yu Yao Yuan Fang ⟪御药院方⟫ |
| Banxia Renshen wine | Pinellia ternata 30 g, Scutellaria baicalensis 3 0 g, Zingiber officinale 20 g, Panax ginseng 20 g, Glycyrrhiza uralensis processing with honey 20 g, Coptis chinensis 6 g, Ziziphus jujuba 10 g, Liquor 700 mL | Decoction | Harmonizing the stomach and subduing rebelliousness, opening up knots and dispersing lumps | Shang Han Lun⟪伤寒论⟫ |
| Banxia powder | Pinellia ternata 60 g, Euodia rutaecarpa (Juss.) Benth. 15 g, Cinnamomum cassia 30 g, Panax ginseng 30 g, Atractylodes macrocephala 30 g, Angelica sinensis 30 g, Magnolia officinalis 45 g, Citrus aurantium 15 g | Powder | warming in the middle and lowering the rebellion | Tai Ping Sheng Hui Fang⟪太平圣惠方⟫ |
| Banxia Shengjiang soup | Zingiber officinale 15 g, Pinellia ternata 10 g | Decoction | Warming the stomach and lowering rebellion | Lei Zheng Huo Ren Shu⟪类证活人书⟫ |
| Banxia xingren soup | Pinellia ternata 3 g, Prunus armeniaca 2.4 g, Citrus aurantium 1.5 g, Platycodon grandiflorum 1.5 g, Scutellaria baicalensis 1.5 g, Perilla frutescens 1.5 g, Ephedra sinica 1.8 g, Glycyrrhiza uralensis 12 g | Decoction | Mainly for wind and phlegm asthma | Xing Yuan Sheng Chun⟪杏苑生春⟫ |
| Banxia yunfang | Pinellia ternata 90 g, Zingiber officinale 90 g, Asarum sieboldii 90 g, Cinnamomum cassia 20 g, Aconitum carmichaelii 10 piece | Decoction | Mainly for pediatric craniosynostosis | Bei Ji Qian Jin Yao Fang⟪备急千金要方⟫ |
| Banxia yinzi | Pinellia ternata 12 g, Magnolia officinalis 9 g, Panax ginseng 9 g, Atractylodes macrocephala 9 g, Zingiber officinale 9 g, Ziziphus jujuba 9 g, Semen Oryzae Sativae 10 g, Citrus reticulata 6 g | Decoction | Benefiting Qi and tonifying the middle Jiao, lowering rebellion and stopping vomiting | Wai Tai Mi Yao⟪外台秘要⟫ |
| Banxia pill | Alumen 450 g, Pinellia ternata 1500 g | Pills | Relieve phlegm and cough | Taiping Huimin Heji Ju Fang⟪太平惠民和剂局方⟫ |
| Buqi Zhenjing soup | Astragalus membranaceus 24 g, Platycladus orientalist 12 g, Ligusticum chuanxiong 6 g, Polygala tenuifolia 10 g, Acorus tatarinowii 10 g, Poria cocos 10 g, Angelica sinensis 10 g, Paeonia lactiflora 10 g, Ziziphus jujuba Mill 10 g, Pinellia ternata 10 g, Arisaema Cum Bile 6 g, Asarum sieboldii 3 g, Glycyrrhiza uralensis 4.5 g | Decoction | Tonifying Qi and expelling blood stasis, relieving spasm and resolving phlegm | Lu Ji Ping Fang ⟪路际平方⟫ |
| Bengtun soup | Glycyrrhiza uralensis 6 g, Angelica sinensis 6 g, Pinellia ternata 12 g, Scutellaria baicalensis 6 g, Pueraria lobata 15 g, Paeonia Lactiflora 6 g, Zingiber officinale 12 g, Euonymus tengyuehensis W. W. Smith 12 g | Decoction | Treatment of Penetrating Dolphin Qi up to the chest and abdominal pain | Jin Kui Yao Lue⟪金匮要略⟫ |
| Bishengyin | Pinellia ternata 6 g, Citrus aurantium 6 g, Gypsum Fibrosum 9 g, Prunus sibirica L. 3 g, Tea 3 g, Ephedra sinica 3 g, Trichosanthes kirilowii 3 g, Glycyrrhiza uralensis 3 g, Zingiber officinale 5 piece | Decoction | Promoting the lowering of the lung and Qi, clearing heat and resolving phlegm | Dan Tai Yu An⟪丹台玉案⟫ |
| Canglian soup | Atractylodes lancea 3 g, Coptis chinensis 3 g, Citrus reticulata 3 g, Pinellia ternata 3 g, Poria cocos 3 g, Massa Medicata Fermentata 3 g, Euodia rutaecarpa 1.5 g, Amomum kravanh 1.5 g, Glycyrrhiza uralensis 0.9 g | Decoction | Drying dampness and resolving phlegm, clearing the stomach and harmonizing the middle | Wan Bing Hui Chun⟪万病回春⟫ |
| Biantong Shiwei Wendan decotion | Citrus reticulata 9 g, Poria cocos 12 g, Pinellia ternata 12 g, Glycyrrhiza uralensis 3 g, Citrus aurantium 6 g, Rehmannia glutinosa 15 g, Ziziphus jujuba 15 g, Polygala tenuifolia 6 g, Acorus tatarinowii Schott 6 g, Succus Bambusae 3 spoons | Decoction | Palpitations and insomnia, mental dementia | Zhong Yi Zhi Fa Yu Fang Ji⟪中医治法与方剂⟫ |
| Canheyin | Terminalia chebula Retz 7.5 g, Atractylodes macrocephala 7.5 g, Astragalus Membranaceus 7.5 g, Poria cocos 7.5 g, Panax ginseng 7.5 g, Rhizoma Pinelliae Fermentata 7.5 g, Citrus reticulata 6 g, Schisandra chinensis 6 g, Glycyrrhiza uralensis 3 g, Aster tataricus 3 g | Decoction | Treatment of cold and phlegm cough | Wei Shi Jia Cang Fang ⟪魏氏家藏方⟫ |
| Biejiajian pill | Trionyx sinensis Wiegmann 90 g, Scutellaria baicalensis 22.5 g, Bupleurum chinense 45 g, Zingiber officinale 22.5 g, Rheum palmatum 22.5 g, Paeonia Lactiflora 37.5 g, Cinnamomum cassia 22.5 g, Lepidium apetalum Willd 7.5 g, Pyrrosia sheareri (Bak.) Ching 22.5 g, Magnolia officinalis 22.5 g, Paeonia Suffruticosa Andr. 37.5 g, Dianthus superbus L. 15 g, Pinellia ternata 7.5 g, Equus asinus Linnaeus 37.5 g, Copris reflexus 45 g, Prunus persica (L.) Batsch 15 g | Pills | Promoting circulation of Qi, resolving blood stasis, softening hardness and dispersing knots | Jin Kui Yao Lue⟪金匮要略⟫ |
| Banqin soup | Pinellia ternata 15 g, Poria cocos 15 g, Coptis chinensis 3 g, Magnolia officinalis Rehd. et Wils. 9 g, Tetrapanax papyrifer (Hook.) K. Koch 24 g | Decoction | Dry dampness and promote water | Wen Bing Tiao Bian⟪温病条辨⟫ |
| Bixaosan | Cortex Hibisci 120 g, Mylabris phalerata Pallas 3 g, Pinellia ternata 15 g, Momordica cochinchinensis (Lour.) Spreng 15 g, Areca catechu L.15 g, Realgar 9 g, Arsenic 3 g | Powder | Treatment of rheumatic scabies and long-standing ringworm | Gu Jin Yi Jian⟪古今医鉴⟫ |
5. Phytochemistry
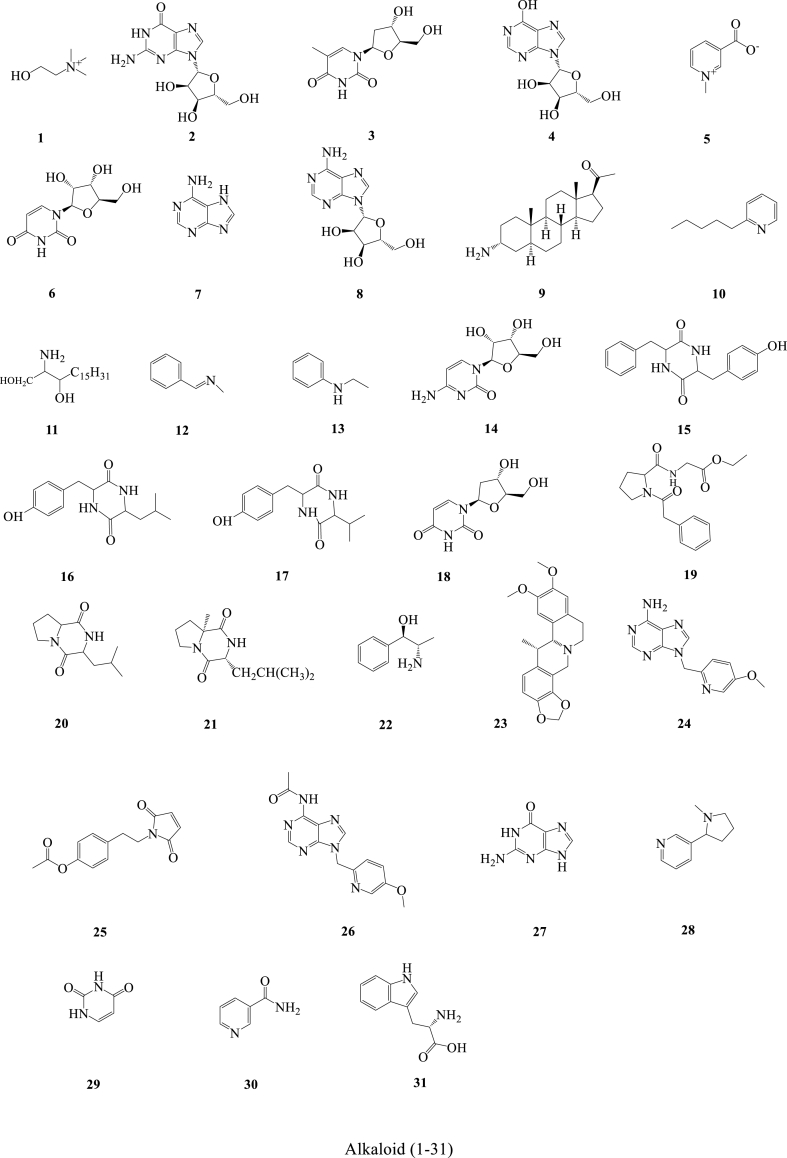
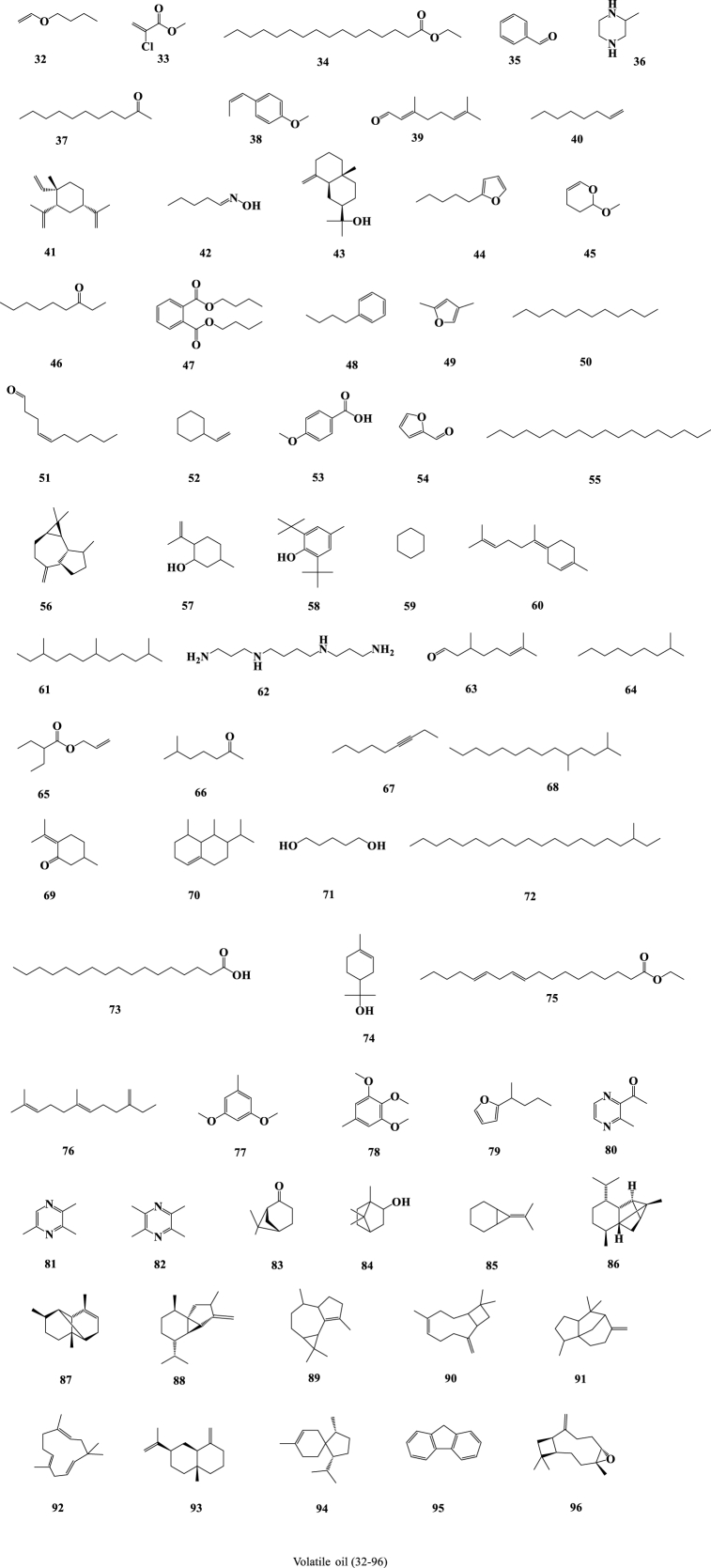
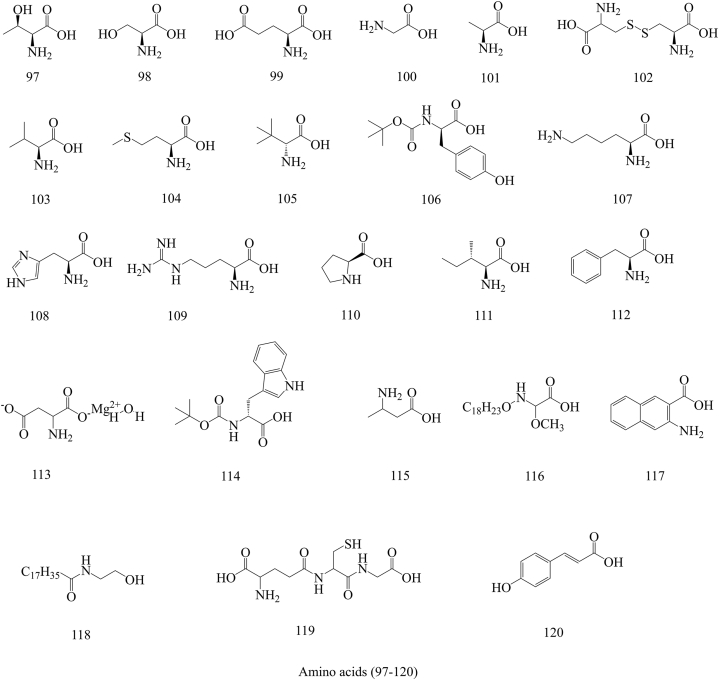
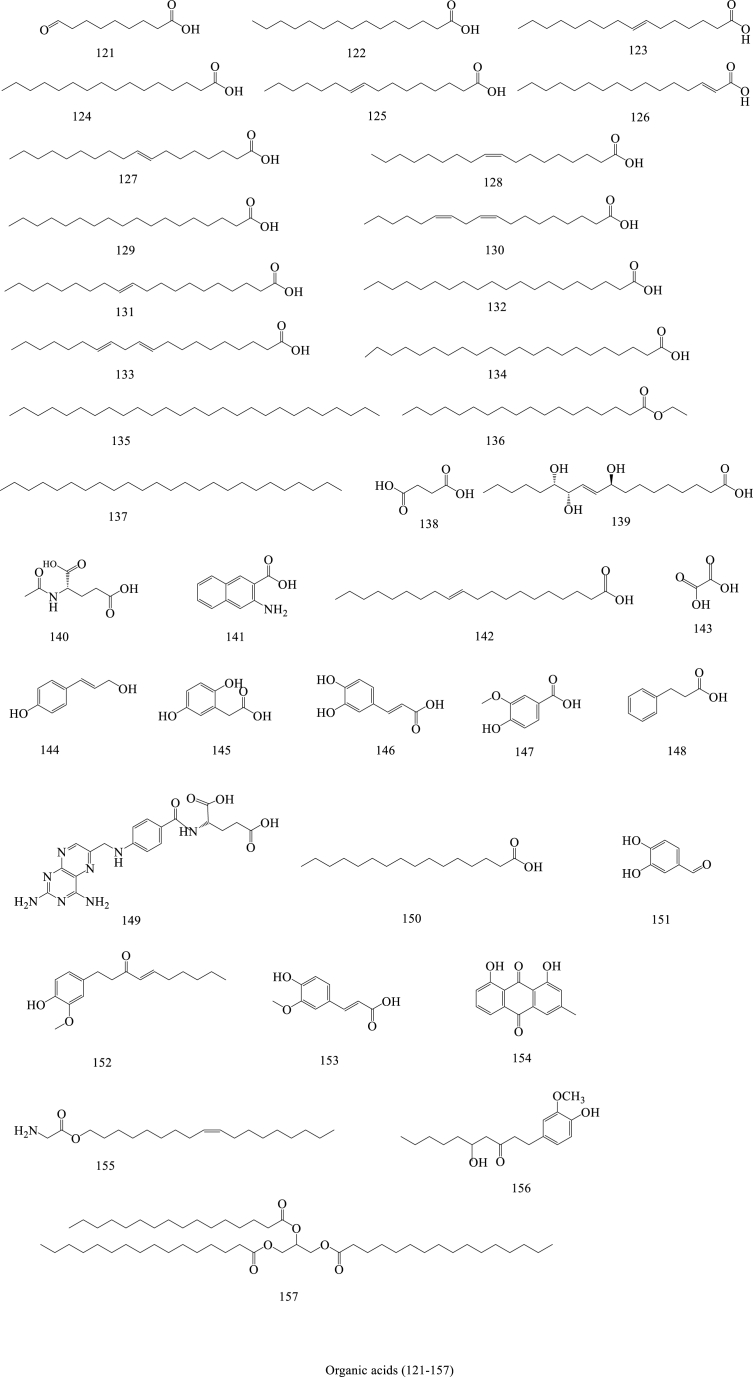
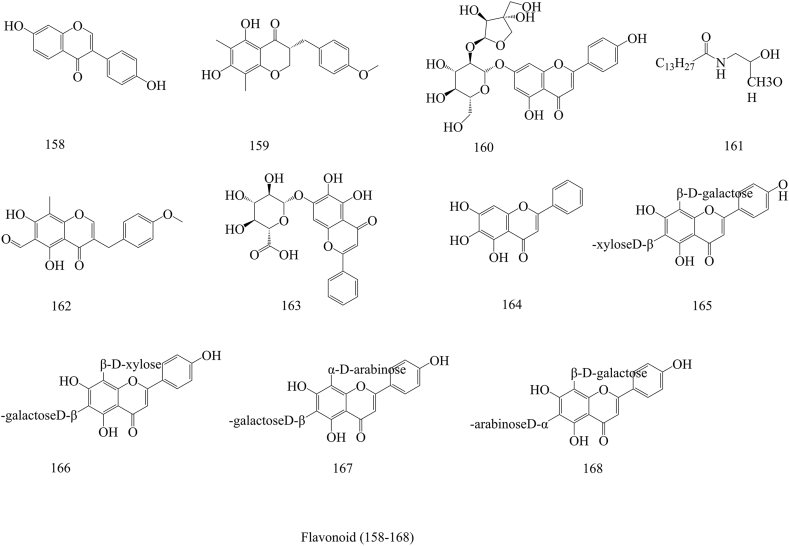
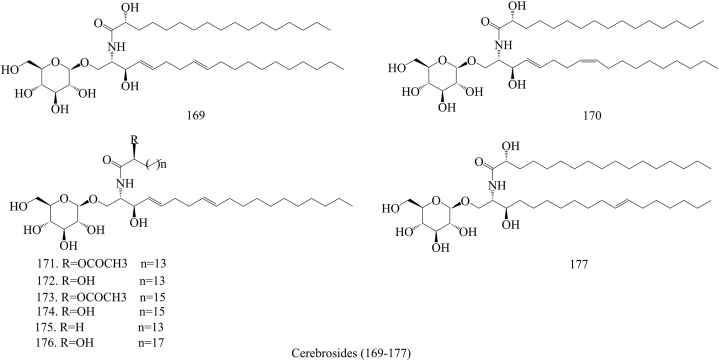
In the past few decades, 212 compounds have been separated and authenticated, predominantly comprising alkaloids (1–31) (Fig. 3), volatile oils (32–96) (Fig. 4), amino acids (97–120) (Fig. 5), organic acids (121–157) (Fig. 6), flavonoids (158–168) (Fig. 7), cerebrosides (169–177) (Fig. 8), phenylpropanoids (178–194) (Fig. 9) and others (195–212) (Fig. 10). Based on previous phytochemical investigations, the compounds have been isolated from different parts of PR (Table 2). Alkaloids, flavonoids, and organic acid are the most important and abundant bioactive constituents in PR extracts. It is considered as a promissing ingredients for future evaluation. Nevertheless, quantitative detection of the active components requires further research [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]].
Fig. 3.
Structures of alkaloid isolated from Pinellia ternata (Thunb.) Breit.
Fig. 4.
Structures of volatile oil isolated from Pinellia ternata (Thunb.) Breit.
Fig. 5.
Structures of Amino acids isolated from Pinellia ternata (Thunb.) Breit.
Fig. 6.
Structures of Organic acid isolated from Pinellia ternata (Thunb.) Breit.
Fig. 7.
Structures of Flavonoids isolated from Pinellia ternata (Thunb.) Breit.
Fig. 8.
Structures of Cerebrosides isolated from Pinellia ternata (Thunb.) Breit.
Fig. 9.
Structures of Phenylpropanoids isolated from Pinellia ternata (Thunb.) Breit.
Fig. 10.
Structures of others isolated from Pinellia ternata (Thunb.) Breit.
Table 2.
Chemical constituents of Pinellia ternata (Thunb.) Breit.
| No. | Chemical component | reference | |
|---|---|---|---|
| Alkaloids | |||
| 1 | Choline | [5] | |
| 2 | Guanosine | [5] | |
| 3 | Thymidine | [5] | |
| 4 | Inosine | [5] | |
| 5 | Trigonelline | [5] | |
| 6 | Uridine | [5] | |
| 7 | Adenine | [6] | |
| 8 | Adenosine | [6] | |
| 9 | 3alpha-amino-5alpha-pregnan-20-one | [6] | |
| 10 | 2-Pentylpyridine | [6] | |
| 11 | (2R,3S,4S,6S)-2-(hydroxymethyl)-6-(2-hydroxytridecyl)-3,4-Piperidinediol | [6] | |
| 12 | N-Benzylidenemethylamine | [6] | |
| 13 | N-Ethylaniline | [6] | |
| 14 | Cytidine | [7] | |
| 15 | Cyclo-(Phe-Tyr) | [8] | |
| 16 | Cyclo-(Leu-Tyr) | [8] | |
| 17 | Cyclo-(Val-Tyr) | [8] | |
| 18 | 2′-deoxyuridine | [8] | |
| 19 | Noopept | [9] | |
| 20 | Cyclo-pro-ile | [9] | |
| 21 | Cyclo-pro-val | [4] | |
| 22 | l-ephedrine | [10] | |
| 23 | Cavidine | [7] | |
| 24 | 9-((5-methoxypyridin-2-yl) methyl)-9 H-purin-6-amine | [11] | |
| 25 | 4-(2-(2,5-dioxopyrrolidin-1-yl) ethyl) phenyl acetate | [11] | |
| 26 | N-(9-((5-methoxypyridin-2-yl) methyl)-9 H-purin-6-yl) acetamide | [11] | |
| 27 | Guanine | [12] | |
| 28 | Nicotine | [12] | |
| 29 | Uracil | [12] | |
| 30 | Nicotinamide | [12] | |
| 31 | tryptophan | [12] | |
| Volatile oil | |||
| 32 | n-Butyl vinyl ether | [13] | |
| 33 | Methyl alpha-chloroacrylate | [13] | |
| 34 | Ethyl palmitate | [13] | |
| 35 | Benzaldehyde | [13] | |
| 36 | 2-Methylpiperazine | [13] | |
| 37 | 2-Undecanone | [13] | |
| 38 | Cis-Anethol | [13] | |
| 39 | Citral | [13] | |
| 40 | Octene | [13] | |
| 41 | Beta-elemene | [13] | |
| 42 | Valeraldehyde oxime | [13] | |
| 43 | Beta-Eudesmol | [13] | |
| 44 | 2-Amylfuran | [13] | |
| 45 | 3,4-Dihydro-2-Methoxy-2H-Pyran | [13] | |
| 46 | Nonan-3-one | [13] | |
| 47 | Dibutyl phthalate | [13] | |
| 48 | Butylbenzene | [13] | |
| 49 | 2,4-dimethylfuran | [13] | |
| 50 | Dodecane | [13] | |
| 51 | Cis-4-decenal | [13] | |
| 52 | Vinylcyclohexane | [13] | |
| 53 | Anisic acid | [13] | |
| 54 | Furfural | [13] | |
| 55 | Octadecane | [13] | |
| 56 | (+)-Aromadendrene | [13] | |
| 57 | Cyclohexanol,5-methyl-2-(1-methylethenyl)- | [13] | |
| 58 | Butylated Hydroxytoluene | [13] | |
| 59 | Cyclohexane | [13] | |
| 60 | Bisabolene | [13] | |
| 61 | 2,6,10-Trimethyldodecane | [13] | |
| 62 | Dipentene | [13] | |
| 63 | Citronellal | [13] | |
| 64 | 2-Methylnonane | [13] | |
| 65 | Allyl 2-ethylbutyrate | [13] | |
| 66 | 6-Methyl-2-heptanone | [13] | |
| 67 | 3-Nonyne | [13] | |
| 68 | 2,5-Dimethyltetradecane | [14] | |
| 69 | Pulegone | [14] | |
| 70 | Octahydro-4α-5-dimethyl-3-isopropyl-naphthalene | [14] | |
| 71 | 1,5-Pentadiol | [14] | |
| 72 | 3-Methyleicosane | [14] | |
| 73 | Heptadecanoic acid | [17] | |
| 74 | Terpineol | [20] | |
| 75 | Octadeca-9,12-dienoicacidethylester | [21] | |
| 76 | (E)-β-farnesene | [21] | |
| 77 | 5-methyl-1,3-dimethoxybenzene | [25] | |
| 78 | 3,4,5-trimethoxytoluene | [25] | |
| 79 | 2-pentyl furan | [25] | |
| 80 | 2-acethyl-3-methyl-pyrazine | [25] | |
| 81 | trimethyl pyrazine | [25] | |
| 82 | 2,3,5,6-tetramethylpyrazine | [25] | |
| 83 | nopinone | [25] | |
| 84 | borneol | [25] | |
| 85 | 7-isopropylidenenorcarane | [25] | |
| 86 | cyclosativene | [25] | |
| 87 | α-ylangene | [25] | |
| 88 | β-cubebene | [25] | |
| 89 | α-gurjunene | [25] | |
| 90 | β-caryophyllene | [25] | |
| 91 | β-cedrene | [25] | |
| 92 | α-humulene | [25] | |
| 93 | β-selinene | [25] | |
| 94 | α-neocallitropsene | [25] | |
| 95 | 9H-fluorene | [25] | |
| 96 | β-caryophyllene oxide | [25] | |
| Amino acids | |||
| 97 | dl-Threonine | [15] | |
| 98 | Serinic acid | [15] | |
| 99 | l-glutamic acid | [15] | |
| 100 | Glycine | [15] | |
| 101 | Alanine | [15] | |
| 102 | Cystine | [15] | |
| 103 | Valine | [15] | |
| 104 | l-Methionine | [15] | |
| 105 | D-tert-Leucine | [15] | |
| 106 | Boc-D-Tyr-OH | [15] | |
| 107 | l-Lysine | [15] | |
| 108 | l-Histidine | [15] | |
| 109 | Argininic acid | [15] | |
| 110 | Proline | [15] | |
| 111 | l-isoleucine | [15] | |
| 112 | Phenylalanine | [15] | |
| 113 | Magnesium 2-aminosuccinate hydrate | [15] | |
| 114 | N-[(tert-Butoxy) carbonyl]-d-tryptophan | [15] | |
| 115 | DL-3-aminobutyric acid | [15] | |
| 116 | N-dodecanoyl-l- serine | [16] | |
| 117 | 3-Amino-2-naphthoic acid | [16] | |
| 118 | N- ([dodecyloxy] carbonyl] valine | [16] | |
| 119 | Glutathione | [16] | |
| 120 | p-hydroxycinnamic acid | [25] | |
| Organic acids | |||
| 121 | Nonanoic acid, 9-oxo- | [17] | |
| 122 | Pentadecanoic acid | [17] | |
| 123 | 7-Hexadecenoic acid | [17] | |
| 124 | Hexadecanoic acid | [17] | |
| 125 | 9-Hexadecenoic acid | [17] | |
| 126 | Hexadecenoic acid | [17] | |
| 127 | 8-Octadecenoic acid | [17] | |
| 128 | Oleic acid | [17] | |
| 129 | Octadecanoic acid | [17] | |
| 130 | Linoleic acid | [17] | |
| 131 | 11-Eicosenoic acid | [17] | |
| 132 | Eicosanoic acid | [17] | |
| 133 | 10,13-Eicosadienoic acid | [17] | |
| 134 | Docosanoic acid | [17] | |
| 135 | Heptacosane | [17] | |
| 136 | Ethyl stearate | [17] | |
| 137 | Pentacosane | [17] | |
| 138 | Succinic acid | [18] | |
| 139 | 9(S),12(S),13(S)-Trihydroxy-10(E)-Octadecenoic acid | [19] | |
| 140 | N-Acetyl-l-glutamic acid | [18] | |
| 141 | 3-Amino-2-naphthoic acid | [12] | |
| 142 | Gondoic acid | [12] | |
| 143 | Dicarboxylic Acid | [12] | |
| 144 | E-P-coumarol | [20] | |
| 145 | Homogentisic acid | [21] | |
| 146 | Caffeic acid | [21] | |
| 147 | Vanillic acid | [21] | |
| 148 | 3-Phenylpropionic acid | [21] | |
| 149 | 12-Octadecadienoic acid | [22] | |
| 150 | Palmitic acid | [22] | |
| 151 | Protocatechualdehyde | [21] | |
| 152 | 6-Shogaol | [21] | |
| 153 | Ferulic acid | [21] | |
| 154 | Chrysophanic acid | [21] | |
| 155 | Oleyl glycine | [16] | |
| 156 | 6-gingerol | [25] | |
| 157 | α-tripalmitin | [25] | |
| Flavonoids | |||
| 158 | Daidzein | [12] | |
| 159 | (3R)-2,3-Dihydro-5,7-dihydroxy-3-[(4-methoxyphenyl) methyl]-6,8-dimethyl-4H-1-benzopyran-4-one | [12] | |
| 160 | Apiin | [12] | |
| 161 | 7- Methyl apigenin | [12] | |
| 162 | 6-Aldehydo-isoophipogonone B | [23] | |
| 163 | Baicalin | [24] | |
| 164 | Baicalein | [23] | |
| 165 | 6C-β-d-Xylopyraose-8C-β-d-galactopyranosyl-5,7,4′-three hydroxyl flavone | [23] | |
| 166 | 6C-β-d-Galactopyranosyl-8C-β-d-xylopyraose-5,7,4′-three hydroxyl flavone | [23] | |
| 167 | 6C-β-Galactose-8C-β-arabinose-5,7,4′-three hydroxyl flavone | [23] | |
| 168 | 6C-β-Arabinose-8C-β-galactose-5,7,4′-three hydroxyl Flavone | [23] | |
| Cerebrosides | |||
| 169 | Soyacerebroside I | [24] | |
| 170 | Soyacerebroside II | [24] | |
| 171 | 1-O-glucosyl-N-2′-acetoxypalmitoyl-4,8-sphingodienine | [23] | |
| 172 | 1-O-glucosyl-N-2′-hydroxypalmitoyl-4,8-sphingodienine | [23] | |
| 173 | 1-O-glucosyl-N-2′-acetoxystearoyl-4,8-sphingodienine | [23] | |
| 174 | 1-O-glucosyl-N-2′-hydroxystearoyl-4,8-sphingodienine | [23] | |
| 175 | 1-O-glucosyl-N-2′-palmitoyl-4,8-sphingodienine | [23] | |
| 176 | 1-O-glucosyl-N-2′-hydroxyeicosanoyl-4,8-sphingodienine | [23] | |
| 177 | Pinelloside | [23] | |
| Phenylpropanoids | |||
| 178 | (E)-Coniferin | [20] | |
| 179 | Sachaliside 1 | [20] | |
| 180 | 3,4-Dihydroxycinnamyl alcohol | [20] | |
| 181 | (+)-Isolariciresinol | [20] | |
| 182 | Tiliamuroside A | [20] | |
| 183 | Wheat sterol | [20] | |
| 184 | Lariciresinol 4-O-β-d-glucopyranoside | [20] | |
| 185 | Terpineol -4-O-β-D- glucoside | [20] | |
| 186 | Erythro-guaiacylglycerol-β-O-4′-sinapyl ether | [20] | |
| 187 | Americanol A | [20] | |
| 188 | Dehydrodocosanol-9-O-β-d-glucopyranoside | [20] | |
| 189 | Dehydrodocosanol-9′-O-β-d-glucopyranoside | [20] | |
| 190 | Dehydrodocosanol-4,9-di-O-β-D-glucoside | [20] | |
| 191 | Neoolivil | [20] | |
| 192 | cyclolignanyingoside A | [25] | |
| 193 | Neo-olivil | [25] | |
| 194 | Cinnacassoside A | [25] | |
| Others | |||
| 195 | Isochrysin 9-O-β-d- glucopyranoside | [20] | |
| 196 | Dehydrodocosanol 4-O-β-d- glucopyranoside | [20] | |
| 197 | erythritol | [25] | |
| 198 | gingerol | [25] | |
| 199 | ammonium glycyrrhizinate | [25] | |
| 200 | Cinnamon A | [20] | |
| 201 | Verti-cillatoside A | [20] | |
| 202 | Daucosterol | [25] | |
| 203 | Campesterol | [25] | |
| 204 | Stigmasta-5,22-dien-3-ol | [25] | |
| 205 | Stigmasta-5,24-dien-3-ol | [25] | |
| 206 | Cycloartanol | [25] | |
| 207 | 5-Hydroxymethylfurfural | [21] | |
| 208 | Beta-sitosterol | [21] | |
| 209 | Eleutheroside A | [22] | |
| 210 | 1,3,12-Nonadecatriene | [22] | |
| 211 | α-monpalmitin | [22] | |
| 212 | Pyrrolidine,1-(1-oxo-7,10-hexadecadienyl) | [22] | |
Lectin is a type of protein or glycoprotein that can agglutinate red blood cells (RBCs). There have been many studies on it, too. Sun et al. [26] isolated and purified for the first time a protein with coagulant activity on rabbit erythrocytes from Pinellia Pedatisecta Agglutinin (PPA), which was confirmed by chemical analysis. PPA was first isolated and purified from PR, and its amino terminus was alanine, and the sugar chain part contained mannose, fucose and ethylphthalazinylglucosamine, as confirmed by chemical analysis. Liu et al. [27] isolated and purified lectin from PR, and analysis showed that it contained homotetramers of similar molecular weight and pI 5.8. Wang et al. [28] human screened seven signature enzyme peptides from toxic proteins by a comparative proteogenomic strategy of open-source transcriptomic data on PR and its Typhonii Rhizoma (TR), Arisaematis Rhizoma (AR) and tubers of Typhonium flagelliforme (TF) and Pinellia pedatisecta (PP). Counterfeit species were well identified. Kuarat.K et al. [29] isolated a glycoprotein named 6 KP, one of the major proteins in Pinellia ternata bulbs. Fan et al. [30] obtained a nodular product containing Pinellia ternata proteins consisting of various amino acids such as cystine, arginine, aspartic acid and alanine from Pinellia ternata tubers.
Polysaccharide is a macromolecular saccharide composed of multiple monosaccharide molecules connected by glycosidic bonds. Plant polysaccharides are widely distributed in nature and are one of the basic substances to maintain the normal operation of life. With the extraction and separation of various effective components in PR. Pinellia ternata polysaccharide has been gradually recognized by researchers. PT-F2-I is a polysaccharide with a molecular weight of about 850,000 isolated from the aqueous extract of PR, consisting of ribose, rhamnose, galactose, glucose, fucose, and arabinose in a molar ratio of 0.05: 0.05: 0.09: 0.10: 0.12: 1.00 [31]. Heteropolysaccharides with a molecular weight of about 10,000 were also extracted from PR, while the acidic polysaccharides obtained consisted of d-galacturonic acid, d-glucuronic acid, l-arabinose, d-galactose, and l-rhamnose (3:3:5:15:1), which had a molecular weight of 118,104, and the main chain consisted of -(1 → 3)-linked d-galactose units, and the side chain consisted of -(1 → 6)-linked d-galactose, while some of the sugar units were connected to the acetyl group and peptide fragments. From Pinellia ternata tubers, -(1 → 4) glucan was also isolated, consisting of D-glucans with some sugar units attached to acetyl groups, containing branched chains, with -(1 → 4)-linked D-glucans in the main chain, and partially -(1 → 3)- and -(4 → 6)-linked, which are straight-chained amylopectin with molecular weight of 4100 [32].
6. Extraction method
At present, the research on extraction methods mainly focuses on flavonoids, alkaloids, polysaccharides, and volatile oils in PR. Most of them use the response surface method to optimize the extraction process, but the extraction time varies considerably. When extracting one of the effective parts, the extraction rate of the other effective parts is not fully considered. The extracted components are relatively simple, but the optimization process of phenylpropanoids, organic acids, and other components is more complex. In the future, more consideration should be given to the comprehensive extraction of various components and the optimization of the PR extraction process. A summary of how the PR methods are extracted is provided in Table 3 [[33], [34], [35], [36], [37], [38], [39], [40], [41], [42]].
Table 3.
Pinellia ternata (Thunb.) Breit. extraction methods.
|
Active ingredients |
Extraction method | Extraction reagent | Extraction temperature (°C) | Extraction time (h) |
Material to liquid ratio (mL/g) Material-to-liquid ratio (mL/g) |
Number of extractions | Extraction rate (%) extraction rate | References |
|---|---|---|---|---|---|---|---|---|
| Polysaccharide | Two-phase extraction method | PEG 6000, (NH4)2SO4 | \ | 44 min | 1:1.77 | \ | 44.76 % | [33] |
| Alkaloids | Chloroform extraction | Chloroform, 12 % ammonia water | 60 °C | 25 h | 18.00 | 6 | 4.56 % | [34] |
| Alkaloids | Ultrasonic Assisted Extraction | Chloroform, 12 % ammonia water | 80 °C | 4 h | 18.00 | 6 | 4.51 % | [34] |
| Total flavonoids | ethanol extraction | 62.54 % ethanol | 62.72 °C | 2.61 h | 1:47.10 | \ | 8.91 % | [35] |
| Polysaccharide | Water extraction and alcohol precipitation | 70 % ethanol | 70 °C | 1 h | 1:30 | 3 | 13.68 % | [36] |
| Guanosine | Ultrasonic extraction | water | \ | 2.25 h | 1:10 | 3 | 16.4 % | [37] |
| Polysaccharide | Compound Enzyme Extraction | complex enzyme | 54 °C | 57 min | 1:27 | \ | 27.98 % | [38] |
| Polysaccharide | Cellulase method | 95 % ethanol | 55 °C | 35 h | The amount of enzyme added is 4 % | \ | 71.75 % | [39] |
| Total free organic acids | heating reflow method | 75 % ethanol | \ | 1 h | 1:10 | 2 | 0.19 % | [40] |
| Polysaccharide | Ultrasonic extraction | water | \ | \ | 1:12 | 3 | 33.3 % | [41] |
| Total flavonoids | Ultrasonic extraction | 50 % ethanol | \ | \ | 1:30 | \ | 1.84 % | [42] |
7. Pharmacological effects
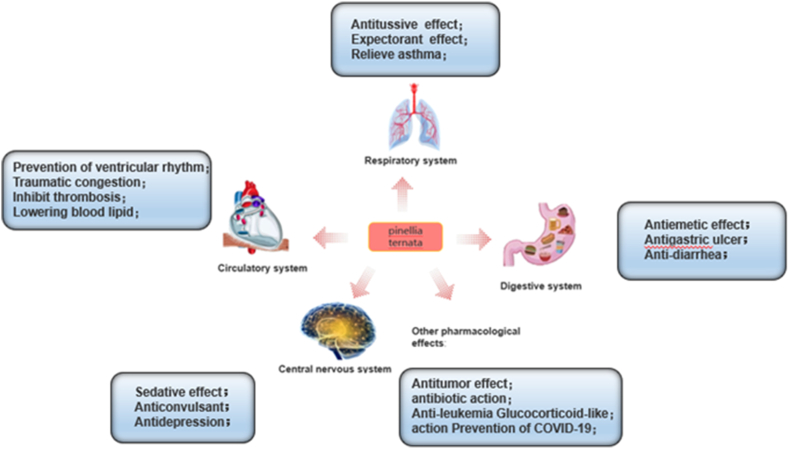
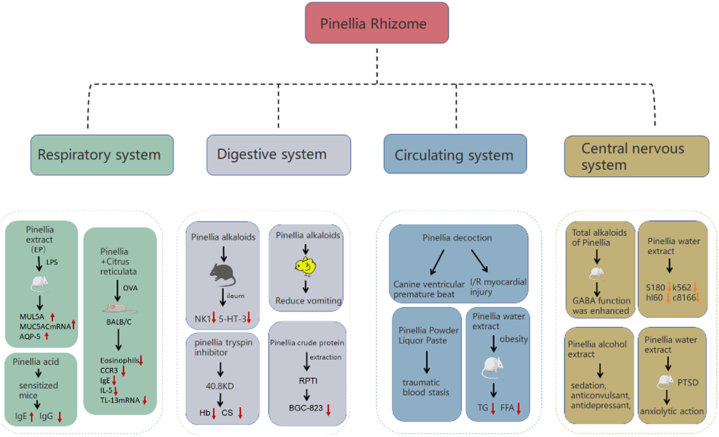
Extracts and certain constituents of PR have progressively exhibited diverse pharmacological properties, among which, antitumor, anticonvulsant, analgesic, and sedative properties are associated with traditional uses. Additionally, several new pharmacological effects have been discovered, including hypolipidemic, anti-gastric ulcer, antidepressant, and other functions. These pharmacological findings are discussed in the following sections and summarized in (Fig. 11, Fig. 12) and Table 4.
Fig. 11.
Pharmacological action diagram of Pinellia ternata (Thunb.) Breit.
Fig. 12.
Pharmacological effect of Pinellia ternata (Thunb.) Breit. Note: Mucin 5AC messenger RNA (MUC5AC mRNA),Aquaporin-5 (AQP-5), Ovalbumin (OVA), Serum CC chemokine receptor 3 (CCR3), Immunoglobulin E (IgE),Immunoglobulin G (IgG), Interleukin-5 (IL-5), Interleukin-13 (IL-13), Thyroglobulin (TG), Post traumatic stress disorder (PTSD), 5-HT receptor 3 (5-HT-3), natural killer cell 1(NK1), a trypsin inhibitor (RPTI).
Table 4.
Pharmacological research on Pinellia ternata (Thunb.) Breit.
| system | effect | Element | dose | inside/outside | references |
| Respiratory system | Antitussive | Total organic acids | 0.41, 0.82 g/kg | In Vivo | [43] |
| Total alkaloids | 1 mL/0.15 g | In Vivo | [44] | ||
| Axpectorant | Pinellia decoction | 1.5, 3 g/kg | In Vivo | [45] | |
| Decoction and alcohol precipitation extract of Pinellia sinensis | 10, 30, 60 g/kg | In Vivo | [46] | ||
| Inhibition of ERK activation protects the airway | Pinellia decoction | 0.3 g/100 g | In Vivo | [47] | |
| Protects against allergic airway inflammation | Pinellia Water Extract | 13.5, 23.3 mg/kg | In Vivo | [48] | |
| Asthma | Pinellia acid | 50 mg/kg | In Vivo | [49] | |
| Digestive system | Anti-vomit | Pinellia alkaloids | 30 mg/kg | In Vivo | [50] |
| Pinellia suspension | 50, 100 g/kg | In Vivo | [51] | ||
| 6 kD globulin | 10, 20, 50 mg/kg | In Vivo | [52] | ||
| Fights vomiting caused by apomorphine | Pinellia polysaccharide PT-F2-1 | In Vivo | [53] | ||
| Can combat vomiting caused by copper sulfate | Methionine | In Vivo | [54] | ||
| Can combat vomiting caused by cedilan-D | Glycine and d-glucuronic acid | In Vivo | [54] | ||
| Anti-stomach ulcer | 5 % ethanol extract of Pinellia chinensis | 5, 15 g/kg | In Vivo | [55] | |
| Pinellia decoction and alcohol precipitation | 10, 20 g/kg | In Vivo | [56] | ||
| Stimulate gastric emptying | Pinellia decoction | 1, 2.5 g/kg | In Vivo | [57] | |
| Pinellia micron powder decoction | 0.75, 1.5, 3 g/kg | In Vivo | [58] | ||
| Gastrointestinal propulsion | Ginger Pinellia Powder Suspension | 2.5 g/kg | In Vivo | [59] | |
| Circulatory system | Prevention of ventricular arrhythmias | Qingpinxia decoction | 53 g/kg | In Vivo | [60] |
| traumatic blood stasis | Pinellia powder white wine paste | 0.15, 0.25 g/mL | In Vitro | [61] | |
| Inhibit thrombosis | 95 % Ethanol Extract | 100 mg/kg | In Vivo | [62] | |
| blood lipid lowering | Pinellia hot water extract | 400 mg/kg | In Vivo | [63] | |
| Central nervous system | Calm down | 75 % ethanol extract | 8, 12 g/kg | In Vivo | [64] |
| anticonvulsant | Pinellia supercritical CO2 ethanol extract | In Vivo | [65] | ||
| Can improve GABAergic nerve function | Pinellia total alkaloids | 0.5, 1.0 g/kg | In Vivo | [66] | |
| antidepressant | Pinellia alcohol extract | 100, 300, 500 mg/kg | In Vivo | [67] | |
| Anxiety | Pinellia no-fried granules suspension | 3.15 g/kg | In Vivo | [68] | |
| Others | Improve Parkinson's disease | Pinellia total alkaloids | 1, 0.5, 0.25 mg/kg | In Vivo | [69] |
| Inhibit tumor growth in the body | Pinellia Water Extract | 100 mg/kg | In Vivo | [70] |
7.1. Respiratory pharmacological effects
7.1.1. Cough suppressant effects
Xiong et al. [71] evaluated the antitussive effect of PR on a mouse cough model induced by ammonia. The results showed that the number of coughs in mice was decreased, and the white blood cell (WBC), lymphocyte (LYM), and monocyte (MON) indices of blood cells was significantly increased. Later research also proved that PR has an antitussive effect through similar experiments also found that PR had an antitussive effect that persisted for more than 5 h by injecting iodine into the chest cavity or electrically stimulating the superior laryngeal nerve of cats [[51], [72], [73]]. Bai et al. [74] found that the antitussive effect of wild PR was obviously better than that of cultivated PR.
Yang [43] studied the antitussive effect of the chemical components of PR and its processed products. Based on the mouse cough model induced by concentrated ammonia water, the antitussive titer of PR and its processed products was determined using the dose-response parallel method, and its efficacy value was determined. Combined with the quantitative determination results of total organic acids, the correlation between total organic acids and biological titer was analyzed. The results showed that the antitussive potency of PR and its processed products was related to the amount of total organic acids. Zhang et al. [18] extracted and separated total free organic acids with 95 % ethanol, and found that total organic acids have an antitussive effect. Zeng et al. [44] used grey correlation analysis to show that the total alkaloids were active constituents that play an antitussive role.
7.1.2. Expectorant effect
Chen et al. [45] evaluated the expected effect of PR by the phenol red secretion in the respiratory tract of mice model. The results showed that the secretion of phenol red in the mouse trachea was increased by PR water extract gavage for seven days. Several studies have also proved that PR has an expectorant effect using similar experiments [44,75]. Deng et al. [46] studied the intervention effect of PR extract on a rat airway mucus hypersecretion model induced by lipopolysaccharide (LPS). One hour before LPS injection every day, rats were given PR extract for four consecutive days. The results showed that the expression of MUC5AC protein in the airway epithelium and MUC5AC mRNA in lung tissue were significantly lower than those in the model group, while AQP-5 in the airway epithelium was significantly higher than that in the model group. Moreover, Du et al. [47] treated COPD rats with budesonide inhalation for two weeks, and then orally administered PR at a dosage of 3 g/kg for four weeks. The results showed that PR could protect the airway from ICS withdrawal-induced mucus hypersecretion and airway inflammation by inhibiting extracellular signal regulated kinase (ERK) activation.
7.1.3. Asthma calming effect
Ok et al. [76] evaluated the asthma calming effect of PR in an ovalbumin (OVA) induced asthma mice model. A combination of PR and citrus reticulata was used for oral administration. The results showed that eosinophil infiltration, chemokine receptor-3, histamine, and OVA-specific IgE production in serum were reduced, while IL-5 and IL-13 mRNA in lung tissue were inhibited. Lee et al. [48] administered PR water extract at 13.5 mg/kg and 23.3 mg/kg for 25 days and found that PR water extract had a protective effect on allergic airway inflammation. Shan et al. [75] gavaged guinea pigs at 0.15, 0.3, and 0.6 g/kg of PR water extract, and found that the water extract of PR had a certain preventive effect on asthma. Subsequently, Nagai et al. [49] found that Pinellia acid was the active ingredient of PR against allergic airway inflammatory asthma, and gavage of Pinellia acid 50 mg/kg to mice decreased the titer of ovalbumin specific immunoglobulin E (IgE) antibody in the bronchoalveolar lavage fluid of sensitized mice. Moreover, the titer of antivirus immunoglobulin G (IgG) antibody in serum and bronchoalveolar lavage fluid increased by three times.
7.2. Digestive system pharmacological effects
7.2.1. Antivomiting effect
Tang et al. [51] found the antivomiting effect of Hemerocallis by gavage with 50, 100 g/kg of PR suspension through CuSO4-induced vomiting of domestic pigeons. Zhao et al. [77] similarly demonstrated the antivomiting effect of PR through a mink locomotor vomiting model. Zhang et al. [50] studied the underlying mechanism of PR in preventing and treating chemotherapy-induced nausea and vomiting by observing the effects of alkaloids in PR on the contraction of isolated guinea pig ileum under the intervention of 5-hydroxytryptophan (5-HT), 2-Methyl-5-HT, substance P, and the selective NK1 receptor agonist GR73632 and measured the peak of contraction force of three-dimensional intestinal tube of guinea pig with an isolated thermostatic bath. The results showed that PR alkaloids had an obvious inhibitory effect on the contraction tension of the guinea pig ileum. The results also showed a certain dose dependence, indicating that PR alkaloids had a certain blocking effect on 5-HT receptor 3 (5-HT3) and natural killer cell 1 (NK1) receptors in the ileum, that is, PR alkaloids were the main effective part of PR for stopping vomiting. Similarly, it was later demonstrated that PR alkaloids are one of the active components of PR antiemetic [[45], [46], [47], [48], [49], [50], [75], [76], [77], [78], [79], [80]].
Kurata et al. [52] isolated a water-soluble globulin with a molecular weight of 6 kD from PR. Moreover, when its content was between 5.75 % and 8.30 %, it had an antiemetic effect. Furthermore, by taking the efferent activity of the gastric branch of the vagus nerve in rats as an index. Ao [81] found that the water-soluble and fat-soluble components of PR have antiemetic effects. Maki et al. [53] found that filling the stomach of chickens with PR polysaccharide PT-F2-1 can prevent vomiting caused by apomorphine. Additionally, Ho et al. [54] found that methionine contained in PR can resist vomiting caused by copper sulfate, while glycine and d-glucuronic acid contained in PR can resist vomiting caused by Cedilanid-D.
7.2.2. Anti-gastric ulcer and improvement in gastric function
Shen et al. [55] found that PR had a strong anti-gastric ulcer effect in a gastric ulcer mouse model following oral administration of 5 g/kg and 15 g/kg of crude drugs from PR with 75 % ethanol. Subsequently, Liu et al. [56] and others used two doses (10, 20 g/kg) of PR decoction and alcohol precipitation solution for intragastric administration, which also proved the anti-gastric ulcer effect of PR. Wu et al. [59] gavaged mice with 2.5 g/kg suspension of raw PR and Jiang-Banxia powder for three consecutive days and found that raw PR had no significant impact in gastric emptying, while Jiang-Banxia inhibited it. In previous studies [57,82], mice were given PR decoction (1, 2.5 g/kg) by intragastric administration, which could promote gastric emptying. Li et al. [58] found that gastric emptying could be dose-dependently promoted by intragastric administration of 0.75, 1.5, and 3 g/kg of Pinellia micro-rice flour decoction to mice.
7.2.3. Antidiarrheal and gastrointestinal propulsive motility modulating effects
Zhang et al. [83] gavaged mice with two doses (5, 15 g/kg) of 75 % ethanol extract of PR and found that the number of instances of diarrhea caused by castor oil and senna leaves were reduced within 4 h, while the inhibition time could last for more than 8 h, thereby showing antidiarrhea effects. Wu et al. [59] found that Jiang-Banxia powder suspension (2.5 g/kg) could inhibit the gastrointestinal propulsion of mice by gavage for three days. Oshio et al. [14] found that both methanol and water extracts of PR showed relaxing and antihistamine-like effects on isolated quail rectum. Liu et al. [60] gavaged rabbits with 60 g/kg of Qing-Banxia decoction for four days and found that there was no obvious correlation between the effect of PR on gastrointestinal propulsion and its antidiarrhea effect.
7.2.4. Other pharmacological effects of the digestive system
Wang et al. [84] isolated a trypsin inhibitor (RPTI) with a molecular weight of approximately 14 kD from an extract of crude PR protein. The results showed that the mass inhibition ratio of RPTI to trypsin was approximately 1:4.72, and it could inhibit the cell proliferation of the human poorly differentiated gastric adenocarcinoma cell line BGC-823, with an IC50 of 121.53 μg/mL; thus, RPTI is a type of antinausea drug. Wu et al. [85] found a PR trypsin inhibitor with a molecular weight of 40.8 kD, which could inhibit the hydrolysis of amide, ester, hemoglobin and casein by trypsin. Li et al. [86] found that PR decoction (10 %) had no significant effect on the dissolution of gallstones in test tubes when they studied the dissolution of gallstones from 43 kinds of TCM in vitro.
7.3. Role of the circulatory system
7.3.1. Cardiovascular system
Liu et al. [60] proved that Qing-Banxia water extract (53 g/kg) administered to rats prevents ventricular arrhythmia. Teng et al. [87] intravenously injected mixed dogs with PR aqueous solution (0.2, 0.3 g/kg) and found that it had an obvious curative effect on canine ventricular premature beats caused by barium chloride. Moreover, Li et al. [88] adopted the model of myocardial ischemia-reperfusion in rats, and orally administered 2.5 g/kg of Jiang-Banxia water extract to rats for 14 days. The results showed that PR had a certain protective effect on I/R myocardial injury. Huang et al. [89] found that PR water extract could stimulate the secretion of vascular repair factors and reendothelialization of blood vessels, and inhibit the proliferation of neointima, thus playing a role in the vascular repair of carotid artery injury in rats. Zhou et al. [90] found that PR could inhibit the secretion of inflammatory factors in mouse aortic endothelial cells. PR was injected intravenously into rats, dogs, rabbits and cats, which had a short-term antihypertensive effect and was quickly tolerated; however, intramuscular injection had no significant effect on blood pressure and respiration. PR also has a certain cardiac inhibitory effect, inhibiting isolated frog and rabbit hearts and increasing the coronary flow and amplitude of the cardiac contraction curve of isolated rabbit hearts Liu et al. [91] Hong et al. [92] gave PR (1.2 mL/day/200 g) to normal rats, and the experiment proved that PR could prevent or delay the formation of food-induced hyperlipidemia, and had a certain therapeutic effect on hyperlipidemia. Kim et al. [63] fed obese Zucker rats with hot water extract of PR 400 mg/kg every day for six weeks and found that it could reduce the levels of triglyceride and free fatty acids in the blood of such obese rats, and slightly reduce their body weight.
7.3.2. Hematological systems
Jiang et al. [93] gavaged rats with PR decoction 5 g/kg for seven days and found that it could increase the whole blood viscosity at high shear rate and decrease the erythrocyte aggregation index and erythrocyte deformation index. Lu et al. [61] used a quantitative heavy object to hit the soft tissue of rats’ right hind limbs, and wet applied PR powder and Chinese liquor paste of 0.15 and 0.25 g/mL (dosage of 0.5 mL/cm2) every day for 6 h and 5 d and found that PR for external use had a good curative effect on traumatic blood stasis. Shi et al. [62] adopted the mouse model of Agkistrodon acutus poisoning, and orally administered PR powder suspension 1 g/kg or PR 95 % ethanol extract 100 mg/kg every day for seven days. The research found that PR not only inhibited thrombosis but also prevented the exhaustion of coagulation factors. Zhang et al. [94] used 75 % ethanol extract of PR (3 g/kg) and 10 g/kg to gavage rats continuously for three days, the results of which showed a significant increase in the time required for electrical stimulation of the common carotid artery to thrombosis, as well as a slightly prolonged coagulation time.
7.4. Central nervous system
7.4.1. Sedation
Liu et al. [60] injected PR water extracts of 30 and 60 g/kg into the abdominal cavity of mice and found that it could significantly reduce the number of independent activities of mice and slightly prolong the sleep time of mice. Zhan et al. [95,64] gavaged mice with 75 % ethanol extract of PR (8, 12 g/kg), and found that it can significantly increase the number of mice sleeping with a subthreshold dose of pentobarbital sodium, and 12 g/kg can also prolong the sleeping time of mice with a subthreshold dose of pentobarbital sodium. You et al. [96] gavaged mice with raw PR decoction of 1.2, 3.6 g/kg for seven days. The results showed that both of them could increase the sleeping rate caused by subthreshold dose of pentobarbital sodium, although they did not cause mice to fall asleep directly. Fang et al. [97] found that raw PR had a good sedative effect in mice following gavage for seven days with 4, 8, and 16 g/kg. Zhou et al. [98] found that fresh PR decoction had an inhibitory effect on the central nervous system by gavage of 10 g/kg for 0.5 and 1.0 h.
7.4.2. Anticonvulsant
Yang et al. [65] found that the supercritical CO2 ethanol extract of PR could resist the convulsion induced by maximal electroconvulsive and pentylenetetrazole and reduce the mortality of convulsed mice. Cheng et al. [99] found that the total alkaloid of PR is the anticonvulsant active part. Gu et al. [66] gavaged mice with 0.5, 1.0 g/kg of total alkaloids of PR for 13 days, and found that total alkaloids of PR could improve GABA nerve function. Deng et al. [100] also confirmed that the total alkaloids of PR have a certain therapeutic effect on epilepsy.
7.4.3. Antidepressant
Zhang et al. [67] gavaged mice with 100, 300, and 500 mg/kg of PR alcohol extract, and the results showed that it could shorten the immobility time during forced swimming and tail suspension; thus, PR had an antidepressant effect. Fang et al. [68] used single-prolonged stress method to establish a posttraumatic stress disorder rat model. PR powder mixture (3.15 g/kg) was administered orally for seven days, and the behavioral tests of open field test and forced swimming test were conducted. The results showed that PR had an antianxiety effect, and its effect was similar to that of 1 mg/kg alprazolam.
7.5. Anti-tumor effects
PR can be used to treat various cancers, such as cervical cancer, ovarian cancer, breast cancer, leukemia, liver cancer, chol-angiocarcinoma, and gastric cancer [22]. Chen et al. [70] found that the concentration of PR water extract was 100 mg/kg in the ascites of S180 sarcoma tumor-bearing mice, which suggests that PR could inhibit tumor growth in vivo. Total protein extracts of PR have a significant inhibitory effect on ovarian cancer cell line SKOV3 by inducing cell apoptosis in a dose- and time-dependent manner. Another recombinant PR agglutinin exhibits anti-proliferative activity on hepatoma cells in a dose dependent manner [101]. Wu et al. [102] found that PR extract could induce apoptosis in tumor cell K562, prolong the G0/G1 phase of K562 cells, and interfere with DNA synthesis, confirming the antitumor properties of PR. Xiong et al. [103] using network pharmacology and molecular docking techniques, found that PR has the prevention and treatment of lung cancer as a potential pharmacological effect. Trypsin inhibitor isolated from PR significantly inhibited the proliferation of human gastric cancer cell line BGC-823 in a concentration- and dose-dependent manner [104]. Xia Lei et al. [105] found that the mechanism by which PR and ginger synergize against SKOV3 in ovarian cancer may be related to the induction of ROS generation, iron death, and inhibition of the P13K/AKT/mTOR signaling pathway. PR fat-soluble extracts combined with cis-dichlorodiammineplatinum-II (CDDP) acted on human cervical cancer cells (Si Ha line and Ca Ski line) to exert antitumor effects by arresting the Si Ha line and Ca Ski line at the cellular G0/G1 and G2/M phases, respectively [106]. Baicalein, the active component of PR, can inhibit tumor angiogenesis by down-regulating the expression of VEGF, fibroblast growth factor receptor-2 (FGFR-2) and up-regulating the expression of RB-1 (a tumor suppressor gene that regulates cell growth and differentiation) [107]. PR extract showed significant growth inhibition and pro-apoptotic effects on cervical cancer HeLa cells, as well as increased the sensitivity of HeLa cells to cisplatin [108]. Moreover, Zhao [109] and Li [110] confirmed that the PR polysaccharide is its antitumor active ingredient. Addition to proteins, various types of secondary metabolites are reported to be involved in various antitumor processes. A water-soluble polysaccharide (RAP-W1) has inhibitory effect on human breast cancer cell line MCF-7 [111].
7.6. Other pharmacological effects
Zhou et al. [69] gavaged MPTP rats with Parkinson's disease with total alkaloids of PR of 1, 0.5, and 0.25 mg/kg for eight weeks and found that total alkaloids of PR could improve the learning and memory function of Parkinson's disease rats. Chen et al. [112] Pinellia glycosides extracted from PR can also inhibit the growth of Candida albicans. Moreover, it has been speculated that 3,4-dihydroxyformaldehyde in PR may be an effective component with an antibacterial effect [113]. Liu [114] found that PR extract could inhibit the proliferation of myeloid leukemia K562 and HL60 and lymphocytic leukemia C8166 cells, and was the most stable at a concentration of 500 μg/mL. Feng et al. [115] found that the anti-leukemia mechanism of PR extract may be related to its regulation of Bax/Bcl-2 and Caspase-3 protein expression. It was later reported that PR had a glucocorticoid-like effect, which was speculated to be an underlying mechanism responsible for the anti-inflammatory effect of PR [116]. It was also reported that PR has certain pharmacological effects in treating COVID-19 [117]. The lysophoSphatidylcholine in PR is also known to cause demyelination, degeneration, and inflammatory reactions of neurons to varying degrees [118].
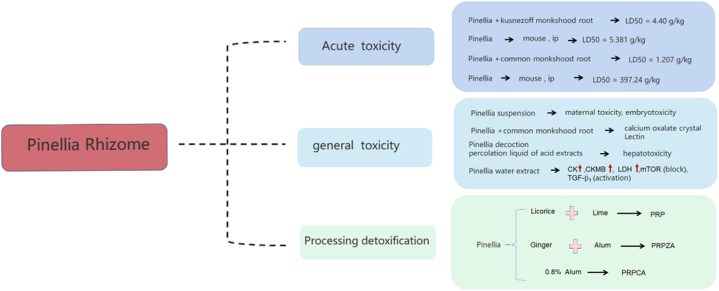
8. Toxicology
In the Chinese Pharmacopoeia (2020 edition), PR is listed as a toxic TCM, and its toxicity has been recorded in the medical books of successive dynasties. The manifestations and mechanisms of its toxicity have gradually been clarified through widespread research. Here, we comprehensively describe the research progress on the toxicology of Pinellia ternata (Fig. 13 and Table 5).
Fig. 13.
Toxicological effect of Pinellia ternata (Thunb.) Breit. Note:Pinelliae Rhizoma Praeparatum Cum Alumine (PRPCA), Pinelliae Rhizoma Praeparatum Cum Zingibere et Alumine (PRPZA), and Pinelliae Rhizoma Praeparatum (PRP). Creatine kinase (CK), Creatine kinase Isozyme (CKMB), Lactate dehydrogenase (LDH), Mammalian target of rapamycin (mTOR), Transforming growth factor-β1 (TGF-β).
Table 5.
Toxic effects of the Pinellia ternata (Thunb.) Breit.
| Toxic effects | Animal/cells | Extracts/compounds | Detail effects | References |
| Causing death | Mice | Born Pinellia (i.p.) | LD50 = 5.381 g/kg | [119] |
| Mice | Born Pinellia (i.g.) | LD50 = 397.24 g/kg | [120] | |
| Mice | Suspension of PR powder (i.g.) | LD50 = 3.359 g/kg | [121] | |
| Mice | Water extracts of PR (i.g.) | No obvious death | [121] | |
| Mice | Suspension of PR powder (i.g.) | LD50 = 0.553 g/kg | [122] | |
| Mice | Suspen sion of PRPZA powder (i.g.) | LD50 = 1.23 g/kg | [122] | |
| Mice | Suspension of PR powder (i.g.) | LD50 = 5.38 g/kg | [119] | |
| Mice | Needle-like calcium oxalate crystal and PR Lectin (12 kD) (i.g.) | LD50 = 15.94 mg/kg | [121] | |
| Inflammation causing effect | Macrophage | Different Concentrations of Poisonous Needle Crystals of Pinellia pedatisecta | Increasing the TGF-α, IL-1β | [123] |
| Mice | Suspension of PR powder (0.2 g/kg, i.g.) | Inducing writhing response and celiac inflammation. | [119] | |
| Mice | Suspension of PR powder (0.4 g/kg, i.g.) | Increasing capillary permeability, PGE 2 and histamine in abdominal cavity; | [121] | |
| Inducing celiac inflammation | ||||
| Conjunctival irritation | Rabbit | Needle-like calcium oxalate crystal (o.a.), 2 drops/rabbit | Tissue swelling and hyperemia | [121] |
| Inflammation causing effect | Rats | PR Lectin (12kD, 0.15, 0.3, 0.6, 1.2, 2.4 mg/kg, i.g.) | Increasing the contents of protein and PGE2 in peritoneal exudate; | [124] |
| Inducing celiac inflammation | ||||
| Mice | Needle-like calcium oxalate crystal and PR Lectin (12kD, i.g.) |
Stabbing into the mesenteric surface of mice and causing tissue swelling and inflammation | [121] | |
| Hepatotoxicity | Mice | pinellia water extraction (62.5 g/kg, i.p.) | Increasing the ALT, AST and liver tissue damage within 2 h | [125] |
| Mice | Pinellia acid water percolation liquid (1.77 g/kg, i.p.) | Increasing the ALT, AST and liver tissue damage within 2–4 h | [125] | |
| HepG2 | Water extracts of PR (10–20 μg/mL) | Inhibiting cell proliferation, and increasing ALT and AST | [126] | |
| Mice | PR powder (25 g/kg, for 10 days, i.g.) | Inducing edma of hepatic cells and steatosis | [127] | |
| Cardiotoxicity | Mice | Water extracts of PR (2.275 g/kg, crude herb mass equivalent, for 14 days, i.g.) | Inducing cardiomyocyte degeneration | [128] |
| Rats | PR powder (6 g/kg, for 14 days, i.g.) | Increasing the LDH, CK, CK-MB in serum | [129] |
| Cardiotoxicity | Rats | PR powder, PRPZA powder (3 g/kg, i.g., for 14 days) | Causing cardiotoxicity via inhibition of mTOR signaling; and activation of the TGF-β pathway, and processing reduced the toxicity via free radical scavenging | [130] |
| Nephrotoxicity | Mice | PR powder (2.275 g/kg/day, i.g.) | Inducing obvious pathological changes of kidney; Inducing obvious pathological changes of heart and kidney in pregnant mice after PR administration in gestation 6.5–15.5 days | [131] |
| Mice | PR powder (25 g/kg, i.g., for 10 days) | Inducing scattered focal lymphocytic infiltration in the renal parenchyma, and tubular formation in the renal tubules | [127] | |
| Mice | Water extracts of PR (2.275 g/kg, crude herb mass equivalent, i.g., for 14 days) | Inducing degeneration of renal tubular cells | [128] | |
| Inducing vomiting | Pigeon | PR powder (i.g.) | ED50 = 0.74 g/kg | [132] |
| Conjunctival irritation | Rabbit | Suspension of PR powder (27 %), Ophthalmic administration (o.a.), 2 drops/rabbit |
Tissue swelling and hyperemia | [121] |
| Rabbit | Raw Pinellia prepared into 30 % suspension with normal saline | 100 % positive rate | [78] | |
| Suspension of PR powder (20 %), o.a., 0.05 mL/rabbit | Serious conjunctival irritation | [128] | ||
| Effect of Pinellia extract on airway mucus hypersecretion | Rat | The extract of PR was given 10,30,60 g/kg by gavage for 4 consecutive days. | Increased MUC5AC content; Significant reduction of AQP-5 mRNA TNF-α | [46] |
| Reproductive-embryonic toxicity | Mice | Suspension of PR powder (36 g/kg) | Mortality 50 % | [133] |
| Mice | Suspension of PR powder (6 g/kg) | Mortality 30 % | [133] | |
| Mice | PR Lectin (4.4 kD, 30 mg/kg, s.c.) | Anti-early pregnancy rate was 100 %; decreasing plasma progesterone level; decreasing endometrial thickness and decidual reaction; inducing embryo arrest and abortion | [3] |
| Mutagenic effect | Mice | Raw Pinellia and PRPCA decoction 10 d (ip) | Equally increased the incidence of chromosomal abnormalities in cells | [134] |
| Pregnant mice | PR and PRPCA (1.434 g/kg) | Glycerophospholipid metabolism, amino acid and carbohydrate metabolism have different degrees of interference and produce toxic effects | [5] | |
| PRPCA (10 g/kg) | Can cause DNA damage, leading to maternal cell genetic material changes, resulting in teratogenic effects | [135] | ||
| Mice | PR decoction (10 g/kg) | embryotoxicity | [134] | |
| Mice | PRPCA (10 g/kg、20 g/kg、30 g/kg) | PRPCA can cause changes in maternal cell genetic material; mutagenic effect on fetal cells through placental barrier | [134] | |
| Reproductive-embryonic toxicity | Rabbit | PR Lectin (4.4kD, 500 μg/rabbit, intrauterine injection) | Inhibitory rate of the rabbit embryo implantation was 100 % | [3] |
| Mice | PR Lectin (4.4kD, 250 μg/mice, s.c.) | Inducing abortion in 50 % mice | [3] |
8.1. Acute toxicity
Wu et al. [119] found obvious acute toxicity after intraperitoneal injection of PR in mice, with an LD50 of 5.381 g/kg. Zhang et al. [120] gavaged 1 mL of PR for seven days in mice and found obvious acute toxicity with LD50 of 397.24 g/kg. Increasing evidence has shown that the powder of PR has obvious toxicity, while its water extract shows almost no toxicity.
8.2. General toxicity
Xu et al. [133] found that raw PR suspension (36 g/kg) had high acute toxicity, while 6 g/kg dose had significant maternal toxicity and embryo toxicity. Zhong et al. [121] found that the stimulating component of PR may be calcium oxalate needle crystal, which is mainly composed of calcium oxalate and protein, with a protein content exceeding 6 %. Zhao [136] and Pan [123] confirmed that toxic needle crystals and lectin proteins of PR can cause cell membrane damage and cell death. Lu et al. [137] found that the toxic components of PR mostly exist in the alcohol-soluble part, with few toxic components in the water-soluble part. Lv et al. [138] found that the toxicity of PR was related to the content of total organic acids and alkaloids. Zhang et al. [125] found that PR water extract (62.5 g/kg) and acid water percolate (1.77 g/kg) had hepatotoxicity. The water extract of PR (0.4 g/mL) could significantly increase the contents of creatine kinase (CK), creatine kinase isozyme (CKMB), and lactate dehydrogenase (LDH) in SD rats, while raw PR could cause cardiotoxicity by blocking the mTOR signal pathway and activating transforming growth factor-β1 (TGF-β1) signaling pathway [136,139]. Additionally, raw PR significantly increased the content of serotonin (5-HT) in serum. It has also been reported that 5-HT can induce cell apoptosis by activating the TGF-β1 signaling pathway and upregulating the expression of TGF-β1 protein, which ultimately results in cardiotoxicity [129,140]. Studies have shown that raw PR (2.25, 4.5, 9 g/kg) can cause compensatory kidney growth and even death in mice [141,131]. It has been reported that PR decoction (25 g/kg) can cause focal infiltration of lymphocytes scattered in the renal parenchyma of mice, resulting in a tubular formation [132].
8.3. Irritant toxicity
In a rabbit eye irritation experiment, Hu et al. [78] found that the positive rate of 30 % PR powder and 0.9 % sodium chloride suspension on rabbit eyelid conjunctiva reached 100 %. Wu et al. [127] found that soaking in 8 % alum water or alkaline water with a pH > 12 could reduce the irritating toxicity of raw PR. Moreover, Zhu et al. [142] found that PR agglutinin can significantly increase PGE 2 and protein content in rat peritoneal exudate and aggravate the irritation of PR poison needle crystal to rabbit conjunctiva. PR poison needle crystal could significantly induce neutrophil migration after stimulating macrophages, while pure PR poison needle crystals or macrophages themselves had no such effect; thus, PR lectin protein has a strong inflammatory effect.
8.4. Mutagenic toxicity
Wang et al. [143] used three doses (10, 20, 30 g/kg) of Jiang-Banxia for 15 consecutive days and conducted micronucleus analysis of the sternum bone marrow of the mother mouse and the liver blood of the fetal mouse and made Single Cell Gel Electrophoresis (SCGE) test of blood lymphocytes. The results showed that Jiang-Banxia may have some mutagenic effects. Xiong et al. [144] administered mice the decoction of PR and Jiang-Banxia for 10 days continuously and found that they all increased the incidence of abnormal chromosome structure cells to the same extent, which was close to that of the 1 mg/kg mitomycin C group.
It has been reported that PR and Jiang-Banxia (1.434 g/kg) have toxic effects on glycerophospholipid metabolism, amino acid, and carbohydrate metabolism in pregnant rats, with varying degrees of interference [145]. Jiang-Banxia (10 g/kg) can cause DNA damage, resulting in the alteration of maternal cell genetic material and teratogenic effects [5]. Moreover, PR decoction administered at 10 g/kg has been shown to be embryotoxic to mice [134].
8.5. Concoction to reduce toxicity
The proper use of PR requires processing using concoctions and related methods before clinical use. Knowledge of empirical practice suggests that processing has a considerable impact on the safety of PR. A logical way to demonstrate detoxification is to check whether the content of toxic components changes significantly after processing. Tao et al. [135] reported that soaking in lime water (pH > 12) caused irreversible denaturation of lectin proteins, resulting in a significant decrease in the inflammation-causing toxicity of PR, which played a key role in the detoxification of the concoction. Ge et al. [146] reported that the use of alum can completely destroy the needle crystal structure and composition of the toxic needle crystal, resulting in a significant reduction in the toxicity of PR. It has also been shown that ginger and licorice can reduce the inflammatory reaction caused by the poisonous needle crystals of PR, thus reducing the irritation induced by PR [[122], [124], [126], [128], [130], [147]].
9. Quality control
9.1. Origins
PR is the tuber of the Pinellia ternata genus in the family Araceae, which is composed of about six species worldwide and five species in China. It can be confirmed that the original plant of the Pinellia ternata herb is consistent with The Ch.P (2020 edition). Tu Jing Ben Cao (⟪图经本草⟫): “February born seedlings, a stem, stem end out of the three leaves, light green, rather like bamboo leaves and light, Jiangnan people like peony leaves.” The “Zhi Wu Ming Shi Tu kao” (⟪植物名实图考⟫): “where all have, have long leaves, round leaves two kinds, the same place.” That is to say, it describes the different leaf shape variations of PR. The former describes that the leaf shape of PR is divided into bamboo leaf shape and peony leaf shape, while the latter is divided into long and round leaf shape. In fact, the leaf shape variations of PR are even more diverse than those mentioned above, and the ratio between the length and width can be from 2:1 to 30:1, which is also significantly different between cultivated and wild Pinellia ternata.
9.2. Cultivation technology
Pinellia ternata root shallow, like mild, humid climate, afraid of drought, avoid high temperature. In recent years, due to changes in land use and cover changes as well as changes in farming practices such as pesticide and herbicide use, which have led to changes in the wild microenvironment of PR, wild resource populations have been greatly reduced or depleted, and cultivation has become the main source of PR. Many factors such as sowing density and depth, seed stem specification, shading intensity, and planting and harvesting patterns have an important effect on Pinellia ternata yield during artificial planting, with sowing density having the greatest impact on Pinellia ternata yield. Sowing density is too small then it will cause land and fertilizer waste, resulting in low yields too high density is prone to nutrient deficiencies, poor ventilation and air permeability, less light, and low photosynthetic products. High temperature and rainy season are serious, only reasonable dense planting to achieve high yield. In addition, the sowing depth should also be appropriate, sowing depth is too shallow, half-summer growth is susceptible to surface temperature and dry early influence, thus inhibiting tuber expansion and plant growth and development, depth is too deep, the soil permeability is poor, half-summer seedling late, development is not complete, low yield. Be careful to keep the indoor temperature not more than 10 °C, to avoid high temperatures to break the dormancy of the seed stem germination. Pinellia ternata seed sources are best from areas of similar ecology and geographic proximity.
9.3. Agricultural management
In the past 10 years (2011–2020), the international trade of Pinellia ternata herbs in China is mainly dominated by export trade, with a total export volume of 15,642.06 tons and a total export value of 22,888.40 million US dollars, while the import trade accounts for a very small proportion. In the past 10 years, our Pinellia ternata herbs have been exported to 33 countries and regions, mainly in East and Southeast Asia. At present, there are more problems in the development of international trade and industrialization of PR medicinal materials, such as limited export targets, difficulties in standardization, decline of wild high-quality resources, many pests and diseases, primitive and crude planting patterns, imperfect industrial chain, and insufficient protection of Taoist brands. We should strengthen the scientific research of Pinellia ternata and establish an internationally unified quality standard system for Pinellia ternata herbs; rely on scientific and technological innovation to empower the development of the Pinellia ternata herb industry; shape the Taoist Pinellia ternata brand, expand and extend the Pinellia ternata industry chain; standardize the international business of Chinese herbal medicines and boost the development of international trade of Pinellia ternata herbs.
9.4. Determination of the content of active ingredients
Developing a quality evaluation system for TCM is necessary to improve the quality standards of TCM. Over-collection of wild and endangered herbs can lead to the depletion of wild medicinal resources, while the global demand for endangered herbs continues to increase, especially in Asian countries. In this context, quality control of herbal medicines is particularly important. With the continuous development and progress of modern science and technology, many analytical tools, such as Thin-layer chromatography (TLC), Ultraviolet–visible spectrometry (UV), High-performance liquid chromatography (HPLC), Ultra-performance liquid chromatography-quadrupole optical-fluorescence mass spectrometry (UPLC-Q-TOF-MS) and Gas chromatography-mass spectrometry (GC-MS) coupling techniques have been used for the qualitative and quantitative analyses of the chemical compositions in the PR, and the relevant analytical methods are summarized in Table 6.
Table 6.
Summary of different methods and representative sample preparation procedures for analysis of markers in Pinellia ternata (Thunb.) Breit.
| Method | Analytes | Sample preparation | Column | Mobile phase | Reference |
| UV | Beta-Sitosterol | 0.4 g of PR powder was mixed with 20 mL ethyl acetate, and cold soak overnight, take the filtrate. The filtrate was extracted twice, the ethyl acetate was recovered, and the residue was diluted to 10 mL with ethyl acetate. | [148] | ||
| TLC |
Pinellia ternata Pinellia pedatisecta |
1 g PR power was mixed with 20 mL of ethanol and then subjected to refluxed extraction for 1 h and evaporated to dryness. The residue was dissolved in 1 mL of ethanol. | Silicone G Thin Layer Plates | Trichloromethane-methanol-water (6:4:0.5) | [149] |
| ELISA | Pinellia ternata lectin | 1 g PR power was mixed with 20 mL of ultrapure water and shaking for 2 h, and centrifuged at 8000 r/min for 15 min. The residue was re-extracted twice under the same conditions and supernatants were combined. | [150] | ||
| ELISA | PTL | 1 g PR power was mixed with 20 mL of PBS and shaking for 1 h, and centrifuged at 8000 r/min for 15 min. The residue was re-extracted twice under the same conditions and supernatants were combined. | [151] | ||
| UPLC-MS/MS | Lysophosphatidylcholines Sanedrine Trigonelline Choline Protocatechuic acid Protamine sulfates Homogentisic acid Chrysophanic acid |
5 g of PR、PRPCA、PRPZA and PRP power ware mixed with 50 mL of methanol and 10 L of internal standard solution, and reaction was extracted twice of 30 min each time, centrifuged at 10000 r/min for 15 min, and then the supernatants were combined. | Waters Acquity UPLC HSST3 (100 mm × 2.1 mm, 1.8 μm) | methanol (A)-water (B) with gradient elution | [152] |
| UPLC-Q-TOF-MS/MS | Alkaloids Flavonoids Amino acids Others |
2 g PR powder was immersed in 70 % ethanol (20 mL) for 0.5 h, then extracted under reflux for 1 h, filtered, extracted with 70 % methanol (10 mL) for 0.5 h, combined filtrate. | shim-pack xR-ODSⅢ column (2.1 mm × 75 mm, 1.6 μm) | acetonitrile (A)-0.1 % formic acid in water (B) with gradient elution | [153] |
| HPLC | Uracil 6-Hydroxypurine Uridine Guanosine Thymidine |
1 g of PR powder was mixed were 50 mL of water, weigh the mass, sonicate for 30 min, filter and take the filtrate. | Agilent Zorbax SB C18 column (4.6 mm × 250 mm, 5 μm) | methanol (A)-water (B) with gradient elution | [154] |
| HPLC | Uracil Cytidine Uridine Inosine Guanosine Thymidine Adenosine |
0.2 g of PR powder was mixed were 10 mL of water, ultrasonic extraction for 1 h, centrifuged at 3000 r/min for 10min, and the supernatant was taken. | Agilent Zorbax SB Cs (4.6 mm × 250 mm, 5 μm) | water (A)-methanol (B) with gradient elution | [155] |
| HPLC | Inosine Guanosine Adenosine Succinic acid Ephedrine Hydrochloride |
2 g of PR powder was mixed with 20 mL of ultrapure water, ultrasonic extraction for 45 min, 10000 r/min−1 centrifugation for 5 min, take the supernatant 10 mL, recover the solvent to dry, add the appropriate amount of ultrapure water to make the dissolution, transferred to a 10 mL measuring flask, with the use of purified water to the gradient of the volume, shaking well, and the extract was then extracted. | Agilent Eclipse XDB C18 column (4.6 mm × 250 mm, 5 μm) | acetonitrile (A)- water (B) with gradient elution | [156] |
| HPLC | Inosine Guanosine Thymidine Adenosine Trigonelline |
1 g of PR powder was mixed with 50 mL ultrapure water, ultrasonic extraction was performed 3 times, each time for 45 min. 12000 r/min centrifugation was performed for 10 min, and the supernatants of the 3 extractions were combined. | C18 column (150 mm × 4.6 mm, 5 μm) | methanol and water (3:97) | [157] |
| UPLC | Calcium oxalate | Precisely weigh the appropriate amount of PR powder, add ultra-pure water vortex mixing, set to 60 °C aqueous solution, set to 60 °C water bath heating and stirring, centrifuged at 8000 r/min for 5 min, aspirate the supernatant, the same method for 2 times: combined supernatant and transferred to a 10 mL measuring flask, add water to set volume. | Acquity UPLC BEH Cl8 column (50 mm × 2.1 mm, 1.7 μm) | 0.5 % (NH4) H2PO4 | [158] |
| RP-HPLC | Oxalic acid Citric acid Malic acid Succinic acid |
2 g of PR powder was mixed with 40 mL ultrapure water, ultrasonic extraction 2 times, each time 2 h, filtration, combined filtrate, with ultrapure water to 100 mL, precision measurement of 20 mL, add concentrated ammonia to adjust pH 11.5, with 3 times the amount of extract of ethyl acetate (60 mL) extraction for 3 times, collection of alkaline solution, acidified with phosphoric acid to pH 2.0, and then 3 times the amount of ethyl acetate extraction for 5 times. Then the extract was extracted with 3 times amount of ethyl acetate for 5 times, collected the ethyl acetate, dried by rotary evaporation, dissolved in ultrapure water and concentrated to 25 mL. | Gemini-C18 column (4.6 mm × 250 mm, 5 μm) | methanol and 0.03 % ammonium dihydrogen phosphate buffer (97:3) | [159] |
| Potentiometric titration | Total acid | 5 g PR power was mixed with 95 % ethanol and then subjected to refluxed extraction for 1 h, extracted three times, filtered and 0.1 mol/L NaOH aqueous solution (10 mL), and then subjected to ultrasonic extraction for 30 min. | NaOH, HCL titration solution | [160] | |
| microchip electrophoresis with electrochemical detection | Guanosine Methionine Glycine 3,4-dihydroxybenzaldehyde Homogentisic acid |
2 g PR power was mixed with 6 mL of the extraction solvent containing C2H5OH/DI H2O (1:1, v/v) and then subjected to ultrasonic extraction for 20 min. | [161] | ||
| HPLC | Uridine Adenine Guanosine Adenosine |
1 g PR powder was mixed with 25 mL of water, sonicate for 30 min, cool to room temperature, shake well and centrifuge at 12000 r/min for 10 min, take the supernatant. | Shim-pack Scepter C18-120 column(250 mm × 4.6 mm, 5 μm) | 10 % acetonitrile (A)-water (B) with gradient elution | [162] |
| GC-MS | Fat-soluble ingredients | 500 g of PR powder, 3 times with 3 L methanol osmosis drip extraction, each 4 d, respectively, combined extracts, 38 concentrated under reduced pressure into a crude extract. The crude extract was dispersed into 600 mL of distilled water, and then extracted with 600 mL of petroleum acyl (60~90 °C) for 3 times, concentrated at 38 °C under reduced pressure, and then freeze-dried by freeze-dryer to obtain the extract. 1 mg of each extract was dissolved in 10 mL of methanol and sonicated. | VARIAN VF-5 ms column (30 m × 0.25 mm × 0.25 μm) | carrier gas: N2 | [163] |
| MLPA | PR Pinellia pedatisecta Pinellia cordata |
The surface of the herbs was scrubbed with 75 % ethanol and the surface cortex was scraped off, 30–50 mg of the dried samples were placed in 2 mL EP tubes and 2 small steel beads were added, and then the samples were ground into powder for 2 min in a high-throughput tissue grinder, and then the samples were extracted by using the Plant Genomic DNA Extraction Kit, and the concentration and purity of the DNA were determined by using the NanoDrop-20O0 Ultra-Micro Spectrophotometer. | [10] | ||
| PCR-RFLP | PR Arisaema erubescens |
By comparing the rbcL sequences of Arisaema erubescens, PR and their mixed pseudo-products, the specific cleavage sites HaeIII and DraⅠ were selected for Arisaema erubescens and PR, respectively, and were identified by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) | [164] | ||
| ATR-FTIR | Pinellia ternata of different origins | The powder of pinellia ternata was finely ground in an agate mortar and pestle under dry conditions. | [165] |
TLC is a chromatographic separation technique for the separation, identification and quantification of mixed samples with support coated on support plate as stationary phase and suitable solvent as mobile phase. The Ch.P (2020 edition) identifies arginine, alanine, valine and leucine, the characteristic components of PR, mainly by TLC. Chen et al. [162] found that the TLC profiles were clearly different between the PR and Pinellia pedatisecta. PR showed only one yellow-green spot on TLC while Pinellia pedatisecta showed two yellow-green spot.
HPLC method is a powerful tool for qualitative and quantitative analysis of PR and its adulterants. Most of chemical profiling and the main chemical component's contents in PR were carried out by using HPLC. Researchers developed an HPLC method for the simultaneous determination of five representative components in PR, which can be used as a rapid and reliable method for the quantitative analysis of active components in PR [150,155]. Liu et al. [151] established a content determination method for simultaneous determination of seven nucleoside constituents in PR, and found that the relative contents of different nucleoside constituents in the same origin of PR also differed considerably. Wang et al. [152] established a method for the determination of uridine, adenine, guanosine and adenosine content in PR and its mixed pseudo-products, Arisaema erubescens, Typhonium flagelliforme and Pinellia pedatisecta by one-measure-multiple-assessment method, and it was found that, except for Arisaema erubescens, the results of the determination of the content of nucleosides based on nucleosides combined with the stoichiometric analysis can well differentiate between Pinellia ternata, Typhonium flagelliforme and Arisaema erubescens. He et al. [158] developed a UPLC method for the determination of calcium oxalate in Pinellia ternata herbs and their concoctions from different origins, which is accurate and reliable, and can provide a reference for the quality control of Pinellia ternata herbs.
UPLC-Q-TOF-MS technology, due to its advantages of rapidity, high resolution and high sensitivity, has been widely used in the quantitative analysis of Chinese medicine ingredients with the advancement of technology, providing more possibilities for quality control. Zhai et al. [10] using UPLC-Q-TOF-MS were isolated and identified 80 compounds, which can be used for basic research on PR. Cui et al. [16] used UPLC-Q-TOF-MS to analyze the chemical composition of multi-material, multi-processed PR and found significant differences in the chemical composition of PR before and after processing. Lu et al. [149] used UPLC-MS/MS to determine the contents of eight potentially toxic components in PR and artillery products, combined with PCA and PLS-DA multivariate statistical analyses, found that choline, ephedrine hydrochloride, and protocatechuic acid were the main differential components. In addition, Xu et al. [154] used GC-MS to analyze the fat-soluble components in PR and identified 27 compounds, which can provide a reference for the standardization of quality standards for PR.
Other analytical methods including microchip electrophoresis, MLPA, UV, PCR-RFLP, ATR-FTIR and ELISA have also been employed to determine the chemical constituents of PR. Shih et al. [164] used microchip electrophoresis to determine five representative ingredients in PR. The method was rapid, accurate and sensitive, and provided a promising alternative analytical platform for rapid analysis of PR. Mo et al. [160] found that PR and its common mixed forgeries (Pinellia pedatisecta and Pinellia cordata) could be clearly identified by using the multiple ligation probe amplification technique. Zhang et al. [156] established a UV method to determine the content of total alkaloids in PR. The results showed that there were significant differences in the alkaloids content of PR from different origins. Xiao et al. [165] used PCR-RFLP technology to identify Arisaematis Rhizoma and PR. Qiao et al. [161] realized rapid identification of PR from different origins based on infrared spectroscopy.
The Chinese Pharmacopoeia (2020 edition) identifies arginine, alanine, valine and leucine, the characteristic components of PR, mainly by TLC, and specifies that the leachate should not be less than 7.5 %, the moisture should not be more than 13.0 %, and the total ash should not be more than 4.0 %. Which detailed description of the nature of the flavour attribution, function and main treatment, usage and dosage, contraindications and storage measures, concoctions and processing methods are also included in the standard. The Ch.P (2015 edition) specifies that the total acids content in PR calculated as succinic acid equivalent should not be less than 0.25 %. However, controlling the total acid content does not reflect the traditional medicinal effects of PR, nor does it control its toxicity. The Ch.P (2020 edition) is a lack of quality standards for quantitative analysis. And the search for quality marker for PR is particularly important for quality control. However, the quality of PR is affected by many factors such as origin, harvesting season, processing, storage method and storage time, as well as climatic and environmental changes, which make the myriad of chemical components contained in PR contribute differently to the clinical efficacy, and it is difficult to achieve quality control by a single indicator only; therefore, to ensure clinical efficacy, the quality standard of PR should be unified and a PR quality standard system should be developed. Furthermore the processing method is also an important factor affecting the content of ingredients. Therefore, we believe that different quality standards should be established for quality control of different processed products based on multiple bioactive ingredients.
10. Conclusions and future perspectives
To summarize, despite showing a certain toxicity, PR is an important TCM with a long history of more than 2000 years. Many chemical constituents have been isolated and identified from the plant, and many experts continually study PR, with great contributions from many aspects. However, there remain problems and challenges that require further research and exploration to meet the clinical needs.
First, although various chemical constituents have been identified from PR, the substances that exert pharmacological effects are still unclear. Therefore, it is necessary to further study the combination of monomer compounds and its clinical effects. Certain methods combined with experiments were used to verify the mechanisms and components of PR. Second, the mechanism of action of Chinese medicine is a hot research topic. Although the pharmacological effects of PR are continuously reported, they are mostly evaluated on the efficacy of animals, with few studies on the targets and pathways of action of PR. Therefore, studies on the mechanism of action at the cellular level should be conducted in the future. Third, although some progress has been made in the study of the toxicity of PR, the toxic substances remain unclear. The reported calcium oxalate needle crystals are only closely related to irritation. In the future, there is a need to further elucidate the toxic substances of PR along with comprehensive toxicity evaluation to improve the safety of its clinical use to lay the foundation for future drug development and clinical use.
Generally, as a commonly used TCM, PR still needs further research. Here, we have systematically and comprehensively introduced the current status of the research on PR at home and abroad in recent years, including traditional applications, phytochemistry, pharmacology, and toxicology. Although great progress has been made in its research, there are still some problems in various aspects; therefore, continuous efforts to develop and exploit PR are required.
Funding statement
This work was supported by National Natural Science Foundation of China (81803732) and Project of Philosophy and Social Science Key Research Base of Shaanxi Provincial Department of Education (20JZ040).
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Ting Zou: Writing – review & editing, Writing – original draft. Jing Wang: Supervision, Validation. Xu Wu: Validation. Kai Yang: Project administration. Qiao Zhang: Methodology. Changli Wang: Formal analysis. Xiao Wang: Data curation, Methodology. Chongbo Zhao: Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Xiao Wang, Email: 2091028@sntcm.edu.cn.
Chongbo Zhao, Email: zhao_chongbo@126.com.
References
- 1.Xue R., Gong J.W., Qu L.Y. Research progress and discussion on processing of toxic traditional Chinese medicine decoction pieces. World J Trad Chinese. 2022;17(9):1193–1201. [Google Scholar]
- 2.Zeng X.Q., Peng Z.S. Growth and propagation of wild Pinellia ternata in cultivation. China J. Chin. Mater. Med. 2008;(8):878–883. [PubMed] [Google Scholar]
- 3.Peng W., Li N., Jiang E. A review of traditional and current processing methods used to decrease the toxicity of the rhizome of Pinellia ternata in traditional Chinese medicine. J. Ethnopharmacol. 2022;299 doi: 10.1016/j.jep.2022.115696. [DOI] [PubMed] [Google Scholar]
- 4.Commission N.P. Beijing; 2020. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- 5.Gong M.F., Zou J. Re-study on teratogenic effects of three kinds of processed Pinellia ternata on pregnant mice. J Beijing Univ Tradit Chin Med. 1990;(1):36–37. [Google Scholar]
- 6.Yang L., Zhou Y., Wang X.M. Research progress on chemical constituents variation in processing of banxia (rhizoma pinelliae) J Liaoning Univ Tradit Chin Med. 2022;24(2):49–53. [Google Scholar]
- 7.Huang H., Zhang M.X., Shen Y. Immune modulation of a novel lipidsoluble extract of Pinellia pedatisecta Schott in the tumor microenvironment of an HPV+tumor-burdened mouse model. J. Ethnopharmacol. 2018:5567. doi: 10.1016/j.jep.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 8.Huang C., Peng W., Wei S.J. Effect of pinelliae rhizoma Praeparatum Cum alumine polysaccharides on MUC5AC mRNA in luna tissues of alleraic asthma model rats. Chin. J. Exp. Trad. Med. Formul. 2019;25(22):15–21. [Google Scholar]
- 9.Liu Y.H., Guo J.H., Liu W.T. Research progress on alkaloids from Pinellia ternate. J North A&F Univ. 2015;43(9):171–177. [Google Scholar]
- 10.Zhai X.Y., Zhang L., Li B.T. Chemical components in pinalliae rhizoma by UPLC-Q-TOF-MS/MS. Chin. J. Exp. Trad. Med. Formul. 2019;25(7):173–183. [Google Scholar]
- 11.Chen P., Li C., Liang S.B. Characterization and quantification of eight water-soluble constituents in tubers of Pinellia ternata and in tea granules from the Chinese multiherb remedy Xiaochaihu-tang. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;843(2):183–193. doi: 10.1016/j.jchromb.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Xu J.K., Zhang T.L., Yi G.Q. Isolation and identification of chemical constituents from bulk Pinellia ternate. J. Shenyang Pharm. Univ. 2010;27(6):429–433. [Google Scholar]
- 13.Xu W.Y., Liu C.S., Feng M.X. Study on the chemical composition of traditional Chinese medicine Pinellia ternata by C18-mixed-stationary phase column LC-MS. Northwest Pharm J. 2015;30(4):354–357. [Google Scholar]
- 14.Oshio H., Tsukui M., Matsuoka T. Isolation of L-ephedrine from pinelliae tuber chemical. Pham Bull. 1978;26(7):2096–2097. doi: 10.1248/cpb.26.2096. [DOI] [PubMed] [Google Scholar]
- 15.DU J., Ding J., Mu Z.Q. Three new alkaloids isolated from the stem tuber of Pinellia pedatisecta. Chin. J. Nat. Med. 2018;16(2):139–142. doi: 10.1016/S1875-5364(18)30040-2. [DOI] [PubMed] [Google Scholar]
- 16.Cui M.N., Zhong L.Y., Lan Z.L. Effect of multi-material and multi-process processing on chemical composition of Pinellia ternata based on UPLC-Q-TOF-MS/MS. Chin Tradit Herbal Drugs. 2021;52(24):7428–7437. [Google Scholar]
- 17.Wu Y.Y., Huang X.X., Zhang M.Y. Chemical constituents from the tubers of pinellia ternata (Araceae) and their chemotaxonomic interest. Biochem Syst Ecol. 2015;(62):236–240. [Google Scholar]
- 18.Zhang K.W., Wu H., Wu L.L. A study on component of aliphatic acid in rhizoma pinelliae ternatae praeparata. J Nanjing Med Univ Tradit Chin Med. 2002;(5):291–292. [Google Scholar]
- 19.Nagai T., Arai Y., Emori M. Anti-allergic activity of a Kampo (Japanese herbal) medicine "Sho-seiryu-to (Xiao-Qing-Long-Tang)" on airway inflammation in a mouse model. Int Immunopharmacol. 2004;4(10–11):1353–1365. doi: 10.1016/j.intimp.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Han M.H., Yang X.W., Zhang M. Phytochemical study of the Rhizome of Pinellia ternata and quantification of phenylpropanoids in commercial Pinellia tuber by RP-LC. J. Chromatogr. A. 2006;64(11–12):647–653. [Google Scholar]
- 21.Zhang Z.H., Dai Z., Hu X.R. Isolation and structure elucidation of chemical constituents from pinellia ternata. J Chin Med Mat. 2013;36(10):1620–1622. [PubMed] [Google Scholar]
- 22.Ji X., Huang B., Wang G. The ethnobotanical, phytochemical and pharmacological profile of the genus Pinellia. Fitoterapia. 2014;(93):1–17. doi: 10.1016/j.fitote.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Niijima A., Okui Y., Kubom Effect of Pinellia ternata tuber on the efferent activity of the gastric vagus nerve in the rat. Brain Res. Bull. 1993;32(2):103–106. doi: 10.1016/0361-9230(93)90063-h. [DOI] [PubMed] [Google Scholar]
- 24.Zheng X.B., Chen K.L., Yin W.Z. Analysis of lipid compounds of high-yielded Rhizoma Pinelliae growing in the west of Hubei province by gas chromatography-mass spectrometry. J Chin Med Mat. 2007;(6):665–667. [PubMed] [Google Scholar]
- 25.Bai J., Qi J.L., Yang L. A comprehensive review on ethnopharmacological, phytochemical, pharmacological and toxicological evaluation, and quality control of Pinellia ternata (Thunb.) Breit. J. Ethnopharmacol. 2022;(29) doi: 10.1016/j.jep.2022.115650. [DOI] [PubMed] [Google Scholar]
- 26.Sun G.X., Ding S.S., Qian Y.J. Isolation, purification and analysis of Pinellia ternata lectin A. Fudan Univ. J. Med. Sci. 1995;22(4):299–302. [Google Scholar]
- 27.Liu X., Tian X., Liu T. Disclosure of the tuberous lectin composed of homogeneous tetramers in Pinellia pedatisecda Schott. Appl. Biochem. Biotechnol. 2010;162(4):1214–1223. doi: 10.1007/s12010-010-8908-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang C.C., Bi Q., Huang D. Identification of Pinelliae Rhizoma and its counterfeit species based on enzymatic signature peptides from toxic proteins. Phytomedicine. 2022;107 doi: 10.1016/j.phymed.2022.154451. [DOI] [PubMed] [Google Scholar]
- 29.Kuata K. Qantitiative analysis of anti-emetic principle in the tubers of pinellia ternate by enzyme immune assay. Pantta Medica. 1998;64(7):645–648. doi: 10.1055/s-2006-957539. [DOI] [PubMed] [Google Scholar]
- 30.Fan M.H., Zhou J.Y. Progress of research on pinellia ternate. Northwest Pharm J. 2004;19(2):90–92. [Google Scholar]
- 31.Luo Q., Niang X.Y., Liu X. Research progress on chemical constituents and pharmacological activities of pinellia ternate. Spec Wild Econ Anim and Plant Res. 2020;42(5):54–60. [Google Scholar]
- 32.Tomoda M., Gonda R., Shimizu N. Characterization of an acidic polysaccharide having immunological activities from the tuber of Alisma orientale. Biol. Pharm. Bull. 1994;17(5):572–576. doi: 10.1248/bpb.17.572. [DOI] [PubMed] [Google Scholar]
- 33.Wei L., Zhou Q., Zhu L.X. A study of optimization of the aqueous two-phase extraction process for Pinellia ternata polysaccharides based on the Box-Behnken response surface method. Hunan J Tradit Chin Med. 2022;38(7):170–175. [Google Scholar]
- 34.You S.B., Han Z.X. J Lanzhou Univ Arts Sci.; 2006. Study on Extraction Technology of Alkaloids from Pinellia Ternata; pp. 69–71. 2. [Google Scholar]
- 35.Chen L., Sun D.M., Lan D. Optimization of extraction of total flavonoids from pinelliae rhizoma by response surface methodology. Guangzhou Chem. Ind. 2020;48(21):54–58. [Google Scholar]
- 36.Wei S.H. Study on extraction process for polysaccharide of pinellia ternata. Biotechnol. 2010;20(2):42–44. [Google Scholar]
- 37.Zhu J.W., Feng G., Feng Y. Optimization of ultrasonic extraction technology of guanosine from Pinellia ternata by orthogonal design. Henan J Tradit Chin Med. 2013;33(4):607–608. [Google Scholar]
- 38.Yang Y.L., Qi W.Q. Study on antioxidant activity of polysaccharide from BanXia and extraction process optimization. West J Tradit Chin Med. 2016;29(7):37–41. [Google Scholar]
- 39.Guo H.J., Wei D.G. Optimization of extraction process of polysaccharide from pinellia ternata and study on its scavenging ability of free radical. J Anhui Agric Sci. 2010;38(34):19341–19342+19345. [Google Scholar]
- 40.Wu H., Wang M., Xu F.Q. Study on optimizing the extraction process of total free organic acids in pinellia ternata Breit. J Chin Med Mat. 2004;(7):524–525. [PubMed] [Google Scholar]
- 41.Weng L.D., Li H., Zhang L. A study on the ultrasonic extracting technology method of polysaccharide in radix pinellia. J Liaoning Univ Tradit Chin Med. 2007;(4):151–152. [Google Scholar]
- 42.Li D.D., Zhang Z.Q., Wang W.Q. Optimization of extraction of total flavonoids from shuangxia decoction by response surface methodology. Chin Arch Tradit Chin Med. 2015;33(9):2140–2142. [Google Scholar]
- 43.Yang B.Y. Research on the efficacy of crude and related processed Rhizoma pinelliae based on material basis and biological activity. Chengdu J. Tradit. Chin. Med. 2014;5:94–98. [Google Scholar]
- 44.Zeng S., Li S.Y., Wu Z.J. Ingredients-effect relationship study on antitussive and expectorant of pinelliae rhizoma. Mod Chin Med. 2013;15(6):452–455. [Google Scholar]
- 45.Shen L.W., Lu X.M., Chen S.M. Experimental study of preliminary evaluation of pharmacodynamics of pinellia rhizome in different habitats. J Chengdu J Tradit Chin Med. 2008;(2):54–57. [Google Scholar]
- 46.Deng Q.N., Zhou J.L., Guo Z.H. Airway mucus hypersecretion in rats is inhibited by the extracts of pinellia. Chin. J. Respir. Crit. Care Med. 2009;8(5):477–481. [Google Scholar]
- 47.Du W., Su J., Ye D. Pinellia ternata attenuates mucus secretion and airway inflammation after inhaled corticosteroid withdrawal in COPD rats. Am. J. Chin. Med. 2016;44(5):1027–1041. doi: 10.1142/S0192415X16500579. [DOI] [PubMed] [Google Scholar]
- 48.Lee M.Y., Shin I.S., Jeon W.Y. Pinellia ternata Breitenbach attenuates ovalbumin-induced allergic airway inflammation and mucus secretion in a murine model of asthma. Immunopharm Immunot. 2013;35(3):410–418. doi: 10.3109/08923973.2013.770522. [DOI] [PubMed] [Google Scholar]
- 49.Nagai T., Arai Y., Emori M. Anti-allergic activity of a Kampo (Japanese herbal) medicine "Sho-seiryu-to (Xiao-Qing-Long-Tang)" on airway inflammation in a mouse model. Int Immunopharmacol. 2004;4(10–11):1353–1365. doi: 10.1016/j.intimp.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q.L., Gong L.L., Li G.S. Effects of alkaloids of pinellia ternata on 5-HT3 receptor and NK1 receptor in isolated Guinea-piglleum. J. Shandong Univ. Tradit. Chin. Med. 2017;41(5):466–468. [Google Scholar]
- 51.Tang Y.M., Zhou X.Y. Comparison of pharmacodynamics of Pinellia ternata before and after processing. Chin Tradit Patent Med. 1994;(9):21–22. [Google Scholar]
- 52.Kurata K., Takaaki T., Ye Y. Quantitative analysis of anti-emetic principle in the tuber of Pinellia ternata by en-zyme immunoassay. Planta Med. 1998;64(7):645–648. doi: 10.1055/s-2006-957539. [DOI] [PubMed] [Google Scholar]
- 53.Maki T., Takahashi K., Shibata S. An anti-emetic principle of Pinellia ternata tuber. Planta Med. 1987;53(5):410–414. doi: 10.1055/s-2006-962759. [DOI] [PubMed] [Google Scholar]
- 54.Ho S.T., Liu T.S. Studies on pinellia ternata breitenbach. Report Ⅰ. Antiemetic action of pinellia ternata breiten-bach and its components. J Taiwan Pharm Ass. 1975;27(1–2):41–46. [Google Scholar]
- 55.Shen Y.Q., Zhang M.F., Zhu Z.P. Analgesic, anti-ulcerative and anti-thrombotic actions of rhizoma pinelliae. Chine J Biol Pharm. 1998;3:141–143. [Google Scholar]
- 56.Liu S.Y., You C.L., Wang Y.M. Experimental study on anti-ulcer effect of Pinellia ternate. Pharm Clin Chin Mat Med. 1993;(3):27–29. [Google Scholar]
- 57.Wu H., Cai B.C., Rong G.X. Effect of Pinellia ternata processed with ginger on gastrointestinal function of animals. China J Chin Mat Med. 1994;(9) 535-537+574. [PubMed] [Google Scholar]
- 58.Li F.R. Comparative study on pharmacodynamics of micron medicinal materials of Pinellia ternata and its traditional decoction pieces. J Pract Tradit Chin Med. 2006;(6):378–379. [Google Scholar]
- 59.Wu H., Wu L.P., Qiu L.Y. Comparison of pharmacological effects of pharmacopoeia Jiang banxia and orthogonal Jiang banxia. Chin Trad Pat Med. 1998;(4) 16-18+50-51. [Google Scholar]
- 60.Liu J.L., Zhong Q. Comparative study on some pharmacological effects of Pinellia ternata and Pinellia ternate. J Chengdu Univ Tradit Chin Med. 1989;(2):41–44. [Google Scholar]
- 61.Lu J.D., Miao J.X., Miao M.S. External curative effect of Pinellia ternata wine paste on traumatic blood stasis rat model. China J Tradit Chin Med Pharm. 2013;28(3):616–619. [Google Scholar]
- 62.Shi Y.N., Li W.P., Tao T. The study of the rhizoma pinelliae cordatae and rhizoma pinelliae against Agkistrodon acutus (Guenther)snake venom poisoning. J Snake. 2012;24(3):233–236. [Google Scholar]
- 63.Kim Y.J., Shin Y.O., Ha Y.W. Anti-obesity effect of Pinellia ternata extract in Zucker rats. Biol. Pharm. Bull. 2006;29(6):1278–1281. doi: 10.1248/bpb.29.1278. [DOI] [PubMed] [Google Scholar]
- 64.Zhan A.P., Wang P., L K. Chen Comparative study on sedative and hypnotic effects of Pinellia ternata, Pinellia palmata and Pinellia ternata on mice. J Chin Med Mat. 2006;(9):964–965. [Google Scholar]
- 65.Yang R., Wang M.Z., Cheng Y.X. Comparison study of supercritical-CO2 fluid extractions of pinellia rhizoma on anticonvulsant action. Chin. J. Exp. Trad. Med. Formul. 2012;18(6):214–219. [Google Scholar]
- 66.Gu Y.T., Ma Y.G., Wang M.Z. The effects of pinellia total alkaloids on the amino acid concentration and the GABAA receptor expression in hippocampus region of epileptic rats. J Chin Pharm Sci. 2009;18(3):252–256. [Google Scholar]
- 67.Zhang Y.U., Ni H.Y. Alleviating effects of ethanol extracts from pinellia ternata and magnolia officinalis on depression model in mice. China Modern Drug Appl. 2013;7(14):245–246. [Google Scholar]
- 68.Fang J.Y., Chen G.Y., Gao J.Q. Study on the regulatory effect of high dose Pinellia ternata on PTSD-like rats based on ethology. Lishizhen Med Mat Med Res. 2017;28(8):1870–1871. [Google Scholar]
- 69.Zhou F., Diao B., Duan K. Effect of total alkaloid derived from Pinellia ternata on learning and memory and preliminary study of its mechanism in rats with Parkinson disease. Chin J Clin Neurosur. 2011;16(7):413–416. [Google Scholar]
- 70.Chen K.S. Screening test of anti-tumor activity of Chinese herbal medicine. Progr Jpn Med. 1980;(6):31. [Google Scholar]
- 71.Xiong F.L., Yang J.H. Experimental study on antitussive effect of Pinellia ternata from two different habitats in Guizhou Province. Chin J Tradit Vet Sci. 2015;(9):16–17. [Google Scholar]
- 72.Ke C.Y. Sputum removing and cough relieving effects of 5 different solvent extracts of pinellia ternata on mice. China Pharm. 2012;23(39):3652–3654. [Google Scholar]
- 73.Xue J.H., Xiao T.H., Wang Q.M. Pharmacological effects of Pinellia ternata. Lishizhen Med Mater Med Res. 1991;(4):153–154. [Google Scholar]
- 74.Bai Q., Li M., Jia M.R. Comparision of expectorant and antitussive actions between pinellia tubers from different areas of production. Chin. Pharmacol. Bull. 2004;(9):1059–1062. [Google Scholar]
- 75.Shan J.S., Li J.X., Jiang P. Effects of compound pinellia water extract in treating cough, asthma and expectoration. Tianjin J Tradit Chin Med. 2009;26(4):338–340. [Google Scholar]
- 76.Ok I.S., Kim S.H., Kim B.K. Mediators Inflamm.; 2009. Pinellia Ternata, Citrus Reticulata, and Their Combinational Prescription Inhibit Eosinophil Infiltration and Airway Hyperresponsiveness by Suppressing CCR3+ and Th2 Cytokines Production in the Ovalbumin-Induced Asthma Model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y.J., Ji Z.Q., Zhang X.L. Effect of rhizoma pinelliae on vomiting in minks. China J Chin Mat Med. 2005;(4):38–40. [PubMed] [Google Scholar]
- 78.Hu C.J., Li G.M., Ma L. Study on the processing technology reform of Pinellia ternate. Chin Tradit Patent Med. 1999;(1):20–23. [Google Scholar]
- 79.Wang L., Zhao Y.J., Zhang X.L. Determination of the total alkaloids of pinellia tuber and its anti-vomiting study in minks. Chin. Pharmacol. Bull. 2005;(7):864–867. [Google Scholar]
- 80.Lu Y., Nie K., Lin Y.Y. Determination of alkaloid content in Pinellia ternata and its effect on intestinal motility in Guinea pigs in vitro. Shandong J. Tradit. Chin. Med. 2013;32(12):916–918. [Google Scholar]
- 81.Yukiya O. Activation of Pinellia ternata on efferent activity of gastric branch of vagus nerve in rats. International J Tradit Chin Med. 1995;(4):30. [Google Scholar]
- 82.Zhang W.W., Sun S.Y., Li Y. Effects of banxia houpu decoction on gastric emptying and intestinal propulsion in mice. Chin J Int Tradit West Med. 1998;(S1) 134-136+374-375. [Google Scholar]
- 83.Zhang M.F., Shen Y.Q. Research progress on pharmacologic actions of extract from pinelliae rhizoma in respiratory and digestive systems. Anti-Infection Pharmacy. 2017;14(8):1457–1462. [Google Scholar]
- 84.Wang H.W., Tian J.Z. Purification and some properties of a trypsin inhibitor from rhizoma pinelliae. Nat Prod Res Dev. 2009;21(5):844–848. [Google Scholar]
- 85.Wu K.Z., Tao Z.J. Trypsin inhibitor extracted from Pinellia ternata and its characteristics. Acta Bioch Bioph Sin. 1981;(3):267–274. [Google Scholar]
- 86.Li Y.X., Wang S.J., Li M.S. Observation on dissolution of gallstones by 43 traditional Chinese medicines in vitro. Beijing J Tradit Chin Med. 1993;(3):35. [Google Scholar]
- 87.Teng S.Z., Han T.Y., Wang G.Z. Experimental study on antiarrhythmic effect of Pinellia ternata extract (abstract) J. Harbin Med. Univ. 1985;(3):75. [Google Scholar]
- 88.Li L., Ma Y.H., Liu X.T. Effects of preconditioning with aconite and Pinellia ternata on cardiac function and myocardial ultrastructure in rats with ischemia-reperfusion injury. Lishizhen Med Mater Med Res. 2014;25(2):325–327. [Google Scholar]
- 89.Huang Z.X., Tao Q., Hang C.F. Experimental study on re-endothelialization effects of Pinellia ternata extracts on carotid artery injury. Mod. Hosp. 2017;17(7):1053–1056. [Google Scholar]
- 90.Zhou X., Zhang X.R., Zhang Q.Y. Effect of different compositions of raw pinellia ternata and processed P.ternatas on secretion of inflammation cytokines of mice aorta endothelial cells. Chin. J. Exp. Trad. Med. Formul. 2013;19(10):261–265. [Google Scholar]
- 91.Liu S.Y., Li Y.X. vol. 12. J Liaoning Med Univ; 1997. p. 38. (Pinellia Ternata Decoction and Alcohol Precipitation Increase Coronary Flow in Isolated Heart). [Google Scholar]
- 92.Hong X.Q., Wo X.D., He Y.Z. J Zhejiang Med Univ.; 1995. Study on Hypolipidemic Effect of Pinellia Ternate; pp. 28–29+56. 2. [Google Scholar]
- 93.Jiang W.Y., Yang Y., Li Y.Y. Effects of some drugs for resolving phlegm on blood rheological property in rats. J. Tradit. Chin. Med. 2002;(3):215–216+225+215. [Google Scholar]
- 94.Zhang M.F., Shen Y.Q., Zhu Z.P. Study on the properties of traditional Chinese medicine with pungent and warm (heat) combined with spleen and stomach meridian-antithrombotic and anticoagulant effects. China J Chin Mat Med. 1997;(11):51–53. [Google Scholar]
- 95.Zhan A.P. Studies of the sedative and hypnotic action on mice of Pinellia ternata with sophora flavascens ait. J Jinggangshan Univ. 2008;(3):81–82. [Google Scholar]
- 96.You Q.Y., Wang P. Study on the sedative-hypnotic effect of the raw pinellia ternata and prepared pinellia tuber aqueous extract. Hubei J Tradit Chin Med. 2013;35(3):3–5. [Google Scholar]
- 97.Fang M.M., Lin S.S., Yuan Z.Z. Comparison of different processed products of rhizoma pinelliae sedative effect on mice. J Med Res. 2016;45(2):71–75. [Google Scholar]
- 98.Zhou X.G., Yan F.G. Inhibitory effect of raw Pinellia ternata water extract on central nervous system in mice. Pract Clin Med. 2011;12(1):4+11. [Google Scholar]
- 99.Cheng Y.X., Wang M.Z., Yang R. Synergistic effect of pinellia total alkaloids and uncaria total alkaloids on anticonvulsant action in mice and rats. J Chin Pharm Sci. 2007;16(2):139–145. [Google Scholar]
- 100.Deng C.X., Z.M Y., Lin P. Effects of pinellia total alkaloids on neuroethology, brain histopathology and BDNF/Trk-B expression in epileptic arts. Jilin J Chin Med. 2021;41(12):1652–1656. [Google Scholar]
- 101.Xu T., Wang B., Wang L. Pinellia ternata agglutinin produced in Bombyx mori cells exhibits bioactivity. Acta Biochim. Pol. 2012;(59):231–236. [PubMed] [Google Scholar]
- 102.Wu M., Chen P. Primary study on apoptosis of K562 cells in vitro induced by water extract of Pinellia ternata (Thunb)Breit. J Clin Hematol. 2014;27(11):983–986. [Google Scholar]
- 103.Xiong C.Z., Han K.Y., Chen Y.P. Action mechanism of pinellia ternata in treating lung cancer based on network pharmacology and molecular docking technology. Inform on Tradit Chinese Med. 2022;39(11):26–34. [Google Scholar]
- 104.Xia L. Shandong Univ Tradit Chin Med.; 2022. Study on the Synergistic Anti-proliferative and Pro-apoptotic Mechanism of Pinellia Ternata and Ginger in Ovarian Carcinoma. [Google Scholar]
- 105.Zu G., Wang H., Wang J. Rhizoma Pinelliae trypsin inhibitor separation, purification and inhibitory activity on the proliferation of BGC-823 gastric adenocarcinoma cells. Exp. Ther. Med. 2014;(8):248–254. doi: 10.3892/etm.2014.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang M.X., Zhang H.W., Yu Y. Synergistic effects of a novel lipid-soluble extract from Pinellia pedatisecta Schott and cisplatin on hu man cervical carcinoma cell lines through the regulation of DNAdamage response signaling pathway. Oncol. Lett. 2017;13(4):2121–2128. doi: 10.3892/ol.2017.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cathcart M.C., Useckaite Z., Drakeford C. Anti-cancer effects of baicalein in non-small cell lung cancer in-vitro and in-vivo. BMC Cancer. 2016;(16):707. doi: 10.1186/s12885-016-2740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li G.L., Gui S.Q., Zhu D.H. Effects of pinellia extraction only or combined with cisplatin on growth of HeLa cells of cervical cancer. Fudan Univ. J. Med. Sci. 2007;34(6):869–872. [Google Scholar]
- 109.Zhao Y.J., Wang L., Hou L. Study on antitumor effect of Pinellia polysaccharide. Chin. Pharmacol. Bull. 2006;(3):368–371. [Google Scholar]
- 110.Li X., Lu P., Zhang W. Study on anti-Ehrlich ascites tumour effect of Pinellia ternata polysaccharide in vivo. Afr J Tradit Complement Altern Med. 2013;10(5):380–385. [PMC free article] [PubMed] [Google Scholar]
- 111.Chen G., Xu J., Miao X. Characterization and antitumor activities of the water-soluble polysaccharide from Rhizoma Arisaematis. Carbohydr. Polym. 2012;90:67–72. doi: 10.1016/j.carbpol.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 112.Chen J.H., Cui G.Y., Liu J.Y. Pinelloside, an antimi-crobial cerebroside from Pinellia ternate. Phytochemistry. 2003;64(4):903–906. doi: 10.1016/s0031-9422(03)00421-7. [DOI] [PubMed] [Google Scholar]
- 113.Wang G.F. Study on in vitro bacteriostasis of the extract from effective parts of Pinellia ternata against common bacteria and yeast-like fungi. P Clin Med. 2009;18(11):1588–1589. [Google Scholar]
- 114.Liu W. Kunming Univ Sci Tech.; 2019. Study of the Proliferation Inhibition and Apoptosis Mechanism of Pinellia Ternata Extract on Leukemia Cells. [Google Scholar]
- 115.Feng J.K., Liu W., Li Z.F. Pinellia ternata extract induces apoptosis of leukemia cells by regulating the expression of Bax, Bcl-2 and Caspase-3 proteins. Tissue Eng Res China. 2020;24(31):5023–5029. [Google Scholar]
- 116.Zhou Q., Wu H., Wang Q.R. Anti-inflammatory effect of total alkaloids part of rhizoma pinelliae ternatae praeparata. J Nanjing Univ Tradit Chin Med. 2006;(2):86–88. [Google Scholar]
- 117.Huang F.Y., Gao J.M., Gong Q.H. Research progress on pharmacological effects and toxicity of Pinellia ternate. Natural Product Research and Development. 2020;32(10):1773–1781. [Google Scholar]
- 118.Liang X.Y., Wang L. Research progress on active components, processing, compatibility and representative formulas of pinellia ternate. J. Ethnobiol. Ethnomed. 2020;29(23):68–73. [Google Scholar]
- 119.Wu Z.J., Feng B.C., Shen Z.B. Acute toxicity study on Araceae toxic herbs and its processed products. J Guangdong Pharm Univ. 2018;34(3):312–315. [Google Scholar]
- 120.Zhang Z., Shan Z.Y., Xiang L.H. Experimental study on the lethal dose of 15 toxic traditional Chinese medicines to mice. J Basic Chin Med. 2005;(6):435–436. [Google Scholar]
- 121.Zhong L.Y. Nanjing Univ Chin Med.; 2007. Studies on Irritant Component of Pinelliae Tuber and the Attenuation Mechanism and Technology of Processing. [Google Scholar]
- 122.Zhou X. Southwest Jiaotong Univ.; 2013. Study on Activity and Toxicity of Raw Pinellia Ternata and its Processed Products. [Google Scholar]
- 123.Pan Y.Z., Yu H.L. Research on the relationship between raphides and lectin from Pinellia pedatisecta induce inflammatory and macrophage. China J Tradit Chin Med Pharm. 2014;29(5):1397–1401. [Google Scholar]
- 124.Yue P., Zhang K., Shan J.J. Effects of shengbanxia decoction and ganjiang renshen banxia tang on general toxicity and embryonic development of mice. Chin. J. Exp. Trad. Med. Formul. 2016;22(19):105–110. [Google Scholar]
- 125.Zhang M.L., Bao B.Y., Huang Y.Y. Experimental study on the "Dosage-Time-Toxicity"Relationship of acute hepatotoxicity induced by water extraction from rhizoma pinelliae in mice. Chine J Pharm. 2011;8(1):11–15. [Google Scholar]
- 126.Zhang Z.H., Zhao Y.Y., Cheng X.L. Metabonomic study of biochemical changes in the rat urine induced by Pinellia ternata (Thunb.) Berit. J. Pharm. Biomed. Anal. 2013;(85):186–193. doi: 10.1016/j.jpba.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 127.Wu H., Zhong L.Y. Study on processing mechanism of Pinellia ternate. China J Chin Mat Med. 2007;(14):1402–1406. [PubMed] [Google Scholar]
- 128.Cheng Y.Q., Yu H.L., Wu H. Irritant toxicity and lectin content of different processed products of Pinelliae Rhizoma. China J Chin Mat Med. 2022;47(17):4627–4633. doi: 10.19540/j.cnki.cjcmm.20211207.301. [DOI] [PubMed] [Google Scholar]
- 129.Su T., Tan Y., Tsui M.S. Metabolomics reveals the mechanisms for the cardiotoxicity of Pinelliae Rhizoma and the toxicity-reducing effect of processing. Sci. Rep. 2016;(6) doi: 10.1038/srep34692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu H., Cai B.C., Shi X.L. Effects of Pinellia ternata processed with ginger on animal irritation and toxicity. China J Chin Mat Med. 1993;(7) 408-410+446-447. [PubMed] [Google Scholar]
- 131.Yang S.Y., Ye W.H., Wu Z.L. Study on acute, subacute and cumulative toxicity of Pinellia ternata before and after processing in mice. Chin Tradit Patent Med. 1988;(7):18–19. [Google Scholar]
- 132.Yan X.Y., Chen J.P., Dong J. Study on the toxicity of decoction of Pinellia ternate. Inform on Tradit Chin Med. 2012;(29):102–105. [Google Scholar]
- 133.Xu J.Y., Xie H.H., Shan J.J. Different effects of rhizoma pinelliae processed by ginger or decocting on gestation and embryonic development of mice. J Nanjing Univ Tradit Chine Med. 2013;29(3):255–258. [Google Scholar]
- 134.Wang Y., Hu S.M., Li P.F. Pinelliae rhizoma reducing toxicity by processing. Jilin J Chin Med. 2019;39(10):1264–1267. [Google Scholar]
- 135.Tao X.B., Wu H. Effect of lime water processing of Pinelliae Rhizoma Praeparatum on toxic component lectin protein. China J Chin Mat Med. 2023;48(4):951–957. doi: 10.19540/j.cnki.cjcmm.20220718.302. [DOI] [PubMed] [Google Scholar]
- 136.Zhao T.F. Nanjing Univ Chin Med; 2013. Study on the Toxicity Mechanism of Pinellia Ternata and Detoxication Mechanism of Ginger. [Google Scholar]
- 137.Lu Y.H., Wang L., Huang Y.Y. Comparative study on acute toxicity of different components of rhizoma pinelliae in mice. Chin. J. Pharm. 2010;7(11):646–649. [Google Scholar]
- 138.Lv L.L., Huang W., Huang Y.Y. Experimental research on influences on the content of toxical substances and acute toxicity of different producing Areas'Rhizoma pinelliae. Chine J Pharm. 2010;7(11):649–652. [Google Scholar]
- 139.Zhang Z.H., Zhao Y.Y., Cheng X.L. Metabonomic study of biochemical changes in the rat urine induced by Pinellia ternata (Thunb.) Berit. J. Pharm. Biomed. Anal. 2013;(85):186–193. doi: 10.1016/j.jpba.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 140.Jian B., Navneet N., Li Q.Y. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann. Thorac. Surg. 2003;75(2):457–466. doi: 10.1016/s0003-4975(02)04312-6. [DOI] [PubMed] [Google Scholar]
- 141.Xu J.Y., Zhang K., Xie H.H. Effects of raw pinellia ternata and Ganjiang Renshen Banxia Pill on reproductive related toxicity of mice. China J Tradit Chin Med Pharm. 2017;32(7):3154–3157. [Google Scholar]
- 142.Zhu F.G., Yu H.L., Wu H. Correlation of Pinellia ternata agglutinin and Pinellia ternata raphides'toxicity. China J Chin Mat Med. 2012;37(7):1007–1011. [PubMed] [Google Scholar]
- 143.Wang X.H., J H., Xia M.Z. Experimental study on mutagenicity of pinellia ternate. Jiangsu Jl Tradit Chin Med. 2002;(8):42–43. [Google Scholar]
- 144.Xiong S.F., Gong M.F., Jiang W.H. Study on teratogenic effects of several different processed Pinellia ternata on pregnant mice. Beijing J Tradit Chin Med. 1987;(6):51–52. [Google Scholar]
- 145.Xie H.H., Xu J.Y., Xie T. Effects of Pinellia ternata (Thunb.) Berit. on the metabolomic profiles of placenta and amniotic fluid in pregnant rats. J. Ethnopharmacol. 2016;183:38–45. doi: 10.1016/j.jep.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 146.Ge X.Y. Nanjing Univ Chin Med; 2009. The Poisonous Components and Common Mechanism of Detoxification Processing of Raceae Herbal Medicine. [Google Scholar]
- 147.Zhu F.G. Nanjing Univ Chin Med; 2012. Study on the Pro-inflammatory Effect of Lectin Protein in Pinellia Ternata and Pinellia Palmatum and the Mechanism of Detoxification by Alumen. [Google Scholar]
- 148.Yu H.L., Xie Y.W., Wu H. Determination of Pinellia ternata lectin in Pinelliae Rhizoma and its processed products by ELISA. Drug Stan China. 2023;24(3):268–274. [Google Scholar]
- 149.Lu Y., Zhang Y.H., Sun Y.T. Simultaneous quantitative analysisof 8 potential toxic components in different processed products of pinellia ternata based on UP-LC-MS/MS. J Anhui Agric Sci. 2023;51(3):192–196+245. [Google Scholar]
- 150.Hao Z., He F.Q., Jin X. Comparative study of the quality of different varieties of pinellia ternata (thunb.) Breit. Heilongjiang Sci. 2022;13(20):5–7. [Google Scholar]
- 151.Liu Y., Bao H.Y., Wang B. Determination of seven nucleosides in Pinellia ternata from different habitats. J Gansu Univ Chin Med. 2022;39(3):32–36. [Google Scholar]
- 152.Wang C.C., Bi Q.R., Zhang J.Q. Quantitative analysis of four nucleosides in Pinellia Rhizoma and its adulterants by the quantitative analysis of multi-components by a single marker. Chin Tradit Herbal Drugs. 2022;53(19):6180–6186. [Google Scholar]
- 153.Xie Y.W., Yu H.L., Wu H. Preparation of monoclonal antibody against Pinellia ternata lectin protein and establishment of double antibody sandwich ELISA method. China J. Chin. Mater. Med. 2022;47(22):6076–6081. doi: 10.19540/j.cnki.cjcmm.20210414.302. [DOI] [PubMed] [Google Scholar]
- 154.Xu J., Zhao X.Q. Analysis and identification of fat-soluble components and amino acids in different concoctions of Pinellia ternata. Mod Chin Med. 2022;42(2):49–53. [Google Scholar]
- 155.Yang B.Y., Jing Y., Lai Y.Y. Simultaneous determination of five representative components in Pinelliae Rhizoma by HPLC. Chin J Pharm Anal. 2019;39(11):1992–1997. [Google Scholar]
- 156.Zhang S.Q., Li M., Yu L. Comparison study on contents of B-sitosterol in three kinds of different processed products of pinellia ternata. Chin Arch Tradit Chin Med. 2018;36(1):42–44. [Google Scholar]
- 157.Zhen Z.X., Ge S.J. Determination of four nucleosides and fenugreek alkaloids in Palm Sempervivum of different origins. J Chin Med Mat. 2017;40(12):2820–2823. [Google Scholar]
- 158.He D., Sun Q., Yang L. Determination of calcium oxalate in Pinellia ternata and the processed products from different habitats by UPLC, West China. J Pharm Sci. 2017;32(4):414–416. [Google Scholar]
- 159.Sun Q., Zhang J.J., Fu Y. Simultaneous determination of four organic acids in Pinelliae Rhizoma by RP-HPLC. Chin J Pharm Anal. 2015;35(6):1062–1066. [Google Scholar]
- 160.Mo J., Wang W.B., Chen H.C. Cheng huachun, authentication of pinellia ternata and its common adulterants by multiplex ligation-dependent probe amplification. Her Med. 2023;42(3):322–328. [Google Scholar]
- 161.Qiao L., Zhang Y.Q., Zhao B.Y. Rapid identification of banxia (pinelliae rhizoma) from different areas by infrared spectroscopy. Chin Arch Tradit Chin Med. 2022;40(11) 73-76+274-277. [Google Scholar]
- 162.Chen Y., Qiu Y.Q., Niang S.W. Identification of pinellia ternata and rhizoma pinelliae with Thin layer chromatography. Asia-Pacific Tradit Med. 2018;14(2):44–46. [Google Scholar]
- 163.Wang B., Li M., Lu D.H. Determination of total acid in Pinellia ternata of various populations by potentiometric titration. Pharm Clin Chin Mater Med. 2010;1(2):15–17. [Google Scholar]
- 164.Shih T.T., Lee H.L., Chen S.C. Rapid analysis of traditional Chinese medicine Pinellia ternata by microchip electrophoresis with electrochemical detection. J Sep Sci. 2018;41(3):740–746. doi: 10.1002/jssc.201700901. [DOI] [PubMed] [Google Scholar]
- 165.Xiao J.C., Yan B.B., Yang J. Identification of Arisaematis rhizoma and pinelliae rhizoma by PCR-RFLP, chin. J. Exp. Trad. Med. Formul. 2023;29(6):194–201. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.