Abstract
The human progesterone receptor (PR) contains multiple Ser-Pro phosphorylation sites that are potential substrates for cyclin-dependent kinases, suggesting that PR activity might be regulated during the cell cycle. Using T47D breast cancer cells stably transfected with an mouse mammary tumor virus (MMTV) chloramphenicol acetyltransferase reporter (Cat0) synchronized in different phases of the cell cycle, we found that PR function and phosphorylation is remarkably cell cycle dependent, with the highest activity in S phase. Although PR expression was reduced in the G2/M phase, the activity per molecule of receptor was markedly reduced in both G1 and G2/M phases compared to the results seen with the S phase of the cell cycle. Although PR is recruited to the MMTV promoter equivalently in the G1 and S phases, recruitment of SRC-1, SRC-3, and, consequently, CBP is reduced in G1 phase despite comparable expression levels of SRC-1 and SRC-3. In G2/M phase, site-specific phosphorylation of PR at Ser162 and at Ser294, a site previously reported to be critical for transcriptional activity and receptor turnover, was abolished. Treatment with the histone deacetylase inhibitor trichostatin A elevated G1 and G2/M activity to that of the S phase, indicating that the failure to recruit sufficient levels of active histone acetyltransferase is the primary defect in PR-mediated transactivation.
In breast cancer cells expressing the progesterone receptor (PR), progesterone induces a biphasic change in cell cycle progression, initially accelerating the cells to progress through the cell cycle and then inducing an arrest in the G0/G1 phase of the subsequent cycle (29). PR, a member of the steroid-thyroid receptor family of ligand-activated transcription factors (6, 40), is expressed as two isoforms, the longer PR-B form, containing 933 amino acids, and PR-A, which lacks the first 164 amino acids of PR-B (17). The isoforms have distinct tissue-specific functions and regulate different subsets of genes (10, 28, 35).
The functions of PR are regulated not only by progesterone but also by modulators of various cell signaling pathways (3, 21). The phosphorylation sites in PR (18), as in those of other steroid receptors, including the estrogen receptor (ER) (23), androgen receptor (AR) (12), and glucocorticoid receptor (GR) (4), are predominantly Ser-Pro motifs suggestive of regulation by proline-directed kinases, which include the cyclin-dependent kinases and the mitogen-activated protein kinases (MAPKs). Moreover, several recent reports have shown that cyclins, which are expressed in a cell cycle-dependent manner, can regulate steroid receptor function and that this regulation is independent of the kinase partner (19, 30, 32, 43). Early studies of GR function suggested reduced transcriptional activity and elevated basal phosphorylation of GR in the G2 phase of the cell cycle (15), although a more recent study failed to detect a reduction in GR activity in G2 phase (1). AR activity is reduced in cells blocked in G1/S phase compared to cells in G0 or S phase, although GR in the same cells did not show a comparable reduction in activity (26). None of the studies have identified the cause(s) of the alterations in activity. Our recent finding that PR activity is stimulated by cyclin A/Cdk2 (30) led us to measure the activity of PR as a function of cell cycle in T47D breast cancer cells, which express endogenous PR-B and PR-A.
Using T47D cells stably transfected with mouse mammary tumor virus (MMTV) chloramphenicol acetyltransferase (CAT) (Cat0) synchronized in the G1, S, and G2/M phases of the cell cycle, we found that PR function and phosphorylation is cell cycle dependent. PR activity was highest in S phase. Although PR expression was reduced in the G2/M phase, the activity per molecule of receptor was reduced in both G1 and G2/M phases compared to that seen in the S phase of the cell cycle. PR is recruited to the MMTV promoter equivalently in the G1 and S phases, but recruitment of SRC-1, SRC-3, and, consequently, CBP is reduced in G1 phase despite comparable expression levels of SRC-1 and SRC-3. Interestingly, the cytoplasmic-to-nuclear distribution of PR in response to hormone in S phase differs from the results seen with G1 and G2/M phase. Hormone treatment causes a marked increase in nuclear PR in S phase but not in the other phases. In addition to the reduction in transcriptional activity in G2/M phase, we found that site-specific phosphorylation of PR at Ser162 and Ser294 was abolished. Treatment with the histone deacetylase (HDAC) inhibitor trichostatin A (TSA) elevated G1 and G2/M activity to that of the S phase with little effect in S phase, indicating that the failure to recruit sufficient levels of active histone acetyltransferases (HATs) is the primary defect in PR-mediated transactivation.
MATERIALS AND METHODS
Materials and plasmids.
All cell culture reagents were obtained from Invitrogen (Carlsbad, Calif.). Indole 3-carbinol and nocodazole were obtained from Calbiochem (La Jolla, Calif.). Cadmium chloride, 4-hydroxy urea, propidium iodide, and trichostatin A were obtained from Sigma (St. Louis, Mo.). R5020 (promegestone) and [3H]R5020 were obtained from NEN Life Science Products (Boston, Mass.). RU486 (mifepristone) was obtained from Siniwest Holdings (San Diego, Calif.). SRC-1, SRC-3, and CBP antibodies were obtained from Santa Cruz Biotechnologies Inc. (Santa Cruz, Calif.). The actin antibody was obtained from Chemicon (Temecula, Calif.), and the phospho-extracellular signal-regulated kinase (pERK) antibody was obtained from New England Biolabs (Beverly, Mass.). Alexa Fluor 488 goat anti-mouse antibody, 4,6-diamidino-2-phenylindole (DAPI), and slow-fade mounting medium were obtained from Molecular Probes (Eugene, Oreg.). Phosphospecific antibodies to Ser190, Ser294, and the total PR antibody 1294 (9) were described earlier.
Cell culture.
T47D cells were purchased from the American Type Culture Collection. T47D cells stably transfected with MMTV CAT (Cat0 cells) were a kind gift from Andrew Cato (7) and have been used previously to study interactions of PR and its coregulators with the MMTV promoter by the use of chromatin immunoprecipitation (ChIP) assays (24). T47D and Cat0 cells were maintained in minimal essential medium-10% fetal bovine serum (FBS) with penicillin-streptomycin and Dulbecco’s modified Eagle medium (DME)-10% FBS with G418, respectively. T47D cells were plated in DME-10% charcoal stripped serum with penicillin and streptomycin (Invitrogen) at 2.3 million cells per 10-cm-diameter dish. Cat0 cells were plated in DME-10% serum with 200 μg of G418/ml at 2.3 million cells per 10-cm-diameter dish, 10 million cells per 150-mm-diameter dish, or 300,000 cells per well of a 6-well plate. All cells were maintained at 37°C with 5% CO2 in a tissue culture incubator.
Synchronization of Cat0 cells.
Cat0 cells were plated in DME supplemented with 10% fetal bovine serum. The synchronization scheme is shown as a diagram in Fig. 1A. To synchronize in the G1 phase, the cells were starved for 48 h, fed with 10% serum-containing medium, and then treated for 24 h with 100 μM indole 3-carbinol. Indole 3-carbinol, a chemical that naturally occurs in Brassica vegetables, induces a G1 cell cycle arrest by inhibiting Cdk6 expression (11). To synchronize in the S phase, cells were serum starved for 48 h to first synchronize in the G0/G1 phase and then synchronized at the G1/S boundary by incubation with 1.5 μg of 4-hydroxy urea/ml for 24 h in 10% serum-containing medium (27). The cells were washed with Hanks buffered saline (HBS) and released into S phase with serum-free medium. Synchronization in the G2/M phase was carried out by synchronizing the cells at the G1/S boundary with 1.5 μg of 4-hydroxy urea/ml in 10% serum-containing medium for 48 h, washing with HBS, and synchronizing in the G2/M phase by treating with nocodazole (1.0 μg/ml) in 10% serum-containing medium for 24 h. Nocodazole is an antimicrotubule agent that arrests cells in the G2/M phase of the cell cycle (8).
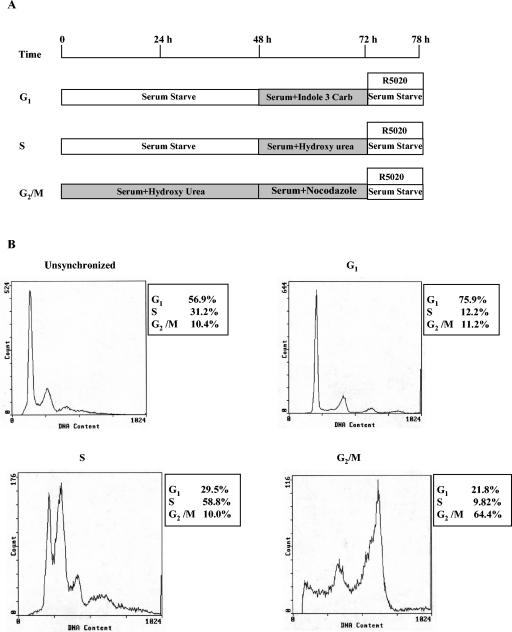
FIG. 1.
Cell synchronization plan and flow cytometry analysis. (A) Schematic for the cell synchronization protocol for T47D cells stably transfected with MMTV CAT (Cat0) that is described in detail in Materials and Methods. (B) Representative FACS analyses obtained from the synchronization protocol. The numbers in the inset next to each FACS analysis represent the percentages of cells synchronized in different phases under the synchronization conditions described in Materials and Methods.
All of the cells were washed with HBS, fed with serum-free medium, and treated as indicated in the figures for 6 h for CAT assays, PR transcription, and translation level measurements and for metallothionein IIA induction. For Western analysis of phosphoproteins, microscopy, and ChIP assays, the cells were transferred to serum-free medium for 6 h and treated with 10 nM R5020 for the final hour only. Synchronization was confirmed by fluorescence-activated cell sorter (FACS) analysis. For experiments involving RU486 and TSA, the cells were treated with RU486 and TSA along with the synchronizing reagents for the final 16 h and then were treated with 10 nM R5020 as indicated above.
Processing of cells for FACS analysis.
Cat0 cells were washed once in phosphate-buffered saline (PBS), scraped in PBS, and pelleted by centrifugation at 340 × g in a clinical centrifuge. The cells were resuspended in 2 ml of 0.9% sodium chloride and fixed for 30 min in 5 ml of 90% ethanol. Ethanol was added dropwise with constant vortexing. After 30 min, the cells were centrifuged out of the fix and resuspended in 1 ml of propidium iodide (50 μg/ml) diluted in PBS. At 30 min before FACS analysis, the cells were incubated at 37°C for 30 min with 100 μg of RNase A (Sigma) and the cell cycle distribution was determined by flow cytometry. FACS analysis was performed at the time of harvesting for each treatment variable to verify the synchronization of cells in different phases of the cell cycle. The cell synchronization in the early G1 phase ranged between 70 and 75% for G1-phase cells compared to about 55% for asynchronous cells. Cell synchronization in S phase ranged between 45 and 60% for S-phase cells compared to about 23 to 30% for asynchronous cells. Cell synchronization in G2/M phase ranged between 53 and 69% for G2/M-phase cells compared for 10% in asynchronous cells. Representative profiles are shown in Fig. 1B.
Cell sorting using Altra FACS.
Cat0 cells were plated at 10 million cells per 150-mm-diameter dish in medium supplemented with fetal calf serum. The cells were treated for 24 h with 10 nM RU486 to reduce background CAT expression, washed, fed with charcoal-stripped serum, and treated with 10 nM R5020 for 6 h or left untreated. The cells were washed with PBS, trypsinized, resuspended in culture medium, and stained with Hoechst 33342 dye (10 μg/ml in water) for 90 min. The cells were spun down, resuspended in cold HBS, and sorted using Altra FACS.
Preparation of Ser162 monoclonal antibody.
Phosphospecific Ser162 monoclonal antibody was generated as described earlier for phosphospecific Ser190 and Ser294 antibodies (9) by the use of a synthetic phosphopeptide conjugated to keyhole limpet hemocyanin-KLH [NH2-CGGATQRVLS(PO3)PLMSRSG-COOH] as the antigen. The PR sequence in the peptide corresponds to amino acids 156 to 169; the N-terminal CGG are extra amino acids added for conjugation to KLH and to separate the receptor sequence from KLH. Sera from the immunized mice were tested for antibody titers by enzyme-linked immunosorbent assays as described earlier (9). Mouse spleen cells were fused with the THT (Fox-NY) myeloma cell line (41), and the hybridomas were screened by enzyme-linked immunosorbent assay for differential reactions against free (nonconjugated) phospho- and dephosphopeptide followed by Western blotting of T47D cell extracts for a monoclonal antibody reaction with the phosphorylated peptide sequence in the context of native PR-B.
Reporter gene analysis.
The cells were harvested by adding TEN (0.15 M NaCl, 0.01 M EDTA, 0.04 M Tris, pH. 8.0) at room temperature for 30 min. The cells were pelleted at 13,000 rpm for 30 sec in an Eppendorf 5415C table top centrifuge. The CAT assay was performed as described earlier (44).
Western analysis.
The cells were rinsed once with cold 1× PBS and then scraped in PBS. The cells were pelleted and extracted in lysis buffer (homogenization buffer: [0.05 M potassium phosphate, 10 mM sodium molybdate, 50 mM sodium fluoride, 2 mM EDTA, 2 mM EGTA, 0.05% monothioglycerol]-0.4 M NaCl-protease inhibitors [1 mg/ml concentrations of aprotinin, leupeptin, antipain, benzamidine HCl, and pepstatin]-0.2 mM phenylmethylsulfonyl fluoride-1 mM sodium vanadate) by three freeze-thaw cycles. The cell debris was pelleted, and protein levels were measured by use of a Bradford reagent. Equal amounts of protein extracts were run on an SDS-PAGE gel, and the proteins were transferred to nitrocellulose overnight at 150 mA. Experiments were performed with three times more protein extract loaded on the gel in the lanes corresponding to the G2/M phase of the cell cycle to compensate for the lower total receptor expression level (see Fig. 4 and 7). Lanes in the pERK Western blot (see Fig. 4) contained equal amounts of protein. After overnight transfer, the proteins on the membrane were analyzed by Western blotting by a method described earlier (30).
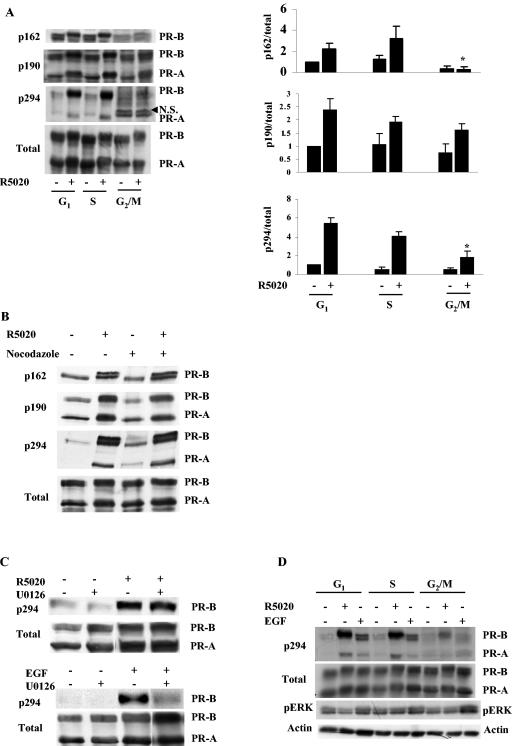
FIG. 4.
Site-specific PR phosphorylation is impaired during the G2/M phase of the cell cycle. (A) R5020-dependent phosphorylation of Ser162 and Ser294 but not Ser190 is impaired during the G2/M phase of the cell cycle. Cat0 cells were synchronized as indicated in Materials and Methods and treated with 10 nM R5020 for 60 min or left untreated. The cells were harvested, and protein was extracted, run on an SDS-6.5% polyacrylamide gel, and blotted with phosphospecific antibodies to Ser162, Ser190, and Ser294 and for total PR as described in Materials and Methods. The Western blots were quantified; values for PR-B are expressed in the panels on the right as phosphorylation of the individual sites normalized to total receptor level. N.S. indicates a nonspecific band that appears above PR-A when blotted with phospho-Ser294 antibody. *, significant difference in hormone-dependent phosphorylation at P < 0.05 in G2/M phase compared to the hormone-dependent phosphorylation in the S and G1 phases. (B) Nocodazole does not inhibit PR phosphorylation in unsynchronized cells. Unsynchronized Cat0 cells were treated or not with 1 μg/ml nocodazole (the level used to synchronize cells in G2/M phase). After 6 h, the cells were treated with 10 nM R5020 for 60 min or left untreated and harvested, and protein was extracted, run on a SDS-6.5% PAGE gel, and blotted with phosphospecific antibodies to Ser162, Ser190, and Ser294 and for total PR. (C) Involvement of different kinases in the R5020- and EGF-induced phosphorylation of Ser294. T47D cells were pretreated with U0126 (MEK inhibitor) for 3 h and then treated with 10 nM R5020 for 60 min or 100 ng of EGF/ml for 15 min. The cells were harvested, and protein was extracted, run on SDS-PAGE gels, and blotted with antibodies for p294 and total PR. (D) EGF-induced phosphorylation of Ser294 is impaired during the G2/M phase of the cell cycle. Synchronized Cat0 cells were treated with vehicle, 10 nM R5020 for 60 min, or 100 ng of EGF/ml for 15 min. The cells were harvested, and protein was extracted, run on an SDS-PAGE gel, and blotted with phosphospecific antibodies to Ser294, ERK, and total PR. Due to the lower receptor levels in the G2/M phase of the cell cycle, a 3× level of protein was loaded in the lanes corresponding to the G2/M phase in panels A and D. Equal amounts of protein were used for the pERK Western blot analysis whose results are shown in panel D. In all the panels, the antibodies used for the respective Western blots are given on the left of the Western blot and the receptor isoforms detected are given to the right. ERK, extracellular signal-regulated kinase.
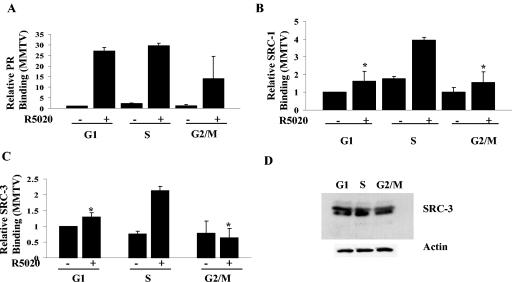
FIG. 7.
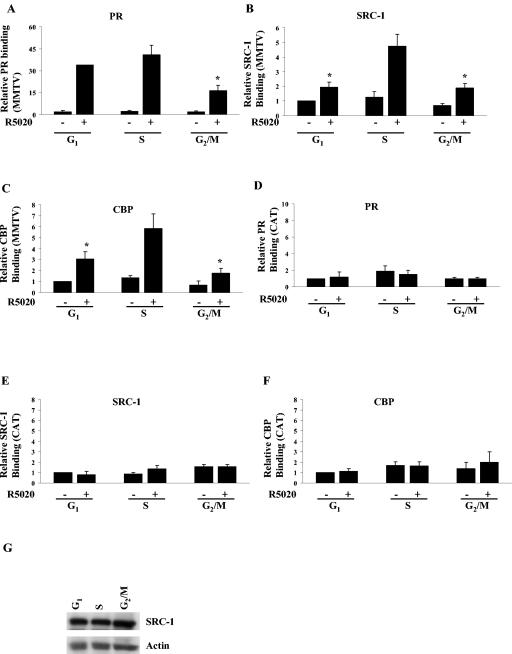
SRC-3 recruitment is maximal during the S phase of the cell cycle. Cat0 cells synchronized in different phases of the cell cycle as described in Materials and Methods were treated with 10 nM R5020 or left untreated and cross-linked, extracts were immunoprecipitated with antibodies to PR (A), SRC-1 (B), and SRC-3 (C), and the recruitment to the MMTV promoter was determined by ChIP assays as indicated in Materials and Methods. (D) SRC-3 levels were determined by loading equal amounts of protein extracts on an SDS-6.5% PAGE gel and blotted with antibodies to SRC-3 and actin. All results are expressed as percentages of input. *, significance at P < 0.05 compared to the recruitment in R5020-treated S-phase cells. The results represent averages from three independent experiments.
Ligand binding assay.
A whole-cell binding assay was performed with Cat0 cells synchronized in the different phases of the cell cycle as described earlier (33). Briefly, cells were treated with 1 nM [3H]R5020 for 6 h. Cells were washed five times with ice-cold PBS, and [3H]R5020 was extracted with ethanol. Samples were counted in scintillation cocktail in a LS6500 counter (Beckman Coulter, Inc., Fullerton, Calif.). Nonspecific counts (from cells treated with a combination of 1 nM [3H]R5020 and 500 nM radioinert R5020) were subtracted from total counts to yield specific counts bound. To normalize the counts to receptor levels, the cell extracts were run on an SDS-PAGE gel and blotted for total PR as mentioned above. The PR bands were densitometrically quantified, and the hormone-bound amount of receptor/total amount of receptor ratio was determined.
Processing of cells for deconvolution microscopy.
All steps were performed at room temperature according to a protocol described earlier (38). The cells were plated on coverslips at 300,000 cells per well in the aforementioned medium, synchronized as described earlier, and treated for 60 min. After treatment, the cells were fixed and processed by a method described earlier (31).
Deconvolution microscopy.
Deconvolution microscopy was performed on the processed and mounted coverslips with a Zeiss AxioVert S100 TV microscope (Carl Zeiss, Thornwood, N.Y.) and a Delta Vision restoration microscopy system (Applied Precision, Inc.). A Z series of focal planes were digitally imaged at constant exposure time and deconvolved with the Delta Vision constrained iterative algorithm to generate high-resolution images (38). The intensity of the staining in the nucleus was determined by quantifying the intensity by the use of Metamorph software.
ChIP assay.
ChIP assays were performed with synchronized cells as described earlier (20, 30).
Real-time PCR.
Real-time PCR was done with an ABI PRISM 7700 sequence detector and Taqman PCR master mix from Applied Biosystems (Foster City, CA). For MMTV, the forward primer was GGT TAC AAA CTG TTC TTA AAA CGA GGA, the reverse primer was AAC ACT AAG AGC TCA GAT CAG AAC ATT T, and the probe was TGA GAC AAG TGG TTT CCT GAC TTG GTT TGG T. For CAT, the forward primer was CGC AAG GCG ACA AGG TG, the reverse primer was CCA TCA CAG ACG GCA TGA TG, and the probe was CCT GAA TCG CCA GCG GCA TCA. The primers and probes were synthesized by Biosource International (Camarillo, Calif.). The TaqMan primers and probes were chosen on the basis of a method described in a previous publication (24). The probes were made with 5′ 6-carboxyfluorescein and 3′ 6-carboxytetramethylrhodamine quencher fluorescence tags. The PCRs were performed under universal conditions of 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min, and the data were collected and processed using sequence detection software from Applied Biosystems.
Real-time RT-PCR analysis.
Total RNA was isolated from the cells using Trizol reagent (Invitrogen). The RNA was diluted 100-fold for MT2A and PR reverse transcriptase PCR (RT-PCR) and 1,500-fold for 18S rRNA detection. The message was analyzed using real-time PCR (ABI PRISM 7700 sequence detector; Applied Biosystems) and one-step real-time RT-PCR mix (Applied Biosystems) with TaqMan primers and probes for the metallothionein IIA gene (forward primer, GGC GTC GGA CAA GTG CAG; reverse primer, TTG TGG AAG TCG CGT TCT TTA C; probe, CTG GGA CAG CCC CGC TCCC [Biosource International]) and PR gene (forward primer, AGA AAT GAC TGC ATC GTT GAT AAA ATC; reverse primer, GGA CCA TGC CAG CCT GAC; probe, TCT GCC CAG CAT GTC GCC TTA GAA AGT GC) and a Taqman primer-probe set for 18S rRNA from Applied Biosystems. The RT-PCR was performed using a one-step RT-PCR reagent from Applied Biosystems under conditions of 48°C for 30 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min, and the data were collected and processed using sequence detection software from Applied Biosystems.
All experiments were performed at least three times. In addition, reporter gene assays were performed in triplicate for each experiment. Statistical analysis was performed by one-way analysis of variance, and when significance (P < 0.05) was revealed, a Holm-Sidak test was performed using Sigma Stat software to identify differences between the groups. All data are represented as means ± standard errors.
RESULTS
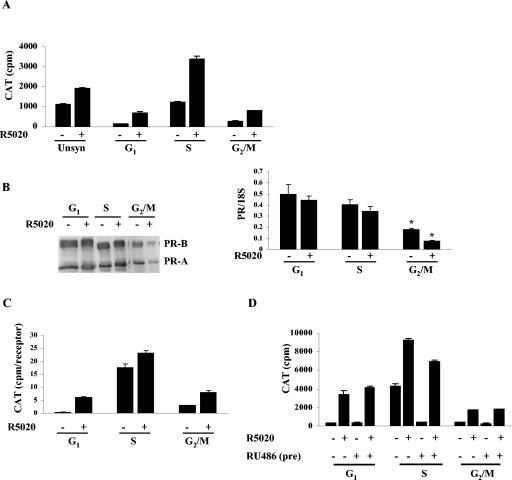
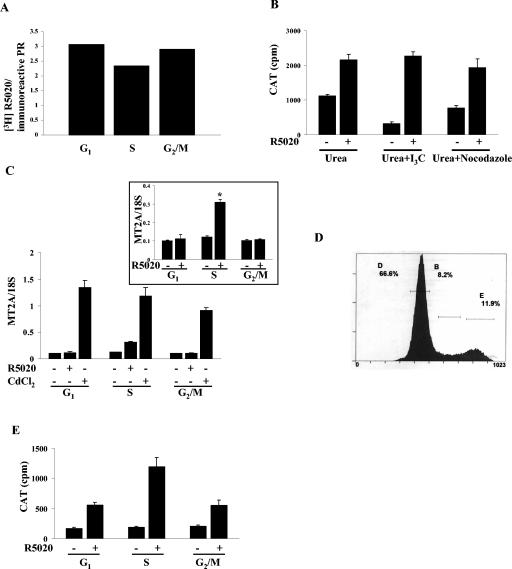
Human PR activity is reduced in the G1 and G2/M phases of the cell cycle. T47D cells stably transfected with MMTV-CAT (Cat0) were synchronized as shown in Fig. 1A, and the synchronization was confirmed by FACS analysis (Fig. 1B). The cells, treated with 10 nM R5020 or left untreated, were harvested, and CAT activity was measured and normalized to total protein levels. Figure 2A shows greatly reduced PR activity, both basal and R5020 dependent, in the G1 and the G2/M phases compared to the results seen with the S phase of the cell cycle or with unsynchronized cells. Analysis of the receptor level indicates equal levels of PR expression in the G1 and S phases but reduced expression in G2/M phase (Fig. 2B, left panel). This reduction is also observed at the mRNA level (Fig. 2B, right panel). However, normalization of PR activity to the receptor levels obtained from densitometric scans of the blots still shows reduced PR activity in both G1 and G2/M phases compared to S-phase results (Fig. 2C).
FIG. 2.
Human progesterone receptor activity is impaired in the G1 and G2/M phases of the cell cycle. (A) PR activity is maximal in the S phase of the cell cycle. Unsynchronized Cat0 cells or cells synchronized in the different phases of the cell cycle were treated with 10 nM R5020 as indicated for 6 h or left untreated. The cells were harvested, protein was extracted, CAT assays were performed, and activity was normalized to the total cellular protein as described in Materials and Methods. Unsyn, unsynchronized cells. (B) Both PR protein and mRNA levels are reduced in the G2/M phase of the cell cycle. PR expression in Cat0 cells synchronized and treated with 10 nM R5020 for 6 h or left untreated was determined both at the protein (left panel) and the mRNA (right panel) level by Western blotting and real-time RT-PCR. *, significance at P < 0.05 with respect to mRNA level compared to the results seen with the corresponding samples in G1 and S phases. (C) PR activity normalized to the receptor levels is reduced in the G1 and G2/M phases of the cell cycle. Normalization of the CAT activity to the receptor level was accomplished by fractionating the cell extracts on an SDS-PAGE gel, blotting with PR antibody, and quantifying by densitometry. (D) The basal activity in S phase is receptor dependent. Cat0 cells were synchronized in different phases as indicated in Materials and Methods. The cells were treated with 10 nM RU486 for the final 24 h or left untreated. The cells were washed and treated with 10 nM R5020 for the final 6 h in serum-free medium or left untreated. CAT assays were performed, and activity levels were normalized to total cellular protein levels as indicated for panel A.
Both basal and hormone-dependent activity appeared to be elevated in S phase. To determine whether the higher basal activity in S phase is receptor dependent, half of the cells were treated with 10 nM RU486 for the final 24 h of synchronization, RU486 was washed out, and the activity in both sets of cells was measured after an additional 6 h in serum-free medium treated with 10 nM R5020 or left untreated. Figure 2D shows a very substantial reduction in the basal transcriptional activity by RU486 treatment in the S phase with minimal effect on hormone-dependent activity, showing that the apparent higher basal activity in S phase is a receptor-dependent carryover from the synchronization procedure in FBS, which contains small amounts of progestin. In contrast, RU486 pretreatment had no effect in the G1 and G2/M phases.
Reduced PR activity in the G1 and G2/M phases is not due to the chemical treatments utilized to synchronize the cells.
To explore the possibility that the reduced transcriptional activity of PR was due to the effects of the chemicals used to synchronize in the G1 and G2/M phases as opposed to cell cycle phase-specific factors, several control studies were done. A comparison of the ratio of whole-cell-specific [3H]R5020 binding to total receptor measured by Western blotting revealed that the same proportion of receptor was capable of binding hormone in all phases of the cell cycle (Fig. 3A).
FIG. 3.
Reduced PR activity in the G1 and G2/M phases is not due to the chemicals used for the synchronization. (A) Levels of hormone binding to the receptor were equal in all phases. Cat0 cells were synchronized in different phases of the cell cycle as indicated in Materials and Methods. The cells were treated with 1 nM [3H]R5020 alone or 1 nM [3H]R5020 and a 500-fold excess of cold R5020 (0.5 μM) to compete with the [3H]R5020. The cells were washed, and bound [3H]R5020 ethanol was extracted and measured. Normalization of the hormone binding activity to the receptor level was accomplished by fractionating the cell extracts on an SDS-PAGE gel, blotting with a PR antibody, and quantifying the signal densitometrically. (B) PR activity is comparable in cells treated with the chemicals. Cat0 cells were synchronized at the G1/S boundary with 4-hydroxy urea and treated with 100 μM indole 3-carbinol or 1 μg of nocodazole/ml in the presence of 4-hydroxy urea for 16 h or left untreated. The cells were washed, treated with 10 nM R5020 for 6 h or left untreated, and harvested, and CAT activity was measured and normalized to the total cellular protein levels. Urea, 4-hydroxy urea; I3C, indole 3-carbinol; CAT, chloramphenicol acetyltransferase. (C) Metallothionein IIA gene transcription induced by R5020 but not by CdCl2 is impaired in the G1 and G2/M phases of the cell cycle. Cat0 cells were synchronized as described for Fig. 1. The cells were treated with vehicle, 10 nM R5020, or 5 μM cadmium chloride for 6 h. RNA was isolated, and a real-time RT-PCR was performed for the metallothionein IIA gene and normalized to total ribosomal 18S RNA. *, significance at P < 0.05 from vehicle-treated S-phase cells and from the R5020-treated cells in G1 and G2/M phases. MT2A, metallothionein IIA. (D) The distribution of Cat0 cells used for cell sorting in different phases (D indicates G1 phase, B indicates S phase, and E indicates G2/M phase) is shown. (E) PR activity is maximal in S-phase Cat0 cells obtained by sorting. Cat0 cells were treated for 24 h with 10 nM RU486 in full serum, washed, and treated with 10 nM R5020 in 10% charcoal stripped serum for 6 h or left untreated. The cells were sorted using an Altra FACS into G1, S, and G2/M phases. Protein from the sorted cells were extracted, and CAT activity was assayed as described for panel A. The results represent averages for four samples.
To determine whether indole 3-carbinol or nocodazole alters PR activity independently of cell cycle distribution, cells were first synchronized at the G1/S boundary with 4-hydroxy urea. The cells were then incubated with indole 3-carbinol or nocodazole in the presence of 4-hydroxy urea (which prevents further cell cycle progression) for 24 h. The cells were washed, fed with fresh serum-free medium, and treated for 6 h, releasing them into S phase. As shown in Fig. 3B, the addition of the chemicals did not impair PR activity, suggesting that the impairment of PR activity in the G1 and G2/M phases is cell cycle phase dependent and is not due to toxicity of the chemicals used. To determine whether similar changes in activity occur with an endogenous gene target and to test whether the reduced activity of PR in the G1 and G2/M phases reflects a primary dependency on PR, we compared cadmium- and progesterone-induced activation levels of metallothionein IIA (MT2A) gene transcription. The promoter of MT2A has a hormone-responsive element and a metal-responsive element; transcription can be induced either by a heavy metal such as cadmium chloride (CdCl2) or by a progestin such as R5020 (16, 37). Cat0 cells were synchronized in the different phases and treated with vehicle, 10 nM R5020, or 5 μM CdCl2, RNA was extracted, transcription of MT2A was measured by real-time RT-PCR, and expression was normalized to 18S. As shown in Fig. 3C, the levels of MT2A gene transcription induced by CdCl2 are comparable in all of the phases whereas R5020-induced MT2A transcription is substantially reduced in the G1 and G2/M phases compared to S-phase results. These results show that altered transcriptional activity of PR during cell cycle progression extends to an endogenous target gene and does not reflect changes in general gene regulation.
As an additional control to test for cell cycle dependence of transcriptional activity, unsynchronized T47D cells pretreated with 10 nM RU486 for 24 h were washed, transferred to medium containing charcoal-stripped serum, and treated with 10 nM R5020 for 6 h or left untreated. These cells were then stained with Hoechst 33342 dye, sorted by FACS, and collected for measurement of CAT activity. The proportions of cells present in the different phases of the cell cycle are shown in Fig. 3D. CAT activity was measured in the sorted cells and normalized to total protein levels. As shown in Fig. 3E, in similarity to our findings presented in Fig. 2A, PR activity was maximal in the S phase of the cell cycle.
Site-specific alterations in PR phosphorylation in the G2/M phase of the cell cycle.
We have shown previously that most of the phosphorylation sites in PR contain Ser-Pro motifs (18), suggesting that the sites are phosphorylated by cyclin-dependent kinases or mitogen-activated kinases. To determine whether PR phosphorylation activities differ during the cell cycle, we assessed the basal and hormone-dependent levels of phosphorylation at three specific sites by the use of phosphorylation site-specific antibodies. Cat0 cells were synchronized as described above and were treated with 10 nM R5020 for the final 60 min of the serum-free period or left untreated. The cells were harvested, and phosphorylation was detected by Western blotting with phosphospecific antibodies to Ser162 (a PR-B specific site), Ser190, and Ser294. Total PR was measured using the 1294 antibody. To ensure equal total PR loading, 3× cell extract from the cells synchronized in the G2/M phase was loaded to compensate for lower receptor expression. As shown in Fig. 4A, phosphorylation of PR at Ser162 and Ser294 is impaired in the G2/M phase whereas the phosphorylation of PR at Ser190 is still robust in the G2/M phase and comparable to the levels in the G1 and S phases. A comparison of the ratios of signals obtained using the phosphorylation-specific antibodies divided by total PR (1294 antibody) revealed that the hormone-dependent phosphorylation of PR-B at Ser162 and Ser294 but not Ser190 was impaired in the G2/M phase compared to the G1 or S phase. Moreover, the differential phosphorylation is not due to the nocodazole treatment, as unsynchronized Cat0 cells treated with nocodazole in the presence or absence of R5020 exhibited levels of phosphorylation comparable to the control cell results (Fig. 4B). Note that the hormone-dependent change in mobility of PR-B still occurs in G2/M phase, indicating that phosphorylation of these sites still occurs.
Phosphorylation of Ser294 can be induced by p42/p44 MAPK (ERK) and by R5020 (22), and the induction by R5020 and epidermal growth factor (EGF) involves different kinases (34). As shown in Fig. 4C, R5020-dependent Ser294 phosphorylation is not inhibited by the MEK inhibitor U0126, whereas EGF-induced Ser294 phosphorylation is reduced by U0126, suggesting that the kinase(s) responsible for hormone-dependent phosphorylation of PR is not ERK. To determine whether reduced phosphorylation of Ser162 and Ser294 in G2/M phase was due to lack of a specific protein kinase activity or to an inaccessibility of these residues in PR, cells were synchronized and treated with EGF for 15 min or with vehicle or 10 nM R5020 for 60 min. Analysis of the cell extracts revealed that EGF-dependent phosphorylation of Ser294 was detected in the G1 and S phases but not in G2/M phase despite the activation of ERK, which was measured using a phosphorylation-specific antibody (Fig. 4D). These results suggest that PR is either in an altered conformation or associated with a protein(s) that occludes the Ser294 site.
Hormone-dependent relocalization of PR occurs in the S phase of the cell cycle.
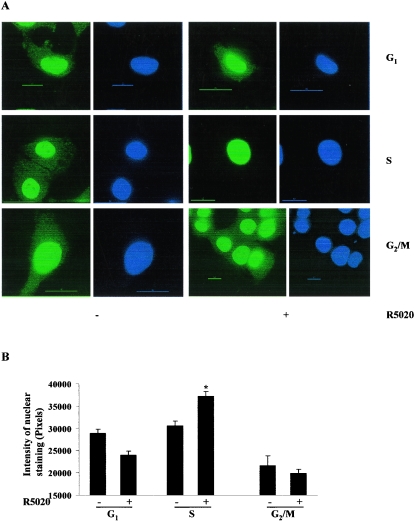
To determine whether altered receptor localization might contribute to reduced activity, synchronized Cat0 cells were treated with 10 nM R5020 for 60 min or left untreated, cells were fixed, and PR was detected by indirect immunofluorescence by the use of a deconvolution microscope. Previous studies had shown that in the absence of hormone, PR-B is found in both the cytoplasm and the nucleus whereas PR-A appears to be exclusively nuclear and treatment with hormone causes essentially complete nuclear localization of both forms (25). Figure 5A shows that R5020-dependent translocation of PR occurs in the S phase of the cell cycle. However, substantial cytoplasmic staining is still observed in the G1 and G2/M phases after hormone treatment. Quantification of the intensity of PR staining in the nucleus of approximately 250 cells in each phase shows a significant increase in R5020-dependent PR staining in the nucleus of cells in the S phase (Fig. 5B) only. Thus, nucleocytoplasmic shuttling of PR in response to progestin varies as a function of cell cycle and the higher nuclear/cytoplasmic ratio of PR in S phase correlates with higher transcriptional activity.
FIG. 5.
Hormone-dependent PR redistribution into the nucleus in the S phase but not in the G1 or G2/M phases of the cell cycle. Cat0 cells plated on coverslips were synchronized as described in Fig. 1. The cells were treated with 10 nM R5020 for 60 min or left untreated, and the coverslips were immunostained with an antibody to total PR followed by an anti-mouse Alexa Fluor 488 antibody and processed as indicated in Materials and Methods. The cells were visualized with a deconvolution microscope, and the images were captured with softWoRx software as described in Materials and Methods. Green fluorescence in the figure is the PR immunofluorescence, and the blue fluorescence is the nucleus stained with DAPI. The intensity of PR staining in the nucleus in different phases of the cell cycle was quantified using Metamorph software and is expressed as a bar graph in panel B. *, significance at P < 0.05 of results from the vehicle-treated S-phase cells and from the R5020-treated cells in other phases. DAPI, 4,6-diamidino-2-phenylindole.
SRC-1, SRC-3, and CBP are maximally recruited in the S phase of the cell cycle.
To identify defects in PR action at the molecular level that might account for lower activity of PR in G1 and G2/M phases, the hormone-dependent recruitment of PR and its associated coactivators to the promoter of a stably integrated MMTV CAT reporter was measured by ChIP assays in synchronized Cat0 cells treated with 10 nM R5020 for 60 min or left untreated. ChIP assays were performed with the integrated MMTV promoter or with the nonspecific CAT coding region in the reporter gene. As shown in Fig. 6, recruitment of SRC-1 and CBP but not PR was reduced in the G1 phase compared to the S phase of the cell cycle. Recruitment of PR, SRC-1, and CBP was impaired in the G2/M phase, although this may have been due in part to the reduced receptor levels. The differential recruitment of SRC-1 was not due to reduced expression of SRC-1, as levels were comparable in all phases of the cell cycle (Fig. 6G). Figure 6D, E, and F show the lack of recruitment of PR, SRC-1, and CBP, respectively, to the CAT region of the integrated MMTV gene, indicating the specificity of PR and coactivator association with the MMTV promoter region. Note the differences in the scales for MMTV and CAT. The range for CAT was adjusted so that the low level of hormone-independent nonspecific binding can be seen.
FIG. 6.
SRC-1 and CBP recruitment are maximal during the S phase of the cell cycle. Cat0 cells synchronized in different phases of the cell cycle as described in Materials and Methods were treated with 10 nM R5020 or left untreated and cross-linked, extracts were immunoprecipitated with antibodies to PR (A), SRC-1 (B), and CBP (C), and the recruitment to the MMTV promoter was determined by ChIP assays as indicated in Materials and Methods. As a measure of nonspecific binding, recruitment of PR (D), SRC-1 (E), and CBP (F) to the CAT coding region (nonspecific region) was determined using the primers generated to the CAT sequence as described in Materials and Methods. SRC-1 levels were determined by loading equal amounts of protein extracts on an SDS-6.5% PAGE gel and blotted with antibodies to SRC-1 and actin (panel G). All results are expressed as percentages of input. *, significance at P < 0.05 compared to the recruitment in R5020-treated S-phase cells. The results represent averages from three independent experiments. SRC-1, steroid receptor coactivator 1; CBP, CREB binding protein.
To address the cell cycle-dependent recruitment of other members of the p160 family of coactivators, another set of ChIP assays were performed with PR, SRC-1, and SRC-3 antibodies. An earlier study by Li et al. showed that although PR recruits SRC-1 and SRC-3 to the MMTV promoter, PR does not recruit TIF2/SRC-2 to this promoter in these cells (24). In similarity to the results seen with SRC-1 and CBP, SRC-3 is also maximally recruited to the MMTV promoter in the S phase of the cell cycle (Fig. 7C) without any alterations in its expression (Fig. 7D).
Inhibition of HDAC increases the PR activity in the G1 and G2/M phases but not in the S phase.
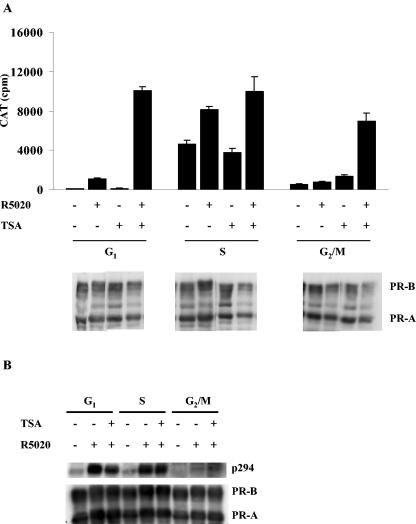
Because the SRC-1/CBP complex contains histone acetyltransferases, we asked what effect inhibition of HDAC activity would have on the reduced activity of PR in G1 and G2/M phases. To test this, synchronized Cat0 cells were treated with TSA, the HDAC inhibitor, for 16 h and then with vehicle or 10 nM R5020 for 6 h or left untreated. The cells were harvested, and CAT activity was measured and normalized to protein levels. As shown in Fig. 8A, TSA treatment elevated PR activity in the G1 and G2/M phases to the level observed in the S phase but had little effect on S-phase activity. Treatment with TSA did not increase receptor protein levels, as determined by Western analysis for PR (Fig. 8A). If anything, PR protein levels were slightly reduced in the TSA-treated cells, showing that the increased PR activity in G1 and G2/M phases was not due to enhanced receptor expression. An earlier report suggested that the transcriptional activity of PR is dependent on the phosphorylation of Ser294 and that mutation of this site leads to decreased PR function (36). Since the presence of TSA increased the PR activity in G2/M phase, we speculated that TSA might be acting by inducing changes that facilitate phosphorylation of Ser294. However, Fig. 8B shows that the level of Ser294 phosphorylation in the G2/M phase remains low in the presence of TSA, suggesting that another mechanism is responsible for activation.
FIG. 8.
Inhibition of histone deacetylase increases the PR activity in the G1 and G2/M phases of the cell cycle. Synchronized Cat0 cells were pretreated with 1 μM trichostatin A (TSA) for 24 h or left untreated and then treated with 10 nM R5020 for 6 h or left untreated as indicated in Materials and Methods. (Upper panel) The cells were harvested, and CAT activity was measured and normalized to the total cellular protein levels. (Lower panels) Equal amounts of the cell extracts were run on an SDS-6.5% PAGE gel and blotted for total PR to determine the levels of PR in the presence or absence of TSA. (B) Phosphorylation of Ser294 in PR-B is not increased by the presence of TSA. Synchronized Cat0 cells were pretreated with 1 μM trichostatin A (TSA) for 24 h or left untreated and then treated with 10 nM R5020 for 1 h or left untreated as indicated in Materials and Methods. The cells were harvested, and Western blotting with a phosphospecific antibody to Ser294 and total PR was performed as described for Fig. 4.
DISCUSSION
Earlier studies from our lab identified PR as an in vitro substrate of cyclin A/Cdk2 (18), and more recent studies show that Cdk2 is important for PR function (30). Since cyclin A/Cdk2 is an S-phase kinase, we speculated that PR would exhibit the cell cycle dependence of its activity. As shown for the first time in this report, PR transcriptional activity is dependent on the phase of the cell cycle, with maximum activity observed in the S phase and much lower activity in the early G1 and G2/M phases of the cell cycle (Fig. 2). The difference observed may be an underestimation, as the synchronization procedures provide great enrichment in the desired phase of the cycle but do not result in 100% synchronization. The differential activation was also observed when the activity of an endogenous target gene, metallothionein IIA (MT2A), was measured (Fig. 3C). That this response is not due to general alterations in transcription was confirmed by measuring the induction of MT2A in response to cadmium. Transcription of MT2A induced by cadmium chloride is not altered by the stages of the cell cycle. An independent assessment of PR activity as a function of cell cycle performed by the isolation of cells from an asynchronous population confirmed that PR activity is highest in S phase.
Only a few studies have analyzed the transcriptional activities of steroid receptors as a function of cell cycle. Earlier reports of studies of AR (26) and GR (15) combined with our study of PR show that the steroid receptors exhibit unique patterns of regulation. AR activity was found to be impaired in the G1/S transition but active in the G0 and S phase of the cell cycle, but GR did not exhibit the same pattern of regulation (26). Although the G2/M phase was not examined in this study, our results indicate that regulation of PR differs from that of either of these. Although our primary G1 synchronization procedure differed, when we starved cells to place them in G0, as was done for AR, PR activity levels were very low and were comparable to our G1 synchronization levels (data not shown). Additionally, AR expression was reduced in G1/S compared to G0 or S phase whereas PR expression is only reduced in G2/M phase. The basis for the reduction in PR expression has not been determined but is likely to be at the transcriptional level, as PR mRNA levels are also reduced. Although PR is generally an estrogen receptor-regulated target, its expression is independent of ER in T47D cells (13, 14).
Earlier reports have also indicated that the function and phosphorylation of glucocorticoid receptor (GR) is impaired in the G2/M phase of the cell cycle (15). However, a more recent report attributed the apparent reduction of GR activity in G2 to the use of the synchronization agent Hoescht H33342 (1). When nocodazole was used to synchronize the cells, GR was active in G2 phase, and although activation of an integrated target was impaired in M phase, a transiently transfected reporter was induced. Our use of nocodazole to synchronize the cells as well as the control treatments of cells in S phase supports the conclusion that the differences in activity observed are a result of the cell cycle synchronization rather than artifacts of exposure to the agents used to synchronize the cells.
Although the earlier studies of GR and AR have shown differences in activity throughout the cell cycle, the basis for these differences has not been identified. We have identified several cell cycle-specific changes that contribute to reduced activity. Although the levels of PR are the same in G1 and S phases and comparable amounts of PR bind to the MMTV promoter in response to the presence of hormone, SRC-1, SRC-3, and CBP are poorly recruited in G1 compared to S phase despite identical levels of SRC-1 and SRC-3. Others have shown that CBP recruitment by PR is dependent on SRC-1 (42). Our previous studies have shown that the interaction between PR and SRC-1 is enhanced by Cdk2 (30), a late G1- and S-phase kinase. The G1 synchronization blocks the cells prior to Cdk2 activation. Thus, the lack of recruitment is likely due to a lack of phosphorylation. SRC-1 itself is a histone acetyltransferase (HAT), as are some of the proteins that it recruits, such as CBP. Moreover, there is a report that the HAT activity of CBP is higher at the G1/S transition than that seen in the early G1, G0, or G2/M phases (2) and that this is due to the phosphorylation of CBP by cyclin E Cdk2, a G1/S kinase. Our finding that TSA, the histone deacetylase inhibitor, restores activity in the G1 phase indicates that the reduced activity is due to inadequate recruitment of HATs and subsequent acetylation. Blocking the deacetylation reactions restores PR function by reducing the rate of deacetylation. No enhancement of coactivator recruitment was observed in any phase with TSA treatment (data not shown).
The alterations in G2 are more complex. First, the expression of PR is reduced and that theoretically might be sufficient to reduce PR activity. Indeed, the average amount of PR bound to the MMTV promoter is reduced compared to the results seen with the S phase, as is the amount of SRC-1, SRC-3, and CBP. Nonetheless, TSA treatment restores PR activity, suggesting that the net activity of the recruited HATs is the limiting factor in activating transcription from the MMTV promoter.
In addition to the changes in cofactor recruitment and transcriptional activity, two additional striking changes were noted. First, PR phosphorylation measured using phosphorylation site-specific antibodies was site specifically reduced in the G2/M phase of the cell cycle. Whereas Ser190 displayed comparable basal and hormone-dependent phosphorylation throughout the cell cycle, the phosphorylation of Ser294 and Ser162 is greatly reduced in G2 phase. Ser294 phosphorylation is strongly hormone dependent (9, 45), and its phosphorylation has been reported to be important both for transcriptional activation and for receptor down-regulation (22, 36). Although the site is present in both PR-B and PR-A, it is preferentially phosphorylated in the PR-B form (9). We (Fig. 4C) and others (36) have found that although this site can be phosphorylated by activation of p42/p44 MAPK, the hormone-dependent phosphorylation is mediated by an unidentified kinase. To distinguish between the possibility that the hormone-dependent kinase simply is not active in G2 and the alternative possibility that the site is inaccessible due either to a conformational change in the receptor or occlusion by another protein, we asked whether activation of p42/p44 MAPK induced phosphorylation of Ser294 and found that although the kinase was effectively activated, Ser294 was not phosphorylated. Thus, the deficiency appears to be in the PR substrate. It is also possible that there is a higher level of a phosphatase that specifically dephosphorylates these sites, although we have no evidence for this. The lack of Ser294 phosphorylation and subsequent actions is potentially an important component in the reduced PR activity in the G2 phase. Interestingly, TSA treatment restored PR activity without concomitant phosphorylation of Ser294. Thus, blocking HDAC activity compensates for the lack of phosphorylation.
Finally, in looking for potential defects in subcellular localization, we noted substantial differences in the levels of distribution of PR in response to the presence of the progestin R5020. Previous studies had shown that whereas PR-A is essentially nuclear in the absence of hormone, PR-B is distributed between the cytoplasm and the nucleus (25). Recent studies have revealed that upon hormone treatment, cytoplasmic PR-B activates src, resulting in activation of p42/p44 MAPK (5). This activity is dependent upon a proline-rich region in the amino terminus of PR (5). The finding that PR retains cytoplasmic expression in the presence of hormone in the G1 and G2/M phases of the cell cycle, but not in the S phase, suggests that PR can continue to activate src in the presence of hormone in some phases but not in others. Thus, the cytoplasmic actions of PR may be strongest in the phases of the cell cycle in which transcriptional activity is weakest. Moreover, the rapid extranuclear signaling induced by progestins in G1 phase could promote progression through the restriction point, increasing the number of cells in S phase and, thus, the number of cells with transcriptionally active PR.
Most functional studies of nuclear receptors and other transcription factors measure the average activity in cells distributed throughout the cell cycle. Studies of nuclear receptors at the single-cell level that utilize techniques such as confocal microscopy have revealed heterogeneity of responses that has been attributed to a variety of factors, including overexpression (39). Our studies highlight the significant differences that occur as a function of cell cycle and suggest that an analysis of receptor behavior as a function of cell cycle distribution would resolve some of these discrepancies.
Acknowledgments
We thank Kurt Christensen and Lori Sherman and the University of Colorado Cancer Center Tissue Culture Core for production of PR Ser162 monoclonal antibody. We thank the Flow Cytometry Core, Baylor College of Medicine, for assistance with the FACS analyses.
This work was supported by Public Health Service grant R01 CA57539 (N.L.W. and D.P.E.) from the National Cancer Institute and Public Health Service training grant T32 HD07165 (R.N.).
REFERENCES
- 1.Abel, G. A., G. M. Wochnik, J. Rüegg, A. Rouyer, F. Holsboer, and T. Rein. 2002. Activity of the GR in G2 and mitosis. Mol. Endocrinol. 16:1352-1366. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Si-Ali, S., S. Ramirez, F.-X. Barre, F. Dkhissi, L. Magnaghi-Jaulin, J. A. Girault, P. Robin, M. Knibiehler, L. L. Pritchard, B. Ducommun, D. Trouche, and A. Harel-Bellan. 1998. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature 396:184-186. [DOI] [PubMed] [Google Scholar]
- 3.Apostolakis, E. M., R. Lanz, and B. W. O'Malley. 30. October 2003, posting date. Pituitary adenylate cyclase activating peptide (PACAP): a pivotal modulator of steroid-induced reproductive behavior in female rodents. Mol. Endocrinol. 18:173-183. [Online.] [DOI] [PubMed] [Google Scholar]
- 4.Bodwell, J. E., J. C. Webster, C. M. Jewell, J. A. Cidlowski, J. M. Hu, and A. Munck. 1998. Glucocorticoid receptor phosphorylation: overview, function and cell cycle-dependence. J. Steroid Biochem. Mol. Biol. 65:91-99. [DOI] [PubMed] [Google Scholar]
- 5.Boonyaratanakornkit, V., M. P. Scott, V. Ribon, L. Sherman, S. M. Anderson, J. L. Maller, W. T. Miller, and D. P. Edwards. 2001. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol. Cell 8:269-280. [DOI] [PubMed] [Google Scholar]
- 6.Carson-Jurica, M. A., W. T. Schrader, and B. W. O'Malley. 1990. Steroid receptor family: structure and functions. Endocr. Rev. 11:201-220. [DOI] [PubMed] [Google Scholar]
- 7.Cato, A. C. B., D. Henderson, and H. Ponta. 1987. The hormone response element of the mouse mammary tumour virus DNA mediates the progestin and androgen induction of transcription in the proviral long terminal repeat region. EMBO J. 6:363-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou, C. F., and M. B. Omary. 1994. Mitotic arrest with anti-microtubule agents or akadaic acid is associated with increased glycoprotein terminal GIcNAc's. J. Cell Sci. 107:1833-1843. [DOI] [PubMed] [Google Scholar]
- 9.Clemm, D. L., L. Sherman, V. Boonyaratanakornkit, W. T. Schrader, N. L. Weigel, and D. P. Edwards. 2000. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site specific monoclonal antibody. Mol. Endocrinol. 14:52-65. [DOI] [PubMed] [Google Scholar]
- 10.Conneely, O. M., B. Mulac-Jericevic, J. P. Lydon, and F. J. DeMayo. 2001. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol. Cell. Endocrinol. 179:97-103. [DOI] [PubMed] [Google Scholar]
- 11.Cram, E. J., B. D. Liu, L. F. Bjeldanes, and G. L. Firestone. 2001. Indole-3-carbinol inhibits CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter. J. Biol. Chem. 276:22332-22340. [DOI] [PubMed] [Google Scholar]
- 12.Gioeli, D., S. B. Ficarro, J. J. Kwiek, D. Aaronson, M. Hancock, A. D. Catling, F. M. White, R. E. Christian, R. E. Settlage, J. Shabanowitz, D. F. Hunt, and M. J. Weber. 2002. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J. Biol. Chem. 277:29304-29314. [DOI] [PubMed] [Google Scholar]
- 13.Hissom, J. R., and M. R. Moore. 1987. Progestin effects on growth in the human breast cancer cell line T-47D—possible therapeutic implications. Biochem. Biophys. Res. Commun. 145:706-711. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz, K. B., M. B. Mockus, and B. A. Lessey. 1982. Variant T47D human breast cancer cells with high progesterone receptor levels despite estrogen and antiestrogen resistance. Cell 28:633-642. [DOI] [PubMed] [Google Scholar]
- 15.Hu, J. M., J. E. Bodwell, and A. Munck. 1994. Cell cycle-dependent glucocorticoid receptor phosphorylation and activity. Mol. Endocrinol. 8:1709-1713. [DOI] [PubMed] [Google Scholar]
- 16.Karin, M., A. Haslinger, H. Holtgreve, R. I. Richards, P. Krauter, H. M. Westphal, and M. Beato. 1984. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature 308:513-519. [DOI] [PubMed] [Google Scholar]
- 17.Kastner, P., A. Krust, B. Turcotte, U. Strupp, L. Tora, H. Gronemeyer, and P. Chambon. 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 9:1603-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knotts, T. A., R. S. Orkiszewski, R. G. Cook, D. P. Edwards, and N. L. Weigel. 2001. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J. Biol. Chem. 276:8475-8483. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen, K. E., W. K. Cavenee, and K. C. Arden. 1999. D-type cyclins complex with the androgen receptor and inhibit its transcriptional transactivation ability. Cancer Res. 59:2297-2301. [PubMed] [Google Scholar]
- 20.Lambert, J. R., and S. K. Nordeen. 2001. Analysis of steroid hormone-induced histone acetylation by chromatin immunoprecipitation assay. Methods Mol. Biol. 176:273-281. [DOI] [PubMed] [Google Scholar]
- 21.Lange, C. A. 16. October 2003, posting date. Making sense of cross-talk between steroid hormone receptor and intracellular signaling pathways: who will have the last word? Mol. Endocrinol. 18:269-278. [Online.]. [DOI] [PubMed] [Google Scholar]
- 22.Lange, C. A., T. Shen, and K. B. Horwitz. 2000. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc. Natl. Acad. Sci. USA 97:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeGoff, P., M. M. Montano, D. J. Schodin, and B. S. Katzenellenbogen. 1994. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J. Biol. Chem. 269:4458-4466. [PubMed] [Google Scholar]
- 24.Li, X., J. Wong, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 2003. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol. Cell. Biol. 23:3763-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim, C. S., C. T. Baumann, H. Htun, W. Xian, M. Irie, C. L. Smith, and G. L. Hager. 1999. Differential localization and activity of the A- and B-forms of the human progesterone receptor using green fluorescent protein chimeras. Mol. Endocrinol. 13:366-375. [DOI] [PubMed] [Google Scholar]
- 26.Martinez, E. D., and M. Danielsen. 2002. Loss of androgen receptor transcriptional activity at the G(1)/S transition. J. Biol. Chem. 277:29719-29729. [DOI] [PubMed] [Google Scholar]
- 27.Maurer-Schultze, B., M. Siebert, and I. D. Bassukas. 1988. An in vivo study on the synchronizing effect of hydroxyurea. Exp. Cell Res. 174:230-243. [DOI] [PubMed] [Google Scholar]
- 28.Mulac-Jericevic, B., J. P. Lydon, F. J. DeMayo, and O. M. Conneely. 2003. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. USA 100:9744-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musgrove, E. A., C. S. L. Lee, and R. L. Sutherland. 1991. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor α, epidermal growth factor receptor, c-fos, and c-myc genes. Mol. Cell. Biol. 11:5032-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayanan, R., A. A. Adigun, D. P. Edwards, and N. L. Weigel. 2004. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol. Cell. Biol. 25:264-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narayanan, R., V. A. Tovar Sepulveda, M. Falzon, and N. L. Weigel. 2004. The functional consequences of cross talk between the vitamin D receptor and ERK signaling pathways are cell specific. J. Biol. Chem. 279:47298-47310. [DOI] [PubMed] [Google Scholar]
- 32.Neuman, E., M. H. Ladha, N. Lin, T. M. Upton, S. J. Miller, J. DiRenzo, R. G. Pestell, P. W. Hinds, S. F. Dowdy, M. Brown, and M. E. Ewen. 1997. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol. Cell. Biol. 17:5338-5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olea-Serrano, N., N. Deuleeschouwer, G. Leclercq, and J. C. Henson. 1985. Assay for estrogen and progesterone receptors of breast cancer cell lines in monolayer culture. Eur. J. Cancer Clin. Oncol. 21:965-973. [DOI] [PubMed] [Google Scholar]
- 34.Qiu, M., A. Olsen, E. Faivre, K. B. Horwitz, and C. A. Lange. 2003. Mitogen activated protein kinase regulates nuclear association of human progesterone receptors. Mol. Endocrinol. 17:628-642. [DOI] [PubMed] [Google Scholar]
- 35.Richer, J. K., B. M. Jacobsen, N. G. Manning, M. G. Abel, D. M. Wolf, and K. B. Horwitz. 2002. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 277:5209-5218. [DOI] [PubMed] [Google Scholar]
- 36.Shen, T., K. B. Horwitz, and C. A. Lange. 2001. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol. Cell. Biol. 21:6122-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slater, E. P., A. C. B. Cato, M. Karin, J. D. Baxter, and M. Beato. 1988. Progesterone induction of metallothionein-IIA gene expression. Mol. Endocrinol. 2:485-491. [DOI] [PubMed] [Google Scholar]
- 38.Stenoien, D. L., M. G. Mancini, K. Patel, E. A. Allegretto, C. L. Smith, and M. A. Mancini. 2000. Subnuclear trafficking of estrogen receptor-α and steroid receptor coactivator-1. Mol. Endocrinol. 14:518-534. [DOI] [PubMed] [Google Scholar]
- 39.Stenoien, D. L., K. Patel, M. G. Mancini, M. Dutertre, C. L. Smith, B. W. O'Malley, and M. Mancini. 2001. FRAP reveals that mobility of oestrogen receptor-α is ligand- and proteasome-dependent. Nat. Cell Biol. 3:15-23. [DOI] [PubMed] [Google Scholar]
- 40.Tsai, M.-J., and B. W. O'Malley. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63:451-486. [DOI] [PubMed] [Google Scholar]
- 41.Weigel, N. L., C. A. Beck, P. A. Estes, P. Prendergast, M. Altmann, K. Christensen, and D. P. Edwards. 1992. Ligands induce conformational changes in the carboxyl-terminus of progesterone receptors which are detected by a site-directed antipeptide monoclonal antibody. Mol. Endocrinol. 6:1585-1597. [DOI] [PubMed] [Google Scholar]
- 42.Xu, Y., L. Klein-Hitpass, and M. K. Bagchi. 2000. E1A-mediated repression of progesterone receptor-dependent transactivation involves inhibition of the assembly of a multisubunit coactivation complex. Mol. Cell. Biol. 20:2138-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto, A., Y. Hashimoto, K. Kohri, E. Ogata, S. Kato, K. Ikeda, and M. Nakanishi. 2000. Cyclin E as a coactivator of the androgen receptor. J. Cell Biol. 150:873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, Y., W. Bai, V. E. Allgood, and N. L. Weigel. 1994. Multiple signaling pathways activate the chicken progesterone receptor. Mol. Endocrinol. 8:577-584. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, Y., C. A. Beck, A. Poletti, D. P. Edwards, and N. L. Weigel. 1995. Identification of a group of Ser-Pro motif hormone-inducible phosphorylation sites in the human progesterone receptor. Mol. Endocrinol. 9:1029-1040. [DOI] [PubMed] [Google Scholar]