Abstract
The peroxisomal docking complex is a key component of the import machinery for matrix proteins. The core protein of this complex, Pex14, is thought to represent the initial docking site for the import receptors Pex5 and Pex7. Associated with this complex is a fraction of Pex13, another essential component of the import machinery. Here we demonstrate that Pex13 directly binds Pex14 not only via its SH3 domain but also via a novel intraperoxisomal site. Furthermore, we demonstrate that Pex5 also contributes to the association of Pex13 with Pex14. Peroxisome function was affected only mildly by mutations within the novel Pex14 interaction site of Pex13 or by the non-Pex13-interacting mutant Pex5W204A. However, when these constructs were tested in combination, PTS1-dependent import and growth on oleic acid were severely compromised. When the SH3 domain-mediated interaction of Pex13 with Pex14 was blocked on top of that, PTS2-dependent matrix protein import was completely compromised and Pex13 was no longer copurified with the docking complex. We conclude that the association of Pex13 with Pex14 is an essential step in peroxisomal protein import that is enabled by two direct interactions and by one that is mediated by Pex5, a result which indicates a novel, receptor-independent function of Pex5.
Peroxisomal matrix protein import occurs posttranslationally and is likely to involve the transfer of folded or even oligomerized proteins across the peroxisomal membrane. The vast majority of peroxisomal matrix proteins possess either of two evolutionarily conserved peroxisomal targeting signals, C-terminal PTS1 and N-terminal PTS2. These signals are recognized in the cytosol by specific import receptors: Pex5 for PTS1 proteins and the PTS2 receptor complex, consisting of Pex7 and species-specific auxiliary factors, for PTS2 proteins. The cargo-loaded receptors then are transported to the peroxisomal membrane, where protein translocation takes place. This process is conceptually divided into three stages: docking, translocation of matrix proteins, and recycling of the unloaded receptors. The key components of this import machinery, the so-called peroxins, were identified in recent years, and some light was recently shed on the organization of the peroxins into subcomplexes within the peroxisomal membrane (for reviews, see references 12, 20, and 40). However, unraveling the actual mechanism of protein translocation remains a major challenge.
This work is concerned with the association of Pex13 with the docking complex. The docking complex is involved in the binding of cargo-laden receptors on the surface of the peroxisomal membrane, which is likely to be accomplished by Pex14, the core component of the docking complex that binds both Pex5 and Pex7 (2, 6). In yeast cells, this core complex additionally contains Pex17, which is tightly associated with Pex14 (1, 29). Pex13, another essential component of the import machinery, is also thought to belong to the docking complex (13, 14, 21), since Pex13 binds both the PTS1 and the PTS2 receptors (19, 38, 45) and is able to interact directly with Pex14 (2, 3). However, purification of Pex13 from rat liver peroxisomes revealed that most of Pex13 is present as a large homooligomeric complex (41). Likewise, only a small fraction (5 to 10%) of Pex13 was found to be copurified with Pex14 from yeast and mammalian peroxisomal membranes (1, 41), indicating that Pex14 and Pex13 interact either weakly or temporarily with each other and probably constitute two distinct subcomplexes.
Interestingly, the PTS1 import receptor Pex5 has also been found in significant amounts in the docking complex. The membrane-bound portion of Pex5 is tightly associated with Pex14 and, at least in mammals, has been demonstrated to behave like an integral membrane protein (1, 10, 23). The respective PTS2 receptor, Pex7, has not been identified in the purified docking complex, suggesting that the amount of membrane-bound Pex5 exceeds the amount of Pex7 or that Pex5 has an additional function as an integral part of the docking complex. A small fraction of the ring finger complex, comprising the integral membrane proteins Pex2, Pex10, and Pex12, and the yeast-specific peroxin Pex8 have also been found in the Pex14 complex (1, 26, 41). The ring finger complex acts at a later stage of the protein import cascade and is thought to interact only transiently with the docking complex (1, 7, 36).
Here we analyzed the interactions of Pex13 with Pex14, the core component of the docking complex, in detail. In addition to the established Pex14 interaction with the SH3 domain of Pex13, a second direct interaction was found to occur between the two peroxins. Moreover, we found that Pex5 also contributes to the in vivo association of Pex13 with Pex14. We studied the impact of each interaction on the association of Pex13 with the Pex14-containing docking complex, on the import of proteins into the peroxisomal matrix, and on peroxisome function. Finally, we discuss our findings in terms of a requirement for a close spatial association of Pex13 and Pex14 to enable peroxisomal matrix protein import.
MATERIALS AND METHODS
Strains and media.
Escherichia coli strain DH5α was used for all plasmid amplifications and isolations. E. coli strain C41(DE3) (J. Walker, MRC, Cambridge, United Kingdom) was used for the heterologous expression of recombinant glutathione S-transferase (GST)-Pex14 (pGEX4T-2-PEX14) and His6-Pex14 (pQE31-PEX14). Yeast strains used included wild-type strain UTL-7A and its otherwise isogenic pex5Δ, pex13Δ, and pex5Δ pex13Δ derivatives. Genomic tagging of the PEX14 locus with protein A was carried out as described previously (1). Standard media for the cultivation of yeast and bacterial strains were prepared as described previously (16, 43). Oleic acid plates contained 0.17% yeast nitrogen base without amino acids and ammonium sulfate, 0.5% ammonium sulfate, amino acids as required, 0.1% yeast extract, 0.5% potassium phosphate buffer (pH 6), 0.1% oleic acid, 0.5% Tween 80, and 2% agar.
Plasmids and oligonucleotides.
The plasmids and oligonucleotides used are listed in Tables 1 and 2, respectively. To generate pGEX4T-2-PEX14, PEX14 was amplified from genomic DNA with primer pair RE705-RE706 and cloned as a BamHI-EcoRI fragment into pGEX4T-2 (Amersham Biosciences). Plasmid pKat129 was generated by cloning a PEX13 fragment containing positions 151 to 264 (PEX13151-264 fragment), amplified from genomic DNA with primer pair RE26-RE34 and cut with SmaI and SpeI, into appropriately cut pPC97. Point mutations in PEX13 were introduced by overlap extension PCR with the following primer pairs in combination with outer primer pair RE421-RE423 and pKat113 as template DNA: L236A (RE748-RE749), I237A (RE750-RE751), F240A (RE752-RE753), and F243A (RE754-RE755). To introduce the E320K (SH3) mutation, PEX13 was amplified by PCR with primer pair RE421-RE423 and pWG13/15 as template DNA. The quadruple L236A-I237A-F240A-F243A (loop) mutation was introduced by overlap extension PCR with primer pair RE822-RE823, outer primer pair RE421-RE423, and PEX13 F243A as template DNA. PEX13 with loop and SH3 mutations was similarly generated but with the PEX13 SH3 domain mutation as template DNA. Subsequently, the PEX13 open reading frame of pKat113 was replaced with the mutant PEX13 alleles as NotI-HindIII fragments (Table 1). These expression cassettes were also cloned as BamHI-HindIII fragments into YCplac111 (18). All PEX13 mutations were also introduced into the PEX13173-258 fragment by PCRs with primer pair RE560-RE564 and the respective mutant alleles of full-length PEX13 as templates. Plasmid pAS81 was constructed by lifting a BamHI-XhoI fragment comprising the PEX5 open reading frame harboring the W204A mutation plus its promoter region from plasmid pWib21 (unpublished data) and ligating the fragment to similarly cut vector pRS414. All mutations were verified by automated sequencing (MWG Biotech, Eberswalde, Germany).
TABLE 1.
Plasmids used in this study
| Plasmid | Descriptiona | Reference or source |
|---|---|---|
| pKat61 | PEX19 in pPC86/SalI-SacI | 42 |
| pPC86-PEX14 | PEX14 in pPC86 | 2 |
| pWG14/6 | PEX14 AXXA in pPC86 | 19 |
| pWG13/15 | PEX13 (1-386) E320K | 19 |
| pKat31 | PEX13 (1-151) in pPC97/SmaI-SpeI | 42 |
| pKat33 | PEX13 (1-264) in pPC97/SmaI-SpeI | 42 |
| pKat129 | PEX13 (151-264) in pPC97/SmaI-SpeI | This study |
| pKat145 | PEX13 (173-258) in pPC97/SalI-NotI | 42 |
| pKat42 | PEX13 (280-386) in pPC97/SmaI-SpeI | 42 |
| pAS40 | PEX13 (173-258) L236A in pPC97 | This study |
| pAS41 | PEX13 (173-258) I237A in pPC97 | This study |
| pAS42 | PEX13 (173-258) F240A in pPC97 | This study |
| pAS43 | PEX13 (173-258) F243A in pPC97 | This study |
| pAS82 | PEX13 (173-258) L236A-I237A-F240A-F243A (loop) in pPC97 | This study |
| pQE31-PEX14 | His6-PEX14 | 45 |
| pGEX4T-2-PEX14 | GST-PEX14 in pGEX4T-2/BamHI-EcoRI | This study |
| pAS61 | PEX13prom-PEX13 E320K (SH3) in YCplac111 | This study |
| pAS62 | PEX13prom-PEX13 in YCplac111 | This study |
| pAS75 | PEX13prom-PEX13 loop + SH3 in YCplac111 | This study |
| pAS76 | PEX13prom-PEX13 loop in YCplac111 | This study |
| pKat113 | PEX13prom-PEX13 in pRSTERM | 45 |
| pAS53 | PEX13prom-PEX13 SH3 in pRSTERM | This study |
| pAS71 | PEX13prom-PEX13 loop in pRSTERM | This study |
| pAS73 | PEX13prom-PEX13 loop + SH3 in pRSTERM | This study |
| pRSPMP27tag | PEX11prom-PEX11-HA in pRS315 | 27 |
| pAS81 | PEX5prom-PEX5 W204A in pRS414 | This study |
Numbers in parentheses indicate amino acids. prom, promoter.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′-3′) |
|---|---|
| RE26 | GTGAATTCGGATCCATATGTTAATAGAAAGTTTGATAGGC |
| RE34 | GCTCTAGAACTAGTGTTTAGTAGATATGGAAAACC |
| RE421 | GCATGCGGCGGCCGCTCATCCACAGCAGTACCA |
| RE423 | AAGCTTCTAGTGTGTACGCGTTTCAT |
| RE560 | GTCGACGATGACACACAACTCGTTTTTC |
| RE564 | GCGGCCGCCTATCTTGTGGCTTTCTCATTAGA |
| RE705 | GGATCCATGAGTGACGTGGTCAGT |
| RE706 | GAATTCCTATGGGATGGAGTCTTC |
| RE748 | GAATCTGAAGGAAGCAAAAATAAAGCAATTGAAGATTTTCAAAAG |
| RE749 | CTTTTGAAAATCTTCAATTGCTTTATTTTTGCTTCCTTCAGATTC |
| RE750 | GAATCTGAAGGAAGCAAAAATAAACTAGCTGAAGATTTTCAAAAG |
| RE751 | CTTTTGAAAATCTTCAGCTAGTTTATTTTTGCTTCCTTCAGATTC |
| RE752 | GGAAGCAAAAATAAACTAATTGAAGATGCTCAAAAGTTTAAT |
| RE753 | CATTAAACTTTTGAGCATCTTCAATTAGTTTATTTTTGCTTC |
| RE754 | GAAGATTTTCAAAAGGCTAATGATAGTGGTACCATAAATTC |
| RE755 | GAATTTATGGTACCACTATCATTAGCCTTTTGAAAATCTTC |
| RE822 | CTGAAGGAAGCAAAAATAAAGCAGCTGAAGATGCTCAAAAGGCTAATGATA |
| RE823 | CTATCATTAGCCTTTTGAGCATCTTCAGCTGCTTTATTTTTGCTTCCTTCA |
Yeast two-hybrid assays.
Various fusions of Pex13 fragments with the DNA-binding domain of Gal4 (Gal4-BD) were tested in combination with previously described plasmids expressing Gal4 activation domain (Gal4-AD) fusions of Pex14, Pex14AXXA (Pex14 mutated in its proline-rich motif), and Pex19 (Table 1) by cotransformation into yeast strain PJ69-4A. All constructs were derived from two-hybrid vectors pPC86 and pPC97 (8). Double transformants selected on synthetic dextrose medium without tryptophan and leucine were tested for histidine and adenine prototrophy by growth on selective plates lacking leucine, tryptophan, histidine, and adenine.
Peptide blot assays.
GST-Pex14 was expressed from plasmid pGEX4T-2-PEX14 in E. coli strain C41(DE3). The soluble fraction was incubated with a glutathione-Sepharose 4B matrix (Amersham Biosciences) at 4°C for 2 h. After the matrix was washed with phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4 · 7H2O, 1.4 mM KH2PO4), the bound protein was eluted with 10 mM glutathione in 50 mM Tris-HCl (pH 8). Eluted GST-Pex14 was purified to apparent homogeneity, as revealed by Coomassie brilliant blue staining (data not shown). The purified protein then was added to a peptide-containing cellulose membrane (see Fig. 1C) at a concentration of 10 μg/ml. As a control, 10 μg of GST (Sigma)/ml was added to a duplicate membrane. Bound protein was immunologically detected by using monoclonal anti-GST antibodies (34). Spot intensities were quantified with a LumiImager (Roche, Basel, Switzerland).
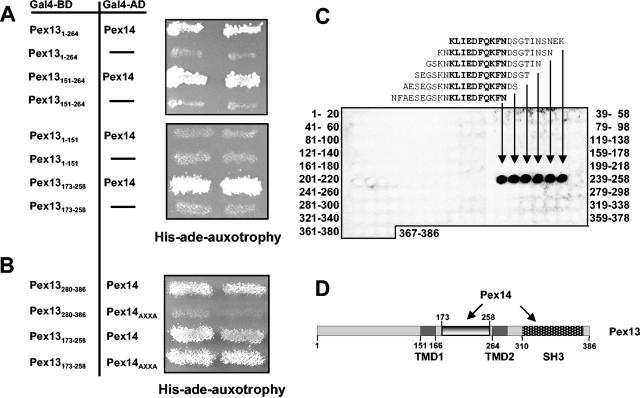
FIG. 1.
Identification of a novel Pex14-binding site in Pex13. (A) Interactions of Pex13 fragments with Pex14 in a yeast two-hybrid assay. Truncations of PEX13 were fused to the GAL4 DNA-binding domain (Gal4-BD) and coexpressed with a PEX14-GAL4 activation domain (Gal4-AD) fusion in yeast strain PJ69-4A. As control, the bare Gal4-AD was coexpressed with the Pex13-Gal4-BD fusion. Two independent transformants were tested for prototrophy on plates lacking both histidine and adenine. The following plasmids were used to express the indicated Pex13 fragments: Pex131-151 (pKat31), Pex131-264 (pKat33), Pex13151-264 (pKat129), and Pex13173-258 (pKat145). (B) Involvement of the proline-rich motif of Pex14 in binding to Pex13. The two Pex14-interacting fragments of Pex13, comprising the SH3 domain (Pex13280-386) and the novel binding site (Pex13173-258), were tested for interactions with Pex14 mutated in its proline-rich motif (Pex14AXXA; pWG14/6) as described for panel A. (C) In vitro binding of Pex14 to the novel binding site of Pex13. Synthetic 20-mer peptides with two-amino-acid shifts between neighboring peptides and representing full-length Pex13 were synthesized on cellulose membranes. The identities of the first and the last peptides in each line of the peptide array are indicated. The membranes were incubated with purified recombinant GST-Pex14 followed by monoclonal anti-GST antibodies. Pex13 peptides that bound to GST-Pex14p were visualized with horseradish peroxidase-conjugated anti-mouse antibodies and ECL reagent. The sequences of the interacting peptides are shown, and the overlapping amino acids are highlighted by bold type. (D) Schematic view of Pex13 and, its proposed transmembrane domains TMD1 and TMD2, the SH3 domain, and the novel Pex14-binding site. Numbers denote amino acid positions.
To other membranes (see Fig. 2A and B), His6-Pex14 was added at a concentration of 10 μg/ml. His-tagged Pex14 was purified essentially as described previously (45). In brief, the soluble fraction of bacterially expressed His6-Pex14 (pQE31-PEX14), which had been induced with 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 20°C for 3 h, was incubated with a nickel-nitrilotriacetic acid matrix (Invitrogen, De Schelp, The Netherlands) at 4°C for 2 h. After the sample was washed with column buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole [pH 8]), His6-Pex14 was eluted with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 300 mM imidazole [pH 8]). Detection was achieved with monoclonal anti-His6 antibodies in combination with an enhanced chemiluminescence (ECL) system (Amersham Biosciences). As a control, a duplicate membrane was incubated with the same antibody-ECL system combination but in the absence of His6-Pex14.
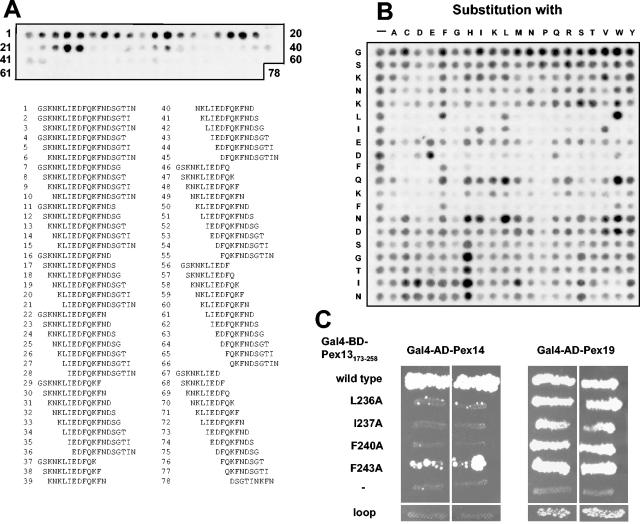
FIG. 2.
Characterization of the second Pex14-binding site in Pex13. (A) Length analysis of the novel Pex14-binding site. Peptides comprising systematic truncations of Pex14-interacting peptide Pex13231-250 down to a length of nine amino acids were synthesized on cellulose membranes and incubated with purified His6-Pex14. Bound His6-Pex14 was visualized immunologically with monoclonal anti-His6 antibodies. The numbered peptide sequences shown below the membrane correlate with the spot numbers on the membrane. (B) Substitution analysis. Pex14 was tested for interactions with mutated variants of Pex13231-250 peptide GSKNKLIEDFQKFNDSGTIN as described for panel A. The first row represents the nonmutated wild-type peptide, whereas peptides in all other rows harbor the indicated single amino acid substitutions. Spots with reduced intensities represent peptides with reduced binding affinities for Pex14. (C) In vivo effect of mutating critical residues of the Pex14-binding site. (Left panels) A yeast two-hybrid assay was used to study the interaction of Pex14 with Pex13173-258 that had been mutated to A at position L236 (pAS40), I237 (pAS41), F240 (pAS42), or F243 (pAS43) or at all four positions (loop; pAS82). (Right panels) As a control, the Pex13 fragments were also assayed for interactions with Pex19, which require amino acids 200 to 220 of Pex13. BD, DNA-binding domain; AD, activation domain.
Fractionation of yeast homogenates.
Preparation and fractionation of yeast homogenates by differential centrifugation at 25,000 × g were performed as described previously (16).
Purification of the docking complex with IgG-Sepharose.
The docking complex was purified essentially as described previously (1). Whole-cell extracts of oleic acid-induced yeast cells were obtained by disintegration with glass beads. The lysis buffer used contained 20 mM HEPES, 100 mM potassium acetate, 5 mM magnesium acetate (pH 7.5), and protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 0.3 μM aprotinin, 1 μM bestatin, 1.5 μM pepstatin, 5 μM leupeptin, 1 μM benzamidine, 8 μM antipain, 5 mM NaF, and 10 μM chymostatin). After the removal of cell debris, samples were centrifuged at 100,000 × g for 1 h at 4°C (Sorvall Ultracentrifuge rotor TH-641). The resulting total membrane pellet fraction was resuspended in 4 ml of lysis buffer containing 10% glycerol and adjusted to 10 mg of total protein in a total volume of 10 ml. Solubilization was started by adding digitonin to a concentration of 1% (wt/vol) and was allowed to proceed at 4°C for 1.5 h. The solubilized membrane proteins were obtained by centrifugation at 100,000 × g for 1 h. The supernatant was incubated overnight with 50 μl of immunoglobulin G (IgG)-Sepharose (Amersham Biosciences) at 4°C. Bound material was collected, washed five times with 500 μl of lysis buffer containing 0.1% digitonin, and eluted with 50 μl of lysis buffer containing 10% sodium dodecyl sulfate (SDS). A total of 10 μl of each solubilized sample (solubilisate) and 5 μl of each eluate were separated by SDS-polyacrylamide gel electrophoresis and subjected to Western blot analysis. Immunoreactive complexes were visualized with antibodies against Pex14 (2) and Pex13 (14), in combination with anti-rabbit IgG-coupled horseradish peroxidase and the ECL system. Signal intensities were quantified with ImageMaster TotalLab version 2.01 software (Amersham Biosciences).
Immunofluorescence microscopy.
Colocalization studies carried out with oleic acid-induced yeast cells were performed with an Axioplan microscope and AxioVision 4.1 software (Zeiss, Jena, Germany) as described previously (19) and with the previously described rabbit polyclonal antibodies against Pex14, Fox3 (15), Pcs60 (4), and Cta1 (25) as well as mouse monoclonal antibodies against the hemagglutinin (HA) epitope (Santa Cruz Biotechnology, Santa Cruz, Calif.). The secondary antibodies applied were anti-mouse antibodies conjugated with Alexa Fluor 488 and anti-rabbit antibodies conjugated with Alexa Fluor 594 (Molecular Probes, Eugene, Oreg.).
RESULTS
Identification of a second Pex14-binding site within Pex13.
The association of Pex14 with Pex13 is achieved via the PXXP motif of Pex14, which is a ligand for the SH3 domain of Pex13 (11, 39). However, since mutating the PXXP motif prevents binding to the Pex13 SH3 domain yet allows Pex14 function to be retained (19), additional interactions between these two proteins should exist. We therefore analyzed whether other regions of Pex13 may also associate with Pex14. A yeast two-hybrid assay showed that Pex14 indeed interacts with Pex131-264, a fragment lacking the entire SH3 domain, as indicated by the growth of the transformed strain on medium lacking both histidine and adenine (Fig. 1A). The potential second Pex14-binding site was narrowed down by dissecting Pex131-264 into an N-terminal fragment of 151 amino acids and a fragment containing amino acids 151 to 264. The latter fragment clearly interacted with Pex14, whereas Pex131-151 failed to do so. The slightly smaller Pex13173-258 fragment also produced a positive result in this assay. This fragment comprises the lumenal part of Pex13 excluding the adjoining two transmembrane domains (Fig. 1D).
To analyze whether Pex14 contacts this binding site through its PXXP motif, Pex14AXXA was used in a two-hybrid assay. This mutant protein possesses alanine residues instead of proline residues at positions 87 and 90 and does not interact with the Pex13 SH3 domain (19). Pex13280-386, comprising the SH3 domain, indeed interacted with wild-type Pex14 but did not interact at all with Pex14AXXA (Fig. 1B). On the other hand, the Pex14 interaction with Pex13173-258 was not affected by the AXXA mutation. These data suggest that two independent domains of Pex13 are recognized by Pex14 and that this recognition is accomplished through distinct binding motifs in Pex14.
The applied yeast-two hybrid assay did not distinguish between direct Pex13-Pex14 interactions and those mediated by a bridging protein. To make this distinction, cellulose membranes that contained an array of synthetic 20-mer peptides representing the entire Pex13 sequence in an overlapping arrangement were synthesized. These membranes were incubated either with recombinant GST or with Pex14 purified as a recombinant GST fusion protein from E. coli (see Materials and Methods). Immunological detection of bound protein by anti-GST monoclonal antibodies revealed that the control incubation with purified GST did not result in significant binding to any of the peptides (data not shown); however, with GST-Pex14, an array of 6 peptides gave rise to strong signals (Fig. 1C). These peptides comprise amino acids 225 to 256 of Pex13 and contain an overlapping 10-amino-acid region from positions 235 to 244. Binding to peptides covering the SH3 domain was not observed in this experiment, a result which indicates that Pex14 recognizes only the folded SH3 domain of Pex13. The Pex14-binding site identified in vitro lies within the Pex13173-258 fragment that tested positive in the two-hybrid assay, indicating that both methods detected the same binding event. Thus, Pex14 directly binds not only to the SH3 domain but also to a linear, intraperoxisomal sequence of Pex13.
Characterization of the luminal Pex14-binding site within Pex13.
To determine the minimal length of this novel Pex14-binding site, the peak interacting Pex13 peptide (amino acids 231 to 250) as well as all possible truncations down to a length of nine amino acids were synthesized and tested for Pex14 binding (Fig. 2A). The 14-mer 233KNKLIEDFQKFNDS246 (spot 24) was identified as the smallest peptide capable of binding to Pex14 with an affinity comparable to that of the starting 20-mer.
To identify invariant or restricted amino acid residues within this sequence, mutant versions of the peptide containing amino acids 231 to 250 (231GSKNKLIEDFQKFNDSGTIN250) were synthesized to harbor a single amino acid exchange at each position. This substitution matrix revealed a sequence-specific core of eight amino acids (236LIEDFQKF243). All other positions were exchangeable without a loss of binding (Fig. 2B). Within the core sequence, residue F240 could not be substituted with any other amino acid, and F243 was exchangeable only with aromatic amino acids. Residues L236 and I237 were also largely invariant, whereas the three charged residues present in the sequence, E238, D239, and K242, appeared to be less critical. Substitution with proline impaired Pex14 binding at any of the positions of the core sequence, suggesting that the binding site is α helical.
To analyze whether the identified critical amino acid residues influence Pex13 binding affinity for Pex14 in vivo as well, each of the four important hydrophobic amino acids was mutated to alanine in the Pex13173-258 fragment. The subsequent two-hybrid assay revealed that the L236A and F243A mutations provoked a significantly reduced affinity for Pex14 and that the I237A and F240A mutations inhibited the Pex14 interaction (Fig. 2C, left panels). All mutated Pex13173-258 fragments retained their propensity to interact with Pex19, which is accomplished through amino acids 200 to 220 of Pex13 (42), indicating that the mutated protein fragments were normally expressed (Fig. 2C, right panels). The quadruple mutant L236A-I237A-F240A-F243A (loop mutant) also still interacted weakly with Pex19 but failed to bind to Pex14. From the combined results, we concluded that the second Pex14-binding site within Pex13 is probably α helical and contains four key hydrophobic side chains at positions 1, 2, 5, and 8 of the core binding site.
The association of Pex13 with the docking complex depends on both Pex14-binding sites.
Current knowledge of the physiological role of the Pex13-Pex14 interaction stipulates that the loose association of the Pex14-Pex17 core of the docking complex with Pex13 is pivotal for peroxisomal matrix protein import (1, 11). To assess the importance of the two Pex14-binding sites within Pex13 for its association with the docking complex, the membrane-bound Pex14 complex was solubilized from total membrane fractions with digitonin and purified with the help of a functional protein A-Pex14 (ProtA-Pex14) fusion as described previously (1). ProtA-Pex14 was isolated from oleic acid-induced pex13Δ mutant cells expressing the wild-type allele of PEX13, the PEX13loop mutation (L236A-I237A-F240A-F243A), the PEX13SH3 mutation (E320K), which specifically inhibits the Pex14-Pex13 SH3 domain interaction (5, 13, 19), and a PEX13 allele with both sites mutated (PEX13loop+SH3). The mutant proteins were stably expressed at comparable levels (Fig. 3, top panels), with the loop mutation causing a slight decrease in the mobility of Pex13 in SDS gels. When the docking complex was purified from wild-type cells, a small but significant amount of Pex13 was recovered (Fig. 3, eluate panels). A comparison of the amounts of Pex13 copurified with ProtA-Pex14 from complemented pex13Δ cells revealed that wild-type Pex13 was about as abundant as the Pex13 variants harboring the SH3 mutation and the loop mutation. In contrast, significantly less Pex13 with both sites mutated was isolated; from a densitometric quantification of the immunoblot, it was estimated that the observed reduction was approximately threefold (Fig. 3). Thus, both Pex14 interaction sites contribute to the physiological association of Pex13 with the docking complex.
FIG. 3.
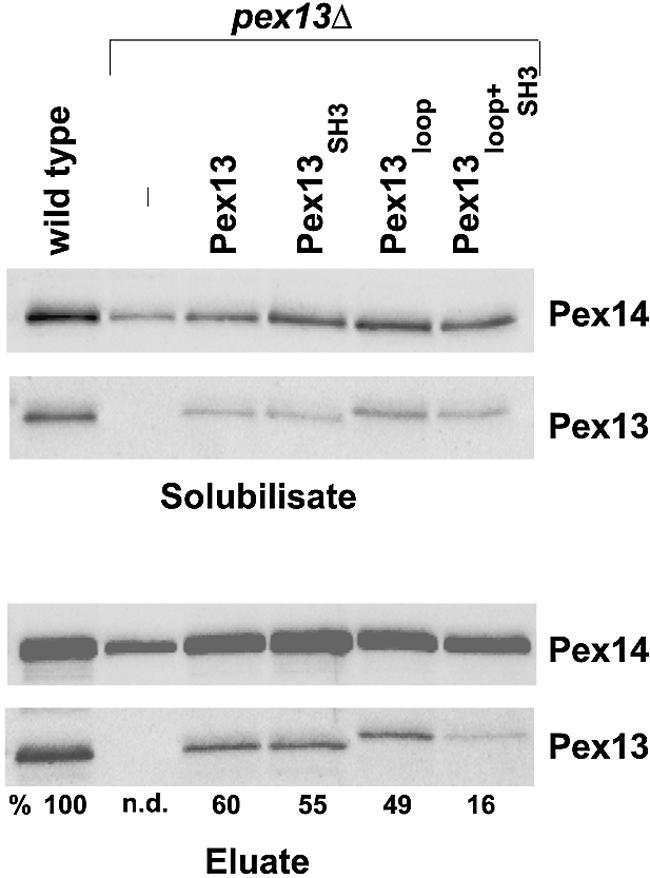
Association of Pex13 mutant proteins with the docking complex. The indicated oleic acid-induced strains containing a chromosomal protein A tag at the PEX14 locus were analyzed for the presence of Pex13 in the docking complex that had been purified with ProtA-Pex14. In particular, the UTL-7A-derived pex13Δ mutant strains harboring plasmids designed to express Pex13 (pAS62), Pex13loop (pAS76), Pex13SH3 (pAS61), and Pex13loop+SH3 (pAS75) were analyzed. (Upper panels) Immunoblots loaded with aliquots of proteins solubilized with 1% digitonin from total membrane fractions and decorated with anti-Pex13 and Pex14 antibodies. (Lower panels) Immunoblots showing the abundance of Pex14 and Pex13 in eluates. Numbers below the bottom panel denote the ratios of the intensities of the Pex13 signals to the corresponding Pex14 signals. The calculated intensity of the wild-type sample was set at 100%. n.d., not determined.
The two Pex14-binding sites within Pex13 are important but not essential for matrix protein import.
To test whether the amount of Pex13 copurified with the docking complex correlates with the ability to import peroxisomal matrix proteins, immunofluorescence microscopy was used to determine the localizations of native PTS1 and PTS2 proteins in a pex13Δ strain expressing mutant alleles of PEX13. In all strains analyzed, peroxisomes were visualized by means of an HA-tagged version of Pex11, a peroxisomal membrane protein whose targeting does not depend on Pex13 or Pex14 (27).
In wild-type cells, punctate staining patterns were observed for the PTS1 protein Pcs60, the PTS2 protein Fox3, and the PTS1-like protein Cta1; these patterns were superimposable with those of Pex11-HA, indicating that all import routes were functional (Fig. 4A to C). As expected for a strain devoid of Pex13, diffuse, cytosolic staining was obtained for all three matrix proteins, commensurate with a general matrix protein import defect. Complementation of the pex13Δ mutant with the wild-type or loop mutant allele of PEX13 resulted in a wild-type-like punctate staining pattern. In agreement with previous work (13), the SH3 mutation resulted in superimposable punctate staining patterns for the three matrix proteins in addition to a diffuse fluorescence background of various intensities, indicative of a partial cytosolic mislocalization of the proteins. Even the strain expressing the Pex13 variant with both sites mutated (Pex13loop+SH3) showed in many cells a punctate staining pattern (above the cytosolic background) for Pcs60 and Fox3 that was superimposable with that of Pex11-HA, whereas the import of catalase appeared to be more severely impaired.
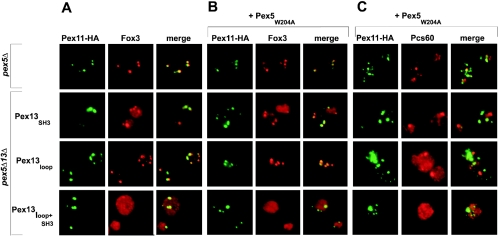
FIG. 4.
Dependence of peroxisomal matrix protein import on direct Pex13-Pex14 interactions. Oleic acid-induced wild-type and pex13Δ cells expressing HA-Pex11 were subjected to double-immunofluorescence microscopy so as to localize HA-Pex11 in tandem with PTS1 protein Pcs60 (A), PTS2 protein Fox3 (B), or PTS1-like protein Cta1 (C). The same procedure was applied to pex13Δ cells expressing Pex13 (pKat113), Pex13SH3 (pAS53), Pex13loop (pAS71), and Pex13loop+SH3 (pAS73). Detection was achieved with mouse monoclonal antibodies against the HA epitope combined with rabbit polyclonal antibodies against the individual matrix proteins. As secondary antibodies, Alexa Fluor 488-labeled anti-mouse IgG and Alexa Fluor 594-labeled anti-rabbit IgG were used. A congruent fluorescence pattern denotes colocalization of HA-Pex11 with the analyzed matrix proteins, a finding which is readily revealed in the merged images. (D) From the same cells, a postnuclear supernatant (PNS) was produced and subfractionated into a 25,000 × g pellet fraction (P) enriched for peroxisomes and a supernatant fraction (SN) enriched for the cytosol. Equivalent volumes of fractions were loaded on the gels, transferred to nitrocellulose membranes, and analyzed for the distributions of the specified proteins.
To underscore the fluorescence microscopy data, postnuclear supernatants from the same strains were subjected to differential centrifugation at 25,000 × g. The resulting pellet fraction contained mainly peroxisomes and mitochondria, whereas the supernatant fraction was enriched with cytosol. Accordingly, in both wild-type and pex13Δ strains complemented with either wild-type Pex13 or Pex13loop, the marker proteins Pcs60, Fox3, and Cta1 were predominantly found in the pellet fraction, whereas in the pex13Δ mutant strain, all three matrix proteins appeared in the supernatant fraction. Upon the expression of Pex13SH3 or Pex13loop+SH3, the portion of matrix proteins in the supernatant fraction was larger than that in the pellet fraction. However, significant amounts of Pcs60 and Fox3 were still detected in the pellet fraction, a result which was in line with a protein import defect that is only partial. The distribution of peroxisomal membrane protein Pex3 was analyzed as a control for a protein whose import does not depend on Pex13. Taken together, the results indicated that blocking of the direct interaction of Pex13 with Pex14 clearly affected but did not abolish matrix protein import. These data suggested that the association of Pex13 with the docking complex either is not essential or is additionally accomplished by a bridging protein(s) that mediates a Pex13-Pex14 interaction.
Pex13 additionally associates with the docking complex via the PTS1 import receptor.
Pex5 was an obvious candidate for a bridging protein, because (i) a fraction of Pex5 copurifies with the docking complex (1), (ii) Pex5 interacts with both Pex13 and Pex14 (2, 6), and (iii) Pex5 forms a ternary complex with Pex14 and the SH3 domain of Pex13 in vitro (3). To test this hypothesis, the docking complex was again purified from strains expressing the PEX13 alleles described above but additionally lacking Pex5. A significant amount of wild-type Pex13 was still coisolated with ProtA-Pex14 in the absence of Pex5, whereas the abundances of Pex13SH3 and Pex13loop in the isolated docking complex were reduced approximately three- and fivefold, respectively (Fig. 5). Importantly, despite being expressed and membrane associated, Pex13loop+SH3 was not detectable in the docking complex when Pex5 was concomitantly absent. These data suggested that Pex13 interacts with Pex14 via one indirect, Pex5-mediated and two direct binding events. Only a block of all three modes of interaction causes the apparent exclusion of Pex13 from the docking complex.
FIG. 5.
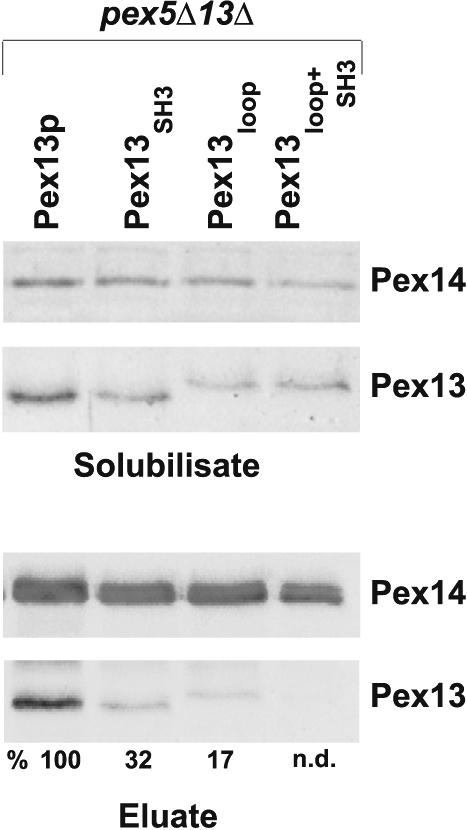
Involvement of Pex5 in the association of Pex13 with the Pex14-containing docking complex. The docking complex was isolated with ProtA-Pex14 from oleic acid-induced cells lacking Pex5 and expressing the indicated mutant versions of Pex13. (Lower panels) The amount of Pex13 coeluting with the docking complex was determined by immunoblot analysis. Numbers below the bottom panel denote the ratios of the intensities of the Pex13 signals to the corresponding Pex14 signals. The calculated intensity of the sample derived from pex5Δ pex13Δ cells expressing wild-type Pex13 was set at 100%. n.d., not determined. (Upper panels) Amounts of Pex14 and Pex13 present in solubilized membrane protein fractions.
The targeting of Pex14 is independent of Pex13SH3+loop and Pex5.
The results described above may also be explained by a mislocalization of Pex14, because it has been shown that Pex14 is localized in nonperoxisomal structures in a pex13Δ deletion strain (19). Thus, the localization of native Pex14 in strains expressing mutant versions of Pex13 was determined by immunofluorescence microscopy and compared to the subcellular distribution of peroxisomal Pex11-HA. Congruent staining patterns for both proteins, indicative of peroxisomal localization, were found for both the wild-type strain and the pex13Δ strain complemented with Pex13SH3 or Pex13loop (Fig. 6). Even the expression of Pex13SH3+loop allowed Pex14 to be retained at the peroxisomal membrane. Importantly, when the distribution of Pex14 in the pex5Δ pex13Δ strain expressing Pex13SH3+loop was analyzed, only some cells showed the typical diffuse staining pattern of a pex13Δ mutant; most cells had Pex14 correctly targeted (Fig. 6). Thus, we concluded that the absence of Pex13SH3+loop from the docking complex was not due to a mislocalization of Pex14.
FIG. 6.
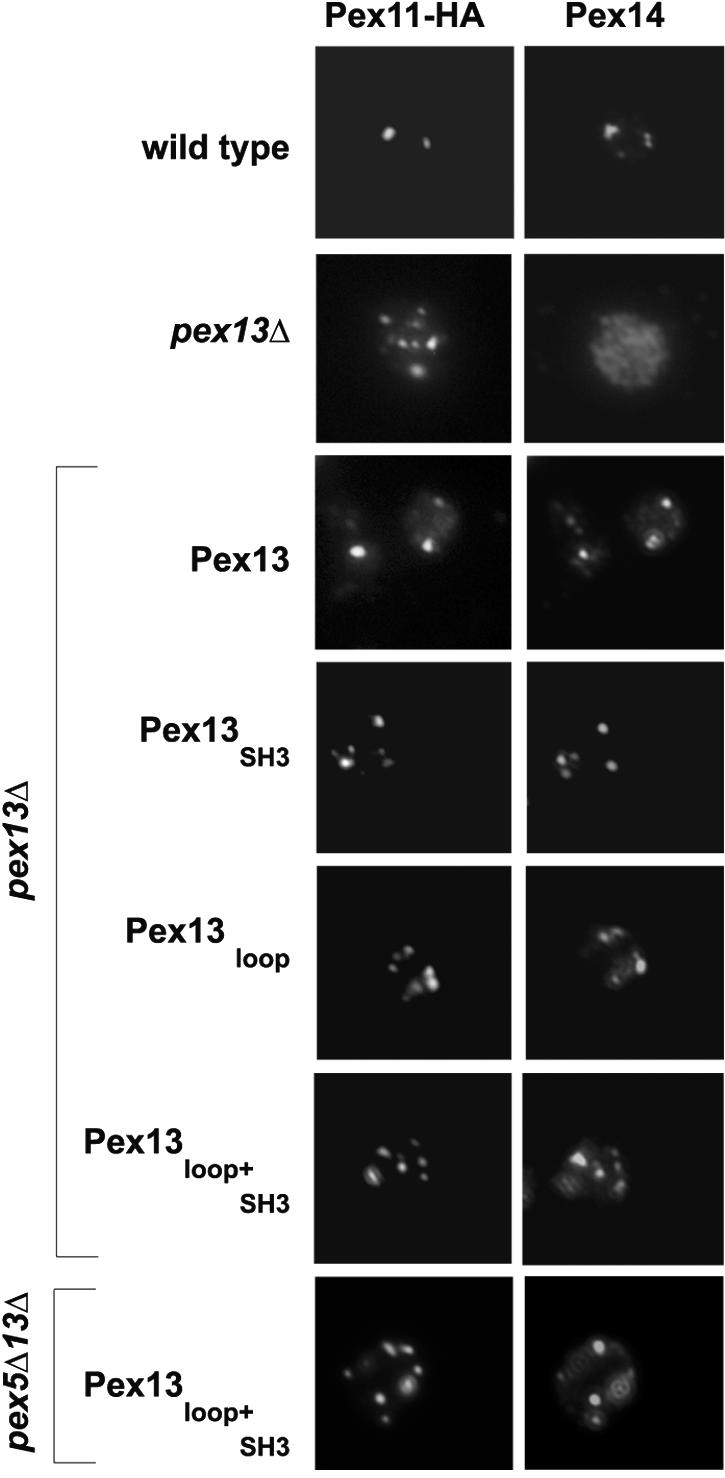
Influence of mutant versions of Pex13 and of Pex5 on Pex14 targeting. The localization of Pex14 in oleic acid-induced pex13Δ and pex5Δ pex13Δ cells expressing wild-type or mutant versions of Pex13 was compared to that of HA-Pex11 by double-immunofluorescence microscopy. Detection was achieved with rabbit polyclonal antibodies against Pex14 and anti-HA monoclonal antibodies followed by Alexa Fluor 488-labeled anti-mouse and Alexa Fluor 594-labeled anti-rabbit secondary antibodies.
The association of Pex13 with the docking complex is mandatory for matrix protein import.
The apparent absence of Pex13loop+SH3 from the docking complex in the pex5Δ strain provoked the question as to whether its absence would also be reflected in a more severe protein import defect. Because yeast PTS2 proteins are normally imported in the absence of Pex5, the import of the PTS2 protein Fox3 was amenable for a meaningful inspection. The well-established Pex5-independent import of Fox3 is shown in Fig. 7A. Strains devoid of Pex5 and expressing Pex13SH3 or Pex13loop were still able to import thiolase, albeit less efficiently, as evidenced by diffuse background staining. Strikingly, the expression of Pex13loop+SH3 in pex5Δ pex13Δ cells resulted in a completely diffuse staining pattern, indicating that Fox3 was not imported at all. This observation was in agreement with the hypothesis that the lack of import was caused directly by the lack of an interaction between Pex13 and Pex14. However, because Pex5 interacts with a number of peroxins, it was by no means clear whether the import defect seen was functionally linked to the Pex13-Pex14 bridging function of the PTS1 import receptor or whether other Pex5-mediated interactions were responsible for the loss of PTS2 import (in the background of inhibited direct binding of Pex13 to Pex14).
FIG. 7.
Dependence of matrix protein import on Pex5 in the absence of direct Pex13-Pex14 interactions. (A) Oleic acid-induced pex5Δ pex13Δ cells expressing mutant versions of Pex13 in combination with HA-Pex11 were processed for double-immunofluorescence microscopy with rabbit antibodies against PTS2 protein Fox3 and anti-HA monoclonal antibodies. The secondary antibodies used were Alexa Fluor 488-labeled anti-mouse IgG and Alexa Fluor 594-labeled anti-rabbit IgG. pex5Δ cells were used as a control for intact PTS2 import. (B and C) The same cells but additionally expressing Pex5W204A were similarly inspected for the localization of Fox3 (B) and Pcs60 (C).
To discriminate between these two possibilities, a version of Pex5 that was specifically impaired in binding to Pex13 was exploited in the following protein import complementation assays. Pex5 contacts the SH3 domain of Pex13 in a nonconventional manner through one of its WXXXF/Y motifs (11, 39). Mutating either the W204 or the F208 residue in this motif abolishes the binding of Pex5 to Pex13 but not to PTS1 proteins or other peroxins (5). Thus, only if Pex5 with the W204A mutation (Pex5W204A) failed to rescue the Fox3 import defect would bridging apply as an explanation for the observed PTS2 import phenotype. Figure 7B shows that this was indeed the case. The expression of Pex5W204A in combination with either Pex13loop or Pex13SH3 led to an aggravation of Fox3 import; in the presence of Pex13loop+SH3, however, import was completely eliminated. Hence, the combined data strongly suggested that Pex5 was required for PTS2 import because it connected Pex13 with Pex14.
With regard to PTS1 proteins, the expression of Pex5W204A in a pex5Δ strain partially restored the import of Pcs60 (Fig. 7C) but not of Cta1 (data not shown). This finding was in agreement with previous work that had shown that the Pex5-Pex13 interaction was not essential for the import of most PTS1 proteins, except for Cta1 (5). However, in the presence of Pex13loop, the expression of Pex5W204A did result in severely compromised Pcs60 import (Fig. 7C). Thus, although both single mutations only mildly affected the peroxisomal import of this PTS1 protein, the mutations in combination abolished protein import. The combined expression of Pex13SH3 or Pex13loop+SH3 and Pex5W204A also abolished protein import. The observed cumulative phenotype supported the notion that Pex5 additionally serves as a mediator of Pex13 binding to Pex14.
A combination of Pex13loop and Pex5W204A prevents growth on oleic acid.
In yeast cells, peroxisomes are the exclusive site for fatty acid β oxidation and are therefore essential for growth on oleic acid as a sole carbon source. To test whether the second Pex14-binding site of Pex13 is relevant for growth on that medium, a PEX13 allele harboring the loop mutation was tested either alone or in combination with the SH3 mutation. No significant retardation of growth was observed with the loop mutation, whereas the SH3 mutation severely impaired growth (Fig. 8A), as reported previously (13). The respective double mutation aggravated growth even further, but this conclusion is arguable in light of the strong phenotype caused by the single SH3 mutation. The PEX5W204A allele affected growth on oleic acid only mildly (Fig. 8B), in agreement with a previous report (5). However, when it was tested in combination with the loop mutation, growth was severely compromised (Fig. 8B). Thus, through a combination of these two single mutations causing weak phenotypes, a synthetic lethal effect was obtained. As expected, combining PEX5W204A with PEX13SH3 or PEX13loop+SH3 also caused a nongrowth phenotype. The observed disturbance of peroxisome function corroborated the physiological relevance of multiple, partially redundant interactions between Pex14 and Pex13.
FIG. 8.

Effect of weakening the Pex13-Pex14 association on peroxisome function. Complementation analysis of pex13Δ cells expressing Pex13, Pex13SH3, Pex13loop, or Pex13loop+SH3 (A) and pex5Δ pex13Δ cells expressing Pex5W204A together with Pex13, Pex13SH3, Pex13ploop, or Pex13loop+SH3 (B) was carried out. The indicated cells were spotted as a series of 10-fold dilutions on oleic acid plates and incubated at 30°C for 5 days.
DISCUSSION
The work described here sheds new light on the association of Pex13 with the docking complex of the peroxisomal protein import machinery. It was well established that Pex14 interacts via its proline-rich motif with the SH3 domain of Pex13 (2, 19); nonetheless, at least partial matrix protein import still occurs with mutated alleles of PEX13 or PEX14 that specifically interfere with this protein-protein interaction (13, 19). We demonstrate the existence of additional contact sites between Pex13 and Pex14 which contribute to tethering of Pex13 to the Pex14-containing docking complex (Fig. 9). These include a novel, direct interaction site and one that is mediated by Pex5. Upon blocking of all known contact sites, matrix protein import was abolished (Fig. 7), and peroxisome function was compromised (Fig. 8). Our data therefore imply that the association of Pex13 with the docking complex is an essential event in peroxisome biogenesis.
FIG. 9.
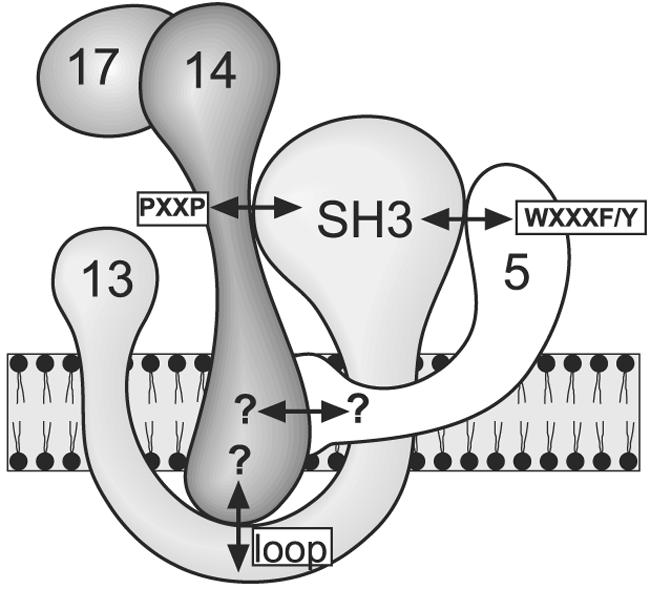
Graphic representation of Pex13-Pex14 interactions. Protein-protein interactions between S. cerevisiae peroxins Pex5, Pex13, and Pex14 are denoted by double-headed arrows. Mapped interactions are labeled according to the designations used in this work. Published interactions that had not yet been mapped are indicated by question marks.
The novel Pex14-binding site was identified in a combination approach of two-hybrid analysis and peptide scanning. The site was limited to a linear sequence element of 14 amino acids, 233KNKLIEDFQKFNDS246, which is characterized by the presence of rather invariant hydrophobic and aromatic amino acids. As proline residues are not allowed within this region, the binding site is likely to acquire an α-helical conformation. Remarkably, this site lies within a region of Pex13 that is localized in the peroxisomal lumen, suggesting that Pex14 spans the peroxisomal membrane at least temporarily. It is worth noting that Pex14 has been reported to reside within the peroxisomal membrane as an integral membrane protein in mammals (17, 37, 44, 50) and the yeasts Hansenula polymorpha (32) and Pichia pastoris (30), whereas for Saccharomyces cerevisiae the topology of Pex14 is under debate. Albertini and colleagues (2) found the wild-type protein to be sensitive to exogenously added protease and completely extractable by carbonate, indicative of a peripheral membrane protein facing the cytosolic site of the peroxisomal membrane. In compliance with Pex14 also being an integral membrane protein in S. cerevisiae, a partially carbonate-resistant Pex14 was reported by Brocard et al. (6), thereby supporting the feasibility of a lumenal Pex14-Pex13 interaction. Which region of Pex14 actually binds this novel site in Pex13 remains to be defined, but it is already clear that it is not the proline-rich motif required for binding of the SH3 domain (Fig. 1).
The second Pex14-binding site of Pex13 is indeed involved in the in vivo association of the two proteins, since mutating both the SH3 domain- and the loop-binding sites resulted in a significant decrease in the association of Pex13 with the Pex14-containing docking complex. Moreover, mutation of both sites also led to a clear import defect for catalase, a PTS1-like protein (24, 31, 33), indicating the requirement of both sites for proper peroxisomal protein import (Fig. 4). However, the residual import of catalase and the obviously less severely affected import of other PTS1 and PTS2 proteins (Fig. 4) suggested that the two proteins might still be tethered together. Since additional direct interactions between Pex13 and Pex14 were not detected with the methods applied, we assumed that another protein might be responsible for the association of Pex13 and Pex14.
Several lines of evidence identified Pex5 as such a bridging protein. The association of Pex14 with Pex13 lacking both Pex14-binding sites was even more reduced when Pex5 was additionally absent (Fig. 5). This result extends the previous finding of a weakened Pex13-Pex14 interaction in the absence of Pex5 obtained in a yeast two-hybrid assay (5) insofar as it also demonstrates a dependence on Pex5 for the formation of the native Pex14-Pex13 complex. The structural basis for the capability of the SH3 domain to simultaneously bind Pex14 and Pex5 was resolved recently; the two proteins bind to the SH3 domain from opposing sides (11, 39).
It has been established that Pex5 interacts with the SH3 domain of Pex13 via one of its WXXXF/Y motifs (5). The significance of this interaction was up to that time limited to the import of peroxisomal catalase (5). We showed here that the PEX5W204A mutation, which specifically prevents the Pex5-Pex13 interaction, also causes a mislocalization of typical PTS1 protein Pcs60 when introduced together with the loop mutation in Pex13 (Fig. 7). Since in this case the SH3 domain of Pex13 remained intact, the importance of the novel Pex14-binding site of Pex13 for matrix protein import was also clearly revealed. The cumulative impairment was also observed in a growth assay on oleic acid-containing medium: although Pex5W204A and Pex13loop were able to complement the growth defect of the corresponding single-mutant strains on oleic acid medium, they failed to do so in combination (Fig. 8).
In yeast cells, Pex5 functions as a signal sequence receptor for PTS1 proteins and is usually not required for the targeting of PTS2 proteins (35, 46, 48, 49). We therefore did not expect to detect the observed complete import defect for PTS2 matrix proteins upon expression of Pex5W204A in a background of Pex13 with mutations in both Pex14-binding sites (Fig. 7). Thus, Pex5 proved capable of fulfilling an essential function that was independent of its role as a PTS1 signal receptor. The most plausible explanation for this observation is that Pex5 served as a bridging factor between Pex14 and Pex13, association of which was required for Pex7 to translocate its cargo proteins. An intriguing question to be answered is whether a pool of docking complex-associated Pex5 normally serves this structural role in a wild-type strain. In any case, Pex7 was apparently not self-sufficient to bridge Pex14 and Pex13, even though it directly interacts with both proteins (45).
We were unable to detect a similar lumenal binding motif in Pex13 proteins from other organisms, thereby leaving open the question as to whether Pex13 orthologues develop multiple Pex14 contacts. Likewise, the Pex5-binding site within the SH3 domain of Pex13 is not entirely conserved; in mammals, the N terminus of Pex13 interacts with Pex5 (38). Nonetheless, this interaction may well serve the same purpose of bringing Pex13 and Pex14 into close contact. It is worth noting that in mammals, prevention of the Pex5-Pex13 interaction results in a catalase import defect, whereas PTS1 and PTS2 proteins are normally imported (38), suggesting that catalase is probably more sensitive to perturbations of the assembled docking complex than other PTS1 proteins.
A growing body of evidence suggests an import cascade model in which the transfer of cargo-loaded Pex5 from the Pex14-containing docking complex to Pex13 is coupled to the translocation of a cargo protein (22, 28, 38, 47). Subsequently, the ring finger complex is thought to be recruited via Pex8, which in turn causes Pex5 to be transferred to the ring finger complex (1, 9, 41). Our data do not indicate whether Pex13 is part of the docking complex or is involved in a step following docking, but they do demonstrate that a close spatial association of Pex13 and Pex14 is at least temporarily required for the function of the two proteins in importing peroxisomal matrix proteins. The association of Pex13 with the core of the docking complex may occur as long as some of the interactions are maintained, in turn allowing ring finger complex recruitment. However, when the association of Pex13 with Pex14 is totally blocked, protein translocation stalls. The proposed dynamics of complex formation can now be studied with the mutant cells generated in this study, in which Pex13 and Pex14 are kept separately within the peroxisomal membrane.
Acknowledgments
We are grateful to Wolfgang Schliebs for providing plasmid pWib21 (PEX5 with a W204A mutation), Henk F. Tabak for providing Cta1 antibodies, and Wolf H. Kunau and Achim Kramer for fruitful discussions.
This work was supported by the Deutsche Forschungsgemeinschaft (grants SFB480 to R.E. and H.R. and SFB449 to R.V.-E.) and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Agne, B., N. M. Meindl, K. Niederhoff, H. Einwachter, P. Rehling, A. Sickmann, H. E. Meyer, W. Girzalsky, and W. H. Kunau. 2003. Pex8p: an intraperoxisomal organizer of the peroxisomal import machinery. Mol. Cell 11:635-646. [DOI] [PubMed] [Google Scholar]
- 2.Albertini, M., P. Rehling, R. Erdmann, W. Girzalsky, J. A. Kiel, M. Veenhuis, and W. H. Kunau. 1997. Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent import pathways. Cell 89:83-92. [DOI] [PubMed] [Google Scholar]
- 3.Barnett, P., G. Bottger, A. T. Klein, H. F. Tabak, and B. Distel. 2000. The peroxisomal membrane protein Pex13p shows a novel mode of SH3 interaction. EMBO J. 19:6382-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blobel, F., and R. Erdmann. 1996. Identification of a yeast peroxisomal member of the family of AMP-binding proteins. Eur. J. Biochem. 240:468-476. [DOI] [PubMed] [Google Scholar]
- 5.Bottger, G., P. Barnett, A. T. Klein, A. Kragt, H. F. Tabak, and B. Distel. 2000. Saccharomyces cerevisiae PTS1 receptor Pex5p interacts with the SH3 domain of the peroxisomal membrane protein Pex13p in an unconventional, non-PXXP-related manner. Mol. Biol. Cell 11:3963-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brocard, C., G. Lametschwandtner, R. Koudelka, and A. Hartig. 1997. Pex14p is a member of the protein linkage map of Pex5p. EMBO J. 16:5491-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, C. C., D. S. Warren, K. A. Sacksteder, and S. J. Gould. 1999. PEX12 interacts with PEX5 and PEX10 and acts downstream of receptor docking in peroxisomal matrix protein import. J. Cell Biol. 147:761-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevray, P. M., and D. Nathans. 1992. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc. Natl. Acad. Sci. USA 89:5789-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, C. S., J. E. Kalish, J. C. Morrell, J. M. McCaffery, and S. J. Gould. 2000. The peroxisome biogenesis factors Pex4p, Pex22p, Pex1p, and Pex6p act in the terminal steps of peroxisomal matrix protein import. Mol. Cell. Biol. 20:7516-7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dammai, V., and S. Subramani. 2001. The human peroxisomal targeting signal receptor, Pex5p, is translocated into the peroxisomal matrix and recycled to the cytosol. Cell 105:187-196. [DOI] [PubMed] [Google Scholar]
- 11.Douangamath, A., F. V. Filipp, A. T. Klein, P. Barnett, P. Zou, T. Voorn-Brouwer, M. C. Vega, O. M. Mayans, M. Sattler, B. Distel, and M. Wilmanns. 2002. Topography for independent binding of alpha-helical and PPII-helical ligands to a peroxisomal SH3 domain. Mol. Cell 10:1007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckert, J. H., and R. Erdmann. 2003. Peroxisome biogenesis. Rev. Physiol. Biochem. Pharmacol. 147:75-121. [DOI] [PubMed] [Google Scholar]
- 13.Elgersma, Y., L. Kwast, A. Klein, T. Voorn-Brouwer, M. van den Berg, B. Metzig, T. America, H. F. Tabak, and B. Distel. 1996. The SH3 domain of the Saccharomyces cerevisiae peroxisomal membrane protein Pex13p functions as a docking site for Pex5p, a mobile receptor for the import PTS1-containing proteins. J. Cell Biol. 135:97-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdmann, R., and G. Blobel. 1996. Identification of Pex13p a peroxisomal membrane receptor for the PTS1 recognition factor. J. Cell Biol. 135:111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdmann, R., and W. H. Kunau. 1994. Purification and immunolocalization of the peroxisomal 3-oxoacyl-CoA thiolase from Saccharomyces cerevisiae. Yeast 10:1173-1182. [DOI] [PubMed] [Google Scholar]
- 16.Erdmann, R., M. Veenhuis, D. Mertens, and W. H. Kunau. 1989. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 86:5419-5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fransen, M., S. R. Terlecky, and S. Subramani. 1998. Identification of a human PTS1 receptor docking protein directly required for peroxisomal protein import. Proc. Natl. Acad. Sci. USA 95:8087-8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527-534. [DOI] [PubMed] [Google Scholar]
- 19.Girzalsky, W., P. Rehling, K. Stein, J. Kipper, L. Blank, W. H. Kunau, and R. Erdmann. 1999. Involvement of Pex13p in Pex14p localization and peroxisomal targeting signal 2-dependent protein import into peroxisomes. J. Cell Biol. 144:1151-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould, S. J., and C. S. Collins. 2002. Opinion: peroxisomal-protein import: is it really that complex? Nat. Rev. Mol. Cell. Biol. 3:382-389. [DOI] [PubMed] [Google Scholar]
- 21.Gould, S. J., J. E. Kalish, J. C. Morrell, J. Bjorkman, A. J. Urquhart, and D. I. Crane. 1996. Pex13p is an SH3 protein of the peroxisome membrane and a docking factor for the predominantly cytoplasmic PTS1 receptor. J. Cell Biol. 135:85-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gouveia, A. M., C. P. Guimaraes, M. E. Oliveira, C. Reguenga, C. Sa-Miranda, and J. E. Azevedo. 2003. Characterization of the peroxisomal cycling receptor, Pex5p, using a cell-free in vitro import system. J. Biol. Chem. 278:226-232. [DOI] [PubMed] [Google Scholar]
- 23.Gouveia, A. M., C. Reguenga, M. E. Oliveira, C. Sa-Miranda, and J. E. Azevedo. 2000. Characterization of peroxisomal Pex5p from rat liver. Pex5p in the Pex5p-Pex14p membrane complex is a transmembrane protein. J. Biol. Chem. 275:32444-32451. [DOI] [PubMed] [Google Scholar]
- 24.Gunkel, K., R. van Dijk, M. Veenhuis, and I. J. van der Klei. 2004. Routing of Hansenula polymorpha alcohol oxidase: an alternative peroxisomal protein-sorting machinery. Mol. Biol. Cell 15:1347-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurvitz, A., H. Rottensteiner, S. H. Kilpelainen, A. Hartig, J. K. Hiltunen, M. Binder, I. W. Dawes, and B. Hamilton. 1997. The Saccharomyces cerevisiae peroxisomal 2,4-dienoyl-CoA reductase is encoded by the oleate-inducible gene SPS19. J. Biol. Chem. 272:22140-22147. [DOI] [PubMed] [Google Scholar]
- 26.Hazra, P. P., I. Suriapranata, W. B. Snyder, and S. Subramani. 2002. Peroxisome remnants in pex3Δ cells and the requirement of Pex3p for interactions between the peroxisomal docking and translocation subcomplexes. Traffic 3:560-574. [DOI] [PubMed] [Google Scholar]
- 27.Hettema, E. H., W. Girzalsky, M. van Den Berg, R. Erdmann, and B. Distel. 2000. Saccharomyces cerevisiae Pex3p and Pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 19:223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holroyd, C., and R. Erdmann. 2001. Protein translocation machineries of peroxisomes. FEBS Lett. 501:6-10. [DOI] [PubMed] [Google Scholar]
- 29.Huhse, B., P. Rehling, M. Albertini, L. Blank, K. Meller, and W. H. Kunau. 1998. Pex17p of Saccharomyces cerevisiae is a novel peroxin and component of the peroxisomal protein translocation machinery. J. Cell Biol. 140:49-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, M. A., W. B. Snyder, J. L. Cereghino, M. Veenhuis, S. Subramani, and J. M. Cregg. 2001. Pichia pastoris Pex14p, a phosphorylated peroxisomal membrane protein, is part of a PTS-receptor docking complex and interacts with many peroxins. Yeast 18:621-641. [DOI] [PubMed] [Google Scholar]
- 31.Klein, A. T., M. van den Berg, G. Bottger, H. F. Tabak, and B. Distel. 2002. Saccharomyces cerevisiae acyl-CoA oxidase follows a novel, non-PTS1, import pathway into peroxisomes that is dependent on Pex5p. J. Biol. Chem. 277:25011-25019. [DOI] [PubMed] [Google Scholar]
- 32.Komori, M., S. W. Rasmussen, J. A. Kiel, R. J. Baerends, J. M. Cregg, I. J. van der Klei, and M. Veenhuis. 1997. The Hansenula polymorpha PEX14 gene encodes a novel peroxisomal membrane protein essential for peroxisome biogenesis. EMBO J. 16:44-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kragler, F., A. Langeder, J. Raupachova, M. Binder, and A. Hartig. 1993. Two independent peroxisomal targeting signals in catalase A of Saccharomyces cerevisiae. J. Cell Biol. 120:665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landgraf, C., S. Panni, L. Montecchi-Palazzi, L. Castagnoli, J. Schneider-Mergener, R. Volkmer-Engert, and G. Cesareni. 2004. Protein interaction networks by proteome peptide scanning. PLoS Biol. 2:E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCollum, D., E. Monosov, and S. Subramani. 1993. The pas8 mutant of Pichia pastoris exhibits the peroxisomal protein import deficiencies of Zellweger syndrome cells—the PAS8 protein binds to the COOH-terminal tripeptide peroxisomal targeting signal and is a member of the TPR protein family. J. Cell Biol. 121:761-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okumoto, K., I. Abe, and Y. Fujiki. 2000. Molecular anatomy of the peroxin Pex12p: ring finger domain is essential for Pex12p function and interacts with the peroxisome-targeting signal type 1-receptor Pex5p and a ring peroxin, Pex10p. J. Biol. Chem. 275:25700-25710. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira, M. E., C. Reguenga, A. M. Gouveia, C. P. Guimaraes, W. Schliebs, W. H. Kunau, M. T. Silva, C. Sa-Miranda, and J. E. Azevedo. 2002. Mammalian Pex14p: membrane topology and characterisation of the Pex14p-Pex14p interaction. Biochim. Biophys. Acta 1567:13-22. [DOI] [PubMed] [Google Scholar]
- 38.Otera, H., K. Setoguchi, M. Hamasaki, T. Kumashiro, N. Shimizu, and Y. Fujiki. 2002. Peroxisomal targeting signal receptor Pex5p interacts with cargoes and import machinery components in a spatiotemporally differentiated manner: conserved Pex5p WXXXF/Y motifs are critical for matrix protein import. Mol. Cell. Biol. 22:1639-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pires, J. R., X. Hong, C. Brockmann, R. Volkmer-Engert, J. Schneider-Mergener, H. Oschkinat, and R. Erdmann. 2003. The ScPex13p SH3 domain exposes two distinct binding sites for Pex5p and Pex14p. J. Mol. Biol. 326:1427-1435. [DOI] [PubMed] [Google Scholar]
- 40.Purdue, P. E., and P. B. Lazarow. 2001. Peroxisome biogenesis. Annu. Rev. Cell Dev. Biol. 17:701-752. [DOI] [PubMed] [Google Scholar]
- 41.Reguenga, C., M. E. Oliveira, A. M. Gouveia, C. Sa-Miranda, and J. E. Azevedo. 2001. Characterization of the mammalian peroxisomal import machinery: Pex2p, Pex5p, Pex12p, and Pex14p are subunits of the same protein assembly. J. Biol. Chem. 276:29935-29942. [DOI] [PubMed] [Google Scholar]
- 42.Rottensteiner, H., A. Kramer, S. Lorenzen, K. Stein, C. Landgraf, R. Volkmer-Engert, and R. Erdmann. 2004. Peroxisomal membrane proteins contain common Pex19p-binding sites that are an integral part of their targeting signals. Mol. Biol. Cell 15:3406-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Shimizu, N., R. Itoh, Y. Hirono, H. Otera, K. Ghaedi, K. Tateishi, S. Tamura, K. Okumoto, T. Harano, S. Mukai, and Y. Fujiki. 1999. The peroxin Pex14p. cDNA cloning by functional complementation on a Chinese hamster ovary cell mutant, characterization, and functional analysis. J. Biol. Chem. 274:12593-12604. [DOI] [PubMed] [Google Scholar]
- 45.Stein, K., A. Schell-Steven, R. Erdmann, and H. Rottensteiner. 2002. Interactions of Pex7p and Pex18p/Pex21p with the peroxisomal docking machinery: implications for the first steps in PTS2 protein import. Mol. Cell. Biol. 22:6056-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szilard, R. K., V. I. Titorenko, M. Veenhuis, and R. A. Rachubinski. 1995. Pay32p of the yeast Yarrowia lipolytica is an intraperoxisomal component of the matrix protein translocation machinery. J. Cell Biol. 131:1453-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urquhart, A. J., D. Kennedy, S. J. Gould, and D. I. Crane. 2000. Interaction of Pex5p, the type 1 peroxisome targeting signal receptor, with the peroxisomal membrane proteins Pex14p and Pex13p. J. Biol. Chem. 275:4127-4136. [DOI] [PubMed] [Google Scholar]
- 48.van der Klei, I. J., R. E. Hilbrands, G. J. Swaving, H. R. Waterham, E. G. Vrieling, V. I. Titorenko, J. M. Cregg, W. Harder, and M. Veenhuis. 1995. The Hansenula polymorpha PER3 gene is essential for the import of PTS1 proteins into the peroxisomal matrix. J. Biol. Chem. 270:17229-17236. [DOI] [PubMed] [Google Scholar]
- 49.Van der Leij, I., M. M. Franse, Y. Elgersma, B. Distel, and H. F. Tabak. 1993. PAS10 is a tetratricopeptide-repeat protein that is essential for the import of most matrix proteins into peroxisomes of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 90:11782-11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Will, G. K., M. Soukupova, X. Hong, K. S. Erdmann, J. A. Kiel, G. Dodt, W. H. Kunau, and R. Erdmann. 1999. Identification and characterization of the human orthologue of yeast Pex14p. Mol. Cell. Biol. 19:2265-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]