Abstract
We aimed to evaluate the blood lactate level in response to two bouts of exercise. First, we hypothesized that blood lactate elevation in response to moderate-intensity aerobic exercise (MIAE) would be lower at the end of the second bout of MIAE than the first bout of MIAE. In this context, we also hypothesized that lactate accumulation at the end of resistance exercise (RE) would be reduced if MIAE is performed before RE (i.e., concurrent exercise; CE). If so, we hypothesized that the order of the CE (i.e., RE + MIAE vs. MIAE + RE) influences blood lactate kinetics. To test the hypotheses, forty-three healthy men participated in three studies. In study 1, 20 men (age 21 ± 2 years) performed two bouts of a 20-min MIAE separated by a 20-min rest interval. In study 2, 11 men (age 22 ± 1 years) performed RE only and CE (MIAE + RE; ARCE) with a 20-min rest interval in a crossover design. In study 3, 12 men (age 21 ± 2 years) performed both CEs, which were ARCE and RE + MIAE (RACE), with a 20-min rest interval in a crossover design. We measured blood lactate before and at the end of each exercise session. In study 1, the blood lactate response to the second bout of MIAE was lower than that of the first bout (P < 0.001, r = 0.68). However, the blood lactate response to the ARCE trial was not lower than the response to the RE trial in study 2 (P = 0.475, r = 0.22). The results of study 3 showed that the RACE and ARCE trials induced a similar lactate response (MIAE P = 0.423, r = 0.28; RE P = 0.766, d = 0.03). These observations indicate that whereas lactate accumulation might be diminished by a second bout of MIAE, a different type of exercise (i.e., aerobic/resistance) did not result in a diminished lactate accumulation in response to a second bout of exercise.
Subject terms: Metabolism, Biomarkers
Introduction
Many studies have demonstrated the health benefits of physical activity and strategic ‘exercise’1–5, and these include preventing muscle atrophy2, weight gain3, and cognitive decline4. Among these benefits, it is widely known that resistance exercise (RE) can effectively induce muscle hypertrophy, while cardiorespiratory aerobic exercise (AE) can reduce body fat due to an increase in energy expenditure2. Given these different merits of RE and AE, it seems reasonable that the combination of RE and AE (called concurrent exercise; CE6) is a beneficial strategy for maintaining and improving health2. Notably, in terms of brain health, both RE (e.g., knee extensions) and AE (e.g., cycling exercise) improve cognitive function7, 8, while a meta-analysis by Colcombe and Kramer9 reported that CE has a greater impact on cognitive improvement than AE alone.
One potential physiological mechanism for the positive impacts of exercise is the bioavailability of lactate, which is produced through anaerobic glycolysis from glucose/glycogen5, 10–14. Lactate serves as not only an energy substrate but also as a myokine at rest and an exerkine during exercise15, 16. During exercise, lactate is primarily secreted by white-glycolytic muscle fibers in an exercise intensity-dependent manner and is distributed to ‘consumers’, such as the brain, heart, and liver, in addition to red-oxidative muscle fibers5, 13–17. When the muscle glycogen content is progressively decreased during prolonged AE, the ability to accumulate lactate may be diminished (i.e., a low blood lactate concentration)18. In addition, our previous studies revealed that blood lactate is lower in response to a second bout of high-intensity interval AE (HIIE) than in response to the first bout of HIIE even though two identical exercise sessions were performed (i.e., the same intensity and duration)13, 19. Potentially, the first bout of HIIE may approach muscle glycogen depletion, thereby creating a small elevation in blood lactate during repeated exercise. For example, during a soccer game, blood lactate elevation is diminished during the second half compared with the first half and is likely due to a decrease in muscle glycogen20. Given the dose-dependent effect of lactate on some health factors11, 21, 22, the positive impact of the second bout of HIIE on health can be lower than for the first bout of HIIE13, 19.
In general, the recommended amount of moderate-intensity physical activity to maintain and improve health is above 150 min/wk, implying that MIAE for at least 30 min/day (5 days/wk) is needed2. Moreover, a recommendation from the American College of Sports Medicine and the American Heart Association suggests that the benefits of MIAE can be accumulated through multiple bouts of short-duration exercise1, 2, indicating that both one bout of AE (i.e., continuous AE) and multiple bouts of AE (i.e., repeated AE) are suitable strategies for promoting health2. However, little is known about the blood lactate response to a second bout of MIAE (i.e., around the lactate threshold). Similar to HIIE session (i.e., above lactate threshold)13, 19, we hypothesized that the second bout of MIAE would result in reduced blood lactate elevation compared with the first bout. In this context, if the first bout of MIAE affects the blood lactate response to the second bout of exercise, we also hypothesized that blood lactate elevation in response to the RE trial would be reduced when the MIAE is performed before the RE. If so, it is possible the CE order influences blood lactate kinetics; namely, lactate accumulation in response to the CE program as MIAE + RE (ARCE) would be lower than that of RE + MIAE (RACE). To advance our understanding of the bioavailability of lactate, we designed three studies that aimed to examine the blood lactate response to the second bout of exercise in two bouts of AE and CE programs. These findings would indicate a new idea to induce further lactate-enhancing accumulation in response to multiple bouts of exercise, which may contribute to health benefits.
Results
Study 1: The first bout versus the second bout of moderate-intensity aerobic exercise
The baseline heart rate (HR), blood glucose, and blood lactate were 67 ± 4 bpm, 97.7 ± 7.7 mg/dL, and 1.0 ± 0.1 mM, respectively.
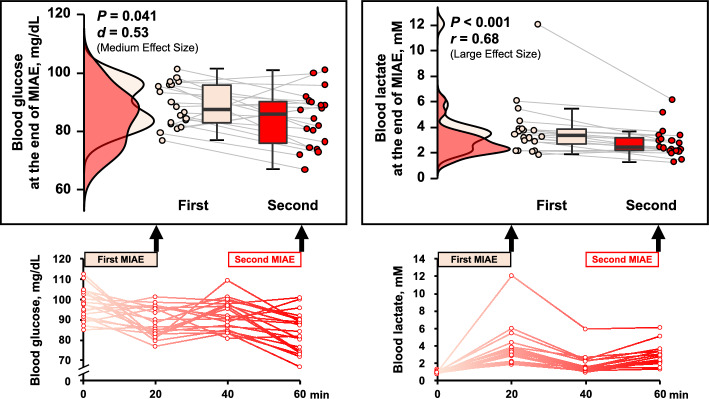
The HR (P < 0.01, d = 0.46) and rating of perceived exertion (RPE) for leg effort (P < 0.05, r > 0.5) in response to the second bout of MIAE were higher than those in response to the first bout of MIAE (Table 1). Compared with the first bout, lower blood glucose (P < 0.05, d = 0.53) and lactate (P < 0.001, r > 0.5) levels were observed at the end of the second bout of MIAE (Fig. 1).
Table 1.
The heart rate (HR) and a rating of perceived exertion (RPE) in response to moderate-intensity aerobic exercise (MIAE) in study 1.
| First MIAE | Repeated MIAE | P-value | Effect size | |
|---|---|---|---|---|
| HR, bpm | 153 ± 12 | 158 ± 10 | 0.009 | d = 0.46 |
| RPE, 6–20 scale | 13.5 (13–15) | 15 (13–15) | 0.068 | r = 0.41 |
| RPE, CR10 scale | 4.5 (4–6) | 6 (4–7) | 0.012 | r = 0.56 |
Values are mean ± SD or median (IQR).
Significant values are in bold.
Figure 1.
Blood glucose and lactate at the end of the first and second bouts of moderate-intensity aerobic exercise (MIAE) in study 1. The raincloud plots show the distribution of both glucose and lactate, circle plots represent individual data, and the box-and-whisker plots are median values (IQR and max/min).
Study 2: Resistance exercise versus moderate-intensity aerobic exercise + resistance exercise
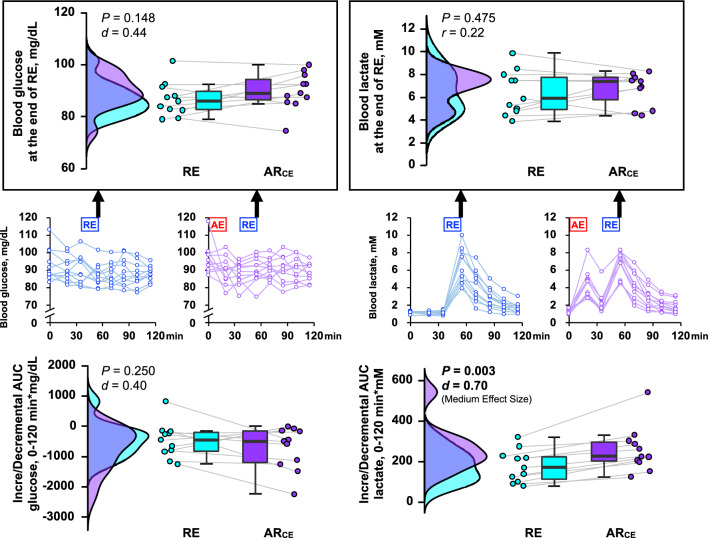
The baseline HR (RE 64 ± 7 bpm vs. ARCE 67 ± 8 bpm, P = 0.058, d = 0.51), blood glucose (RE 88.5 (87–101) md/dL vs. ARCE 95.5 (90–99.5) mg/dL, P = 0.059, r = 0.57), and blood lactate (RE 1.2 ± 0.1 mM vs. ARCE 1.2 ± 0.3 mM, P = 0.334, d = 0.36) were similar between the RE and ARCE trials.
Whereas the HR in response to RE in the ARCE trial was higher than that in the RE trial (P < 0.05, d = 0.55), a similar RPE was observed in both trials (Table 2). Both glucose (P = 0.148, d = 0.44) and lactate (P = 0.475, r = 0.22) responses to RE were not different between the RE and ARCE trials (Fig. 2). Additionally, the glucose area under the curve (AUC) throughout the RE trial was identical to that of the ARCE trial (P = 0.250, d = 0.40). In contrast, the lactate AUC throughout the experiment was larger in the ARCE trial than in the RE trial (P < 0.01, d = 0.70).
Table 2.
The heart rate (HR) and a rating of perceived exertion (RPE) in response to the resistance exercise (RE) in study 2.
| RE | ARCE | P-value | Effect size | |
|---|---|---|---|---|
| HR, bpm | 121 ± 16 | 130 ± 15 | 0.020 | d = 0.55 |
| RPE, 6–20 scale | 17 (15–19) | 17 (14–18) | 0.564 | r = 0.17 |
| RPE, CR10 scale | 9 (7–10) | 9 (7–10) | 1.000 | r = 0.00 |
Values are mean ± SD or median (IQR). ARCE, concurrent exercise (MIAE + RE).
Significant values are in bold.
Figure 2.
Blood glucose and lactate responses in study 2. The upper illustrations in the black boxes represent the data at the end of the resistance exercise (RE), while the lower illustrations represent the area under the curve (AUC) throughout the experiment. The raincloud plots show the distribution of both glucose and lactate. The circle plots represent individual data, and the box-and-whisker plots are median values (IQR and max/min). AE; aerobic exercise, ARCE; concurrent exercise (MIAE + RE).
Study 3: Moderate-intensity aerobic exercise + resistance exercise versus resistance exercise + moderate-intensity aerobic exercise
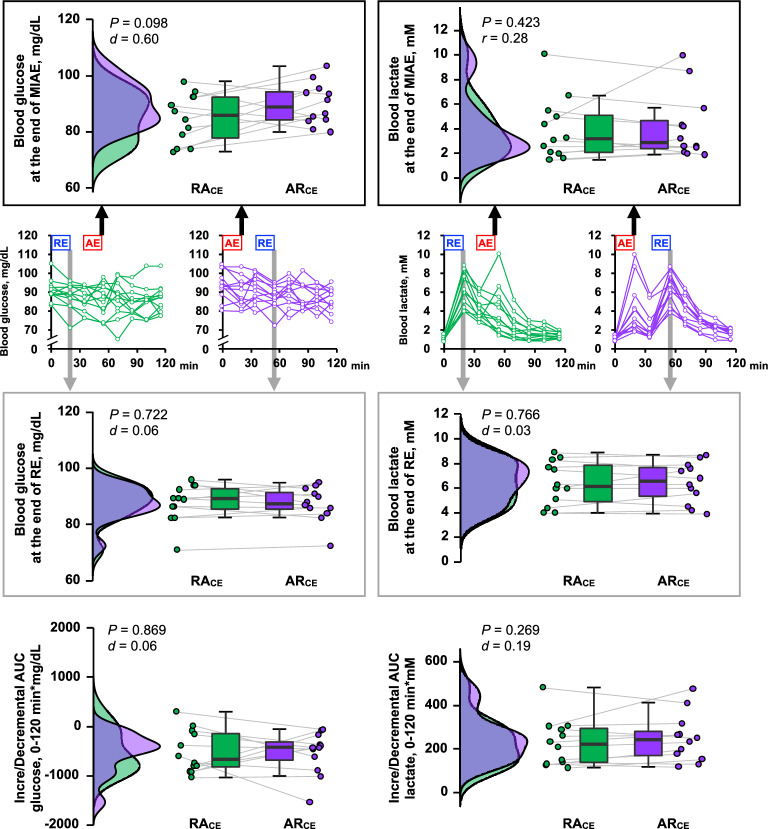
The baseline HR (RACE 67 ± 6 bpm vs. ARCE 69 ± 5 bpm, P = 0.175, d = 0.42), blood glucose (RACE 91 ± 6 mg/dL vs. ARCE 94 ± 8 mg/dL, P = 0.087, d = 0.44), and blood lactate (RACE 1.1 (1.0–1.5) mM vs. ARCE 1.2 (1.1–1.4) mM, P = 0.326, r = 0.27) measurements were similar between the RACE and ARCE trials.
In the same way as in study 2, the RE-enhanced HR was higher in the ARCE trial than in the RACE trial (P < 0.01, d = 0.54). Meanwhile, the HR response to MIAE was not different between the RACE and ARCE trials (P = 0.455, d = 0.18). In terms of RPE, there were no differences between the RACE and ARCE trials (Table 3). Additionally, blood glucose (e.g., AUC RACE − 507 ± 436 min*mg/dL vs. ARCE − 533 ± 429 min*mg/dL, P = 0.869, d = 0.06) and lactate (e.g., AUC RACE 230 ± 108 min*mM vs. ARCE 250 ± 109 min*mM, P = 0.269, d = 0.19) levels were not affected by the order of CE (Fig. 3).
Table 3.
The heart rate (HR) and a rating of perceived exertion (RPE) in responses to moderate-intensity aerobic exercise (MIAE) and resistance exercise (RE) in study 3.
| RACE | ARCE | P-value | Effect size | |
|---|---|---|---|---|
| HR, bpm | ||||
| MIAE | 161 ± 11 | 159 ± 16 | 0.455 | d = 0.18 |
| RE | 127 ± 17 | 136 ± 15 | 0.007 | d = 0.54 |
| RPE, 6–20 scale | ||||
| MIAE | 15 (14–17) | 15 (14–16) | 0.470 | r = 0.21 |
| RE | 15 (15–17) | 16.5 (13–17) | 0.784 | r = 0.08 |
| RPE, CR10 scale | ||||
| MIAE | 6 (4–8) | 5 (4–6) | 0.188 | r = 0.38 |
| RE | 7.5 (6–8) | 7.5 (6–9) | 0.595 | r = 0.15 |
Values are mean ± SD or median (IQR). RACE, concurrent exercise (RE + MIAE); ARCE, concurrent exercise (MIAE + RE).
Significant values are in bold.
Figure 3.
Blood glucose and lactate responses in study 3. The upper illustrations in the black boxes represent the data at the end of the MIAE, while the middle illustrations in gray boxes represent the data at the end of the RE. The lower illustrations represent the AUC throughout the experiment. The raincloud plots show the distribution of both glucose and lactate. The circle plots represent individual data, and the box-and-whisker plots are median values (IQR and max/min).
Discussion
In study 1, we examined the impacts of two bouts of MIAE on blood lactate. Compared with the first bout, the blood lactate response was reduced during the second bout of MIAE. Thus, consistent with our hypothesis, lactate accumulation was reduced in the second bout of AE even at a moderate intensity. Nevertheless, contrary to our hypothesis, blood lactate elevation was not reduced during RE when RE was performed after MIAE as the CE protocol. Similarly, blood lactate elevation during MIAE was not influenced when MIAE was performed after RE. These observations highlighted that the order of the CE trial is not an important consideration for the bioavailability of lactate.
The impact of the second bout of moderate-intensity aerobic exercise on blood lactate elevation
In the present study, blood glucose was reduced during the second bout of MIAE, implying that gluconeogenesis may not be markedly increased in the second bout of MIAE. Febbraio and Dancey18 demonstrated that the muscle glycogen level is associated with MIAE-induced lactate accumulation. In this context, blood lactate is increased during MIAE for 20 min, whereas blood lactate levels can disappear during MIAE at 40 min with muscle glycogen depletion, indicating that the duration of MIAE is a key factor in lactate accumulation18. We previously reported similar blood lactate responses to one 40-min bout of MIAE (i.e., continuous MIAE) and two 20-min bouts of MIAE (i.e., repeated MIAE)23. Given the results of study 1, the duration of the total exercise period, rather than each bout, may determine blood lactate kinetics when MIAE is separately performed. In other words, AE-induced lactate elevation can be an inaccurate marker of exercise intensity.
During exercise, lactate is distributed as an energy substrate to the brain, heart, and red-oxidative muscle fibers, etc.5, 13–17. For instance, Rasmussen et al.24 suggested that lactate metabolism in the human brain during exercise is associated with blood lactate levels and accelerated by blood lactate levels ≥ 2 mM. In the present study 1, the blood lactate level at the end of the first bout of MIAE was 3.4 (2.4–3.9) mM [median (IQR)], and at the end of the second bout of MIAE, it was 2.4 (2.2–3.3) mM (see Fig. 1). In addition to being an energy substrate, in vivo and in vitro studies have shown the positive impact of lactate stimulation on mitochondrial biogenesis in skeletal muscle cells11, adipocytes10, and the brain25. Moreover, the level of blood lactate elevation may play an important role in signaling muscle-brain crosstalk for health5, 14, 15. For instance, peroxisome proliferator activated-receptor γ coactivator-1α (PGC-1α) mRNA in skeletal muscle cells is increased by lactate administration in a dose-dependent manner11. PGC-1α is not only a transcriptional coactivator of mitochondrial biogenesis but also leads to increased fibronectin type III domain-containing 5 (FNDC5) expression26. FNDC5, as irisin, is released into the blood, and circulating irisin can contribute to an increase in brain-derived neurotrophic factor (BDNF)27, 28, which is capable of inducing neurogenesis in the brain and is associated with cognitive function28–30. Indeed, blood lactate elevation in response to AE can be correlated with exercise-enhanced circulating BDNF21, 22. Schiffer et al.31 demonstrated that circulating BDNF is increased immediately after lactate infusion in humans.
The impact of concurrent exercise on blood lactate elevation
Inconsistent with our hypothesis based on study 1, in studies 2 and 3, RE-enhanced lactate accumulation was not reduced in the second bout of exercise in the ARCE protocol even though MIAE was performed as the first bout of exercise. In studies 2 and 3, the participants performed cycling exercise as the AE and knee extensions as the RE. Previous studies have indicated that both cycling and knee extensions use mainly lower-limb muscles, such as the vastus lateralis (VL) and rectus femoris7, 32, suggesting similar muscle activation. However, based on the degree of lactate elevation, it is assumed that the activation of white-glycolytic muscle fiber (i.e., greater motor-unit recruitment) is more necessary during RE than MIAE. MIAE may not reduce the glycogen in the white-glycolytic fibers, which are used during RE for tetanic contraction. Therefore, the decreased muscle glycogen after MIAE potentially did not affect lactate accumulation in response to RE. Meanwhile, it remains unknown whether high-intensity AE + RE reduces the blood lactate response to RE. Notably, the AUC for lactate was higher in the ARCE trial (i.e., two bouts of exercise) than in the RE trial (i.e., one bout of exercise). From the perspective of lactate bioavailability, CE is a more effective strategy than RE alone.
Creer et al.33 demonstrated that glycogen content in human VL muscle is decreased by knee extensions consisting of three sets of 10 repetitions at 70% 1-RM with 2-min recovery intervals. Based on this previous finding, it is assumed that RE in the present study reduced muscle glycogen content. Nevertheless, the RACE trial did not influence the lactate response to MIAE as the second bout of exercise in study 3. Given that the AUC did not differ between the RACE and ARCE trials, the order of the CE trial is not an important consideration for the bioavailability of lactate. In contrast to two bouts of MIAE (i.e., the same exercise modality), blood lactate levels in the second bout of exercise may not be affected when performing a different type of exercise (i.e., RE and MIAE).
Perspective
It is well known that ‘exercise is medicine’. In particular, a CE program may offer easy access to health benefits. The results of the present study suggest that the negative impact of the second bout of exercise on lactate accumulation may not occur when a CE program focusing on the lower-limb muscles is performed, regardless of the exercise order. Given that lactate is one of the indicators of the positive impact of exercise on health5, 10–14, these observations may partly explain why the CE program is an easy way to improve health.
In contrast to the CE program, the second bout of AE had a reduced blood lactate response even at a moderate intensity, which is the most common AE prescription for health. Lecoultre et al.34 demonstrated that fructose and glucose coingestion enhances blood lactate elevation during MIAE; in other words, it is possible that energy intake can manipulate lactate kinetics in response to MIAE. To make efficient use of intervals in multiple bouts of the MIAE protocol, a nutritional strategy before performing MIAE may compensate for the lack of lactate accumulation in the multiple bouts of MIAE. Taken together, two bouts of MIAE without energy intake in the interval reduced blood lactate elevation during the second bout of MIAE, which may dampen the positive impact of exercise on some health factors.
Conclusions
The blood lactate response to a second bout of MIAE was reduced. On the other hand, the blood lactate response to RE was not affected when MIAE was performed before RE, and the blood lactate elevation during the RACE trial was also at a similar level as that in the ARCE trial. Thus, blood lactate kinetics are not altered by the combined performance of the different types of exercise focusing on the lower-limb muscles (i.e., knee extensions as RE and cycling exercise as AE).
Methods
Ethics and participants
All procedures conformed to the Declaration of Helsinki and were approved by the Ethics Committee of Ritsumeikan University (BKC-2017-078). Forty-three healthy men participated in the studies after providing written informed consent. All participants were free of neurologic, cardiovascular, or pulmonary disorders, did not take any medication and were nonsmokers. Participants were instructed to avoid strenuous physical activity in the 24 h preceding each experiment visit. Each participant was also asked to abstain from food, alcohol, and caffeine intake for 12 h before each experiment. Experiments were performed at 22–24 °C. Compared with the volume-matched 40-min MIAE, data from study 1 on HR, blood pressure, blood metabolites, cognitive function, and psychological parameters, after only the second bout of the 20-min MIAE, are already published23.
Experimental procedure and trials
For studies 2 and 3, a one-repetition maximum (1-RM) was determined on the first visit to calculate the intensity of bilateral knee extensions at least 7 days before the third and fourth visits. The 1-RM trial was designed using increments of 10 kg until 60–80% of the perceived maximum was achieved. Subsequently, the load was incrementally increased by 1–5 kg until failure, which was indicated by the inability to maintain proper form or complete the repetition. The last acceptable lift with the highest possible load was defined as the 1-RM7.
Approximate peak oxygen consumption (VO2 peak) was determined to calculate the intensity of cycling the MIAE on the first visit for study 1 and the second visit for studies 2 and 3, at least 4 days before the next visit. The fitness test began at a power of 30 W for 3 min. Subsequently, the workload was increased by 30 W/min until the participants were not able to maintain a cadence of 60 rpm (task failure of a pedaling rate of at least 55 rpm over 5 s despite maximal effort). During the test, breath-by-breath pulmonary gas-exchange data were collected and averaged every 10 s (AE-310S; Minato Medical Science, Japan). Additionally, HR was checked continuously via telemetry (RS400; Polar Electro, Finland). The VO2 peak was determined as the highest 30-s value attained prior to exhaustion8, 19.
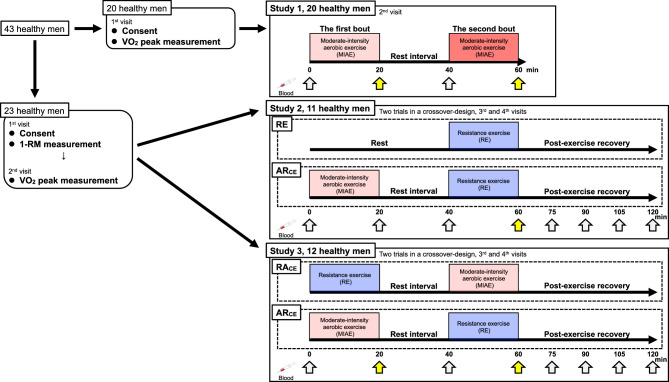
In study 1, twenty participants (age 21 ± 2 years, height 173 ± 3 cm, weight 64 ± 9 kg, and VO2 peak 45.9 ± 4.6 ml/kg/min; means ± SDs) performed two identical bouts of the MIAE trial23. In study 2, eleven participants (age 22 ± 1 years, height 170 ± 3 cm, weight 62 ± 8 kg, 1-RM 118 ± 21 kg, and VO2 peak 47.0 ± 4.3 ml/kg/min) performed two separate trials (RE and ARCE trials) on the third and fourth visits in a randomized, counterbalanced order. In study 3, twelve participants (age 21 ± 2 years, height 172 ± 4 cm, weight 62 ± 6 kg, 1-RM 121 ± 22 kg, and VO2 peak 46.9 ± 4.0 ml/kg/min) performed two separate trials (RACE and ARCE trials) on the third and fourth visits in a randomized, counterbalanced order. For studies 2 and 3, each experimental day was separated by at least 72 h.
In all trials (Fig. 4), every participant attended the laboratory at 0800–1100. Upon arrival, a nurse inserted an 18-gauge cannula in the cephalic vein of the nondominant arm for blood sampling. Afterward, all participants rested in a seated upright position for at least 10 min before data collection began. Blood was collected after measuring HR at baseline. Subsequently, the participants carried out each intervention.
Figure 4.
Schematic representation of the experimental design. The yellow arrows indicated the measurement point to compare lactate accumulation in response to each exercise protocol.
Study 1 The participants performed two bouts of MIAE. Before starting the second bout of MIAE, the participants took a 20-min rest interval. The HR, RPE, and blood were collected before and at the end of the first and second bouts.
Study 2 In the RE trial, the participants performed RE after a seated upright position for 40-min. In the ARCE trial, the participants performed MIAE and RE with 20 min intervals in a seated upright position. The HR, RPE, and blood were collected before and at the end of each MIAE and RE. To estimate the bioavailability of lactate, blood was collected 4 times at 15-min intervals following RE. At the same time point as the ARCE trial, blood in the RE trial was collected 3 times before the RE trial (i.e., in a seated upright position for 40 min).
Study 3 The ARCE trial was performed in the same way as study 2. In the RACE trial, the participants performed RE and MIAE with 20 min intervals in a seated upright position. The HR, RPE, and blood were collected at the same time point as in study 2.
Exercise protocols
The MIAE protocol consisted of cycling exercise at an estimated 60% VO2 peak (Study 1: 148 ± 16 watts; Study 2: 146 ± 21 watts; Study 3: 148 ± 20 watts) for 20 min8.
The RE protocol used bilateral knee extensions at 80% 1-RM (Study 2: 91 ± 13 kg; Study 3: 93 ± 16 kg) and was programmed for 6 sets with 10 repetitions (1-s concentric/1-s eccentric contraction) per set. The participants rested for a 3 min before starting each set of knee extensions5; therefore, the total RE protocol time was also 20 min.
Measurements
The HR was checked via telemetry during each trial (RS400; Polar Electro, Finland).
The psychological response to the MIAE/RE, RPEs for breathing and leg effort were evaluated using the Borg 6–20 scale and the Borg CR10 scale, respectively. The Borg 6–20 scale ranges from 6 (no exertion) to 20 (maximal exertion). The Borg CR10 scale ranges from 0 (nothing at all) to 10 (almost maximum)35.
Blood was collected into 1-ml syringes to determine blood glucose and lactate levels, which were measured using glucose (Medisafe FIT Blood Glucose Meter; Terumo, Japan) and lactate analyzers (Lactate Pro 2; Arkray, Japan), respectively.
Statistical analysis
In the figures, the individual, box-and-whisker, and raincloud plots were created using JASP software (version 0.16.4, University of Amsterdam, Netherlands)36. The other data are expressed as the means ± SDs if a normal data distribution was confirmed using the Shapiro‒Wilk test; if not, the data are expressed as medians (IQRs). According to our hypothesis, data at the end of the MIAE/RE were analyzed using a paired t test after normal data distribution was confirmed, whereas the Wilcoxon signed-rank test was used if normal data distribution was not confirmed. Similarly, the estimated AUC was also analyzed using a paired t test because all AUCs were normally distributed. Statistical significance was indicated by P < 0.05. For normal data distribution, Cohen’s d effect size using the means and pooled SD were calculated, along with the 95% confidence interval to determine the magnitude of differences. The strength of the effect size of Cohen’s d was interpreted as weak (0.20 ≤ d < 0.50), medium (0.50 ≤ d < 0.80), and large (0.80 ≤ d)37. For nonnormal data distribution, the effect size, as r, was estimated using the Z score for the Wilcoxon signed-rank test. The strength of the effect size of r was interpreted as weak (0.10 ≤ r < 0.30), medium (0.30 ≤ r < 0.50), and large (0.50 ≤ r)37. All statistical analyses were conducted using IBM SPSS software (version 27, Chicago, IL, United States).
Acknowledgements
The authors appreciate the time and effort expended by the volunteer subjects. We are also grateful to Nurse Kayoko Naito for the experimental support.
Author contributions
Conceived and designed research, H.T. and T.Suga; Performed experiments, H.T., T.Suga, K.D., T.Sugi, and K.T.; Analyzed data, H.T., T.Suga, and T.Sugi; Interpreted results of experiments, H.T. and T.H.; Prepared figures, H.T.; drafted manuscript, H.T.; Edited and revised manuscript, H.T., T.I., and T.H. All authors have approved the final version of the manuscript.
Funding
This research was funded by the Nakatomi Foundation (to H.T.), the Center of Innovation Program from the Japan Science and Technology Agency (JPMJCE1306 to T. Suga and T.I.), and the Japan Society for the Promotion of Science KAKENHI Grant Number 21K17695 to H.T.
Data availability
The data that support the findings of this study are available from the corresponding author, H.T., upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haskell WL, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 2.Garber CE, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for pre-scribing exercise. Med. Sci. Sport. Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 3.Jakicic JM, et al. Physical activity and the prevention of weight gain in adults: A systematic review. Med. Sci. Sport. Exerc. 2019;51:1262–1269. doi: 10.1249/MSS.0000000000001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson KI, et al. Physical activity, cognition, and brain outcomes: A review of the 2018 Physical activity guidelines. Med. Sci. Sport. Exerc. 2019;51:1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto T, Tsukamoto H, Ando S, Ogoh S. Effect of exercise on brain health: The potential role of lactate as a myokine. Metabolites. 2021;11:813. doi: 10.3390/metabo11120813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glowacki SP, et al. Effects of resistance, endurance, and concurrent exercise on training outcomes in men. Med. Sci. Sport. Exerc. 2004;36:2119–2127. doi: 10.1249/01.MSS.0000147629.74832.52. [DOI] [PubMed] [Google Scholar]
- 7.Tsukamoto H, et al. An acute bout of localized resistance exercise can rapidly improve inhibitory control. PLoS One. 2017;12:e0184075. doi: 10.1371/journal.pone.0184075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsukamoto H, et al. Impact of exercise intensity and duration on postexercise executive function. Med. Sci. Sport. Exerc. 2017;49:774–784. doi: 10.1249/MSS.0000000000001155. [DOI] [PubMed] [Google Scholar]
- 9.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, Sato K, Iemitsu M. Exercise-inducible factors to activate lipolysis in adipocytes. J. Appl. Physiol. 2013;115:260–267. doi: 10.1152/japplphysiol.00427.2013. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: Activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 12.Oishi Y, et al. Mixed lactate and caffeine compound increases satellite cell activity and anabolic signals for muscle hypertrophy. J. Appl. Physiol. 2015;118:742–749. doi: 10.1152/japplphysiol.00054.2014. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto T, et al. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. 2018;32:1417–1427. doi: 10.1096/fj.201700381RR. [DOI] [PubMed] [Google Scholar]
- 14.Brooks GA, et al. Lactate in contemporary biology: A phoenix risen. J. Physiol. 2022;600:1229–1251. doi: 10.1113/JP280955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks GA, et al. Lactate as a myokine and exerkine: Drivers and signals of physiology and metabolism. J. Appl. Physiol. 2023;134:529–548. doi: 10.1152/japplphysiol.00497.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. 2018;27:757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 2010;199:499–508. doi: 10.1111/j.1748-1716.2010.02122.x. [DOI] [PubMed] [Google Scholar]
- 18.Febbraio MA, Dancey J. Skeletal muscle energy metabolism during prolonged, fatiguing exercise. J. Appl. Physiol. 1999;87:2341–2347. doi: 10.1152/jappl.1999.87.6.2341. [DOI] [PubMed] [Google Scholar]
- 19.Tsukamoto H, et al. Repeated high-intensity interval exercise shortens the positive effect on executive function during post-exercise recovery in healthy young males. Physiol. Behav. 2016;160:26–34. doi: 10.1016/j.physbeh.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Krustrup P, et al. Muscle and blood metabolites during a soccer game: Implications for sprint performance. Med. Sci. Sport. Exerc. 2006;38:1165–1174. doi: 10.1249/01.mss.0000222845.89262.cd. [DOI] [PubMed] [Google Scholar]
- 21.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sport. Exerc. 2007;39:728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 22.Reycraft JT, et al. Exercise intensity and recovery on circulating brain-derived neurotrophic factor. Med. Sci. Sport. Exerc. 2020;52:1210–1217. doi: 10.1249/MSS.0000000000002242. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto T, et al. Effect of repeated bouts versus a single bout of moderate-intensity exercise on postexercise inhibitory control. Physiol. Rep. 2020;8:e14528. doi: 10.14814/phy2.14528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen P, Wyss MT, Lundby C. Cerebral glucose and lactate consumption during cerebral activation by physical activity in humans. FASEB J. 2011;25:2865–2873. doi: 10.1096/fj.11-183822. [DOI] [PubMed] [Google Scholar]
- 25.L E, Lu J, Selfridge JE, Burns JM, Swerdlow RH. Lactate administration reproduces specific brain and liver exercise-related changes. J. Neurochem. 2013;127:91–100. doi: 10.1111/jnc.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boström P, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wrann CD, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen BK. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019;15:383–392. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 29.Griffin ÉW, et al. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Shimada H, et al. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014;6:69. doi: 10.3389/fnagi.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiffer T, et al. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci. Lett. 2011;488:234–237. doi: 10.1016/j.neulet.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 32.Rouffet DM, Hautier CA. EMG normalization to study muscle activation in cycling. J. Electromyogr. Kinesiol. 2008;18:866–878. doi: 10.1016/j.jelekin.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Creer A, et al. Influence of muscle glycogen availability on ERK1/2 and Akt signaling after resistance exercise in human skeletal muscle. J. Appl. Physiol. 2005;99:950–956. doi: 10.1152/japplphysiol.00110.2005. [DOI] [PubMed] [Google Scholar]
- 34.Lecoultre V, et al. Fructose and glucose co-ingestion during prolonged exercise increases lactate and glucose fluxes and oxidation compared with an equimolar intake of glucose. Am. J. Clin. Nutr. 2010;92:1071–1079. doi: 10.3945/ajcn.2010.29566. [DOI] [PubMed] [Google Scholar]
- 35.Borg GA. Psychophysical bases of perceived exertion. Med. Sci. Sport. Exerc. 1982;14:377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Allen M, Poggiali D, Whitaker K, Marshall TR, Kievit RA. Raincloud plots: A multi-platform tool for robust data visualization. Wellcome Open Res. 2019;4:63. doi: 10.12688/wellcomeopenres.15191.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen J. A power primer. Psychol. Bull. 1992;112:115–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, H.T., upon reasonable request.