Abstract
A monumental diversity of catalytic methods imparts the ability to select one of two configurations of tetravalent stereocentres. Conversely, catalyst control over pentavalent stereocentres, where a fifth moiety bound to the central atom encodes an expanded stereochemical space, remained a challenge to be accomplished. Herein, we report the feasibility of the catalytic tractability of pentavalent stereocentres. A bifunctional iminophosphorane thiourea catalyst enables enantio- and diastereocontrol over pentavalent phosphoranes to differentiate configurationally stable enantiomers and ensembles of diastereomers which emerge together from a single stereocentre. The desired dioxophosphorane stereoisomers are obtained with excellent yield and selectivity (up to 99% yield, 96:4 e.r. and 99:1 d.r.), while stereodivergent catalysis reroutes the reaction for selective access to each of the viable stereoisomeric states of pentavalent phosphoranes. Considering the diversity of high-valent main group species, it is expected that catalyst control over pentavalent stereocentres significantly increases the synthetically addressable stereochemical space.
Subject terms: Stereochemistry, Synthetic chemistry methodology
Pentavalent stereocentres encode an expanded stereochemical space, but their catalytic tractability remained unprecedented. In this report, the authors report that catalyst control over pentavalent stereocentres is feasible.
Introduction
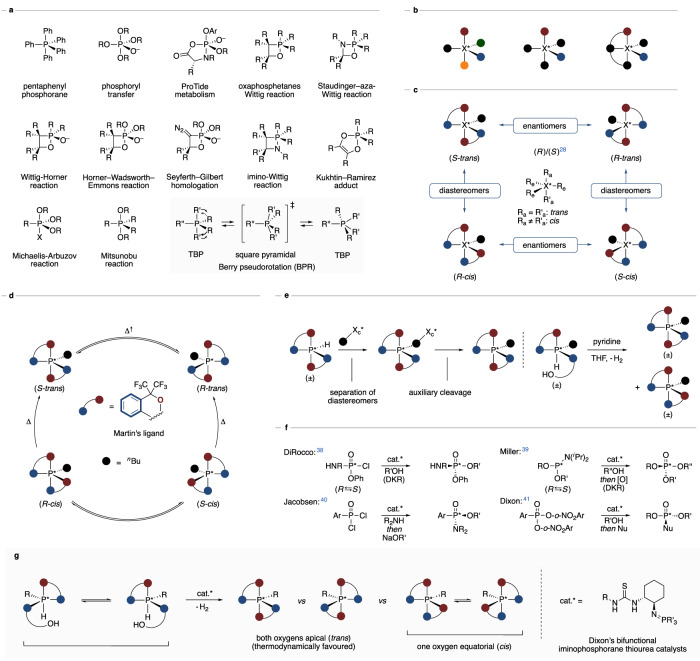
Endeavoured by Staudinger1, a pentavalent phosphorane (Ph5P) was first prepared by Wittig and Rieber to validate their stability in 1949 (Fig. 1a)2. The intermediacy of pentavalent phosphoranes for phosphoryl transfer was thereupon proposed by Westheimer3, which recently received further significance by the metabolism of ProTides4. In current synthetic methodology, a great number of indispensable transformations proceed through pentavalent phosphorane intermediates, such as the oxaphosphetanes of the Wittig reaction5, the oxazaphosphetidines of the Staudinger–aza-Wittig reaction6, or the phosphoranes of the Wittig–Horner7, Horner–Wadsworth–Emmons8 and Seyferth–Gilbert reaction9. These validated or assumed species furthermore include the azaphosphetanes of the imino-Wittig reaction10–12, the Kukhtin–Ramirez adduct13–15, the phosphoranes of the Michaelis–Arbuzov16 and the Mitsunobu reaction17, besides intermediates of other pertinent transformations.18–20 Despite the ability of pentavalent phosphoranes with their trigonal bipyramidal (TBP) structure to undergo Berry pseudorotations through square pyramidal transition states21–24, a broad variety of configurationally stable phosphoranes was obtained for relatively congested compounds or upon bidentate binding to the phosphorus centre18,19,25, for instance with the Martin ligand26–28. In contrast to the classical Le Bel–van ’t Hoff stereoisomerism of tetravalent stereocentres that results in twofold stereogenicity29,30, pentavalent stereocentres commonly give rise to an increased number of isomers emerging from a single stereocentre (Fig. 1b)31. Even with two identical unsymmetric bidentate ligands and a fifth equatorial residue, four isomers emanate from a single phosphorane stereocentre in the form of two diastereomeric pairs of enantiomers (Fig. 1c, TBP: Ra = apical, Re = equatorial). Notably, in their pioneering work, Akiba and co-workers established the configurational stability of pentavalent trans-dioxophosphoranes and further isolated the thermodynamically disfavoured anti-apicophilic cis-isomer as an ensemble of equilibrating enantiomers (Fig. 1d)32–37. Moreover, an auxiliary strategy with diastereomer separation allowed to prepare enantioenriched phosphoranes32, while hydridophosphoranes treated with pyridine were observed to provide a mixture of racemic dioxophosphorane diastereomers (Fig. 1e)33. In contrast to pentavalent stereocentres, remarkable recent strategies by the DiRocco38, Miller39, Jacobsen40 and Dixon41 groups enable to catalytically control tetravalent phosphorous (V) stereocentres by dynamic kinetic resolution (DKR) or desymmetrisation approaches (Fig. 1f).
Fig. 1. Background and concept.
a Selected pentavalent phosphoranes. b Pentavalent stereocentres. c Fourfold stereogenicity in pentavalent stereocentres. d Stereoisomerisation of pentavalent dioxophosphoranes32–37. e Auxiliary (Xc*) approaches & dehydrogenation of hydridophosphoranes. f Recent strategies to catalytically control tetravalent phosphorus stereocentres. g Catalyst control over pentavalent phosphorus stereocentres (this work).
From this background and with our interest in catalyst control over higher-order stereogenicity42, with previous emphasis on atropisomers43,44, overcrowded alkenes45 and efforts on Co-complexes46, we questioned if pentavalent stereocentres that encode more than two stereoisomers per stereogenic unit are catalytically addressable (Fig. 1g). More specifically, we envisioned a catalytic activation of rapidly racemising hydridophosphoranes37 to allow enantio- and diastereocontrol to govern the configuration of pentavalent phosphorus stereocentres. To study the tractability of pentavalent stereocentres within their extended stereochemical space, we resorted to chiral thioureas and in particular Dixon’s bifunctional iminophosphoranes47, as a multitude of diversified catalysts are accessible due to their high modularity and in situ accessibility.
Using iminophosphorane thioureas, we report herein that catalyst control over pentavalent phosphorus stereocentres is feasible by means of DKR with enantio- and diastereoselectivity, while rerouting allows to stereodivergently address all stable stereoisomeric forms of the desired dioxophosphoranes.
Results
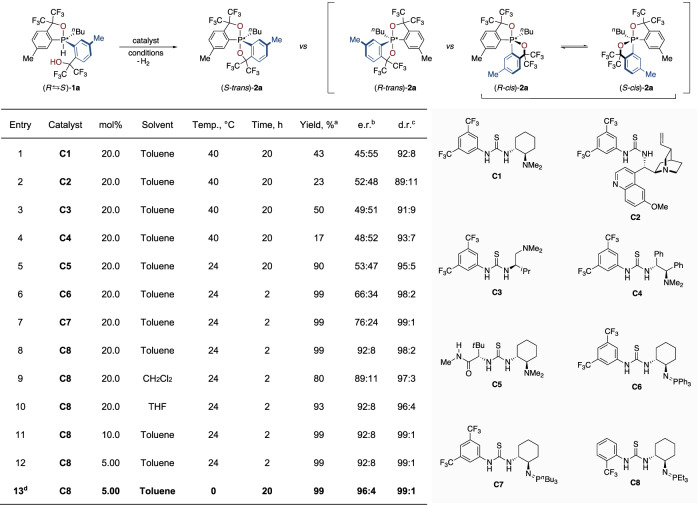
In the method development, control over the configuration of pentavalent stereocentres involved overall the evaluation of 23 known and 103 unprecedented chiral catalysts for the stereoselective dehydrogenation of hydridophosphoranes (Fig. 2 & ESI).
Fig. 2. Optimisation of the reaction conditions.
Conditions: 1a (30.1 mg, 50.0 μmol), with indicated catalysts in 5.0 mL toluene. aIsolated yield. bDetermined as (S):(R) by HPLC of isolated product on a chiral stationary phase. cDetermined as (trans):(cis) by 19F NMR analysis of the crude reaction mixture. d2.5 mL instead of 5.0 mL toluene. Optimised conditions in bold.
The Takemoto bifunctional thiourea catalyst C148 (entry 1) initially provided dioxophosphorane 2a in moderate yield (43%) and good diastereoselectivity (92:8 d.r.) in favour of the trans isomer with a slight enantioenrichment (e.r. 45:55). An improvement by variation of the linkage between the thiourea and basic amine moiety (C2–C4, entries 2–4) was not observed. The Jacobsen-type catalysts possessing an additional stereocentre (C549, entry 5) provided the product in high yield (90%), but the enantioenrichment remained modest (53:47 e.r.). However, changing the tertiary amine to Dixon’s superbasic iminophosphorane catalyst C647 (entry 6) resulted in an increased enantioselectivity (66:34 e.r.), excellent yield (99%) and a diastereoselectivity of 98:2. Briefly, further optimisation revealed that stereocontrol correlates with the catalyst’s basicity, with more basic and reactive iminophosphoranes bearing aliphatic groups giving superior results (C7, entry 7, 76:24 e.r., ESI). Gratifyingly, the introduction of a key CF3 ortho-substituted aryl moiety to the thiourea and a change to a small triethyl iminophosphorane culminated in excellent selectivities (catalyst C8, 99% yield, 92:8 e.r., 98:2 d.r., entry 8). Variation of the solvent confirmed toluene as optimal, with CH2Cl2 and THF providing slightly diminished enantio- and diastereoselectivities (entries 9–10, ESI). We were pleased to observe that high stereocontrol was maintained with a reduced catalyst loading of 5.00 mol% (entries 11–12) and that decreasing the reaction temperature to 0 °C led to further improvement of the selectivity (96:4 e.r.) (entry 13).
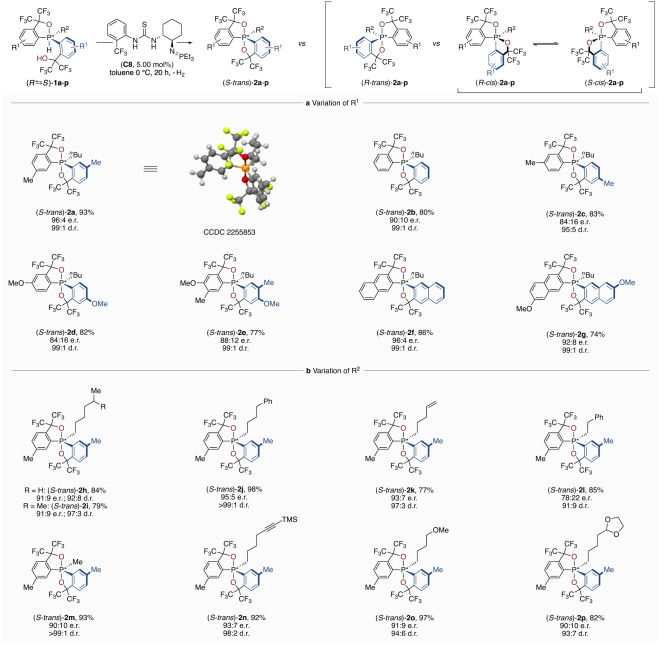
With optimised conditions in hand, we explored the scope of the catalyst control over pentavalent phosphorane stereocentres (Fig. 3). The reaction was successfully scaled to 100 μmol without affecting selectivity, providing the dioxophosphorane 2a in 93% yield with 96:4 e.r. and 99:1 d.r. X-ray crystallographic analysis of a single crystal revealed the absolute configuration as (S-trans)-2a. We continued by exploring substrates with different aryl moieties at the Martin ligand to study the influence of steric and electronic effects on the outcome of the reaction. Unsubstituted (S-trans)-2b was obtained in excellent yield and diastereoselectivity with an e.r. of 90:10, whereas other positions at the aryl ring for (S-trans)-2c-e led to a somewhat compromised level of stereocontrol. A slightly lower enantioselectivity was observed for disubstituted (S-trans)-2e (88:12 e.r., 99:1 d.r.), whereas excellent stereocontrol was reached for the naphthyl congeners (S-trans)-2f (96:4 e.r., 99:1 d.r.) and (S-trans)-2g (92:8 e.r., 99:1 d.r.). We next investigated the impact of the fifth moiety bound to the phosphorus stereocentre (Fig. 3b). High yields and selectivities were obtained for the phosphoranes bearing long primary alkyl chains ((S-trans)-2h-k, (S-trans)-2n-p). Interestingly, contraction of the linkage of (S-trans)-2l as compared to (S-trans)-2j revealed that shorter chains impact the enantiodifferentiation and yield (85%). In contrast, the reaction was highly efficient (93% yield) and selective with a small methyl substituent ((S-trans)-2m, 90:10 e.r., >99:1 d.r.). Furthermore, the method is mild and compatible with several functionalities, including alkenes ((S-trans)-2k), alkynes ((S-trans)-2n), as well as ether and acetal groups ((S-trans)-2p,o), providing diverse phosphoranes with high yield and stereoselectivity.
Fig. 3. Scope of the method.
Conditions: hydridophosphorane substrate 1a-p (100 μmol), catalyst C8 (5.00 μmol), 5.0 mL toluene, 0 °C, 20 h. Yields are given for isolated products. The d.r. was determined as (trans):(cis) by 19F NMR of the crude reaction mixture and the e.r. as (S):(R) by HPLC on a chiral stationary phase of the isolated product.
To confirm the feasibility of complete catalyst control over high-valent stereocentres, we proceeded by investigating the possibility for stereodivergent catalysis (Fig. 4). As expected, with the enantiomer of the catalyst ent-C8, the product with the opposite absolute configuration was readily obtained with inverted enantioselectivity ((R-trans)-2a, 3:97 e.r., 99:1 d.r.). In strong contrast, redirecting the diastereoselectivity proved to be distinctly challenging. After an extensive assessment of catalysts and conditions, we found that catalyst C9 with electron-poor aryl groups at the iminophosphorane moiety gives rise to reversed diastereoselectivity, albeit with a comparatively low yield and level of diastereocontrol (details in the ESI). However, upon validating the generation of H2 by NMR, we observed that palladium catalysis (Pd/C), as an alternative dehydrogenation manifold, enables a remarkable selectivity for the (cis)-2a diastereomer. Under optimised conditions, palladium on charcoal ultimately furnished the thermodynamically disfavoured diastereomer with excellent yield and diastereoselectivity (98%, 6:94 d.r.). With access to both enantiomers of the trans-configured phosphorane as well as the ensemble of equilibrating enantiomers of the anti-apicophilic (cis)-2a diastereomer, the ability for stereodivergent catalyst control over pentavalent stereocentres was conclusively verified.
Fig. 4. Stereodivergent catalyst control over pentavalent phosphorus stereocentres and stereodynamic behaviour.
The d.r. was determined as (trans): (cis) by 19F NMR of the crude reaction mixture and the e.r. as (S):(R) by HPLC on a chiral stationary phase of the isolated product.
The nature of the obtained dioxophosphoranes was next investigated by assessing their stereodynamic behaviour. To determine the rate of enantiomerisation of the thermodynamically more stable trans-isomer with both oxygens placed in apical positions, a sample of enantioenriched ((S-trans)-2a) (94:6 e.r., 99:1 d.r.) was heated to 180 °C to measure a ΔG‡180 °C of 161 kJ mol–1. The diastereoisomerisation of (cis)-2a to (trans)-2a in heptane at 30 °C proceeds with a barrier of ΔG‡30 °C = 103 kJ mol–1, whereas no reaction was observed when (cis)-2a was subjected to identical trans-selective reaction conditions, substantiating that the cis-configured phosphorane is not an intermediate in the stereocontrolled synthesis of (trans)-2a. The enantiomerisation barrier of (cis)-2a determined by 19F VT-NMR gave a ΔG‡–20 °C of 48 kJ mol–1, which underscores the rapid interconversion of this ensemble of enantiomers in agreement with the data reported by Akiba and co-workers37. Moreover, the interconversion rate of the two enantiomers of the starting material 1a was measured by coalescence 19F VT-NMR, giving a ΔG‡93 °C = 72 kJ mol–1 to verify that a DKR takes place during the catalyst-controlled synthesis of pentavalent phosphorane stereocentres.
In conclusion, the feasibility of catalyst control over pentavalent stereocentres was established by employing bifunctional iminophosphorane thiourea catalysis for the stereoselective synthesis of dioxophosphoranes, rendering an extended stereochemical space emerging from a single stereocentre synthetically addressable. Control over each viable stereoisomer was attained by a stereodivergent approach, with the diastereo- and enantiomers selectable by the choice of catalyst. Furthermore, the DKR of the substrates and the stereodynamic nature of the products were validated. It is thus anticipated that the significantly increased stereochemical space of the diverse high-valent main group species is rendered synthetically accessible with catalyst control over pentavalent stereocentres.
Methods
Enantio- and trans-diastereoselective catalyst control over pentavalent stereocentres
To a mixture of the hydridophosphorane substrate 1a-p (100 μmol, 1.00 eq.) and catalyst C8 (2.17 mg, 5.00 μmol, 5.00 mol%) in a dried 20 mL crimp cap vial under an Ar atmosphere at 0 °C was added toluene (5.0 mL). The mixture was stirred at 0 °C for 20 h and the solvent removed under reduced pressure at 10 °C to 20 °C. The d.r. was determined by 19F NMR of the residue, before it was purified by silica gel flash column chromatography (230–400 mesh) to isolate the desired product. The e.r. of the isolated product was measured by HPLC on a chiral stationary phase.
Stereodivergent cis-selective catalyst control
To a mixture of the hydridophosphorane substrate 1a (60.2 mg, 100 μmol, 1.00 eq.) and Pd/C (5.00 % wt, 42.6 mg, 20.0 μmol, 20.0 mol%) in a 20 mL crimp cap vial at 0 °C under ambient atmosphere was added MeOH (HPLC-grade, 10 mL). Three needles were inserted through the cap of the vial for gas exchange with the open atmosphere. The mixture was stirred at 0 °C for 4 days, filtered through a short pad of silica gel (230 – 400 mesh) and the silica gel was washed with MeOH (2 ×5.0 mL). The solvent was removed under reduced pressure at 10 °C–20 °C to afford the title compound as a white solid (59.0 mg, 98.3 μmol, 98%, 6:94 d.r.). At room temperature, the product undergoes gradual interconversion to (±-trans)-2a and was therefore stored at −20 °C.
Supplementary information
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 101002471). We thank T. A. Schmidt, the other members of the Sparr group and Prof. T. R. Ward for helpful discussions and Dr. A. Prescimone for X-ray crystallography.
Author contributions
C.S., A.B. and J.D. conceived the study, designed the experiments, and analysed the data. A.B. and J.D. performed the experiments and D.H. the NMR studies. C.S. wrote the manuscript with input from all authors.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
All data are available in the main text and Supplementary Information, including supplementary methods, experimental details, NMR spectra and crystallographic data. Supplementary crystallographic data for this paper can be obtained from the Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/structures (CCDC 2255853).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-43750-w.
References
- 1.Staudinger H, Meyer J. Über neue organische Phosphorverbindungen. Helv. Chim. Acta. 1919;2:612–618. doi: 10.1002/hlca.19190020162. [DOI] [Google Scholar]
- 2.Wittig G, Rieber M. Darstellung und Eigenschaften des Pentaphenyl-phosphors. Justus Liebigs Ann. Chem. 1949;562:187–192. doi: 10.1002/jlac.19495620304. [DOI] [Google Scholar]
- 3.Westheimer FH. Pseudo-rotation in the hydrolysis of phosphate esters. Acc. Chem. Res. 1968;1:70–78. doi: 10.1021/ar50003a002. [DOI] [Google Scholar]
- 4.Procházková E, Navrátil R, Janeba Z, Roithová J, Baszczyňski O. Reactive cyclic intermediates in the ProTide prodrugs activation: trapping the elusive pentavalent phosphorane. Org. Biomol. Chem. 2019;17:315–320. doi: 10.1039/C8OB02870B. [DOI] [PubMed] [Google Scholar]
- 5.Kawashima T, Kato K, Okazaki R. Novel Synthetic Route to Isolable Pentacoordinate 1,2-Oxaphosphetanes and Mechanism of Their Thermolysis, the Second Step of the Wittig Reaction. J. Am. Chem. Soc. 1992;114:4008–4010. doi: 10.1021/ja00036a078. [DOI] [Google Scholar]
- 6.Kano N, Hua XJ, Kawa S, Kawashima T. Synthesis, structure, and thermolysis of pentacoordinate 1,3,2λ5-oxazaphosphetidines: the intermediates of aza-Wittig reactions. Tet. Lett. 2000;41:5237–5241. doi: 10.1016/S0040-4039(00)00829-7. [DOI] [Google Scholar]
- 7.Horner L., Hoffmann, H. & Wippel H. G. Phosphororganische Verbindungen, XIII. Darstellung von Phosphinsäuren aus Phosphinoxyden. Chem. Ber. 91, 64–67 (1958).
- 8.Ando K. A mechanistic Study of the Horner−Wadsworth−Emmons reaction: computational investigation on the reaction pass and the stereochemistry in the reaction of lithium enolate derived from trimethyl phosphonoacetate with acetaldehyde. J. Org. Chem. 1999;64:6815–6821. doi: 10.1021/jo9909150. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert JC, Weerasooriya U. Diazoethenes: their attempted synthesis from aldehydes and aromatic ketones by way of the Horner-Emmons modification of the Wittig reaction. A facile synthesis of alkynes. J. Org. Chem. 1982;47:1837–1845. doi: 10.1021/jo00349a007. [DOI] [Google Scholar]
- 10.Bestmann HJ, Seng F. Reaction of Alkylenetriphenylphosphoranes with Schiff Bases. Angew. Chem. Int. Ed. 1963;2:393. doi: 10.1002/anie.196303931. [DOI] [Google Scholar]
- 11.Kawashima T, Soda T, Okazaki R. Synthesis, Structure, and Thermolysis of N-Apical 1,2λ5-Azaphosphetidines with a Pentacoordinate P Center and the First Observation of Their N-Equatorial Pseudorotamers. Angew. Chem. Int. Ed. 1996;35:1096–1098. doi: 10.1002/anie.199610961. [DOI] [Google Scholar]
- 12.Dong D-J, Li H-H, Tian S-K. A highly tunable stereoselective olefination of semistabilized triphenylphosphonium ylides with N-sulfonyl imines. J. Am. Chem. Soc. 2010;132:5018–5020. doi: 10.1021/ja910238f. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez F. Oxyphosphoranes. Acc. Chem. Res. 1968;1:168–174. doi: 10.1021/ar50006a002. [DOI] [Google Scholar]
- 14.Miller EJ, Zhao W, Herr JD, Radosevich AT. A nonmetal approach to α-heterofunctionalized carbonyl derivatives by formal reductive X–H insertion. Angew. Chem. Int. Ed. 2012;51:10605–10609. doi: 10.1002/anie.201205604. [DOI] [PubMed] [Google Scholar]
- 15.Calcatelli A, Denton RM, Ball LT. Modular synthesis of α,α-Diaryl α‐Amino Esters via Bi(V)-Mediated Arylation/SN2‐Displacement of Kukhtin−Ramirez Intermediates. Org. Lett. 2022;24:8002–8007. doi: 10.1021/acs.orglett.2c03201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granoth I. A stable bromoalkoxyphosphorane, a model for the postulated five-co-ordinate intermediate in the Arbuzov and related reactions. Chem. Soc., Perkin Trans. 1982;1982:735–740. doi: 10.1039/p19820000735. [DOI] [Google Scholar]
- 17.Satish Kumar N, Kommana P, Vittal JJ, Kumara Swamy KC. Pentacoordinate phosphoranes with reversed apicophilicity as stable intermediates in a Mitsunobu-type reaction. J. Org. Chem. 2002;67:6653–6658. doi: 10.1021/jo011150a. [DOI] [PubMed] [Google Scholar]
- 18.Kawashima T. In Chemistry of Hypervalent Compounds (Ed. Akiba K.-Y.) 171–210 (John Wiley & Sons Inc., 1998).
- 19.Yoshifuji M. In Organophosphorus Chemistry: From Molecules to Applications (Ed. Iaroshenko V.) 219–237 (Wiley-VCH, 2019).
- 20.Zhang X, et al. Phosphorus-mediated sp2–sp3 couplings for selective C–H fluoroalkylation of azines. Nature. 2021;594:217–222. doi: 10.1038/s41586-021-03567-3. [DOI] [PubMed] [Google Scholar]
- 21.Berry RS. Correlation of Rates of Intramolecular Tunneling Processes, with Application to Some Group V Compounds. J. Chem. Phys. 1960;32:933–938. doi: 10.1063/1.1730820. [DOI] [Google Scholar]
- 22.Meakin P, Muetterties EL, Jesson JP. Intramolecular rearrangement mechanisms in five-coordinate complexes. J. Am. Chem. Soc. 1972;94:5271–5285. doi: 10.1021/ja00770a022. [DOI] [Google Scholar]
- 23.Couzijn EPA, Slootweg JC, Ehlers AW, Lammertsma K. Stereomutation of pentavalent compounds: validating the Berry pseudorotation, redressing Ugi’s turnstile rotation, and revealing the two- and three-arm turnstiles. J. Am. Chem. Soc. 2010;132:18127–18140. doi: 10.1021/ja105306s. [DOI] [PubMed] [Google Scholar]
- 24.Mislow K. Role of pseudorotation in the stereochemistry of nucleophilic displacement reactions. Acc. Chem. Res. 1970;3:321–331. doi: 10.1021/ar50034a001. [DOI] [Google Scholar]
- 25.Hellwinkel D. Über ein erstes optisch aktives Pentaarylphosphoran. Chem. Ber. 1966;99:3642–3659. doi: 10.1002/cber.19660991132. [DOI] [Google Scholar]
- 26.Granoth I, Martin JC. A phosphoranoxide anion – direct observation and isolation of a stable model for the postulated intermediate in nucleophilic substitution at tetracoordinated phosphinoyl phosphorus. J. Am. Chem. Soc. 1978;100:5229–5230. doi: 10.1021/ja00484a064. [DOI] [Google Scholar]
- 27.Martin JC. “Frozen” Transition States: Pentavalent Carbon et al. Science. 1983;221:509–514. doi: 10.1126/science.221.4610.509. [DOI] [PubMed] [Google Scholar]
- 28.Martin JC, Balthazor TM. Stereochemical course of an associative displacement at tetracoordinate sulfur(IV) in a sulfurane of known absolute configuration. A proposed system of nomenclature for optically active pentacoordinate species. J. Am. Chem. Soc. 1977;99:152–162. doi: 10.1021/ja00443a029. [DOI] [Google Scholar]
- 29.Le Bel JA. Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bull. Soc. Chim. Fr. 1874;22:337–347. [Google Scholar]
- 30.Van ‘t Hoff JH. Sur les formules de structure dans l’espace. Arch. Neerl. Sci. Exactes Nat. 1874;9:445–454. [Google Scholar]
- 31.Dunitz JD, Prelog V. Ligand Reorganization in the Trigonal Bipyramid. Angew. Chem. Int. Ed. 1968;7:659–746. doi: 10.1002/anie.196807251. [DOI] [Google Scholar]
- 32.Kojima S, Kajiyama K, Akiba K-Y. Characterization of an optically active pentacoordinate phosphorane with asymmetry only at phosphorus. Tet. Lett. 1994;35:7037–7040. doi: 10.1016/0040-4039(94)88219-3. [DOI] [Google Scholar]
- 33.Kojima S, Kajiyama K, Nakamoto M, Akiba K-Y. First Characterization of a 10-P-5 Spirophosphorane with an Apical Carbon−Equatorial Oxygen Ring. Kinetic Studies on Pseudorotation of Stereoisomers. J. Am. Chem. Soc. 1996;118:12866–12867. doi: 10.1021/ja9621408. [DOI] [Google Scholar]
- 34.Kajiyama K, et al. Selective One-Pot Synthesis of Spirophosphoranes Exhibiting Reversed Apicophilicity by Oxidation of Dianions Generated from P−H Spirophosphorane. Org. Lett. 2001;3:1873–1875. doi: 10.1021/ol015927y. [DOI] [PubMed] [Google Scholar]
- 35.Kojima S, Sugino M, Matsukawa S, Nakamoto M, Akiba K-Y. First Isolation and Characterization of an Anti-Apicophilic Spirophosphorane Bearing an Oxaphosphetane Ring: A Model for the Possible Reactive Intermediate in the Wittig Reaction. J. Am. Chem. Soc. 2002;124:7674–7675. doi: 10.1021/ja0170145. [DOI] [PubMed] [Google Scholar]
- 36.Kajiyama K, Yoshimune M, Kojima S, Akiba K-Y. A new method for the formation of anti-apicophilic (O-cis) spirophosphoranes – kinetic studies on the stereomutation of O-cis arylspirophosphoranes to their O-trans isomers. Eur. J. Org. Chem. 2006;2006:2739–2746. doi: 10.1002/ejoc.200600031. [DOI] [Google Scholar]
- 37.Kojima S, Kajiyama K, Nakamoto M, Matsukawa S, Akiba K-Y. The ligand-exchange process of P–Hapical Phosphoranes and the thermal formation and pseudorotation of anti-apicophilic Spiro-phosphoranes. Eur. J. Org. Chem. 2006;2006:218–234. doi: 10.1002/ejoc.200500510. [DOI] [Google Scholar]
- 38.DiRocco DA, et al. A multifunctional catalyst that stereoselectively assembles prodrugs. Science. 2017;356:426–430. doi: 10.1126/science.aam7936. [DOI] [PubMed] [Google Scholar]
- 39.Featherston AL, et al. Catalytic asymmetric and stereodivergent oligonucleotide synthesis. Science. 2021;371:702–707. doi: 10.1126/science.abf4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forbes KC, Jacobsen EN. Enantioselective hydrogen-bond-donor catalysis to access diverse stereogenic-at-P(V) compounds. Science. 2022;376:1230–1236. doi: 10.1126/science.abp8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Formica M, et al. Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters. Nat. Chem. 2023;15:714–721. doi: 10.1038/s41557-023-01165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The stereogenicity of (irreducible) stereogenic units allows the prediction of the number of stereoisomers according to an extended Le Bel–Van ’t Hoff rule, as: ... (s: stereogenicity, n: number of stereogenic units with the specific stereogenicity). Schmidt T. A. & Sparr C. Catalyst Control over Twofold and Higher-Order Stereogenicity by Atroposelective Arene Formation. Acc. Chem. Res. 54, 2764–2774 (2021). [DOI] [PubMed]
- 43.Wu X, et al. Catalyst control over sixfold stereogenicity. Nat. Catal. 2021;4:457–462. doi: 10.1038/s41929-021-00615-z. [DOI] [Google Scholar]
- 44.Schmidt TA, Schumann S, Ostertag A, Sparr C. Catalyst control over threefold stereogenicity: selective synthesis of atropisomeric sulfones with stereogenic C−S axes. Angew. Chem. Int. Ed. 2023;62:e202302084. doi: 10.1002/anie.202302084. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt TA, Sparr C. Catalyst-controlled stereoselective Barton–Kellogg Olefination. Angew. Chem. Int. Ed. 2021;60:23911–23916. doi: 10.1002/anie.202109519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt TA, Sparr C. Photocatalytic deracemisation of cobalt(III) complexes with fourfold stereogenicity. Chem. Commun. 2022;58:12172–12175. doi: 10.1039/D2CC05196F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Formica M, Rozsar D, Su G, Farley AJM, Dixon DJ. Bifunctional iminophosphorane superbase catalysis: applications in organic synthesis. Acc. Chem. Res. 2020;53:2235–2247. doi: 10.1021/acs.accounts.0c00369. [DOI] [PubMed] [Google Scholar]
- 48.Okino T, Hoashi Y, Takemoto Y. Enantioselective Michael reaction of malonates to nitroolefins catalyzed by bifunctional organocatalysts. J. Am. Chem. Soc. 2003;125:12672–12673. doi: 10.1021/ja036972z. [DOI] [PubMed] [Google Scholar]
- 49.Fuerst DE, Jacobsen EN. Thiourea-catalyzed enantioselective cyanosilylation of ketones. J. Am. Chem. Soc. 2005;127:8964–8965. doi: 10.1021/ja052511x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text and Supplementary Information, including supplementary methods, experimental details, NMR spectra and crystallographic data. Supplementary crystallographic data for this paper can be obtained from the Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/structures (CCDC 2255853).